Abstract
Background
Severe and critical COVID-19 disease is characterized by hyperinflammation involving pro-inflammatory cytokines, particularly IL-6. Tocilizumab is a monoclonal antibody that blocks IL-6 receptors.
Objectives
This study evaluated the efficacy of tocilizumab in Filipino patients with severe to critical COVID-19 disease.
Methods
This phase 3 randomized double-blind trial, included patients hospitalized for severe or critical COVID-19 in a 1:1 ratio to receive either tocilizumab plus local standard of care or placebo plus standard of care. Patients were eligible for a repeat IV infusion within 24-48 hours if they deteriorated or did not improve. Treatment success or clinical improvement was defined as at least two categories of improvement from baseline in the WHO 7-point Ordinal Scale of patient status, in an intention-to-treat manner.
Results
Forty-nine (49) patients were randomized in the tocilizumab arm and 49 in the placebo arm. There was no significant difference in age, comorbidities, COVID-19 severity, need for mechanical ventilation, presence of acute respiratory distress syndrome, or biomarker levels between groups. Use of adjunctive therapy was similar between groups, with corticosteroid used in 91.8% in tocilizumab group and 81.6% in the placebo group, while remdesivir was used in 98% of participants in both groups.
There was no significant difference between groups in terms of treatment success in both the intention-to-treat analysis (relative risk=1.05, 95% CI: 0.85-1.30) and per-protocol analysis (relative risk=0.98, 95% CI: 0.80 to 1.21). There was no significant difference in time to improvement of at least two categories relative to baseline on the 7-point Ordinal Scale of clinical status.
Conclusion
The use of tocilizumab on top of standard of care in the management of patients with severe to critical COVID-19 did not result in significant improvement as defined by the WHO 7-point Ordinal Scale of patient status, nor in significant improvement in incidence of mechanical ventilation, incidence of ICU admission, length of ICU stay, and mortality rate.
Keywords: COVID-19, tocilizumab, IL-6, randomized trial
INTRODUCTION
COVID-19, the disease caused by the novel coronavirus SARS-CoV-2, has emerged as the most recent pandemic, affecting all parts of the world. As of April 27, 2022, more than 508,827,830 confirmed cases from more than two hundred countries and territories have been reported, and more than 6,227,291 patients have died from the disease worldwide.1 In the Philippines, the Department of Health (DOH) reported the country’s first confirmed case on January 30, 2020. As of March 6, 2022, the Philippines has already had more than 3,667,542 confirmed COVID-19 cases, with more than 57,023 deaths.2
The deterioration in severe to critical patients with COVID-19 infection has been associated with abnormal cytokine levels,3,4 especially that of Interleukin-6 (IL-6), a pro-inflammatory cytokine significantly elevated in COVID-19 patients.4 Overproduction of IL-6 has been shown to correlate negatively with both the expression of HLA-DR on CD14 monocytes and the absolute lymphocyte count in plasma from COVID-19 patients with immune dysregulation.5 A direct correlation, on the other hand, has been reported between SARS-CoV-2 RNA load and IL-6 levels in critical COVID-19 cases.6
Tocilizumab is a monoclonal antibody known to block the IL-6 receptor associated with inflammatory and autoimmune diseases. Tocilizumab has been shown to improve HLA-DR expression in plasma media and to increase the absolute lymphocyte counts of COVID-19 patients.5 The use of intravenous tocilizumab for COVID-19 patients with pneumonia has been evaluated in several international studies, both clinical trials and observational studies, and have shown variable effects in improving clinical status and reducing mortality.7-10 This study aimed to evaluate the effectiveness of tocilizumab plus local standard of care versus standard of care among Filipino patients with severe to critical COVID-19 disease admitted in a COVID-19 referral center.
MATERIALS AND METHODS
Study Design and Participants
This investigator-initiated study was a phase 3, double-blind, randomized, single center, placebo-controlled study, conducted at the University of the Philippines - Philippine General Hospital (UP-PGH), the largest government tertiary hospital and among the first DOH-designated COVID-19 referral centers in the Philippines.
Protocol development adhered to the Standard Protocol Items: Recommendations for Intervention Trials (SPIRIT) 2013 checklist guidelines.11 This study was designed, implemented, executed, and reported in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice (GCP),12 with applicable local regulation by the FDA, and with the ethical principles laid down in the Declaration of Helsinki. Approval from the UP Manila Research Ethics Board was obtained prior to the commencement of enrollment. A study investigator invited eligible patients, explained the nature of the study, discussed the contents of the informed consent in detail, and clarified all questions and concerns. A signed informed consent form was obtained before randomization and proceeding with study procedures. In cases where written consent could not be given by the patient, their legally authorized representative provided verbal and written consent which the investigator documented.
We enrolled all laboratory-confirmed COVID-19 patients who met the World Health Organization (WHO) criteria for severe or critical disease, aged 18 years and older, admitted at UP-PGH from January 7, 2021 until July 31, 2021. All patients had positive SARS-CoV-2 RT-PCR of any specimen including respiratory, blood, urine, stool, or other bodily fluid, and pneumonia confirmed by chest X-ray or CT scan, as well as oxygen saturations (SpO2) ≤93% or a ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) <300 mmHg. Patients were included if they had at least one of the following elevated biomarker levels upon admission: D-dimer >1000 mg/mL or a two-fold increase in value from baseline; C-reactive peptide (CRP) >40 mg/L; or Ferritin >400 mg/mL or a two-fold increase in value from baseline.
We excluded patients who had active tuberculosis (TB) infection, or other suspected active bacterial, fungal, or non-COVID-19 viral infection; those who received oral anti-rejection or immunomodulatory drugs (except corticosteroids) within the previous three months; or if per investigator’s evaluation, the patient had a high risk of mortality within the next 24 hours, irrespective of the provision of treatments. We also excluded pregnant or breastfeeding patients and patients with abnormal laboratory tests such as elevated liver enzymes (i.e., alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >5x the upper limit of normal), an absolute neutrophil count (ANC) <1000/mL, or a platelet count <50,000/mL detected within 24 hours at screening or at baseline.
Local standard of care treatment included glucocorticoids, antivirals such as remdesivir, convalescent plasma, and supportive care. Concomitant treatment with another investigational agent was prohibited.
Sample Size
At the time of the study conception, the only available estimates for relevant outcomes were reported by Weiss and Murdoch, and Zhou et al., wherein the largest estimate of mortality was at 28%, plus 12% more patients who do not improve.13,14 Based on these, we assumed a failure rate of 40% and that IV tocilizumab can reduce mortality to 15% (Relative Risk of 0.375). Given these assumptions, 49 patients per group or a total of 98 patients were adjudged to be required to reach a level of significance of 5% (two-tailed) with power of 80%. EpiInfo Statcalc and Fleiss Method were used in the above calculations.
Randomization and Masking
We randomized the recruited patients using the randomization module of Research Electronic Data Capture (REDCap), a secure web-based application in managing databases, to achieve a 1:1 allocation between intravenous (IV) tocilizumab plus standard of care or IV placebo (95% sodium chloride) plus standard of care. An independent biostatistician created the allocation sequence. This was encoded and concealed in the REDCap software, which was only accessible to a few co-investigators (FMMC, NNIP, PMGMC). The allocation sequence was not accessible to the research staff in charge of participant enrolment and assignment to interventions.
Once the patient or his/her caregiver consented to enrollment, an unblinded sub-investigator was in charge of entering the patient’s baseline details into the electronic data capture application (REDCap). The web-based application centrally randomized the patients into either the treatment or placebo arm. The said sub-investigator then informed the sub-investigators tasked with the administration of the medications of the assigned study drug code.
The trial patients, the rest of the investigators, care providers, and outcome assessors were masked to patient assignment. To preserve the blinding, the sub-investigators who supervised administration did not disclose whether IV tocilizumab or placebo was infused. In the event of an adverse effect that could be attributed to the study drug received, and upon the responsible investigator's approval, blinding was removed.
Procedures
Demographics and baseline clinical conditions of eligible patients were obtained upon enrollment. The following biomarkers were measured at baseline and every three days during admission: serum concentration of IL-6, ferritin, CRP, lactate dehydrogenase (LDH), and D-dimer. Data collected were encoded into REDCap using an online data collection form that mirrored the study case report form.
Enrolled patients were randomized to receive either a 60-minute intravenous placebo infusion (0.9% sodium chloride) plus standard of care treatment (as determined by the Internal Medicine service, as guided by UP-PGH Internal Medicine COVID-19 Pathway and the Interim Guidance on the Clinical Management of Adult Patients with Suspected or Confirmed COVID-19 Infection15) or a 60-minute 400 mg intravenous tocilizumab infusion diluted in 0.9% sodium chloride plus standard of care treatment. The placebo and tocilizumab infusion systems are identical in appearance.
Patients were eligible for a repeat IV infusion given 24-48 hours after the initial infusion in cases where there was note of clinical deterioration, or the absence of improvement, together with at least one of the following biomarker criteria: progressive elevation of D-dimer >1000 mg/mL, CRP >40 mg/L, Ferritin >400 mg/mL, or IL-6 >7 pg/mL. Patients with acute respiratory distress syndrome were eligible for a second infusion if they had satisfied one biomarker criteria and one of the following clinical criteria: progression of radiologic findings in the absence of any other cause, progressive increase in PEEP requirement, progressive increase in FiO2 requirement, peak flow ratio <150 liters per min or inability to maintain adequate oxygenation upon return to supine of patients who were on prone positioning; or if they fulfill two of the above clinical criteria.
Outcomes
The primary outcome of this study was treatment success or clinical improvement (defined as at least two categories of improvement from baseline in the WHO 7-point Ordinal Scale of patient status) at the end of admission according to the following categories:
Discharged or ready for discharge
Hospitalization in a non–intensive care unit (ICU) without supplemental oxygen
Non–ICU hospitalization with supplemental oxygen
ICU or non–ICU hospitalization with noninvasive ventilation or high-flow oxygen
ICU hospitalization with intubation and mechanical ventilation
ICU hospitalization with extracorporeal membrane oxygenation or mechanical ventilation and additional organ support
Death
Data using this 7-point Ordinal Scale was recorded at baseline on Day 1 (day of randomization and before treatment is given) and then again once daily every morning (between 8 am and 12 pm) while hospitalized.
The secondary outcomes evaluated were time to improvement of at least two categories relative to baseline on a 7-category Ordinal Scale of clinical status, incidence of mechanical ventilation, duration of ICU stay, and mortality rate.
Statistical analysis for outcomes
Relative risks for primary outcome assessment were calculated using 2 x 2 contingency table. The relative risk was crudely calculated as the ratio between the proportion of those with outcome among the intervention arm and the proportion of those with outcome among the comparator arm. Its 95% confidence interval was calculated according to Altman.16
The log-rank test and Kaplan-Meier plot were used for time-to-event analysis at the end of admission as part of secondary outcome assessment on time-to-clinical improvement. A p-value of less than 0.05 was considered as statistically significant.
Ethical considerations
This study was approved by the UP Manila Research Ethics Board (UPMREB code 2020-382-01). This protocol was registered in the Philippine Health Research Registry (Registry number: PHRR201005-003010).
RESULTS
Patient Enrollment and Randomization
From January 7, 2021 until July 31, 2021, 572 patients were evaluated for eligibility (Figure 1). Ninety-eight patients eligible for inclusion were randomized in a 1:1 manner. Three patients in the placebo arm did not receive study intervention: one patient withdrew consent prior to administration, one died prior to administration, and one had exclusion-level thrombocytopenia on a post-screening blood test prior to administration. Forty-five patients (91.8%) completed the study in the tocilizumab plus standard of care group and 39 patients (79.5%) completed the study in the placebo plus standard of care group. In addition, of the 49 patients in placebo arm, seven patients who did not improve after the initial dose were given out-of-trial tocilizumab by the attending physician and were thus considered as withdrawals as per-protocol analysis or treatment failure in intention-to-treat analysis.
Figure 1.
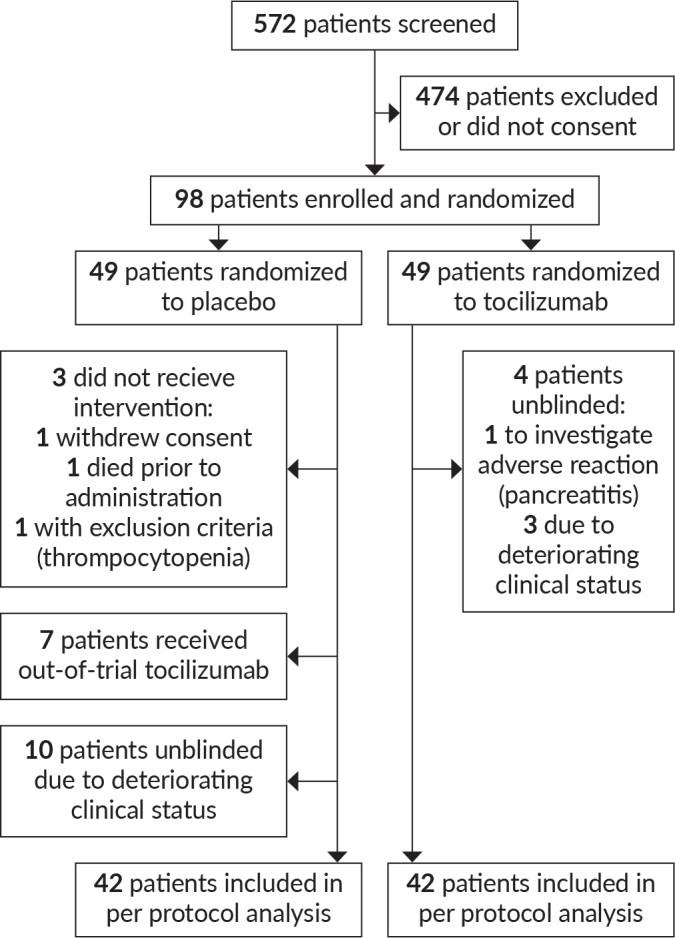
Number of patients screened, enrolled, randomized, and included in the analysis of this study.
Baseline characteristics were similar between groups (Table 1). There was no significant difference in age, frequency of co-morbidities, COVID-19 severity upon enrollment, need for mechanical ventilation, or presence of acute respiratory distress syndrome between groups. Proportions of patients given adjunctive treatment (corticosteroids, remdesivir) also did not differ significantly between treatment groups. Baseline biomarker levels such as serum Il-6, ferritin, CRP, LDH and D dimer likewise did not differ significantly between groups (Table 2).
Table 1.
Baseline Characteristics
| Characteristics | Tocilizumab (n=49) | Placebo (n=49) | p-value |
|---|---|---|---|
| Age, mean (SD) | 58.6 (14.2) | 57.8 (14.3) | 0.79 |
|
| |||
| Males, n (%) | 28 (45.2) | 34 (54.8) | 0.21 |
|
| |||
| With comorbidities, n (%) | |||
| Diabetes | 20 (40.8) | 15 (30.6) | 0.29 |
| Hypertension | 31 (63.3) | 31 (63.3) | 1.00 |
| Heart Disease | 4 (8.2) | 3 (6.1) | 0.70 |
| Liver Disease | 2 (4.1) | 0 (0) | 0.15 |
| Kidney Disease | 6 (12.2) | 3 (6.1) | 0.29 |
| Asthma | 1 (2) | 6 (12.2) | 0.05 |
| Cancer | 1 (2) | 0 (0) | 0.32 |
|
| |||
| COVID-19 Severity on Admission, n (%) | 0.65 | ||
| Mild | 1 (2) | 1 (2) | |
| Moderate | 8 (16.3) | 7 (14.3) | |
| Severe | 28 (57.1) | 33 (67.3) | |
| Critical | 12 (24.5) | 8 (16.3) | |
|
| |||
| COVID-19 Severity on Enrollment, n (%) | 0.68 | ||
| Severe | 29 (59.2) | 32 (65.3) | |
| Critical | 20 (40.8) | 17 (34.7) | |
|
| |||
| On oxygen support, n (%) | |||
| Low flow | 27 (55.1) | 32 (65.3) | 0.30 |
| High flow | 11 (22.4) | 10 (20.4) | 0.81 |
| On invasive ventilation, n (%) | 10 (20.4) | 5 (10.2) | 0.16 |
| Acute Respiratory Distress Syndrome, n (%) | 8 (16.3) | 5 (10.2) | 0.37 |
| On vasopressor, n (%) | 1 (2) | 1 (2) | 1.00 |
|
| |||
| Other treatment received, n (%) | |||
| Steroids | 45 (91.8) | 40 (81.6) | 0.23 |
| Remdesivir | 48 (98.0) | 48 (98.0) | 1.00 |
Table 2.
Baseline Biomarkers
| Tocilizumab | Placebo | p-value | |
|---|---|---|---|
| Mean Serum Concentration of IL-6 (pg/mL) | 294.94 (n=33) | 199.91 (n=33) | 0.51 |
| Serum Concentration of Ferritin (μg/mL) | 1390.59 (n=48) | 1522.22 (n=47) | 0.62 |
| Serum Concentration of hsCRP (mg/dL) | 131.11 (n=39) | 113.07 (n=41) | 0.34 |
| Serum Concentration of LDH (U/L) | 500.27 (n=49) | 444.47 (n=48) | 0.23 |
| Serum Concentration of D-dimer (μg/mL) | 2.9 (n=47) | 2.81 (n=45) | 0.89 |
Primary Outcome: Treatment success or Clinical improvement
The primary outcome of treatment success or clinical improvement did not significantly differ between groups as evident by the estimated relative risk ratios of 0.98 (95% CI: 0.80 to 1.21) and 1.05 (95% CI: 0.85 to 1.30) based on per-protocol (Table 3) or intention-to-treat [ITT] (Table 4) analyses, respectively.
Table 3.
Primary Outcome: Treatment success or Clinical Improvement (Per-Protocol Analysis)
| Treatment Success | Treatment Failure | Total | |
|---|---|---|---|
| Tocilizumab (n) | 39 | 10 | 49 |
| Placebo (n) | 34 | 8 | 42 |
| Total | 91 | ||
| Placebo | Tocilizumab | p-value | |
| Two proportion Z-test | 0.81 | 0.80 | 0.90 |
Table 4.
Primary Outcome: Treatment Success or Clinical Improvement (Intention-to-Treat Analysis)
| Treatment Success | Treatment Failure | Total | |
|---|---|---|---|
| Tocilizumab (n) | 39 | 10 | 49 |
| Placebo (n) | 37 | 12 | 49 |
| Total | 76 | 22 | 98 |
| Placebo | Tocilizumab | p-value | |
| Two proportion Z-test | 0.75 | 0.80 | 0.81 |
Secondary Outcome: Time to Improvement
There was no significant difference in time to improvement of at least two categories relative to baseline on the 7-category Ordinal Scale of clinical status on both per-protocol (Table 5) and intention-to-treat analyses (Table 6).
Table 5.
Secondary Outcome: Time to Improvement of at least 2 Categories Relative to Baseline on a 7-Category Ordinal Scale of Clinical Status (Per-Protocol Analysis)
| (Number of Days) | Placebo | Tocilizumab | p-value |
|---|---|---|---|
| Mean | 11.24 | 11.33 | 0.91 |
| Median | 11 | 10 | |
| Interquartile Range (IQR) | (7 to 14) | (8 to 13) |
Table 6.
Secondary Outcome: Time to Improvement of at least 2 Categories Relative to Baseline on a 7-Category Ordinal Scale of Clinical Status (Intention-to-Treat Analysis)
| (Number of Days) | Placebo | Tocilizumab | p-value |
|---|---|---|---|
| Mean | 11.49 | 11.33 | 0.90 |
| Median | 11 | 10 | |
| Interquartile Range (IQR) | (7 to 14) | (8 to 13) |
Likewise, other secondary outcomes such as incidence of mechanical ventilation, incidence of ICU admission, duration of ICU stay, and mortality rate were also not significantly different between groups (Tables 7 and 8).
Table 7.
Comparison of Other Secondary Outcomes (Per-Protocol Analysis)
| Tocilizumab | Placebo | Effect Estimate (95% CI) | p-value | |
|---|---|---|---|---|
| Incidence of Mechanical Ventilation, n (%) | 13 (26.5) | 7 (16.7) | RR = 1.59 (0.70 to 3.62) | 0.27 |
| Ventilator-Free Days (mean number of days) | 11.76 | 10.41 | MD = -1.35 (-2.65 to 2.73) | 0.33 |
| Incidence of Intensive Care Unit (ICU) Admission, n (%) | 13 (26.5) | 7 (16.7) | RR = 1.59 (0.70 to 3.62) | 0.27 |
| Duration of ICU Stay (mean number of days) | 1.47 | 0.55 | MD = -0.92 (-1.99 to 0.15) | 0.09 |
| Mortality, n (%) | 10 (20.4) | 8 (19.0) | RR = 1.07 (0.47 to 2.47) | 0.87 |
| Time to Hospital Discharge (mean number of days) | 5.64 | 5.06 | MD = -0.58 (-2.36 to 1.19) | 0.52 |
| Duration of Time on Supplemental Oxygen (mean number of days) | 6.47 | 5.37 | MD = -1.10 (-2.72 to 0.51) | 0.18 |
RR - relative risk, MD - mean difference
Table 8.
Comparison of Other Secondary Outcomes (Intention-to-Treat Analysis)
| Tocilizumab | Placebo | Effect Estimate (95% CI) | p-value | |
|---|---|---|---|---|
| Incidence of Mechanical Ventilation, n (%) | 13 (26.5) | 9 (18.4) | RR =0.90 (0.73 to 1.12) | 0.33 |
| Ventilator-Free Days (mean number of days) | 11.76 | 11.80 | MD = 0.041 (-2.65 to 2.73) | 0.98 |
| Incidence of Intensive Care Unit (ICU) Admission, n (%) | 13 (26.5) | 9 (18.4) | RR =0.90 (0.73 to 1.12) | 0.34 |
| Duration of ICU Stay (mean number of days) | 1.47 | 1 | MD = -0.47 (-1.70 to 0.76) | 0.45 |
| Mortality, n (%) | 10 (20.4) | 12 (24.5) | RR = 1.05 (0.8515 to 1.3048) | 0.63 |
| Time to Hospital Discharge (mean number of days) | 5.64 | 5.81 | MD = 0.17 (-1.77 to 2.11) | 0.86 |
| Duration of Time on Supplemental Oxygen (mean number of days) | 6.47 | 6.78 | MD = 0.31 (-1.56 to 2.18) | 0.74 |
RR - relative risk, MD - mean difference
Safety
One patient from the tocilizumab arm was reported to have acute pancreatitis, from which he recovered.
No other serious adverse events were reported.
DISCUSSION
In this single center, phase 3, placebo-controlled, randomized clinical trial in hospitalized patients with severe to critical COVID-19 pneumonia, we found no significant difference in treatment success or clinical improvement between the tocilizumab and placebo groups. There was also no significant difference between groups in mortality rate, although the study was not powered to assess this outcome. Per protocol analysis did not yield a significant difference between groups, either.
Markedly elevated inflammatory markers and elevated pro-inflammatory cytokines (including interleukin-6) are associated with critical and fatal COVID-19, and blocking the inflammatory pathway may prevent disease progression.
This result is similar to the findings of a meta-analysis of seven RCTs in 2021, involving 5,585 participants, which showed that tocilizumab results in little to no increase in clinical improvement at day 28 (RR= 1.06, 95%CI: 1.00 to 1.13; I2 = 40.9%), but showed a mortality benefit with tocilizumab.17 In another meta-analysis of eight randomized trials involving 6,263 COVID-19 patients, all-cause mortality was significantly lower among those who received tocilizumab compared with placebo or standard of care (odds ratio = 0.83, 95% CI 0.74 to 0.92).18
The largest trial included in the meta-analysis is the RECOVERY Trial that included patients with severe COVID-19 and elevated inflammatory markers. There were 4,116 patients with hypoxemia and a CRP level ≥75 mg/L. The study showed that adding tocilizumab to usual care reduced the 28-day mortality rate compared with usual care alone (31 versus 35 percent, relative risk 0.85, 95% CI 0.76-0.94). Most patients were also using glucocorticoids, mainly dexamethasone.10
Our study did not show a significant difference in the outcome of mortality. It may be due to the small number of patients included, which could have underestimated the true effects of the treatment. The treatment failure rate in our study was 22.4%, which is much less than our initial estimate of 40% used in the computation of sample size.
An important limitation of our trial is that the standard of care has evolved throughout the duration of the trial and that attending physicians had the discretion to administer the prevailing standard of care. As of July 2021, tocilizumab is strongly recommended in addition to steroids for patients with severe or critical COVID-19. Nevertheless, the distribution of patients in the treatment and control groups that received additional interventions did not differ.
Another limitation was the availability of the non-study drug tocilizumab in the institution and its inclusion in local COVID-19 guidelines later in the course of the study. A few patients from each group were withdrawn from the study due to non-improvement, and given out-of-trial tocilizumab. However, per protocol analysis done to account for this still did not reveal any significant difference in primary and secondary outcomes between groups.
Our study used a composite outcome that encompassed several important measures in the treatment of COVID-19. However, the study was not powered to assess significant differences in mortality between groups.
Further studies are recommended with larger populations to evaluate the true effect of IV tocilizumab in serious to critical COVID-19 patients receiving the current standard of care.
CONCLUSION
Administration of tocilizumab did not result in better treatment success or clinical improvement, in use of mechanical ventilation, length of ICU stay, and mortality rate among patients with severe or critical COVID-19. While existing guidelines strongly recommend the administration of tocilizumab to severe and critical COVID-19 patients with pneumonia, more studies on proper timing of administration using larger sample size may be pursued to determine the role of tocilizumab in the management of severe and critical COVID-19 patients.
Acknowledgments
The authors would like to thank the DOST - Philippine Council for Health Research and Development (PCHRD) and Roche Philippines, Inc. for funding this clinical trial, Mr Patrick Wincy Reyes for contributions to statistical analysis, as well as Dr. Mark Joseph Abaca, Dr. Kenzle Denise Monsanto, Dr. Angelito Flora Jr., Dr. Jana Cortez, and Dr. Blessie Marie Perez for their assistance in recruiting and monitoring participants for this study.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
Author Disclosure
All authors declared no conflicts of interest.
REFERENCES
- 1.World Health Organization , Coronavirus Disease (COVID-19) Dashboard [Internet]. 2021. [cited 2022 May]. Available from: https://covid19.who.int
- 2.Department of Health , Covid-19 Case Tracker [Internet]. 2022. [cited 2022 May]. Available from: https://doh.gov.ph/covid-19/case-tracker
- 3.Zhu J, Pang J, Ji P, Zhong Z, Li H, Li B, et al. Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis. J Med Virol. 2021. Jan; 93(1):35-7. doi: 10.1002/jmv.26085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udomsinprasert W, Jittikoon J, Sangroongruangsri S, Chaikledkaew U. Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-alpha, as potential biomarkers of severity and mortality for COVID-19: systematic review with meta-analysis. J Clin Immunol. 2021. Jan;41(1):11-22. doi: 10.1007/s10875-020-00899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020. Jun;27(6):992-1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum Severe Acute Respiratory Syndrome Coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with Coronavirus Disease 2019. Clin Infect Dis. 2020. Nov;71(8):1937-42. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe COVID-19 pneumonia. N Engl J Med. 2021. Apr;384(16):1503-16. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with COVID-19 pneumonia. N Engl J Med. 2021. Jan;384(1):20-30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P; CORIMUNO-19 Collaborative Group . Effect of Tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021. Jan 1;181(1):32-40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021. May;397(10285):1637-45. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013. Feb;158(3):200–7. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) . ICH Harmonised Guideline: Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2) [Internet]. 2016. [cited 2022 Dec]. Available from: https://database.ich.org/sites/default/files/E6_R2_Addendum.pdf.
- 13.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020. Mar; 395(10229):1014-5. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. Mar;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Philippine Society for Microbiology and Infectious Diseases, Philippine College of Chest Physicians, Philippine College of Physicians, Philippine Rheumatology Association, Philippine College of Hematology and Transfusion Medicine . Interim guidance on the clinical management of adult patients with suspected or confirmed COVID-19 infection [Internet]. 2020. [cited 2021 Dec].Available from: https://www.psmid.org/wp-content/uploads/2020/07/Final-PCP-PSMID-PCCP-COVID-19-Guidelines-20July2020b.pdf
- 16.Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991. [Google Scholar]
- 17.Cabaluna IT, Garcia A, Bayona HHG. Should Tocilizumab be used for the treatment of hospitalized patients with COVID-19? [Internet]. 2021. [cited 2021 Dec]. Available from: https://www.psmid.org/wp-content/uploads/2021/04/Tocilizumab-Living-CPG-March-2021.pdf
- 18.Ghosn L, Chaimani A, Evrenoglou T, Davidson M, Graña C, Schmucker C, et al. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021. Mar;3(3):CD013881. doi: 10.1002/14651858.CD013881. [DOI] [PMC free article] [PubMed] [Google Scholar]


