Abstract
Retrospective tracing of somatic mutations predicted that most cells in the human body could be traced back to a single cell of the 2-cell stage embryo. Accordingly, a recent prospective study of the developmental trajectory of blastomeres in human embryos confirmed that progeny of the first 2-cell stage blastomere to divide generates more epiblast cells (future body). How the 2-cell blastomeres differ is unknown. Here, we show that 2-cell stage blastomeres in human embryos are asymmetric; they differ in size and the bigger blastomere divides first to 4-cell stage. We propose that this asymmetry might originate differences in cell fate.
Figure 1. Analysis of blastomere size and time of division in 2-cell human embryos .
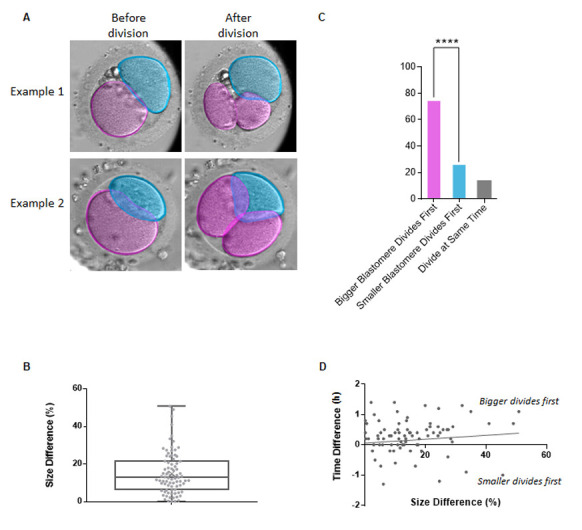
A. Schematic of the analysis used to generate data from human Embryoscope TM time-lapse movies. Two examples (single plane snapshots) from the movies are shown: before and after division of the bigger blastomere, pseudocolored in pink. The smaller blastomere is pseudocolored in blue. B. Quantification of the size difference between the two blastomeres of the 2-cell stage human embryo. Each datapoint represents the percentile difference between the two cells within individual 2-cell stage embryos. The centre line marks the median, the box contains the 25 th -75 th centile of the dataset, the whiskers mark the highest and lowest value, and the cross sign marks the mean. C. Quantification of order of division of blastomeres in 2-cell human embryos in relation to their size. Student’s t-test, p = 0.0000096 (****). n = 83 embryos. D . Scatter plot showing the correlation between % size difference (bigger/smaller) of the blastomeres of two-cell stage human embryos vs difference in time of their division (in hours, time of smaller blastomere division – time of bigger blastomere division). R = 0.04, p = 0.71.
Description
Whether blastomeres of mammalian embryos show any tendency to follow a particular developmental pathway has been under debate for many years. During the first cleavage divisions of fertilized mouse eggs, blastomeres have similar morphology which is not indicative of their future fate. Cell fate specification becomes apparent in development when asymmetric cell divisions direct one daughter cell to the inside of the embryo and the other to the outside. Inside cells generate the inner cell mass (ICM) of the blastocyst, which gives rise to the pluripotent epiblast (the future body) and primitive endoderm/hypoblast (the future yolk sac), while the outer cells differentiate into extraembryonic trophectoderm (TE) (the future placenta) (Molè et al., 2020).
Developmental plasticity of mouse embryos led to a conclusion that all early blastomeres contribute equally to development, as removing an individual cell from the embryo (Tarkowski, 1959; Tarkowski et al., 2001, 2005, 2010) or cutting away their animal or vegetal poles is not detrimental for subsequent development (Zernicka-Goetz, 1998) . This contrasts to similar experiments in the deterministic, lineage-based development of many non-mammalian species, for example C.elegans (Munro & Bowerman, 2009) , Drosophila (St Johnston & Niisslein-Volhardt, 1992) and Xenopus (Houston, 2017) , where removing part of the embryo compromises specific aspects of development. Indeed both 2-cell stage, and most 4-cell stage, blastomeres of the mouse embryo contribute to both ICM and TE lineages. However, lineage tracing studies and gene expression analysis unexpectedly showed that 2- or 4-cell blastomeres have differing developmental potential, depending on their orientation and order of cell divisions (Bischoff et al., 2008; Casser et al., 2017; Gardner, 1997, 2001; Goolam et al., 2016; Krawczyk et al., 2021; Piotrowska et al., 2001; Piotrowska-Nitsche et al., 2005; Sun et al., 2020; Torres-Padilla et al., 2007; White et al., 2016) . Moreover, it was found that cells internalized at the 8- to 16-cell stage tended to contribute to the embryonic epiblast, whereas those internalized at the 16- to 32- or 32- to 64-cell stage tended to contribute to the extra-embryonic primitive endoderm (future yolk sac) (Morris et al., 2010) . The balance of inside and outside cells and so the composition of embryonic and extra-embryonic tissues is thus influenced by the number and timing of symmetric and asymmetric divisions in mouse embryos (Fleming, 1987; Johnson & Ziomek, 1981; Morris et al., 2010, 2013) . The unexpected nature of these findings made them contentious (Dietrich & Hiiragi, 2007) . However, support was gained from the discovery of molecular heterogeneities in gene expression and epigenetic modifications in mouse embryos that were shown to affect the contribution of a blastomere to specific lineages (Goolam et al., 2016; Lim et al., 2020; Panamarova et al., 2016; Torres-Padilla et al., 2007; J. Wang et al., 2018) .
In humans, the retrospective tracking of somatic mutations and lineage reconstruction recently predicted that in adults, the majority of human organs are derived from only one of two blastomeres at the 2-cell stage (Coorens et al., 2021; Fasching et al., 2021; Park et al., 2021; Spencer Chapman et al., 2021) . Similarly, retrospective tracking of mutations in the placenta and analysis of lineage contribution imbalances also led back to the 2-cell stage embryo (Coorens et al., 2021) . However, due to the retrospective nature of these studies, how these clonal differences arise remained unclear. To gain insight into this question, a recent study followed the developmental trajectory of blastomeres in live human embryos from the first cleavage division until the establishment of the three lineages in the blastocyst (Junyent et al., 2024) . This work showed that only one of the two 2-cell stage blastomeres contributes the majority of cells to the future body. Specifically, the first 2-cell blastomere to divide to the 4-cell stage has a higher likelihood of undertaking more asymmetric cell divisions, generating more of the epiblast, that will form the future body (Junyent at al., 2024) .
The above findings raise the question of whether, and if so how, the first two blastomeres differ to give this advantage in the timing of cell division. It was shown that in invertebrates asymmetric cell divisions in early development may play a role in establishing cell fate (Aoki & Shimizu, 2017; Munro & Bowerman, 2009; Ren & Weisblat, 2006; Shimizu et al., 1998; Wang & Seydoux, 2013) . Similarly, asymmetric cell divisions at the early stages of development are present in Xenopus laevis (Tassan et al., 2017) , which is an example of a vertebrate with deterministic type development. However, there are currently no well-documented examples of mammalian embryos displaying consistent blastomere size differences at the early cleavage stages. Although, in contrast to mouse, it has been observed that human blastomeres of the 2-cell stage embryo may differ in size, this observation has been linked to embryo quality, not the fate of the individual blastomeres (Hardarson et al., 2001; Holte et al., 2007) . We wished to test a hypothesis that differences in blastomere size at the 2-cell stage could be linked to their order of division. However, there are several limitations for studies using early human embryos. First, access to human embryos at the 2-cell stage is extremely limited. Research-consented embryos can be sourced from in vitro fertilization (IVF) clinics and this process relies on patient donation. However, early embryo freezing is no longer common clinical practice as embryos are commonly cultured to the blastocyst stage and only then would these be frozen for future use.
Thus, to approach this question, we acquired access to time-lapse movies of human embryo development obtained from an IVF clinic. The movies were acquired with the Embryoscope TM system and covered development from the zygote to the blastocyst stage. The movies used in this analysis were multi-focal time-lapse movies allowing us to trace blastomere division over time in several focal planes. Use of brightfield microscopy has limited potential for accurate segmentation and volumetric quantifications. Thus, as a proxy for overall blastomere size, we had to rely upon cell area at its mid-point, which approximates the maximal measured area between focal planes ( Fig. 1A, Methods ). We analysed movies of 83 dividing 2-cell stage embryos and measured the area of each blastomere 5 frames before division, recording which cell divided first ( Fig. 1A, Methods ).
We observed that overall, one blastomere had an area that was 15.3% (+/- 1.37%) larger than the other cell ( Fig. 1B ). Importantly, in 68.7% (57/83 embryos) of all embryos, the larger of the two blastomeres divided first ( Fig. 1C ). In the remaining 12.0% (10/83 embryos), the blastomeres divided at the same time and only in 19.3% (16/83 embryos) the smaller blastomere divided first. We did not observe a clear correlation between blastomere size difference and the degree of asynchrony ( Fig. 1D ).
The occurrence of unevenly sized blastomeres in early human development has been linked to potential aneuploidies and embryo quality (Hardarson et al., 2001; Holte et al., 2007) . However, our analysis included only movies of embryos that resulted in successful live births, which validates the quality of the embryos analysed and shows that differences in blastomere size in human embryos are not detrimental to embryo development.
The implication of these observations is that the first cleavage of the human zygote tends to divide the cell asymmetrically to generate daughter cells of differing size, which endows the larger cell with the capability of dividing ahead of its smaller sister cell at the next cleavage. In Junyent et al. (2024), it has been shown that the first blastomere to divide at the 2-cell stage generates daughter cells that also tend to divide first at the 4-cell and 8-cell stages, effectively giving it a higher probability to undergo the first asymmetric cell division and contribute preferentially to the ICM and so the future body. Although the quality of Embryoscope TM movies obtained from the clinic do not permit an accurate measure of cell volume, the result is nevertheless clear from these recordings. This finding also accords with earlier findings in the mouse embryo, where the 2-cell blastomere that divides first was found to contribute preferentially to the embryonic part of the blastocyst which contains the ICM (Piotrowska et al., 2001) , raising the possibility that this may be a common feature of mammalian embryos.
The mechanism behind the asymmetry of the first cleavage responsible for the difference in size between 2-cell blastomeres remains unknown. It was previously observed that the position and orientation of the first cleavage plane in the mouse embryo could be influenced by both the site of sperm entry into the egg (Piotrowska & Zernicka-Goetz, 2001; Plusa et al., 2002) and the site of the meiotic division in the oocyte (Plusa et al., 2002) , events which influence the organisation of the cortical cytoskeleton. In this case, it is possible that the zygote carries positional information established in the highly asymmetric meiotic divisions that influences subsequent cleavage events. However, a full mechanistic explanation of this phenomenon is still lacking. It cannot be discounted that the generation of 2-cell blastomeres of differing size is a stochastic event and that it is cell size per se that influences the timing of cleavage. It is plausible to imagine that the bigger blastomere may contain more organelles or other factors, such as mitochondria, which may result in a higher energetic status of this cell. Indeed, it has been shown that selective inheritance of distinct mitochondrial age-classes might act as a cell fate determinant in the asymmetric division of epithelial stem cell-like cells (Döhla et al., 2022). On the other hand, in the bigger blastomere some factors may be less concentrated in comparison to the smaller blastomere due to higher cytoplasmic volume. It has been shown human cell growth dilutes cell cycle inhibitor Retinoblastoma protein to trigger division (Zatulovskiy et al., 2020) . If similar inhibitors were present in the cytoplasm of 2-cell human embryos, they may be less concentrated in the bigger blastomeres. However, at these stage, the cells are not growing but beginning rounds of division into smaller cells at the onset of cleavage.
It is postulated that even small asymmetries between the blastomeres at the 2-cell stage can be amplified with time and translate to different cell fate (Chen et al., 2018) . This is consistent with the observations that asymmetries in the timing of division in 2-cell embryos could be linked to the asymmetries that contribute to the ICM during the 8- to 16-cell stage division (Junyent et al., 2024) . Our current observations point towards the influence of size differences between 2-cell blastomeres upon division timing. Future studies will be required to understand both the mechanisms whereby cell size exerts this effect, and the events that lead to the asymmetry of the first cleavage division of the zygote, resulting in this differential size between daughter cells.
Methods
Embryoscope TM time-lapse movies
Embryoscope TM time-lapse movies capturing preimplantation development of human embryos from the zygote to blastocyst stage were provided by IVIRMA-Valencia (IVI Foundation, Spain). These movies were generated during routine IVF clinical practice, when the embryos were placed in time-lapse incubators. The use of movies for retrospective analysis was approved by the Research Ethics Committee of IVI Valencia (IRB protocol number 2203-VLC-028-MD). The identities of embryos that had been filmed were anonymized to the research team. Each movie contained transmitted light images on 11 focal planes with an average imaging frequency of 15 minutes. All samples represented embryos that resulted in successful pregnancies and live births, which validates the quality of the sample analysed.
Measurements and quantification:
Fiji (ImageJ) (Schindelin et al., 2012) was used to manually track blastomeres, annotate timing of the division and measure blastomere area. Movies with embryos positioned in a way that would make measurements inaccurate, embryos with blastomere morphology not allowing for accurate measurements, and low-quality movies were rejected from the analysis. Blastomeres were considered to have divided when the first frame with full completion of the cytokinesis could be observed. This timepoint was annotated as the timing of the division for each individual blastomere. Blastomere size measurements were taken 5 frames before the first sign of entry into mitosis could be observed (i.e. nuclear envelope breakdown and entry of nuclei into prophase, which can be clearly observed in bright field). The area of each 2-cell stage blastomere was used as a proxy for overall blastomere size. The focal plane with the largest blastomere area was always selected for the measurements.
Acknowledgments
Acknowledgments
We thank Magdalena Zernicka-Goetz, David Glover and Sergi Junyent for invaluable discussions. We thank Maria Luisa Pardinas and Maria José De los Santos Molina and the team at IVIRMA Valencia (IVI Foundation) for granting us access to the Embryoscope TM human embryo movies.
Funding Statement
<p>We thank Wellcome Trust funding (PMAG/636) that funds research in Zernicka-Goetz lab.</p>
References
- Aoki Momoe, Shimizu Takashi. Transcriptional control of unequal cleavage in early Tubifex embryos. Development Genes and Evolution. 2017 Jun 17;227(4):279–287. doi: 10.1007/s00427-017-0584-5. [DOI] [PubMed] [Google Scholar]
- Bischoff Marcus, Parfitt David-Emlyn, Zernicka-Goetz Magdalena. Formation of the embryonic-abembryonic axis of the mouse blastocyst:relationships between orientation of early cleavage divisions and pattern of symmetric/asymmetric divisions. Development. 2008 Mar 1;135(5):953–962. doi: 10.1242/dev.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casser E., Israel S., Witten A., Schulte K., Schlatt S., Nordhoff V., Boiani M. Totipotency segregates between the sister blastomeres of two-cell stage mouse embryos. Scientific Reports. 2017 Aug 15;7(1) doi: 10.1038/s41598-017-08266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Shi J, Tao Y, Zernicka-Goetz M. Tracing the origin of heterogeneity and symmetry breaking in the early mammalian embryo. Nat Commun. 2018 May 8;9(1):1819–1819. doi: 10.1038/s41467-018-04155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorens Tim H. H., Moore Luiza, Robinson Philip S., Sanghvi Rashesh, Christopher Joseph, Hewinson James, Przybilla Moritz J., Lawson Andrew R. J., Spencer Chapman Michael, Cagan Alex, Oliver Thomas R. W., Neville Matthew D. C., Hooks Yvette, Noorani Ayesha, Mitchell Thomas J., Fitzgerald Rebecca C., Campbell Peter J., Martincorena Iñigo, Rahbari Raheleh, Stratton Michael R. Extensive phylogenies of human development inferred from somatic mutations. Nature. 2021 Aug 25;597(7876):387–392. doi: 10.1038/s41586-021-03790-y. [DOI] [PubMed] [Google Scholar]
- Coorens Tim H. H., Oliver Thomas R. W., Sanghvi Rashesh, Sovio Ulla, Cook Emma, Vento-Tormo Roser, Haniffa Muzlifah, Young Matthew D., Rahbari Raheleh, Sebire Neil, Campbell Peter J., Charnock-Jones D. Stephen, Smith Gordon C. S., Behjati Sam. Inherent mosaicism and extensive mutation of human placentas. Nature. 2021 Mar 10;592(7852):80–85. doi: 10.1038/s41586-021-03345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich Jens-Erik, Hiiragi Takashi. Stochastic patterning in the mouse pre-implantation embryo. Development. 2007 Dec 1;134(23):4219–4231. doi: 10.1242/dev.003798. [DOI] [PubMed] [Google Scholar]
- Döhla J, Kuuluvainen E, Gebert N, Amaral A, Englund JI, Gopalakrishnan S, Konovalova S, Nieminen AI, Salminen ES, Torregrosa Muñumer R, Ahlqvist K, Yang Y, Bui H, Otonkoski T, Käkelä R, Hietakangas V, Tyynismaa H, Ori A, Katajisto P. Metabolic determination of cell fate through selective inheritance of mitochondria. Nat Cell Biol. 2022 Feb 14;24(2):148–154. doi: 10.1038/s41556-021-00837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasching, Liana, Jang, Yeongjun, Tomasi, Simone, Schreiner, Jeremy, Tomasini, Livia, Brady, Melanie V, et al., Vaccarino, Flora M. 2021. Early developmental asymmetries in cell lineage trees in living individuals. [DOI] [PMC free article] [PubMed]
- Fleming, Tom P 1987. A Quantitative Analysis of Cell Allocation to Trophectoderm and Inner Cell Mass in the Mouse Blastocyst. DEVELOPMENTAL BIOLOGY. 119: 520. [DOI] [PubMed]
- Gardner R. L. The early blastocyst is bilaterally symmetrical and its axis of symmetry is aligned with the animal-vegetal axis of the zygote in the mouse. Development. 1997 Jan 15;124(2):289–301. doi: 10.1242/dev.124.2.289. [DOI] [PubMed] [Google Scholar]
- Gardner R. L. Specification of embryonic axes begins before cleavage in normal mouse development. Development. 2001 Mar 15;128(6):839–847. doi: 10.1242/dev.128.6.839. [DOI] [PubMed] [Google Scholar]
- Goolam Mubeen, Scialdone Antonio, Graham Sarah J.L., Macaulay Iain C., Jedrusik Agnieszka, Hupalowska Anna, Voet Thierry, Marioni John C., Zernicka-Goetz Magdalena. Heterogeneity in Oct4 and Sox2 Targets Biases Cell Fate in 4-Cell Mouse Embryos. Cell. 2016 Mar 1;165(1):61–74. doi: 10.1016/j.cell.2016.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardarson, Thorir, Hanson, Charles, Sjögren, Anita, Lundin, Kersti 2001. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Human Reproduction. 16: 313. [DOI] [PubMed]
- Holte J., Berglund L., Milton K., Garello C., Gennarelli G., Revelli A., Bergh T. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Human Reproduction. 2006 Nov 9;22(2):548–557. doi: 10.1093/humrep/del403. [DOI] [PubMed] [Google Scholar]
- Houston Douglas W. Vertebrate Axial Patterning: From Egg to Asymmetry. Advances in Experimental Medicine and Biology. 2016 Dec 15;:209–306. doi: 10.1007/978-3-319-46095-6_6. [DOI] [PMC free article] [PubMed]
- Johnson, Martin H, Ziomek, Carol Ann 1981. The Foundation of Two Distinct Cell Lineages within the Mouse Morula. Cell. 24: 71. [DOI] [PubMed]
- Junyent Sergi, Meglicki Maciej, Vetter Roman, Mandelbaum Rachel, King Catherine, Patel Ekta M., Iwamoto-Stohl Lisa, Reynell Clare, Chen Dong-Yuan, Rubino Patrizia, Arrach Nabil, Paulson Richard J., Iber Dagmar, Zernicka-Goetz Magdalena. The first two blastomeres contribute unequally to the human embryo. Cell. 2024 May 1; doi: 10.1016/j.cell.2024.04.029. [DOI] [PubMed] [Google Scholar]
- Krawczyk Katarzyna, Kosyl Ewa, Częścik-Łysyszyn Karolina, Wyszomirski Tomasz, Maleszewski Marek. Developmental capacity is unevenly distributed among single blastomeres of 2-cell and 4-cell stage mouse embryos. Scientific Reports. 2021 Nov 2;11(1) doi: 10.1038/s41598-021-00834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Hui Yi Grace, Alvarez Yanina D., Gasnier Maxime, Wang Yiming, Tetlak Piotr, Bissiere Stephanie, Wang Hongmei, Biro Maté, Plachta Nicolas. Keratins are asymmetrically inherited fate determinants in the mammalian embryo. Nature. 2020 Aug 26;585(7825):404–409. doi: 10.1038/s41586-020-2647-4. [DOI] [PubMed] [Google Scholar]
- Molè Matteo A., Weberling Antonia, Zernicka-Goetz Magdalena. Comparative analysis of human and mouse development: From zygote to pre-gastrulation. Gastrulation: From Embryonic Pattern to Form. 2020:113–138. doi: 10.1016/bs.ctdb.2019.10.002. [DOI] [PubMed]
- Morris Samantha A., Graham Sarah J. L., Jedrusik Agnieszka, Zernicka-Goetz Magdalena. The differential response to Fgf signalling in cells internalized at different times influences lineage segregation in preimplantation mouse embryos. Open Biology. 2013 Nov 1;3(11):130104–130104. doi: 10.1098/rsob.130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris Samantha A., Teo Roy T. Y., Li Huiliang, Robson Paul, Glover David M., Zernicka-Goetz Magdalena. Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo. Proceedings of the National Academy of Sciences. 2010 Mar 22;107(14):6364–6369. doi: 10.1073/pnas.0915063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro E., Bowerman B. Cellular Symmetry Breaking during Caenorhabditis elegans Development. Cold Spring Harbor Perspectives in Biology. 2009 Sep 16;1(4):a003400–a003400. doi: 10.1101/cshperspect.a003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panamarova Maryna, Cox Andy, Wicher Krzysztof, Butler Richard, Bulgakova Natalia, Jeon Shin, Rosen Barry, Seong Rho H., Skarnes William, Crabtree Gerald, Zernicka-Goetz Magdalena. BAF chromatin remodelling complex is an epigenetic regulator of lineage specification in the early mouse embryo. Development. 2016 Jan 1; doi: 10.1242/dev.131961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Seongyeol, Mali Nanda Maya, Kim Ryul, Choi Jeong-Woo, Lee Junehawk, Lim Joonoh, Park Jung Min, Park Jung Woo, Kim Donghyun, Kim Taewoo, Yi Kijong, Choi June Hyug, Kwon Seong Gyu, Hong Joo Hee, Youk Jeonghwan, An Yohan, Kim Su Yeon, Oh Soo A, Kwon Youngoh, Hong Dongwan, Kim Moonkyu, Kim Dong Sun, Park Ji Young, Oh Ji Won, Ju Young Seok. Clonal dynamics in early human embryogenesis inferred from somatic mutation. Nature. 2021 Aug 25;597(7876):393–397. doi: 10.1038/s41586-021-03786-8. [DOI] [PubMed] [Google Scholar]
- Piotrowska Karolina, Wianny Florence, Pedersen Roger A., Zernicka-Goetz Magdalena. Blastomeres arising from the first cleavage division have distinguishable fates in normal mouse development. Development. 2001 Oct 1;128(19):3739–3748. doi: 10.1242/dev.128.19.3739. [DOI] [PubMed] [Google Scholar]
- Piotrowska Karolina, Zernicka-Goetz Magdalena. Role for sperm in spatial patterning of the early mouse embryo. Nature. 2001 Jan 1;409(6819):517–521. doi: 10.1038/35054069. [DOI] [PubMed] [Google Scholar]
- Piotrowska-Nitsche Karolina, Perea-Gomez Aitana, Haraguchi Seiki, Zernicka-Goetz Magdalena. Four-cell stage mouse blastomeres have different developmental properties. Development. 2005 Feb 1;132(3):479–490. doi: 10.1242/dev.01602. [DOI] [PubMed] [Google Scholar]
- Piotrowska-Nitsche Karolina, Zernicka-Goetz Magdalena. Spatial arrangement of individual 4-cell stage blastomeres and the order in which they are generated correlate with blastocyst pattern in the mouse embryo. Mechanisms of Development. 2005 Apr 1;122(4):487–500. doi: 10.1016/j.mod.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Plusa Berenika, Piotrowska Karolina, Zernicka‐Goetz Magdalena. Sperm entry position provides a surface marker for the first cleavage plane of the mouse zygote. genesis. 2002 Feb 27;32(3):193–198. doi: 10.1002/gene.10027. [DOI] [PubMed] [Google Scholar]
- Plusa Berenika, Grabarek Joanna B., Piotrowska Karolina, Glover David M., Zernicka-Goetz Magdalena. Site of the previous meiotic division defines cleavage orientation in the mouse embryo. Nature Cell Biology. 2002 Sep 23;4(10):811–815. doi: 10.1038/ncb860. [DOI] [PubMed] [Google Scholar]
- Ren Xiaoyun, Weisblat David A. Asymmetrization of first cleavage by transient disassembly of one spindle pole aster in the leech Helobdella robusta. Developmental Biology. 2006 Apr 1;292(1):103–115. doi: 10.1016/j.ydbio.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Schindelin Johannes, Arganda-Carreras Ignacio, Frise Erwin, Kaynig Verena, Longair Mark, Pietzsch Tobias, Preibisch Stephan, Rueden Curtis, Saalfeld Stephan, Schmid Benjamin, Tinevez Jean-Yves, White Daniel James, Hartenstein Volker, Eliceiri Kevin, Tomancak Pavel, Cardona Albert. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012 Jun 28;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Takashi, Ishii Ryuichi, Takahashi Hirokazu. Unequal cleavage in the early Tubifex embryo . Development, Growth & Differentiation. 1998 Jun 1;40(3):257–266. doi: 10.1046/j.1440-169x.1998.00001.x. [DOI] [PubMed] [Google Scholar]
- Spencer Chapman Michael, Ranzoni Anna Maria, Myers Brynelle, Williams Nicholas, Coorens Tim H. H., Mitchell Emily, Butler Timothy, Dawson Kevin J., Hooks Yvette, Moore Luiza, Nangalia Jyoti, Robinson Philip S., Yoshida Kenichi, Hook Elizabeth, Campbell Peter J., Cvejic Ana. Lineage tracing of human development through somatic mutations. Nature. 2021 May 12;595(7865):85–90. doi: 10.1038/s41586-021-03548-6. [DOI] [PubMed] [Google Scholar]
- St Johnston, Daniel, Niisslein-Volhardt, Christiane 1992. The Origin of Pattern and Polarity in the Drosophila Embryo Review. Cell. 68: 201. [DOI] [PubMed]
- Reproduction. 2013 Nov 18; doi: 10.1530/rep. [DOI] [Google Scholar]
- TARKOWSKI ANDRZEJ K. Experiments on the Development of Isolated Blastomeres of Mouse Eggs. Nature. 1959 Oct 1;184(4695):1286–1287. doi: 10.1038/1841286a0. [DOI] [PubMed] [Google Scholar]
- Tarkowski, Andrzej K, Ozdzenski, Waclaw, Czolowska, Renata 2001. Mouse singletons and twins developed from isolated diploid blastomeres supported with tetraploid blastomeres. Int. J. Dev. Biol. 45: 591. [PubMed]
- Tarkowski Andrzej K., Ozdzenski Waclaw, Czolowska Renata. Identical triplets and twins developed from isolated blastomeres of 8- and 16-cell mouse embryos supported with tetraploid blastomeres. The International Journal of Developmental Biology. 2005;49(7):825–832. doi: 10.1387/ijdb.052018at. [DOI] [PubMed] [Google Scholar]
- Tarkowski Andrzej K., Suwińska Aneta, Czołowska Renata, Ożdżeński Wacław. Individual blastomeres of 16- and 32-cell mouse embryos are able to develop into foetuses and mice. Developmental Biology. 2010 Dec 1;348(2):190–198. doi: 10.1016/j.ydbio.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Tassan Jean-Pierre, Wühr Martin, Hatte Guillaume, Kubiak Jacek. Asymmetries in Cell Division, Cell Size, and Furrowing in the Xenopus laevis Embryo. Results and Problems in Cell Differentiation. 2017:243–260. doi: 10.1007/978-3-319-53150-2_11. [DOI] [PubMed]
- Torres-Padilla Maria-Elena, Parfitt David-Emlyn, Kouzarides Tony, Zernicka-Goetz Magdalena. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature. 2007 Jan 1;445(7124):214–218. doi: 10.1038/nature05458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Jennifer T., Seydoux Geraldine. Germ Cell Specification. Germ Cell Development in C. elegans. 2012 Jun 28;:17–39. doi: 10.1007/978-1-4614-4015-4_2. [DOI] [PMC free article] [PubMed]
- Wang Jiaqiang, Wang Leyun, Feng Guihai, Wang Yukai, Li Yufei, Li Xin, Liu Chao, Jiao Guanyi, Huang Cheng, Shi Junchao, Zhou Tong, Chen Qi, Liu Zhonghua, Li Wei, Zhou Qi. Asymmetric Expression of LincGET Biases Cell Fate in Two-Cell Mouse Embryos. Cell. 2018 Dec 1;175(7):1887–1901.e18. doi: 10.1016/j.cell.2018.11.039. [DOI] [PubMed] [Google Scholar]
- White Melanie D., Angiolini Juan F., Alvarez Yanina D., Kaur Gurpreet, Zhao Ziqing W., Mocskos Esteban, Bruno Luciana, Bissiere Stephanie, Levi Valeria, Plachta Nicolas. Long-Lived Binding of Sox2 to DNA Predicts Cell Fate in the Four-Cell Mouse Embryo. Cell. 2016 Mar 1;165(1):75–87. doi: 10.1016/j.cell.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Zatulovskiy E, Zhang S, Berenson DF, Topacio BR, Skotheim JM. Cell growth dilutes the cell cycle inhibitor Rb to trigger cell division. Science. 2020 Jul 24;369(6502):466–471. doi: 10.1126/science.aaz6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernicka-Goetz Magdalena. Fertile offspring derived from mammalian eggs lacking either animal or vegetal poles. Development. 1998 Dec 1;125(23):4803–4808. doi: 10.1242/dev.125.23.4803. [DOI] [PubMed] [Google Scholar]


