Abstract
Body size is a fundamental biological trait shaping ecological interactions, evolutionary processes, and our understanding of the structure and dynamics of marine communities on a global scale. Accurately defining a species' body size, despite the ease of measurement, poses significant challenges due to varied methodologies, tool usage, and subjectivity among researchers, resulting in multiple, often discrepant size estimates. These discrepancies, stemming from diverse measurement approaches and inherent variability, could substantially impact the reliability and precision of ecological and evolutionary studies reliant on body size data across extensive species datasets. This study examines the variation in reported maximum body sizes across 69,570 individual measurements of maximum size, ranging from <0.2 μm to >45 m, for 27,271 species of marine metazoans. The research aims to investigate how reported maximum size variations within species relate to organism size, taxonomy, habitat, and the presence of skeletal structures. The investigation particularly focuses on understanding why discrepancies in maximum size estimates arise and their potential implications for broader ecological and evolutionary studies relying on body size data. Variation in reported maximum sizes is zero for 38% of species, and low for most species, although it exceeds two orders of magnitude for some species. The likelihood of zero variation in maximum size decreased with more measurements and increased in larger species, though this varied across phyla and habitats. Pelagic organisms consistently had low maximum size range values, while small species with unspecified habitats had the highest variation. Variations in maximum size within a species were notably smaller than interspecific variation at higher taxonomic levels. Significant variation in maximum size estimates exists within marine species, and partially explained by organism size, taxonomic group, and habitat. Variation in maximum size could be reduced by standardized measurement protocols and improved meta‐data. Despite the variation, egregious errors in published maximum size measurements are rare, and their impact on comparative macroecological and macroevolutionary research is likely minimal.
Keywords: body size, macroecology, macroevolution, maximum size, variation
This research investigates maximum body sizes in 27,581 marine species from 24 phyla globally in the modern oceans. Analyzing size data and factors like taxonomy, habitat, and measurement methods revealed significant variation in reported maximum sizes. Despite variations, only about 5% of species experienced significant errors, emphasizing the need for standardized measurement protocols to minimize discrepancies in maximum size reporting without significantly altering species rankings in macroecological and macroevolutionary studies.
1. INTRODUCTION
Body size is a fundamental biological trait and has a long‐history of intensive study across many biological disciplines, including ecology, evolution, physiology, and medicine (Bonner, 2007). Body size can affect an organism's resource use (Brown et al., 2004), level of success in competition (Grant, 1968; Hutchinson, 1959; Wilson, 1975), and interactions with predators and prey (Barnes et al., 2010; Costa, 2009). Differently sized animals may be able to use different resources in the landscape and at different timescales (Cooke et al., 2022; McClain et al., 2006; Ritchie & Olff, 1999). In addition, body size can influence the efficiency of movement, which can be important in determining an animal's ability to disperse, migrate, or hunt (Goldbogen et al., 2012). Body size is subject to natural selection (Nagel & Schluter, 1998; Schluter & Smith, 1986), with different body sizes being favored in different environments (Aava, 2001; Gearty et al., 2018; Gearty & Payne, 2020; Knope & Scales, 2013; Knouft, 2004; Lomolino, 2005; Poulin, 1996). The adaptive importance of body size is also strengthened by the direct link of body size to reproductive success, where larger individuals of a species may have higher reproductive output (Bosch & Vicens, 2006; Wiklund & Kaitala, 1995). Thus, by analyzing body‐size data, scientists can uncover patterns in the structure and dynamics of communities, and make predictions about how organisms may respond to changing environments (Hunt & Roy, 2006; Millien, 2004; Sheridan & Bickford, 2011), and gain a deeper understanding of the evolutionary history of life on Earth (Alroy, 1998; Heim et al., 2015; Payne et al., 2008).
One reason for the popularity of body size as a research topic, aside from its fundamental importance in many biological processes, is that it is relatively easy to measure and straightforward to compare across the tree of life from viruses and archaea to blue whales (Brown, 1995). Due to the ease of measuring body size, large amounts of data exist for many different species, making it a valuable trait for comparative studies and meta‐analyses (Bloom et al., 2018; DeLong et al., 2010; Harmon et al., 2010; Heim et al., 2017; Hillebrand & Azovsky, 2001; Thornton & Fletcher Jr, 2014). Furthermore, the abundance of data on body size allows for detailed analyses of patterns and trends in body size across different ecological, geographical, and evolutionary scales.
Despite the ease of taking body‐size measurements, the accurate characterization of body size, including assigning a single, appropriate value to a species, can be challenging. Multiple estimates of body size often arise for a single species. In part, this situation reflects biologically important ontogenetic and intraspecific variation. However, some of this size variation arises from multiple attempts to characterize the size of a species using a single size metric. For example, “maximum size,” which is quite often a target measurement for both biological and practical reasons, maybe collected by different researchers, at different places or times, making different measurement choices, or subject to other methodological issues. In addittion, many tools (e.g., rulers, calipers, lasers, scanners, scales), and measures (e.g., length, area, volume, mass), exist to quantify body size. Errors can also easily arise, such as those due to the use of inaccurate instruments, measurement variability among observers, and measurement bias due to subjectivity or inappropriate scaling methods, in addition to simple typographical errors that can propagate as data are transcribed from one source to another. For example, the Australian trumpet snail (Syrinx aruanus) has a reported maximum length value in the literature, databases, and websites of either 91.4 cm or 72.2 cm (McClain et al., 2015). Further research showed that both of these measurements are attributed to the same specimen and collector and that the larger measurement (91.4 cm) is an error (McClain et al., 2015). In addition, for some taxa, standards on measurement do not exist. For example, for species of wood‐boring bivalves in the families Xylophagiidae and Terenidae, reported length measurements can reflect the shell alone, often millimeters to centimeters in length, or include the siphons that reach a meter in some species (Hanks et al., in review). Thus, multiple estimates of body size for a single species can differ substantially and potentially affect the accuracy and reproducibility of ecological and evolutionary studies that rely on body size data. In studies that address size evolution across hundreds to thousands or tens of thousands of species, it may not be realistic or even possible to vet all size data from other sources to identify and address these sources of error or variability. Moreover, biologists currently lack a comprehensive understanding of what factors may bias the size measurements due to a lack of research.
Here, we examine variability in reported maximum size measurements within species across marine Metazoa. Multiple estimates of maximum size can occur tied to real intraspecific variation coded into the literature as holotypes, paratypes, and neotypes, or reflecting differences in body size varying environmentally and recorded in different regional inventories. For each species in our dataset, we characterize the largest maximum size reported (maxsizelargest), smallest maximum size reported (maxsizesmallest), and the difference between the two (maxsizerange). We analyze maximum size measurements for 27,271 marine species with multiple available estimates of maximum size. These species range in reported total length from the smallest value of maxsizesmallest of 0.195 microns (Batillipes tubernatis, a benthic tardigrade) to the largest value of maxsizelargest of 45.7 meters (Praya dubia, the giant siphonophore). We specifically test how ranges in maximum size within marine species varies with: (1) the reported size of the organism, (2) taxonomic group, (3) habitat, and (4) presence of exo‐ or endo‐skeleton. We chose maximum size because it is commonly used in broad‐scale studies of body‐size evolution, often with the justification of avoiding the inclusion of juveniles, providing a consistent approach to species with indeterminate growth (Heim et al., 2015), and difficulty in estimating the entire size distribution within a single species across habitats. We hypothesize the range in (log‐transformed) maximum size estimates is: (1) greater for smaller organisms because of greater error in measurement relative to body size; (2) greater in some taxonomic groups due to either complex bauplans, including coloniality, or measurement standards; (3) greater in pelagic organisms given their often‐gelatinous nature, indeterminate growth, and difficulty in collection; and (4) greater in those organisms lacking hard skeletons, which makes measurement more difficult and variable, leading to greater variation in measured lengths due to the ease of body deformation. Finally, we examine those species with extreme (>2 orders of magnitude) range in maximum size measurements and consider the impacts that errors in maximum size estimates may have on comparative macroecological and macroevolutionary studies that rely on collations of body size data from the literature.
2. METHODS
Maximum size as the largest linear dimension was collected for marine metazoans from 356 online databases and published literature. We choose linear dimension (e.g., height, length, width, and diameter), for this study because it is the most commonly reported measure of size in the literature. While mass, rather than length, scales proportionally with energetics and metabolic rate, length scales with mass in higher taxa (Benke et al., 1999; Gaspar et al., 2001; Méthot et al., 2012; Rosati et al., 2012; Santini et al., 2018; Seebacher, 2001; Trites & Pauly, 1998). A complete set of references for the dataset is provided in Appendix S1. A standardized taxonomy, including unique species identifiers (AphiaID), synonymized names, and taxonomy was based on the World Register of Marine Species (WoRMS; WoRMS Editorial Board, 2023). In total, at least two estimates of maximum size were collected for 27,271 marine species from 24 phyla for a total of 69,570 size measurements. No quality control was conducted on size measurements. For example, if a species had a reported size well outside logical size range, we kept this error in the database because our objective was to specifically examine the influence of all errors, including typographical errors. As set out below, our results give some insight into the likely prevalence of such errors in large body size databases, and guidance for how to identify them. All data and code are available at https://anon.to/yrHI74.
For each species, the range among multiple measurements of maximum size was quantified as:
| (1) |
Note that this measure of range is the log‐transformed ratio of the maximum and minimum maximum size for each species. This calculation of range allows us to include in analyses species whose smallest and largest maximum sizes are equal (i.e., maxsizerange = 0).
Higher level taxonomy and broad habitat classifications (termed “functional groups” in WoRMS) for each species were taken from WoRMS (WoRMS Editorial Board, 2023). For the analyses, we combined habitat information into groups of benthic, pelagic, and unspecified/unknown (Table 1). WoRMS functional group data were compiled by expert taxonomic editors, with additional input from targeted pilot projects on specific taxa. Classification is at the species level, although can be at higher taxonomic levels for groups where all members are known to have the same broad functional group. Designations are typically unambiguous and in very few species is their disagreement between experts. For each species, we also coded whether it has an exoskeleton, endoskeleton, or no skeleton based on taxonomy and known invertebrate anatomy (Table 1). Count was taken as the number of maximum size estimates for each species.
TABLE 1.
Summary of number of species per phylum, habitat group, and type of skeleton.
| Phylum | Total count | Benthic | Pelagic | Unspecified | None | Exo | Endo |
|---|---|---|---|---|---|---|---|
| Annelida | 379 | 372 | 1 | 6 | 379 | 0 | 0 |
| Arthropoda | 2601 | 594 | 1888 | 119 | 0 | 2601 | 0 |
| Bryozoa | 237 | 220 | 0 | 17 | 237 | 0 | 0 |
| Chordata | 13,080 | 9975 | 2927 | 178 | 0 | 0 | 13,080 |
| Cnidaria | 707 | 379 | 285 | 43 | 687 | 20 | 0 |
| Echinodermata | 234 | 228 | 0 | 6 | 0 | 234 | 0 |
| Mollusca | 9367 | 8600 | 235 | 532 | 763 | 8604 | 0 |
| Nematoda | 666 | 653 | 0 | 13 | 666 | 0 | 0 |
| Totals | 27,271 | 21,021 | 5336 | 914 | 2732 | 11,459 | 13,080 |
For our main analyses, we limited the dataset to only include phyla with at least 100 species in our dataset to allow for robust statistical analysis (Table 1). This resulted in a final dataset of n = 27,271 species with a total of 69,570 measurements. The overall distribution of maxsizerange was heavily‐right skewed (most species have small ranges of maxsizerange but some have very large ranges) and also zero‐inflated (38% of species have zero variation in maxsizerange measures; Figure 1). Given this distribution of maxsizerange, we analyzed the data using zero‐inflated gamma hurdle models, first using a binomial model with logit link to test which factors were associated with a species having zero or non‐zero size range, and then using a gamma model with log link to model the non‐zero values of size range (Brooks et al., 2017). We fitted these zero‐inflated gamma hurdle models using the function glmmTMB in the R package glmmTMB (Brooks et al., 2017), setting family to ziGamma with a log link, and using identical sets of predictors for both the zero and the non‐zero components of the model. Preliminary analyses showed that treating count as a continuous variable caused very high uncertainty in parameter estimates, especially at high values of count—which are rare in our dataset (the median value for count is 2, and only 755 (2.8%) species have >5 maximum size estimates). For all analyses, we therefore treated count as a four‐level categorical variable, with values 2, 3, 4–5, and ≥6.
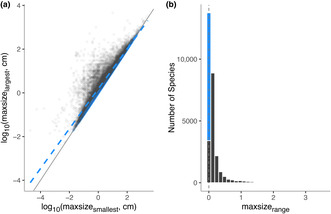
FIGURE 1.
(a) Largest versus smallest reported maximum sizes for each individual species, that is, log10(maxsizelargest, cm) versus log10(maxsizesmallest, cm) for each individual species. Solid gray line represents 1:1 relationship between largest and smallest maximum reported size. Blue dash line is the linear model log10maxsizelargest = 0.17 + 0.91* log10maxsizesmallest between the two variables with zero uncertainty included, that is, log10maxsizelargest = log10maxsizesmallest. (b) Distribution of size range, maxsizerange, in length measurements (log10maxsizelargest–log10maxsizesmallest). Blue color represents species with no variation in measurements (maxsizerange = 0, n = 10,345 species).
Our models took the form:
| (2) |
where variable is either phylum, habitat, invertebrate, or skeleton, or phylum and habitat, invertebrate and habitat, or skeleton and habitat. The categories were highly collinear (Table 1), particularly with phylum because the values of skeleton and invertebrate are largely conserved at high taxonomic scales. Because of this collinearity, we limited the set of predictors in any one model but ran all factors in different models. We compared separate models using sample‐corrected Aikake Information Criterion (AICc) values.
In all models, post hoc comparisons were conducted by computing estimated marginal means for specified factors or factor combinations in the general linear model and conducting contrasts among them using the function eemeans in the eemeans R package (Lenth, 2022). The function automatically adjusts for multiple comparisons using a Tukey adjustment.
To examine the impact of intraspecific variation in maximum size in broad‐scale comparative summaries of body size across all species, we compared the intraspecific variation in maximum size to variation observed at higher taxonomic levels, for the exemplar group of Gastropoda. We then considered the extent to which a species' position in the rank order of body sizes across all 27,271 species changes depending on which estimate of maximum size was chosen. We generated 1000 pairs of body size rankings, with each ranking obtained by drawing a single maximum size for each species. For each pair of rankings, we calculated the overall correlation in ranks, as well as the median and maximum changes in species rank position. We also identified the species with the largest change in body size ranking for each of the 1000 randomizations. Finally, we identified all species with a maxsizerange in excess of two orders of magnitude and investigated the reason for this large range.
3. RESULTS
A positive relationship, with a slope significantly less than one existed between maxsizelargest and maxsizesmallest for the full dataset of 27,581 species (maxsizelargest = 0.18 + 0.91* maxsizesmallest, t‐ratio(1,27,579) = −52.94, p < .0001, Adj. R 2 = .91 Figure 1a). After removing 10,345 species with no variation in measurements, the slope remained significantly less than one, with a slightly elevated intercept compared to the full dataset (maxsizelargest = 0.25 + 0.90* maxsizesmallest, t‐ratio(1,17,234) = −40.59, p < .0001, Adj. R 2 = .88 Figure 1a). Both the zero‐variation included and excluded datasets were significantly right‐skewed (D'Agostino skewness test, with zeros: skew = 4.76, z = 136.4, p‐value <.001; non‐zeros: skew = 4.03, z = 100.8, p‐value <.001, Figure 1b).
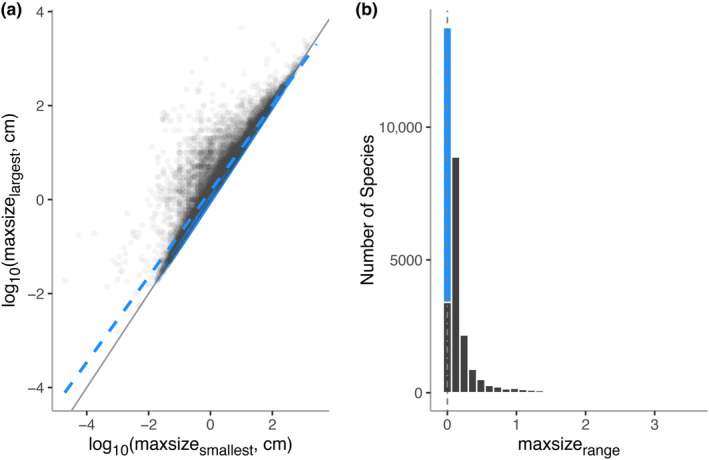
Initial exploration of the eight phyla that have >100 species in our dataset suggested that range in maxsizerange varies with measurement count, phylum, and habitat (Figure 2). From the set of gamma hurdle models we fitted to test this, the model with the lowest AICc contained the predictors minimum size, count, phylum, and habitat, and the two‐way interactions between minimum size and each of the other variables (Table 2). This model substantially outperformed the second‐best model, which excluded habitat (Table 2). Models with skeleton performed worse than models with phylum.
FIGURE 2.
(a) Size range, maxsizerange, in length measurements (log10maxsizelargest–log10maxsizesmallest) versus the number of measurements per species (b). Violin plots of maxsizerange by phylum. (c) Violin plots of maxsizerange by habitat.
TABLE 2.
AICc for various Hurdle models to predict maxsizerange. All Hurdle models also include maxsizesmallest, count of observations, and interaction terms.
| Model | df | AICc |
|---|---|---|
| Phylum + Habitat | 53 | −841.44 |
| Phylum | 45 | 486.00 |
| Skeleton + Habitat | 33 | 1684.91 |
| Invertebrate + Habitat | 29 | 2452.69 |
| Habitat | 25 | 3532.82 |
| Skeleton | 25 | 3816.72 |
| Invertebrate | 21 | 4591.70 |
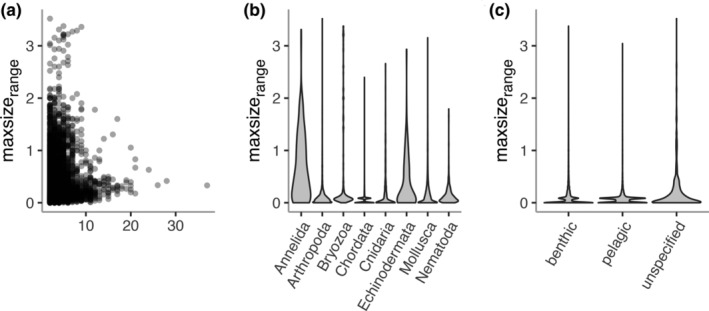
The zero‐inflation component of the hurdle model (Table 3A) showed that the likelihood of a species having zero maxsizerange decreases with the number of maximum size estimates, but this relationship varied with maxsizesmallest: the smallest species are very unlikely to have zero maxsizerange regardless of the value of count, whereas in larger species the likelihood of having zero maxsizerange approaches 100% for species with count = 2, declining to approximately 0% for species with high values of count (Figure 3a). In most phyla, the likelihood of having zero maxsizerange increases with increasing maxsizesmallest (Figure 3b), although note that the total range of sizes observed within most phyla spans only a part of the entire axis of maxsizesmallest (in particular, log10(maxsizesmallest) is ≤0.38 for all nematodes). The exceptions to the general pattern are Mollusca and Bryozoa. For bryozoans, this exception is likely a consequence of small numbers of species with zero maxsizerange, (2 species, or 0.8% of bryozoans). For mollusks however, the increased likelihood of zero maxsizerange in smaller species appears to be a genuine trend across the range of log10(maxsizesmallest) observed in this group (c. −2 to +2.5). The relationship between the likelihood of zero maxsizerange and habitat also varied with maxsizesmallest: at small sizes, zero maxsizerange is highly unlikely across all habitats, whereas at larger body sizes, zero maxsizerange is most likely for benthic species, followed by pelagic and then species with unspecified habitat (Figure 3c).
TABLE 3.
Results of best Hurdle model to predict maxsizerange. maxsizerange ~ maxsizesmallest + count + phylum + habitat + phylum* maxsizesmallest + habitat* maxsizesmallest. Significant variables at the Bonferroni corrected value of α = .009 are in bold. Those variables significant at α = .05 are italicized. A. The zero‐inflation binomial model to test the association between each predictor and the likelihood of a species having zero variation in maximum size across all species. B. The conditional gamma model to test the association between each predictor and the value of maxsizerange across the species with non‐zero variation in maximum size.
| A. Zero‐inflation model (Binary Logistic Regression All Data) | Estimate | Std. Error | z Value | Pr(>|z|) |
|---|---|---|---|---|
| Intercept | −0.74 | 0.050 | −14.8 | <.0001 |
| Maxsizesmallest | −0.61 | 0.064 | −9.6 | <.0001 |
| Count3 | 0.34 | 0.019 | 18.2 | <.0001 |
| Count4‐5 | 0.60 | 0.021 | 29.1 | <.0001 |
| Count6+ | 0.97 | 0.034 | 28.2 | <.0001 |
| Arthropoda | −1.38 | 0.056 | −24.7 | <.0001 |
| Bryozoa | −0.54 | 0.087 | −6.1 | <.0001 |
| Chordata | −0.91 | 0.056 | −16.1 | <.0001 |
| Cnidaria | −0.27 | 0.070 | −3.9 | .0001 |
| Echinodermata | 0.10 | 0.097 | 1.0 | .3003 |
| Mollusca | −0.92 | 0.051 | −18.2 | <.0001 |
| Nematoda | −1.96 | 0.090 | −21.8 | <.0001 |
| Pelagic | −0.53 | 0.024 | −21.8 | <.0001 |
| Unspecified Habitat | 0.31 | 0.037 | 8.4 | <.0001 |
| Maxsizesmallest:Count3 | 0.03 | 0.019 | 1.3 | .1848 |
| Maxsizesmallest:Count4‐5 | 0.08 | 0.024 | 3.4 | .0006 |
| Maxsizesmallest:Count6+ | 0.03 | 0.041 | 0.8 | .4406 |
| Maxsizesmallest:Arthropoda | 0.21 | 0.069 | 3.1 | .0022 |
| Maxsizesmallest:Bryozoa | −0.36 | 0.078 | −4.6 | <.0001 |
| Maxsizesmallest:Chordata | 0.10 | 0.067 | 1.4 | .1546 |
| Maxsizesmallest:Cnidaria | 0.11 | 0.089 | 1.2 | .2191 |
| Maxsizesmallest:Echinodermata | −0.34 | 0.124 | −2.8 | .0056 |
| Maxsizesmallest:Mollusca | 0.14 | 0.066 | 2.1 | .0361 |
| Maxsizesmallest:Nematoda | 0.28 | 0.097 | 2.9 | .0043 |
| Maxsizesmallest:Pelagic | 0.41 | 0.021 | 19.5 | <.0001 |
| Maxsizesmallest:Unspecified Habitat | 0.00 | 0.028 | 0.0 | .9615 |
| B. Conditional model (Gamma Regression Model to Non‐Zeros) | Estimate | Std. Error | z Value | Pr(>|z|) |
|---|---|---|---|---|
| Intercept | −1.50 | 0.205 | −7.29 | <.0001 |
| Maxsizesmallest | 1.88 | 0.293 | 6.40 | <.0001 |
| Count3 | −1.83 | 0.058 | −31.5 | <.0001 |
| Count4‐5 | −3.72 | 0.143 | −26.0 | <.0001 |
| Count6+ | −6.34 | 1.24 | −5.09 | <.0001 |
| Arthropoda | 1.01 | 0.221 | 4.58 | <.0001 |
| Bryozoa | −2.18 | 0.980 | −2.23 | .0260 |
| Chordata | −0.72 | 0.216 | −3.36 | .0008 |
| Cnidaria | 2.14 | 0.225 | 9.51 | <.0001 |
| Echinodermata | −0.60 | 0.441 | −1.37 | .1704 |
| Mollusca | 1.42 | 0.207 | 6.88 | <.0001 |
| Nematoda | −0.73 | 0.644 | −1.13 | .2582 |
| Pelagic | 0.26 | 0.080 | 3.27 | .0011 |
| Unspecified Habitat | −0.09 | 0.085 | −1.04 | .2969 |
| Maxsizesmallest:Count3 | −0.14 | 0.052 | −2.62 | .0089 |
| Maxsizesmallest:Count4‐5 | −0.23 | 0.140 | −1.66 | .0979 |
| Maxsizesmallest:Count6+ | −1.85 | 1.42 | −1.30 | .1937 |
| Maxsizesmallest:Arthropoda | −0.84 | 0.309 | −2.73 | .0063 |
| Maxsizesmallest:Bryozoa | −2.32 | 0.749 | −3.09 | .0020 |
| Maxsizesmallest:Chordata | 0.12 | 0.297 | 0.42 | .6747 |
| Maxsizesmallest:Cnidaria | −1.01 | 0.318 | −3.17 | .0015 |
| Maxsizesmallest:Echinodermata | −0.01 | 0.472 | −0.02 | .9822 |
| Maxsizesmallest:Mollusca | −2.39 | 0.297 | −8.06 | <.0001 |
| Maxsizesmallest:Nematoda | −0.98 | 0.718 | −1.37 | .1718 |
| Maxsizesmallest:Pelagic | −0.87 | 0.061 | −14.2 | <.0001 |
| Maxsizesmallest:Unspecified Habitat | −1.04 | 0.090 | −11.6 | <.0001 |
FIGURE 3.
Predicted values (marginal effects) for specific model terms for the Hurdle model predicting range in maximum size estimates (maxsizerange). (a) Effects of measurement count and log10maxsizesmallest on the probability of non‐zero maxsizerange in the zero‐inflation model (binary logistic regression) fit to all data. (b) Effects of phyla and log10maxsizesmallest on the probability of non‐zero maxsizerange in the zero‐inflation model (binary logistic regression) fit to all data. (c) Effects of habitat and log10maxsizesmallest on the probability of non‐zero maxsizerange in the zero‐inflation model (binary logistic regression) fit to all data. (d) Effects of measurement count and log10maxsizesmallest) on maxsizerange in the conditional model (gamma regression model) fit to non‐zeros maxsizerange data. (e) Effects of phyla and log10maxsizesmallest on maxsizerange in the conditional model (gamma regression model) fit to non‐zeros maxsizerange data. (f) Effects of habitat and log10maxsizesmallest on maxsizerange in the conditional model (gamma regression model) fit to non‐zeros maxsizerange data.
The gamma model for non‐zero values of maxsizerange (Table 3B) showed that maxsizerange decreases with increasing maxsizesmallest and increases with increasing number of measurements (Figure 3d, Table S1). Values of maxsizerange were particularly high and variable in Annelida, Echinodermata, and Cnidaria, and lowest in Nematoda (Figure 3e, Appendix S2: Table S1). Values of maxsizerange were low in pelagic organisms regardless of size, and highest in small species with unspecified habitat, with benthic organisms intermediate (Figure 3f, Appendix S2: Table S2). These differences between habitats largely disappear among larger organisms (Figure 3f).
Significant differences occurred between cumulative frequency distributions based on using maxsizesmallest, maxsizemean, or maxsizelargest for a species (Figure 4, Table 4). Standard deviation of the maxsize distributions was the greatest in minimum and smallest in maximum measurements (Table 4). Distributions, once mean centered, also exhibited significant differences in variance (Table 4) except for between maxsizelargest and maxsizemean. However, visually the three distributions vary little from one another and Spearman's Rank Order Correlations are all highly significant, with rho >0.96 (Table 4 ). Moreover, these variations in maxsize within a species are far less than the interspecific variation within genera, families, and orders, as exemplified for gastropods (Figure 5).
FIGURE 4.
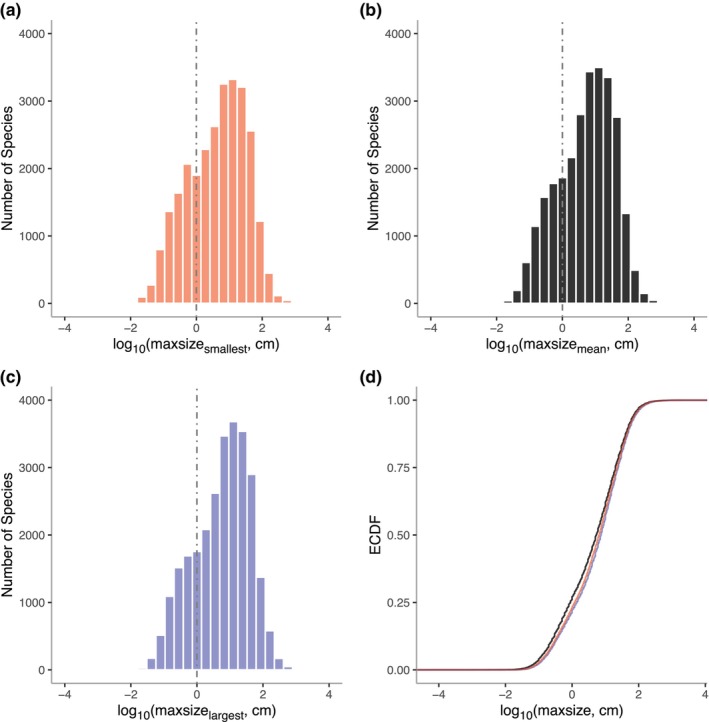
Distributions of reported sizes (a) log10maxsizesmallest (b) log10maxsizemean (c) log10maxsizelargest for the complete dataset, (d) Empirical cumulative distribution function (ECDF) for log10maxsizesmallest (red), log10maxsizemean (black), and log10maxsizelargest (blue).
TABLE 4.
Results of statistical tests between frequency distributions based minimum, mean, and maxsize.
| Comparison | Mean (SD) | Kolmogorov–Smirnov Test (D, p‐value) | F test (on centered data) (F, p‐value) | Spearman's rank correlation (rho, p‐value) |
|---|---|---|---|---|
| Mean vs. Minimum | 0.6846 (0. 8582) | 0.0423, .001 | 1.0787, <.0001 | .982, <.0001 |
| 0.6010 (0.8948 | ||||
| Mean vs. Maximum | 0.6846 (0.8582) | 0.0280, .001 | 1.0129, .2914 | .998, <.0001 |
| 0.7297 (0.8526) | ||||
| Minimum vs. Maximum | 0.6010 (0.8948) | 0.0621, .001 | 1.0926, < .0001 | .967, <.0001 |
| 0.7297 (0.8526) |
FIGURE 5.
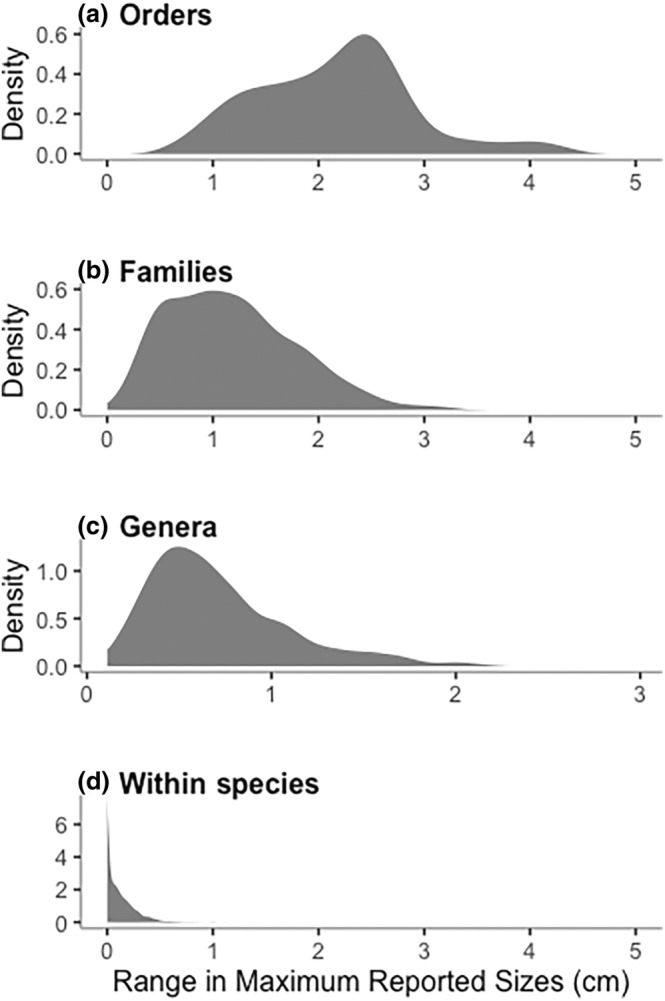
Distributions of size range, maxsizerange, within gastropod (a) orders (n = 21) (b) families (n = 227) (c) genera (n = 944) (d) and species (n = 7059). Species size ranges reflect different reports of maximum size from different literature sources and databases. Size ranges for orders, families, and genera is the interspecific range between the largest and smallest species in the taxon.
More generally, randomly drawing a single maximum size estimate for each species barely changes the overall rank order of species body sizes: the mean correlation between two such sets of rankings is 0.98 (n = 1000 randomizations), with the typical species changing body size rank due to intraspecific variation in maximum size only by around 24 places in the full rank order of all 27,571 species (Appendix S2: Figure S1). However, species with a very high maxsizerange can shift by 25,000 or more places in the body size rank order (Appendix S2: Table S3). We identified these species with very high maxsizerange. Only 44 species (0.16%) varied in maximum size by over two orders of magnitude, 492 (1.8%) by one order of magnitude, and 1424 (5.2%) by half an order of magnitude. For the top 44 species (greater than two orders of magnitude in maxsizerange), we further investigated the sources of variation (Appendix S2: Table S3). Bryozoa accounted for 19 of the 44 species with maxsizerange > 2, with a Bryozoan also the species with the biggest change in body size rank order in 916 of the 1000 randomizations described above. In these species, maxsizelargest represented a colony size and maxsizesmallest the dimension of an individual zooid. A similar issue arose with the six Bivalvia in this subset; all are members of wood‐boring families Xylophagiidae and Terenidae, where maxsizesmallest quantified the shell size and maxsizelargest the length of the foot. Unusually large maxsizerange occurred in two species of Echinodermata due to different measurements being the diameter of the central disc versus including arm length. This discrepancy was particularly noticeable in long‐armed Asteroidea. Five Polychaeta worms also had large maxsizerange due to both width and length measurements being reported as maxima. Three species of Chordata, all fishes, and five species of Cnidaria, all medusae or pelagic forms, had large maxsizerange that incorporated intraspecific size differences between adults or between adults and larvae. For example, Praya dubia, the giant siphonophore, has a maximum reported length of 45.72 m but also occurs in the database at 10 cm, a reasonable length for a small adult. Pleuronectes platessa, the flatfish European plaice, has a verifiable maximum size of 1.22 m but also occurs in the dataset as unlabeled juvenile fish of 1.1 cm. The remaining four species of the 44 represent true errors in size including two species Polychaeta, a species of Chordata, and one species of Copepoda. For example, one of the Polychaeta, Polyophthalmus mauliola, is a small worm from subtidal mudflats with the holotype measuring 7.5 mm (MagalhÃes et al., 2019) but occurs in the dataset here as 51.83 m long. The Chordata species, Fraser's dolphin, Lagenodelphis hosei has a maxsizesmallest of 2.6 cm. In both cases, the error is most likely to result from incorrect specification of measurement units.
4. DISCUSSION
Here, we analyzed reported maximum size measurements of 27,271 marine species and aimed to understand the factors influencing variation in these estimates. Range in maximum size within a species scaled from zero, that is, multiple sources gave identical measurements for maximum size, to over three orders of magnitude. The results highlight the complexity and potential sources of variability in body size measurements emphasizing the influence of: (1) organism size, (2) taxonomic group, (3) habitat, and (4) measurement methodology on the reported values of maximum linear dimension within marine species.
4.1. Organism size
The relationship between the smallest and largest maximum size per species had an observed slope less than one (b = 0.91), indicating greater variation associated with size measurements in smaller species, which may be attributed to several factors. First, measurement error becomes more pronounced as it approaches the scale of measurement, leading to increased variability in size estimates. From our experience with extracting maximum size data, sizes of smaller organisms are often rounded off or not measured to a level of precision corresponding with the size of the organism. However, this phenomenon of increased uncertainty due to rounding appears to be limited to organisms below 100 micrometers (equivalent to a log10 size of −2 in Figure 3a). Another possibility is that smaller organisms may have less mature taxonomy, implying a less refined categorization compared to vertebrates and macroinvertebrates. This potential disparity in taxonomic knowledge could arise from the relatively younger field and involvement of fewer researchers. Certainly, the most recently described marine species tend to be relatively small, with the modal size class of marine species described between 2013 and 2017 at 2–10 mm (Bouchet et al., 2023). Bouchet et al. (2023) also document high rates of synonymy across marine species, up to 25% for species described between 1910 and 1950. If these rates of synonymy were biased toward smaller species, this phenomenon could lead to a greater maxsizerange once aggregated to the newly synonymized species level. Regardless, taxonomic uncertainty and revision can lead to substantial variation in maxsize: eight currently accepted marine species are listed in Hayward and Ryland (1990) under two or more synonyms with separate estimates of maxsize, with this issue of synonymy alone resulting in maxsizerange values of up to 0.56, a c. 3.6× difference between smallest and largest maximum size (TJW unpublished analyses).
4.2. Taxonomic group
Echinoderms, annelids, and bryozoans exhibited significantly greater variation in maxsizerange when compared to other phyla (Figure 3 ). Notably, mollusks, chordates, arthropods, and nematodes displayed the smallest differences in maxsizerange. The variation in measures of maxsize within echinoderms might be attributed to the way size is recorded. For instance, Hayward and Ryland (1990) list maxsize for the classes within Echinodermata as variously “arm length” (Crinoidea), “diameter” (Asteroidea), “disc diameter” (Ophiuroidea) “test diameter” (Echinoidea), and “total length” (Holothurioidea). Similarly, it is plausible that the same consideration regarding measurement techniques applies to cnidarians, and perhaps certain annelids such as the feather‐duster (family Sabellidae). Another factor potentially influencing the observed maxsizeranges is the preservation of small organisms within these phyla. Preservation methods may have a differential impact on size estimation, leading to variations in the recorded size range. Importantly, the influence of phylum on observed size ranges is larger than the influences of other contributing factors. Further investigation and understanding of these differences can shed light on the underlying mechanisms shaping size variations within and among phyla.
4.3. Habitat type
The analysis of maxsizerange across different habitat types revealed notable distinctions. Interestingly, benthic organisms displayed a relatively higher level of maxsizerange compared to other habitat types. It is possible that this discrepancy arises from the challenge of distinguishing measurement errors from ecophenotypic variations, given the inherent variability of benthic habitats. These habitats may exhibit greater habitat diversity compared to pelagic or demersal environments. Benthic species dominate marine biodiversity and occur in more animal phyla than other functional groups (Webb & Vanhoorne, 2020). The range of body forms and morphologies this encompasses likely exacerbates some of the methodological issues previously raised. For instance, almost all Echinodermata in our dataset (228 of 234 species) are benthic, and the use of different length measurements by different researchers may increase variation within this group. Species with unspecified habitats exhibited the highest maxsizerange; the lack of comprehensive ecological data suggests these organisms have been poorly explored, likely leading to less‐well‐constrained size measurements.
4.4. Measurement methodology
Increasing the number of measurements decreases the likelihood of zero variation, and once non‐zero variation exists, a further increase in measurements can lead to an increase in overall variation. While this observation may present challenges, it also highlights the need to carefully consider measurement strategies and their impact on size estimates. The persistence of this issue rests on the absence of a centralized repository for size data, resulting in multiple measurements scattered across separate databases and publications. To address this dispersion of information, the creation of a curated, centralized, industry‐standard database for size data among marine species would prove highly beneficial, enabling the reconciliation of various maximum size measures and facilitating error detection and correction processes.
4.5. Can we use maximum size in macro‐ecological and ‐evolutionary research?
An analysis of cumulative frequency distributions based on maxsizesmallest, maxsizelargest, maxsizerange estimates for each species revealed significant differences among these distributions. Specifically, the maxsize values exhibited a consistent increase in discrepancy from the smallest to the mean estimates, and further to the largest estimates. Additionally, the distributions displayed variation in terms of their variance. The distinction between the largest and the smallest reported maximum size as measures of size range deserves consideration. Although the use of maximum size has been criticized in interspecific comparative analyses, it remains a common practice due to limitations in available data and the assumption that among‐species differences outweigh within‐species differences. This study provides empirical evidence that choice of measurement can change the nature of the distribution, that is, picking the largest known measurement for each species may significantly shift the distribution.
However, we also show that these differences in size distributions, while significant, are minor and subtle. We also demonstrate that these differences in maximum size estimates also do not significantly change the rank order sizes of species, which would allow for robust eco‐evolutionary examination. The variation in maximum size estimates is also far less than the natural variation in maximum size within even small, low‐diversity clades, and the data are suitable for evaluating changes in mean with large sample sizes, particularly in changes larger than one log unit or more. For any particular case, our data provide guidance to assess whether the signal being assessed is likely to exceed the noise inherent in using maximum size values. We do identify some species with very large ranges in maximum size (Appendix S2: Table S3), however these are unusual cases and do not preclude the use of large compilations of species‐level maximum size in comparative macroecology and macroevolution.
5. CONCLUSIONS
Our results indicate that actual errors in estimates of maximum body size reported in the literature are rare (<2%) and many inconsistences in maximum size can be accounted for with better annotation. This variation in reports of maximum size within species is also far less than interspecific variation and in most macroecological and macroevolutionary studies it is unlikely to impact the results. However, it is essential to address the practical utility of current data and identify the specific research questions and effect sizes for which the available variation and uncertainty can still provide meaningful insights. Clarifying these aspects will contribute to a more nuanced understanding of the signal‐to‐noise issue in body size datasets and foster better‐informed interpretations of study outcomes. Further, we reiterate that establishing standardized measurement protocols and promoting the sharing of size data through a curated centralized repository, would represent a substantial advantage for the research community.
AUTHOR CONTRIBUTIONS
Craig R. McClain: Conceptualization (lead); data curation (lead); investigation (lead); methodology (lead); project administration (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Thomas J. Webb: Data curation (supporting); formal analysis (supporting); investigation (supporting); methodology (supporting); validation (supporting); visualization (supporting); writing – original draft (supporting); writing – review and editing (supporting). Noel A. Heim: Data curation (supporting); formal analysis (supporting); methodology (supporting); validation (supporting); writing – original draft (supporting); writing – review and editing (supporting). Matthew L. Knope: Conceptualization (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). Pedro M. Monarrez: Conceptualization (supporting); data curation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). Jonathan L. Payne: Conceptualization (supporting); data curation (supporting); methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting).
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
None Declared.
Supporting information
Appendix S1.
Appendix S2.
ACKNOWLEDGEMENTS
We thank the editor and two reviewers for their insights and edits. Michelle Gaither‐McClain provided loving patience with the first author.
McClain, C. R. , Webb, T. J. , Heim, N. A. , Knope, M. L. , Monarrez, P. M. , & Payne, J. L. (2024). Navigating uncertainty in maximum body size in marine metazoans. Ecology and Evolution, 14, e11506. 10.1002/ece3.11506
DATA AVAILABILITY STATEMENT
Data are available at https://github.com/crmcclain/MOBS_OPEN/.
REFERENCES
- Aava, B. (2001). Primary productivity can affect mammalian body size frequency distributions. Oikos, 93, 205–212. [Google Scholar]
- Alroy, J. (1998). Cope's rule and the dynamics of body mass evolution in North American fossil mammals. Science, 280, 731–734. [DOI] [PubMed] [Google Scholar]
- Barnes, C. , Maxwell, D. , Reuman, D. C. , & Jennings, S. (2010). Global patterns in predator–prey size relationships reveal size dependency of trophic transfer efficiency. Ecology, 91, 222–232. [DOI] [PubMed] [Google Scholar]
- Benke, A. C. , Huryn, A. D. , Smock, L. A. , & Wallace, J. B. (1999). Length‐mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. Journal of the North American Benthological Society, 18, 308–343. [Google Scholar]
- Bloom, D. D. , Burns, M. D. , & Schriever, T. A. (2018). Evolution of body size and trophic position in migratory fishes: a phylogenetic comparative analysis of Clupeiformes (anchovies, herring, shad and allies). Biological Journal of the Linnean Society, 125, 302–314. [Google Scholar]
- Bonner, J. T. (2007). Why size matters: from bacteria to blue whales. Princeton University Press. [Google Scholar]
- Bosch, J. , & Vicens, N. (2006). Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behavioral Ecology and Sociobiology, 60, 26–33. [Google Scholar]
- Bouchet, P. , Decock, W. , Lonneville, B. , Vanhoorne, B. , & Vandepitte, L. (2023). Marine biodiversity discovery: the metrics of new species descriptions. Frontiers in Marine Science, 10, 929989. [Google Scholar]
- Brooks, M. E. , Kristensen, K. , Van Benthem, K. J. , Magnusson, A. , Berg, C. W. , Nielsen, A. , Skaug, H. J. , Machler, M. , & Bolker, B. M. (2017). glmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling. The R journal, 9, 378–400. [Google Scholar]
- Brown, J. H. (1995). Macroecology. University of Chicago Press. [Google Scholar]
- Brown, J. H. , Gillooly, J. F. , Allen, A. P. , Savage, V. M. , & West, G. B. (2004). Toward a metabolic theory of ecology. Ecology, 85, 1771–1789. [Google Scholar]
- Cooke, R. , Gearty, W. , Chapman, A. S. , Dunic, J. , Edgar, G. J. , Lefcheck, J. S. , Rilov, G. , McClain, C. R. , Stuart‐Smith, R. D. , & Kathleen Lyons, S. (2022). Anthropogenic disruptions to longstanding patterns of trophic‐size structure in vertebrates. Nature Ecology & Evolution, 6, 684–692. [DOI] [PubMed] [Google Scholar]
- Costa, G. C. (2009). Predator size, prey size, and dietary niche breadth relationships in marine predators. Ecology, 90, 2014–2019. [DOI] [PubMed] [Google Scholar]
- DeLong, J. P. , Okie, J. G. , Moses, M. E. , Sibly, R. M. , & Brown, J. H. (2010). Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proceedings of the National Academy of Sciences, 107, 12941–12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar, M. , Santos, M. , & Vasconcelos, P. (2001). Weight–length relationships of 25 bivalve species (Mollusca: Bivalvia) from the Algarve coast (southern Portugal). Journal of the Marine Biological Association of the United Kingdom, 81, 805–807. [Google Scholar]
- Gearty, W. , McClain, C. R. , & Payne, J. L. (2018). Energetic tradeoffs control the size distribution of aquatic mammals. Proceedings of the National Academy of Sciences, 115, 4194–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearty, W. , & Payne, J. L. (2020). Physiological constraints on body size distributions in Crocodyliformes. Evolution, 74, 245–255. [DOI] [PubMed] [Google Scholar]
- Goldbogen, J. A. , Calambokidis, J. , Croll, D. A. , McKenna, M. F. , Oleson, E. , Potvin, J. , Pyenson, N. D. , Schorr, G. , Shadwick, R. E. , & Tershy, B. R. (2012). Scaling of lunge‐feeding performance in rorqual whales: Mass‐specific energy expenditure increases with body size and progressively limits diving capacity. Functional Ecology, 26, 216–226. [Google Scholar]
- Grant, P. R. (1968). Bill size, body size, and the ecological adaptations of bird species to competitive situations on islands. Systematic Biology, 17, 319–333. [PubMed] [Google Scholar]
- Harmon, L. J. , Losos, J. B. , Jonathan Davies, T. , Gillespie, R. G. , Gittleman, J. L. , Bryan Jennings, W. , Kozak, K. H. , McPeek, M. A. , Moreno‐Roark, F. , & Near, T. J. (2010). Early bursts of body size and shape evolution are rare in comparative data. Evolution, 64, 2385–2396. [DOI] [PubMed] [Google Scholar]
- Hayward, P. J. , & Ryland, J. S. (1990). The marine fauna of the British Isles and north‐west Europe. Oxford University Press. [Google Scholar]
- Heim, N. A. , Knope, M. L. , Schaal, E. K. , Wang, S. C. , & Payne, J. L. (2015). Cope's rule in the evolution of marine animals. Science, 347, 867–870. [DOI] [PubMed] [Google Scholar]
- Heim, N. A. , Payne, J. L. , Finnegan, S. , Knope, M. L. , Kowalewski, M. , Lyons, S. K. , McShea, D. W. , Novack‐Gottshall, P. M. , Smith, F. A. , & Wang, S. C. (2017). Hierarchical complexity and the size limits of life. Proceedings of the Royal Society B: Biological Sciences, 284, 20171039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand, H. , & Azovsky, A. I. (2001). Body size determines the strength of the latitudinal diversity gradient. Ecography, 24, 251–256. [Google Scholar]
- Hunt, G. , & Roy, K. (2006). Climate change, body size evolution, and Cope's Rule in deep‐sea ostracods. Proceedings of the National Academy of Science, U.S.A, 103, 1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson, G. E. (1959). Homage to Santa Rosalia, or why are there so many kinds of animals. American Naturalist, 93, 145–159. [Google Scholar]
- Knope, M. , & Scales, J. (2013). Adaptive morphological shifts to novel habitats in marine sculpin fishes. Journal of Evolutionary Biology, 26, 472–482. [DOI] [PubMed] [Google Scholar]
- Knouft, J. H. (2004). Latitudinal variation in the shape of the species body size distribution: an analysis using freshwater fishes. Oecologia, 139, 408–417. [DOI] [PubMed] [Google Scholar]
- Lenth, R. (2022). emmeans: estimated marginal means, aka least‐squares means. R package version 1.4. 7. 2020 .
- Lomolino, M. V. (2005). Body size evolution in insular vertebrates: generality of the Island rule. Journal of Biogeography, 32, 1683–1699. [Google Scholar]
- MagalhÃes, W. F. , Rizzo, A. E. , & Bailey‐Brock, J. H. (2019). Opheliidae (Annelida: Polychaeta) from the western Pacific islands, including five new species. Zootaxa, 4555, 209–235. [DOI] [PubMed] [Google Scholar]
- McClain, C. R. , Balk, M. A. , Benfield, M. C. , Branch, T. A. , Chen, C. , Cosgrove, J. , Dove, A. D. M. , Gaskins, L. C. , Helm, R. R. , Hochberg, F. G. , Lee, F. B. , Marhsall, A. , McMurray, S. E. , Schanche, C. , Stone, S. N. , & Thaler, A. D. (2015). Sizing ocean giants: patterns of intraspecific size variation in marine megafauna. PeerJ, 3, e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain, C. R. , Boyer, A. , & Rosenberg, G. (2006). The Island rule and the evolution of body size in the deep sea. Journal of Biogeography, 33, 1578–1584. [Google Scholar]
- Méthot, G. , Hudon, C. , Gagnon, P. , Pinel‐Alloul, B. , Armellin, A. , & Poirier, A.‐M. T. (2012). Macroinvertebrate size–mass relationships: how specific should they be? Freshwater Science, 31, 750–764. [Google Scholar]
- Millien, V. (2004). Relative effects of climate change, isolation and competition on body‐size evolution in the Japanese field mouse, Apodemus argenteus . Journal of Biogeography, 31, 1267–1276. [Google Scholar]
- Nagel, L. , & Schluter, D. (1998). Body size, natural selection, and speciation in sticklebacks. Evolution, 52, 209–218. [DOI] [PubMed] [Google Scholar]
- Payne, J. L. , Boyer, A. G. , Browh, J. H. , Finnegan, S. , Kowalewski, M. , Krause, R. A., Jr. , Lyons, S. K. , McClain, C. R. , McShea, D. W. , Novack‐Gottshall, P. M. , Smith, F. A. , Stempien, J. A. , & Wang, S. C. (2008). Two‐phase increase in maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. Proceedings of the National Academy of Science, U.S.A, 106, 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin, R. (1996). The evolution of body size in the Mongenea: the role of host size and latitude. Canadian Journal of Zoology, 74, 726–736. [Google Scholar]
- Ritchie, M. E. , & Olff, H. (1999). Spatial scaling laws yield a synthetic theory of biodiversity. Nature, 400, 557–560. [DOI] [PubMed] [Google Scholar]
- Rosati, I. , Barbone, E. , & Basset, A. (2012). Length–mass relationships for transitional water benthic macroinvertebrates in Mediterranean and Black Sea ecosystems. Estuarine, Coastal and Shelf Science, 113, 231–239. [Google Scholar]
- Santini, L. , Benítez‐López, A. , Ficetola, G. F. , & Huijbregts, M. A. (2018). Length–mass allometries in amphibians. Integrative Zoology, 13, 36–45. [DOI] [PubMed] [Google Scholar]
- Schluter, D. , & Smith, J. N. (1986). Natural selection on beak and body size in the song sparrow. Evolution, 40, 221–231. [DOI] [PubMed] [Google Scholar]
- Seebacher, F. (2001). A new method to calculate allometric length‐mass relationships of dinosaurs. Journal of Vertebrate Paleontology, 21, 51–60. [Google Scholar]
- Sheridan, J. A. , & Bickford, D. (2011). Shrinking body size as an ecological response to climate change. Nature Climate Change, 1, 401–406. [Google Scholar]
- Thornton, D. H. , & Fletcher, R. J., Jr. (2014). Body size and spatial scales in avian response to landscapes: a meta‐analysis. Ecography, 37, 454–463. [Google Scholar]
- Trites, A. W. , & Pauly, D. (1998). Estimating mean body masses of marine mammals from maximum body lengths. Canadian Journal of Zoology, 76, 886–896. [Google Scholar]
- Webb, T. J. , & Vanhoorne, B. (2020). Linking dimensions of data on global marine animal diversity. Philosophical Transactions of the Royal Society B, 375, 20190445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund, C. , & Kaitala, A. (1995). Sexual selection for large male size in a polyandrous butterfly: the effect of body size on male versus female reproductive success in Pieris napi . Behavioral Ecology, 6, 6–13. [Google Scholar]
- Wilson, D. S. (1975). The adequacy of body size as a niche difference. American Naturalist, 109, 769–784. [Google Scholar]
- WoRMS Editorial Board . (2023). World Register of Marine Species (WoRMS). WoRMS Editorial Board. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Appendix S2.
Data Availability Statement
Data are available at https://github.com/crmcclain/MOBS_OPEN/.


