Abstract
Two cellular receptors for adenovirus, coxsackievirus-adenovirus receptor (CAR) and major histocompatibility complex class I (MHC-I) α2, have recently been identified. In the absence of CAR, MHC-I α2 has been suggested to serve as a cellular attachment protein for subgenus C adenoviruses, while members from all subgenera except subgenus B have been shown to interact with CAR. We have found that adenovirus type 37 (Ad37) attachment to CAR-expressing CHO cells was no better than that to CHO cells lacking CAR expression, suggesting that CAR is not used by Ad37 during attachment. Instead, we have identified sialic acid as a third adenovirus receptor moiety. First, Ad37 attachment to both CAR-expresing CHO cells and MHC-I α2-expressing Daudi cells was sensitive to neuraminidase treatment, which eliminates sialic acid on the cell surface. Second, Ad37 attachment to sialic acid-expressing Pro-5 cells was more than 10-fold stronger than that to the Pro-5 subline Lec2, which is deficient in sialic acid expression. Third, neuraminidase treatment of A549 cells caused a 60% decrease in Ad37 replication in a fluorescent-focus assay. Moreover, the receptor sialoconjugate is most probably a glycoprotein rather than a ganglioside, since Ad37 attachment to sialic acid-expressing Pro-5 cells was sensitive to protease treatment. Ad37 attachment to Pro-5 cells occurs via α(2→3)-linked sialic acid saccharides rather than α(2→6)-linked ones, since (i) α(2→3)-specific but not α(2→6)-specific lectins blocked Ad37 attachment to Pro-5 cells and (ii) pretreatment of Pro-5 cells with α(2→3)-specific neuraminidase resulted in decreased Ad37 binding. Taken together, these results suggest that, unlike Ad5, Ad37 makes use of α(2→3)-linked sialic acid saccharides on glycoproteins for entry instead of using CAR or MHC-I α2.
The adenovirus family consists of 49 known serotypes, which fall into six subgenera, A to F (40, 52). Subgenus D consists of 31 serotypes, and several members of this subgenus are poorly characterized. It is known that adenovirus type 9 (Ad9) and Ad15 infrequently cause acute follicular conjunctivitis and can also infect the respiratory tract (8, 33). Ad19p has been isolated only once, according to the literature, in Saudi Arabia in 1955 and, to the best of our knowledge, never since (7). Ad8 was the main causative agent of epidemic keratoconjunctivitis (EKC) until 1973, when Ad19a emerged (24). In 1976, another new adenovirus, Ad37, emerged. Like Ad19a, Ad37 was also found to cause EKC (17). In addition, Ad37, Ad19a and, less frequently, Ad8 have been isolated from the human genital tract (22, 34, 46). Molecular characterizations of Ad19a (51) and Ad37 (53) have been described earlier.
Adenovirus uses its protruding fibers to attach to a primary cellular receptor (21, 30, 44). Two fiber receptors, the coxsackievirus-adenovirus receptor (CAR) (10, 48) and the major histocompatibility complex class I (MHC-I) α2 subunit, have been described to date (24). Both receptors have been shown to have affinity for subgenus C adenovirus fibers (10, 24). However, CAR has been suggested to mediate high-affinity binding to subgenus C adenovirus fibers, while MHC-I α2 has been suggested to mediate adenovirus attachment to and permissiveness of cells expressing no or low amounts of CAR (15). CAR has also been shown to serve as a fiber attachment molecule for certain members of most subgenera, including Ad9 and Ad19p of subgenus D, but not for members of subgenus B (38). Ad19a and Ad37 have identical fiber proteins. In the fiber knob, they differ by only 2 amino acids from Ad19p (4).
Ad9, of subgenus D, was shown by Roelvink et al. (39) to use the same fiber receptor as Ad2, but unlike Ad2, Ad9 needs another viral component, the penton base, for optimal virion binding to target cells. The relative impact of the penton base during attachment has been suggested to be a function of the length of the fiber: a shorter fiber shaft, as in Ad9, with only 8 repeats, was suggested to allow penton base interaction with RGD motif-interacting integrins during attachment, while a longer fiber shaft, as in Ad2, with 22 repeats, might hamper the interaction between the penton base and integrins during attachment (38, 39). Apart from this function, the adenovirus penton base has an important function during the phases of virus internalization and release from cellular endosomes (6, 54). Adenovirus serotypes different from Ad2 and Ad9 have also been shown to interact with αv integrins (31, 32).
Adenoviruses are used to deliver and express foreign genes in human cells and tissues (14, 20). The commonly used adenovirus vectors are based on Ad5. Some lack of success in the use of Ad5 vectors can be explained partly by existing host immunity (41) and partly by the inefficiency of Ad5 in entering target cells (3). In this paper, we demonstrate that sialic acids α(2→3) linked to neighboring saccharides localized on cellular glycoproteins serve as cellular receptor moieties for Ad37 virions but not for Ad5 or Ad19p virions.
MATERIALS AND METHODS
Cells and viruses.
A549 and HEp-2 cells were grown as monolayers in Dulbecco's modified Eagle's medium (DMEM; Sigma Chemical Co., St. Louis, Mo.) supplemented with 10% fetal calf serum (FCS; Sigma). MHC-I α2-expressing B-lymphoblastoid E8.1 cells of Daudi cell origin (37) were grown in suspension in RPMI medium (Sigma) supplemented with 10% FCS and 10 mM sodium pyruvate. Human corneal epithelial (HCE) cells (2) and Chinese hamster ovary (CHO) cells expressing CAR (10) or human α2 integrin (9) were grown as monolayers as described previously. Lec2 cells, defective in sialic acid expression, and the Lec2 parental cell line Pro-5 (expressing sialic acid), both of CHO cell line origin, were grown as monolayers as described previously (18, 42, 43). All cell lines used in this study and their expression phenotypes for known adenovirus receptors are listed in Table 1.
TABLE 1.
Phenotypes of cell lines used in this study
| Cell linea | Phenotypeb
|
||
|---|---|---|---|
| CAR | Sialic acid | αv Integrin | |
| A549 | + | + | + |
| E8.1 | + | + | − |
| HCE | + | + | + |
| CHO-CAR | + | + | − |
| CHO-α2 | − | + | − |
| Pro-5 | − | + | − |
| Lec2 | − | − | − |
CHO-CAR and CHO-α2, CHO cells expressing CAR and α2 integrin, respectively.
+, expression; −, no expression.
Ad5 (strain F2853-5b), Ad37 (strain 1477), and Ad19p (prototype strain 587) were propagated with or without 3H-thymidine labeling (TRK 120; Amersham Pharmacia Biotech) in A549 (Ad5 and Ad19p) or HEp-2 (Ad37) cells as described previously (16). Cells were harvested 40 to 48 h postinfection, and labeled virions were purified as described previously (23). The specific radioactivity was 4 × 10−5 (Ad5) and 6.8 × 10−6 (Ad37) cpm/particle.
Binding and binding inhibition assays.
All cells grown as monolayers were harvested with phosphate-buffered saline (PBS)–EDTA only. All binding and binding inhibition assays were carried out in triplicate with 1.5-ml Eppendorf microtubes. Epithelial cells of different origins were washed twice in binding buffer (BB; DMEM plus 2% FCS) and diluted with BB to 2 × 105 cells/100 μl. E8.1 cells growing in suspension were washed twice in BB and diluted with BB to 2 × 105 cells/100 μl. For binding inhibition assays, cells were pelleted and resuspended in BB including different inhibitors. For binding assays, 5 μl of labeled Ad37 virions (in total, 94,000 cpm of Ad37, corresponding to 6.9 × 104 particles/cell) or Ad5 virions (in total, 550,000 cpm of Ad5, corresponding to 6.9 × 104 particles/cell) was added to the tubes, which were incubated on ice for 1 h. Unbound virions were removed by washing the cells twice in BB. Cell pellets were resuspended in 100 μl of BB prior to the addition of 2 ml of scintillation fluid. Thereafter, the cell-associated radioactivity was counted with a Wallac 1409 scintillation counter.
Prior to the addition of 100 μl of neuraminidase, 2 × 105 A549, E8.1, HCE, or CAR-expressing CHO cells were washed in PBS containing 2% FCS and pelleted. Neuraminidases from Clostridium perfringens (Sigma), Arthrobacter ureafaciens (Sigma), and Vibrio cholerae (Sigma and Boehringer Mannheim GmbH, Mannheim, Germany) were diluted in PBS containing 2% FCS, added to cell pellets, and incubated at 37°C. After 1 h, the cells were washed twice in BB. Five microliters of Ad37 or Ad5 virions was added to the cells and incubated on ice for 1 h. The cells were washed twice in BB, pelleted, and resuspended in 100 μl of BB. Two milliliters of scintillation fluid was added, and the cell-associated radioactivity was counted.
Prior to the addition of 100 μl of α(2→3)-specific neuraminidase (Sigma), 2 × 105 Pro-5 cells were washed twice with 50 mM NaPO4 (pH 6) containing 2% FCS and pelleted. The α(2→3)-specific neuraminidase was diluted in 50 mM NaPO4 (pH 6) containing 2% FCS, added to cell pellets, and incubated at 37°C for 1 h. Thereafter, the cells were washed twice in BB, and 5 μl of labeled Ad37 virions was added. The cells were incubated on ice for 1 hour, washed twice in BB, and mixed with 2 ml of scintillation fluid, and the cell-associated radioactivity was counted.
Prior to the addition of 100 μl of different proteases, 106 Pro-5 cells were washed twice in PBS and pelleted. Three different proteases, bromelain (a pineapple stem cysteine protease), ficin (a fig tree latex cysteine protease), and V8 protease (a Glu- or Asp-specific protease from Staphylococcus aureus) (all purchased from Sigma), were dissolved and diluted in PBS, added to cell pellets, and incubated at 37°C for 30 min. The cells were washed carefully in ice-cold BB to remove all traces of protease. Labeled Ad37 virions were added to the cells, and the mixture was incubated on ice for 1 h and washed twice in BB. Pellets were resuspended in BB and diluted in scintillation fluid, and the cell-bound radioactivity was counted. Cell viability was determined by the trypan blue method.
For the blocking assays, 100 μg of glycophorin A (GpA; molecular mass, 30 kDa; Sigma) or asialo-glycophorin A (asialo-GpA; Sigma) glycoprotein per ml was incubated with 5 μl of labeled Ad37 virions on ice. After 1 h, 2 × 105 prewashed Pro-5 cells were pelleted, resuspended in the virion-inhibitor mixture, and incubated on ice for 1 h. Cells were washed twice in BB, pelleted, and resuspended in 100 μl of BB. Scintillation fluid was added, and the cell-associated radioactivity was counted.
In a similar blocking assay, 100 μg of wheat germ agglutinin (WGA; molecular mass, 36 kDa; Sigma), Sambucus nigra (molecular mass, 140 kDa; Sigma), or Maackia amurensis (molecular mass, 130 kDa; Sigma) lectin per ml was dissolved in BB and added to 2 × 105 prewashed Pro-5 cells. The cells were incubated on ice for 1 h, and 5 μl of labeled Ad37 virions was added. After 1 h on ice, the cells were washed, pelleted, and resuspended in 100 μl of BB. Scintillation fluid was added, and the cell-associated radioactivity was counted.
FACS.
For fluorescence-activated sell sorting (FACS) analysis, a total of 106 Lec2 or Pro-5 cells were incubated with 0.1 μg of fluorescein isothiocyanate (FITC)-labeled WGA on ice for 1 h. Unbound WGA was washed away, and the number of cells positive for WGA binding was assayed with a FACScan (Becton Dickinson) flow cytometer.
Replication assay.
A total of 2 × 105 A549 cells/well in 24-well plates were incubated at 37°C with 10-fold serial dilutions of V. cholerae neuraminidase, starting with 10 mU. One hour later, the cells were washed twice in BB. Virus stocks prepared from Ad5- or Ad37-infected A549 cells were added at dilutions corresponding to 5,000 focus-forming units (FFU)/well. Thus, the dilutions of each virus were adjusted so that 100 FFU/view field was obtained in the control. After 1 h of incubation at 37°C, noninternalized viruses were removed by washing with DMEM supplemented with 2% FCS. At 40 h postinfection, the cells were rinsed in PBS, fixed with 99% methanol, and incubated with rabbit polyclonal anti-Ad5 or anti-Ad37 antibodies diluted 1:200. Thereafter, the cells were washed twice for 15 min each time in PBS, stained with FITC-labeled swine anti-rabbit immunoglobulin G antibodies (Dakopatts, Glostrup, Denmark), washed as before, and examined with an immunofluorescence microscope (magnification, ×100; Xiovert 25; Carl Zeiss, Jena GmbH, Jena, Germany). The results are presented as the mean of three independent experiments.
RESULTS
Ad37 does not use CAR as a primary receptor.
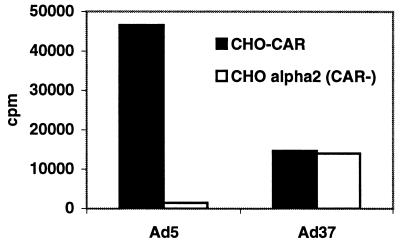
Since CAR has been shown to function as a cellular attachment molecule for human adenoviruses, including the subgenus D adenovirus Ad19p and Ad9, we wanted to ascertain whether Ad37 makes use of CAR or if it uses a distinct cellular receptor. The α2-integrin-expressing CHO cell line lacking CAR expression was used to compare Ad37 binding to that of the CAR-expressing CHO cell line. We found that Ad37 bound with equal efficiency to both types of cells (Fig. 1). Ad5, on the other hand, bound significantly more strongly to CAR-expressing CHO cells than to α2-integrin-expressing cells. These results show that CAR is used as a cellular attachment molecule for Ad5 but not for Ad37.
FIG. 1.
Binding of 3H-labeled Ad5 or Ad37 virions to CHO cells expressing CAR (CHO-CAR) or human α2 integrin (CHO alpha2). Pelleted cells (2 × 105) were resuspended in 100 μl of BB containing Ad5 or Ad37 virions. Cells were incubated on ice for 1 h, and nonbound virions were washed away. Cell-associated radioactivity was counted as described in Materials and Methods.
Neuraminidase treatment of epithelial cells inhibits virus binding.
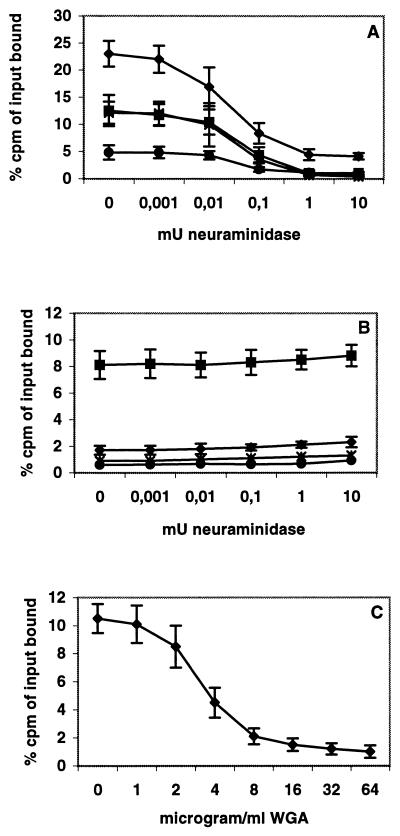
It is known that only a limited number of adenoviruses, including Ad37, are able to agglutinate human type O erythrocytes (28). Furthermore, subgenus D adenovirus-mediated agglutination of human erythrocytes has been found to be sensitive to cholera filtrate (i.e., neuraminidase) treatment (50). Consequently, we tested the ability of Ad37 virions to bind to different cell lines pretreated with different concentrations of V. cholerae neuraminidase (Sigma). The binding of 3H-thymidine-labeled Ad37 virions to CAR-expressing CHO cells and MHC-I α2-expressing Daudi cells decreased with increasing neuraminidase concentration in a dose-dependent manner (Fig. 2A). The reduction in Ad37 binding was similar to that obtained with neuraminidase-treated A549 and HCE cells. Labeled Ad37 virions were also tested for binding to CAR-expressing CHO cells treated with two different neuraminidases (from C. perfringens or A. ureafaciens) and a protease-free V. cholerae neuraminidase from another manufacturer (Boehringer Mannheim), all with similar results (data not shown), suggesting that the reduced binding of Ad37 is not simply due to traces of proteases and/or N-acetyl neuraminic acid-aldolase found in some of the enzyme preparations. A total of 23% of the labeled Ad37 virions added were found to remain at the cell surface of A549 cells after washing, whereas only 2% of Ad5 virions added remained cell associated (Fig. 2A and B). Neuraminidase treatment did not reduce Ad5 binding to any of the cells tested.
FIG. 2.
(A and B) Binding of 3H-labeled Ad37 (A) or Ad5 (B) virions to 2 × 105 V. cholerae neuraminidase-treated A549 (⧫), CAR-expressing CHO (■), HCE (●), or E8.1 (∗) cells. (C) Binding of labeled Ad37 to 2 × 105 Pro-5 cells premixed with different concentrations of WGA. Error bars show standard errors.
WGA lectin, which has a strong affinity for a broad range of sialoconjugates, was used to test the blockage of Ad37 binding to Pro-5 cells. We found that WGA blocked Ad37 binding in a dose-dependent manner, less than 10 μg/ml being sufficient to reduce binding to near negligible levels (Fig. 2C).
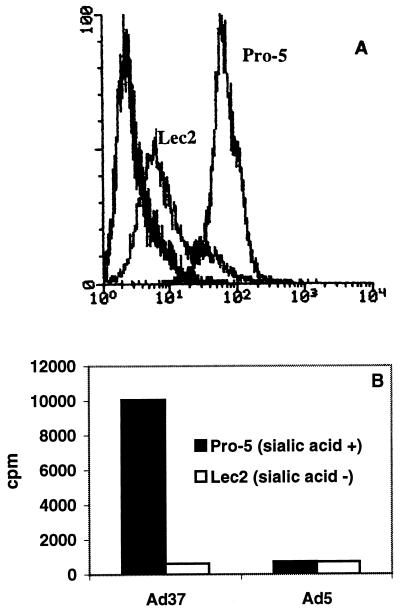
The Lec2 cell line is a subline of CHO cell line Pro-5. It was chosen on the basis of its resistance to a WGA interaction and is characterized by a defect in the Golgi body-dependent sialylation of carbohydrate moieties, resulting in more than 90% of the total amount of sialic acid saccharides on the Lec2 cell membrane being lost, compared to the amount in the sialic acid-expressing parental cell line Pro-5. These results are shown in Fig. 3A, where the relative expression of sialic acid was measured by use of FITC-labeled WGA in a FACS assay. These two cell lines were used to investigate whether sialic acid could serve as a receptor for Ad37. Weak binding of Ad37 to Lec2 cells, corresponding to less than 10% of the binding of Ad37 to Pro-5 cells, was seen (Fig. 3B). The binding of Ad5 to sialylated Pro-5 cells and that to nonsialylated Lec2 cells were equally low, suggesting that sialic acid is not used by Ad5 during the binding step. We have also found that neuraminidase treatment of human type O erythrocytes abolishes Ad37-mediated hemagglutination, suggesting that sialic acid is also the receptor for Ad37 on human erythrocytes (data not shown). Taken together, these experiments suggest that sialic acid is used as a cellular attachment molecule by Ad37 but not by Ad5.
FIG. 3.
(A) Expression of sialic acid on sialic acid-lacking Lec2 cells and Pro-5 cells (parental cells for Lec2 cells), which express sialic acid, as measured by use of FITC-labeled WGA in a FACS assay. (B) Binding of 3H-labeled Ad37 or Ad5 to Pro-5 cells or to Lec2 cells. Cell-associated radioactivity was counted as described in Materials and Methods.
Neuraminidase treatment of A549 cells reduces the infectivity of Ad37.
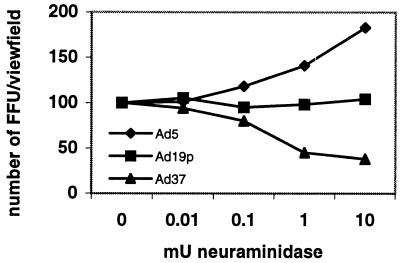
To investigate whether sialic acid is a functional receptor for Ad37 or whether Ad37 is trapped by cell surface sialic acid in a dead-end pathway, we treated A549 cells with V. cholerae neuraminidase and assayed Ad37 replication by using a fluorescent-focus assay. We found that V. cholerae neuraminidase was efficient in reducing the number of Ad37-infected cells (FFU) (Fig. 4). The infectivity was reduced in a neuraminidase dose-dependent manner but was not completely abolished. Approximately 40% of the Ad37 added to the A549 cells could still enter the cells. This fraction of replicating Ad37 may have entered through an as-yet-unknown route of infection. This result indicates that sialic acid is a functional cellular receptor for Ad37 on human cell line A549. Ad19p infection was not affected by neuraminidase treatment of the cells, suggesting that sialic acid is not used as a receptor by Ad19p. The infectivity of Ad5 as a reference was not decreased but rather was increased nearly 100% in A549 cells treated with 10 mU of neuraminidase. The mechanism for the enhanced entry of Ad2 and Ad5 into neuraminidase-treated target cells has been discussed by others (3, 10).
FIG. 4.
Infectivity of Ad5, Ad19p, or Ad37 in 2 × 105 A549 cells in 24-well plates. Prior to infection, the cells were incubated at 37°C for 1 h with different concentrations of V. cholerae neuraminidase diluted in PBS containing 2% FCS. Virus stocks were added in dilutions corresponding to 5,000 FFU/well and incubated for 1 h at 37°C. Nonbound virions were washed away. At 40 h postinfection, the cells were stained and examined as described in Materials and Methods.
The sialic acid moiety recognized by Ad37 is localized on glycoproteins rather than on gangliosides.
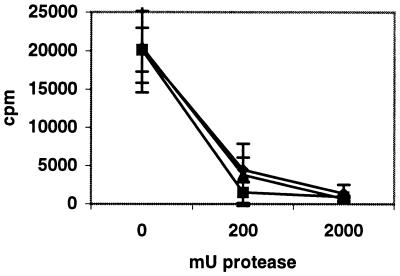
Since sialic acid saccharides are expressed on both glycoproteins and glycolipids (gangliosides), we treated Pro-5 cells expressing sialic acid with two different cysteine proteases, bromelain or ficin, or V8 protease, which is specific for cleaving polypeptides at Glu or Asp residues, to determine whether glycoproteins carrying terminal sialic acid saccharides are involved in Ad37 binding to these cells (Fig. 5). Two different protease concentrations were used: 200 and 2,000 mU. A strong inhibitory effect was seen with all three proteases at concentrations of 200 mU. Digestion with 2,000 mU reduced Ad37 binding at least 20-fold, independently of the type of protease. Cell viability was assessed immediately after proteolytic digestion, and no cytotoxic effect mediated by the protease treatment could be seen, compared to the results for untreated control cells. This experiment indicated that the sialic acid saccharides which bind Ad37 are localized on glycoproteins rather than on gangliosides.
FIG. 5.
Ad37 binding to Pro-5 cells pretreated with different proteases. Pelleted Pro-5 cells (106) were resuspended in PBS containing 0, 200, or 2,000 mU of protease and incubated at 37°C. The proteases bromelain (⧫), V8 protease (▴), and ficin (■) were used. After 1 h, the proteases were washed away, and the cells were pelleted. Cells were resuspended in 100 μl of BB containing 3H-labeled Ad37. After 1 h of incubation on ice, nonbound virions were washed away. Cell-associated radioactivity was counted as described in Materials and Methods. Error bars show standard errors.
Nature of the glycoprotein receptor.
The next step in identifying the Ad37 receptor was to block Ad37 binding to sialic acid-expressing Pro-5 cells. GpA glycoprotein at 100 μg/ml was sufficient to block more than 80% of Ad37 binding to Pro-5 cells (Fig. 6A), while 10 μg of GpA per ml blocked Ad37 binding only weakly (data not shown). The molecular mass of GpA is 30 kDa, which means that the total number of GpA molecules required to inhibit Ad37 binding with 80% efficiency corresponded to 2 × 1014. Since the total number of Ad37 virions in one reaction corresponded to 1.38 × 1010, the GpA/virion ratio was approximately 14,500 and the GpA/fiber ratio was approximately 1,200 (assuming 12 fibers per virion). On the other hand, the same amount of asialo-GpA did not inhibit Ad37 binding to Pro-5 cells at all (Fig. 6A). These data further support the statement that sialic acid serves as an Ad37 cell surface receptor moiety. They also support the suggestion that the Ad37 receptor is a sialic acid saccharide localized on glycoproteins rather than on gangliosides.
FIG. 6.
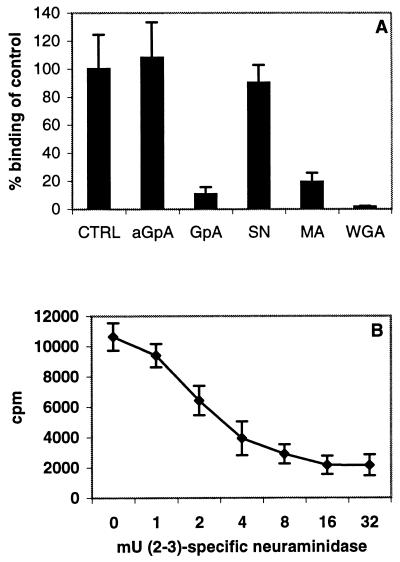
Ad37 binds to Pro-5 cells via (2→3)-linked sialic acids, and the binding is inhibited by GpA but not by aGpA. (A) 3H-labeled Ad37 virions were incubated on ice in 100 μl of BB with 10 μg of GpA or asialo-GpA (aGpA) per ml. After 1 h, the mixtures were added to 2 × 105 pelleted Pro-5 cells. The resuspended cells were incubated on ice for 1 h. Alternatively, 2 × 105 Pro-5 cells were mixed with 100 g of lectin from M. amurensis [MA, specific for (2→3)-linked sialic acid], S. nigra [SN, specific for (2→6)-linked sialic acid], or WGA per ml. After 1 h on ice, 3H-labeled Ad37 virions were added to make a final volume of 100 μl, and the mixtures were incubated on ice for 1 h. Nonbound virions were washed away, and cell-associated radioactivity was measured as described in Materials and Methods. CTRL, control. (B) Pro-5 cells (2 × 105) were incubated at 37°C with different concentrations of (2-3)-specific neuraminidase. After 1 h, the neuraminidase was washed away, and the pelleted cells were resuspended in 100 μl of BB containing 3H-labeled Ad37 virions. The mixtures were incubated on ice for 1 h. Nonbound virions were washed away, and cell-associated radioactivity was measured as described in Materials and Methods. Error bars show standard errors.
To further characterize the sialylated glycoprotein, we pretreated 2 × 105 Pro-5 cells with 100 μg of three different lectins per ml: (i) WGA, with the capacity to interact with a broad range of sialoconjugates; (ii) M. amurensis, which interacts specifically with α(2→3)-linked sialic acid saccharides; and (iii) S. nigra, which interacts specifically with α(2→6)-linked sialic acid saccharides. As expected, cellular incubation with WGA incubation Ad37 binding to Pro-5 cells almost completely (Fig. 6A).
Furthermore, we found that the Ad37 interaction with Pro-5 cells occurs mainly through α(2→3)-linked sialic acid saccharides rather than α(2→6)-linked ones. At 100 μg/ml, the α(2→3)-specific M. amurensis lectin (130 kDa) blocked Ad37 binding with almost 90% efficiency, while the same concentration of the α(2→6)-specific S. nigra lectin (140 kDa) was inefficient in blocking Ad37 binding to Pro-5 cells (Fig. 6A). The latter lectin interacts specifically with α-NeuNAc-α(2→6)Gal or GalNAc sialic acid constellations, indicating that α(2→6)-linked sialic acid units are not used in Ad37 binding to Pro-5 cells. To test this conclusion further, we treated 2 × 105 Pro-5 cells with up to 32 mU of α(2→3)-specific neuraminidase. We found that approximately 80% of Ad37 binding to Pro-5 cells is mediated by α(2→3)-linked carbohydrates.
DISCUSSION
A number of virus families have been shown to use sialic acid-containing molecules as cellular receptors. Most viruses that use sialic acid as a receptor interact with sialylated glycolipids (gangliosides) on target cell membranes. For example, the different types of influenza virus use gangliosides as cellular receptors (45). Bovine parvovirus uses GpA as a receptor on erythrocytes (47), and canine parvovirus uses a sialylated glycoprotein as a primary receptor on A72 cells (5). Polyomaviruses have been shown to interact with a tunicamycin-induced receptor on 3T3 cells which can be either α(2→3) or α(2→6) linked (12), and the same viruses use α(2→6)-linked sialoconjugates solely as receptors on glial cells (29).
Although the penton base of certain adenovirus types has been shown to be of importance during virus-cell interactions (39), the fiber protein has been shown to mediate adenovirus attachment to target cells (21, 30, 44). We have reported previously that the fiber knob of Ad37 differs from the fiber knob of Ad19p (the prototype strain) by 2 amino acids: Ad37 fibers contain Lys240 and Asn340, whereas Ad19p fibers contain Glu240 and Asp340 (4). Recently, Huang et al. showed that Ad37 but not Ad19p was able to attach to immortalized conjunctival epithelial cells (26). An exchange in the Ad19p fiber knob from Glu240 to Lys conferred Ad19p binding to the conjunctival cells, while the reverse mutation in the Ad37 fiber knob abrogated binding. Point mutation studies of subgenus D adenovirus fibers showed conclusively that these are also responsible for hemagglutination (19, 36). Taken together, these results suggest that the fiber protein of Ad37 mediates binding to erythrocytes as well as to conjunctival cells.
When this study was started, CAR and MHC-I α2 had been identified as cellular receptors for subgenus C adenoviruses. CAR appears to be the main high-affinity adenovirus receptor (15). CAR has also been shown to serve as a cellular attachment molecule for the subgenus D adenovirus members Ad9 and Ad19p (38). Ad37 also belongs to subgenus D but has a tropism which is different from that of Ad9. To test whether CAR could serve as a receptor for Ad37, we tested the ability of Ad37 to bind to CAR-expressing versus non-CAR-expressing cells. The results indicated that CAR plays a minor role, if any, in Ad37 attachment to target cells. Hence, it is likely that CAR can serve as a receptor for some subgenus D adenoviruses but not for Ad37.
In this study, we have presented a number of lines of evidence indicating that sialic acid serves as a cellular receptor for Ad37. (i) The enzymatic removal of sialic acid from target cell surface membranes resulted in a significant decrease in Ad37 virion attachment to all cell lines tested, including CAR-expressing CHO and E8.1 cells. (ii) The sialic acid-interacting WGA lectin strongly blocked Ad37 virion attachment to Pro-5 cells. (iii) Ad37 virions attached more strongly (more than 10-fold) to sialic acid-expressing Pro-5 cells than to nonexpressing Lec2 cells. (iv) Ad37 replication in A549 cells was inhibited when the cells were treated with neuraminidase prior to infection. Moreover, the function of sialic acid as an adenovirus receptor seemed to be specific for Ad37 virions, since Ad5 virion attachment to the cells used in this study was independent of the presence of sialic acid.
The Ad37 receptor appears to be a glycoprotein rather than a ganglioside, since protease treatment of Pro-5 cells resulted in a decrease in Ad37 binding to these cells. The presence of a protein component in the Ad37 receptor has been reported by others (26). This notion is further supported by the finding that the GpA glycoprotein inhibits Ad37 binding to Pro-5 cells. It is also possible, but less likely, that the receptor functions as a coreceptor for a protein. The finding that Ad37 uses α(2→3)-linked rather than α(2→6)-linked sialoconjugates suggests that the sialic acid saccharide must be localized within a specific spatial context in order to be recognized by Ad37.
Several members of the adenovirus family have been shown to interact with CAR (38). However, certain types with a distinct tropism may interact with receptors different from CAR. In addition to Ad37, both Ad8 and Ad19a are known to cause EKC (27, 28). We have found previously that Ad37 and Ad19a have identical fiber genes (4), suggesting that Ad19a may also interact with sialic acid. As expected, we found that Ad19a agglutination of human type O erythrocytes was neuraminidase sensitive (data not shown). Ad8, which also causes EKC, has been found to partially compete with Ad37 for binding to human conjunctival cells (26), suggesting that Ad8 may also interact with sialic acid. Ad19p has not been reported to share tropism with Ad37 but has been shown to interact with CAR (38). This finding is in agreement with our observation that Ad19p infection of A549 cells is not neuraminidase sensitive (Fig. 4). Consequently, it is likely that both Ad19a and Ad8 but not Ad19p can also use sialic acid for attachment to target cells.
It has been shown previously that the number of Ad2 and Ad3 receptors on both KB and A549 cells ranges between 5,000 (Ad2) and 7,000 (Ad3) per cell (16). Furthermore, it has been demonstrated that 2% of input Ad5 virions and 4% of input Ad7 virions remain attached to A549 cells after 1 h of incubation on ice and subsequent washing steps (21). It was recently reported that Chang conjunctival cells express 2.4 × 104 Ad37 receptors/cell (26). Here, we found a strikingly high ratio of bound virions to input virions for Ad37 attachment to A549 cells (23%), compared to the results obtained for Ad5 (2%). Consequently, it is possible that the Ad37 receptor moiety is present in large amounts on A549 cells, since the ratio of bound virions to input virions reflects the number of receptors on one cell. Furthermore, we found that as many as 8.4 × 107 WGA molecules/cell were required to achieve significant inhibition of Ad37 attachment to Pro-5 cells. However, we cannot exclude the possibility that the different ratios for Ad37 and Ad5 are a consequence of different affinities of these adenovirus types for their respective receptors.
Ad5-based vectors do not enter target cells with sufficient efficiency (35, 55). One of the reasons appears to be the existence of charge-dependent repulsions between the Ad5 hexon protein, which is unusually acidic in the exposed loops (1, 13, 49), and cell surface sialic acid (3, 11). It has been suggested that the removal of sialic acid from the cell surface by neuraminidase could result in a higher efficiency of subgenus C adenoviruses encountering the target cell and attaching to its receptor (3). In this study, this feature was reflected by a twofold increase in the number of infected cells when neuraminidase-treated cells were exposed to Ad5. Ad37, however, makes use of the cell surface molecules that apparently hamper Ad5 entry into target cells in vivo. Moreover, the Ad37 receptor is probably expressed in larger amounts than the Ad5 receptor. Consequently, when adenoviruses are used to deliver genetic material to sialic acid-expressing cells, entry efficiency may be improved if (i) the receptor-recognizing protein has a naturally evolved ability to interact with sialic acid and (ii) the choice of vector is based on the charge in the hexon loops. Ad37 and possibly some other subgenus D adenoviruses use sialic acid to attach to and enter target cells. Furthermore, none of the subgenus D adenovirus hexons sequenced so far have been found to contain the acidic loops of subgenus C adenoviruses (13), a fact which may make them better candidates as vectors for gene therapy.
In conclusion, we believe that this novel information on sialic acid as a receptor for Ad37 will further the understanding of adenovirus tropisms and may contribute substantially to the development of functional and efficient adenovirus vectors for gene therapy.
ACKNOWLEDGMENTS
We are indebted to P. Boulanger for the suggestion of using neuraminidase, to J. M. Bergelson and R. W. Finberg for CHO transfectants, and to D. Fradelizi for Daudi MHCI-α2-expressing cells.
This work was supported by the Swedish Medical Research Council (grant 16x-05688) and by the Fund for Advanced Research at Umeå University Hospital.
REFERENCES
- 1.Akusjärvi G, Aleström P, Pettersson M, Lager M, Jörnvall H, Pettersson U. The gene for adenovirus 2 hexon protein. J Biol Chem. 1984;25:13976–13979. [PubMed] [Google Scholar]
- 2.Araki-Sasaki K, Ohasi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 3.Arcasoy S M, Latoche J, Gondor M, Watkins S C, Henderson R A, Hughey R, Finn O J, Pilewski J M. MUC1 and other sialoglycoconjugates inhibit adenovirus-mediated gene transfer to epithelial cells. Am J Respir Cell Mol Biol. 1997;17:422–435. doi: 10.1165/ajrcmb.17.4.2714. [DOI] [PubMed] [Google Scholar]
- 4.Arnberg N, Mei Y, Wadell G. Fiber genes of adenoviruses with tropism for the eye and the genital tract. Virology. 1997;227:239–244. doi: 10.1006/viro.1996.8269. [DOI] [PubMed] [Google Scholar]
- 5.Basak S, Turner H, Parr S. Identification of a 40–42 kDa attachment polypeptide for canine parvovirus in A72 cells. Virology. 1994;205:7–16. doi: 10.1006/viro.1994.1614. [DOI] [PubMed] [Google Scholar]
- 6.Belin M-T, Boulanger P. Involvement of cellular adhesion sequences in attachment of adenovirus to the HeLa cell surface. J Gen Virol. 1993;74:1485–1497. doi: 10.1099/0022-1317-74-8-1485. [DOI] [PubMed] [Google Scholar]
- 7.Bell S D, Rondon Rota T, McComb D E. Adenoviruses isolated from Saudi Arabia. Six new serotypes. J Virol. 1960;9:523–526. [Google Scholar]
- 8.Bennett F M, Law B B, Hamilton W, MacDonald A. Adenovirus eye infection in Aberdeen. Lancet. 1957;ii:670–673. doi: 10.1016/s0140-6736(57)92109-8. [DOI] [PubMed] [Google Scholar]
- 9.Bergelson J M, St. John N, Kawaguchi S, Chan M, Stubdal H, Modlin J, Finberg R W. Infection by echoviruses 1 and 8 depends on the α2 subunit of human VLA-2. J Virol. 1993;67:6847–6852. doi: 10.1128/jvi.67.11.6847-6852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 11.Boulanger P A, Houdret N, Scharfman A, Lemay P. The role of surface sialic acid in adenovirus-cell adsorption. J Gen Virol. 1972;16:429–434. doi: 10.1099/0022-1317-16-3-429. [DOI] [PubMed] [Google Scholar]
- 12.Chen M H, Benjamin T. Roles of N-glycans in α2,6 as well as α2,3 linked sialic acid in infection by polyoma virus. Virology. 1997;233:440–442. doi: 10.1006/viro.1997.8596. [DOI] [PubMed] [Google Scholar]
- 13.Crawford-Miksza L, Schnurr D P. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol. 1996;70:1836–1844. doi: 10.1128/jvi.70.3.1836-1844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crystal R G, Jaffe A, Brody S, Mastrangeli A, McElvaney N G, Rosenfeld M E, Chu C, Danel C, Hay J, Eissa T. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Hum Gene Ther. 1995;6:643–666. doi: 10.1089/hum.1995.6.5-643. [DOI] [PubMed] [Google Scholar]
- 15.Davidson E, Kirby I, Elliott T, Santis G. The human HLA-A*0201 allele, expressed in hamster cells, is not a high-affinity receptor for adenovirus type 5 fiber. J Virol. 1999;73:4513–4517. doi: 10.1128/jvi.73.5.4513-4517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Defer C, Belin M, Caillet-Boudin M, Boulanger P. Human adenovirus-host interactions: comparative study with members from subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong J C, Wigand R, Wadell G, Keller D, Muzerie C J, Wermenbol A G, Schaap G J P. Adenovirus 37: identification and characterization of a medically important new adenovirus type of subgroup D. J Med Virol. 1981;7:105–118. doi: 10.1002/jmv.1890070204. [DOI] [PubMed] [Google Scholar]
- 18.Deutscher S L, Nuwayhid N, Stanley P, Barak Briles E I, Hirschberg C B. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984;39:295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- 19.Eiz B, Pring-Åkerblom P. Molecular characterization of the type-specific γ-determinant located on the adenovirus fiber. J Virol. 1997;71:6576–6581. doi: 10.1128/jvi.71.9.6576-6581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelhardt J F, Yang Y, Stratford-Perricaudet L D, Allen E D, Kozarsky K, Perricaudet M, Yankaskas J R, Wilson J M. Direct gene transfer of human CFTR into bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993;4:27–34. doi: 10.1038/ng0593-27. [DOI] [PubMed] [Google Scholar]
- 21.Gall J, Kass-Eisler A, Leinwand L, Falck-Pedersen E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J Virol. 1996;70:2116–2123. doi: 10.1128/jvi.70.4.2116-2123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harnett G B, Phillips P A, Gollow M M. Association of genital adenovirus infection with urethritis in men. Med J Aust. 1984;141:337–338. doi: 10.5694/j.1326-5377.1984.tb132799.x. [DOI] [PubMed] [Google Scholar]
- 23.Hennache B, Boulanger P. Biochemical study of KB-cell receptor for adenovirus. Biochem J. 1977;166:237–247. doi: 10.1042/bj1660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hierholzer J C, Guyer B, O'Day D, Schaffner W. Adenovirus type 19 keratoconjunctivitis. N Engl J Med. 1974;290:1436. doi: 10.1056/nejm197406202902512. [DOI] [PubMed] [Google Scholar]
- 25.Hong S S, Karayan L, Tournier J, Curiel D T, Boulanger P A. Adenovirus type 5 fiber knob binds to MHC class I α2 domain on the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang S, Reddy V, Dasgupta N, Nemerow G R. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J Virol. 1999;73:2798–2802. doi: 10.1128/jvi.73.4.2798-2802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jawetz E, Kimura S, Nicholas A N, Thygeson P, Hanna L. New type of APC virus from keratoconjunctivitis. Science. 1955;122:1190–1192. doi: 10.1126/science.122.3181.1190-a. [DOI] [PubMed] [Google Scholar]
- 28.Kemp M C, Hierholzer J C, Cabradilla C P, Obijeski J F. The changing etiology of epidemic keratoconjunctivitis: antigenic and restriction enzyme analyses of adenovirus types 19 and 37 isolated over a 10-year period. J Infect Dis. 1983;148:24–33. doi: 10.1093/infdis/148.1.24. [DOI] [PubMed] [Google Scholar]
- 29.Liu C K, Wei G, Atwood W J. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing α(2→6)-linked sialic acids. J Virol. 1998;72:4643–4649. doi: 10.1128/jvi.72.6.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louis N, Fender P, Barge A, Kitts P, Chroboczek J. Cell-binding domain of adenovirus serotype 2 fiber. J Virol. 1994;68:4104–4106. doi: 10.1128/jvi.68.6.4104-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathias P, Galleno M, Nemerow G R. Interactions of soluble recombinant integrin αvβ5 with human adenoviruses. J Virol. 1998;72:8669–8675. doi: 10.1128/jvi.72.11.8669-8675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murray E S, Chang R S, Bell S D, Jr, Tarizzo M L, Snyder J C. Agents recovered from acute conjunctivitis cases in Saudi Arabia. Am J Opththalmol. 1957;4:32. [Google Scholar]
- 34.Phillips P A, Harnett G B, Gollow M M. Adenovirus type 19 and a closely related new serotype in genital infections. Br J Vener Dis. 1982;58:131–132. doi: 10.1136/sti.58.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pring-Åkerblom P, Heim A, Trijssenaar F E J. Molecular characterization of hemagglutination domains on the fibers of subgenus D adenoviruses. J Virol. 1998;72:2297–2304. doi: 10.1128/jvi.72.3.2297-2304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quillet A, Presse F, Marchiol-Fournigault C, Harel-Bellan A, Benbunan M, Ploegh H, Fradelizi D. Increased resistance to non-MHC-restricted cytotoxicity related to HLA A, B expression: direct demonstration using β2-microglobulin-transfected Daudi cells. J Immunol. 1988;141:17–20. [PubMed] [Google Scholar]
- 38.Roelvink P W, Lizonova A, Lee J G M, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roelvink P W, Kovesdi I, Wickham T J. Comparative analysis of adenovirus fiber-cell interaction: adenovirus type 2 (Ad2) and Ad9 utilize the same cellular fiber receptor but use different binding strategies for attachment. J Virol. 1996;70:7614–7621. doi: 10.1128/jvi.70.11.7614-7621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenk T. Adenoviridae: the viruses and their replication. In: Fields B, editor. Virology. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. pp. 2111–2148. [Google Scholar]
- 41.Smith C A, Woodruff L S, Rooney C, Kitchingman G R. Extensive cross-reactivity of adenovirus-specific cytotoxic T cells. Hum Gene Ther. 1998;9:1419–1427. doi: 10.1089/hum.1998.9.10-1419. [DOI] [PubMed] [Google Scholar]
- 42.Stanley P, Callibot V, Siminovitch L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cells. Cell. 1975;6:121–128. doi: 10.1016/0092-8674(75)90002-1. [DOI] [PubMed] [Google Scholar]
- 43.Stanley P, Siminovitch L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 1977;3:391–405. doi: 10.1007/BF01542968. [DOI] [PubMed] [Google Scholar]
- 44.Stevenson S C, Rollence M, White B, Weaver L, McClelland A. Human adenovirus serotypes 3 and 5 bind to two different cellular receptors via the fiber head domain. J Virol. 1995;69:2850–2857. doi: 10.1128/jvi.69.5.2850-2857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki Y. Gangliosides as influenza virus receptors. Variation of influenza viruses and their recognition of the receptor sialo-sugar chains. Prog Lipid Res. 1994;33:429–457. doi: 10.1016/0163-7827(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 46.Swenson P D, Lowens M S, Celum C L, Hierholzer J C. Adenovirus types 2, 8, and 37 associated with genital infections in patients attending a sexually transmitted disease clinic. J Clin Microbiol. 1995;33:2728–2731. doi: 10.1128/jcm.33.10.2728-2731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thacker T C, Johnson F B. Binding of bovine parvovirus to erythrocyte membrane sialylglycoproteins. J Gen Virol. 1998;79:2163–2169. doi: 10.1099/0022-1317-79-9-2163. [DOI] [PubMed] [Google Scholar]
- 48.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toogood C I, Murali R, Burnett R M, Hay R T. The adenovirus type 40 hexon: sequence, predicted structure and relationship to other adenovirus hexons. J Gen Virol. 1989;70:3203–3214. doi: 10.1099/0022-1317-70-12-3203. [DOI] [PubMed] [Google Scholar]
- 50.Wadell G. Hemagglutination with adenoviruses belonging to Rosen's subgroups II and III. Proc Soc Exp Biol Med. 1969;132:413–421. doi: 10.3181/00379727-132-34227. [DOI] [PubMed] [Google Scholar]
- 51.Wadell G, de Jong J C. Restriction endonucleases in identification of genome type of adenovirus 19 associated with keratoconjunctivitis. Infect Immun. 1980;27:292–296. doi: 10.1128/iai.27.2.292-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wadell G, Hammarskjöld M-L, Winberg G, Varsanyi T M, Sundell G. Genetic variability of adenoviruses. Ann N Y Acad Sci. 1980;354:16–42. doi: 10.1111/j.1749-6632.1980.tb27955.x. [DOI] [PubMed] [Google Scholar]
- 53.Wadell G, Sundell G, de Jong J C. Characterization of candidate adenovirus 37 by SDS-polyacrylamide gel electrophoresis of virion polypeptides and DNA restriction site mapping. J Med Virol. 1981;7:119–125. doi: 10.1002/jmv.1890070205. [DOI] [PubMed] [Google Scholar]
- 54.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 55.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]