Abstract
Background
Ongoing debate remains regarding optimal antithrombotic therapy in patients with atrial fibrillation (AF) and coronary artery disease.
Methods
We performed a systematic review and meta-analysis to synthesize randomized controlled trials (RCTs) comparing the following: (i) dual-pathway therapy (DPT; oral anticoagulant [OAC] plus antiplatelet) vs triple therapy (OAC and dual-antiplatelet therapy) after percutaneous coronary intervention (PCI) or acute coronary syndrome (ACS), and (iii) OAC monotherapy vs DPT at least 1 year after PCI or ACS. Following a 2-stage process, we identified systematic reviews published between 2019 and 2022 on these 2 clinical questions, and we updated the most comprehensive search for additional RCTs published up to October 2022. Outcomes of interest were major adverse cardiovascular events (MACE), death, stent thrombosis, and major bleeding. We estimated risk ratios (RRs) and 95% confidence intervals (CIs) using a random-effects model.
Results
Based on 6 RCTs (n = 10,435), DPT reduced major bleeding (RR 0.62, 95% CI 0.52-0.73) and increased stent thrombosis (RR 1.55, 95% CI 1.02-2.36), vs triple therapy after PCI or medically-managed ACS, with no significant differences in MACE and death. In 2 RCTs (n = 2905), OAC monotherapy reduced major bleeding (RR 0.66, 95% CI 0.49-0.91) vs DPT in AF patients with remote PCI or ACS, with no significant differences in MACE or death.
Conclusions
In patients with AF and coronary artery disease, using less-aggressive antithrombotic treatment (DPT after PCI or ACS, and OAC alone after remote PCI or ACS) reduced major bleeding, with an increase in stent thrombosis with recent PCI. These results support a minimalist yet personalized antithrombotic strategy for these patients.
Résumé
Contexte
La question du traitement antithrombotique optimal chez les personnes présentant une fibrillation auriculaire (FA) et une coronaropathie demeure controversée.
Méthodologie
Nous avons réalisé une revue systématique et une méta-analyse pour synthétiser les essais contrôlés randomisés ayant comparé i) la bithérapie (anticoagulant oral et antiplaquettaire) et la trithérapie (anticoagulant oral et bithérapie antiplaquettaire) après une intervention coronarienne percutanée (ICP) ou un syndrome coronarien aigu (SCA), et ii) un anticoagulant oral en monothérapie et la bithérapie au moins 1 an après une ICP ou un SCA. Nous avons procédé en 2 temps, d’abord en répertoriant les revues systématiques publiées entre 2019 et 2022 sur ces 2 questions cliniques, puis en effectuant la recherche la plus exhaustive possible pour trouver d’autres essais contrôlés randomisés publiés jusqu’en octobre 2022. Les paramètres qui nous intéressaient étaient les événements cardiovasculaires indésirables majeurs (ECIM), le décès, la thrombose de l’endoprothèse et l’hémorragie majeure. Nous avons estimé les rapports de risques (RR) et les intervalles de confiance (IC) à 95 % à l’aide d’un modèle à effets aléatoires.
Résultats
D’après 6 essais contrôlés randomisés (n = 10 435), la bithérapie a réduit les hémorragies majeures (RR : 0,62; IC à 95 % : 0,52 à 0,73) et augmenté les thromboses de l’endoprothèse (RR : 1,55; IC à 95 % : 1,02 à 2,36), comparativement à la trithérapie après une ICP ou un SCA ayant fait l’objet d’une prise en charge médicale, tandis qu’aucune différence significative n’a été observée quant aux ECIM et aux décès. Dans 2 essais contrôlés randomisés (n = 2 905), un anticoagulant oral en monothérapie a réduit les hémorragies majeures (RR : 0,66; IC à 95 % : 0,49 à 0,91) comparativement à la bithérapie chez des patients présentant une FA après une ICP ou un SCA plus lointain, sans différence significative quant aux ECIM et aux décès.
Conclusions
Chez les patients présentant une FA et une coronaropathie, l’utilisation d’un traitement antithrombotique moins agressif (bithérapie après un ICP ou un SCA, et anticoagulant oral en monothérapie après une ICP ou un SCA plus lointain) réduit les hémorragies majeures, mais s’accompagne d’une augmentation des thromboses de l’endoprothèse en cas d’ICP récente. Ces résultats plaident en faveur d’une stratégie antithrombotique minimaliste, mais personnalisée chez ces patients.
Coronary artery disease (CAD) and atrial fibrillation (AF) are disease states that are pathophysiologically linked and often coexist in a patient.1 In AF, anticoagulation is more efficacious than antiplatelet therapy in preventing thromboembolic events,2,3 whereas dual-antiplatelet therapy is the preferred treatment for CAD, based on trials conducted in the early era of coronary stenting.4,5 This difference poses a management dilemma, as finding the optimal balance in patients with concomitant indications for different antithrombotic agents remains a conundrum. Based on randomized controlled trials (RCTs), European and North American guidelines have been aligned in several respects regarding the management of patients with concomitant AF and CAD. These include favouring direct oral anticoagulants over vitamin K antagonists (VKAs), favouring clopidogrel over potent P2Y12 inhibitors (ticagrelor and prasugrel), and omitting acetylsalicylic acid (ASA) in long-term treatment.6, 7, 8, 9, 10, 11 However, these studies were heterogeneous, and they were insufficiently powered for thrombotic events. To address this, meta-analyses have been carried out that show a decrease in bleeding with dual-pathway therapy (DPT) consisting of an oral anticoagulant (OAC) and single-antiplatelet vs triple-antithrombotic therapy with dual-antiplatelet therapy plus OAC.12,13 However, these meta-analyses were methodologically classified as being of low quality according to the A Measurement Tool to Assess Systematic Reviews (AMSTAR) 2 criteria. Additionally, recent evidence has emerged, challenging the practice of combining antiplatelet therapy and OAC beyond 1 year for patients with AF and percutaneous coronary intervention (PCI) or acute coronary syndrome (ACS).14,15
We aimed to synthesize evidence from RCTs in patients with AF and either of the following: (i) recent PCI or ACS, with a particular emphasis on addressing pertinent and specific management issues; or (ii) chronic CAD without recent PCI or ACS. The goal was to provide evidence-based guidance for the management of antithrombotic agents in patients with concomitant AF and CAD, throughout their journey.
Methods
We conducted a systematic review and meta-analysis to support the development of recommendations for the 2023 Canadian Cardiovascular Society /Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. We followed a 2-stage process, as follows. First, we searched for all recent systematic reviews that addressed any of the predefined clinical questions for this topic (outlined below) and extracted all relevant RCTs from these systematic reviews. We rated all identified systematic reviews using the AMSTAR 2 criteria.16 We subsequently identified and updated the most-comprehensive search strategies from available systematic reviews. Two of the current authors (B.J.M. and R.D.T.) re-extracted all data from the original RCT articles. This report follows the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.17
Search strategies and data source
To identify relevant systematic reviews, we searched PubMed (for the period January 1, 2019 to October 1, 2022). We selected 2019 to ensure that identified systematic reviews would include the most-recent large studies addressing these clinical questions— Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (ENTRUST-AF-PCI) and Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease (AFIRE)—which were both published in 2019. We then refined and updated (using minor optimizations to increase search specificity) the most comprehensive search among these meta-analyses, for each clinical question, and we updated these searches in PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL), to identify subsequent RCTs. Supplemental Appendix S1 describes the full search strategies.
We included parallel RCTs that evaluated the following clinical issues, and reported on at least one of the outcomes of interest (listed in Outcomes section below): (i) DPT (direct oral anticoagulant [DOAC] and P2Y12 inhibitor) vs triple-antithrombotic therapy (ASA, P2Y12 inhibitor, and a VKA or DOAC) in patients with AF and PCI (for ACS or stable CAD) or medically-managed ACS; and (ii) comparison of OAC monotherapy (DOAC or VKA) to DPT (OAC plus one antiplatelet agent) in patients with AF and CAD with remote (> 1 year) ACS and/or PCI.
One reviewer (B.J.M.) performed all database searches and imported the records into Covidence. Using Covidence, 2 reviewers (B.J.M. and R.D.T.) independently screened article titles and abstracts, and reviewed full-text articles for inclusion. One reviewer (B.J.M.) extracted data using a standardized data collection form, with replication of the data extraction by a second reviewer (R.D.T.).
Assessment of risk of bias (RoB) and certainty of evidence
Two reviewers (B.J.M. and R.D.T.) independently evaluated trial-level RoB using the Cochrane RoB 2 tool.18 The same reviewers then rated outcome-level certainty of evidence using the Grading Recommendations Assessment, Development and Evaluation (GRADE) framework, which incorporates the risk of bias, imprecision, inconsistency, indirectness, and publication bias.19 No discrepancies between the reviewers occurred.
Outcomes
The outcomes of interest were as follows: (i) major adverse cardiovascular events (MACE; composite of death from any cause, myocardial infarction [MI], or stroke—when this composite was not available, we used the composite of cardiovascular death, MI, or stroke, or a broader composite that encompasses additional components, as reported); (ii) all-cause death; (iii) stent thrombosis (definite or probable based on original Academic Research Consortium (ARC) or ARC-2 definition)20,21; and (iv) major bleed (Bleeding ARC [BARC] 3 or 5 bleed22; when information using this classification was not available, we preferentially reported bleeding based on the International Society on Thrombosis and Haemostasis definition, followed by the Thrombolysis in Myocardial Infarction [TIMI] definition, as used in the respective trials).
Statistical analysis
We pooled dichotomous outcomes as risk ratios (RRs) with 95% confidence intervals (CIs) using a DerSimonian-Laird inverse variance random-effects model for all outcomes. We evaluated statistical heterogeneity with visual inspection of the forest plot, and quantified the percentage of variability due to heterogeneity between trials using the I2 statistic.
We prespecified the following subgroups of interest for the comparison of DPT vs triple-antithrombotic therapy in recent PCI: choice of P2Y12 inhibitor; and early vs late effect (with respect to the duration of ASA). We did not conduct any subgroup analyses for the comparison of OAC monotherapy to DPT in AF with remote PCI and/or ACS.
We conducted all analyses using Review Manager version 5.4 (Cochrane, Copenhagen, Denmark).
Results
The initial search strategy identified 30 recent meta-analyses (with or without systematic reviews) addressing comparisons of interest (Fig. 1).12,13,23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 All meta-analyses except for one Cochrane review26 were ranked as having low or critically low quality levels according to the AMSTAR 2 criteria (Supplemental Table S1). The high-quality Cochrane review compared DOACs to VKAs post-PCI, which was not a focus for this review. Consequently, the reviews of next-highest quality level were selected instead.
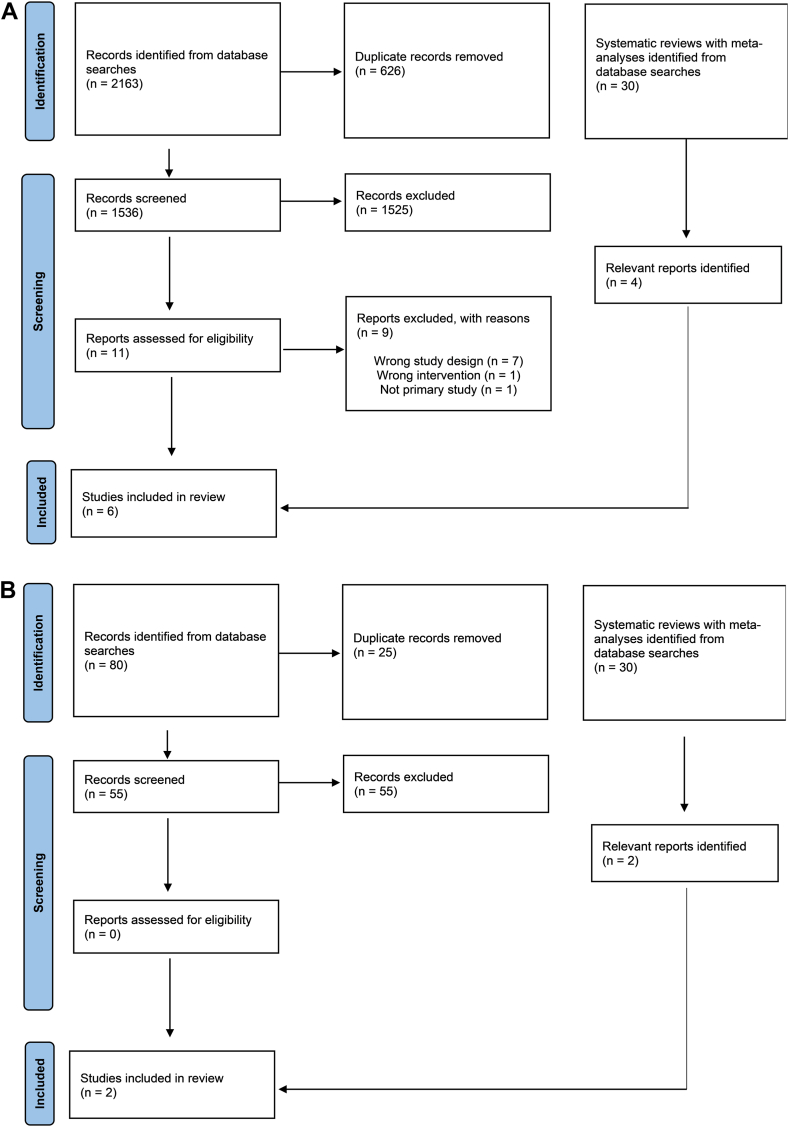
Figure 1.
Study flow diagram for comparing the following: (A) dual-pathway therapy vs triple-antithrombotic therapy; and (B) oral anticoagulant monotherapy vs dual-pathway therapy.
DPT vs triple-antithrombotic therapy in AF after PCI or medically-managed ACS
Characteristics of included studies
We selected the highest-quality systematic review for this question and updated their searches to extend them to October 2022.13 From 1536 records screened, we identified 2 additional RCTs,50,51 for a total of 6 RCTs (n = 11,156). Table 1 describes key study and participant characteristics. In brief, across all trials, the weighted mean age was 70 years; 41% of participants were women; the median CHA2DS2-VASc (Congestive Heart Failure, Hypertension, Age [≥ 75 years] [doubled], Diabetes Mellitus, Stroke/Transient Ischemic Attack [doubled], Vascular Disease, Age [65-74] Years, Sex Category [female]) score was 4; and clopidogrel was the predominant P2Y12 inhibitor (91%). Time from PCI to randomization ranged from a mean of 1.9 days (ENTRUST-AF-PCI) to 6 days (Apixaban Versus Warfarin in Patients With AF and ACS or PCI [AUGUSTUS]). In the AUGUSTUS trial,9 1097 patients (23.9% of the total population) had medically-managed ACS, and 1714 (37.3%) had ACS managed with PCI.
Table 1.
Patient’s characteristics across all included studies in atrial fibrillation after PCI or medically-managed ACS
| AUGUSTUS (PCI subgroup, n = 3498) | AUGUSTUS (n = 1097, medically managed subgroup) | ENTRUST-AF-PCI (n = 1506) | PIONEER-AF PCI (n = 2124) | RE-DUAL PCI (n = 2725) | Bai et al.50 (2022) (n = 100) |
Liu et al.51 (2021) (n = 106) |
|
|---|---|---|---|---|---|---|---|
| Age, mean, y | 70.7 | 69.6 | 70 | 70.2 | 70.8 | 58.0 | 78.5 |
| Female, % | 25.8 | 39.0 | 25.6 | 26.1 | 24.0 | 37.0 | 40.6 |
| CHAD2DS2VASc, mean | 3.9 | 4.1 | 4 | 4 | 3.6 | 3 | 4.7 |
| HAS-BLED, mean | 2.6 | 2.5 | 3 | 3 | 2.7 | 2 | 3.9 |
| ACS, % | 50.0 | 100 | 51.6 | 51 | 50.5 | 100 | 58.5 |
| NSTE-ACS/STEMI, % | — | — | — | 39.2/11.5 | 39.5/14.8 | — | — |
| DES, % | — | 0 | — | 67.7 | 84.1 | 100 | 100 |
| Days from index event to randomization | < 14 (mean 6.0) | < 14 (mean 8.5) | ≤ 5 (mean 1.9) | ≤ 3 | ≤ 5 | — | ≤ 3 |
| P2Y12i at baseline | |||||||
| Clopidogrel, % | 91.0 | 97.7 | 92.4 | 94.7 | 88.0 | — | — |
| Prasugrel/ticagrelor, % | 1.5/7.5 | 0.2/2.1 | 0/7 | 1.2/4.1 | -/10.7 | — | — |
| Dual therapy | |||||||
| OAC | Apixaban 5 mg BID or warfarin (INR 2-3) | Edoxaban 60 mg daily | Rivaroxaban 15 mg daily | Dabigatran 110 mg or 150 mg BID | Rivaroxaban 15 mg daily | Rivaroxaban 15 mg daily | |
| P2Y12i | Clopidogrel or ticagrelor or prasugrel | Clopidogrel or ticagrelor or prasugrel | Clopidogrel or ticagrelor or prasugrel | Clopidogrel or ticagrelor | Clopidogrel | Ticagrelor | |
| Triple therapy | |||||||
| OAC | Apixaban 5 mg BID or warfarin (INR 2-3) | Warfarin (INR 2-3) | Warfarin (INR 2-3) | Warfarin (INR 2-3) | Warfarin (INR 2-3) | Warfarin (INR 1.6-2.5) | |
| P2Y12i | Clopidogrel or ticagrelor or prasugrel | Clopidogrel or ticagrelor or prasugrel | Clopidogrel or ticagrelor or prasugrel | Clopidogrel or ticagrelor | Clopidogrel | Clopidogrel | |
| Outcome definitions | |||||||
| MACE | Death, MI, stroke, stent thrombosis (definite or probable), or urgent revascularization | Death, MI, stroke, stent thrombosis (definite), or systemic embolism | CV death, MI, or stroke | Death, MI, stroke, systemic embolism | Death, MI, stroke, stent thrombosis, unplanned revascularization | CV death, MI, stroke, or stent thrombosis | |
| Major bleeding | ISTH | BARC (3 or 5) | ISTH | ISTH | TIMI | TIMI | |
| Stent thrombosis | Reported: Definite/probable | Reported: Definite/probable | Reported | Reported: Definite | Reported | Reported | |
| Follow-up duration, mo | 6 | 12 | 12 | 14 | 12 | 12 | |
ACS, acute coronary syndrome; AUGUSTUS, Apixaban Versus Warfarin in Patients With AF and ACS or PCI; BARC, Bleeding Academic Research Consortium; BID, twice a day; CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age [≥ 75 years] [doubled], Diabetes Mellitus, Stroke/Transient Ischemic Attack [doubled], Vascular Disease, Age [65-74] Years, Sex Category [female]); CV, cardiovascular; DES, drug-eluting stent; ENTRUST-AF-PCI, Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention; HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol Concomitantly; INR, international normalized ratio; MACE, major adverse cardiovascular events; OAC, oral anticoagulant; MI, myocardial infarction; ISTH, International Society on Thrombosis and Haemostasis; NSTE-ACS, non-ST-elevation ACS; PCI, percutaneous coronary intervention; PIONEER-AF PCI, Exploration of Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo a PCI; P2Y12i, P2Y12 inhibitor; RE-DUAL PCI, Randomized Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting; STEMI, ST-elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction.
RoB and certainty of evidence
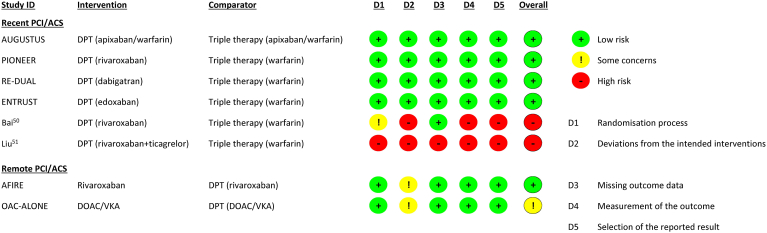
We rated 4 trials at a low risk of bias, and 2 trials at a high risk of bias (Fig. 2). AUGUSTUS employed a 2-by-2 factorial design to compare the use of ASA vs placebo (double-blind), as well as apixaban vs VKA (open-label); all other trials were open-label. Notably, we rated the open-label comparisons within AUGUSTUS, ENTRUST-AF, Exploration of Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo a Percutaneous Coronary Intervention (PIONEER-AF PCI), and Randomized Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting (RE-DUAL PCI) as having a low RoB, given the limited evidence of trial protocol deviations, and the use of relatively bias-proof outcomes combined with blinded outcome adjudication.
Figure 2.
Cochrane Risk of Bias 2 tool summary for considered trials. ACS, acute coronary syndrome; AFIRE, Atrial Fibrillation and Ischemic Events with Rivaroxaban in Patients with Stable Coronary Artery Disease; AUGUSTUS, Apixaban Versus Warfarin in Patients with AF and ACS or PCI; DOAC, direct oral anticoagulant; DPT, dual-platelet therapy; ENTRUST, Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (ENTRUST-AF-PCI); ID, identification; OAC-ALONE, Optimizing Antithrombotic Care in Patients With Atrial Fibrillation and Coronary Stent; PCI, percutaneous coronary intervention; PIONEER, Exploration of Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo a Percutaneous Coronary Intervention (PIONEER-AF PCI); RE-DUAL, Randomized Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting (RE-DUAL PCI); VKA, vitamin-K antagonist.
We rated the certainty of evidence as moderate for MACE, death, and stent thrombosis, due to serious imprecision, whereas we rated the certainty of evidence as high for major bleeding. GRADE certainty of evidence ratings, along with relative and absolute estimates of effect, are provided in Table 2.
Table 2.
Summary-of-findings table
| Outcome | Certainty of evidence | Effect estimate | |
|---|---|---|---|
| RR (95% CI) | Absolute difference, per 1000 | ||
| DOAC-based dual-pathway therapy vs triple-antithrombotic therapy in AF after PCI or medically-managed ACS | |||
| MACE | Moderate∗ | 1.12 (0.97–1.28) | 8 more (from 2 fewer to 19 more) |
| Death | Moderate∗ | 1.07 (0.88–1.30) | — |
| Stent thrombosis | Moderate∗ | 1.55 (1.02–2.36) | 4 more (from 0 to 9 more) |
| Major bleed | High | 0.62 (0.52–0.73) | 23 fewer (from 16 to 29 fewer) |
| OAC monotherapy vs OAC plus single antiplatelet in AF plus remote (> 1 y) ACS/PCI | |||
| MACE | Very low†,‡ | 0.91 (0.58–1.41) | — |
| Death | Very low†,‡ | 0.85 (0.37–1.92) | — |
| Stent thrombosis | Low‡ | 5.03 (0.24–104.37) | — |
| Major bleed | High | 0.66 (0.49–0.91) | 22 fewer (from 6 to 33 fewer) |
ACS, acute coronary syndrome; AF, atrial fibrillation; CI, confidence interval; DOAC, direct OAC; MACE, major adverse cardiovascular events; OAC, oral anticoagulation; PCI, percutaneous coronary intervention; RR, risk ratio.
Rated down 1 category for serious imprecision.
Rated down 1 category for serious inconsistency.
Rated down 2 categories for very serious imprecision.
Outcomes and subgroup analyses
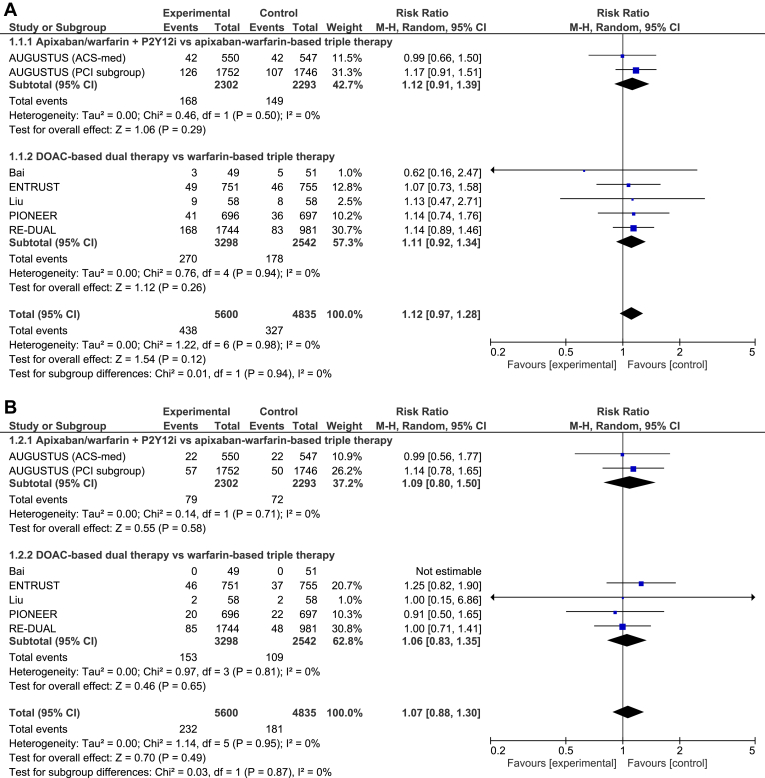
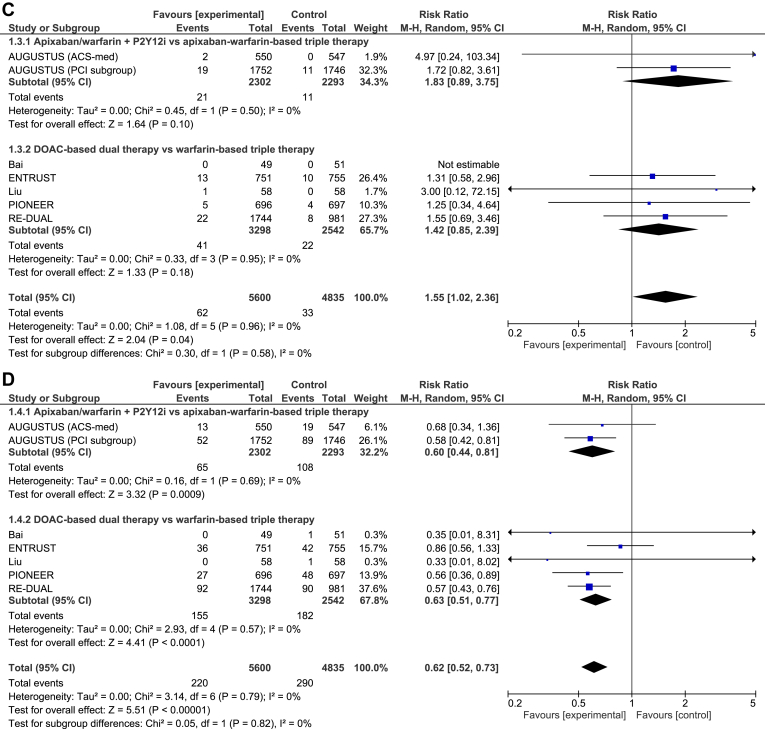
All 6 trials reported on all outcomes of interest. The differences in MACE (RR 1.12, 95% CI 0.97-1.28, I2 = 0%; Fig. 3A) and death (RR 1.07, 95% CI 0.88-1.30, I2 = 0%; Fig. 3B) between DPT and triple-antithrombotic therapy were not statistically significant. DPT increased the risk of stent thrombosis, compared with triple-antithrombotic therapy (RR 1.55, 95% CI 1.02-2.36, I2 = 0%; Fig. 3C), translating to a 0.4% absolute risk increase. Conversely, DPT reduced the risk of major bleeding, compared with triple-antithrombotic therapy (RR 0.62, 95% CI 0.52-0.73, I2 = 0%; Fig. 3D), translating to a 2.1% absolute risk reduction.
Figure 3.
Forest plot for (A) major adverse cardiovascular events, (B) death, (C) stent thrombosis, and (D) bleeding. ACS, acute coronary syndrome; AUGUSTUS, Apixaban Versus Warfarin in Patients with AF and ACS or PCI; CI, confidence interval; DOAC, direct oral anticoagulant; ENTRUST, Edoxaban Treatment Versus Vitamin K Antagonist in Patients With Atrial Fibrillation Undergoing Percutaneous Coronary Intervention (ENTRUST-AF-PCI); M-H, Mantel-Haenszel; PCI, percutaneous coronary intervention; PIONEER, Exploration of Two Treatment Strategies of Rivaroxaban and a Dose-Adjusted Oral Vitamin K Antagonist Treatment Strategy in Subjects With Atrial Fibrillation Who Undergo a Percutaneous Coronary Intervention (PIONEER-AF PCI); P2Y12i, P2Y12 inhibitor; RE-DUAL, Randomized Evaluation of Dual Therapy With Dabigatran vs Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting (RE-DUAL PCI).
No statistical heterogeneity and no significant subgroup interaction was present in the results for any outcome between patients managed with PCI (elective PCI or ACS treated with PCI) or medically-managed ACS in the AUGUSTUS trial.9
Exploration of the timing of treatment effects regarding ASA duration was variably reported and could not be meta-analyzed, so this is reported separately. In a post hoc landmark analysis of AUGUSTUS,52 ASA was compared to placebo (ie, DPT vs triple-antithrombotic therapy) in the first 30 days after randomization, and days 30-180 after randomization. Within 30 days, ASA increased bleeding events (fatal, intracranial, and major) by an absolute 0.97% (95% CI 0.23%-1.70%), and reduced thrombotic events (cardiovascular death, MI, stroke, and stent thrombosis) by 0.91% (-1.74% to -0.08%). After day 30, an increase in bleeding occurred with ASA (1.25%, 95% CI 0.23%-2.27%) and no significant reduction occurred in thrombotic events (-0.17%, 95% CI -1.33%-0.98%). In a similar analysis in RE-DUAL PCI,53 a consistent reduction occurred in major bleeding < 30 days and > 30 days after randomization with dabigatran-based DPT, compared with warfarin-based triple-antithrombotic therapy, with no statistically significant difference in thrombotic events at either timepoint. Landmark analysis of ENTRUST-AF-PCI11 at 14 days demonstrated heterogeneity of treatment effect, suggesting an increased risk of major or clinically-relevant major bleeding with edoxaban-based DPT vs warfarin up to day 14, followed by a reduction in bleeding events after 14 days. Notably, this analysis could not disentangle the effect of different OACs (with warfarin taking time to achieve therapeutic range, and spending a low amount of time in the therapeutic range in this early phase) and cessation of ASA.
OAC monotherapy vs combination therapy (OAC plus ASA) in AF patients with remote ACS or PCI
Characteristics of included studies
We selected one of the highest-quality systematic reviews for this question, and updated their search to extend it to October 2022.23 From 68 records screened, we identified no additional trials for inclusion. Because this previous systematic review pooled RCTs with observational studies, without analyses limited to randomized data, we replicated the meta-analyses including only RCTs. Both RCTs (n = 2932) were conducted exclusively in Japan; the mean age was 74.6 years; and 19.5% of participants were women (Table 3). The Optimizing Antithrombotic Care in Patient With Atrial Fibrillation and Coronary Stent (OAC-ALONE) trial54 compared OAC monotherapy to OAC plus an antiplatelet agent in patients with AF and CAD beyond 1 year after stenting. The AFIRE study55 compared rivaroxaban monotherapy to rivaroxaban plus a single antiplatelet. The choice of the antiplatelet agent, which was left to the discretion of the physician, was consistent between the trials, with the majority using ASA (85.9% in the OAC-ALONE trial, and 70.2% in the AFIRE study). In the OAC-ALONE trial, all patients had received coronary stents > 1 year prior. whereas in the AFIRE study, 781 patients (70.6%) had a history of PCI (723 of them with stent implantation), and 125 patients (11.3%) had a history of coronary artery bypass grafting.
Table 3.
Patient characteristics across all included studies for atrial fibrillation plus remote (> 1 year) ACS and/or PCI
| AFIRE (n = 2236) | OAC-ALONE (n = 696) | ||
|---|---|---|---|
| Country | Japan | Japan | |
| Age, mean, y | 74.4 | 75.1 | |
| Female sex, % | 21.0 | 14.8 | |
| CHADS2, mean | 2 | 2.5 | |
| CHA2DS2-VASc, mean | 4 | 4.6 | |
| HAS-BLED, mean | 2 | 2 | |
| ACS, % | 35.1 | 38.6 | |
| PCI, % | 70.7 | 100 | |
| DES, % | 67.7 | 70.4 | |
| Time from index event, y | > 1 | > 1 (median 4.5) | |
| Baseline antiplatelet, % | |||
| ASA | 56.7 | 85.9 | |
| Clopidogrel | 23.3 | 14.5 | |
| Other | 7.8 | - | |
| Baseline anticoagulant, % | |||
| Apixaban | 10.6 | 10 | |
| Dabigatran | 5.7 | 5.9 | |
| Edoxaban | 2.8 | 2.9 | |
| Rivaroxaban | 60.9 | 5.9 | |
| Warfarin | 13 | 75.2 | |
| Study OAC in both groups | Rivaroxaban 15 mg | Warfarin or DOAC | |
| Antiplatelet in comparator group | ASA or P2Y12 inhibitor | ASA or clopidogrel | |
| Post-randomization antiplatelet | |||
| OAC monotherapy | Dual-pathway therapy | ||
| ASA | 0.7 | 70.2 | — |
| Clopidogrel | 0.1 | 25.4 | — |
| Prasugrel | 0.1 | 1.5 | — |
| MACE definition | Death, MI, stroke, systemic embolism, unstable angina requiring revascularization | Death, MI, stroke, systemic embolism | |
| Major bleeding definition | ISTH | ISTH | |
| Non-major clinically-relevant bleeding definition | ISTH | TIMI | |
| Follow-up duration, mo | 24.1 | 30 | |
ACS, acute coronary syndrome; AFIRE, Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease; ASA, acetylsalicylic acid; CHADS2, Congestive Heart Failure, Hypertension, Age ≥ 75, Diabetes, and Prior Stroke/Transient Ischemic Attack (doubled); CHA2DS2-VASc, Congestive Heart Failure, Hypertension, Age [≥ 75 years] [doubled], Diabetes Mellitus, Stroke/Transient Ischemic Attack [doubled], Vascular Disease, Age [65-74] Years, Sex Category [female]); DES, drug-eluting stent; HAS-BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly (> 65 Years), Drugs/Alcohol Concomitantly; ISTH, International Society on Thrombosis and Haemostasis; MACE, major adverse cardiovascular events; MI, myocardial infarction; OAC, oral anticoagulant; OAC-ALONE, Optimizing Antithrombotic Care in Patients With Atrial Fibrillation and Coronary Stent; PCI, percutaneous coronary intervention; TIMI; thrombolysis in myocardial infarction.
RoB and certainty of evidence
We rated the AFIRE study as having a low RoB. We rated the OAC-ALONE trial as having some concerns for bias arising from deviations from intended interventions (12% of the patients in the intervention group added an antiplatelet agent due to a coronary procedure, and 9% of the patients in the comparator group stopped their antiplatelet therapy due to a bleeding event).
We rated the certainty of evidence as very low for MACE and death, due to serious inconsistency and very serious imprecision. Inconsistency (explored by contrasting both studies’ characteristics) between the AFIRE and OAC-ALONE studies for these 2 outcomes could not be readily explained; however, the AFIRE study had a lower RoB and demonstrated a statistically significant reduction in both outcomes. We rated the certainty of evidence for stent thrombosis as low, due to very serious imprecision, and rated major bleeding as having high certainty of evidence.
Outcomes
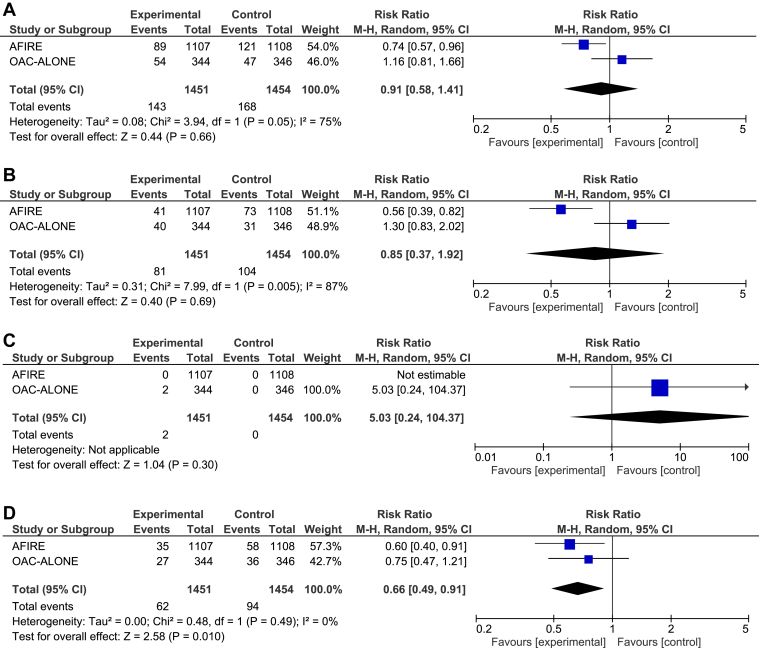
Both trials reported on all outcomes of interest. The differences in MACE (RR 0.91, 95% CI 0.58-1.41, I2 = 75%; Fig. 4A), death (RR 0.85, 0.37-1.92, I2 = 87%; Fig. 4B), and stent thrombosis (RR 5.03, 95% CI 0.24-104.37; Fig. 4C) between OAC monotherapy and the combination therapy were not statistically significant. OAC monotherapy reduced the risk of major bleeding, compared with the combination therapy (RR 0.66, 95% CI 0.49-0.91, I2 = 0%; Fig. 4D), translating to a 2.2% absolute risk reduction.
Figure 4.
Forest plot for (A) major adverse cardiovascular events; (B) death, (C) stent thrombosis, and (D) bleeding 1 year after percutaneous coronary intervention. AFIRE, Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease; CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; OAC-ALONE, Optimizing Antithrombotic Care in Patients With Atrial Fibrillation and Coronary Stent.
Discussion
The main findings of this systematic review with meta-analysis are as follows: (i) in patients with AF and CAD and/or ACS medically treated or with PCI (< 1 year), DOAC-based DPT regimens reduced rates of bleeding and increased in-stent thrombosis, compared to triple-antithrombotic therapy, with no significant differences in MACE or death; (ii) in patients with AF and remote PCI and/or ACS (> 1 year), OAC monotherapy reduced major bleeding, compared to OAC plus a single antiplatelet agent.
Finding the balance between thrombosis and bleeding risks in this high-risk population remains a clinical challenge. The What Is the Optimal Antiplatelet and Anticoagulation Therapy in Patients With Oral Anticoagulation and Coronary Stenting (WOEST) trial56 was the first randomized trial to explore a “less-is-more” approach to antithrombotic therapy in patients with AF and CAD, by comparing DPT (warfarin plus clopidogrel) vs triple-antithrombotic therapy, and demonstrating lower rates of bleeding and death with DPT. The Intracoronary Stenting and Antithrombotic Regimen: Triple Therapy in Patients on Oral Anticoagulation After Drug Eluting Stent Implantation (ISAR-TRIPLE) trial57 further suggested that 6 weeks, compared with 6 months, of VKA-based triple-antithrombotic therapy had similar efficacy and safety. In light of DOACs having replaced VKA as the first-line OAC choice for nonvalvular AF, trials evaluating DOAC-based regimens are more applicable to contemporary practice than the VKA-based trials.
Regarding the first study question regarding antithrombotic regimens early after PCI or ACS, meta-analyses demonstrated that 23 fewer patients had major bleeds, at the expense of 4 more stent thromboses, for every 1000 patients treated with DPT instead of triple therapy (ie, 6 major bleeds avoided for each stent thrombosis caused by a more conservative approach). In other words, in the study by Marquis-Gravel et al.,58 a major bleeding event experienced after ACS was associated with an increase in death at 1 year, similar to the incidence of death following recurrent MI. From a patient-oriented perspective, the goal is to reduce mortality and maintain quality of life, and given the similar impact of the 2 outcomes, avoiding bleeding after PCI should be considered as critical as reducing ischemic events. That said, the patient’s perspective is important to consider in the risk-benefit balance, as they may value these outcomes differently than do clinicians, and their buy-in is important to ensure patient-centred care and treatment adherence.
Additional analyses from the included trials provide insights into the optimal timing of ASA discontinuation. The post hoc analysis of AUGUSTUS59 showed a narrow tradeoff of an ∼0.9% absolute reduction in thrombotic events and an ∼0.9% increase in major bleeding events with ASA continuation up to 1 month among trial all-comers, followed by net harm after 30 days. Routine ASA discontinuation before 1 month should be the default approach, with the potential to continue ASA for 1 month or longer among patients at higher risk of stent thrombosis with low bleeding risk who may yet derive net benefit. Specifically, patients with a history of stent thrombosis, who were systematically excluded from all identified trials, or multiple thrombotic high-risk clinical or angiographic features, such as those outlined in the Canadian Cardiovascular Society antiplatelet guidelines, may benefit.60, 61, 62 This approach is also in line with the latest European recommendations for patients with high-risk ischemic features.60
In terms of P2Y12 inhibitor selection, clopidogrel was the predominant P2Y12 inhibitor used in the included RCTs, and no trial randomized patients to different P2Y12 inhibitors. One prior meta-analysis included observational comparisons of potent P2Y12 inhibitors (prasugrel and ticagrelor) to clopidogrel and found an associated increased risk of major bleeding without reducing MACE.33 In the absence of evidence for their superiority in this population, use of potent P2Y12 inhibitors should be restricted to those patients with clopidogrel intolerance or allergy, documented clopidogrel resistance identified by platelet-function or pharmacogenomic testing, recurrent MACE on clopidogrel, or who are part of a research protocol.
Our results are in accordance with previous meta-analyses on this topic. Overall, the most recent12,13,24,46 showed similar results, with a significant and conclusive reduction of bleeding with DPT, compared to triple therapy. We found a small but significant increase in stent thrombosis among those randomized to DPT, with a similar signal identified in prior meta-analyses.43,46 The study by Potpara et al.12 showed a decrease in bleeding and an increase in MACE with the use of DPT, and Khan et al.24 also found a decrease in bleeding, but results regarding ischemic events were less convincing. Capodanno et al.1 encouraged the use of an initial course of triple-antithrombotic therapy in selected patients, whereas Lopes et al.46 emphasized the importance of avoiding such an association. However, some methodological differences should be highlighted when comparing the present study to the most recent meta-analysis including the 4 major RCTs. First, RoB assessments in the present study were conducted by independent reviewers rather than a trial author. Second, the present study employed the GRADE rating of certainty of evidence, which was not done in prior reviews. Third, we added a summary-of-findings table, putting the results in context in absolute terms, to properly weigh tradeoffs. Fourth, the current analysis brings additional information regarding medically treated patients with no PCI.
As for our second question, few trials have assessed antithrombotic strategies in patients with chronic CAD and remote ACS or revascularization. For a long time, debate remained as to whether antiplatelet therapy should be continued chronically after the index event, or whether OAC monotherapy would suffice. Only the OAC-ALONE54 and AFIRE55 studies have addressed this question directly, with conflicting results. The main differences between the 2 trials are the premature termination of the OAC-ALONE trial, and the use of OAC in only one-quarter of the patients, in contrast to the exclusive use of rivaroxaban in the AFIRE study. Both trials were conducted in Japan, where OAC dosing differs from that in non-Asian countries, particularly for rivaroxaban, due to population-level pharmacokinetic and pharmacodynamic differences. 63 Overall, the AFIRE study had a lower RoB and provided high-certainty evidence for the superiority of OAC monotherapy, compared with DPT, with respect to bleeding. This finding is further supported by indirect evidence from the pre-stent era demonstrating similar thrombotic outcomes and fewer bleeds with VKA monotherapy, compared with ASA plus VKA post-MI.64 Overall, 1 year after the index procedure, OAC monotherapy with the omission of an antiplatelet agent seems to decrease the risk of bleeding. However, only low-certainty evidence remains for death and MACE.
Limitations
Our study results should be contextualized based on several limitations. First, we only had access to published trial-level data, and therefore certain subgroup analyses, such as analyses based on thrombotic risk or PCI complexity, were not possible. Second, only one included trial (AUGUSTUS) directly compared DOAC to VKA therapy, and DPT to triple-antithrombotic therapy, whereas all others compared DOAC-based DPT to VKA-based triple antithrombotic therapy. Despite this issue, results were consistent between trials. Finally, only 2 conflicting trials (with data limited to Japanese populations) were available for the comparison of patients with AF and remote ACS and/or PCI, and further trials may meaningfully change the pooled estimates for MACE. Future trials are required to address the following knowledge gaps: timing of aspirin discontinuation after PCI, as part of the DPT strategy; safety of potent P2Y12 inhibitors in combination with chronic OAC; safety and efficacy of DPT in patients undergoing complex PCI (who were underrepresented in trials); and development of tools to tailor antithrombotic strategies based on individual ischemic and bleeding risk in this population.
Conclusion
In patients with AF and recent PCI or ACS, DPT reduces major bleeding, but it increases stent thrombosis without increasing MACE or all-cause death. A tailored approach should be considered, as special attention should be given to patients with high ischemic risk in whom triple-antithrombotic therapy could be a reliable initial option. In patients with AF and remote PCI or ACS, OAC monotherapy reduces major bleeding and seems not to increase thrombotic events. After 1 year, an OAC may be continued as a monotherapy.
Acknowledgments
Ethics Statement
The research being reported has adhered to the relevant ethical guidelines. This study did not require approval by an ethics review board as no individual patient data was used.
Patient Consent
The authors confirm that patient consent is not applicable to this article. This meta-analysis used publicly-available aggregate data; therefore, patient consent was not required.
Funding Sources
The present work was funded by the Canadian Cardiovascular Society (CCS) to support the development of the 2023 Canadian Cardiovascular Society Antiplatelet Guidelines Update.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 718 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2024.01.001.
Supplementary Material
References
- 1.Capodanno D., Lip G.Y.H., Windecker S., et al. Triple antithrombotic therapy in atrial fibrillation patients with acute coronary syndromes or undergoing percutaneous coronary intervention or transcatheter aortic valve replacement. EuroIntervention. 2015;10:1015–1021. doi: 10.4244/EIJV10I9A174. [DOI] [PubMed] [Google Scholar]
- 2.Mant J., Hobbs F.D.R., Fletcher K., et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 3.Connolly S.J., Eikelboom J., Joyner C., et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe H., Domei T., Morimoto T., et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019;321:2414–2427. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubboli A., Milandri M., Castelvetri C., Cosmi B. Meta-analysis of trials comparing oral anticoagulation and aspirin versus dual antiplatelet therapy after coronary stenting. Clues for the management of patients with an indication for long-term anticoagulation undergoing coronary stenting. Cardiology. 2005;104:101–106. doi: 10.1159/000086918. [DOI] [PubMed] [Google Scholar]
- 6.Angiolillo D.J., Bhatt D.L., Cannon C.P., et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583–596. doi: 10.1161/CIRCULATIONAHA.120.050438. [DOI] [PubMed] [Google Scholar]
- 7.Neumann F.J., Sechtem U., Banning A.P., et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 8.Gibson C.M., Mehran R., Bode C., et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 9.Lopes R.D., Heizer G., Aronson R., et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083. [DOI] [PubMed] [Google Scholar]
- 10.Cannon C.P., Bhatt D.L., Oldgren J., et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 11.Vranckx P., Valgimigli M., Eckardt L., et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–1343. doi: 10.1016/S0140-6736(19)31872-0. [DOI] [PubMed] [Google Scholar]
- 12.Potpara T.S., Mujovic N., Proietti M., et al. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta-analysis of pooled data from the PIONEER AF-PCI, RE-DUAL PCI, and AUGUSTUS trials. Europace. 2020;22:33–46. doi: 10.1093/europace/euz259. [DOI] [PubMed] [Google Scholar]
- 13.Capodanno D., Di Maio M., Greco A., et al. Safety and efficacy of double antithrombotic therapy with non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation undergoing percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rein N., Heide-Jørgensen U., Lijfering W.M., et al. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy. Circulation. 2019;139:775–786. doi: 10.1161/CIRCULATIONAHA.118.036248. [DOI] [PubMed] [Google Scholar]
- 15.Lamberts M., Gislason G.H., Lip G.Y.H., et al. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients taking an oral anticoagulant: a nationwide cohort study. Circulation. 2014;129:1577–1585. doi: 10.1161/CIRCULATIONAHA.113.004834. [DOI] [PubMed] [Google Scholar]
- 16.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358 doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 19.Balshem H., Helfand M., Schünemann H.J., et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Cutlip D.E., Windecker S., Mehran R., et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Garcia H.M., McFadden E.P., Farb A., et al. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 consensus document. Circulation. 2018;137:2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289. [DOI] [PubMed] [Google Scholar]
- 22.Mehran R., Rao S.V., Bhatt D.L., et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 23.Malladi S., Hamid K., Pendyala N.C., et al. Management of stable coronary artery disease and atrial fibrillation with anti-thrombotic therapy: a systematic review and meta-analysis. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000027498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan S.U., Osman M., Khan M.U., et al. Dual versus triple therapy for atrial fibrillation after percutaneous coronary intervention: a systematic review and meta-analysis. Ann Intern Med. 2020;172:474–483. doi: 10.7326/M19-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shurrab M., Danon A., Alnasser S., et al. Dual-antithrombotic therapy with DOACs after acute coronary syndrome or percutaneous coronary intervention in atrial fibrillation: a meta-analysis of randomized controlled trials. Can J Cardiol. 2020;36:135–142. doi: 10.1016/j.cjca.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Al Said S., Alabed S., Kaier K., et al. Non-vitamin K antagonist oral anticoagulants (NOACs) postpercutaneous coronary intervention: a network meta-analysis. Cochrane Database Syst Rev. 2019;12:CD013252. doi: 10.1002/14651858.CD013252.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupercio F., Giancaterino S., Villablanca P.A., et al. P2Y12 inhibitors with oral anticoagulation for percutaneous coronary intervention with atrial fibrillation: a systematic review and meta-analysis. Heart. 2020;106:575–583. doi: 10.1136/heartjnl-2019-315963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W., Huang Q., Pan D., Zheng W., Zheng S. The optimal duration of triple antithrombotic therapy in patients with atrial fibrillation and acute coronary syndrome or undergoing percutaneous coronary intervention: a network meta-analysis of randomized clinical trials. Int J Cardiol. 2022;357:33–38. doi: 10.1016/j.ijcard.2022.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Altoukhi R.M., Alshouimi R.A., Al Rammah S.M., et al. Safety and efficacy of dual versus triple antithrombotic therapy (DAT vs TAT) in patients with atrial fibrillation following a PCI: a systematic review and network meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-036138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mainka F.F., Ferreira V.L., Mendes A.M., et al. Safety and efficacy of oral anticoagulants therapies in patients with atrial fibrillation undergoing percutaneous coronary intervention: a network meta-analysis. J Cardiovasc Pharmacol Ther. 2020;25:399–408. doi: 10.1177/1074248420930136. [DOI] [PubMed] [Google Scholar]
- 31.Liang B., Zhu Y.C., Gu N. Comparative safety and efficacy of eight antithrombotic regimens for patients with atrial fibrillation undergoing percutaneous coronary intervention. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.832164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colleran R., Byrne R.A., Ndrepepa G., et al. Antithrombotic therapy with or without aspirin after percutaneous coronary intervention or acute coronary syndrome in patients taking oral anticoagulation: a meta-analysis and network analysis of randomized controlled trials. Cardiovasc Revasc Med. 2022;36:99–106. doi: 10.1016/j.carrev.2021.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Casula M., Fortuni F., Ferlini M., et al. Meta-analysis comparing potent oral P2Y12 inhibitors versus clopidogrel in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am J Cardiovasc Drugs. 2021;21:231–240. doi: 10.1007/s40256-020-00436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuno T., Ueyama H., Takagi H., et al. Meta-analysis of antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am J Cardiol. 2020;125:521–527. doi: 10.1016/j.amjcard.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 35.Kuno T., Ueyama H., Ando T., Briasoulis A., Takagi H. Antithrombotic therapy in patients with atrial fibrillation and acute coronary syndrome undergoing percutaneous coronary intervention; insights from a meta-analysis. Coron Artery Dis. 2021;32:31–35. doi: 10.1097/MCA.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 36.De Rosa S., Sabatino J., Polimeni A., Sorrentino S., Indolfi C. Dual anti-thrombotic treatment with direct anticoagulants improves clinical outcomes in patients with atrial fibrillation with ACS or undergoing PCI. A systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0235511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoppellaro G., Marchese G.M., Squizzato A., et al. Benefit of dual antithrombotic therapy with direct oral anticoagulants in patients with atrial fibrillation undergoing percutaneous coronary intervention: a systematic review and metaanalysis of randomized clinical trials. Intern Emerg Med. 2020;15:1093–1104. doi: 10.1007/s11739-020-02402-3. [DOI] [PubMed] [Google Scholar]
- 38.Cen Z., Meng Q., Cui K. New oral anticoagulants for nonvalvular atrial fibrillation with stable coronary artery disease: a meta-analysis. Pacing Clin Electrophysiol. 2020;43:1393–1400. doi: 10.1111/pace.14081. [DOI] [PubMed] [Google Scholar]
- 39.Cordero A., Ferreiro J.L., Bertomeu-González V., et al. Direct oral anticoagulants versus vitamin-K antagonist after PCIs in patients with AF: a meta-analysis of cardiac ischemic events. J Cardiovasc Pharmacol. 2021;77:164–169. doi: 10.1097/FJC.0000000000000938. [DOI] [PubMed] [Google Scholar]
- 40.Wang S., Liu Y., Wang L., et al. Optimisation of oral anticoagulants for patients with atrial fibrillation within 12 months after percutaneous coronary intervention: a meta-analysis and systematic review. Int J Cardiol Heart Vasc. 2021;36 doi: 10.1016/j.ijcha.2021.100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saglietto A., D’Ascenzo F., Errigo D., et al. Antithrombotic strategies in patients needing oral anticoagulation undergoing percutaneous coronary intervention: a network meta-analysis. Catheter Cardiovasc Interv. 2021;97:581–588. doi: 10.1002/ccd.29192. [DOI] [PubMed] [Google Scholar]
- 42.Qiu M., Liu S.Y., Zhou H.R. Double antithrombotic therapy for prevention of bleeding and ischemic events after percutaneous coronary intervention in patients with atrial fibrillation: a meta-analysis. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000024188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galli M., Andreotti F., D’Amario D., et al. Dual therapy with direct oral anticoagulants significantly increases the risk of stent thrombosis compared to triple therapy. Eur Hear J Cardiovasc Pharmacother. 2020;6:128–129. doi: 10.1093/ehjcvp/pvz030. [DOI] [PubMed] [Google Scholar]
- 44.Gargiulo G., Goette A., Tijssen J., et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019;40:3757–3767. doi: 10.1093/eurheartj/ehz732. [DOI] [PubMed] [Google Scholar]
- 45.Goel S., Pasam R.T., Sharma A., Gidwani U. Dual versus triple antithrombotic therapy after acute coronary syndrome or percutaneous coronary intervention in patients with atrial fibrillation: an updated meta-analysis. Cardiovasc Revasc Med. 2020;21:239–241. doi: 10.1016/j.carrev.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Lopes R.D., Hong H., Harskamp R.E., et al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: a network meta-analysis of randomized controlled trials. JAMA Cardiol. 2019;4:747–755. doi: 10.1001/jamacardio.2019.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopes R.D., Hong H., Harskamp R.E., et al. Optimal antithrombotic regimens for patients with atrial fibrillation undergoing percutaneous coronary intervention: an updated network meta-analysis. JAMA Cardiol. 2020;5:582–589. doi: 10.1001/jamacardio.2019.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kheiri B., Simpson T.F., Stecker E.C., et al. Antithrombotic therapy for atrial fibrillation with stable coronary artery disease: a meta-analysis of randomized controlled trials. J Thromb Thrombolysis. 2020;50:395–398. doi: 10.1007/s11239-020-02041-7. [DOI] [PubMed] [Google Scholar]
- 49.Ullah W., Sattar Y., Shaukat M., Fischman D.L. Safety and efficacy of anticoagulant monotherapy in atrial fibrillation and stable coronary artery disease: a systematic review and meta-analysis. Eur J Intern Med. 2020;81:54–59. doi: 10.1016/j.ejim.2020.06.035. [DOI] [PubMed] [Google Scholar]
- 50.Bai L., Yang X.H., Zhou Y.Q., et al. Safety and efficacy evaluation of antithrombotic therapy with rivaroxaban and clopidogrel after PCI in Chinese patients. Clin Appl Thromb Hemost. 2022;28 doi: 10.1177/10760296221074681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X., Wang L., Zhou M., Feng L. Efficacy and safety of rivaroxaban and ticagrelor in elderly patients with atrial fibrillation undergoing percutaneous coronary intervention. Contemp Clin Trials. 2021;104 doi: 10.1016/j.cct.2021.106365. [DOI] [PubMed] [Google Scholar]
- 52.Alexander J.H., Wojdyla D., Vora A.N., et al. Risk/benefit tradeoff of antithrombotic therapy in patients with atrial fibrillation early and late after an acute coronary syndrome or percutaneous coronary intervention: insights from AUGUSTUS. Circulation. 2020;141:1618–1627. doi: 10.1161/CIRCULATIONAHA.120.046534. [DOI] [PubMed] [Google Scholar]
- 53.Peterson B.E., Bhatt D.L., Gabriel Steg P., et al. Evaluation of dual versus triple therapy by landmark analysis in the RE-DUAL PCI trial. JACC Cardiovasc Interv. 2021;14:768–780. doi: 10.1016/j.jcin.2021.02.022. [DOI] [PubMed] [Google Scholar]
- 54.Matsumura-Nakano Y., Shizuta S., Komasa A., et al. Open-label randomized trial comparing oral anticoagulation with and without single antiplatelet therapy in patients with atrial fibrillation and stable coronary artery disease beyond 1 year after coronary stent implantation. Circulation. 2019;139:604–616. doi: 10.1161/CIRCULATIONAHA.118.036768. [DOI] [PubMed] [Google Scholar]
- 55.Yasuda S., Kaikita K., Akao M., et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019;381:1103–1113. doi: 10.1056/NEJMoa1904143. [DOI] [PubMed] [Google Scholar]
- 56.Dewilde W.J.M., Oirbans T., Verheugt F.W.A., et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–1115. doi: 10.1016/S0140-6736(12)62177-1. [DOI] [PubMed] [Google Scholar]
- 57.Fiedler K.A., Maeng M., Mehilli J., et al. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE trial. J Am Coll Cardiol. 2015;65:1619–1629. doi: 10.1016/j.jacc.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 58.Marquis-Gravel G., Dalgaard F., Jones A.D., et al. Post-discharge bleeding and mortality following acute coronary syndromes with or without PCI. J Am Coll Cardiol. 2020;76:162–171. doi: 10.1016/j.jacc.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 59.Lopes R.D., Leonardi S., Wojdyla D.M., et al. Stent thrombosis in patients with atrial fibrillation undergoing coronary stenting in the AUGUSTUS trial. Circulation. 2020;141:781–783. doi: 10.1161/CIRCULATIONAHA.119.044584. [DOI] [PubMed] [Google Scholar]
- 60.Collet J.P., Thiele H., Barbato E., et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 61.Ueki Y., Karagiannis A., Zanchin C., et al. Validation of high-risk features for stent-related ischemic events as endorsed by the 2017 DAPT guidelines. JACC Cardiovasc Interv. 2019;12:820–830. doi: 10.1016/j.jcin.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Valgimigli M., Bueno H., Byrne R.A., et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2018;53:34–78. doi: 10.1093/ejcts/ezx334. [DOI] [PubMed] [Google Scholar]
- 63.Hori M., Matsumoto M., Tanahashi N., et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation—the J-ROCKET AF study. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454. [DOI] [PubMed] [Google Scholar]
- 64.Anand S.S., Yusuf S. Oral anticoagulants in patients with coronary artery disease. J Am Coll Cardiol. 2003;41(suppl 4):S62–S69. doi: 10.1016/s0735-1097(02)02776-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.