Abstract
Background
Balancing the effects of dual antiplatelet therapy (DAPT) in the era of potent purinergic receptor type Y, subtype 12 (P2Y12) inhibitors remains a challenge in the management of acute coronary syndrome (ACS).
Methods
We conducted a systematic review and meta-analysis following a 2-stage process consisting of searching for systematic reviews published between 2019 and November 2022. We included randomized controlled trials (RCTs) of ACS patients treated with percutaneous coronary intervention comparing (i) ticagrelor- vs prasugrel-based DAPT and (ii) P2Y12 inhibitor de-escalation strategies. Outcomes of interest were major adverse cardiovascular events (MACE), all-cause death, stent thrombosis, and major bleeding. We estimated risk ratios (RRs) and 95% confidence intervals (CIs) using a random-effects model.
Results
Eight RCTs (n = 5571) compared ticagrelor to prasugrel. Ticagrelor was associated with an increased risk of MACE compared to prasugrel (RR 1.23, 95% CI 1.01-1.49, moderate certainty), without significant differences in death, stent thrombosis, or major bleeding. In 2 RCTs (n = 3343) comparing clopidogrel-based DAPT de-escalation after 1 month to potent P2Y12 inhibitor–based DAPT continuation, clopidogrel de-escalation did not significantly alter the incidence of MACE, death, or stent thrombosis, but reduced that of major bleeding (RR 0.51, 95% CI 0.28-0.92, high certainty). The effect of prasugrel dose de-escalation was inconclusive for all outcomes based on one trial.
Conclusions
Ticagrelor was associated with an increase in MACE compared with prasugrel, based on low-certainty evidence, whereas de-escalation to clopidogrel after 1 month of potent P2Y12 inhibitor was associated with a decrease in incidence of major bleeding without increasing thrombotic outcomes in ACS patients post-percutaneous coronary intervention.
Résumé
Contexte
Équilibrer les effets de la bithérapie antiplaquettaire (BTAP) à l’ère des puissants inhibiteurs du récepteur purinergique de type Y, sous-type 12 (P2Y12) demeure un défi dans la prise en charge du syndrome coronarien aigu (SCA).
Méthodologie
Nous avons procédé à un examen systématique et à une méta-analyse, tout d’abord en recherchant les revues systématiques publiées entre 2019 et 2022, puis en mettant à jour la recherche la plus complète de ces revues jusqu’en novembre 2022. Nous avons inclus des essais contrôlés randomisés (ECR) menés chez des patients ayant subi un SCA traité par intervention coronarienne percutanée qui comparaient i) une BTAP comportant du ticagrélor à une BTAP à base de prasugrel, et ii) les stratégies de réduction graduelle de la dose de l’inhibiteur de P2Y12. Les résultats d’intérêt comprenaient les événements cardiovasculaires indésirables majeurs (ECIM), les décès toutes causes confondues, les thromboses de l’endoprothèse et les saignements majeurs. Nous avons estimé les rapports de risques (RR) et les intervalles de confiance (IC) à 95 % à l’aide d’un modèle à effets aléatoires.
Résultats
Huit ECR (n = 5 571) ont comparé le ticagrélor au prasugrel. Le ticagrélor était associé à un risque accru d’ECIM comparativement au prasugrel (RR de 1,23, IC à 95 % de 1,01 à 1,49, certitude modérée), sans différence significative quant au décès, à la thrombose de l’endoprothèse ou au saignement majeur. Dans deux ECR (n = 3 343) comparant la réduction graduelle de la BTAP à base de clopidogrel après 1 mois à la poursuite de la BTAP comportant un inhibiteur puissant de P2Y12, la réduction graduelle de la dose de clopidogrel n’a pas modifié de manière significative la fréquence des ECIM, des décès ou des thromboses de l’endoprothèse, mais a réduit celle des saignements majeurs (RR de 0,51, IC à 95 % de 0,28 à 0,92, certitude élevée). L’effet de la réduction graduelle de la dose de prasugrel n’a pas été concluant pour tous les résultats sur la base d’un seul essai.
Conclusions
Si l’on se fie aux données probantes de faible certitude, le ticagrélor a été associé à une augmentation des ECIM comparativement au prasugrel, tandis que la réduction graduelle de la dose de clopidogrel après 1 mois d’administration d’un puissant inhibiteur de P2Y12 a été associée à une diminution de la fréquence des saignements majeurs sans augmentation des événements thrombotiques chez les patients ayant subi une intervention coronarienne percutanée pour traiter un SCA.
Dual antiplatelet therapy (DAPT) combining acetylsalicylic acid and a purinergic receptor type Y, subtype 12 (P2Y12) inhibitor is the treatment of choice in patients with acute coronary syndrome (ACS) treated with percutaneous coronary intervention (PCI).1 Current guidelines recommend the use of the potent oral P2Y12 inhibitors (ticagrelor and prasugrel) over clopidogrel in the first year after ACS in patients treated with PCI,1, 2, 3 as these agents have stronger platelet inhibition activity, leading to reductions in ischemic events at the expense of an increased bleeding risk.4,5 However, Canadian guidelines do not make specific recommendations favouring one potent P2Y12 inhibitor over another. Additionally, emerging strategies that use a potent P2Y12 inhibitor-based DAPT followed by de-escalation to clopidogrel-based DAPT or a lower dose of a potent P2Y12 inhibitor-based DAPT may optimally balance thrombotic and bleeding risk.
Consequently, the optimal choice and duration of potent P2Y12 inhibitor therapy in ACS patients post-PCI remains unclear. Therefore, we conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to compare the efficacy and safety of (i) ticagrelor- vs prasugrel-based DAPT, and (ii) P2Y12 inhibitor de-escalation-DAPT strategies in ACS patients treated with PCI.
Methods
We conducted an abbreviated systematic review and meta-analysis following a 2-stage process. First, we searched for all recent systematic reviews that addressed any of the predefined clinical questions for this topic (outlined below), and extracted all relevant RCTs from these systematic reviews. We subsequently identified and updated the most comprehensive search strategies from available systematic reviews. Two authors re-extracted all data from the original RCT articles. This report follows the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.6
Search strategies and data source
To identify relevant systematic reviews, we searched PubMed covering the period from January 1, 2019 to November 22, 2022 (the search strategy is given in Supplemental Appendix S1). We then refined and updated the most comprehensive search among these meta-analyses for each clinical question, and then updated these searches to identify RCTs published after the search date of those respective reviews. Minor optimizations were made to these searches, and RCT filters were used to increase the specificity of results. Supplemental Appendix S1 describes the full search strategies for systematic reviews and additional RCTs.
Study selection, eligibility criteria, and data extraction
We included RCTs that enrolled patients who underwent PCI for ACS and made one of the following comparisons: (i) ticagrelor- vs prasugrel-based DAPT; or (ii) initial potent P2Y12 inhibitor for 1 month followed by de-escalation (either de-escalation to clopidogrel or to a lower-dose P2Y12 inhibitor) vs continuation of the potent P2Y12 inhibitor. We reported on at least one outcome of interest (outlined below) at up to 12 months of follow-up.
One reviewer performed all database searches and imported the records into Covidence (Covidence, Australia). Using Covidence, 2 reviewers independently screened article titles and abstracts, and reviewed full-text articles for inclusion. The same 2 reviewers independently extracted the following data from each study, using a standardized data-collection form: study acronym; lead author; publication year; sample size; baseline characteristics; intervention characteristics; and data on all outcomes (number of participants with events and total number of participants in each group in the intention-to-treat population). No instances of disagreement between the reviewers occurred.
Assessment of risk of bias and certainty of evidence
Two reviewers independently evaluated trial-level risk of bias (RoB) using the Cochrane RoB 2 tool.7 Once again, no instances of disagreement between the reviewers occurred. One reviewer then rated outcome-level certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework,8 which incorporates risk of bias, imprecision, inconsistency, indirectness, and publication bias.
Outcomes
The outcomes of interest were as follows: (i) major adverse cardiovascular events (MACE; composite of death from any cause, myocardial infarction [MI], or stroke—when this composite was not available, we used the composite of cardiovascular death, MI, or stroke, or a broader composite that encompasses additional components); (ii) all-cause mortality; (iii) stent thrombosis (definite and/or probable based on original Academic Research Consortium (ARC)9 or ARC-210 definition); and (iv) major bleed (Bleeding Academic Research Consortium [BARC]11 3 or 5 bleed; when information using this classification was not available, we preferentially extracted bleeding information based on the International Society on Thrombosis and Haemostasis [ISTH],12 followed by the Thrombolysis in Myocardial Infarction [TIMI] definition).4,13
Statistical analysis
We pooled dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs) using a DerSimonian-Laird inverse variance random-effects model for all outcomes. We evaluated statistical heterogeneity with visual inspection of the forest plot and quantified the percentage of the variability that is due to heterogeneity between trials using the I2 statistic.
We prespecified sex as a subgroup of interest; however, sex was reported in only 1 trial (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5 [ISAR-REACT 5]) and could therefore not be meta-analyzed. In a post hoc sensitivity analysis, we compared the results of the ISAR-REACT 5 trial to those of the remaining trials.14 As the ISAR-REACT 5 trial also contained subgroup data on those who did not receive PCI, we included this subgroup analysis within the post hoc sensitivity analysis.
We conducted all analyses using Review Manager version 5.4 (Cochrane, Copenhagen, Denmark).
Results
Ticagrelor- vs prasugrel-based DAPT
Characteristics of included studies
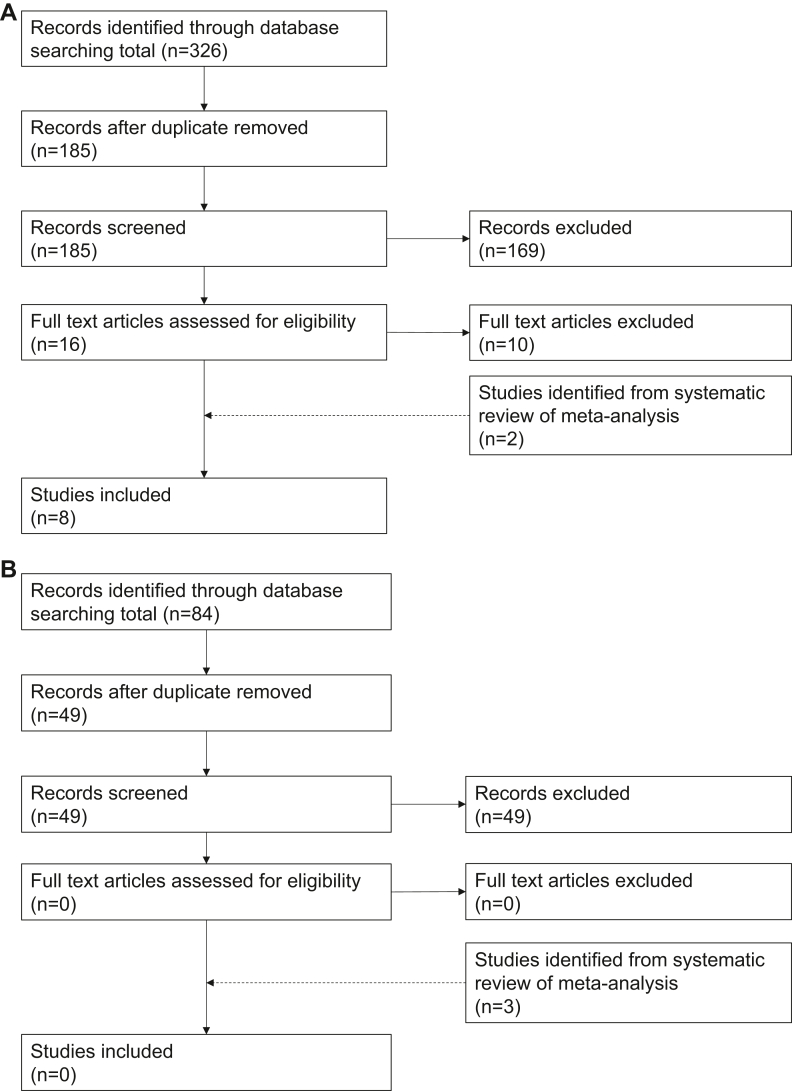
The initial search identified 9 systematic reviews with meta-analysis comparing ticagrelor- to prasugrel-based DAPT in ACS patients undergoing PCI (Fig. 1).15, 16, 17, 18, 19, 20, 21, 22, 23 From these systematic reviews, we identified 6 relevant RCTs.14,24, 25, 26, 27, 28 We updated the search strategy from the most recent and comprehensive systematic review15 to November 2022. From the 326 additional records identified, we identified 2 additional RCTs for inclusion.29,30 We contacted and obtained additional outcome data from the authors of the Downstream vs Upstream Strategy for the Administration of P2Y12 Receptor Blockers in Non-ST Elevated Acute Coronary Syndromes With Initial Invasive Indication (DUBIUS) trial.29 Across all 8 included RCTs (n = 5571), the mean age was 64 years, and 42% of participants were women (see Table 1 for key characteristics).
Figure 1.
Study selection for comparison of (A) ticagrelor vs prasugrel, and (B) purinergic receptor type Y, subtype 12 (P2Y12) inhibitor de-escalation vs continuation of potent P2Y12 inhibitor.
Table 1.
Characteristics of trials comparing ticagrelor to prasugrel
| Characteristic | ISAR-REACT 5 (PCI subgroup, n = 3377) | ISAR-REACT 5 (Non-PCI subgroup, n = 641) | PRAGUE-18 (n = 1230) | DUBIUS trial∗ (n = 438) | Bonello et al.25 (2015) (n = 213) |
REDUCE-MVI trial (n = 108) | Laine et al.26 (2014) (n = 100) |
Alexopoulos et al.24 (2012) (n = 55) |
RAPID trial (n = 50) |
|---|---|---|---|---|---|---|---|---|---|
| Age, y, mean | 64.6 | 64.8 | 61.8 | 65.0 | 60.7 | 60.6 | 63.8 | 59.4 | 67.0 |
| Women | 21.2 | 37.9 | 24.5 | 24.4 | 25.2 | 15.4 | 24 | 19.9 | 22.0 |
| Prior MI | 15.5 | 16.9 | 8.3 | 17.5 | 11.5 | — | — | — | 8.0 |
| DES | 89.5 | — | 65.2 | — | 96 | 100.0 | 79.5 | — | — |
| Index event | |||||||||
| Unstable angina | 8.2 | 36.3 | — | 21.0 | — | — | 19.4 | — | — |
| NSTEMI | 45.4 | 50.4 | 5.4 | 79.0 | 49.8 | — | 80.6 | — | — |
| STEMI | 46.5 | 13.3 | 89.5 | — | — | 100.0 | 100.0 | 100.0 | |
| MACE definition | All-cause death, MI, or stroke | CV death, nonfatal MI, or nonfatal stroke | Death from vascular causes, non-fatal MI, or non-fatal stroke | CV death, MI, urgent revascularization, and stroke | All cause death, MI | CV death, MI, and stroke | — | MI | |
| Major bleeding definition | BARC 3–5 | BARC 3–5 | BARC 3–5 | BARC 3–5 | —– | BARC 3–5 | — | TIMI Major | |
| Follow-up duration | 12 mo | 12 mo | 1 mo | 1 mo | 12 mo | in-hospital (mean, 3 d) | 5 d | in-hospital | |
Values are %, unless otherwise indicated.
BARC, Bleeding Academic Research Consortium; CV, cardiovascular; DES, drug-eluting stent; DUBIUS, Downstream vs Upstream Strategy for the Administration of P2Y12 Receptor Blockers In Non-ST Elevated Acute Coronary Syndromes With Initial Invasive Indication; ISAR-REACT 5, Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5; MACE, major adverse cardiac events; MI, myocardial infarction; NSTEMI, non-STEMI; PCI, percutaneous coronary intervention; PRAGUE-18, Comparison of Prasugrel and Ticagrelor in the Treatment of Acute Myocardial Infarction; RAPID, Rapid Activity of Platelet Inhibitor Drugs; REDUCE-MVI, Reducing Microvascular Dysfunction in Revascularized STEMI Patients by Off-Target properties of Ticagrelor; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Baseline characteristics from the total DUBIUS trial cohort; only 30% of these patients were treated with PCI.
Risk of bias and certainty of evidence
We rated one RCT as having a low risk of bias,14 6 RCTs as having some concerns regarding bias,24, 25, 26,28, 29, 30 and 1 RCT as having a high risk of bias (Supplemental Fig. S1).27 The ISAR-REACT 5 trial,14 which contributed ≥ 60% of the weight for all outcomes, was rated as having a low risk of bias. The Comparison of Prasugrel and Ticagrelor in the Treatment of Acute Myocardial Infarction (PRAGUE-18)27 trial received the second-highest weight and had some concerns regarding bias due to a lack of information on allocation concealment and possible deviations from the intended intervention.
We rated the certainty of evidence as follows: low for MACE, due to serious imprecision as well as serious inconsistency between the ISAR-REACT 5 trial and other trials (Supplemental Fig. S2); moderate for all-cause death due to serious imprecision; low for stent thrombosis due to very serious imprecision; and moderate for major bleeding due to serious imprecision. The GRADE certainty of evidence, along with relative and absolute estimates of effect, is provided in Table 2.
Table 2.
Summary-of-findings table
| Effect estimate | |||
|---|---|---|---|
| Outcome | Certainty of evidence | RR (95% CI) | Absolute change, per 1000 |
| Ticagrelor vs prasugrel | |||
| MACE | Low∗† | 1.23 (1.01–1.49) | 15 more (from 1 to 31 more) |
| Death | Moderate∗ | 1.12 (0.86–1.46) | — |
| Stent thrombosis | Low‡ | 1.21 (0.74–1.98) | — |
| Major bleed | Moderate† | 1.01 (0.78–.132) | — |
| De-escalation to clopidogrel vs potent P2Y12 inhibitor | |||
| MACE | Moderate∗ | 0.76 (0.55–1.06) | — |
| Death | Low‡ | 0.75 (0.21–2.69) | — |
| Stent thrombosis | Low‡ | 1.17 (0.39–3.47) | — |
| Major bleed | High | 0.51 (0.28–0.92) | 9 fewer (from 2 to 14 fewer) |
| De-escalation of prasugrel dose to 5 mg daily vs continuation of prasugrel 10 mg daily | |||
| MACE | Low‡ | 0.76 (0.40–1.45) | — |
| Death | Low‡ | 0.71 (0.32–1.60) | — |
| Stent thrombosis | Low‡ | 0.33 (0.03–3.19) | — |
| Major bleed | Low‡ | 1.12 (0.43–2.90) | — |
CI, confidence interval, MACE, major adverse cardiac events; P2Y12, purinergic receptor type Y, subtype 12; RR, risk ratio.
Rated down 1 category for serious imprecision.
Rated down 1 category for serious inconsistency.
Rated down 2 categories for very serious imprecision.
Outcomes
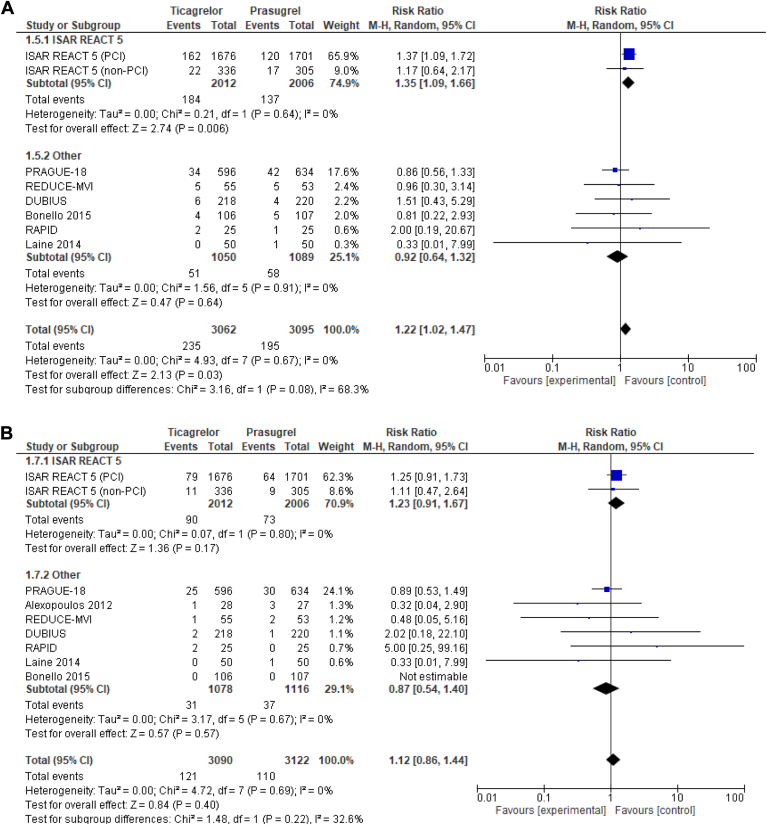
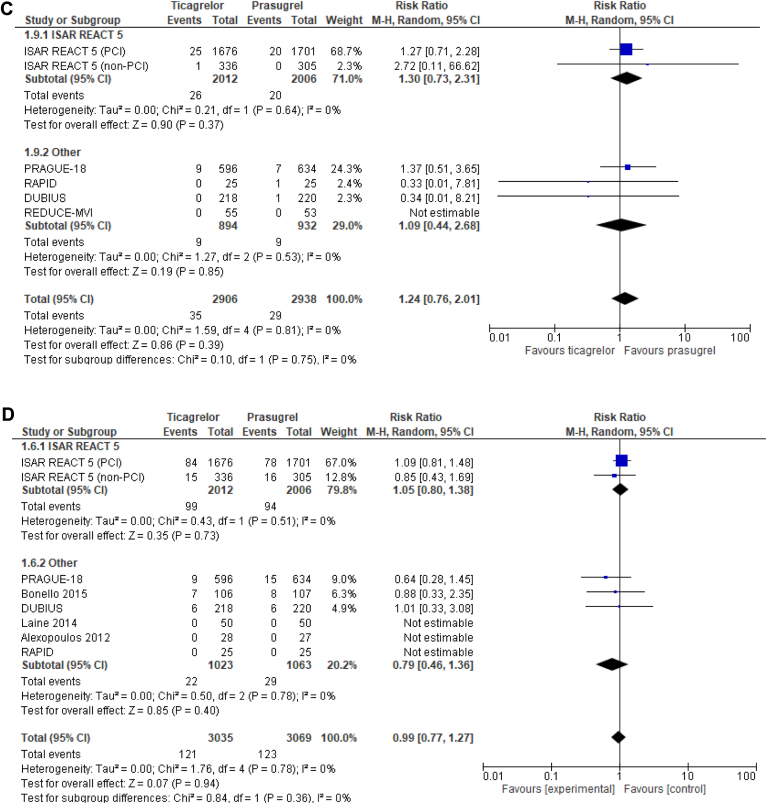
Seven trials reported on the risk of MACE (Fig. 2A). Ticagrelor was associated with an increased risk of MACE, compared to prasugrel (RR 1.23, 95% CI 1.01-1.49, I2 = 0%). No significant differences were present between ticagrelor and prasugrel in all-cause death (RR 1.12; 95% CI 0.86-1.46, I2 = 0%; Fig. 2B), stent thrombosis (RR 1.21; 95% CI 0.74-1.98, I2 = 0%; Fig. 2C), or major bleeding (RR 1.01, 95% CI 0.78-1.32, I2 = 0%; Fig. 2D).
Figure 2.
Forest plot of (A) major adverse cardiovascular events, (B) all-cause death, (C) stent thrombosis, and (D) major bleeding with ticagrelor-, compared with prasugrel-based, dual antiplatelet therapy in patients with acute coronary syndrome treated with percutaneous coronary intervention (PCI). CI, confidence interval; df, degrees of freedom; DUBIUS, Downstream vs Upstream Strategy for the Administration of P2Y12 Receptor Blockers In Non-ST Elevated Acute Coronary Syndromes With Initial Invasive Indication; ISAR-REACT 5, Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 5; M-H, Mantel–Haenszel; PRAGUE-18, Comparison of Prasugrel and Ticagrelor in the Treatment of Acute Myocardial Infarction; RAPID, Rapid Activity of Platelet Inhibitor Drugs; REDUCE-MVI, Reducing Microvascular Dysfunction in Revascularized STEMI Patients by Off-Target properties of Ticagrelor.
Results did not differ when the ISAR-REACT 5 trial non-PCI subgroup was added, and no significant interaction occurred based on this subgroup in the ISAR-REACT 5 trial for MACE, all-cause death, or major bleeding (Supplemental Fig. S2).
DAPT dose and conversion de-escalation strategies
Characteristics of included studies
We identified 7 systematic reviews addressing empiric P2Y12 inhibitor dose and conversion de-escalation strategies in patients with ACS treated with PCI (Fig. 1).31, 32, 33, 34, 35, 36, 37 From these meta-analyses, we identified 2 RCTs (Ticagrelor vs Clopidogrel in Stabilized Patients With Acute Myocardial Infarction [TALOS-AMI] and Timing of Platelet Inhibition After ACS [TOPIC])38,39 that address de-escalation of potent P2Y12 inhibitors to clopidogrel at 1 month post-ACS (DAPT conversion de-escalation), and 1 trial that addresses prasugrel dose de-escalation (5 mg daily) after 1 month of prasugrel 10 mg daily (Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases Trial - Comparison of Reduction of Prasugrel Dose or Polymer Technology in ACS Patients [HOST-REDUCE-POLYTECH-ACS]) (DAPT dose de-escalation).40 No trials evaluated dose de-escalation of ticagrelor. An updated search (Supplemental Appendix S1) including 84 records identified no additional RCTs. Across all 3 included de-escalation RCTs, the mean age was 60 years, and 14% of participants were women. Key characteristics of the 3 RCTs are shown in Table 3.
Table 3.
Characteristics of purinergic receptor type Y, subtype 12 (P2Y12) inhibitor de-escalation trials
| Characteristic | TALOS-AMI trial (n = 2697) | TOPIC trial (n = 646) | HOST-REDUCE-POLYTECH-ACS trial (n = 2338) |
|---|---|---|---|
| Age, y, mean | 60 | 60 | 58.8 |
| Women | 16.8 | 17.6 | 10.7 |
| PCI received | 100 | 97.5 | 100 |
| DES | 100 | 90.6 | 100 |
| Index event | |||
| Unstable angina | 0 | 60.2 | 60.8 |
| NSTEMI | 45.6 | 25.3 | |
| STEMI | 54 | 39.8 | 14 |
| Timing from PCI to de-escalation, mo | 1 | 1 | 1 |
| Treatment prior to de-escalation | Ticagrelor 90 mg BID | Ticagrelor 90 mg BID or prasugrel 10 mg | Prasugrel 10 mg |
| De-escalation intervention | Clopidogrel 75 mg | Clopidogrel 75 mg | Prasugrel 5 mg |
| MACE definition | Cardiovascular death, MI, or stroke | Cardiovascular death, unplanned revascularisation, stroke | All-cause death, MI, repeat revascularization |
| Major bleed definition | BARC 3,5 | TIMI Major | BARC 3–5 |
| Follow-up duration, months | 12 | 12 | 12 |
Values are %, unless otherwise indicated.
BARC, Bleeding Academic Research Consortium; BID, twice a day; DES, drug-eluting stent; HOST-REDUCE-POLYTECH-ACS, Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases Trial - Comparison of Reduction of Prasugrel Dose or Polymer Technology in ACS Patients; MACE, major adverse cardiac events; MI, myocardial infarction; NSTEMI, non-STEMI; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TALOS-AMI, Ticagrelor vs Clopidogrel in Stabilized Patients With Acute Myocardial Infarction; TIMI, Thrombolysis in Myocardial Infarction; TOPIC, Timing of Platelet Inhibition After Acute Coronary Syndrome.
Risk of bias and certainty of evidence
We rated the TALOS-AMI trial39 as having a low risk of bias, despite its being an open-label trial, due to the employment of bias-proof outcomes, high adherence to assigned treatment, and the use of blinded outcome adjunction (Supplemental Fig. S1). We rated the TOPIC trial38 as having a high risk of bias, due to concerns regarding performance bias (86% adherence in the de-escalation group vs 75% in the continuation group). Notably, patients in the de-escalation group received clopidogrel plus acetylsalicylic acid in a combination tablet, whereas those in the usual-care group received either ticagrelor or prasugrel in combination with aspirin as separate tablets, which could have influenced adherence. The HOST-REDUCE-POLYTECH-ACS trial had some concerns regarding bias, due to possible nonprotocol deviations from the intended treatment (6% of patients did not de-escalate therapy despite their assignation to receive de-escalation).40
For DAPT-conversion de-escalation, we rated the certainty of evidence as follows: moderate for MACE, due to serious imprecision; low for death and stent thrombosis, due to very serious imprecision; and high for major bleeding (Table 2). For DAPT-dose de-escalation, we rated all outcomes as having low certainty of evidence, due to very serious imprecision (Table 2).
Outcomes
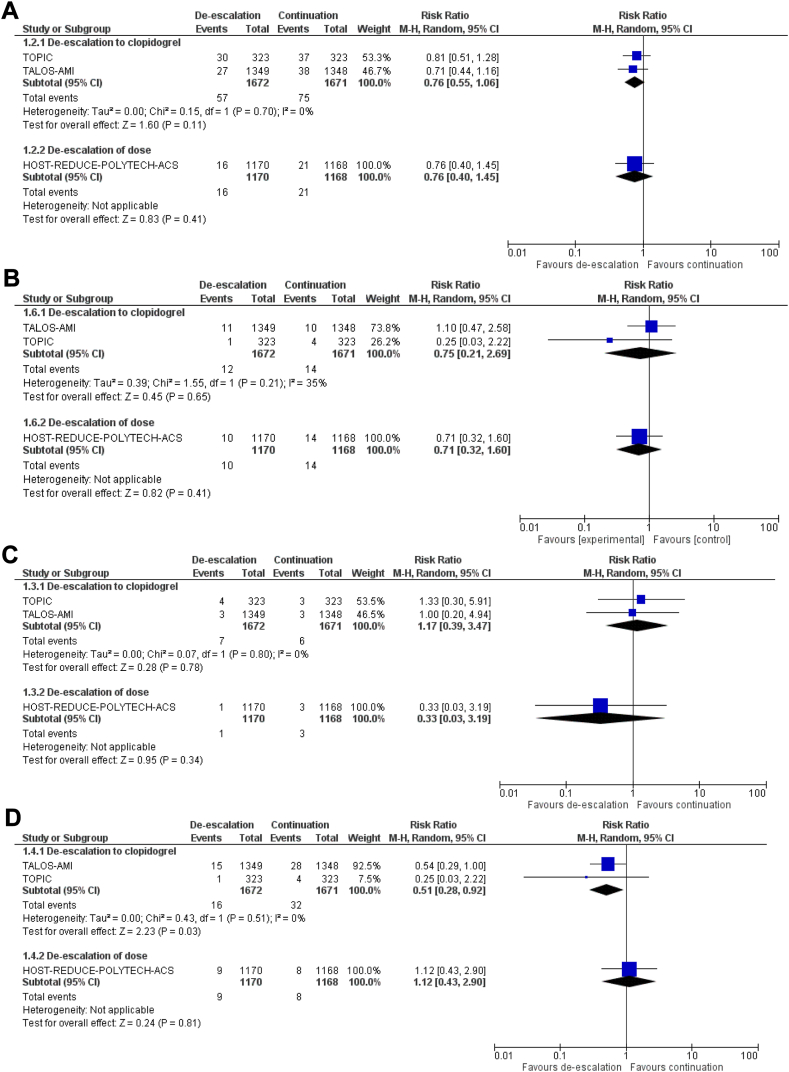
DAPT-conversion de-escalation did not increase the risk of MACE (RR 0.76, 95% CI 0.55-1.06, I2 = 0%; Fig. 3A), all-cause death (RR 0.75, 95% CI 0.21-2.69, I2 = 35%; Fig. 3B), or stent thrombosis (RR 1.17, 95% CI 0.39-3.47, I2 = 0%; Fig. 3C), compared to continuation of potent P2Y12 inhibitor. DAPT-conversion de-escalation reduced the risk of major bleeding (RR 0.51, 95% CI 0.28-0.92; I2 = 0%; Fig. 3D), compared to continuation of a potent P2Y12 inhibitor.
Figure 3.
Forest plot of (A) major adverse cardiovascular events, (B) all-cause death, (C) stent thrombosis, and (D) major bleeding with purinergic receptor type Y, subtype 12 (P2Y12) inhibitor de-escalation vs continuation of potent P2Y12 inhibitor, stratified by de-escalation strategy. CI, confidence interval; df, degrees of freedom; HOST-REDUCE-POLYTECH-ACS, Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases Trial - Comparison of Reduction of Prasugrel Dose or Polymer Technology in ACS Patients; M-H, Mantel–Haenszel; TALOS-AMI, Ticagrelor Versus Clopidogrel in Stabilized Patients With Acute Myocardial Infarction; TOPIC, Timing of Platelet Inhibition After Acute Coronary Syndrome.
DAPT-dose de-escalation was evaluated in only 1 trial of 2338 patients comparing de-escalation to prasugrel 5 mg daily 1 month after PCI for ACS, vs continuation of prasugrel 10 mg daily.40 No differences between groups occurred in MACE (RR 0.76, 95% CI 0.40-1.45; Fig. 3A), all-cause death (RR 0.71, 95% CI 0.32-1.60; Fig. 3B), stent thrombosis (RR 0.33, 95% CI 0.03-3.19; Fig. 3C), or major bleeding (RR 1.12, 95% CI 0.43-2.90; Fig. 3D).
Discussion
This meta-analysis addressed the optimal choice and duration of potent P2Y12 inhibitor therapy in ACS patients post-PCI. First, ticagrelor-based DAPT was associated with an increased risk of MACE, without increasing major bleeding, compared to prasugrel-based DAPT in ACS patients treated with PCI, driven by the results of the ISAR-REACT 5 trial. Second, DAPT-conversion de-escalation 1 month post-PCI in ACS patients reduced the risk of major bleeding without increasing the risk of thrombotic events. Third, only 1 trial assessed DAPT-dose de-escalation, which provided inconclusive effects on thrombotic and bleeding outcomes.
The results of the meta-analysis comparing ticagrelor to prasugrel were driven by the ISAR-REACT 5 trial, which found an increased risk of MACE with ticagrelor vs prasugrel.14 However, several limitations in the study design and its organization must be acknowledged, including the following: the open-label design; the fact that it was performed in only 2 countries; the differential medication discontinuation (due to more intolerable adverse effects with ticagrelor); and the high proportion of patients who were not treated with the assigned medication (∼20% of patients not discharged on their assigned P2Y12 inhibitor).14 The ISAR-REACT 5 trial also included patients without PCI for the index event, which represented about 16% of the population, although results were consistent without subgroup interaction.14 The reduction in MACE with prasugrel, as compared to ticagrelor, in the ISAR-REACT 5 trial has yet to be replicated. A recent observational analysis of the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART) registry did not find a difference between prasugrel and ticagrelor,41 although this study was limited by exposure ascertainment being based only on P2Y12 inhibitor prescribed at discharge. Other trials included in this systematic review were underpowered and had short follow-up relative to that in the ISAR-REACT 5 trial. The second largest trial, PRAGUE-18, did not find a significant difference in any outcome with ticagrelor- or prasugrel-based DAPT at 30 days or 1 year.27 However, the PRAGUE-18 trial was stopped early owing to futility. Additionally, groups differed in their need to switch to clopidogrel because of financial strain (34% with prasugrel and 44% with ticagrelor), as some centres provided reimbursement for prasugrel only.2 The ongoing registry-based, step-wedge, cluster randomized Switching from Ticagrelor to Prasugrel in Patients with Acute Coronary Syndrome (SWITCH)-SWEDEHEART trial may provide a more definitive comparison of prasugrel vs ticagrelor.42 Two recent meta-analyses examined ticagrelor- vs prasugrel-based DAPT in ACS patients (independently of whether PCI was performed) and found conflicting results. Farmakis et al.23 found that, in non ST-elevation-ACS patients, prasugrel-based DAPT is more efficacious than ticagrelor-based DAPT, whereas Fong et al.21 found that, in ACS patients, prasugrel- and ticagrelor-based DAPT had a similar efficacy as ticagrelor-based DAPT. By including 2 additional RCTs,29,30 this present meta-analysis represents the most comprehensive and updated evidence from RCTs assessing prasugrel-based DAPT vs ticagrelor-based DAPT specifically in ACS patients post-PCI.
Current clinical guidelines recommend the use of DAPT with potent P2Y12 inhibitors (ticagrelor or prasugrel) for 1 year after PCI in ACS patients.1 However, the greatest benefit regarding prevention of ischemic events lies in the initial phase (30 days post-ACS), whereas bleeding events accrue in both the acute and chronic (beyond 30 days post-ACS) phases.43,44 These observations underscore the need to identify optimal DAPT strategies for balancing ischemic and bleeding risk, which may include more-potent DAPT initially, with earlier de-escalation. The small TOPIC trial was the first to evaluate de-escalation of clopidogrel, and it did not find any statistically significant differences in ischemic events, but it did find a reduction in major bleeding with this strategy 1 month post-PCI for patients with ACS.38 However, this study had differential study drug discontinuation, which may have been attributable to ticagrelor-related dyspnea and a greater risk of major bleeding in the comparator group, as well as use of a fixed-dose combination antiplatelet pill in the intervention group.38 Reassuringly, the TALOS-AMI trial replicated this reduction in major bleeding without an increase in thrombotic events in the setting of > 97% adherence to allocated treatment.39 The HOST-REDUCE-POLYTECH-ACS trial was the only one that assessed the safety and efficacy of prasugrel dose de-escalation to 5 mg daily starting 1 month after PCI, in South Korean ACS patients; it found that this therapy was noninferior to continuation of prasugrel 10 mg daily in preventing the composite of all-cause death, nonfatal MI, stroke, and major bleeding (attributable to the reduction in major bleeding without an increase in ischemic events).40 However, the conclusion of noninferiority was based on a generous noninferiority margin that allowed an up to 2.5% absolute risk increase in this primary outcome, which included competing thrombotic and bleeding events, biasing the results toward noninferiority. Limitations of this study are that the median body weight was around 72 kg, and all patients were East Asians. Whether prasugrel-dose de-escalation to 5 mg daily 1 month after PCI would have the same efficacy among a more diverse population is unclear.45 Therefore, this question warrants further study in North American and European populations. A recent meta-analysis by Khan et al. examined randomized and nonrandomized evidence of DAPT de-escalation to monotherapy with a single P2Y12 inhibitor or aspirin 1-3 months post-PCI (independently of ACS status).33 They found that de-escalation of DAPT (1-3 months) to monotherapy with a P2Y12 inhibitor, instead of aspirin, did not increase cardiovascular mortality, but it did significantly decrease bleeding events, compared with 12 months of DAPT in patients with PCI and a drug-eluting stent.33 Thus, our meta-analysis represents the most updated comprehensive evidence from RCTs assessing DAPT dose and de-escalation strategies specifically in ACS patients post-PCI.
Our study does have limitations. First, this is a meta-analysis of trial-level data, which limited our ability to explore patient subgroups, such as comparisons based clinical presentation and procedural characteristics. Second, we excluded studies that evaluated selection of P2Y12 inhibitors guided by pharmacogenomics or platelet function testing, as these are not recommended by guidelines and are not routinely used in Canadian practice. Third, we did not identify any trial to date that assessed the use of de-escalation to low-dose ticagrelor. One ongoing trial (Ticagrelor De-escalation Strategy in East Asian Patients With AMI [EASTYLE]; NCT04755387) is investigating this strategy, with study completion planned for January 2024. Fourth, ethnic differences were not considered in this meta-analysis. Fifth, the definition of MACE varied across the included trials.
Conclusion
In ACS patients treated with PCI for whom DAPT with potent P2Y12 inhibitors is initially considered, ticagrelor-based DAPT is associated with an increased risk of MACE, compared with prasugrel-based DAPT, based on low-certainty evidence. De-escalation from a potent P2Y12 inhibitor to clopidogrel at 1 month was associated with a decreased risk of major bleeding without increasing thrombotic events, whereas evidence was insufficient for de-escalating the dose of a potent P2Y12 inhibitor in ACS patients initially treated with a full-dose potent P2Y12 inhibitor.
Acknowledgments
Ethics Statement
The research reported has adhered to the relevant ethical guidelines.
Patient Consent
The authors confirm that patient consent is not applicable to this article. There is no identifiable patient information reported.
Funding Sources
Laurie-Anne Boivin-Proulx is supported by a grant from the University of Ottawa Heart Institute Foundation and the Whit & Heather Tucker Endowed Funds (Ottawa, Canada). The other authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Editorial Disclaimer
Given her role as Editor-in-Chief, Michelle Graham had no involvement in the peer review of this article and has no access to information regarding its peer review.
Footnotes
See page 686 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2023.11.024.
Supplementary Material
References
- 1.Mehta S.R., Bainey K.R., Cantor W.J., et al. 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol. 2018;34:214–233. doi: 10.1016/j.cjca.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Valgimigli M., Bueno H., Byrne R.A., et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for Dual Antiplatelet Therapy in Coronary Artery Disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 3.Levine G.N., McEvoy J.W., Fang J.C., et al. Management of patients at risk for and with left ventricular thrombus: a scientific statement from the American Heart Association. Circulation. 2022;146:e205–e223. doi: 10.1161/CIR.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott S.D., Antman E.M., Gibson C.M., et al. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel thrombolysis in myocardial infarction 38 (TRITON-TIMI 38) Am Heart J. 2006;152:627–635. doi: 10.1016/j.ahj.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Wallentin L., Becker R.C., Budaj A., et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 6.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 8.Balshem H., Helfand M., Schünemann H.J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip D.E., Windecker S., Mehran R., et al. Clinical end points in coronary stent trials. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Garcia H.M., McFadden E.P., Farb A., et al. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 consensus document. Circulation. 2018;137:2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 11.Mehran R., Rao S.V., Bhatt D.L., et al. Standardized bleeding definitions for cardiovascular clinical trials. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 12.Schulman S., Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 13.Bovill E.G., Terrin M.L., Stump D.C., et al. Hemorrhagic events during therapy with recombinant tissue-type plasminogen activator, heparin, and aspirin for acute myocardial infarction. Ann Intern Med. 1991;115:256–265. doi: 10.7326/0003-4819-115-4-256. [DOI] [PubMed] [Google Scholar]
- 14.Schüpke S., Neumann F.J., Menichelli M., et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381:1524–1534. doi: 10.1056/NEJMoa1908973. [DOI] [PubMed] [Google Scholar]
- 15.Ullah W., Ali Z., Sadiq U., et al. Meta-analysis comparing the safety and efficacy of prasugrel and ticagrelor in acute coronary syndrome. Am J Cardiol. 2020;132:22–28. doi: 10.1016/j.amjcard.2020.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Fei Y., Lam C.K., Cheung B.M.Y. Efficacy and safety of newer P2Y(12) inhibitors for acute coronary syndrome: a network meta-analysis. Sci Rep. 2020;10 doi: 10.1038/s41598-020-73871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarese E.P., Khan S.U., Kołodziejczak M., et al. Comparative efficacy and safety of oral P2Y(12) inhibitors in acute coronary syndrome: network meta-analysis of 52,816 patients from 12 randomized trials. Circulation. 2020;142:150–160. doi: 10.1161/CIRCULATIONAHA.120.046786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahid I., Nizam M.A., Motiani V., et al. Efficacy and safety of oral P2Y12 inhibitors in older patients with acute coronary syndrome: a frequentist network meta-analysis. Drugs Aging. 2021;38:1003–1016. doi: 10.1007/s40266-021-00896-w. [DOI] [PubMed] [Google Scholar]
- 19.Montalto C., Morici N., Munafò A.R., et al. Optimal P2Y12 inhibition in older adults with acute coronary syndromes: a network meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. 2022;8:20–27. doi: 10.1093/ehjcvp/pvaa101. [DOI] [PubMed] [Google Scholar]
- 20.Khan M.S., Memon M.M., Usman M.S., et al. Prasugrel vs. ticagrelor for acute coronary syndrome patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Am J Cardiovasc Drugs. 2019;19:465–476. doi: 10.1007/s40256-019-00337-5. [DOI] [PubMed] [Google Scholar]
- 21.Fong L.C.W., Lee N.H.C., Yan A.T., Ng M.Y. Comparison of prasugrel and ticagrelor for patients with acute coronary syndrome: a systematic review and meta-analysis. Cardiology. 2022;147:1–13. doi: 10.1159/000520673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray A., Najmi A., Khandelwal G., Jhaj R., Sadasivam B. Prasugrel versus ticagrelor in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a systematic review and meta-analysis of randomized trials. Cardiovasc Drugs Ther. 2021;35:561–574. doi: 10.1007/s10557-020-07056-z. [DOI] [PubMed] [Google Scholar]
- 23.Farmakis I.T., Zafeiropoulos S., Doundoulakis I., et al. Comparative efficacy and safety of oral P2Y(12) inhibitors after non-ST-elevation acute coronary syndromes: a network meta-analysis. Open Heart. 2022;9 doi: 10.1136/openhrt-2021-001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexopoulos D., Xanthopoulou I., Gkizas V., et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2012;5:797–804. doi: 10.1161/CIRCINTERVENTIONS.112.972323. [DOI] [PubMed] [Google Scholar]
- 25.Bonello L., Laine M., Cluzel M., et al. Comparison of ticagrelor versus prasugrel to prevent periprocedural myonecrosis in acute coronary syndromes. Am J Cardiol. 2015;116:339–343. doi: 10.1016/j.amjcard.2015.04.050. [DOI] [PubMed] [Google Scholar]
- 26.Laine M., Frère C., Toesca R., et al. Ticagrelor versus prasugrel in diabetic patients with an acute coronary syndrome. A pharmacodynamic randomised study. Thromb Haemost. 2014;111:273–278. doi: 10.1160/TH13-05-0384. [DOI] [PubMed] [Google Scholar]
- 27.Motovska Z., Hlinomaz O., Kala P., et al. One-year outcomes of prasugrel versus ticagrelor in acute myocardial infarction treated with primary angioplasty: the PRAGUE-18 study. J Am Coll Cardiol. 2017 doi: 10.1161/CIRCULATIONAHA.117.027476. [DOI] [PubMed] [Google Scholar]
- 28.Parodi G., Valenti R., Bellandi B., et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol. 2013;61:1601–1606. doi: 10.1016/j.jacc.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Tarantini G., Mojoli M., Varbella F., et al. Timing of oral P2Y(12) inhibitor administration in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2020;76:2450–2459. doi: 10.1016/j.jacc.2020.08.053. [DOI] [PubMed] [Google Scholar]
- 30.van der Hoeven N.W., Janssens G.N., Everaars H., et al. Platelet inhibition, endothelial function, and clinical outcome in patients presenting with ST-segment-elevation myocardial infarction randomized to ticagrelor versus prasugrel maintenance therapy: long-term follow-up of the REDUCE-MVI trial. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdelazeem B., Shehata J., Abbas K.S., et al. De-escalation from prasugrel or ticagrelor to clopidogrel in patients with acute coronary syndrome managed with percutaneous coronary intervention: an updated meta-analysis of randomized clinical trials. Am J Cardiovasc Drugs. 2021;22:287–298. doi: 10.1007/s40256-021-00504-7. [DOI] [PubMed] [Google Scholar]
- 32.Shoji S., Kuno T., Fujisaki T., et al. De-escalation of dual antiplatelet therapy in patients with acute coronary syndromes. J Am Coll Cardiol. 2021;78:763–777. doi: 10.1016/j.jacc.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Khan S.U., Khan M.Z., Khan M.S., et al. De-escalation of antiplatelets after percutaneous coronary intervention: a Bayesian network meta-analysis of various de-escalation strategies. Eur Heart J Cardiovasc Pharmacother. 2021;7:209–215. doi: 10.1093/ehjcvp/pvaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kheiri B., Osman M., Abdalla A., et al. De-escalation of antiplatelet therapy in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a meta-analysis of randomized clinical trials. J Cardiovasc Pharmacol Ther. 2018;24:153–159. doi: 10.1177/1074248418809098. [DOI] [PubMed] [Google Scholar]
- 35.Hong J., Turgeon R.D., Pearson G.J. Switching to clopidogrel in patients with acute coronary syndrome managed with percutaneous coronary intervention initially treated with prasugrel or ticagrelor: systematic review and meta-analysis. Ann Pharmacother. 2019;53:997–1004. doi: 10.1177/1060028019845334. [DOI] [PubMed] [Google Scholar]
- 36.Guo C., Li M., Lv Y.H., Zhang M.B., Wang Z.L. De-escalation versus standard dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Platelets. 2020;31:15–25. doi: 10.1080/09537104.2019.1574969. [DOI] [PubMed] [Google Scholar]
- 37.Hu M.J., Tan J.S., Gao X.J., Yang J.G., Yang Y.J. De-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome: an updated meta-analysis and trial sequential analysis of 21 studies and 38,741 patients. J Cardiovasc Pharmacol. 2022;79:873–886. doi: 10.1097/FJC.0000000000001252. [DOI] [PubMed] [Google Scholar]
- 38.Cuisset T., Deharo P., Quilici J., et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070–3078. doi: 10.1093/eurheartj/ehx175. [DOI] [PubMed] [Google Scholar]
- 39.Kim C.J., Park M.W., Kim M.C., et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet. 2021;398:1305–1316. doi: 10.1016/S0140-6736(21)01445-8. [DOI] [PubMed] [Google Scholar]
- 40.Kim H.S., Kang J., Hwang D., et al. Durable polymer versus biodegradable polymer drug-eluting stents after percutaneous coronary intervention in patients with acute coronary syndrome: the HOST-REDUCE-POLYTECH-ACS trial. Circulation. 2021;143:1081–1091. doi: 10.1161/CIRCULATIONAHA.120.051700. [DOI] [PubMed] [Google Scholar]
- 41.Venetsanos D., Träff E., Erlinge D., et al. Prasugrel versus ticagrelor in patients with myocardial infarction undergoing percutaneous coronary intervention. Heart. 2021;107:1145–1151. doi: 10.1136/heartjnl-2020-318694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omerovic E., Erlinge D., Koul S., et al. Rationale and design of Switch Swedeheart: a registry-based, stepped-wedge, cluster-randomized, open-label multicenter trial to compare prasugrel and ticagrelor for treatment of patients with acute coronary syndrome. Am Heart J. 2022;251:70–77. doi: 10.1016/j.ahj.2022.05.017. [DOI] [PubMed] [Google Scholar]
- 43.Antman E.M., Wiviott S.D., Murphy S.A., et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction) analysis. J Am Coll Cardiol. 2008;51:2028–2033. doi: 10.1016/j.jacc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Becker R.C., Bassand J.P., Budaj A., et al. Bleeding complications with the P2Y12 receptor antagonists clopidogrel and ticagrelor in the platelet inhibition and patient outcomes (PLATO) trial. Eur Heart J. 2011;32:2933–2944. doi: 10.1093/eurheartj/ehr422. [DOI] [PubMed] [Google Scholar]
- 45.Shoji S., Sawano M., Sandhu A.T., et al. Ischemic and bleeding events among patients with acute coronary syndrome associated with low-dose prasugrel vs standard-dose clopidogrel treatment. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.