ABSTRACT
Peritoneal dialysis (PD) and home hemodialysis (HHD) are the two home dialysis modalities offered to patients. They promote patient autonomy, enhance independence, and are generally associated with better quality of life compared to facility hemodialysis. PD offers some advantages (enhanced flexibility, ability to travel, preservation of residual kidney function, and vascular access sites) but few patients remain on PD indefinitely due to peritonitis and other complications. By contrast, HHD incurs longer and more intensive training combined with increased upfront health costs compared to PD, but is easier to sustain in the long term. As a result, the integrated home dialysis model was proposed to combine the advantages of both home-based dialysis modalities. In this paradigm, patients are encouraged to initiate dialysis on PD and transfer to HHD after PD termination. Available evidence demonstrates the feasibility and safety of this approach and some observational studies have shown that patients who undergo the PD-to-HHD transition have clinical outcomes comparable to patients who initiate dialysis directly on HHD. Nevertheless, the prevalence of PD-to-HHD transfers remains low, reflecting the multiple barriers that prevent the full uptake of home-to-home transitions, notably a lack of awareness about the model, home-care “burnout,” clinical inertia after a transfer to facility HD, suboptimal integration of PD and HHD centers, and insufficient funding for home dialysis programs. In this review, we will examine the conceptual advantages and disadvantages of integrated home dialysis, present the evidence that underlies it, identify challenges that prevent its success and finally, propose solutions to increase its adoption.
Keywords: dialysis, home hemodialysis, integrated home dialysis, peritoneal dialysis, transition
INTRODUCTION
With effects ranging from quality of life (QOL) to clinical outcomes and health expenditures, the selection of a kidney replacement modality (KRT) is crucial for patients suffering from end-stage kidney disease (ESKD), caregivers, and their clinicians [1, 2]. Nevertheless, dialysis techniques are not lifelong, and many patients will need to transition from a modality to another during their dialysis course [3]. In that spirit, the concept of “integrated dialysis care” was introduced >20 years ago. It postulates that pre-dialysis care, dialysis initiation, and transition between modalities constitute a planned continuum in which patients use the dialysis modality that offers them the most benefits at a given moment [4–7]. Initially, this paradigm referred to the initiation of KRT with peritoneal dialysis (PD), to benefit from its initial lifestyle benefits, with a subsequent transition toward facility hemodialysis (HD). Given the growing interest in home hemodialysis (HHD) and its advantages in terms of clinical outcomes and QOL [8–12], the concept of integrated dialysis was rapidly amended to propose a PD-to-HHD transition, which was termed “integrated home dialysis” (Fig. 1) [13–15]. This new model offers patients a durable home dialysis option after PD, while maintaining the lifestyle benefits of PD at dialysis initiation. In this article, we will discuss the rationale of integrated home dialysis, review the evidence that underlies it, identify barriers that prevent its uptake, and propose future directions to maximize its success.
Figure 1:
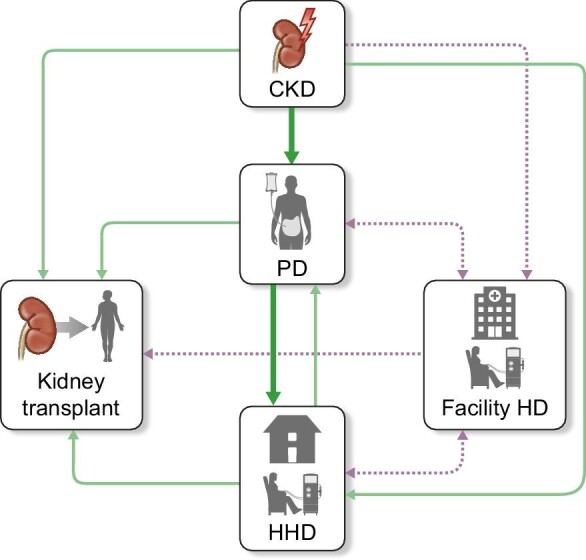
Kidney failure pathways with the integrated home dialysis model highlighted in green, other preferred transitions in light green and less preferred transitions in gray. CKD: chronic kidney disease, PD: peritoneal dialysis, HHD: home hemodialysis, HD: hemodialysis.
RATIONALE AND BENEFITS
Home-based dialysis: a cornerstone of ESKD
Home-based dialysis (namely HHD and PD) is associated with medical, economic, and lifestyle advantages, and is the preferred dialysis option for most ESKD patients [2]. Home modalities directly address patients’ and caregivers’ priorities for KRT by allowing autonomy, treatment ownership, and flexibility [16–20]. Furthermore, they are generally associated with improvements in QOL [20–25] and healthcare costs [26–32] compared with facility HD. Therefore, when provided with adequate education and shared decision-making, most patients will select HHD or PD as their modality of choice [17, 33–36]. Home dialysis is also a governmental priority, with the USA targeting 80% of incident ESKD patients to receive a kidney transplant or initiate KRT in home dialysis for 2025 and the UK aiming for 20% of prevalent KRT patients to be on PD or HHD [37, 38]. Nevertheless, except for rare jurisdictions with high PD rates (such as El Salvador, Hong Kong, Colombia, Australia, New Zealand, and Canada), home dialysis prevalence remains below 15% in most countries [39–41].
Initial advantages of PD
While PD and HHD share benefits compared to facility HD, initiating KRT in PD offers some unique advantages [42, 43]. Among these, PD offers a continuous and more gentle ultrafiltration than HD and, consequently, protects residual kidney function (RKF) for longer and at higher levels, with estimated rates of decline 20 to 80% less than with HD [44–48]. Preservation of RKF has been repeatedly linked to improved survival in both HD and PD [49–54], potentially due to improved blood pressure management, phosphate control, nutritional status, and cytokine elimination [48, 55]. This smoother ultrafiltration and ensuing hemodynamic stability also allow easier volume removal, especially in patients with heart failure for whom poor tolerance for higher-rate ultrafiltration may lead to intradialytic hypotension and volume overload [56]. Similarly, PD protects potential vascular access sites for eventual hemodialysis and is associated with reduced procedures to maintain access patency compared to HD [57]. PD has also been linked to reduction in bacteremia episodes and hepatitis transmission in comparison with HD [58–60].
PD as the initial dialysis modality might also enhance QOL and lifestyle compared to HHD. While formal comparisons of QOL between PD and HHD remain scarce [25, 61, 62], PD has the theoretical benefits of allowing travel and improving schedule flexibility and mobility compared to HHD [63]. Likewise, encouraging PD as the initial KRT modality might offer economic benefits, as shown by several studies observing financial advantages to PD in terms of overall costs and cost per quality-adjusted life years (QALY) [32, 64–67].
Moreover, PD can ease the transition into dialysis for patients and healthcare systems. For example, the flexibility and preservation of RKF associated with PD facilitate incremental dialysis, which may be more burdensome to implement with HHD [68–71]. Furthermore, the learning curve for PD is gentler than HHD, easing their entry into KRT. Accumulating evidence also suggests that PD can be safely employed in “urgent-start” settings when pre-dialysis planning has not been completed [72–77]. In this context, PD allows patients to start dialysis directly in the desired modality, rather than in facility HD with a planned transfer to home dialysis that can be compromised by clinical inertia.
The need for a transition toward home hemodialysis
Although PD is advantageous in its initial years, it is associated with uncertain long-term modality survival and recent studies report that only 14 to 27% of patients remain on PD after 5 years of treatment [78, 79]. In one of these studies using USRDS data, death during PD explained 30% of PD terminations while kidney transplantation was responsible for another 20%. Notably, jurisdictions with higher rates of transplant during PD have been associated with shorter median times on PD [80]. Nevertheless, declining RKF and changes in peritoneal function may occur with time on PD, while repeated peritonitis episodes and changes in both clinical and psychosocial characteristics may further compromise the maintenance of PD [55, 81, 82]. As a result, 40% of patients initiating PD had transferred to HD after 5 years in USRDS data, representing half of PD terminations [79]. Similar findings were observed in ERA and Peritoneal Dialysis Outcomes and Practice Patterns Study studies in which 20 to 40% of PD patients had transferred into facility HD after 5 years [80, 83]. Data from Australia and New Zealand also highlighted how causes of PD ending varied through time on therapy, with mechanical complications being more common during the first 9 months and infectious complications more frequently associated with transfer to HD afterward [84].
The initial advantages of PD, combined with its sometime limited longevity, have led several authors to suggest a transition to HHD after PD termination [13–15]. In this paradigm, termed the integrated home dialysis model, patients initiate KRT on PD and, when necessary, experience a timely transition toward HHD. This way, home dialysis patients benefit from the initial advantages of PD while maintaining dialysis at home through HHD after PD. Furthermore, if PD ending is anticipated and the transition planned accordingly, vascular access and HHD training can be completed before the need to cease PD. Thus, patients can avoid altogether the morbid and often permanent transfer into facility HD (Table 1) [85, 86].
Table 1:
Conceptual benefits of integrated home dialysis.
| Preservation of schedule flexibility during PD |
| Possibility to travel during PD |
| Ability to use incremental PD |
| Possibility of “urgent-start” home dialysis using PD |
| Easier training in home dialysis (for PD as initial modality but also for HHD considering previous self-care experience) |
| Protection of RKF |
| Preservation of vascular access sites |
| Reduced risk of bacteremia and hepatitis transmission during PD |
| Lower healthcare costs in PD compared to HHD (and in HHD compared to HD as a 2nd modality) |
| Technique longevity in HHD after the transfer from PD |
Drawbacks of the integrated home dialysis model
Among potential drawbacks of the integrated home dialysis model is the concern for patient's and/or caregiver's burnout after years on PD that might discourage the more complex learning of HHD [87]. Similarly, patients fit for HHD at dialysis initiation may deteriorate medically and cognitively during PD and could become ineligible for HHD at PD termination. Some patients might alternatively consider HHD training futile since they accumulated enough dialysis vintage to expect a kidney transplant in foreseeable future, resulting in a transfer toward facility HD rather than HHD. These concerns are especially important knowing that the first year of HHD may be more demanding, with 1-year HHD failure rates ranging up to 25% especially in older or comorbid patients [88–90]. Moreover, for patients who will terminate PD before they obtain a kidney transplant, the integrated approach implies a second home dialysis training that would not be necessary if they began KRT with HHD.
Another drawback is the hypothetical concern that patients eligible for both PD and HHD might experience worse outcomes in PD than in HHD. Past observational studies have indeed observed increased hospitalizations and decreased survival in PD compared with HHD [78, 89–92]. Nevertheless, these studies can be affected by residual confounding that cannot be eliminated by statistical methods. As an example, a Canadian study observed that the contemporary increase in HHD prevalence (and more liberal criteria for HHD initiation) led to higher rates of transfer to in-center hemodialysis in the corresponding HHD cohorts [93]. In another study, the survival “advantage” of HHD (compared to PD) was not observed in patients initiating dialysis in recent years [89]. As a whole, these findings suggest that case-mix differences in HHD and PD cohorts might confound the association between PD and adverse clinical outcomes, which is reassuring for patients contemplating integrated home dialysis.
FIRST RESULTS AND CLINICAL OUTCOMES
First clinical results
The first feasibility study of PD-to-HHD transitions was published in 2007 [14]. In this study from two Canadian dialysis centers, 69 patients terminated PD between 2003 and 2005 and eight successively transferred to nocturnal HHD (NHD) after a mean PD vintage of 4.8 years. All these patients remained on NHD without adverse events for the duration of the study and improvement in stdKt/V, blood pressure control, nutrition, anemia, and phosphate levels were observed. One of these centers recently published a longer follow-up of their PD program (826 patients terminating PD between 1996 and 2019) and reported 24 successful PD-to-HHD transfers [94]. In this study from Elbokl and colleagues (including authors from this review), technique survival in transferred patients was 86% and all remained eligible for transplantation (or were transplanted). In another study from a Canadian center conducted between 2000 and 2010, 12 PD-to-HHD transitions occurred from the 75 patients who terminated PD (mean PD vintage 2.8 years at transition) [95]. Aside from Canada, one Japanese study also reported 10 PD-to-HHD transitions in a feasibility analysis [96].
Prevalence and predictors of transition
The prevalence of PD-to-HHD transitions is difficult to establish accurately due to the scarceness of national dialysis databases and various definitions of transitions. In the three previously described studies (all conducted in Toronto, Canada), the prevalence of PD-to-HHD transitions ranged from 3% to 16% [14, 94, 95]. At the national level, a study using the Australia and New Zealand Dialysis and Transplant (ANZDATA) registry (with authors from this review) revealed that 5.4% of patients terminating PD transferred to HHD in <180 days while another study using USRDS data reported a transfer incidence of 1.6% in <90 days [97, 98]. More recently, our analysis using the Canadian Organ Replacement Registry from 2005 to 2018 found that 3.6% of terminating PD patients transferred to HHD in <90 days and 5.8% in <days [99]. However, it is worth mentioning that these studies were not designed to evaluate all transferring patients and used a 90- or 180-day window to define the PD-to-HHD transfer.
These reports also identified factors predictive of a transition to HHD after PD and the reasons for PD termination in these patients. Younger age, male sex, increased distance from facility HD centers, longer PD vintage, obesity, and white ethnicity were associated with increased odds of a PD-to-HHD transfer [95, 97, 98]. By contrast, indigenous ethnicity, cardiovascular comorbidities, and diabetic or hypertensive renal diseases were linked to a lower chance of PD-to-HHD transfer. In most studies, inadequate dialysis (ultrafiltration failure or insufficient solute clearance) was the principal cause for PD termination [14, 95, 97] while one study reported peritonitis and inadequate dialysis as similarly prevalent [94]. Although these findings contrast with the usual breakdown of reasons for PD failure (in which peritonitis and catheter-related infections predominate) [100–104], this discrepancy is expected: inadequate dialysis is clinically predictable and probably enhances the probability of a successful transfer to HHD, while peritonitis often leads to urgent-start facility HD.
Long-term clinical outcomes
Four observational studies have reported long-term clinical outcomes associated with a PD-to-HHD transition. In one US study, USRDS and NxStage databases were linked to identify patients who underwent PD in the 90 days preceding HHD [98]. These PD-to-HHD patients had technique failure rates of 19% (1 year) and 30% (3 years), which stabilized afterwards. In a matched analysis, PD-to-HHD patients had a significant survival advantage compared to patients transferring from PD-to-facility HD. As noted by authors, these two groups are nevertheless markedly different and residual confounding could not be eliminated by the propensity-score matching.
Analysis of the ANZDATA registry identified 84 PD-to-HHD patients and compared them with those undergoing HHD or PD as first home-based KRT modality using a propensity score [105]. While patient and technique survival were similar in PD-to-HHD and HHD patients, they were inferior in the PD-only group. Similar findings were obtained in our previous single-center Canadian study in which HHD patients with or without previous exposure to PD had similar patient and technique survival [106]. In a recent Canadian registry study, we compared 163 PD-to-HHD patients with a matched sample of incident HHD patients [99]. The two groups had similar technique survival, despite the longer dialysis vintage of PD-to-HHD patients. In contrast, PD-to-HHD patients had a survival advantage when compared to HHD patients with an equivalent dialysis vintage. Hospitalization risk was similar between the groups in the two analyses.
These studies are, however, subject to inherent biases of their observational design. Notably, they are subject to potential selection and indication bias since patients who are eligible for a PD-to-HHD transition have an intrinsically better predicted long-term prognosis than patients who are not eligible for the transition. Although statistical adjustment and propensity-score matching can attenuate these biases, these methods cannot eliminate them completely. Furthermore, these studies do not assess patients who entered PD with the intent of transferring to HHD but could not complete the transfer (either because they encountered medical complications, became too frail or died while on PD) and hence, the findings should not be generalized to the entire PD population.
Patient-reported outcomes
Patient-reported outcomes (PROs) have not been directly studied in integrated home dialysis and direct comparisons of PROs between PD and HHD are scarce [25, 62]. In one head-to-head study, 36 patients on nocturnal HD were compared with 57 PD patients [61]. While the two modalities led to similar scores in the Kidney Disease Quality of Life-Short Form and Beck Depression Inventory, PD patients had better scores for social support and burden of kidney disease while HHD patients reported better sexual function. In contrast, QOL has been repeatedly compared between facility HD and HHD in past studies, with most of them reporting improved PROs in HHD [20, 22, 23, 107, 108]. Hence, although QOL studies in integrated home dialysis are lacking, available data suggest that QOL is not markedly different between PD and HHD and that a transition to HHD after PD termination is likely favorable compared to facility HD.
Cost effectiveness
While no study has directly compared the cost effectiveness of a PD-to-HHD transition with a ‘HHD only’ approach, the known cost advantage of PD compared to facility HD and HHD allows us to hypothesize that the integrated approach is more cost-effective [32]. By contrast, a British study has compared the costs of a PD-to-HHD transition with the ones of PD-to-facility HD [109]. Although the PD-to-HHD transition was not shown to be cost-effective overall (additional cost of £46 920 per QALY), this finding was explained by the increased healthcare costs of enhanced survival in HHD, and secondary analyses restricted to dialysis costs revealed a financial advantage to the integrated home dialysis paradigm.
THE UNCOMMON TRANSITION: HHD-TO-PD TRANSFERS
While integrated home dialysis usually refers to a PD to HHD, the inverse scenario (HHD to PD) is feasible but less conceptually intuitive. HHD patients often lose their RKF which may threaten long-term PD success [110]. Furthermore, medical and social reasons that underlie HHD termination often preclude the continuation of home dialysis in PD. Nevertheless, some clinical scenarios might lend themselves to an HHD-to-PD transition: (i) patients failing HHD training might still be candidates for PD; (ii) patients with vascular access issues in HHD could transfer to PD; (iii) patients who cannot perform HHD independently can consider assisted PD; and (v) change in lifestyle or goals of care (e.g. desire to travel). While not precisely known, the incidence of HHD-to-PD transitions has been previously reported. In a single-center Canadian report, four patients (from 85 initiating HHD) transferred from HHD to PD [94]. Two had failed HHD training: one transferred for failure to cope and another for lifestyle reasons. Likewise, in a registry study using ANZDATA, 21 patients accomplished an HHD-to-PD transition from the 685 patients undergoing HHD (incidence of 3.0%) [105].
BARRIERS TO INTEGRATED HOME DIALYSIS
The prevalence of integrated home dialysis remains low despite several benefits, reflecting the numerous barriers that hinder home-to-home transitions. Indeed, to be successful in this model, patients and clinicians must overcome barriers of both PD and HHD and some unique to the PD-to-HHD transition (Table 2).
Table 2:
Challenges and mitigation strategies in integrated home dialysis.
| Challenge | Mitigation strategies |
|---|---|
| Patient-level | |
| Lack of awareness of the PD-to-HHD transition | Education programs across the pre-dialysis and dialysis pathway Enroll home-eligible patients in TCUs |
| Appropriate patient selection for the transition | Multidisciplinary patient evaluations, notably including social worker input |
| Identifying an optimal moment for a PD-to-HHD transition | Systematic tools to predict the risk of PD failure Enhanced shared decision-making with patients Consider transferring a patient near PD failure to a TCU if HHD not available |
| Patients’ fears or concerns about home dialysis and the PD-to-HHD transition | Strong and empathic patient–clinician relationships Psychological support Use of patient partners and peer-support groups Patient education programs Recognizing and treating comorbid mental illnesses |
| Potential home-care burnout after PD failure | Respite programs and temporary semi-autonomous HD Assisted HHD programs |
| Caregiver burden | Monitoring of caregiver fatigue signs Professional home nursing support Financial remuneration or fiscal advantages for care given by family members |
| Financial burden of home modalities and inappropriate house setting for home dialysis | Financial support programs for dialysis equipment and home adaptation Community dialysis houses |
| Center-level | |
| Insufficient physician training and experience in home dialysis and PD-to-HHD transitions | Mandatory and sufficient home dialysis exposure during nephrology fellowship Continuous professional development targeted for home dialysis and home-to-home transitions Mentoring centers and widespread adoption of ECHO initiatives |
| Insufficient surgical training and poor availability for PD catheters, AV access placement | Enhance training in PD catheter insertion and AVF-AVG placement during surgical and radiological residencies Ensure sufficient access to surgical suites and appropriate payment for these procedures for clinicians performing dialysis center installation |
| Nursing shortage in home dialysis centers | Develop telehealth and remote monitoring solutions Ensure the attractiveness of dialysis nurse positions Consider and promote the contribution of non-healthcare workers in assisted dialysis programs |
| Lack of integration between PD and HHD centers | Promote integrated dialysis centers that offer multiple modalities Establish dialysis center networks and integrated care pathways between centers Technological solutions to allow remote visits and monitoring for patients living far away from their dialysis center |
| System-level | |
| Appropriate payment systems for home dialysis centers | Ensure fair payment schemes for each dialysis modalities that recognize the extra costs associated with home modalities Systematically monitor the impact of payment systems on home dialysis incidence Reimbursement of telehealth visits |
| Patient volume requirement for financial viability | Appropriately fund fixed costs associated with dialysis centers Adapt payment schemes to ensure financial viability at smaller patient volumes |
| Performance-related payment discouraging the entry of comorbid patients in home dialysis | Avoid performance-related payments schemes that can discourage the enrollment of comorbid patients Adapt dialysis reimbursement to patient complexity and comorbidities |
| Systematic discrepancies in home dialysis access | Promote home dialysis in underrepresented communities with education programs and financial support Personalize and individualize training programs (notably to work schedules and literacy level) |
AVF, arteriovenous fistula; AVG, arteriovenous graft; ECHO HD, hemodialysis
Patient-related barriers
Patient awareness
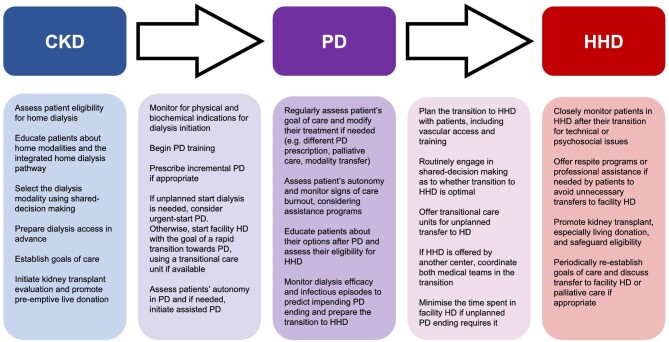
Education is crucial to maximize the uptake of home dialysis and most patients will opt to dialyze at home when provided with enough counseling [17, 33–36]. Despite years of improvement, 42% of patients were still not presented HHD at KRT initiation in a recent European study [111]. Effective education is especially crucial for integrated home dialysis, since it implies a more complex pathway for patients and caregivers. This counseling should be comprehensive, accessible, and provided through the patient journey (before KRT, during PD, and when the transition toward HHD is considered) [112–116]. Clinicians must also strike a balance in the way they inform PD patients about a transition toward HHD: awareness of the transition should allow mental and medical preparation for the transfer but should not convey a sense of futility and discouragement toward PD or foster patients’ anxiety about PD potential “failure.” Indeed, it is best to avoid the word “failure” in discussions about transition between modalities (Fig. 2).
Figure 2:
Multidisciplinary approach for optimal patient-centered care across the different steps of the integrated home dialysis model.
Appropriate patient selection
Optimal patient selection is another challenge of integrated home dialysis. While patients with technique-related PD termination (such as infections and inadequate dialysis) will generally be eligible for a PD-to-HHD transfer, most cases of patient-related PD termination (notably due to frailty, new medical comorbidities, lack of social support) preclude a transfer to HHD. Furthermore, HHD training is longer and more complex than PD and not all patients that succeed in PD will be able to pursue HHD [117]. However, several perceived barriers to HHD (hygiene, housing, literacy, cognitive, or visuospatial capacities) can be overcome with solutions such as care partners, assisted HHD or the concept of “dialysis houses” [118–122]. Clinicians must therefore balance overly enthusiastic or restrictive inclusion criteria into the PD to HHD and recognize patients’ unique abilities and challenges regarding the transition. In addition, patients who prefer HHD as their initial home dialysis modality (notably for aesthetic, convenience, or scheduling reasons) must be respected in their decision and should never be forced into a “theoretical” paradigm such as integrated home dialysis. Patients’ priorities and preferences for KRT should always remain the central factor when selecting a dialysis modality.
Identifying an optimal transfer moment
Some PD interruptions (such as ultrafiltration and adequacy issues) can be anticipated, which generally allows for a smooth transition toward HHD and an adequate time period to create a vascular access. However, most PD endings are caused by “unplanned” events such as peritonitis [100–104]. These unexpected circumstances complexify the transition toward HHD and may lead to permanent transfers in facility HD due to patient and clinician inertia [20]. Hence, identifying an optimal moment to undertake the PD-to-HHD transition can prove difficult for clinicians. On one side, transferring too early can deprive patients from several months of lifestyle flexibility and vascular access protection. Conversely, an overly prolonged time in PD may bring “unnecessary” infectious episodes or worsen medical comorbidities that hinder HHD eligibility and training. In addition, patients terminating PD in an unplanned fashion will most often transition to hemodialysis without an arteriovenous (AV) access. Although home hemodialysis using tunneled catheters has been associated with increased mortality and infectious episodes in observational studies [123–125], the lack of a suitable AV access at PD termination should not preclude a transition to HHD. “Backup” AV access creation during PD has been historically proposed as a solution to prevent transfers using tunneled catheters, but current evidence is lacking to support this approach [126–128]. Therefore, in most cases, clinicians should probably elect to prepare an AV access in PD patients with a very high likelihood of transfer to HHD within 3 to 6 months, while recognizing that such an approach will may to several transfers using tunneled catheters.
Altogether, kidney transplant remains the best KRT option for most patients undergoing home dialysis although the timing of PD-to-HHD transfers in patients on the waitlist may appear challenging. For example, clinicians and patients might at first be reluctant to transfer to HHD if they expect a kidney offer in the foreseeable future, to avoid an unnecessary training for HHD. Nevertheless, this approach should not be preconized since kidney offers are often unpredictable. Hence, except for patients in which living kidney transplant is expected in the immediate future, all eligible patients should be offered HHD at PD completion to avoid unplanned facility HD initiation.
Psychosocial factors and home-care burnout
Patients’ and caregivers’ psychosocial concerns toward home-based dialysis can inflict mental distress and lead to modality transfer [89, 129–131]. They have been extensively reviewed and include: feeling unqualified to perform dialysis, anticipation of catastrophic complications, social isolation, feeling like a burden, anxiety of remote monitoring, fear of self-cannulation, and home “medicalization” [20, 132–134]. In addition, transitioning from PD to HHD brings some unique psychosocial challenges. Terminating PD might lead to feeling guilt or low self-efficacy, making patients less likely to accept the transition toward another (and more complex) dialysis modality. Other patients may be in denial about the need to end PD and be reluctant to consider a transfer to HHD. Several years of PD and its complications may also lead to self-care burnout and diminish patients’ interest toward HHD training. Finally, PD patients may be reluctant about the increased responsibilities required for HHD or doubt the benefits of transferring. While these concerns cannot be entirely eliminated, patient education, peer-support groups, and empathic patient–clinician relationships are attenuating strategies to enhance PD-to-HHD success [135].
The PD-to-HHD model also represents a greater burden on caregivers than each modality alone, especially around the transition period. Clinical teams should therefore monitor signs of caregiver fatigue to prevent otherwise avoidable home dialysis terminations. Furthermore, respite programs might be offered to reduce patient and caregiver fatigue around the transition and maximize long-term technique survival in HHD [136]. Integrated home dialysis also imposes a larger financial burden to patients and their families. Indeed, both modalities involve training time (with potential revenue loss) and home adaptations (water, electricity, renovations), especially for HHD. These costs might discourage patients at lower socioeconomic levels and thus, financial support programs should be available to allow all interested patients to successfully initiate home modalities.
Center-related barriers
The ideal model for integrated home dialysis is a single center that offers both HHD and PD with enough experience and volume in each modality to allow smooth PD-to-HHD transitions. Yet USRDS data reveal that respectively 47% and 70% of US dialysis centers do not offer PD and HHD, with most centers having fewer than 10 patients in each modality [40]. Realistically, in some jurisdictions (especially if dialysis care is not under governmental funding), dialysis centers may have to decide between offering both home modalities at lower volumes or focusing on one modality at higher volumes (leading to financial viability and medical expertise). This is especially true in remote communities, in which centers are likely to offer only one home dialysis modality, typically PD. Hence, the optimal setting in which to practice integrated home dialysis needs to be adapted to regional specificities and can notably take the form of distinct clinics in the same geographical area, dedicated transition clinics, self-care dialysis units or transitional care units (TCUs). TCUs are standalone units or integrated into established centers and offer incident dialysis patients education, training, and allied health professional support [137–139]. They facilitate shared decision-making and are shown to increase the selection of home dialysis. TCUs could prove particularly useful for integrated home dialysis by allowing a structured environment to anticipate PD termination, educate patients about PD-to-HHD transitions, and implement an organized transfer and training toward HHD.
Similarly, clinicians often do not have sufficient training to confidently offer both home dialysis modalities, as revealed by numerous surveys [140–142]. They might also lack awareness of home-to-home transitions that can lead to missed transfer opportunities, in which eager PD patients are not transitioned to HHD in due time. Potential solutions to these issues include: regionalization of dialysis care, enhanced training in home dialysis during nephrology fellowship, continuous professional development, use of systematic tools to identify and follow PD-to-HHD candidates, development of telehealth strategies and finally, “mentoring centers” such as the ECHO (Extension for Community Healthcare Outcomes) initiative [143, 144].
System-related barriers
The various payment systems for dialysis can influence physician behavior and incentivize the selection of modalities, as shown in multiple jurisdictions in which changes in reimbursement schemes have altered clinical practice and improved home dialysis uptake [145–149]. Nevertheless, coverage for home dialysis in some countries (notably the USA) remains incomplete and the patient volume necessary for financial viability may drive dialysis centers to enroll incident home-eligible patients directly in HHD or, conversely, prevent appropriate transfers from PD to HHD. Coverage of nursing assistance for home dialysis might also be limited in some jurisdictions, especially in HHD for which the feasibility and benefits have not been as thoroughly studied [118–120, 150]. By contrast, assisted PD is available in several countries and has been associated with increase home dialysis uptake, and favorable infectious and treatment longevity outcomes [151–154]. As such, patients receiving assisted PD may often not considered as eligible for a transfer to HHD, with perhaps a few program-specific exceptions. Similarly, performance-related payment linked to clinical outcomes may discourage the enrollment of comorbid patients in home dialysis. By contrast, government-level priorities (such as ‘PD-first’ policies) may facilitate a sequential aspect to home dialysis, assuming that HHD is readily available after PD termination.
Policymakers should also consider systemic barriers in the access to home dialysis care. At the social level, patients without elementary education, unmarried, unemployed, and living alone are known to have lower odds of PD initiation compared to HD [113]. Systemic racial differences also exist, as patients of black or Hispanic ethnicity have lower rates of PD or HHD initiation in the USA [155]. Governments should therefore focus on these patient groups with education programs, adapted training policies, and financial initiatives to ensure equal access to home dialysis modalities and home-to-home transitions.
KNOWLEDGE GAPS AND FUTURE DIRECTIONS
Although some studies have reported the incidence of home-to-home transitions, it remains poorly known and should be specifically evaluated in national databases. Further studies should also assess and compare the clinical outcomes of integrated home dialysis care with “HHD only,” especially regarding PROs and caregiver burden. Additional work should be devoted to better predict PD failure and identify the optimal time for a PD-to-HHD transition and AV access creation. To avoid missed transfer opportunities, tools should be developed to systematically follow PD patients and target candidates for HHD. Optimal education approaches for patients and clinicians concerning integrated dialysis care are also poorly known and should be identified. Likewise, the ideal integration of PD and HHD in dialysis centers according to their size and location could be further studied, especially for remote communities. The advent of new HHD technologies (notably low-flow dialysate systems) and their impact on HHD eligibility after PD termination should also be recognized and evaluated. Ultimately, one would hope that new HHD technologies would broader HHD eligibility (notably with shorter and easier training) and facilitate the implementation of an integrated home dialysis approach. Finally, since appropriate access to PD and HHD is essential for integrated home dialysis, efforts to enhance the uptake of these modalities should be encouraged, with a particular effort in populations underrepresented in home dialysis.
CONCLUSION
Integrated home dialysis proposes the initiation of dialysis in PD with a subsequent transition toward HHD after PD termination. It combines the initial lifestyle advantages of PD with the technique longevity of HHD. Its feasibility has been established for more than 15 years and recent studies have shown that patients who transition from PD to HHD have similar clinical outcomes than patients who initiate dialysis directly in HHD. It is therefore an efficient and attractive option for patients living with ESKD. Nevertheless, multiple barriers impair its uptake, and the prevalence of home-to-home transfers remains low. All stakeholders should be mobilized to promote this novel dialysis model to achieve better patient care. Furthermore, integrated home dialysis represents one paradigm among several acceptable dialysis trajectories and patients’ preferences must remain central in the individualization of KRT.
Contributor Information
Louis-Charles Desbiens, Department of Medicine, Université de Montréal, Montreal, Canada; Department of Medicine, Hôpital Maisonneuve-Rosemont, Montreal, Canada.
Joanne M Bargman, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Christopher T Chan, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Annie-Claire Nadeau-Fredette, Department of Medicine, Université de Montréal, Montreal, Canada; Department of Medicine, Hôpital Maisonneuve-Rosemont, Montreal, Canada.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
FUNDING
This paper was published as part of a supplement financially supported by Baxter Healthcare.
AUTHORS’ CONTRIBUTIONS
L.-C. D. was responsible for the conceptualization, evidence collection, writing of the original draft, and review and editing. J.B. was responsible for review and editing of the paper. C.C. was responsible for review and editing of the paper. A.-C.N.-F. was responsible for conceptualization, evidence collection, writing the original draft, review and editing of the paper, and supervision.
CONFLICT OF INTEREST STATEMENT
A.C.N.F. received speaker honoraria by Baxter Healthcare and holds a FRQS scholarship. J.B. serves as consultant to and received speaker honoraria with both DaVita Healthcare Partners and Baxter Healthcare. C.C. holds the R Fraser Elliott Chair in Home Dialysis and serves as consultant to Medtronic, Quanta, and Dialco Inc. He received an investigator—initiated grant from Medtronic ERP program. He also serves as the President of the International Society for Hemodialysis. L.C.D. has no disclosures relevant to the current submission.
REFERENCES
- 1. Chan CT, Blankestijn PJ, Dember LM et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2019;96:37–47. 10.1016/j.kint.2019.01.017 [DOI] [PubMed] [Google Scholar]
- 2. Perl J, Brown EA, Chan CT et al. Home dialysis: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2023;103:842–58. 10.1016/j.kint.2023.01.006 [DOI] [PubMed] [Google Scholar]
- 3. Marshall MR, Polkinghorne KR, Kerr PG et al. Temporal changes in mortality risk by dialysis modality in the Australian and New Zealand dialysis population. A J Kidney Dis 2015;66:489–98. 10.1053/j.ajkd.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 4. Coles GA, Williams JD. What is the place of peritoneal dialysis in the integrated treatment of renal failure? Kidney Int 1998;54:2234–40. 10.1046/j.1523-1755.1998.00183.x [DOI] [PubMed] [Google Scholar]
- 5. Biesen WV, Vanholder RC, Veys N et al. An evaluation of an integrative care approach for end-stage renal disease patients. J Am Soc Nephrol 2000;11:116–25. 10.1681/ASN.V111116 [DOI] [PubMed] [Google Scholar]
- 6. Lameire N, Van Biesen W, Vanholder R. The role of peritoneal dialysis as first modality in an integrative approach to patients with end-stage renal disease. Peritoneal Dialysis Int 2000;20 Suppl 2:S134–141. 10.1177/089686080002002S26 [DOI] [PubMed] [Google Scholar]
- 7. Van Biesen W, Vanholder R, Lameire N. The role of peritoneal dialysis as the first-line renal replacement modality. Perit Dial Int 2000;20:375–83. 10.1177/089686080002000401 [DOI] [PubMed] [Google Scholar]
- 8. Mucsi I, Hercz G, Uldall R et al. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int 1998;53:1399–404. 10.1046/j.1523-1755.1998.00875.x [DOI] [PubMed] [Google Scholar]
- 9. Pierratos A. Nocturnal home haemodialysis: an update on a 5-year experience. Nephrol Dial Transplant 1999;14:2835–40. 10.1093/ndt/14.12.2835 [DOI] [PubMed] [Google Scholar]
- 10. Blagg C. What went wrong with home hemodialysis in the United States and what can be done now? Hemodial Int 2000;4:55–58. 10.1111/hdi.2000.4.1.55 [DOI] [PubMed] [Google Scholar]
- 11. Mohr PE, Neumann PJ, Franco SJ et al. The case for daily dialysis: its impact on costs and quality of life. Am J Kidney Dis 2001;37:777–89. 10.1016/S0272-6386(01)80127-X [DOI] [PubMed] [Google Scholar]
- 12. Culleton BF, Walsh M, Klarenbach SW et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. J Am Med Assoc 2007;298:1291–9. 10.1001/jama.298.11.1291 [DOI] [PubMed] [Google Scholar]
- 13. Mendelssohn DC, Pierratos A. Reformulating the integrated care concept for the new millennium. Peritoneal Dialysis Int 2002;22:5–8. 10.1177/089686080202200101 [DOI] [PubMed] [Google Scholar]
- 14. Wong JH, Pierratos A, Oreopoulos DG et al. The use of nocturnal home hemodialysis as salvage therapy for patients experiencing peritoneal dialysis failure. Peritoneal Dialysis Int 2007;27:669–74. 10.1177/089686080702700613 [DOI] [PubMed] [Google Scholar]
- 15. Burkart J. Transitions from PD are expected. Why not continue at home? Perit Dial Int 2007;27:645–6. 10.1177/089686080702700608 [DOI] [PubMed] [Google Scholar]
- 16. Dahlerus C, Quinn M, Messersmith E et al. Patient perspectives on the choice of dialysis modality: results from the empowering patients on choices for renal replacement therapy (EPOCH-RRT) study. Am J Kidney Dis 2016;68:901–10. 10.1053/j.ajkd.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 17. Morton RL, Snelling P, Webster AC et al. Dialysis modality preference of patients with CKD and family caregivers: a discrete-choice study. Am J Kidney Dis 2012;60:102–11. 10.1053/j.ajkd.2011.12.030 [DOI] [PubMed] [Google Scholar]
- 18. Morton RL, Tong A, Webster AC et al. Characteristics of dialysis important to patients and family caregivers: a mixed methods approach. Nephrol Dial Transplant 2011;26:4038–46. 10.1093/ndt/gfr177 [DOI] [PubMed] [Google Scholar]
- 19. Morton RL, Devitt J, Howard K et al. Patient views about treatment of stage 5 CKD: a qualitative analysis of semistructured interviews. Am J Kidney Diseases 2010;55:431–40. 10.1053/j.ajkd.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 20. Walker RC, Hanson CS, Palmer SC et al. Patient and caregiver perspectives on home hemodialysis: a systematic review. Am J Kidney Dis 2015;65:451–63. 10.1053/j.ajkd.2014.10.020 [DOI] [PubMed] [Google Scholar]
- 21. Chuasuwan A, Pooripussarakul S, Thakkinstian A et al. Comparisons of quality of life between patients underwent peritoneal dialysis and hemodialysis: a systematic review and meta-analysis. Health Qual Life Outcomes 2020;18:191. 10.1186/s12955-020-01449-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller BW, Himmele R, Sawin DA et al. Choosing home hemodialysis: a critical review of patient outcomes. Blood Purif 2018;45:224–9. 10.1159/000485159 [DOI] [PubMed] [Google Scholar]
- 23. Bonenkamp AA, van Eck van der Sluijs A, Hoekstra T et al. Health-related quality of life in home dialysis patients compared to In-center hemodialysis patients: a systematic review and meta-analysis. Kidney Med 2020;2:139–54. 10.1016/j.xkme.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finkelstein FO, Finkelstein SH, Wuerth D et al. Effects of home hemodialysis on health-related quality of life measures. Semin Dial 2007;20:265–8. 10.1111/j.1525-139X.2007.00287.x [DOI] [PubMed] [Google Scholar]
- 25. Ishani A, Slinin Y, Greer N et al. VA evidence-based synthesis program reports. Comparative Effectiveness of Home-Based Kidney Dialysis versus in-Center or Other Outpatient Kidney Dialysis Locations—A Systematic Review. Department of Veterans Affairs (US): Washington (DC); 2015. [PubMed] [Google Scholar]
- 26. Klarenbach S, Tonelli M, Pauly R et al. Economic evaluation of frequent home nocturnal hemodialysis based on a randomized controlled trial. J Am Soc Nephrol 2014;25:587–94. 10.1681/ASN.2013040360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker R, Marshall MR, Morton RL et al. The cost-effectiveness of contemporary home haemodialysis modalities compared with facility haemodialysis: a systematic review of full economic evaluations. Nephrology 2014;19:459–70. 10.1111/nep.12269 [DOI] [PubMed] [Google Scholar]
- 28. Kroeker A, Clark WF, Heidenheim AP et al. An operating cost comparison between conventional and home quotidian hemodialysis. Am J Kidney Dis 2003;42:49–55. 10.1016/S0272-6386(03)00538-9 [DOI] [PubMed] [Google Scholar]
- 29. McFarlane PA, Pierratos A, Redelmeier DA. Cost savings of home nocturnal versus conventional in-center hemodialysis. Kidney Int 2002;62:2216–22. 10.1046/j.1523-1755.2002.00678.x [DOI] [PubMed] [Google Scholar]
- 30. Klarenbach SW, Tonelli M, Chui B et al. Economic evaluation of dialysis therapies. Nat Rev Nephrol 2014;10:644–52. 10.1038/nrneph.2014.145 [DOI] [PubMed] [Google Scholar]
- 31. Hornberger J, Hirth RA. Financial implications of choice of dialysis type of the revised Medicare payment system: an economic analysis. Am J Kidney Dis 2012;60:280–7. 10.1053/j.ajkd.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 32. Lee H, Manns B, Taub K et al. Cost analysis of ongoing care of patients with end-stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis 2002;40:611–22. 10.1053/ajkd.2002.34924 [DOI] [PubMed] [Google Scholar]
- 33. Heaf J, Heiro M, Petersons A et al. Choice of dialysis modality among patients initiating dialysis: results of the peridialysis study. Clin Kidney J 2021;14:2064–74. 10.1093/ckj/sfaa260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manns BJ, Taub K, Vanderstraeten C et al. The impact of education on chronic kidney disease patients' plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int 2005;68:1777–83. 10.1111/j.1523-1755.2005.00594.x [DOI] [PubMed] [Google Scholar]
- 35. Goovaerts T, Jadoul M, Goffin E. Influence of a pre-dialysis education programme (PDEP) on the mode of renal replacement therapy. Nephrol Dial Transplant 2005;20:1842–7. 10.1093/ndt/gfh905 [DOI] [PubMed] [Google Scholar]
- 36. Ribitsch W, Haditsch B, Otto R et al. Effects of a pre-dialysis patient education program on the relative frequencies of dialysis modalities. Perit Dial Int 2013;33:367–71. 10.3747/pdi.2011.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abra G, Schiller B. Public policy and programs—missing links in growing home dialysis in the United States. Semin Dial 2020;33:75–82. 10.1111/sdi.12850 [DOI] [PubMed] [Google Scholar]
- 38. Lipkin G, McKane W. Renal Medicine GIRFT Programme National Specialty Report. National Health Service; 2021. [Google Scholar]
- 39. Jain AK, Blake P, Cordy P et al. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol 2012;23:533–44. 10.1681/ASN.2011060607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. United States Renal Data System . 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2022. [Google Scholar]
- 41. ANZDATA Registry . 45th Report, Chapter 2: Prevalence of Kidney Failure with Replacement Therapy. Adelaide, Australia: Australia and New Zealand Dialysis and Transplant Registry; 2022. [Google Scholar]
- 42. Chaudhary K, Sangha H, Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol 2011;6:447–56. 10.2215/CJN.07920910 [DOI] [PubMed] [Google Scholar]
- 43. Ghaffari A, Kalantar-Zadeh K, Lee J et al. PD first: peritoneal dialysis as the default transition to dialysis therapy. Semin Dial 2013;26:706–13. 10.1111/sdi.12125 [DOI] [PubMed] [Google Scholar]
- 44. Jansen MA, Hart AA, Korevaar JC et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002;62:1046–53. 10.1046/j.1523-1755.2002.00505.x [DOI] [PubMed] [Google Scholar]
- 45. Moist LM, Port FK, Orzol SM et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000;11:556–64. 10.1681/ASN.V113556 [DOI] [PubMed] [Google Scholar]
- 46. Misra M, Vonesh E, Van Stone JC et al. Effect of cause and time of dropout on the residual GFR: a comparative analysis of the decline of GFR on dialysis. Kidney Int 2001;59:754–63. 10.1046/j.1523-1755.2001.059002754.x [DOI] [PubMed] [Google Scholar]
- 47. Lang SM, Bergner A, Topfer M et al. Preservation of residual renal function in dialysis patients: effects of dialysis-technique-related factors. Peritoneal Dialysis Int 2001;21:52–57. 10.1177/089686080102100108 [DOI] [PubMed] [Google Scholar]
- 48. Patel N, Hu SL. Preserving residual renal function in dialysis: what we know. Semin Dial 2015;28:250–8. 10.1111/sdi.12302 [DOI] [PubMed] [Google Scholar]
- 49. Termorshuizen F, Dekker FW, van Manen JG et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 2004;15:1061–70. 10.1097/01.ASN.0000117976.29592.93 [DOI] [PubMed] [Google Scholar]
- 50. Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001;12:2158–62. 10.1681/ASN.V12102158 [DOI] [PubMed] [Google Scholar]
- 51. Diaz-Buxo JA, Lowrie EG, Lew NL et al. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis 1999;33:523–34. 10.1016/S0272-6386(99)70190-3 [DOI] [PubMed] [Google Scholar]
- 52. Paniagua R, Amato D, Vonesh E et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002;13:1307–20. 10.1681/ASN.V1351307 [DOI] [PubMed] [Google Scholar]
- 53. Shemin D, Bostom AG, Laliberty P et al. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis 2001;38:85–90. 10.1053/ajkd.2001.25198 [DOI] [PubMed] [Google Scholar]
- 54. Termorshuizen F, Korevaar JC, Dekker FW et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis 2003;41:1293–302. 10.1016/S0272-6386(03)00362-7 [DOI] [PubMed] [Google Scholar]
- 55. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis 2009;53:1068–81. 10.1053/j.ajkd.2009.02.012 [DOI] [PubMed] [Google Scholar]
- 56. Sarnak MJ, Auguste BL, Brown E et al. Cardiovascular effects of home dialysis therapies: a scientific statement from the American Heart Association. Circulation 2022;146:e146–64. 10.1161/CIR.0000000000001088 [DOI] [PubMed] [Google Scholar]
- 57. Oliver MJ, Verrelli M, Zacharias JM et al. Choosing peritoneal dialysis reduces the risk of invasive access interventions. Nephrol Dial Transplant 2012;27:810–6. 10.1093/ndt/gfr289 [DOI] [PubMed] [Google Scholar]
- 58. Powe NR, Jaar B, Furth SL et al. Septicemia in dialysis patients: incidence, risk factors, and prognosis. Kidney Int 1999;55:1081–90. 10.1046/j.1523-1755.1999.0550031081.x [DOI] [PubMed] [Google Scholar]
- 59. Foley RN, Guo H, Snyder JJ et al. Septicemia in the United States dialysis population, 1991 to 1999. J Am Soc Nephrol 2004;15:1038–45. 10.1097/01.ASN.0000119144.95922.C4 [DOI] [PubMed] [Google Scholar]
- 60. Li PK, Chow KM. Infectious complications in dialysis—epidemiology and outcomes. Nat Rev Nephrol 2011;8:77–88. 10.1038/nrneph.2011.194 [DOI] [PubMed] [Google Scholar]
- 61. Fong E, Bargman JM, Chan CT. Cross-sectional comparison of quality of life and illness intrusiveness in patients who are treated with nocturnal home hemodialysis versus peritoneal dialysis. Clin J Am Soc Nephrol 2007;2:1195–200. 10.2215/CJN.02260507 [DOI] [PubMed] [Google Scholar]
- 62. Budhram B, Sinclair A, Komenda P et al. A comparison of patient-reported outcome measures of quality of life by dialysis modality in the treatment of kidney failure: a systematic review. Canad J Kidney Health Dis 2020;7:2054358120957431. 10.1177/2054358120957431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Manera KE, Johnson DW, Craig JC et al. Patient and caregiver priorities for outcomes in peritoneal dialysis: multinational nominal group technique study. Clin J Am Soc Nephrol 2019;14:74–83. 10.2215/CJN.05380518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Karopadi AN, Mason G, Rettore E et al. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dialysis Transplant 2013;28:2553–69. 10.1093/ndt/gft214 [DOI] [PubMed] [Google Scholar]
- 65. Wong CKH, Chen J, Fung SKS et al. Lifetime cost-effectiveness analysis of first-line dialysis modalities for patients with end-stage renal disease under peritoneal dialysis first policy. BMC Nephrol 2020;21:42. 10.1186/s12882-020-1708-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mohnen SM, van Oosten MJM, Los J et al. Healthcare costs of patients on different renal replacement modalities—analysis of Dutch health insurance claims data. PLoS ONE 2019;14:e0220800. 10.1371/journal.pone.0220800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ferguson TW, Whitlock RH, Bamforth RJ et al. Cost-utility of dialysis in Canada: hemodialysis, peritoneal dialysis, and nondialysis treatment of kidney failure. Kidney Med 2021;3:20–30.e21. 10.1016/j.xkme.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Auguste BL, Bargman JM. Incremental peritoneal dialysis: new ideas about an old approach. Semin Dial 2018;31:445–8. 10.1111/sdi.12712 [DOI] [PubMed] [Google Scholar]
- 69. Blake PG, Dong J, Davies SJ. Incremental peritoneal dialysis. Perit Dial Int 2020;40:320–6. 10.1177/0896860819895362 [DOI] [PubMed] [Google Scholar]
- 70. Cheetham MS, Cho Y, Krishnasamy R et al. Incremental versus standard (full-dose) peritoneal dialysis. Kidney Int Rep 2022;7:165–76. 10.1016/j.ekir.2021.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Golper TA. Incremental hemodialysis: how I do it. Semin Dial 2016;29:476–80. 10.1111/sdi.12530 [DOI] [PubMed] [Google Scholar]
- 72. Bhalla NM, Arora N, Darbinian JA et al. Urgent start peritoneal dialysis: a population-based cohort study. Kidney Med 2022;4:100414. 10.1016/j.xkme.2022.100414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ding X, Gao W, Guo Y et al. Comparison of mortality and complications between urgent-start peritoneal dialysis and urgent-start hemodialysis: a systematic review and meta-analysis. Semin Dial 2022;35:207–14. 10.1111/sdi.13001 [DOI] [PubMed] [Google Scholar]
- 74. Htay H, Johnson DW, Craig JC et al. Urgent-start peritoneal dialysis versus haemodialysis for people with chronic kidney disease. Cochrane Database Syst Rev 2021;1:Cd012899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Karpinski S, Sibbel S, Cohen DE et al. Urgent-start peritoneal dialysis: association with outcomes. Perit Dial Int 2023;43:186–9. 10.1177/08968608221083781 [DOI] [PubMed] [Google Scholar]
- 76. Parapiboon W, Sangsuk J, Nopsopon T et al. Randomized study of urgent-start peritoneal dialysis versus urgent-start temporary hemodialysis in patients transitioning to kidney failure. Kidney Int Rep 2022;7:1866–77. 10.1016/j.ekir.2022.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tunbridge M, Cho Y, Johnson DW. Urgent-start peritoneal dialysis: is it ready for prime time? Curr Opin Nephrol Hypertens 2019;28:631–40. 10.1097/MNH.0000000000000545 [DOI] [PubMed] [Google Scholar]
- 78. Nadeau-Fredette AC, Tennankore KK, Perl J et al. Home hemodialysis and peritoneal dialysis patient and technique survival in Canada. Kidney Int Rep 2020;5:1965–73. 10.1016/j.ekir.2020.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McGill RL, Weiner DE, Ruthazer R et al. Transfers to hemodialysis among US patients initiating renal replacement therapy with peritoneal dialysis. Am J Kidney Dis 2019;74:620–8. 10.1053/j.ajkd.2019.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lambie M, Zhao J, McCullough K et al. Variation in peritoneal dialysis time on therapy by country: results from the peritoneal dialysis outcomes and practice patterns study. Clin J Am Soc Nephrol 2022;17:861–71. 10.2215/CJN.16341221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Goldberg R, Yalavarthy R, Teitelbaum I. Adequacy of peritoneal dialysis: beyond small solute clearance. Contrib Nephrol 2009;163:147–54. 10.1159/000223793 [DOI] [PubMed] [Google Scholar]
- 82. Li PK, Chow KM, Cho Y et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int 2022;42:110–53. 10.1177/08968608221080586 [DOI] [PubMed] [Google Scholar]
- 83. van de Luijtgaarden MW, Jager KJ, Segelmark M et al. Trends in dialysis modality choice and related patient survival in the ERA-EDTA Registry over a 20-year period. Nephrol Dial Transplant 2016;31:120–8. 10.1093/ndt/gfv295 [DOI] [PubMed] [Google Scholar]
- 84. See EJ, Johnson DW, Hawley CM et al. Risk predictors and causes of technique failure within the first year of peritoneal dialysis: an Australia and New Zealand Dialysis and Transplant registry (ANZDATA) study. Am J Kidney Dis 2018;72:188–97. 10.1053/j.ajkd.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 85. Nadeau-Fredette AC, Sukul N, Lambie M et al. Mortality trends after transfer from peritoneal dialysis to hemodialysis. Kidney Int Rep 2022;7:1062–73. 10.1016/j.ekir.2022.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lan PG, Clayton PA, Johnson DW et al. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Peritoneal Dialysis Int 2016;36:623–30. 10.3747/pdi.2015.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shah N, Reintjes F, Courtney M et al. Quality assurance audit of technique failure and 90-day mortality after program discharge in a Canadian home hemodialysis program. Clin J Am Soc Nephrol 2017;12:1259–64. 10.2215/CJN.00140117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Seshasai RK, Mitra N, Chaknos CM et al. Factors associated with discontinuation of home hemodialysis. Am J Kidney Dis 2016;67:629–37. 10.1053/j.ajkd.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Trinh E, Hanley JA, Nadeau-Fredette AC et al. A comparison of technique survival in Canadian peritoneal dialysis and home hemodialysis patients. Nephrol Dial Transplant 2019;34:1941–9. 10.1093/ndt/gfz075 [DOI] [PubMed] [Google Scholar]
- 90. Weinhandl ED, Gilbertson DT, Collins AJ. Mortality, hospitalization, and technique failure in daily home hemodialysis and matched peritoneal dialysis patients: a matched cohort study. Am J Kidney Dis 2016;67:98–110. 10.1053/j.ajkd.2015.07.014 [DOI] [PubMed] [Google Scholar]
- 91. Nadeau-Fredette AC, Hawley CM, Pascoe EM et al. An incident cohort study comparing survival on home hemodialysis and peritoneal dialysis (Australia and New Zealand Dialysis and Transplantation Registry). Clin J Am Soc Nephrol 2015;10:1397–407. 10.2215/CJN.00840115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rydell H, Ivarsson K, Almquist M et al. Fewer hospitalizations and prolonged technique survival with home hemodialysis—a matched cohort study from the Swedish Renal Registry. BMC Nephrol 2019;20:480. 10.1186/s12882-019-1644-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Perl J, Na Y, Tennankore KK et al. Temporal trends and factors associated with home hemodialysis technique survival in Canada. Clin J Am Soc Nephrol 2017;12:1248–58. 10.2215/CJN.13271216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Elbokl MA, Kennedy C, Bargman JM et al. Home-to-home dialysis transition: a 24-year single-centre experience. Perit Dial Int 2022;42:324–7. 10.1177/08968608211029213 [DOI] [PubMed] [Google Scholar]
- 95. Cina DP, Dacouris N, Kashani M et al. Use of home hemodialysis after peritoneal dialysis technique failure. Peritoneal Dialysis Int 2013;33:96–99. 10.3747/pdi.2012.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Suzuki H, Hoshi H, Inoue T et al. New modality of dialysis therapy: peritoneal dialysis first and transition to home hemodialysis. Adv Perit Dial 2012;28:106–11. [PubMed] [Google Scholar]
- 97. Nadeau-Fredette AC, Hawley C, Pascoe E et al. Predictors of transfer to home hemodialysis after peritoneal dialysis completion. Peritoneal Dialysis Int 2016;36:547–54. 10.3747/pdi.2015.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kansal SK, Morfin JA, Weinhandl ED. Survival and kidney transplant incidence on home versus In-center hemodialysis, following peritoneal dialysis technique failure. Perit Dial Int 2019;39:25–34. 10.3747/pdi.2017.00207 [DOI] [PubMed] [Google Scholar]
- 99. Desbiens L-C, Tennankore KK, Goupil R et al. Outcomes of integrated home dialysis care: results from the Canadian Organ Replacement Register. Am J Kidney Dis 2024;83:47–57.e1. 10.1053/j.ajkd.2023.05.011 [DOI] [PubMed] [Google Scholar]
- 100. Lan PG, Clayton PA, Saunders J et al. Predictors and outcomes of transfers from peritoneal dialysis to hemodialysis. Peritoneal Dialysis Int 2015;35:306–15. 10.3747/pdi.2013.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jaar BG, Plantinga LC, Crews DC et al. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: a prospective study. BMC Nephrol 2009;10:3. 10.1186/1471-2369-10-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kolesnyk I, Dekker FW, Boeschoten EW et al. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Peritoneal Dialysis Int 2010;30:170–7. 10.3747/pdi.2008.00277 [DOI] [PubMed] [Google Scholar]
- 103. Workeneh B, Guffey D, Minard CG et al. Causes for withdrawal in an urban peritoneal dialysis program. Int J Nephrol 2015;2015:652953. 10.1155/2015/652953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Htay H, Cho Y, Pascoe EM et al. Multicenter registry analysis of center characteristics associated with technique failure in patients on incident peritoneal dialysis. Clin J Am Soc Nephrol 2017;12:1090–9. 10.2215/CJN.12321216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nadeau-Fredette AC, Chan CT, Cho Y et al. Outcomes of integrated home dialysis care: a multi-centre, multi-national registry study. Nephrol Dial Transplant 2015;30:1897–904. [DOI] [PubMed] [Google Scholar]
- 106. Nadeau-Fredette AC, Bargman JM, Chan CT. Clinical outcome of home hemodialysis in patients with previous peritoneal dialysis exposure: evaluation of the integrated home dialysis model. Perit Dial Int 2015;35:316–23. 10.3747/pdi.2013.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wong B, Collister D, Muneer M et al. In-center nocturnal hemodialysis versus conventional hemodialysis: a systematic review of the evidence. Am J Kidney Dis 2017;70:218–34. 10.1053/j.ajkd.2017.01.047 [DOI] [PubMed] [Google Scholar]
- 108. Kraus MA, Fluck RJ, Weinhandl ED et al. Intensive hemodialysis and health-related quality of life. Am J Kidney Dis 2016;68:S33–s42. 10.1053/j.ajkd.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 109. Erbe AW, Kendzia D, Busink E et al. Value of an integrated home dialysis model in the United Kingdom: a cost-effectiveness analysis. Value Health 2023;26:984–94. 10.1016/j.jval.2023.02.009 [DOI] [PubMed] [Google Scholar]
- 110. Daugirdas JT, Greene T, Rocco MV et al. Effect of frequent hemodialysis on residual kidney function. Kidney Int 2013;83:949–58. 10.1038/ki.2012.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. de Jong RW, Stel VS, Rahmel A et al. Patient-reported factors influencing the choice of their kidney replacement treatment modality. Nephrol Dial Transplant 2022;37:477–88. 10.1093/ndt/gfab059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shukla AM, Easom A, Singh M et al. Effects of a comprehensive predialysis education program on the home dialysis therapies: a retrospective cohort study. Perit Dial Int 2017;37:542–7. 10.3747/pdi.2016.00270 [DOI] [PubMed] [Google Scholar]
- 113. Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. J Am Soc Nephrol 2002;13:1279–87. 10.1681/ASN.V1351279 [DOI] [PubMed] [Google Scholar]
- 114. Lameire N, Van Biesen W. The pattern of referral of patients with end-stage renal disease to the nephrologist—a European survey. Nephrol Dial Transplant 1999;14 Suppl 6:16–23. 10.1093/ndt/14.suppl_6.16 [DOI] [PubMed] [Google Scholar]
- 115. Chan CT, Wallace E, Golper TA et al. Exploring barriers and potential solutions in home dialysis: an NKF-KDOQI Conference Outcomes report. Am J Kidney Dis: J Natl Kidney Fdn 2019;73:363–71. 10.1053/j.ajkd.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 116. Rioux JP, Cheema H, Bargman JM et al. Effect of an in-hospital chronic kidney disease education program among patients with unplanned urgent-start dialysis. Clin J Am Soc Nephrol 2011;6:799–804. 10.2215/CJN.07090810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rioux JP, Marshall MR, Faratro R et al. Patient selection and training for home hemodialysis. Hemodial Int 2015;19 Suppl 1:S71–79. [DOI] [PubMed] [Google Scholar]
- 118. Reddy NC, Korbet SM, Wozniak JA et al. Staff-assisted nursing home haemodialysis: patient characteristics and outcomes. Nephrol Dial Transplant 2007;22:1399–406. 10.1093/ndt/gfl809 [DOI] [PubMed] [Google Scholar]
- 119. Pommer W, Wagner S, Müller D et al. Attitudes of nephrologists towards assisted home dialysis in Germany. Clin Kidney J 2018;11:400–5. 10.1093/ckj/sfx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Agraharkar M, Du Y, Ahuja T et al. Comparison of staff-assisted home hemodialysis with in-center hemodialysis and In-hospital hemodialysis. Hemodial Int 2002;6:58–62. [DOI] [PubMed] [Google Scholar]
- 121. Marshall MR, van der Schrieck N, Lilley D et al. Independent community house hemodialysis as a novel dialysis setting: an observational cohort study. Am J Kidney Dis 2013;61:598–607. 10.1053/j.ajkd.2012.10.020 [DOI] [PubMed] [Google Scholar]
- 122. Walker RC, Tipene-Leach D, Graham A et al. Patients' experiences of community house hemodialysis: a qualitative study. Kidney Med 2019;1:338–46. 10.1016/j.xkme.2019.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hayes WN, Tennankore K, Battistella M et al. Vascular access-related infection in nocturnal home hemodialysis. Hemodial Int 2014;18:481–7. 10.1111/hdi.12140 [DOI] [PubMed] [Google Scholar]
- 124. Perl J, Nessim SJ, Moist LM et al. Vascular access type and patient and technique survival in home hemodialysis patients: the Canadian Organ Replacement Register. Am J Kidney Dis 2016;67:251–9. 10.1053/j.ajkd.2015.07.032 [DOI] [PubMed] [Google Scholar]
- 125. Rivara MB, Soohoo M, Streja E et al. Association of vascular access type with mortality, hospitalization, and transfer to in-center hemodialysis in patients undergoing home hemodialysis. Clin J Am Soc Nephrol 2016;11:298–307. 10.2215/CJN.06570615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Beckingham IJ, O'Rourke JS, Bishop MC et al. Are backup arteriovenous fistulae necessary for patients on continuous ambulatory peritoneal dialysis? Lancet 1993;341:1384–6. 10.1016/0140-6736(93)90951-C [DOI] [PubMed] [Google Scholar]
- 127. Nezakatgoo N, Ndzengue A, Ramaiah M et al. Outcomes of simultaneous peritoneal dialysis and arteriovenous fistula placement in end-stage renal disease patients. Peritoneal Dialysis Int 2017;37:658–61. 10.3747/pdi.2017.00072 [DOI] [PubMed] [Google Scholar]
- 128. Ferreira H, Nunes A, Oliveira A et al. Planning vascular access in peritoneal dialysis-defining high-risk patients. Perit Dial Int 2018;38:271–7. 10.3747/pdi.2017.00180 [DOI] [PubMed] [Google Scholar]
- 129. Weinhandl ED, Collins AJ. Relative risk of home hemodialysis attrition in patients using a telehealth platform. Hemodial Int 2018;22:318–27. 10.1111/hdi.12621 [DOI] [PubMed] [Google Scholar]
- 130. Schachter ME, Tennankore KK, Chan CT. Determinants of training and technique failure in home hemodialysis. Hemodial Int 2013;17:421–6. 10.1111/hdi.12036 [DOI] [PubMed] [Google Scholar]
- 131. Paterson B, Fox DE, Lee CH et al. Understanding home hemodialysis patient attrition: a cohort study. Can J Kidney Health Dis 2021;8:20543581211022195. 10.1177/20543581211022195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pauly RP, Eastwood DO, Marshall MR. Patient safety in home hemodialysis: quality assurance and serious adverse events in the home setting. Hemodial Int 2015;19 Suppl 1:S59–70. [DOI] [PubMed] [Google Scholar]
- 133. Cafazzo JA, Leonard K, Easty AC et al. Patient-perceived barriers to the adoption of nocturnal home hemodialysis. Clin J Am Soc Nephrol: 2009;4:784–9. 10.2215/CJN.05501008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Muringai T, Noble H, McGowan A et al. Dialysis access and the impact on body image: role of the nephrology nurse. Br J Nurs 2008;17:362–6. 10.12968/bjon.2008.17.6.28900 [DOI] [PubMed] [Google Scholar]
- 135. Wong J, Eakin J, Migram P et al. Patients' experiences with learning a complex medical device for the self-administration of nocturnal home hemodialysis. Nephrol Nurs J 2009;36:27–32. [PubMed] [Google Scholar]
- 136. Malavade TS, Dey A, Chan CT. Nocturnal hemodialysis: why aren't more people doing it? Adv Chronic Kidney Dis 2021;28:184–9. 10.1053/j.ackd.2021.04.003 [DOI] [PubMed] [Google Scholar]
- 137. Morfin JA, Yang A, Wang E et al. Transitional dialysis care units: a new approach to increase home dialysis modality uptake and patient outcomes. Semin Dial 2018;31:82–87. 10.1111/sdi.12651 [DOI] [PubMed] [Google Scholar]
- 138. Eschbach JW, Seymour M, Potts A et al. A hemodialysis orientation unit. Nephron 1983;33:106–10. 10.1159/000182922 [DOI] [PubMed] [Google Scholar]
- 139. Bowman B, Zheng S, Yang A et al. Improving incident ESRD care via a transitional care unit. Am J Kidney Dis 2018;72:278–83. 10.1053/j.ajkd.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 140. Rope RW, Pivert KA, Parker MG et al. Education in Nephrology Fellowship: a survey-based needs assessment. J Am Soc Nephrol 2017;28:1983–90. 10.1681/ASN.2016101061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Berns JS. A survey-based evaluation of self-perceived competency after nephrology fellowship training. Clin J Am Soc Nephrol 2010;5:490–6. 10.2215/CJN.08461109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Beaton TJ, Krishnasamy R, Toussaint ND et al. Nephrology training in Australia and New Zealand: a survey of outcomes and adequacy. Nephrology (Carlton) 2017;22:35–42. 10.1111/nep.12720 [DOI] [PubMed] [Google Scholar]
- 143. Arora S, Kalishman SG, Thornton KA et al. Project ECHO: a telementoring network model for continuing professional development. J Contin Educ Health Prof 2017;37:239–44. 10.1097/CEH.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 144. Abra G, Poyan Mehr A, Chan CT et al. The implementation of a virtual home dialysis mentoring program for nephrologists. Kidney360 2022;3:734–6. 10.34067/KID.0000202022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Manns B, Agar JWM, Biyani M et al. Can economic incentives increase the use of home dialysis? Nephrol Dial Transplant 2019;34:731–41. 10.1093/ndt/gfy223 [DOI] [PubMed] [Google Scholar]
- 146. Emrani Z, Amiresmaili M, Daroudi R et al. Payment systems for dialysis and their effects: a scoping review. BMC Health Serv Res 2023;23:45. 10.1186/s12913-022-08974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Trinh E, Chan CT. The rise, fall, and resurgence of home hemodialysis. Semin Dial 2017;30:174–80. 10.1111/sdi.12572 [DOI] [PubMed] [Google Scholar]
- 148. Wilkie M. Home dialysis-an international perspective. NDT Plus 2011;4:iii4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lin E, Cheng XS, Chin KK et al. Home dialysis in the prospective payment system era. J Am Soc Nephrol 2017;28:2993–3004. 10.1681/ASN.2017010041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Giuliani A, Karopadi AN, Prieto-Velasco M et al. Worldwide experiences with assisted peritoneal dialysis. Perit Dial Int 2017;37:503–8. 10.3747/pdi.2016.00214 [DOI] [PubMed] [Google Scholar]
- 151. Brown EA, Wilkie M. Assisted peritoneal dialysis as an alternative to in-center hemodialysis. Clin J Am Soc Nephrol 2016;11:1522–4. 10.2215/CJN.07040716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Oliver MJ, Al-Jaishi AA, Dixon SN et al. Hospitalization rates for patients on assisted peritoneal dialysis compared with in-center hemodialysis. Clin J Am Soc Nephrol 2016;11:1606–14. 10.2215/CJN.10130915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bevilacqua MU, Turnbull L, Saunders S et al. Evaluation of a 12-month pilot of long-term and temporary assisted peritoneal dialysis. Peritoneal Dialysis Int 2017;37:307–13. 10.3747/pdi.2016.00201 [DOI] [PubMed] [Google Scholar]
- 154. Boyer A, Solis-Trapala I, Tabinor M et al. Impact of the implementation of an assisted peritoneal dialysis service on peritoneal dialysis initiation. Nephrol Dial Transplant 2020;35:1595–601. [DOI] [PubMed] [Google Scholar]
- 155. Mehrotra R, Soohoo M, Rivara MB et al. Racial and ethnic disparities in use of and outcomes with home dialysis in the United States. J Am Soc Nephrol 2016;27:2123–34. 10.1681/ASN.2015050472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.