Key Points
Question
Which neoadjuvant regimen is associated with the highest pathological complete response (pCR) in the treatment of locally advanced rectal cancer?
Findings
In this systematic review and network meta-analysis of 27 randomized clinical trials (including a total of 13 413 individuals), the pCR rates achieved with total neoadjuvant therapy regimens outperformed standard long-course chemoradiotherapy, with long-course chemoradiotherapy plus consolidation chemotherapy showing the greatest effect estimate.
Meaning
The findings of this study suggest that total neoadjuvant therapy regimens should be recognized as first-line treatments when aiming at increasing pCR rates in locally advanced rectal cancer.
Abstract
Importance
Treatment of locally advanced rectal cancer (LARC) involves neoadjuvant chemoradiotherapy plus total mesorectal excision and adjuvant chemotherapy. However, total neoadjuvant therapy (TNT) protocols (ie, preoperative chemotherapy in addition to radiotherapy) may allow better adherence and early treatment of distant micrometastases and may increase pathological complete response (pCR) rates.
Objective
To assess the efficacy and tolerability of TNT protocols for LARC.
Data Sources
MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science Core Collection electronic databases and ClinicalTrials.gov for unpublished studies were searched from inception to March 2, 2024.
Study Selection
Randomized clinical trials including adults with LARC who underwent rectal resection as a final treatment were included. Studies including nonoperative treatment (watch-and-wait strategy), treatments other than rectal resection, immunotherapy, or antiangiogenic agents were excluded. Among the initially identified studies, 2.9% met the selection criteria.
Data Extraction and Synthesis
Two authors independently screened the records and extracted data. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)–compliant pairwise and network meta-analyses with a random-effects model were performed in a frequentist framework, and the certainty of evidence was assessed according to the confidence in network meta-analysis approach.
Main Outcomes and Measures
The primary outcome was pCR, defined as the absence of residual tumor at pathological assessment after surgery. Secondary outcomes included tolerability, toxic effects, perioperative outcomes, and long-term survival.
Results
Of 925 records identified, 27 randomized clinical trials, including 13 413 adults aged 18 years or older (median age, 60.0 years [range, 42.0-63.5 years]; 67.2% male) contributed to the primary network meta-analysis. With regard to pCR, long-course chemoradiotherapy (L-CRT) plus consolidation chemotherapy (relative risk [RR], 1.96; 95% CI, 1.25-3.06), short-course radiotherapy (S-RT) plus consolidation chemotherapy (RR, 1.76; 95% CI, 1.34-2.30), and induction chemotherapy plus L-CRT (RR, 1.57; 95% CI, 1.09-2.25) outperformed standard L-CRT with single-agent fluoropyrimidine-based chemotherapy. Considering 3-year disease-free survival, S-RT plus consolidation chemotherapy (RR, 1.08; 95% CI, 1.01-1.14) and induction chemotherapy plus L-CRT (RR, 1.12; 95% CI, 1.01-1.24) outperformed L-CRT, in spite of an increased 5-year locoregional recurrence rate of S-RT plus consolidation chemotherapy (RR, 1.65; 95% CI, 1.03-2.63).
Conclusions and Relevance
In this systematic review and network meta-analysis, 3 TNT protocols were identified to outperform the current standard of care in terms of pCR rates, with good tolerability and optimal postoperative outcomes, suggesting they should be recognized as first-line treatments.
This systematic review and network meta-analysis assesses which total neoadjuvant treatment regimens are associated with the highest pathological complete response rates compared with standard long-course chemoradiotherapy in the treatment of locally advanced rectal cancer.
Introduction
Treatment of locally advanced rectal cancer (LARC) involves a multidisciplinary approach. The standard of care in most high-income countries consists of neoadjuvant chemoradiotherapy followed by total mesorectal excision and adjuvant chemotherapy.1,2,3,4 However, only two-thirds of patients receive planned adjuvant chemotherapy because of postoperative or ostomy-related complications or patients’ preference.5 Some pilot and phase 2 single-arm studies6,7,8 followed by randomized clinical trials (RCTs)9,10,11,12 investigated the role of total neoadjuvant therapy (TNT), which is preoperative chemotherapy in addition to radiotherapy. According to the rationale of these studies, the advantages of preoperative chemotherapy include better adherence, early treatment of micrometastases, and higher pathological complete response (pCR) rates.11,13 The initial results of these studies were extremely encouraging, showing high pCR rates, and TNT protocols were rapidly incorporated in some US guidelines,2,14 even though results on locoregional and distant recurrence rates, disease-free survival (DFS), and overall survival (OS) were not yet available.
Some systematic reviews and meta-analyses assessed the efficacy and tolerability of TNT protocols compared with standard treatment,15,16,17,18 but they used standard pairwise meta-analyses. More importantly, all TNT protocols were grouped together, regardless of the timing of chemotherapy and the type of radiotherapy. Current literature has not yet clarified which TNT protocol bears the best results in terms of pathological and long-term outcomes.
This study aimed to assess the efficacy of available neoadjuvant strategies in individuals with LARC by applying a network meta-analysis (NMA) approach, which permits incorporation of evidence from both direct and indirect comparisons. The primary outcome chosen was pCR, since it is unequivocally measurable and it is associated with improved long-term outcomes.19,20,21
Methods
This systematic review and NMA was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline.22 The study protocol was registered in advance with PROSPERO (CRD42023406169).
Study Selection and Data Extraction
We searched for RCTs including adults aged 18 years or older of both sexes who were diagnosed with LARC and scheduled to undergo rectal resection as a final treatment. Studies were considered eligible if they reported data on the primary outcome (ie, pCR). Studies including participants undergoing nonoperative treatment (watch-and-wait strategy) or treatments other than rectal resection (ie, local excision) were excluded. Studies involving immunotherapy or antiangiogenic agents were also excluded, since the use of these is limited to individuals with peculiar molecular features that could hinder the generalizability of results.
We searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science Core Collection electronic databases and ClinicalTrials.gov for unpublished studies from database inception to March 2, 2024 (eAppendix 1 in Supplement 1). Two authors independently screened the records and extracted data (G.T. and G.O.).
All neoadjuvant strategies were eligible, namely induction chemotherapy plus long-course chemoradiotherapy (L-CRT; induction + L-CRT); L-CRT plus consolidation chemotherapy (L-CRT + consolidation); short-course radiotherapy (S-RT) plus consolidation chemotherapy (S-RT + consolidation); neoadjuvant chemotherapy alone (CHT); S-RT plus early rectal resection (7-10 days; S-RTearly); S-RT plus delayed rectal resection (4-6 weeks; S-RTdelayed); long-course RT (L-RT); L-CRT with single-agent fluoropyrimidine-based chemotherapy (L-CRT1); and L-CRT with duplex chemotherapy drug (fluoropyrimidine plus oxaliplatin; L-CRT2). The L-CRT1 was selected as the common comparator, since it is the recommended treatment in international guidelines1,2,4 and it was established as standard of care in most of the included RCTs.
Outcomes
The primary outcome was the number of participants achieving pCR, defined as the absence of residual tumor at pathological assessment after surgery (ypT0N0). Secondary outcomes included tolerability (rate of participants who received the complete planned treatment dose); toxic effects (rate of participants experiencing chemotherapy- or radiotherapy-associated adverse events of grade 3 or above, in which adverse events were assessed and graded from 1 to 5 by the investigators using Common Terminology Criteria for Adverse Events, version 4, with grade 5 indicating death); dropouts by any cause; preoperative treatment–related deaths; rate of participants undergoing surgery after neoadjuvant treatment; rate of potentially curative resections (R0); rate of negative circumferential resection margins; rate of participants who were node negative on pathological examination (ypN0); rate of severe postoperative complications, graded as Clavien-Dindo grade III or above (which ranges from grades I to V, with higher numbers indicating more severe adverse events)23; anastomotic leak rate; locoregional recurrence rate, defined as local recurrence after R0 to R1 resection at 3 and 5 years; distant recurrence rate at 3 and 5 years; locoregional failure, defined as locally progressive disease leading to an unresectable tumor, R2 resection, or locoregional recurrence at 3 and 5 years; DFS at 3 and 5 years; and OS at 3 and 5 years.
Statistical Analysis
For each outcome, we performed both pairwise and NMA with a random-effects model in a frequentist framework using RStudio, version 2023.06.0-421 (R Project for Statistical Computing) using package netmeta, version 2.9-0 and Stata, version 17.0 (StataCorp LLC) using package mvmeta, version 2.3. We calculated dichotomous data on a strict intention-to-treat basis, considering all randomized participants as the denominator and calculated pooled relative risks (RRs) with 95% CIs. For the secondary outcomes’ postoperative complications and anastomotic leak rate, we conducted per-protocol analyses, considering patients undergoing surgery as the denominator. For the primary outcome, we assumed that participants excluded from the trial had experienced a negative outcome (ie, no pCR). In case of missing data, we contacted trial authors or, alternatively, used validated statistical methods of imputation.24 For the primary outcome, we calculated the number needed to treat, defined as the number of individuals needed to be treated with 1 treatment vs another for 1 individual to have an additional desirable (number needed to treat to benefit) or undesirable (number needed to treat to harm) outcome.25,26 We assessed global heterogeneity using τ2 (low: τ2 ≤ 0.010, moderate: 0.010 < τ2 ≤ 0.242, and high: τ2 > 0.242) and I2 (low: 0%-40%, moderate: 30%-60%, substantial: 50%-90%, and considerable: 75%-100%).27 For the NMA, common heterogeneity across all comparisons28 was assumed and estimated in each network.
To assess transitivity assumption (ie, when effect modifiers are equally distributed across the comparisons), we extracted key potential effect modifiers, namely study design (open label or double blind), sample size, definition of LARC, doses and cycles of chemotherapy agents, doses and modality of radiotherapy, months of follow-up, median year of study conduct, participants discontinuing treatment before the end point, sex, mean age, percentage of clinical T4 (cT4), participants with clinically suspected nodal metastases, mean distance from the anal verge, and percentage of pathological T4 (ypT4). By comparing their distribution across comparisons, we formulated a judgment on whether differences in their distributions were large enough to threaten the validity of the analysis.29 We considered such differences as relevant when significant imbalances emerged according to the Kruskal-Wallis test (continuous variables) and meta-regression analyses showing an association with the treatment effect.30,31 For the primary outcome, we calculated mean ranks of treatments using the R gemtc package, version 1.0-2 (R Project for Statistical Computing).
If more than 10 studies were included in the primary outcome, we assessed publication bias by visually inspecting the funnel plot and performing the Egger’s regression test (eAppendix 3 in Supplement 1).32 For the primary outcome, we assessed the confidence of evidence according to the confidence in network meta-analysis (CINeMA) method (eAppendix 5 in Supplement 1).33,34 For the primary outcome, we conducted sensitivity analyses excluding trials with an overall high risk of bias according to Risk of Bias, version 2.0 (Cochrane Methods),27 a high risk of indirectness, and CHT as 1 of the treatment arms. We also conducted an additional analysis for the primary outcome using the per-protocol population as the denominator. A 2-sided P < .05 was the threshold for statistical significance. Complete statistical methods are reported in the eMethods in Supplement 1.
Results
We identified 925 records after a database and hand search. After removing duplicates and examining titles and abstracts, we selected 80 records for full-text assessment. Of these, 27 studies (2.9%) were eligible for inclusion,10,11,12,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68 accounting for 13 413 participants aged 18 years or older (median age, 60.0 years [range, 42.0-63.5 years]; 32.8% female and 67.2% male) (eFigure 1 in Supplement 1). The full list of studies is provided in eTables 1 and 2 in Supplement 1.
Primary Outcome
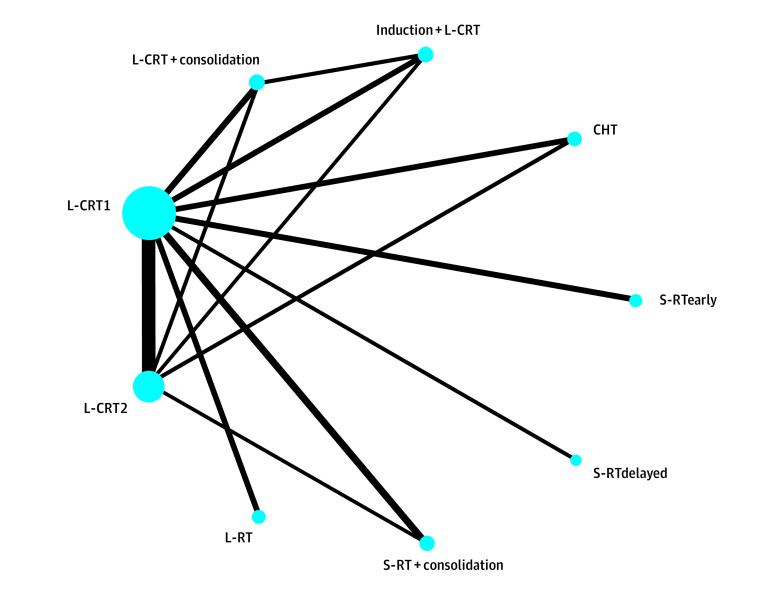
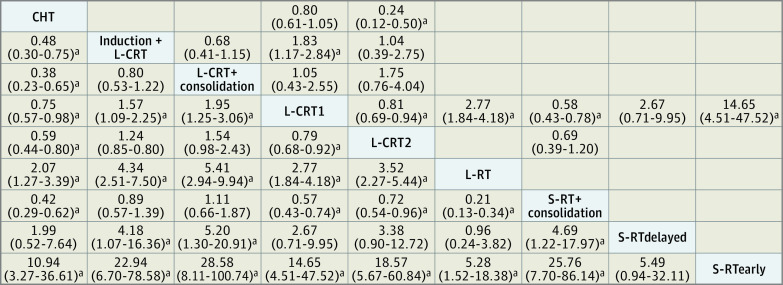
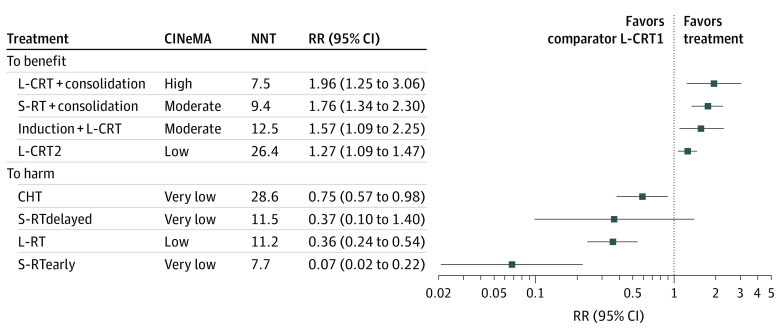
For the primary outcome, pCR, the transitivity assumption was not violated for any of the potential effect modifiers analyzed (eFigures 2 and 3 in Supplement 1). The network plot (Figure 1) shows that all interventions were compared with L-CRT1 in at least 1 study. The league table (Figure 2) shows all head-to-head comparisons between treatments according to the network and the pairwise meta-analyses. Figure 3 shows a more detailed comparison between each treatment and the common comparator L-CRT1, which was outperformed (by decreasing effect size) by L-CRT + consolidation (RR, 1.96; 95% CI, 1.25-3.06, high CINeMA certainty), S-RT + consolidation (RR, 1.76; 95% CI, 1.34-2.30, moderate certainty), induction + L-CRT (RR, 1.57; 95% CI, 1.09-2.25, moderate certainty), and L-CRT2 (RR, 1.27; 95% CI, 1.09-1.47, low certainty). No significant differences emerged between S-RTdelayed and L-CRT1 (RR, 0.37; 95% CI, 0.10-1.40, very low certainty), while CHT (RR, 0.75; 95% CI, 0.57-0.98, very low certainty), L-RT (RR, 0.36; 95% CI, 0.24-0.54, low certainty), and S-RTearly (RR, 0.07; 95% CI, 0.02-0.22, very low certainty) were outperformed by L-CRT1. The rank test supported the following ranking of treatments, ordered from the best performing to worst: L-CRT + consolidation, S-RT + consolidation, induction + L-CRT, L-CRT2, L-CRT1, CHT, S-RTdelayed, L-RT, and S-RTearly (eAppendix 2 in Supplement 1). The weighted mean absolute risks of pCR were 21.5% for induction + L-CRT, 21.5% for L-CRT + consolidation, 18.6% for S-RT + consolidation, 17.2% for L-CRT2, 14.9% for CHT, 14.3% for L-CRT1, 4.3% for L-RT, 4.0% for S-RTdelayed, and 0.9% for S-RTearly. Overall, the NMA showed moderate heterogeneity (τ2 = 0.019; I2 = 26.3%; 95% CI, 0%-56.8%) and inconsistency according to the global approach (Cochran Q = 15.18; df, 6; P = .02). The local approach showed significant inconsistency for 2 of 10 comparisons (namely, L-CRT1 vs CHT and L-CRT2 vs CHT) (eAppendix 2 in Supplement 1). Sensitivity analyses provided results largely consistent with the primary analysis. The analysis excluding CHT arms showed no heterogeneity and inconsistency, and overall results were not affected. Similarly, the additional analysis on the per-protocol population showed results that were overall consistent with those from the primary analysis (eAppendix 6 in Supplement 1).
Figure 1. Network Plot Comparing Each Treatment With the Common Comparator Long-Course Chemoradiotherapy With Single-Agent Fluoropyrimidine (L-CRT1) for Primary Outcome Pathological Complete Response.
The thickness of lines is proportional to the number of studies comparing the 2 treatments, and the size of circles is proportional to the number of individuals for each treatment. CHT indicates chemotherapy; induction + L-CRT, induction CHT plus consolidation L-CRT; L-CRT + consolidation, L-CRT plus consolidation CHT; L-CRT2, L-CRT with duplex CHT drug (fluoropyrimidine plus oxaliplatin); L-RT, long-course radiotherapy; S-RT + consolidation, short-course RT plus consolidation CHT; S-RTdelayed, S-RT plus delayed rectal resection; S-RTearly, S-RT plus early rectal resection.
Figure 2. League Table for the Primary Outcome of Pathological Complete Response.
Treatments included in the analysis are shown in boldface on a diagonal in alphabetical order. Results of the network meta-analysis are reported in the lower left part of the matrix, and results from the pairwise meta-analysis are reported in the upper right matrix of the table. Each cell presents the relative risk (RR) and the corresponding 95% CI, and RRs greater than 1 favor the column-defining treatment (ie, the left-most cell on the diagonal).
aSignificant RR (95% CI).
Figure 3. Forest Plot Comparing Each Treatment With the Common Comparator Long-Course Chemoradiotherapy With Single-Agent Fluoropyrimidine (L-CRT1) for the Primary Outcome Pathological Complete Response.
Relative risks (RRs) greater than 1 favor the treatment over L-CRT1. Squares indicate RRs; horizontal lines, 95% CIs for RRs. CHT indicates chemotherapy; CINeMA, confidence in network meta-analysis; induction + L-CRT, induction CHT plus consolidation long-course chemoradiotherapy; L-CRT + consolidation, L-CRT plus consolidation CHT; L-CRT2, L-CRT with duplex CHT drug (fluoropyrimidine plus oxaliplatin); L-RT, long-course radiotherapy; NNT, number needed to treat (for details, see eAppendix 4 in Supplement 1); S-RT + consolidation, short-course RT plus consolidation CHT; S-RTdelayed, S-RT plus delayed rectal resection; S-RTearly, S-RT plus early rectal resection.
Secondary Outcomes
Toxic Effects and Tolerability
The results of the NMA for toxic effects and tolerability outcomes are reported in Table 1 (see details in eAppendices 7 and 8 in Supplement 1). In comparison with L-CRT1, S-RT + consolidation (RR, 0.90; 95% CI, 0.82-0.99) and L-CRT2 (RR, 0.91; 95% CI, 0.86-0.97) showed better tolerability, while L-RT showed worse tolerability (RR, 1.23; 95% CI, 1.10-1.40). Nevertheless, S-RT + consolidation (RR, 2.01; 95% CI, 1.39-2.91), L-CRT2 (RR, 1.81; 95% CI, 1.44-2.27), CHT (RR, 1.67; 95% CI, 1.04-2.64), and induction + L-CRT (RR, 1.64; 95% CI, 1.05-2.56) showed higher toxic effects, while L-RT (RR, 0.19; 95% CI, 0.08-0.44) and S-RTearly (RR, 0.12; 95% CI, 0.05-0.28) showed reduced toxic effects compared with L-CRT1. No differences were found in terms of dropping out for any reason (eAppendix 9 in Supplement 1), preoperative treatment–related deaths (eAppendix 10 in Supplement 1), and number of patients who underwent surgery (eAppendix 11 in Supplement 1).
Table 1. Preoperative and Short-Term Secondary Outcomes.
| Characteristics and treatments | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tolerability of treatment | Toxic effects of treatmenta | Dropped out for any reason | Treatment-related deaths | Underwent surgery | Rate of R0 | Rate of negative CRM | Rate of ypN0 | Severe postoperative complicationsb | Anastomotic leak | |
| Network characteristics | ||||||||||
| Studies, No. | 25 | 22 | 27 | 24 | 27 | 18 | 11 | 21 | 9 | 17 |
| Participants, No. | 11 987 | 11 568 | 13 383 | 11 963 | 13 413 | 9145 | 4963 | 10 070 | 3525 | 8333 |
| τ2 | 0.007 | 0.080 | 0.003 | 0 | <0.001 | <0.001 | 0.008 | 0.002 | 0.006 | 0 |
| Consistency | ||||||||||
| CnG, Cochran Q; df | 3.73; 6 | 2.00; 4 | 8.56; 6 | 0.98; 6 | 8.50; 6 | 4.55; 5 | 11.68; 1 | 6.19; 4 | 2.50; 1 | 4.88; 5 |
| P value | .71 | .74 | .20 | .99 | .20 | .47 | <.001 | .18 | .11 | .43 |
| CnL, comparisons, No./total No.c | 0/10 | 0/8 | 0/10 | 0/10 | 0/10 | 0/9 | 3/3 | 0/8 | 0/3 | 0/9 |
| Comparison vs L-CRT1, RR (95% CI) | ||||||||||
| L-CRT + consolidation | 0.93 (0.86-1.03) | 1.65 (0.93-2.93) | 1.03 (0.51-2.09) | 1.19 (0.29-4.93) | 1.01 (0.96-1.06) | 0.95 (0.87-1.03) | 1.00 (0.83-1.20) | 1.14 (0.98-1.33) | 0.99 (0.53-1.83) | 1.04 (0.21-5.07) |
| S-RT + consolidation | 0.90 (0.82-0.99)d | 2.01 (1.39-2.91)d | 0.91 (0.67-1.23) | 0.71 (0.25-1.99) | 1.02 (0.98-1.05) | 1.04 (0.99-1.09) | 1.03 (0.85-1.25) | 1.08 (1.00-1.18)d | 0.92 (0.61-1.41) | 1.29 (0.63-2.63) |
| Induction + L-CRT | 0.95 (0.86-1.06) | 1.64 (1.05-2.56)d | 1.42 (0.76-2.66) | 1.30 (0.35-4.77) | 0.99 (0.96-1.03) | 0.97 (0.91-1.03) | 1.05 (0.91-1.22) | 1.08 (0.96-1.22) | NA | 0.90 (0.53-1.52) |
| L-CRT2 | 0.91 (0.86-0.97)d | 1.81 (1.44-2.27)d | 1.14 (0.88-1.50) | 1.48 (0.76-2.88) | 0.99 (0.98-1.01) | 1.00 (0.97-1.02) | 1.02 (0.90-1.16) | 1.02 (0.95-1.09) | 1.13 (0.87-1.47) | 1.08 (0.85-1.36) |
| CHT | 1.01 (0.91-1.11) | 1.67 (1.04-2.64)d | 1.05 (0.74-1.48) | 1.90 (0.49-7.39) | 0.99 (0.96-1.03) | 1.00 (0.96-1.04) | 1.01 (0.84-1.22) | 0.93 (0.85-1.02) | NA | 0.62 (0.39-0.96)d |
| S-RTdelayed | NA | NA | 2.33 (0.60-9.07) | NA | 0.94 (0.86-1.03) | 0.89 (0.76-1.05) | 0.94 (0.74-1.18) | 0.80 (0.62-1.03) | NA | 0.85 (0.24-3.02) |
| L-RT | 1.23 (1.10-1.40)d | 0.19 (0.08 to 0.44)d | 0.59 (0.33-1.08) | 1.01 (0.14-7.17) | 1.02 (0.99-1.04) | NA | NA | 0.92 (0.83-1.01) | NA | 1.00 (0.48-2.06) |
| S-RTearly | 1.05 (0.88-1.25) | 0.12 (0.05-0.28)d | 0.50 (0.23-1.06) | 0.68 (0.11-4.12) | 1.02 (0.98-1.06) | 1.00 (0.93-1.07) | 0.94 (0.76-1.16) | 0.94 (0.77-1.14) | 1.39 (0.68-2.82) | 1.74 (0.52-5.82) |
Abbreviations: CHT, chemotherapy; CnG, consistency–global test; CnL, consistency–local test; CRM, circumferential resection margin; induction + L-CRT, induction CHT plus consolidation long-course chemoradiotherapy; L-CRT + consolidation, L-CRT plus consolidation CHT; L-CRT1, L-CRT with single-agent fluoropyrimidine-based CHT; L-CRT2, L-CRT with duplex CHT drug (fluoropyrimidine plus oxaliplatin); L-RT, long-course radiotherapy; NA, not available; R0, potentially curative resections; RR, relative risk; S-RT + consolidation, short-course RT plus consolidation CHT; S-RTdelayed, S-RT plus delayed rectal resection; S-RTearly, S-RT plus early rectal resection; ypN0, node-negative tumor on a pathological examination.
Rate of participants experiencing chemotherapy- or radiotherapy-associated adverse events of grade 3 or above, in which adverse events were assessed and graded from 1 to 5 by the investigators using Common Terminology Criteria for Adverse Events, version 4, with grade 5 indicating death.
Graded as Clavien-Dindo grade III or above; scores range from grades I to V, with higher numbers indicating more severe adverse events.23
The denominator is the total number of comparisons included in the analysis; the numerator is the number of inconsistencies between direct and indirect estimates.
Significant RR (95% CI).
Pathological and Surgical Outcomes
No significant differences were found in terms of negativity of circumferential resection margins, and the rate of curative resections (Table 1 and eAppendices 12 and 13 in Supplement 1). With regard to ypN0 rates, S-RT + consolidation (RR, 1.08; 95% CI, 1.00-1.18) showed better results compared with L-CRT1 (eAppendix 14 in Supplement 1). None of the treatments showed a higher risk of severe postoperative complications graded as a Clavien-Dindo score of III or above (eAppendix 15 in Supplement 1). Considering anastomotic leak, only CHT showed better performance (RR, 0.62; 95% CI, 0.39-0.96), while other treatments did not show any differences (eAppendix 16 in Supplement 1).
Recurrence
Data for the NMA on long-term outcomes were available from a limited number of studies (Table 2). As compared with L-CRT1, L-RT (RR, 2.08; 95% CI, 1.34-3.22) and S-RT + consolidation (RR, 1.65; 95% CI, 1.03-2.63) showed significantly higher risk of locoregional recurrence at 5 years (eAppendices 17 and 18 in Supplement 1). With regard to locoregional failure, L-CRT + consolidation (RR, 0.41; 95% CI, 0.22-0.78), induction + L-CRT (RR, 0.48; 95% CI, 0.27-0.87), and L-CRT2 (RR, 0.72; 95% CI, 0.53-0.98) showed better results at 3 years compared with L-CRT1 (eAppendix 19 in Supplement 1), while only L-RT (RR, 1.70; 95% CI, 1.22-2.36) showed a worse performance at 5 years (eAppendix 20 in Supplement 1). In contrast, S-RT + consolidation (RR, 0.83; 95% CI, 0.71-0.98) and L-CRT2 (RR, 0.80; 95% CI, 0.69-0.93) showed significantly lower risk of distant recurrence at 3 years compared with L-CRT1 (eAppendix 21 in Supplement 1). The result for S-RT + consolidation was supported at 5 years (RR, 0.76; 95% CI, 0.61-0.95) (eAppendix 22 in Supplement 1).
Table 2. Long-Term Secondary Outcomes.
| Characteristic or treatment | Time of outcome, y | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Locoregional recurrence | Locoregional failure | Distant recurrence | Disease-free survival | Overall survival | ||||||
| 3 | 5 | 3 | 5 | 3 | 5 | 3 | 5 | 3 | 5 | |
| Studies, No. | 11 | 7 | 10 | 6 | 8 | 5 | 13 | 7 | 11 | 8 |
| Participants, No. | 5749 | 4886 | 5234 | 5234 | 4811 | 3016 | 7409 | 5302 | 5576 | 5625 |
| τ2 | 0 | 0 | 0 | NA | 0 | 0 | 0 | 0 | 0.002 | 0.005 |
| Consistency | ||||||||||
| CnG, Cochran Q; df | 0.88; 3 | NA | 0.44; 2 | NA | 0.24; 1 | NA | 0.66; 3 | NA | 2.06; 3 | NA |
| P value | .83 | NA | .80 | NA | .62 | NA | .88 | NA | .56 | NA |
| CnL, comparisons, No./total No.a | 0/8 | NA | 0/6 | NA | 0/3 | NA | 0/8 | NA | 0/8 | NA |
| Comparison vs L-CRT1, RR (95% CI) | ||||||||||
| L-CRT + consolidation | 0.59 (0.25-1.25) | NA | 0.41 (0.22-0.78)b | NA | 0.68 (0.38-1.23) | NA | 1.15 (0.99-1.33) | NA | 1.08 (0.96-1.21) | NA |
| S-RT + consolidation | 1.08 (0.76-1.53) | 1.65 (1.03-2.63)b | 1.04 (0.74-1.45) | 1.43 (0.96-2.15) | 0.83 (0.71-0.98)b | 0.76 (0.61-0.95)b | 1.08 (1.01-1.14)b | 1.10 (1.00-1.20)b | 1.07 (1.01-1.14)b | 1.02 (0.97-1.19) |
| Induction + L-CRT | 0.76 (0.37-1.54) | 2.10 (0.22-19.99) | 0.48 (0.27-0.87)b | 2.70 (0.55-13.35) | 0.76 (0.55-1.05) | 0.98 (0.48-2.03) | 1.12 (1.01-1.24)b | 0.98 (0.74-1.30) | 1.06 (0.97-1.16) | 0.96 (0.75-1.24) |
| L-CRT2 | 0.67 (0.49-0.93)b | 0.77 (0.55-1.08) | 0.72 (0.53-0.98)b | 0.85 (0.52-1.38) | 0.80 (0.69-0.93)b | 0.92 (0.77-1.09) | 1.05 (1.01-1.08)b | 1.02 (0.97-1.08) | 1.00 (0.95-1.05) | 1.04 (0.95-1.14) |
| CHT | 0.96 (0.46-2.00) | 1.13 (0.47-2.72) | 1.05 (0.60-1.84) | 1.07 (0.51-2.23) | NA | NA | 1.00 (0.89-1.12) | 1.03 (0.97-1.09) | 1.01 (0.92-1.11) | 0.99 (0.86-1.15) |
| S-RTdelayed | NA | NA | NA | NA | NA | NA | 0.79 (0.62-0.99)b | NA | 0.94 (0.78-1.12) | NA |
| L-RT | NA | 2.08 (1.34-3.22)b | NA | 1.70 (1.22-2.36)b | NA | NA | NA | 0.93 (0.83-1.06) | NA | 1.01 (0.85-1.19) |
| S-RTearly | 1.72 (0.70-4.27) | 1.34 (0.58-3.10) | 1.72 (0.70-4.27) | 1.34 (0.58-3.10) | NA | 0.92 (0.65-1.30) | NA | NA | NA | 1.07 (0.88-1.30) |
Abbreviations: CHT, chemotherapy; CnG, consistency–global test; CnL, consistency–local test; induction + L-CRT, induction CHT plus consolidation long-course chemoradiotherapy; L-CRT + consolidation, L-CRT plus consolidation CHT; L-CRT1, L-CRT with single-agent fluoropyrimidine-based CHT; L-CRT2, L-CRT with duplex CHT drug (fluoropyrimidine plus oxaliplatin); L-RT, long-course radiotherapy; NA, not available; RR, relative risk; S-RT + consolidation, short-course RT plus consolidation CHT; S-RTdelayed, S-RT plus delayed rectal resection; S-RTearly, S-RT plus early rectal resection.
The denominator is the total number of comparisons included in the analysis; the numerator is the number of inconsistencies between direct and indirect estimates.
Significant RR (95% CI).
Survival
Considering 3-year DFS, induction + L-CRT (RR, 1.12; 95% CI, 1.01-1.24), S-RT + consolidation (RR, 1.08; 95% CI, 1.01-1.14), and L-CRT2 (RR, 1.05; 95% CI, 1.01-1.08) showed significantly better results, while S-RTdelayed (RR, 0.79; 95% CI, 0.62-0.99) showed worse 3-year DFS (eAppendix 23 in Supplement 1). Short-course RT + consolidation showed better outcomes in terms of 5-year DFS (RR, 1.10; 95% CI, 1.00-1.20) (eAppendix 24 in Supplement 1). With regard to 3-year OS, S-RT + consolidation (RR, 1.07; 95% CI, 1.01-1.14) showed better results (eAppendix 25 in Supplement 1), but no treatment outperformed the others at 5 years (eAppendix 26 in Supplement 1).
Discussion
This is the first study, to our knowledge, comparing all available RCTs on neoadjuvant treatments for LARC using the NMA technique. In the era of TNT and personalized medicine, it is of utmost importance to clearly define the efficacy, tolerability, and oncologic benefits of the new protocols against current standard, L-CRT.
Considering the primary outcome pCR, our study showed significantly higher rates with L-CRT + consolidation, S-RT + consolidation, induction + L-CRT, and L-CRT2 compared with L-CRT1. According to our results, we expect that 7.5 individuals should be treated with L-CRT + consolidation to obtain 1 more individual with pCR compared with the common comparator (Figure 3). This number needed to treat should be regarded as clinically relevant, considering an association with significant improvement in long-term survival of patients reaching pCR19,20,21 and that the active comparator is currently considered the gold standard in many countries.1,4,69 Although the Clinical Practice Guidelines from the National Comprehensive Cancer Network and the American Society of Colon and Rectal Surgeons suggest TNT as the preferred treatment for patients with LARC as an alternative to L-CRT1,2,14 European and Eastern guidelines still suggest standard chemoradiotherapy.1,4,69 Despite some increase in toxic effects with TNT, there were no significant differences in terms of the number of patients undergoing surgery, postoperative complications, and pathological outcomes, including the rate of R0 and circumferential resection margin–negative specimens. Only the CHT arm showed a decreased anastomotic leak rate.
The encouraging results obtained for pCR did not completely translate into survival benefits. Only S-RT + consolidation showed better 5-year distant recurrence and DFS, with the drawback of a higher locoregional recurrence rate. In contrast, L-CRT + consolidation, induction + L-CRT, and L-CRT2 showed better locoregional control at 3 years, but the results were not supported at 5 years. Further studies as well as long-term follow-up data of the included RCTs are awaited to assess whether the encouraging results on pCR can be translated into improved OS.
Pathological complete response was chosen as the primary outcome of our NMA, since it is unequivocally measured and reported in all RCTs as a primary or early end point to assess response to treatment. Nevertheless, it may not represent the best surrogate for OS70 or the desired outcome in all patients. As evidence accumulates on the encouraging results of watch-and-wait strategies,71,72,73 some clinicians may choose TNT with the aim of pursuing complete clinical response and rectal preservation. However, there is no consensus on the definition of complete clinical response, limiting consistency and comparisons among trials. Future analyses should investigate DFS as the primary clinical end point in patients undergoing operative and nonoperative management, after an ultimate definition of complete clinical response and publications of long-term results of watch-and-wait trials.
Strengths and Limitations
This study has strengths. To our knowledge, this is by far the largest and most updated systematic review on this important clinical issue and the first using an NMA method. Second, the evidence before our study did not permit the defining of the optimal sequence, type, and duration of neoadjuvant treatment. We used the NMA technique to indirectly compare various protocols, enabling us to identify the ones with the highest pCR. By using the CINeMA appraisal, we found that the most positive results were supported by high to moderate certainty of evidence, which strongly supports their generalizability and applicability. Furthermore, results for the primary outcome were largely supported by sensitivity and meta-regression analyses. Finally, we analyzed data for several efficacy and tolerability outcomes, allowing for an accurate profiling of each treatment in terms of balance between desirable and undesirable effects.
This study also has some limitations. First, most of the analyses were computed on the intention-to-treat population. Although this approach has the advantage of preserving the benefit of randomization in terms of comparability of study arms, 1 possible shortcoming is that individuals who did not receive surgery due to treatment-related complications or disease progression were pooled together with participants who underwent surgery and did not reach pCR. Although technically debatable, this choice is consistent with a conservative approach that might underestimate the actual benefit of treatments. Even though most of the TNT protocols were expected to deliver both radiation and systemic chemotherapy only before surgery, it should be noted that some of them also included adjuvant chemotherapy12,37,38,51,63 with potential effects on long-term survival. Further analyses will be required to investigate the role of adjuvant chemotherapy in participants who already received systemic chemotherapy within TNT. Also, since TNT involves compound treatment, future analyses may involve component analyses to elucidate which element plays the most relevant role. Moreover, there was a temporal gap among the analyzed studies, introducing potential bias related to the evolution in staging and radiation technology. However, we only included RCTs with similar inclusion criteria, and the planned doses of radiotherapy were comparable among studies. The primary analysis was burdened by moderate heterogeneity and inconsistency according to the global approach. Although the assessment of transitivity did not highlight relevant differences on several variables measuring baseline severity, we cannot exclude that subtle clinical differences between studies might have contributed to overall heterogeneity. For example, the PROSPECT (Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery) trial enrolled patients at lower risk and excluded cT4,62 whereas some studies on S-RT + consolidation showed higher percentages of cT4 at diagnosis.11,35,68 Nevertheless, clinical heterogeneity at baseline did not differ for the distribution of the proportion of clinically suspected nodal metastases, the proportion of pathological T4, and the mean anal verge distance.
Conclusions
This systematic review and NMA found that TNT protocols compared with standard treatment (L-CRT1) were associated with significantly better results in terms of pCR, with L-CRT + consolidation having the best RR as well as being the protocol with the least toxic effects. Importantly, TNT protocols demonstrated feasibility and were not associated with poorer pathological curability and surgical outcomes, with promising results in terms of DFS. None of the TNT protocols were associated with an increased distant recurrence rate compared with the standard, but S-RT + consolidation was associated with a higher locoregional recurrence rate. These results suggest that TNT regimens should be recognized as first-line treatments when aiming at increased pCR. Further data on long-term follow-up might increase insight into long-term survival outcomes.
eFigure 1. PRISMA Flowchart
eAppendix 1. Search Strategy and Data Extraction
eMethods. Complete Statistical Methodology
eTable 1. List of Studies Included/Excluded/Ongoing/Awaiting Assessment
eTable 2. Characteristics of Included Studies
eTable 3. Summary of Inclusion and Exclusion Criteria of Included Studies
eFigure 2. Risk of Bias of Included Studies
eFigure 3. Transitivity Assessment and Meta-Regression
eAppendix 2. Primary Outcome: Patients With Pathologic Complete Response
eAppendix 3. Primary Outcome: Assessment of Publication Bias
eAppendix 4. Primary Outcome: Number Needed to Treat
eAppendix 5. Primary Outcome: CINeMA
eAppendix 6. Primary Outcome: Sensitivity Analyses
eAppendix 7. Tolerability of Treatment
eAppendix 8. Toxicity of Treatment
eAppendix 9. Dropouts by Any Cause
eAppendix 10. Preoperative Treatment-Related Deaths
eAppendix 11. Rate of Randomized Patients Who Underwent Surgery
eAppendix 12. Rate of R0 Resections
eAppendix 13. Rate of Negative CRM
eAppendix 14. Rate of ypN0
eAppendix 15. Rate of Postoperative Complications Clavien-Dindo III or Greater
eAppendix 16. Rate of Anastomotic Leak
eAppendix 17. Locoregional Recurrence at 3 Years
eAppendix 18. Locoregional Recurrence at 5 Years
eAppendix 19. Locoregional Failure at 3 Years
eAppendix 20. Locoregional Failure at 5 Years
eAppendix 21. Distant Recurrence at 3 Years
eAppendix 22. Distant Recurrence at 5 Years
eAppendix 23. Disease-Free Survival at 3 Years
eAppendix 24. Disease-Free Survival at 5 Years
eAppendix 25. Overall Survival at 3 Years
eAppendix 26. Overall Survival at 5 Years
eReferences
Data Sharing Statement
References
- 1.Glynne-Jones R, Wyrwicz L, Tiret E, et al. ; ESMO Guidelines Committee . Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv22-iv40. doi: 10.1093/annonc/mdx224 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): rectal cancer. Version 3.2022. October 27, 2022. Accessed November 20, 2022. https://www.nccn.org/guidelines/category_1
- 3.You YN, Hardiman KM, Bafford A, et al. ; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons . The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of rectal cancer. Dis Colon Rectum. 2020;63(9):1191-1222. doi: 10.1097/DCR.0000000000001762 [DOI] [PubMed] [Google Scholar]
- 4.Hashiguchi Y, Muro K, Saito Y, et al. ; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33(16):1797-1808. doi: 10.1200/JCO.2014.60.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markovina S, Youssef F, Roy A, et al. Improved metastasis- and disease-free survival with preoperative sequential short-course radiation therapy and FOLFOX chemotherapy for rectal cancer compared with neoadjuvant long-course chemoradiotherapy: results of a matched pair analysis. Int J Radiat Oncol Biol Phys. 2017;99(2):417-426. [DOI] [PubMed] [Google Scholar]
- 7.Marco MR, Zhou L, Patil S, et al. ; Timing of Rectal Cancer Response to Chemoradiation Consortium . Consolidation mFOLFOX6 chemotherapy after chemoradiotherapy improves survival in patients with locally advanced rectal cancer: final results of a multicenter phase II trial. Dis Colon Rectum. 2018;61(10):1146-1155. doi: 10.1097/DCR.0000000000001207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Aguilar J, Chow OS, Smith DD, et al. ; Timing of Rectal Cancer Response to Chemoradiation Consortium . Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957-966. doi: 10.1016/S1470-2045(15)00004-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciseł B, Pietrzak L, Michalski W, et al. ; Polish Colorectal Study Group . Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol. 2019;30(8):1298-1303. doi: 10.1093/annonc/mdz186 [DOI] [PubMed] [Google Scholar]
- 10.Bujko K, Wyrwicz L, Rutkowski A, et al. ; Polish Colorectal Study Group . Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834-842. doi: 10.1093/annonc/mdw062 [DOI] [PubMed] [Google Scholar]
- 11.Bahadoer RR, Dijkstra EA, van Etten B, et al. ; RAPIDO collaborative investigators . Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29-42. doi: 10.1016/S1470-2045(20)30555-6 [DOI] [PubMed] [Google Scholar]
- 12.Conroy T, Bosset JF, Etienne PL, et al. ; Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group . Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(5):702-715. doi: 10.1016/S1470-2045(21)00079-6 [DOI] [PubMed] [Google Scholar]
- 13.Goffredo P, Khan A, Mott SL, et al. Total neoadjuvant therapy versus standard neoadjuvant chemoradiation in patients with locally advanced rectal cancer: a comparison of short- and long-term oncologic outcomes. Ann Surg. 2022;276(6):e819-e824. doi: 10.1097/SLA.0000000000005141 [DOI] [PubMed] [Google Scholar]
- 14.Langenfeld SJ, Davis BR, Vogel JD, et al. ; Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons . The American Society of Colon and Rectal Surgeons clinical practice guidelines for the management of rectal cancer 2023 supplement. Dis Colon Rectum. 2024;67(1):18-31. doi: 10.1097/DCR.0000000000003057 [DOI] [PubMed] [Google Scholar]
- 15.Petrelli F, Trevisan F, Cabiddu M, et al. Total neoadjuvant therapy in rectal cancer: a systematic review and meta-analysis of treatment outcomes. Ann Surg. 2020;271(3):440-448. doi: 10.1097/SLA.0000000000003471 [DOI] [PubMed] [Google Scholar]
- 16.Kasi A, Abbasi S, Handa S, et al. Total neoadjuvant therapy vs standard therapy in locally advanced rectal cancer: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12):e2030097. doi: 10.1001/jamanetworkopen.2020.30097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabbani M, Giorgi C, Napoli G, et al. Outcomes of locally advanced rectal cancer patients treated with total neoadjuvant treatment: a meta-analysis of randomized controlled trials. Clin Colorectal Cancer. 2022;21(4):297-308. doi: 10.1016/j.clcc.2022.07.005 [DOI] [PubMed] [Google Scholar]
- 18.Kong JC, Soucisse M, Michael M, et al. Total neoadjuvant therapy in locally advanced rectal cancer: a systematic review and metaanalysis of oncological and operative outcomes. Ann Surg Oncol. 2021;28(12):7476-7486. doi: 10.1245/s10434-021-09837-8 [DOI] [PubMed] [Google Scholar]
- 19.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835-844. doi: 10.1016/S1470-2045(10)70172-8 [DOI] [PubMed] [Google Scholar]
- 20.On J, Shim J, Mackay C, et al. Pathological response post neoadjuvant therapy for locally advanced rectal cancer is an independent predictor of survival. Colorectal Dis. 2021;23(6):1326-1333. doi: 10.1111/codi.15512 [DOI] [PubMed] [Google Scholar]
- 21.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99(7):918-928. doi: 10.1002/bjs.8702 [DOI] [PubMed] [Google Scholar]
- 22.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. doi: 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thomas J, Chandler J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019.
- 25.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452-454. doi: 10.1136/bmj.310.6977.452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veroniki AA, Bender R, Glasziou P, Straus SE, Tricco AC. The number needed to treat in pairwise and network meta-analysis and its graphical representation. J Clin Epidemiol. 2019;111:11-22. doi: 10.1016/j.jclinepi.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3. Cochrane; 2022. Accessed February 1, 2023. http://www.training.cochrane.org/handbook
- 28.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105-3124. doi: 10.1002/sim.1875 [DOI] [PubMed] [Google Scholar]
- 29.Cipriani A, Higgins JPT, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159(2):130-137. doi: 10.7326/0003-4819-159-2-201307160-00008 [DOI] [PubMed] [Google Scholar]
- 30.Ostuzzi G, Schneider-Thoma J, Tedeschi F, Leucht S, Barbui C. Crossroads of methodological choices in research synthesis: insights from two network meta-analyses on preventing relapse in schizophrenia. BMJ Ment Health. 2023;26(1):e300677. doi: 10.1136/bmjment-2023-300677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostuzzi G, Bertolini F, Tedeschi F, et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry. 2022;21(2):295-307. doi: 10.1002/wps.20972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682. doi: 10.1371/journal.pone.0099682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4):e1003082. doi: 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakrabarti D, Rajan S, Akhtar N, et al. Short-course radiotherapy with consolidation chemotherapy versus conventionally fractionated long-course chemoradiotherapy for locally advanced rectal cancer: randomized clinical trial. Br J Surg. 2021;108(5):511-520. doi: 10.1093/bjs/znab020 [DOI] [PubMed] [Google Scholar]
- 36.Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34(27):3300-3307. doi: 10.1200/JCO.2016.66.6198 [DOI] [PubMed] [Google Scholar]
- 37.Deng Y, Chi P, Lan P, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the Chinese FOWARC trial. J Clin Oncol. 2019;37(34):3223-3233. doi: 10.1200/JCO.18.02309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26(8):1722-1728. doi: 10.1093/annonc/mdv223 [DOI] [PubMed] [Google Scholar]
- 39.Fokas E, Allgäuer M, Polat B, et al. ; German Rectal Cancer Study Group . Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ArO/AIO-12. J Clin Oncol. 2019;37(34):3212-3222. doi: 10.1200/JCO.19.00308 [DOI] [PubMed] [Google Scholar]
- 40.Fokas E, Schlenska-Lange A, Polat B, et al. ; German Rectal Cancer Study Group . Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the CAO/ARO/AIO-12 randomized clinical trial. JAMA Oncol. 2022;8(1):e215445-e215445. doi: 10.1001/jamaoncol.2021.5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620-4625. doi: 10.1200/JCO.2006.06.7629 [DOI] [PubMed] [Google Scholar]
- 42.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28(10):1638-1644. doi: 10.1200/JCO.2009.25.8376 [DOI] [PubMed] [Google Scholar]
- 43.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30(36):4558-4565. doi: 10.1200/JCO.2012.42.8771 [DOI] [PubMed] [Google Scholar]
- 44.Azria D, Doyen J, Jarlier M, et al. Late toxicities and clinical outcome at 5 years of the ACCORD 12/0405-PRODIGE 02 trial comparing two neoadjuvant chemoradiotherapy regimens for intermediate-risk rectal cancer. Ann Oncol. 2017;28(10):2436-2442. doi: 10.1093/annonc/mdx351 [DOI] [PubMed] [Google Scholar]
- 45.Haddad P, Miraie M, Farhan F, et al. Addition of oxaliplatin to neoadjuvant radiochemotherapy in MRI-defined T3, T4 or N+ rectal cancer: a randomized clinical trial. Asia Pac J Clin Oncol. 2017;13(6):416-422. doi: 10.1111/ajco.12675 [DOI] [PubMed] [Google Scholar]
- 46.Jiao D, Zhang R, Gong Z, et al. Fluorouracil-based preoperative chemoradiotherapy with or without oxaliplatin for stage II/III rectal cancer: a 3-year follow-up study. Chin J Cancer Res. 2015;27(6):588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin J, Tang Y, Hu C, et al. Multicenter, randomized, phase III trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J Clin Oncol. 2022;40(15):1681-1692. doi: 10.1200/JCO.21.01667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim SY, Joo J, Kim TW, et al. A randomized phase 2 trial of consolidation chemotherapy after preoperative chemoradiation therapy versus chemoradiation therapy alone for locally advanced rectal cancer: KCSG CO 14-03. Int J Radiat Oncol Biol Phys. 2018;101(4):889-899. doi: 10.1016/j.ijrobp.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 49.Latkauskas T, Pauzas H, Gineikiene I, et al. Initial results of a randomized controlled trial comparing clinical and pathological downstaging of rectal cancer after preoperative short-course radiotherapy or long-term chemoradiotherapy, both with delayed surgery. Colorectal Dis. 2012;14(3):294-298. doi: 10.1111/j.1463-1318.2011.02815.x [DOI] [PubMed] [Google Scholar]
- 50.Latkauskas T, Pauzas H, Kairevice L, et al. Preoperative conventional chemoradiotherapy versus short-course radiotherapy with delayed surgery for rectal cancer: results of a randomized controlled trial. BMC Cancer. 2016;16(1):927. doi: 10.1186/s12885-016-2959-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maréchal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23(6):1525-1530. doi: 10.1093/annonc/mdr473 [DOI] [PubMed] [Google Scholar]
- 52.Mei WJ, Wang XZ, Li YF, et al. Neoadjuvant chemotherapy with CAPOX versus chemoradiation for locally advanced rectal cancer with uninvolved mesorectal fascia (CONVERT): initial results of a phase III trial. Ann Surg. 2023;277(4):557-564. doi: 10.1097/SLA.0000000000005780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohiuddin M, Winter K, Mitchell E, et al. ; Radiation Therapy Oncology Group Trial 0012 . Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol. 2006;24(4):650-655. doi: 10.1200/JCO.2005.03.6095 [DOI] [PubMed] [Google Scholar]
- 54.Moore J, Price T, Carruthers S, et al. Prospective randomized trial of neoadjuvant chemotherapy during the “wait period” following preoperative chemoradiotherapy for rectal cancer: results of the WAIT trial. Colorectal Dis. 2017;19(11):973-979. doi: 10.1111/codi.13724 [DOI] [PubMed] [Google Scholar]
- 55.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773-2780. doi: 10.1200/JCO.2010.34.4911 [DOI] [PubMed] [Google Scholar]
- 56.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30(31):3827-3833. doi: 10.1200/JCO.2012.42.9597 [DOI] [PubMed] [Google Scholar]
- 57.Ansari N, Solomon MJ, Fisher RJ, et al. Acute adverse events and postoperative complications in a randomized trial of preoperative short-course radiotherapy versus long-course chemoradiotherapy for T3 adenocarcinoma of the rectum: Trans-Tasman Radiation Oncology Group trial (TROG 01.04). Ann Surg. 2017;265(5):882-888. doi: 10.1097/SLA.0000000000001987 [DOI] [PubMed] [Google Scholar]
- 58.O’Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32(18):1927-1934. doi: 10.1200/JCO.2013.53.7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rödel C, Graeven U, Fietkau R, et al. ; German Rectal Cancer Study Group . Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979-989. doi: 10.1016/S1470-2045(15)00159-X [DOI] [PubMed] [Google Scholar]
- 60.Schmoll HJ, Stein A, Van Cutsem E, et al. Pre- and postoperative capecitabine without or with oxaliplatin in locally advanced rectal cancer: PETACC 6 trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J Clin Oncol. 2021;39(1):17-29. doi: 10.1200/JCO.20.01740 [DOI] [PubMed] [Google Scholar]
- 61.Wang J, Guan Y, Gu W, et al. Long-course neoadjuvant chemoradiotherapy with versus without a concomitant boost in locally advanced rectal cancer: a randomized, multicenter, phase II trial (FDRT-002). Radiat Oncol. 2019;14(1):215. doi: 10.1186/s13014-019-1420-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schrag D, Shi Q, Weiser MR, et al. Preoperative treatment of locally advanced rectal cancer. N Engl J Med. 2023;389(4):322-334. doi: 10.1056/NEJMoa2303269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aschele C, Lonardi S, Cionini L, et al. Final results of STAR-01: a randomized phase III trial comparing preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer. J Clin Oncol. 2016;34(15)(suppl):3521-3521. doi: 10.1200/JCO.2016.34.15_suppl.3521 [DOI] [PubMed] [Google Scholar]
- 64.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results—EORTC 22921. J Clin Oncol. 2005;23(24):5620-5627. doi: 10.1200/JCO.2005.02.113 [DOI] [PubMed] [Google Scholar]
- 65.Bosset JF, Calais G, Mineur L, et al. ; EORTC Radiation Oncology Group . Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184-190. doi: 10.1016/S1470-2045(13)70599-0 [DOI] [PubMed] [Google Scholar]
- 66.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72(1):15-24. doi: 10.1016/j.radonc.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 67.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93(10):1215-1223. doi: 10.1002/bjs.5506 [DOI] [PubMed] [Google Scholar]
- 68.Bujko K, Nasierowska-Guttmejer A, Wyrwicz L, et al. ; Polish Colorectal Study Group . Neoadjuvant treatment for unresectable rectal cancer: an interim analysis of a multicentre randomized study. Radiother Oncol. 2013;107(2):171-177. doi: 10.1016/j.radonc.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 69.Chinese Society of Clinical Oncology CSCO Diagnosis and Treatment Guidelines for Colorectal Cancer Working Group. Chinese Society of Clinical Oncology (CSCO) Diagnosis and Treatment Guidelines for Colorectal Cancer 2018 (English version). Chin J Cancer Res. 2019;31(1):117-134. doi: 10.21147/j.issn.1000-9604.2019.01.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fokas E, Glynne-Jones R, Appelt A, et al. Outcome measures in multimodal rectal cancer trials. Lancet Oncol. 2020;21(5):e252-e264. doi: 10.1016/S1470-2045(20)30024-3 [DOI] [PubMed] [Google Scholar]
- 71.Habr-Gama A, Perez RO, Sabbaga J, Nadalin W, São Julião GP, Gama-Rodrigues J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52(12):1927-1934. doi: 10.1007/DCR.0b013e3181ba14ed [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Aguilar J, Patil S, Gollub MJ, et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J Clin Oncol. 2022;40(23):2546-2556. doi: 10.1200/JCO.22.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Valk MJM, Hilling DE, Bastiaannet E, et al. ; IWWD Consortium . Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch and Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537-2545. doi: 10.1016/S0140-6736(18)31078-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flowchart
eAppendix 1. Search Strategy and Data Extraction
eMethods. Complete Statistical Methodology
eTable 1. List of Studies Included/Excluded/Ongoing/Awaiting Assessment
eTable 2. Characteristics of Included Studies
eTable 3. Summary of Inclusion and Exclusion Criteria of Included Studies
eFigure 2. Risk of Bias of Included Studies
eFigure 3. Transitivity Assessment and Meta-Regression
eAppendix 2. Primary Outcome: Patients With Pathologic Complete Response
eAppendix 3. Primary Outcome: Assessment of Publication Bias
eAppendix 4. Primary Outcome: Number Needed to Treat
eAppendix 5. Primary Outcome: CINeMA
eAppendix 6. Primary Outcome: Sensitivity Analyses
eAppendix 7. Tolerability of Treatment
eAppendix 8. Toxicity of Treatment
eAppendix 9. Dropouts by Any Cause
eAppendix 10. Preoperative Treatment-Related Deaths
eAppendix 11. Rate of Randomized Patients Who Underwent Surgery
eAppendix 12. Rate of R0 Resections
eAppendix 13. Rate of Negative CRM
eAppendix 14. Rate of ypN0
eAppendix 15. Rate of Postoperative Complications Clavien-Dindo III or Greater
eAppendix 16. Rate of Anastomotic Leak
eAppendix 17. Locoregional Recurrence at 3 Years
eAppendix 18. Locoregional Recurrence at 5 Years
eAppendix 19. Locoregional Failure at 3 Years
eAppendix 20. Locoregional Failure at 5 Years
eAppendix 21. Distant Recurrence at 3 Years
eAppendix 22. Distant Recurrence at 5 Years
eAppendix 23. Disease-Free Survival at 3 Years
eAppendix 24. Disease-Free Survival at 5 Years
eAppendix 25. Overall Survival at 3 Years
eAppendix 26. Overall Survival at 5 Years
eReferences
Data Sharing Statement