The COVID-19 virus has been associated with heart disease. Reports have shown an increased incidence of arrhythmias following COVID-19. In fact, cardiac injury is suggested to be a driver of the arrhythmogenic substrate, and some case reports showed development of ventricular arrhythmias following COVID-19 in acute settings. However, recent literature is defining a new entity: “long-haul” COVID, as many patients are reporting at least one residual symptom after COVID-19 resolution. 1 In this context, we report the case of a patient who developed ventricular tachycardia after COVID-19 resolution, presenting as a cardiac manifestation of long-haul COVID-19.
Case Presentation
A 29-year-old female patient with a prior medical history of multiple sclerosis (relapsing-remitting, last treated in the 10 years prior) presented for new-onset palpitations a few days following recovery from COVID-19 infection. She had no smoking history, denied illicit substance use, and used alcohol occasionally. Family history was positive for diabetes and hyperlipidemia. She did not have any family history of arrhythmia.
The patient was first diagnosed with COVID-19 in 2020 when she presented with flu-like symptoms (fever, myalgias, nasal congestion, and cough with productive sputum). Diagnosis was confirmed with a polymerase chain reaction (PCR) test for COVID-19. She received only symptomatic treatment (ondansetron and ibuprofen), without any antiviral medication, and was symptomatic for 9 days. She had a residual cough that disappeared on day 14. She was not hospitalized for her symptoms.
A few days following the resolution of her COVID-19 symptoms, she presented to her primary care physician for new-onset intermittent palpitations that occurred spontaneously without precipitating factors. Given that she had had persistent symptoms for the preceding 7 months, she was referred to a pulmonologist and a cardiologist. She underwent pulmonary function tests and spirometry, all of which had normal results. Primary pulmonary conditions were ruled out by her pulmonologist, and a 14-day Holter monitor (iRhythm Technologies, Inc, San Francisco, CA) recording was performed prior to her cardiology appointment.
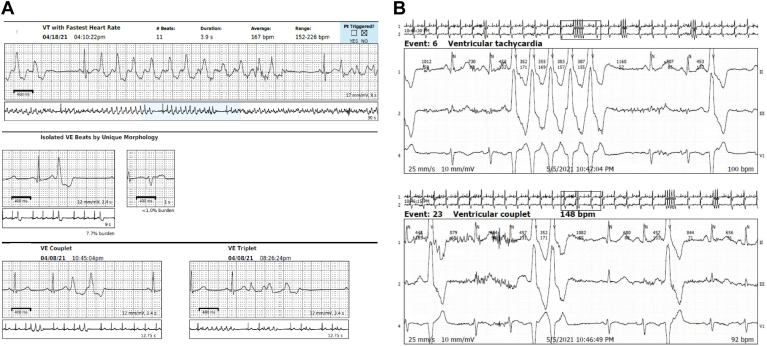
The patient was fitted with a rhythm monitor (Zio XT patch; iRhythm Technologies, Inc) for 2 weeks that showed 13,485 ventricular tachycardia (VT) runs, the longest lasting 30.5 seconds, with an average rate of 181 beats per minute. VT was detected within +/- 45 seconds of symptomatic patient event(s). Isolated ventricular ectopics were frequent (premature ventricular contractions [PVCs] burden = 6.2%). The patient’s average heart rate on the monitor was 79 beats per minute (minimum 45, maximal 231). The patient subsequently consulted a cardiologist and was started on metoprolol XL. She additionally underwent delayed-enhancement magnetic resonance imaging, which revealed mild delayed enhancement at the basal-to-mid right ventricle insertion point in a nonischemic pattern, thought to be nonspecific (Supplemental Fig. S1). A repeat Zio XT patch showed 23,567 VT runs, and the PVC burden was 7.7% (Fig. 1A). Following these results, the patient was hospitalized (14 months post-COVID).
Figure 1.
Strips from the (A) Zio XT patch (iRhythm Technologies, Inc, San Francisco, CA) and (B) the Holter monitor (iRhythm Technologies, Inc) showing episodes of ventricular tachycardia (VT) and premature ventricular contractions (PVCs). bpm, beats per minute.
During her hospitalization, an 18-hour Holter monitor showed 37 VT runs, with the longest lasting 6 beats (Fig. 1B). Flecainide was added and titrated up to 75 mg twice a day. A repeat 22.5-hour Holter monitor on flecainide showed 0 VT runs, with a PVC burden decrease to 2%. The patient underwent repeat cardiac magnetic resonance imaging, with findings similar to those in her previous study. The delayed enhancement was nonspecific. A positron emission tomography scan showed no evidence of scarred myocardium, and sarcoid positron emission tomography showed no evidence of active inflammation, with diffuse myocardial fluorodeoxyglucose uptake, no myocardial scar, and normal left ventricle ejection fraction. The patient was hospitalized for a total of 6 days. Note that the patient initially did not want to undergo an ablation.
One month after hospitalization, the patient had a repeat Zio XT patch, off flecainide, as she had discontinued the drug for personal reasons. The latter showed 1263 VT runs, with a PVC burden of 6.5% burden. She was reinstated on flecainide 75 mg twice a day. Due to persistent symptoms, the dose of flecainide was eventually increased to 150 mg, 20-months post-COVID. A repeat Zio XT patch, on flecainide 150 mg, showed 0 VT runs, with a PVC burden of 7.8%.
Two years after COVID-19 infection, and 10 months after flecainide initiation, the patient has only occasional palpitations, with a drastic improvement of symptoms. A repeat Zio XT patch, on flecainide 150 mg, showed 0 VT runs, with a PVC burden of 4.5%. After an asymptomatic period, the patient had an increased PVC burden eventually on flecainide, estimated on a Holter monitor at 30%. An electrocardiogram capturing the PVCs is shown in Supplemental Figure S2. The patient eventually underwent an electrophysiology study followed by successful electrophysiology ablation. The area of earliest activation was localized in the septal right ventricular outflow tract (RVOT) that was ablated. No PVCs were detected after ablation, even after atropine administration.
Snapshots from the Zio XT patch and the Holter monitors are included in Supplemental Figure S3.
A full timeline of the patient’s presentation is summarized in Table 1.
Table 1.
Timeline of patient’s presentation from COVID-19 diagnosis until the latest cardiology encounter
| Day 0 |
COVID-19 onset FLU-like symptoms managed by symptomatic treatment |
| Day 9 |
COVID-19 resolution Flu-like symptoms resolution with residual cough |
| Day 14 | Cough resolution |
| Month 1 |
Palpitations onset Palpitations ≈ 5 times / day, unrelated to activity or any other precipitating factor |
| Month 7 |
Primary care physician encounter Patient first presented to a physician as symptoms became uncontrollable Referral to a pulmonologist: physical examination, and spirometry and pulmonary function tests were normal Continuous Holter monitor ordered and referral to cardiology |
| Month 7.5–8 |
First Zio XT patch Report: 13,485 VT runs +/- 45 s of symptom onset, PVC burden 6.2% |
| Month 9 |
First cardiology encounter Given Zio XT patch report, metoprolol 25 mg XL was started Cardiac DE-MRI: mild delayed enhancement at the basal-to-mid RV insertion points in a nonischemic pattern |
| Month 12.5 |
Follow-up appointment Persistent palpitations → metoprolol increased to 37.5 mg XL |
| Month 13–13.5 |
Second Zio XT patch Report: 23,567 VT runs +/- 45 s of symptom onset, PVC burden 7.7% |
| Month 14 |
6-days hospitalization 18-hour Holter: 37 VT runs, PVC burden 7.7% Flecainide initiation and titration to 75 mg twice a day Repeat 22.5-h Holter: 0 VT runs, PVC burden 2% Repeat DE-MRI: mild delayed enhancement at the basal-to-mid RV insertion points in a nonspecific, nonischemic pattern PET/CT cardiac perfusion: no evidence of scarred myocardium PET/CT cardiac viability: no evidence of active inflammation with diffuse myocardial FDG uptake and no myocardial scar |
| Month 15–15.5 |
Repeat Zio XT patch off-flecainide Report: 1263 VT runs, PVC burden 6.5% |
| Month 16.5 |
Follow-up cardiology encounter Flecainide 75 mg twice a day maintenance |
| Month 18–22 |
Palpitations persistence Palpitations still bothersome for the patient Flecainide dose increased to 150 mg twice a day |
| Month 23– 23.5 |
Repeat Zio XT patch Report: O VT runs, PVC burden 7.8% |
| Month 24 |
Echocardiography Report: Normal with mild LV dilation, EF = 65% |
| Month 24.5–25 |
Repeat Zio XT patch Report: 0 VT runs, PVC burden 4.5 % |
| Month 28 |
Latest cardiology encounter Symptoms resolution—very occasional palpitations on flecainide 150 mg |
| Month 35 |
48-h Holter monitor Report: 0 VT runs, PVC burden 30% |
| Month 43 |
Electrophysiology with ablation Catheter ablation around septal RVOT |
Manufacturer Information: Zio XT patch (iRhythm Technologies, Inc, San Francisco, CA); Holter monitor (iRhythm Technologies, Inc, San Francisco, CA).
CT, computed tomography; DE-MRI, delayed enhancement magnetic resonance imaging; EF, ejection fraction; FDG, fluorodeoxyglucose; LV, left ventricle; PET: positron emission tomography; PVC, premature ventricular contraction; RV, right ventricle; RVOT, right ventricle outflow tract; VT, ventricular tachycardia.
Discussion
More and more data are emerging regarding the incidence of new-onset cardiovascular conditions in patients previously infected with COVID-19. Furthermore, the information on COVID-19 causing arrhythmogenesis is quite meager. Some authors suggested that the virus has a myocardial tropism and can cause ventricular dysfunction, including ventricular arrhythmias.2 Some papers report an increased incidence of arrhythmias in critically ill patients, owing to COVID-19.3 Some cases were reported of new-onset VT in patients infected with COVID-19, occurring during infection, with most having underlying comorbidities, such as coronary artery disease, left ventricle dysfunction, atrial arrhythmias, thyroid conditions, and myocarditis. Those cases are summarized in Supplemental Table S1. Most of the VT runs occurred following the administration of QTc-prolonging medications, such as hydroxychloroquine, and resolved after cessation of the drugs. Existing literature documents primarily cases of ventricular arrhythmias manifesting in the acute phase of COVID-19, either during the infection or shortly thereafter. The case presented here, however, is distinctive due to the delayed onset of arrhythmia, representing the phenomenon of long-haul COVID. Furthermore, this case illustrates a gradual evolution of ventricular arrhythmia over a 3-year period, suggesting the potential for long-term cardiac sequelae after COVID infection in a previously healthy patient. At this time, little is known regarding VT incidence in long-haul COVID-19 patients and its management. Previously, palpitations were reported to be frequent residual findings in patients previously diagnosed with COVID-19,4 but without confirmation of arrhythmia on Holter monitoring.
Our case report is unique, as it describes a patient with documented VT runs as a long-term complication of COVID-19 infection. Furthermore, our case report is the first to show the incidence of VT in a patient with no history of cardiovascular disease. Three previously reported cases had cardiovascular comorbidities; one had fulminant myocarditis at the time of VT occurrence; one had a masked arrhythmogenic right ventricular cardiomyopathy (ARVC); and the last one had thyroid imbalance when VT occurred. The timing of symptom onset in our patient, immediately after COVID-19 symptoms resolution, and the absence of any previous cardiovascular history suggest that COVID-19 could be the cause of her VT onset. Another hypothesis is that the patient had an idiopathic RVOT-related arrhythmia that was precipitated by her COVID-19 infection and presented as a long-haul COVID symptom. Although the patient did not exhibit overt symptoms of autonomic dysfunction, a careful analysis of her monitoring data revealed significant heart rate fluctuations. This observation is particularly relevant in the context of COVID-19, which has been linked to dysautonomia and disruptions in autonomic tone, including heightened adrenergic activity. Outflow tract VTs (OT VTs), known for their sensitivity to autonomic tone variations, may be implicated in this scenario. Additionally, a plausible possibility to consider is that an increase in adrenergic stimulation could exacerbate any underlying ventricular arrhythmia burden. Therefore, a reasonable hypothesis is that the patient's COVID-19 infection may have precipitated a latent RVOT-related arrhythmia.
Many mechanisms are hypothesized to be involved in cardiac injury and arrhythmia occurrence after COVID 19 infection. First, the virus is thought to have a direct toxic effect on the myocardial cells, causing infective myocarditis. In fact, pathologic reports on COVID-19-infected hearts showed few interstitial mononuclear inflammatory infiltrates in the myocardial interstitium.5 Another theory regarding the effect of the virus on the heart is its tropism to angiotensin-converting enzyme.6 Higher angiotensin levels in COVID patients might lead to cardiac fibrosis. Finally, the cytokine storm, triggered by imbalanced type 1 and 2 T-helper cell activity, and the inflammatory environment might be involved in the development of structural remodelling in the heart and subsequent arrhythmia.7 However, a succinct explanation of COVID-19-mediated cardiac effects remains elusive.
Novel Teaching Points.
The case of a patient who presented for palpitations with prior medical history of COVID-19 infection aids in the following:
-
•
making differential diagnoses of arrhythmias associated with long COVID-19 infection;
-
•
using new digital health tools such as the Zio XT patch to provide continuous rhythm monitoring, and to incorporate those recordings in guiding therapeutic management;
-
•
treatment of long COVID-19-associated arrhythmias to provide symptoms relief.
Acknowledgments
Ethics Statement
The report adhered to institutional review board and ethics committee.
Patient Consent
The authors confirm that a patient consent form has been obtained for this article.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 724 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2024.01.010.
Supplementary Material
References
- 1.Sykes D.L., Holdsworth L., Jawad N., et al. Post-COVID-19 symptom burden: What is long-COVID and how should we manage it? Lung. 2021;199:113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu K., Fang Y.Y., Deng Y., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson P.J., Liu-Fei F., Ng C., et al. Characterization of COVID-19-associated cardiac injury: evidence for a multifactorial disease in an autopsy cohort. Lab Invest. 2022;102:814–825. doi: 10.1038/s41374-022-00783-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beyerstedt S., Casaro E.B., Rangel É.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.