Abstract
Purpose of the Review
Systemic sclerosis (scleroderma) is a complex autoimmune disease that commonly involves the cardiovascular system. Even if often subclinical, cardiac involvement is considered a poor prognostic factor as it is a leading cause of death in scleroderma patients. We review the cardiac manifestations of scleroderma, the diagnostic methods useful in detection, and current advances in therapeutic management.
Recent Findings
Beside the routine exams for the assessment of cardiac status (including EKG, standard echocardiography, provocative tests) novel techniques such as myocardial strain imaging on echocardiography, cardiac magnetic resonance imaging, invasive hemodynamic assessment, and endomyocardial biopsy have been demonstrated to be useful in understanding the cardiac alterations that typically affect scleroderma patients.
Summary
Recent application of novel cardiac detection strategies is providing increased insight into the breadth and pathogenesis of cardiac complications of scleroderma. Further studies coupling exercise provocation, invasive and imaging assessment, and mechanistic studies in scleroderma cardiac tissue are needed to develop the optimal approach to early detection of cardiac disease in scleroderma and targeted therapies.
Keywords: Cardiac complications, Systemic sclerosis, Scleroderma, Diagnosis, Management
Introduction
Scleroderma (systemic sclerosis, SSc) is a rare heterogeneous autoimmune disease that results from a complex relationship of exaggerated fibrosis, vasculopathy, and immune dysregulation. It can affect multiple organ systems, and among the rheumatologic diseases, has the highest rates of case-specific mortality and substantial non-lethal complications [1]. The heterogeneity and patient-to-patient variability inherent in the clinical manifestations of SSc, including autoantibody profiles, speed of disease progression, constellation of target organ effects, and response to treatment and survival, extend to its cardiac manifestations. Cardiac involvement is common in this disease, with a majority of patients having post-mortem pathologic findings [2], but often this remains clinically silent until manifesting in later stages of the disease. SSc can cause pathology in all aspects of the heart, including the pericardium, myocardium, conduction system, vasculature, and less commonly, the valves [3]. It is estimated that only 10–30% of cases with cardiac alterations are symptomatic, whereas the majority of cases (approximately 70%) are subclinical [4]. It is important to note that almost a third of deaths related to SSc are attributed to cardiac causes. Independent of mortality, cardiac involvement in SSc is indicative of aggressive systemic disease.
Among the demographic risk factors that are associated with a higher risk of cardiac involvement in SSc, race has been found to affect outcomes and prevalence. A recent single-center study comparing African Americans to other racial groups over 20 years demonstrated significantly increased rates of diffuse cutaneous disease, as compared to white patients, who tended to have more anti-centromere antibody and limited cutaneous disease [5]. Notably, African Americans experienced an increase in prevalence of cardiac, renal, digital ischemia, muscle, and restrictive lung disease. Compared to whites, African American SSc patients experienced an 80% increased mortality, even after adjusting for age at onset and disease duration. A significantly larger multicenter study comparing an African American cohort to other cohorts confirmed many of these findings, including the tendency of African American patients to have earlier onset, more diffuse cutaneous disease, more severe disease manifestations, including pulmonary arterial hypertension (PAH), and greater mortality [6, 7].
Cardiac manifestations in SSc are varied and are either due to primary effects of the fibrotic disease process underlying SSc on the heart or secondary due to pulmonary arterial hypertension, interstitial lung disease, or SSc renal crisis [8–10]. We will review the broad categories of cardiac manifestations including involvement of the pericardium and myocardium, vascular disease, conduction disease/arrhythmia followed by secondary cardiac manifestations.
Cardiac Manifestations
Pericardial Diseases
Pericardial involvement is more common than significant myocardial fibrosis (62% vs. 30%) but is often limited to asymptomatic effusions [11]. Some effusions in SSc may be secondary (transudative) in the setting of right-heart failure, and recent studies have demonstrated that effusions in SSc patients with PAH is more common than that in idiopathic PAH patients [12, 13]. When secondary to right-heart failure, SSc patients tend to respond more to diuresis rather than immunosuppression. Other less common pericardial manifestations include pericardial inflammation, fibrinous pericarditis, fibrous pericarditis, pericardial adhesions, cardiac tamponade, and constrictive pericarditis, but these are generally less common. When clinically overt, pericardial disease can cause considerable morbidity and is associated with symptoms in 5–16% [11–13]. SSc patients are at risk for development of restrictive cardiomyopathy from fibrosis of the myocardium and constrictive cardiomyopathy from chronic inflammation of the pericardium [11].
Myocardial Diseases
Myocardial involvement is more common and more severe in the diffuse cutaneous variant, though patients with the limited cutaneous and sine SSc variants also have significant cardiac disease [14]. The hallmark pathophysiological mechanism underlying cardiac disease in systemic sclerosis begins with a microvascular disease, which leads to ischemia-reperfusion injury, subsequent necrosis, and eventual fibrosis [15]. These hypothesized vascular mechanisms are based on early autopsy studies in which focal myocardial lesions were found, varying from contraction band necrosis to regional fibrotic scarring, and unrelated to any associated obstructive coronary artery disease [16]. The common triad of SSc pathogenesis involves microvascular abnormalities, immune system activation, and tissue fibrosis [8–10, 15].
In terms of primary cardiac involvement of SSc, similar to that observed in histologic studies, imaging findings demonstrate patchy myocardial fibrosis [16]. Based on a 2015 study of 62 SSc patients, the prevalence of myocardial fibrosis on magnetic resonance imaging (MRI) was estimated to be 45%; myocardial fibrosis was also more frequent and severe in diffuse cutaneous SSc patients, and unrelated to obstructive coronary artery disease [17]. Clinically, diastolic dysfunction of both ventricles is significantly more common than systolic dysfunction [18]. Left ventricular (LV) diastolic dysfunction is an early noninvasive marker of underlying myocardial fibrosis and may occur early in diffuse SSc independent of comorbid cardiac abnormalities, such as essential hypertension [19]. Diastolic dysfunction underlies the clinical syndrome of heart failure preserved ejection fraction (HFpEF), which is highly prevalent in SSc [20]. Diastolic dysfunction reflecting impaired ventricular filling represents a stiff or fibrotic ventricle, which may eventually lead to upstream effects, such as atrial enlargement and associated dysrhythmias, pulmonary venous congestion and edema, and/or ventricular systolic dysfunction [19, 20]. However, the precise diagnosis of HFpEF is a significant challenge in SSc patients given comorbid pulmonary disease which may also cause nonspecific symptoms.
LV systolic dysfunction is far less common in SSc when compared to diastolic dysfunction, with an estimated incidence of 11–15% depending on diagnostic technique [8]. Impaired stroke volume was found to be present at rest and during exercise in patients with SSc [21]. Overt clinical systolic heart failure (heart failure with reduced ejection fraction, HFrEF) in patients with SSc typically presents insidiously and is thought to be due to focal ischemia from microvascular disease, leading to inflammation and myocardial fibrosis [222]. However, some patients with SSc develop acute or subacute myocarditis with a more rapidly progressive clinical course.
Right ventricular (RV) dysfunction in SSc may be the result of HFpEF or HFrEF involving the left ventricle, primary abnormalities of the right ventricle, or secondary to PAH [23].
Vascular Dysfunction
Raynaud’s phenomenon, a sign of vascular dysfunction in SSc, can be found in more than 90% of patients, and can be associated with ischemic digital ulcers or digital loss. The anti-centromere antibody is associated with not only PAH but also increased risk of digital loss. The results of recent studies suggest that double positivity for anti-interferon inducible protein 16 (IFI-16) and anti-centromere antibodies is associated with increased risk of digital gangrene [24, 25].
Cardiac Conduction Disease and Arrhythmias
Arrhythmias are also common, and mechanistically due to autonomic cardiac neuropathy, myocardial fibrosis into the conduction system, as well as microvascular injury. Approximately 25–75% of SSc patients manifest with an abnormal electrocardiogram [26]. The most common electrophysiologic abnormalities include premature ventricular contractions, PR prolongation, left anterior fascicular block, and intraventricular conduction defects [27].
Valvular Disease
There is a low incidence of valvular disease in SSc; the most common valvular abnormality is nodular thickening of mitral and aortic valves, which may be associated with valvular regurgitation that is usually not hemodynamically significant. However, with an increasingly aged population, SSc patients may also develop aortic stenosis, the diagnosis of which may be confounded by multifactorial dyspnea.
Secondary Cardiac Manifestations
Secondary cardiac manifestations of systemic sclerosis are those induced by pulmonary hypertension (PH), interstitial lung disease (ILD), and renal disease [10]. PH can be classified into pre-capillary, capillary, and post-capillary etiologies [28]. It is often difficult to differentiate between PH as a result of pulmonary arteriopathy versus as a consequence of ILD and/or left heart involvement. A recent study was able to deconstruct the wide heterogeneity of pre-capillary pulmonary hypertension in SSc into four clinically relevant clusters [28, 29]. Cluster 1 corresponded to mild and moderate risk PAH without extensive ILD and with a low diffusing capacity of the lungs for carbon monoxide (DLCO). Cluster 2 was characterized by pre-capillary PH due to extensive ILD and with a low DLCO. Cluster 3 was characterized by severe PAH without extensive ILD and with a low DLCO. Cluster 4 was described as mild to moderate risk PAH without extensive ILD and with a normal DLCO. A source of confusion arises from the uncertainty of the degree to which pulmonary hypertension is a marker of severity of underlying left ventricular (LV) dysfunction versus as a target for treatment. A precision medicine approach similar to the one done by Launay et al., may improve and advance the phenotyping that is necessary to have appropriate and effective therapeutic interventions, rather than the current “one-size-fits-all” PH strategy [29]. PH is commonly diagnosed in later stages of the disease process, owing to the non-specific symptoms, such as exertional dyspnea, and the inability of traditional monitoring, such as standard 2D echocardiography, to detect the disease process in its early stages. SSc patients with ILD and associated PH have particularly poor survival, with an estimated median survival time between 1 and 3 years [30]. Because the mortality of PH in the context of SSc is high and occurs rapidly [12, 31], there has been an impetus to diagnose PH in earlier stages of the disease [32]. The DETECT study established an evidenced-based algorithm that minimized missed diagnoses of PAH in SSc, with only a 4% false negative rate [33]. PAH occurs in an estimated 10–12% of SSc patients and is a leading cause of early death in this population, with a median estimated survival of 4 years after diagnosis [34, 35]. PAH is more commonly associated with the limited cutaneous variant and the anti-centromere antibody [36]. Despite this association, an association between serum autoantibodies and survival in patients with SSc-PAH was not identified in the PHAROS cohort [36]. Increasing number of studies suggest that the RNA polymerase III antibody may be associated with both PH and interstitial lung disease in SSc [37]. SSc patients with PH and HFpEF appear to have a twofold increased risk of death compared to SSc-PAH when adjusted for hemodynamic factors [31]. From a pathologic perspective, PAH leads to increased afterload and overload of the RV, leading to RV remodeling and failure [38]. The sarcomere has been shown in molecular analyses to be the cause of RV dysfunction in SSc patients [39••]. SSc patients also demonstrate less increase in RV mass with increasing PVR than do patients with idiopathic PAH, suggesting that patients with SSc-PAH may manifest less adaptive hypertrophy [40]. SSc-PAH carries a significantly worse prognosis compared with any other form of PAH in Group 1, including idiopathic PAH [12, 41]. For prognostication, the ESC/ERS risk prediction model appears to be accurate at predicting survival in newly diagnosed SSc-PAH [42].
Diagnosis
In Fig. 2, we summarized the diagnostic algorithm and emerging research modalities for the assessment of cardiac involvement in systemic sclerosis that will be discussed in the following sections.
Fig. 2.
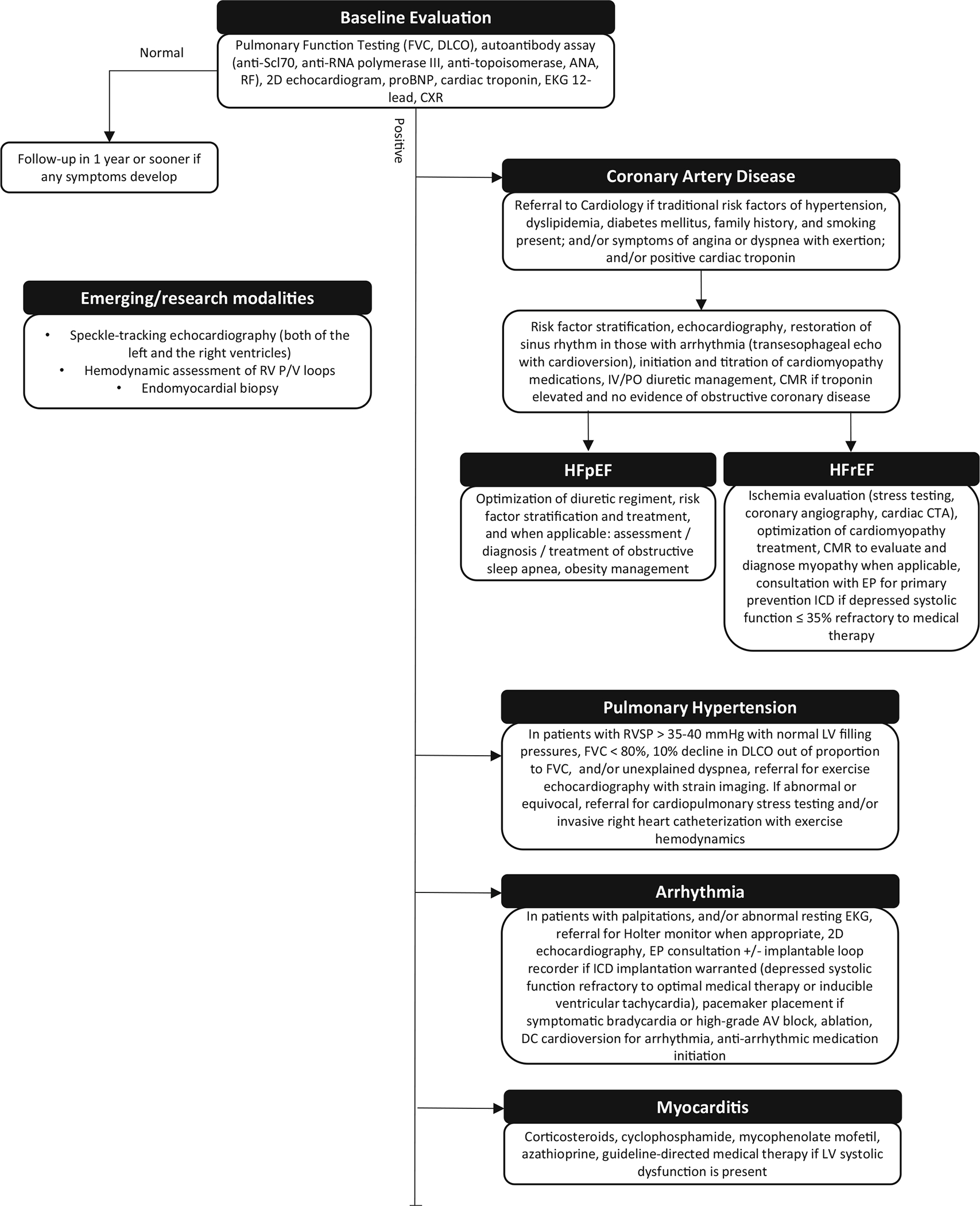
Diagnostic algorithm and emerging research modalities for the assessment of cardiac involvement in systemic sclerosis
Initial Steps and Biomarkers
On history and physical, most patients with cardiac involvement from SSc will manifest with dyspnea, decreased exercise tolerance, fatigue, and/or chest discomfort. First-line testing typically involves an electrocardiogram, chest x-ray, and two-dimensional echocardiogram (2D echo), and subsequent laboratory measurement of troponin, NTpBNP, and CK-MB [43, 44]. It is important to realize that the sensitivity of just an EKG and chest X-ray are low for SSc, and that laboratory testing and imaging will ultimately yield definitive diagnoses. Troponin and NTpBNP have been proven to risk stratify SSc patients, in particular, those at risk of pulmonary hypertension and ventricular arrhythmias [45–48]. In situations with a high suspicion for myocarditis, serum creatine kinase MB isoenzyme elevation diagnoses and monitors myocarditis. sFlt-1, PIGF, and a high RDW on CBC may signify the presence of pulmonary arterial hypertension in SSc, but further studies need to corroborate these findings [49, 50].
Chest X-ray
On chest x-ray, findings that may be associated with SSc include atrial and ventricular enlargement, vascular congestion, interstitial lung disease, and pleuropericardial abnormalities. A pericardial effusion may be visualized as enlargement of the cardiac silhouette [51].
Assessment of Cardiac Conduction System
On EKG, the most common abnormalities include PR prolongation, left anterior fascicular block, and intraventricular conduction defects [26, 52]. In fact, right bundle branch block is a predictor of early mortality in systemic sclerosis [53], and QTc prolongation is associated with longer disease duration, greater disease severity, and the presence of anti-RNA polymerase III antibodies [54]. Diffuse PR depression and ST elevation can be seen in acute pericarditis. An abnormal EKG is typically followed by ambulatory Holter monitor or implanted loop recorder placement [26, 52]. A standard 12-lead EKG is typically insufficient to diagnose arrhythmias and conduction disease; extended monitoring will reveal arrhythmias not apparent in the brief snapshot in time that the 12-lead EKG captures [27, 55].
The most common findings on Holter monitoring are supraventricular tachycardias [52], and a high frequency of ventricular ectopic beats correlates with life-threatening arrhythmic complications [55]. Prolonged QTwas thought to be associated with patients with more severe vascular and fibrotic complications of SSc, but this was later disproved in a larger cohort [55–57]. Other less common manifestations on extended electrophysiologic monitoring include ventricular dysrhythmias, including multiform or coupled ventricular premature contractions, and ventricular tachycardias. Recent studies suggest that impairment of heart rate variability (obtainable with high resolution electrophysiologic monitoring) with respect to time and frequency is a marker of cardiac dysautonomia in SSc [58]. Although the incidence of atrial arrhythmias and their correlation with hemodynamics and clinical parameters has not been well studied, recent data suggest that the occurrence of atrial arrhythmias has prognostic implications [59]. This aligns with its pathophysiology, as intact atrioventricular synchrony optimizes biventricular filling, and is an important determinant of systemic output in patients with RV compromise. An adjunct method for evaluating conduction disease is the exercise treadmill electrocardiogram to detect exertional arrhythmia but is not a standard part of an electrophysiological workup. A Holter monitor or implantable loop recorder will provide the most utility for the vast majority of these patients.
Echocardiography
Echocardiography remains the gold-standard for assessment of the myocardium, owing to its relative accuracy, reproducibility, sensitivity, and widespread availability. On standard doppler echocardiography, findings in SSc include pericardial effusion, diastolic dysfunction (less commonly systolic dysfunction), RVimpairment, abnormal atrial and ventricular volumes, and the presence of PH [60]. Tissue Doppler imaging, which provides direct measurement of myocardial tissue velocities and patterns of tissue movement, allows for the measurement of a myocardial strain rate (SR), which is a sensitive method for identifying myocardial contraction, independent of myocardial translational motion [61]. This is a robust indicator of contractility and stiffness. However, measurement of velocities are insufficient to characterize cardiac mechanics. Strain, which is the fractional change in length of a segment of myocardium compared to its original length, provides an additional parameter to describe cardiac deformation that is particularly valuable in the study of the fibrotic sequelae of SSc. Due to the complex geometric shape of the right ventricle, the ability to measure myocardial strain through tissue Doppler is difficult because this technique requires alignment of the Doppler beam with the direction of myocardial motion [62].
More recently, a new imaging modality called speckle tracking echocardiology (STE), used in conjunction with conventional 2D echo, provides a precise measure of regional and global systolic function through direct measurement of strain that is not user-dependent or angle-dependent, unlike previous Doppler-derived techniques [61]. Recent studies have demonstrated the ability of STE to detect LVand RV dysfunction that precedes abnormalities detected by conventional echocardiographic methods [63••]. STE has also been able to discern subtleties in RV dysfunction strain parameters that help distinguish between different types of pulmonary hypertension [64•].
Echocardiograms are recommended yearly to screen for PAH in SSc. Using data from yearly echocardiographic screening, a recent study demonstrated that the rate of increase in RV systolic pressure is a risk factor for mortality and PAH even after adjustment for clinical characteristics and longitudinal pulmonary function tests (PFT) data [65].
Cardiac MRI
Although cardiac MRI has a sensitivity advantage over current 2D echo techniques, and is less operator dependent than echocardiography, it is often only available at tertiary or quaternary care centers. Gadolinium-delayed contrast enhancement allows for visualization of myocardial fibrosis [17, 66, 67], and T2-weighted imaging identifies inflammatory lesions, as well as measures of ejection fraction and chamber size. For quantitative evaluation of RV function and structural changes, cardiac MRI has become the gold standard, as it provides accurate and reproducible measurements without geometric assumptions. Newer perfusion MRI techniques can also identify perfusion defects through measure of a global perfusion index. Myocardial stress perfusion defects detected by pharmacological stress perfusion CMR have been shown to be reliable and sensitive for non-invasive evaluation of SSc heart disease, even in early stages of the disease [17, 68–71]. Cardiac MRI can also visualize a myriad of additional pathologies, including the ability to detect silent myocarditis through the Lake Louise criteria [72–74], as well as epicardial fat volume, which may be an easily quantifiable cardiovascular risk marker in systemic sclerosis and other rheumatic diseases [75, 76]. Because of its wide-ranging capabilities, there is debate over how early in the diagnostic process it should be utilized [77]. Despite the many capabilities of cardiac MRI, it is limited by ferro-magnetic implants, patient-to-patient variability in respirations, the ability of patients to breath-hold, arrhythmia-induced cardiac motion variability, as well as acquisition time and cost [78].
Nuclear Imaging (PET, SPECT)
Single-photon emission computed tomography can also be used to assess for myocardial perfusion/ischemia [79]. SPECT is widely available, non-invasive, and demonstrates areas of reduced myocardial perfusion during stress. In a study of 35 patients with SSc, 60% manifested signs of reduced blood supply on SPECT, likely unrelated to occlusive coronary artery disease [80]. Hybrid scanners combining PET and MRI can help differentiate scar from active inflammation within myocardium [81, 82].
Increased FDG uptake in the right ventricle correlates with earlier onset of clinical worsening and higher mortality rates, but it is not clear the usefulness of RV FDG uptake as a biomarker in PAH [82–84]. Although nuclear imaging such as SPECT and PET are sensitive for detection of perfusion defects, these techniques are limited and inferior in the imaging of subendocardial defects when compared to cardiac MRI [18, 22, 85]. MRI has largely supplanted the utilization of thallium-201 SPECT, in part due to the lack of radiation exposure, and the additional data regarding chamber volumes and fibrosis [86, 87].
Exercise Testing: 6-Min Walk Test
There are conflicting opinions on the utility of the 6-min walk test (6MWT) in systemic sclerosis [88–90]. Although data show that the results of the 6MWT strongly correlate with overall prognosis [91, 92], many non-cardiac and non-pulmonary aspects of SSc contribute to the results including the integumentary and musculoskeletal system [93, 94]. Despite this controversy, the DIBOSA scoring system, taking into account the 6MWT, has been shown to be a sensitive tool for predicting mortality in SSc-PH, and strongly correlates with pulmonary pressures [95]. An increase in the ratio of mPAP/Q obtained by stress echo during the 6MWT appears to provide a clue to future PH and is a potential indicator of subclinical pulmonary circulatory impairment [91, 96].
Exercise Testing: Cardiopulmonary Exercise Testing
Cardiopulmonary exercise testing (CPET) response in SSc is characterized by reduced peak oxygen consumption, early anaerobic threshold, reduced oxygen pulse, high minute ventilation to carbon dioxide production ratio, low end-tidal pCO2, and variable oxygen desaturation [97–99]. CPET has not been widely used or studied in screening studies, in part because testing and interpretation are complex, costly, time-consuming processes that may not be standardized across centers [94, 100]. However, a recent study demonstrated the capability of CPET to non-invasively detect pulmonary arterial hypertension in a safe manner, thus potentially reducing unnecessary right heart catheterization procedures [101]. CPET has the capability of distinguishing between causes of exercise limitation through characterization of the pattern of gas exchange limitation [102]. Integrating CPET with imaging or invasive hemodynamics is a recent development in the field, and is the subject of ongoing research, particularly because of the potential to assess cardiac “functional reserve” and provide new insights into systemic and pulmonary hemodynamics [103]. These recent developments will be detailed in the next two sections.
Exercise Testing: Exercise Echocardiography
Comparison of normal echocardiographic variables to echocardiographic variables obtained during exercises have yielded insights into SSc heart disease. In particular, exercise testing appears to unmask abnormalities not apparent in resting studies. A study of 25 patients with normal resting PAP demonstrated reduced RV contractile reserve in exercise, suggesting that subclinical RV dysfunction during exercise can be a surrogate for early pulmonary vascular disease [104]. RV dilation visualized by ECHO during exercise can predict adverse ventricular-vascular coupling in PAH patients [105]. Another study found impaired increase in stroke volume in patients with SSc and PH, as compared to those without PH [21]. Increases in measured systolic pulmonary artery pressure on exercise echocardiography can be measured over time, indicating the progression of pulmonary vascular disease [98].
Exercise Testing: Exercise Right Heart Catheterization
Comparison of cardiac catheterization parameters obtained at rest to those obtained during exercise have yielded insights into SSc heart disease. A sensitive set of criteria to define exercise induced pulmonary hypertension have been proposed and include a mPAP > 30mmHg and TPR > 3 WU at maximal exercise [106, 107]. The gradual deterioration of pulmonary exercise hemodynamics and exercise capacity can be tracked by exercise right heart catheterization [90]. Recent studies suggest that exercise PH may be a precursor of resting PH [108, 109]. Thus, exercise testing appears capable of revealing hidden precursor disease states that would not otherwise be apparent with resting standard right heart catheterization. Exercise during cardiac catheterization also elucidates the pathophysiology of dyspnea and distinguishes exercise-induced PAH from the more common diastolic dysfunction in SSc patients [110].
Right Heart Catheterization
Right heart catheterization (RHC) is the gold standard and required to confirm a diagnosis of PAH [111]. A core set of criteria for referral for RHC has been defined by Delphi consensus methods, and these include clinical criteria (progressive dyspnea over 3 months, unexplained dyspnea, worsened of WHO dyspnea functional class, any finding on physical exam suggestive of elevated right heart pressures or right heart failure), echocardiography (systolic pulmonary artery pressure > 45 mmHg and right ventricle dilation) and PFTs (DLCO < 50% without pulmonary fibrosis) [112].
A recent study by Tedford et al. established a protocol for invasive measurements of pressure-volume relations in the right ventricle in humans, along with the use of a Valsalva maneuver to lower preload and generate end-systolic pressure-volume relationships effectively [113••]. Although this is technically difficult and requires skilled operators, this offers an avenue for research that allows advanced physiologic characterization RV dysfunction using methods traditionally reserved for animal models of the left ventricle.
Left Heart Catheterization, Coronary Evaluation
Suspicion for coronary artery disease is evaluated by coronary angiography, and coronary score by CT. Moderate to severe coronary calcifications are seen in up to two-thirds of patients with SSc. There was initial uncertainty over the relation of macrovascular atherosclerotic cardiovascular disease to SSc, but a large study in Hong Kong demonstrated that SSc was an independent risk factor for coronary calcification [114, 115]. On cardiac catheterization, atherosclerotic lesions of small coronary arteries may be seen, but may be better evaluated by SPECT or MRI. SPECT or MRI can demonstrate stress-induced perfusion defects thought to represent fibrotic or microvasculature changes. Microvascular coronary artery disease in SSc has an estimated prevalence of over 60%.
Endomyocardial Biopsy
Endomyocardial biopsy (EMBx) can be performed as part of a cardiac catheterization. On endomyocardial biopsy, fibrotic tissue with concentric intimal hyperplasia of intramural coronary arteries may be seen if affected areas of the heart are sampled. The degree of cardiac inflammation and fibrosis seen on EMBx is associated with adverse cardiac event rate and can be seen in an estimated 80 to 100% of SSc patients [116]. The major utility of endomyocardial biopsy is as a tool to exclude differential diagnoses, such as hydroxychloroquine toxicity and cardiac amyloid, and to distinguish between inflammatory and fibrotic forms of heart involvement in SSc [4]. The observed fibrosis of the myocardium of SSc differs from atherosclerotic coronary artery disease and does not correspond to the regional distribution of a single coronary artery, rather involving the subendocardium. On histology, there is diffuse patchy fibrosis, contraction band necrosis, concentric intimal hypertrophy associated with fibrinoid necrosis of intramural coronary arteries, as shown in Fig. 1. A recent study suggested that endomyocardial biopsy can detect earlier stages of the disease, before any abnormalities manifest on EKG or ECHO [116]. Endomyocardial biopsy, however, is subject to sampling error, as not all areas of the heart may manifest with fibrosis [51].
Fig. 1.
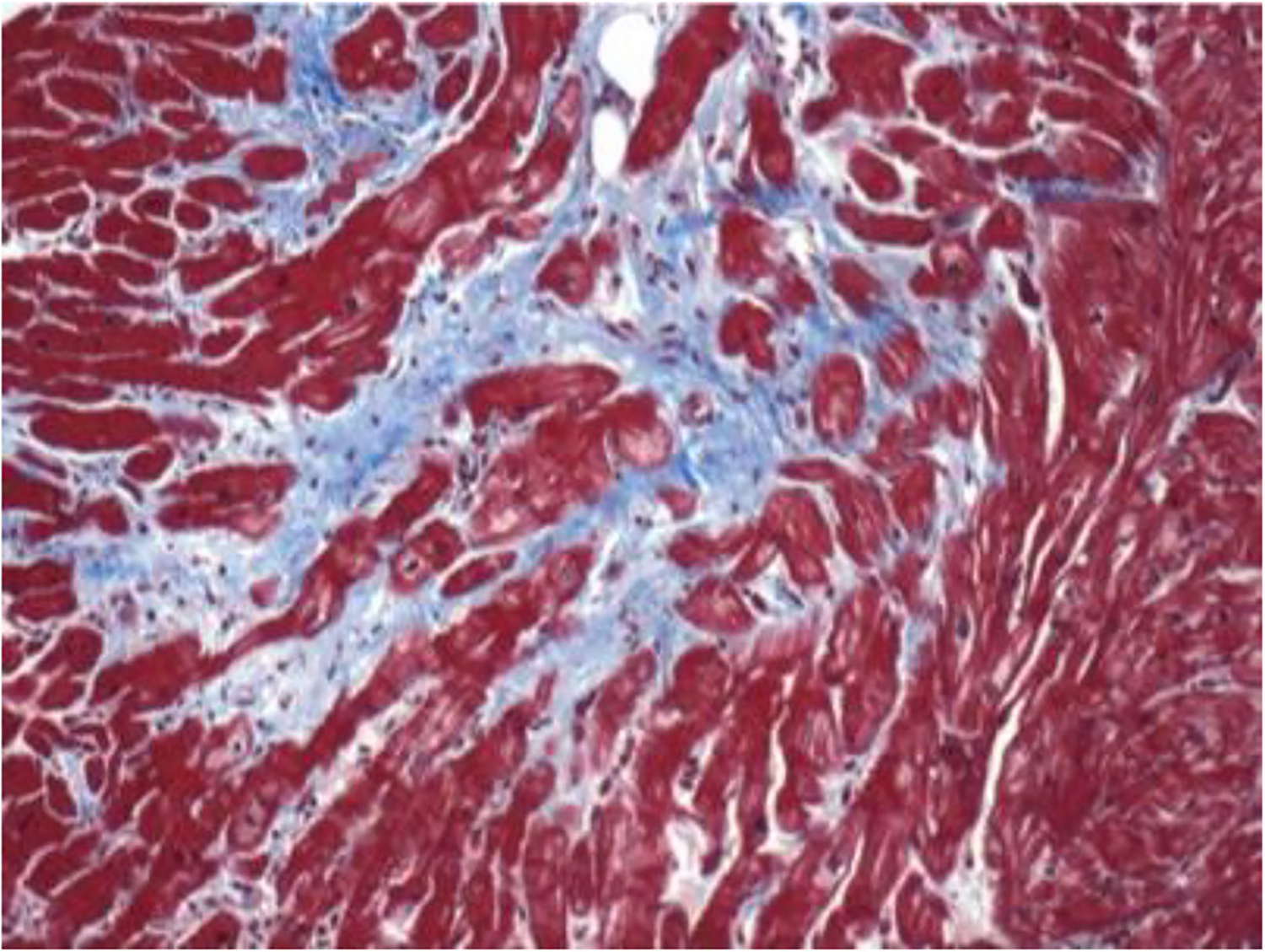
The pathologic hallmark of SSc cardiac involvement is patchy myocardial fibrosis as shown here with trichrome blue staining. Note fibrotic tissue abutting normal cardiac myocardial tissue. Histologic image courtesy of Dr. Fredrick Wigley
Management
In Table 1, we summarized the diagnostic tools and the current treatment of cardiac manifestations in SSc.
Table 1.
Prevalence, diagnostic tools, and treatment of cardiac complications of systemic sclerosis
| Cardiac manifestation | Diagnostic tools | Treatment |
|---|---|---|
|
| ||
| Pericardial diseases • Acute/chronic pericarditis • Pericardial effusions • Constrictive pericarditis |
EKG typically demonstrates sinus tachycardia; may demonstrate pulsus alternans if effusion is present and large Imaging studies: • 2D echocardiography to evaluate for cardiac tamponade or hemodynamic compromise • CT/CMR may demonstrate pericardial thickening in the setting of chronic pericarditis, constrictive pericarditis Right Heart Catheterization: • Invasive hemodynamics may be indicated in cases of discordant findings and will demonstrate discordance of peak LV and RV pressures at peak inspiration Laboratory Studies: elevated NT-proBNP |
Pericarditis: NSAIDs and colchicine; If constriction, then treat right heart failure symptoms with diuretics, sodium and fluid restriction; pericardial stripping surgery is contraindicated in most cases Pericardial effusion: treat only if symptomatic; rule our renal crisis; cautious diuretics in the setting of right heart failure; Pericardiocentesis if severely symptomatic or evidence of cardiac tamponade. Pericardiocentesis is contraindicated in patients with significant pulmonary arterial hypertension or RV dysfunction |
| Primary left and right ventricular myocardial dysfunction | Imaging Studies: • 2D echocardiography is the mainstay in diagnosis of LV/RV systolic or diastolic dysfunction. Tissue Doppler analysis may demonstrate diastolic dysfunction with elevated LV filling pressures. Noninvasive estimation of RV filling pressures may demonstrate evidence of pulmonary hypertension • Speckle tracking echocardiography can be used to identify subclinical abnormalities in contractility CMR for inflammation or fibrosis Right Heart Catheterization: • Invasive hemodynamics for confirmatory Laboratory Studies: troponin, NT-proBNP |
LV systolic dysfunction: GDMT ± CRT, mechanical support devices, cardiac transplantation LV diastolic dysfunction: Symptomatic treatment (i.e. diuretics), adequate blood pressure control RV dysfunction: Symptomatic treatment (i.e., diuretics), Digoxin, invasive hemodynamic testing to rule out pulmonary arterial hypertension |
| Cardiac conduction disease and arrhythmias | Resting EKG or 24-hour ambulatory Holter monitor. In patients where symptoms are less frequent, and/or are associated with cardiogenic syncope, a 30-day event monitor may be indicated. In cases where a 30-day event monitor is unrevealing, implantable loop recorders may extend the ability to detect arrhythmias over a longer period of time | Bradyarrhythmias: pacemaker according to standard guidelines Tachyarrhythmias: Nondihydropyridine calcium channel blockers, avoid betablockers if Raynaud’s present, cautious use of antiarrhythmics consider ablation or ICD in select patients |
| Imaging Studies: • CMR for myocardial fibrosis |
||
| Ischemic cardiomyopathy | Imaging Studies: • Myocardial perfusion imaging (SPECT or CMR imaging) • Cardiac CT with coronary artery calcium score may be useful for screening Left heart catheterization/coronary angiography: • gold standard for assessment and management of obstructive epicardial CAD |
Microvascular CAD: Calcium channel blockers, ACE-inhibitors/angiotensin receptor antagonists; consider ranolazine if angina is present; statins Macrovascular/epicardial CAD: Coronary stenting or standard medical management of coronary artery disease; statins |
Abbreviations: EKG electrocardiogram, NSAIDs non-steroidal anti-inflammatory drugs, CT computed tomography, CMR cardiac magnetic resonance, RV right ventricular, LV left ventricular, GDMT guideline-directed medical therapy, CRT cardiac resynchronization therapy, ICD implantable cardioverter-defibrillator, SPECT single-photon emission computed tomography, CAD coronary artery disease
Ischemic Cardiomyopathy
In patients with suspected ischemic cardiomyopathy, referral to cardiology should be initiated in the setting of new echocardiographic evidence of left ventricular EF < 45% or interval change in EF associated with new ischemic changes on EKG, and/or symptoms of angina or dyspnea with exertion, and/or positive cardiac troponin in setting of traditional risk factors of hypertension, dyslipidemia, diabetes mellitus, family history, and smoking. Referral to cardiology would likely entail risk factor modification, echocardiography, exercise or pharmacologic stress testing, coronary evaluation with coronary CT angiography, or invasive coronary angiography if indicated by symptoms or positive stress testing [117].
Heart Failure
In patients with suspected heart failure, referral to cardiology should be initiated in the setting of traditional risk factors and signs/symptoms, including dyspnea, orthopnea, paroxysmal nocturnal dyspnea, peripheral edema, and elevation in pro-BNP [118]. Referral to cardiology would likely entail risk factor stratification, echocardiography, re-establishment of sinus rhythm in those with arrhythmia, initiation and titration of cardiomyopathy medications, IV/PO diuretic management, and CMR if troponin elevated and no evidence of obstructive coronary disease [118]. For HFpEF, special considerations would include workup and treatment for obstructive sleep apnea and obesity, and referral for cardiac catheterization if symptoms refractory or disproportionate to noninvasive findings or symptoms [118]. For heart failure with reduced ejection fraction, special considerations would include ischemia evaluation, CMR to evaluate and diagnose overlap inflammatory myopathy when applicable, and consultation with EP for primary prevention ICD if depressed left ventricular systolic function EF < 35% refractory to medical therapy [118].
Treatment for systolic dysfunction in systemic sclerosis mirrors traditional guideline directed medical therapy for heart failure, including diuretics, ACE inhibitors, angiotensin receptor blockers, and aldosterone antagonists. A calcium channel blocker can be added, because the pathophysiology of systolic dysfunction in SSc is usually underlying microvascular and diastolic dysfunction. Treatment for diastolic dysfunction in systemic sclerosis also mirrors traditional guideline directed medical therapy, most commonly being diuretics.
Pulmonary Hypertension
SSc patients with suspected PH should be screened with annual PFTs with DLCO measurement and echocardiography [119]. Current standard of treatment includes endothelin receptor antagonists, and PDE5 inhibitors such as sildenafil and tadalafil. Recent studies have demonstrated the benefits of earlier dual therapy of ambrisentan and tadalafil [120, 121•]. It is important to note that analysis of PHAROS data demonstrated a significantly worsened time to clinical worsening in patients initially treated with an endothelin receptor antagonist as monotherapy as compared to patients treated with a phosphodiesterase 5 inhibitor or combination therapy with an endothelin receptor antagonist and PDE5 inhibitor [122]. Riociguat is a soluble guanylyl cyclase stimulator first approved in 2013 for the treatment of different forms of pulmonary hypertension, including pulmonary arterial hypertension associated with CTD [123]. The PATENT-1 and PATENT-2 trials demonstrated that riociguat was well tolerated and associated with positive trends in6 MWand other endpoints [124]. The recent RISE-SSc trial aims to evaluate the use of riociguat in diffuse cutaneous systemic sclerosis [125].
In patients with suspected PH, referral for exercise echo with strain imaging would be initiated in the setting of RVSP greater than 35–40 mmHg with normal LV filling pressures, FVC < 80%, 10% decline in DLCO out of proportion to FVC, and/or unexplained dyspnea. If abnormal or equivocal, referral for cardiopulmonary stress testing and/or invasive RHC with exercise hemodynamics can be considered. Provocative NO testing is not currently recommended in SSc patients. Based on studies of patients enrolled in the PHAROS registry [126–129], a DLCO < 50%, exercise oxygen desaturation, or pericardial effusions should prompt a right heart catheterization and subsequent appropriate intervention if pulmonary hypertension is confirmed [130, 131].
Arrhythmias
For arrhythmias, there are no specialized indications for pacemaker or AICD implantation specific to SSc; traditional guideline-directed electrophysiologic approaches are used. Referral for Holter monitoring or implanted loop recorder and 2D echo would be appropriate in the setting of palpations and/or abnormal resting EKG. As discussed previously, electrophysiology consultation for defibrillator implantation is warranted for depressed systolic function refractory to optimal medical therapy or inducible ventricular tachycardia. Sudden cardiac death is a risk in these patients, and those who might benefit from an ICD should undergo evaluation for placement [26]. EP consultation for pacemaker placement is warranted for symptomatic bradycardia or high-grade AV block.
Pericardial Disease
Treatment of pericarditis in SSc general mirrors standard therapy, including NSAID therapy, and colchicine for 1–3 month duration [11, 132]. Corticosteroids are avoided because of increased risk of transformation to chronic symptomatic pericarditis and can precipitate SSc renal crisis. Corticosteroids are reserved for refractory cases.
Pericardial effusions are typically only treated if symptomatic. As part of the management of pericardial effusions, renal crisis should be ruled out. If there is suspicion for right heart failure with concomitant pericardial effusion, diuresis should be done cautiously. Pericardiocentesis can be performed if severely symptomatic or with tamponade physiology; however, pericardiocentesis is contraindicated in patients with significant pulmonary arterial hypertension or RV dysfunction, due to the concern for acute right heart decompensation [13, 87]. At some centers, RHC is recommended in patients with concomitant pulmonary arterial hypertension and large pericardial effusions to better characterize hemodynamics prior to making the decision between medical vs. surgical management [132].
Arterial Line Placement
For patients requiring arterial line placement, it is important to note that radial arterial line placement may trigger critical ischemic events in SSc patients [133]. Thus, alternatives to arterial lines should be considered prior to insertion in SSc patients.
Broad Perspectives
There are no currently accepted disease-modifying therapies that directly target the heart and its underlying pathophysiology, such as inflammation and/or fibrosis, at this time, though regenerative research into stem cell therapy is ongoing [134]. Recently, results from a multicenter observational long-term study demonstrated that the use of vasodilators (i.e., calcium channel blockers and/or ACE inhibitors or angiotensin II receptor blockers, or even pulmonary vasodilators) and low-dose acetylsalicylic acid was associated with a decrease in the incidence of distinct myocardial manifestations (including arrhythmias, need of pacemaker implantation, reduction of left ventricular ejection fraction, and/or development of chronic heart failure), thus suggesting a possible role of these therapeutic options in preventing the development of SSc-related cardiac complications [135]. Exercise itself may become an adjunct element to treating not just SSc, but rheumatic diseases in general [136]. Future directions are directed at development of novel immunomodulators to counteract the pathologic processes and improving diagnosis through discovery of novel biomarkers from advances in proteomics [137]. Future directions in the realm of pulmonary hypertension are focused on establishing a definitive consensus for a hemodynamic definition, elucidating the pathobiology of lung capillaries and small arteries, standardizing methods for assessment of right ventricular function, and searching for new treatments to improve lung vessels and the remodeled right ventricle [138]. For example, recent developments in therapeutics include macitentan, riociguat, and selexipag [139]; a recent large three-cohort study demonstrated the utility of pulmonary effective arterial elastance as an upcoming noninvasive metric to measure total RV afterload and index ventricular function to RV afterload [140].
Conclusion
Beside the routine exams for the assessment of cardiac status, data from recent studies on novel techniques including myocardial strain imaging on echocardiography, cardiac magnetic resonance imaging, invasive hemodynamic assessment, and endomyocardial biopsy gave relevant insights in the understanding of the SSc-related cardiac alterations (Table 2). Nevertheless, we acknowledge that it is difficult to apply all these evaluations on the daily routine care of SSc patients. Further studies are needed in order to improve the treatment and the early diagnosis of cardiac involvement in SSc.
Table 2.
Summary of the key points of this review on cardiovascular complications in systemic sclerosis
| Key points |
|---|
|
|
| • Cardiac involvement in SSc is common and significantly heterogeneous concerning both prevalence and clinical presentation |
| • Cardiac alterations in SSc could also be secondary to other SSc complications (due to pulmonary arterial hypertension, interstitial lung disease, or SSc renal crisis) with relevant prognostic implications |
| • Cardiac conduction disease and arrhythmias are quite common, probably related to due to autonomic cardiac neuropathy, myocardial fibrosis into the conduction system, as well as microvascular injury |
| • Guideline-directed medical therapy is the current treatment for cardiac complications in SSc |
| • To date no specific treatment is available to prevent cardiac complications in SSc |
| • Speckle-tracking echocardiography (both of the left and the right ventricles), hemodynamic assessment of RV P/V loops, and endomyocardial biopsy can help in better understanding of the SSc-related cardiac alterations |
Grants
MM (Scleroderma Foundation, CHEST Foundation, Chresanthe Staurulakis Memorial Discovery Fund, NIH/NHLBI U01HL175125, R01HL114910-06), VM (European Respiratory Society Research Fellowship), SCM (NIH/NHLBI U01HL175125; Ann Dana Kusch Multidisciplinary Program for Interstitial Lung Disease and Pulmonary Hypertension), AAS (NIH/NIAMS R01AR073208, Chresanthe Staurulakis Memorial Discovery Fund).
Footnotes
Conflict of Interest The authors declare that they have no conflicts of interest.
People N/A
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Lancet The. Systemic sclerosis: advances and prospects. Lancet. 2017;390:1624. 10.1016/S0140-6736(17)32594-1. [DOI] [PubMed] [Google Scholar]
- 2.Sandmeier B, Jäger VK, Nagy G, Carreira PE, Tzankov A, Widuchowska M, et al. Autopsy versus clinical findings in patients with systemic sclerosis in a case series from patients of the EUSTAR database. Clin Exp Rheumatol. 2015;33(4 Suppl 91): S75–9. [PubMed] [Google Scholar]
- 3.Ross L, Prior D, Proudman S, Vacca A, Baron M, Nikpour M. Defining primary systemic sclerosis heart involvement: a scoping literature review. Semin Arthritis Rheum. 2019;48(5):874–87. 10.1016/j.semarthrit.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Boueiz A, Mathai SC, Hummers LK, Hassoun PM. Cardiac complications of systemic sclerosis: recent progress in diagnosis. Curr Opin Rheumatol. 2010;22:696–703. 10.1097/BOR.0b013e32833dfbd8. [DOI] [PubMed] [Google Scholar]
- 5.Gelber AC, Manno RL, Shah AA, Woods A, Le EN, Boin F, et al. Race and association with disease manifestations and mortality in scleroderma: a 20-year experience at the Johns Hopkins Scleroderma Center and review of the literature. Medicine (Baltimore). 2013;92:191–205. 10.1097/MD.0b013e31829be125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco I, Mathai S, Shafiq M, Boyce D, Kolb TM, Chami H, et al. Severity of systemic sclerosis-associated pulmonary arterial hypertension in African Americans. Medicine (Baltimore). 2014. Jul;93(5):177–85. 10.1097/MD.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan ND, Shah AA, Mayes MD, Domsic RT, Medsger TA, Steen VD, et al. Clinical and serological features of systemic sclerosis in a multicenter African American cohort: analysis of the genome research in African American scleroderma patients clinical database. Medicine (Baltimore). 2017;96:e8980. 10.1097/MD.0000000000008980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parks JL, Taylor MH, Parks LP, Silver RM. Systemic sclerosis and the heart. Rheum Dis Clin N Am. 2014;40:87–102. 10.1016/j.rdc.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Desai CS, Lee DC, Shah SJ. Systemic sclerosis and the heart: current diagnosis and management. Curr Opin Rheumatol. 2011;23:545–54. 10.1097/BOR.0b013e32834b8975.u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga J, Denton C, Wigley F. Scleroderma. New York: Springer. 2012. 10.1007/978-3-319-31407-5. [DOI] [Google Scholar]
- 11.Byers RJ, Marshall DA, Freemont AJ. Pericardial involvement in systemic sclerosis. Ann Rheum Dis. 1997;56:393–4. 10.1136/ard.56.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum. 2006. Sep;54(9):3043–50. [DOI] [PubMed] [Google Scholar]
- 13.Hosoya H, Derk CT. Clinically symptomatic pericardial effusions in hospitalized systemic sclerosis patients: demographics and management. Biomed Res Int. 2018;2018:6812082. 10.1155/2018/6812082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucharz EJ, Kopeć-Mędrek M. Systemic sclerosis sine scleroderma. Adv Clin Exp Med. 2017;26:875–80. 10.17219/acem/64334. [DOI] [PubMed] [Google Scholar]
- 15.MokMY Lau CS. The burden and measurement of cardiovascular disease in systemic sclerosis. Nat Rev Rheumatol. 2010;6:430–4. 10.1038/nrrheum.2010.65. [DOI] [PubMed] [Google Scholar]
- 16.Bulkley BH, Ridolfi RL, Salyer WR, Hutchins GM. Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction. Circulation. 1976;53:483–90. 10.1161/01.cir.53.3.483. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Reyna TS, Morelos-Guzman M, Hernández-Reyes P, Montero-Duarte K, Martínez-Reyes C, Reyes-Utrera C, et al. Assessment of myocardial fibrosis and microvascular damage in systemic sclerosis by magnetic resonance imaging and coronary angiotomography. Rheumatology (Oxford). 2015;54:647–54. 10.1093/rheumatology/keu350. [DOI] [PubMed] [Google Scholar]
- 18.Meune C, Khanna D, Aboulhosn J, Avouac J, Kahan A, Furst DE, et al. A right ventricular diastolic impairment is common in systemic sclerosis and is associated with other target-organ damage. Semin Arthritis Rheum. 2016;45:439–45. 10.1016/j.semarthrit.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Hinchcliff M, Desai CS, Varga J, Shah SJ. Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol. 2012;30:S30–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Allanore Y, Meune C, Vonk MC, Airo P, Hachulla E, Caramaschi P, et al. Prevalence and factors associated with left ventricular dysfunction in the EULAR Scleroderma Trial and Research group (EUSTAR) database of patients with systemic sclerosis. Ann Rheum Dis. 2010;69:218–21. 10.1136/ard.2008.103382. [DOI] [PubMed] [Google Scholar]
- 21.Someya F, Mugii N, Oohata S. Factors relating to impaired stroke volume during the 6-minute walk test in patients with systemic sclerosis. Clin Exp Rheumatol. 2016;34(Suppl 100):152–6. [PubMed] [Google Scholar]
- 22.Allanore Y, Meune C. Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol. 2010;28:S48–53. [PubMed] [Google Scholar]
- 23.Maron BA. Independence day: separating right ventricular function from pulmonary arterial hypertension in systemic sclerosis. Circulation. 2016. Jun 14;133(24):2345–7. 10.1161/CIRCULATIONAHA.116.023237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahan ZH, Shah AA, Vaidya D, Wigley FM, Rosen A, Casciola-Rosen L. Anti-interferon-inducible protein 16 antibodies associate with digital gangrene in patients with scleroderma. Arthritis Rheum. 2016;68:1262–71. 10.1002/art.39558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahan ZH, Wigley FM, Casciola-Rosen L. Risk of digital vascular events in scleroderma patients who have both anticentromere and anti-interferon-inducible protein 16 antibodies. Arthritis Care Res. 2017;69:922–6. 10.1002/acr.22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muresan L, Petcu A, Pamfil C, Muresan C, Rinzis M, Mada RO, et al. Cardiovascular profiles of scleroderma patients with arrhythmias and conduction disorders. Acta Reumatol Port. 2016;41:26–39. [PubMed] [Google Scholar]
- 27.Vacca A, Meune C, Gordon J, Chung L, Proudman S, Assassi S, et al. Cardiac arrhythmias and conduction defects in systemic sclerosis. Rheumatology (Oxford). 2014;53:1172–7. 10.1093/rheumatology/ket377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elinoff JM, Agarwal R, Barnett CF, Benza RL, Cuttica MJ, Gharib AM, et al. Challenges inpulmonary hypertension: controversies in treating the tip of the iceberg. A Joint National Institutes of Health Clinical Center and Pulmonary Hypertension Association Symposium Report. Am J Respir Crit Care Med. 2018;198:166–74. 10.1164/rccm.201710-2093PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Launay D, Montani D, Hassoun PM, Cottin V, LePavec J, Clerson P, et al. Clinical phenotypes and survival of pre-capillary pulmonary hypertension in systemic sclerosis. PLoS One. 2018;13:e0197112. 10.1371/journal.pone.0197112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Pavec J, Girgis RE, Lechtzin N, Mathai SC, Launay D, Hummers LK, et al. Systemic sclerosis-related pulmonary hypertension associated with interstitial lung disease: impact of pulmonary arterial hypertension therapies. Arthritis Rheum. 2011;63:2456–64. 10.1002/art.30423. [DOI] [PubMed] [Google Scholar]
- 31.Bourji KI, Kelemen BW, Mathai SC, Damico RL, Kolb TM, Mercurio V, et al. Poor survival in patients with scleroderma and pulmonary hypertension due to heart failure with preserved ejection fraction. Pulm Circ. 2017;7:409–20. 10.1177/2045893217700438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwaiger JP, Khanna D, Gerry CJ. Screening patients with scleroderma for pulmonary arterial hypertension and implications for other at-risk populations. Eur Respir Rev. 2013;22:515–25. 10.1183/09059180.00006013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coghlan JG, Denton CP, Grünig E, Bonderman D, Distler O, Khanna D, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73:1340–9. 10.1136/annrheumdis-2013-203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambova S Cardiac manifestations in systemic sclerosis. World J Cardiol. 2014;6:993–1005. 10.4330/wjc.v6.i9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–99. 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 36.Hinchcliff M, Khanna S, Hsu VM, Lee J, Almagor O, Chang RW, et al. Survival in systemic sclerosis-pulmonary arterial hypertension by serum autoantibody status in the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) Registry. Semin Arthritis Rheum. 2015;45:309–14. 10.1016/j.semarthrit.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mecoli CA, Shah AA, Boin F, Wigley FM, Hummers LK. Vascular complications in systemic sclerosis: a prospective cohort study. Clin Rheumatol. 2018;37:2429–37. 10.1007/s10067-018-4148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonk Noordegraaf A, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. 2019;53(1). 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.••.Hsu S, Kokkonen-Simon KM, Kirk JA, Kolb TM, Damico RL, Mathai SC, et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation. 2018;137:2360–70. 10.1161/CIRCULATIONAHA.117.033147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelemen BW, Mathai SC, Tedford RJ, Damico RL, Corona-Villalobos C, Kolb TM, et al. Right ventricular remodeling in idiopathic and scleroderma-associated pulmonary arterial hypertension: two distinct phenotypes. Pulm Circ. 2015;5:327–34. 10.1086/680356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassoun PM. The right ventricle in scleroderma (2013 Grover Conference Series). Pulm Circ. 2015;5:3–14. 10.1086/679607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mercurio V, Diab N, Peloquin G, Housten-Harris T, Damico R, Kolb TM, et al. Risk assessment in scleroderma patients with newly diagnosed pulmonary arterial hypertension: application of the ESC/ERS risk prediction model. Eur Respir J. 2018;52. 10.1183/13993003.00497-2018. [DOI] [PubMed] [Google Scholar]
- 43.Mathai SC, Hassoun PM. N-terminal brain natriuretic peptide in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2007;28(1):140–1 author reply 141. [DOI] [PubMed] [Google Scholar]
- 44.Hughes M, Lilleker JB, Herrick AL, Chinoy H. Cardiac troponin testing in idiopathic inflammatory myopathies and systemic sclerosis-spectrum disorders: biomarkers to distinguish between primary cardiac involvement and low-grade skeletal muscle disease activity. Ann Rheum Dis. 2015;74:795–8. 10.1136/annrheumdis-2014-206812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avouac J, Meune C, Chenevier-Gobeaux C, Borderie D, Lefevre G, Kahan A, et al. Cardiac biomarkers in systemic sclerosis: contribution of high-sensitivity cardiac troponin in addition to N-terminal pro-brain natriuretic peptide. Arthritis Care Res. 2015;67:1022–30. 10.1002/acr.22547. [DOI] [PubMed] [Google Scholar]
- 46.Nordin A, Svenungsson E, Björnådal L, Elvin K, Larsson A, Jensen-Urstad K. Troponin I and echocardiography in patients with systemic sclerosis and matched population controls. Scand J Rheumatol. 2017;46:226–35. 10.1080/03009742.2016.1192217. [DOI] [PubMed] [Google Scholar]
- 47.Muresan L, Petcu A, Muresan C, Rinzis M, Gusetu G, Pop D, et al. The role of NT-proBNP in the diagnosis of ventricular arrhythmias in patients with systemic sclerosis. Iran J Public Health. 2017;46:906–16. [PMC free article] [PubMed] [Google Scholar]
- 48.Pathak V, Aris R, Jensen BC, Huang W, Ford HJ. Effect of 6-min walk test on pro-BNP levels in patients with pulmonary arterial hypertension. Lung. 2018;196:315–9. 10.1007/s00408-018-0111-0. [DOI] [PubMed] [Google Scholar]
- 49.McMahan Z, Schoenhoff F, Van Eyk JE, Wigley FM, Hummers LK. Biomarkers of pulmonary hypertension in patients with scleroderma: a case-control study. Arthritis Res Ther. 2015;17:201. 10.1186/s13075-015-0712-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao J, Mo H, Guo X, Wang Q, Xu D, Hou Y, et al. Red blood cell distribution width as a related factor of pulmonary arterial hypertension in patients with systemic sclerosis. Clin Rheumatol. 2018;37:979–85. 10.1007/s10067-017-3918-9. [DOI] [PubMed] [Google Scholar]
- 51.Chapin R, Hant FN. Imaging of scleroderma. Rheum Dis Clin N Am. 2013;39:515–46. 10.1016/j.rdc.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 52.Nordin A, Björnådal L, Larsson A, Svenungsson E, Jensen-Urstad K. Electrocardiography in 110 patients with systemic sclerosis: a cross-sectional comparison with population-based controls. Scand J Rheumatol. 2014;43:221–5. 10.3109/03009742.2013.843720. [DOI] [PubMed] [Google Scholar]
- 53.Draeger HT, Assassi S, Sharif R, Gonzalez EB, Harper BE, Arnett FC, et al. Right bundle branch block: a predictor of mortality in early systemic sclerosis. PLoS One. 2013;8:e78808. 10.1371/journal.pone.0078808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Massie C, Hudson M, Tatibouet S, Steele R, Huynh T, Fritzler MJ, et al. Absence of an association between anti-Ro antibodies and prolonged QTc interval in systemic sclerosis: a multicenter study of 689 patients. Semin Arthritis Rheum. 2014;44:338–44. 10.1016/j.semarthrit.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 55.De Luca G, Bosello SL, Gabrielli FA, Berardi G, Parisi F, Rucco M, et al. Prognostic role of ventricular ectopic beats in systemic sclerosis: a prospective cohort study shows ECG indexes predicting the worse outcome. PLoS One. 2016;11:e0153012. 10.1371/journal.pone.0153012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Luca G, Bosello SL, Canestrari G, Cavalli G, Dagna L, Ferraccioli G. QTc interval prolongation in systemic sclerosis: correlations with clinical variables and arrhythmic risk. Int J Cardiol. 2017;239:33. 10.1016/j.ijcard.2017.03.088. [DOI] [PubMed] [Google Scholar]
- 57.Rosato E, Gigante A, Liberatori M, Gasperini ML, Sardo L, Amoroso A, et al. QTc interval prolongation in systemic sclerosis: correlations with clinical variables. Int J Cardiol. 2015;182:20–2. 10.1016/j.ijcard.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 58.Tadic M, Zlatanovic M, Cuspidi C, Stevanovic A, Celic V, Damjanov N, et al. Systemic sclerosis impacts right heart and cardiac autonomic nervous system. J Clin Ultrasound. 2018;46:188–94. 10.1002/jcu.22552. [DOI] [PubMed] [Google Scholar]
- 59.Mercurio V, Peloquin G, Bourji KI, Diab N, Sato T, Enobun B, et al. Pulmonary arterial hypertension and atrial arrhythmias: incidence, risk factors, and clinical impact. Pulm Circ. 2018;8:2045894018769874. 10.1177/2045894018769874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gopal DM, Doldt B, Finch K, Simms RW, Farber HW, Gokce N. Relation of novel echocardiographic measures to invasive hemodynamic assessment in scleroderma-associated pulmonary arterial hypertension. Arthritis Care Res (Hoboken). 2014;66:1386–94. 10.1002/acr.22307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blessberger H, Binder T. NON-invasive imaging: two dimensional speckle tracking echocardiography: basic principles. Heart. 2010;96(9):716–22. 10.1136/hrt.2007.141002. [DOI] [PubMed] [Google Scholar]
- 62.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. [DOI] [PubMed] [Google Scholar]
- 63. Mukherjee M, Chung SE, Ton VK, Tedford RJ, Hummers LK, Wigley FM, et al. Unique abnormalities in right ventricular longitudinal strain in systemic sclerosis patients. Circ Cardiovasc Imaging. 2016;9(6). 10.1161/CIRCIMAGING.115.003792 •• This study demonstrates the ability of speckle-tracking echocardiography in detecting a heterogeneous pattern of alteration in regional right ventricular contractility that is suggestive of occult RV myocardial disease.
- 64. Mukherjee M, Mercurio V, Tedford RJ, Shah AA, Hsu S, Mullin CJ, et al. Right ventricular longitudinal strain is diminished in systemic sclerosis compared with idiopathic pulmonary arterial hypertension. Eur Respir J. 2017;50(5). 10.1183/13993003.01436-2017 • This article highlights the presence of a worse right ventricular function in patients with SSc-PAh in comparison to idiopathic PAH that is not detectable by means of standard echocardiography.
- 65.Shah AA, Chung SE, Wigley FM, Wise RA, Hummers LK. Changes in estimated right ventricular systolic pressure predict mortality and pulmonary hypertension in a cohort of scleroderma patients. Ann Rheum Dis. 2013;72:1136–40. 10.1136/annrheumdis-2012-201861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hromádka M, Seidlerová J, Suchý D, Rajdl D, Lhotský J, Ludvík J, et al. Myocardial fibrosis detected by magnetic resonance in systemic sclerosis patients - relationship with biochemical and echocardiography parameters. Int J Cardiol. 2017;249:448–53. 10.1016/j.ijcard.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 67.Muresan L, Oancea I, Mada RO, Petcu A, Pamfil C, Muresan C, et al. Relationship between ventricular arrhythmias, conduction disorders, and myocardial fibrosis in patients with systemic sclerosis. J Clin Rheumatol. 2018;24:25–33. 10.1097/RHU.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 68.Gyllenhammar T, Kanski M, Engblom H, Wuttge DM, Carlsson M, Hesselstrand R, et al. Decreased global myocardial perfusion at adenosine stress as a potential new biomarker for microvascular disease in systemic sclerosis: a magnetic resonance study. BMC Cardiovasc Disord. 2018;18:16. 10.1186/s12872-018-0756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giacomelli R, Di Cesare E, Cipriani P, Ruscitti P, Di Sibio A, Liakouli V, et al. Pharmacological stress, rest perfusion and delayed enhancement cardiac magnetic resonance identifies very early cardiac involvement in systemic sclerosis patients of recent onset. IntJ Rheum Dis. 2017;20:1247–60. 10.1111/1756-185X.13107. [DOI] [PubMed] [Google Scholar]
- 70.Schicchi N, Valeri G, Moroncini G, Agliata G, Salvolini L, Gabrielli A, et al. Myocardial perfusion defects in scleroderma detected by contrast-enhanced cardiovascular magnetic resonance. Radiol Med. 2014;119:885–94. 10.1007/s11547-014-0419-7. [DOI] [PubMed] [Google Scholar]
- 71.Mavrogeni SI, Bratis K, Karabela G, Spiliotis G, Wijk K, Hautemann D, et al. Cardiovascular magnetic resonance imaging clarifies cardiac pathophysiology in early, asymptomatic diffuse systemic sclerosis. Inflamm Allergy Drug Targets. 2015;14:29–36. [DOI] [PubMed] [Google Scholar]
- 72.Mavrogeni S, Koutsogeorgopoulou L, Karabela G, Stavropoulos E, Katsifis G, Raftakis J, et al. Silent myocarditis in systemic sclerosis detected by cardiovascular magnetic resonance using Lake Louise criteria. BMC Cardiovasc Disord. 2017;17:187. 10.1186/s12872-017-0619-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mavrogeni S, Schwitter J, van Rossum A, Nijveldt R, Aletras A, Kolovou G, et al. Cardiac magnetic resonance imaging in myocardial inflammation in autoimmune rheumatic diseases: an appraisal of the diagnostic strengths and limitations of the Lake Louise criteria. Int J Cardiol. 2018;252:216–9. 10.1016/j.ijcard.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 74.Pieroni M, De Santis M, Zizzo G, Bosello S, Smaldone C, Campioni M, et al. Recognizing and treating myocarditis in recent-onset systemic sclerosis heart disease: potential utility of immunosuppressive therapy in cardiac damage progression. Semin Arthritis Rheum. 2014;43:526–35. 10.1016/j.semarthrit.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Long BD, Stojanovska J, Brown RKJ, Attili AK, Jackson EA, Ognenovski V. Increased epicardial fat volume is independently associated with the presence and severity of systemic sclerosis. Acad Radiol. 2017;24:1473–81. 10.1016/j.acra.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Budoff MJ. Increased epicardial fat volume in systemic sclerosis: a new cardiovascular risk marker? Acad Radiol. 2017;24:1471–2. 10.1016/j.acra.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 77.De Luca G, Campochiaro C, Cavalli G, Dagna L. Cardiac magnetic resonance in systemic sclerosis patients with cardiac symptoms: do we really need it? Eur Rev Med Pharmacol Sci. 2018;22: 2189–90. 10.26355/eurrev_201804_14801. [DOI] [PubMed] [Google Scholar]
- 78.Mavrogeni SI, Schwitter J, Gargani L, Pepe A, Monti L, Allanore Y, et al. Cardiovascular magnetic resonance in systemic sclerosis: “pearls and pitfalls”. Semin Arthritis Rheum. 2017;47:79–85. 10.1016/j.semarthrit.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 79.Dimitroulas T, Mavrogeni S, Kitas GD. Imaging modalities for the diagnosis of pulmonary hypertension in systemic sclerosis. Nat Rev Rheumatol. 2012;8:203–13. 10.1038/nrrheum.2012.2. [DOI] [PubMed] [Google Scholar]
- 80.Papagoras C, Achenbach K, Tsifetaki N, Tsiouris S, Fotopoulos A, Drosos AA. Heart involvement in systemic sclerosis: a combined echocardiographic and scintigraphic study. Clin Rheumatol. 2014;33:1105–11. 10.1007/s10067-014-2666-3. [DOI] [PubMed] [Google Scholar]
- 81.Redureau E, Lairez O, Hitzel A, Pugnet G. Can positron emission tomography be useful to manage systemic sclerosis cardiac involvement? J Nucl Cardiol. 2017;24:1814–5. 10.1007/s12350-016-0649-2. [DOI] [PubMed] [Google Scholar]
- 82.Vonk Noordegraaf A, Haddad F, Bogaard HJ, Hassoun PM. Noninvasive imaging in the assessment of the cardiopulmonary vascular unit. Circulation. 2015;131:899–913. 10.1161/CIRCULATIONAHA.114.006972. [DOI] [PubMed] [Google Scholar]
- 83.Tatebe S, Fukumoto Y, Oikawa-Wakayama M, Sugimura K, Satoh K, Miura Y, et al. Enhanced [18F]fluorodeoxyglucose accumulation in the right ventricular free wall predicts long-term prognosis of patients with pulmonary hypertension: a preliminary observational study. Eur Heart J Cardiovasc Imaging. 2014;15:666–72. 10.1093/ehjci/jet276. [DOI] [PubMed] [Google Scholar]
- 84.Bourji KI, Hassoun PM. Right ventricle dysfunction in pulmonary hypertension: mechanisms and modes of detection. Curr Opin Pulm Med. 2015;21:446–53. 10.1097/MCP.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 85.Kahan A, Devaux JY, Amor B, Menkes CJ, Weber S, Foult JM, et al. Pharmacodynamic effect of dipyridamole on thallium-201 myocardial perfusion in progressive systemic sclerosis with diffuse scleroderma. Ann Rheum Dis. 1986;45:718–25. 10.1136/ard.45.9.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis. 2010;103:46–52. 10.1016/j.acvd.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 87.Rangarajan V, Matiasz R, Freed BH. Cardiac complications of systemic sclerosis and management: recent progress. Curr Opin Rheumatol. 2017;29:574–84. 10.1097/BOR.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 88.Vandecasteele E, De Pauw M, De Keyser F, Decuman S, Deschepper E, Piette Y, et al. Six-minute walk test in systemic sclerosis: a systematic review and meta-analysis. Int J Cardiol. 2016;212:265–73. 10.1016/j.ijcard.2016.03.084. [DOI] [PubMed] [Google Scholar]
- 89.Santus P, Radovanovic D, Frassanito F, Cristiano A, Rizzi M. Is the six-minute walk test useful or useless in systemic sclerosis? Eur J Intern Med. 2017;43:e37–9. 10.1016/j.ejim.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 90.Impens AJ, Wangkaew S, Seibold JR. The 6-minute walk test in scleroderma–how measuring everything measures nothing. Rheumatology (Oxford). 2008;47(Suppl 5):v68–9. 10.1093/rheumatology/ken273. [DOI] [PubMed] [Google Scholar]
- 91.Kusunose K, Yamada H, Hotchi J, Bando M, Nishio S, Hirata Y, et al. Prediction of future overt pulmonary hypertension by 6-min walk stress echocardiography in patients with connective tissue disease. J Am Coll Cardiol. 2015;66:376–84. 10.1016/j.jacc.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 92.Pugnet G, Marjanovic Z, Deligny C, Boussardon K, Benzidia I, Puyade M, et al. Reproducibility and utility of the 6-minute walk test in systemic sclerosis. J Rheumatol. 2018;45:1273–80. 10.3899/jrheum.170994. [DOI] [PubMed] [Google Scholar]
- 93.Schoindre Y, Meune C, Dinh-Xuan AT, Avouac J, Kahan A, Allanore Y. Lack of specificity of the 6-minute walk test as an outcome measure for patients with systemic sclerosis. J Rheumatol. 2009;36:1481–5. 10.3899/jrheum.081221. [DOI] [PubMed] [Google Scholar]
- 94.Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J. 2007;83:675–82. 10.1136/hrt.2007.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gadre A, Ghattas C, Han X, Wang X, Minai O, Highland KB. Six-minute walk test as a predictor of diagnosis, disease severity, and clinical outcomes in scleroderma-associated pulmonary hypertension: the DIBOSA Study. Lung. 2017;195:529–36. 10.1007/s00408-017-0034-1. [DOI] [PubMed] [Google Scholar]
- 96.Chin K, Mathai SC. Exercise echocardiography in connective tissue disease. J Am Coll Cardiol. 2015;66:385–7. 10.1016/j.jacc.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 97.Gigante A, Rosato E, Liberatori M, Sardo L, Di Paolo M, Marinelli P, et al. In systemic sclerosis prolonged QTc interval is associated with reduced exercise tolerance. Int J Cardiol. 2016;203:570–2. 10.1016/j.ijcard.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Kovacs G, Avian A, Wutte N, Hafner F, Moazedi-Fürst F, Kielhauser S, et al. Changes in pulmonary exercise haemodynamics in scleroderma: a 4-year prospective study. Eur Respir J. 2017;50(1). 10.1183/13993003.01708-2016. [DOI] [PubMed] [Google Scholar]
- 99.Milani RV, Lavie CJ, Mehra MR, Ventura HO. Understanding the basics of cardiopulmonary exercise testing. Mayo Clin Proc. 2006;81:1603–11. 10.4065/81.12.1603. [DOI] [PubMed] [Google Scholar]
- 100.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. American Heart Association Exercise CrR, and Prevention Committee of the Council on Clinical Cardiology, Prevention CoEa, Disease CoPV and Research ICoQoCaO. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 101.Dumitrescu D, Nagel C, Kovacs G, Bollmann T, Halank M. Winkler J, et al. Cardiopulmonary exercise testing for detecting pulmonary arterial hypertension in systemic sclerosis. Heart. 2017;103:774–82. 10.1136/heartjnl-2016-309981. [DOI] [PubMed] [Google Scholar]
- 102.Boutou AK, Pitsiou GG, Siakka P, Dimitroulas T, Paspala A, Sourla E, et al. Phenotyping exercise limitation in systemic sclerosis: the use of cardiopulmonary exercise testing. Respiration. 2016;91:115–23. 10.1159/000442888. [DOI] [PubMed] [Google Scholar]
- 103.Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol. 2017;70:1618–36. 10.1016/j.jacc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 104.Chia EM, Lau EM, Xuan W, Celermajer DS, Thomas L. Exercise testing can unmask right ventricular dysfunction in systemic sclerosis patients with normal resting pulmonary artery pressure. Int J Cardiol. 2016;204:179–86. 10.1016/j.ijcard.2015.11.186. [DOI] [PubMed] [Google Scholar]
- 105.Hsu S, Houston BA, Tampakakis E, Bacher AC, Rhodes PS, Mathai SC, et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation. 2016;133:2413–22. 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Herve P, Lau EM, Sitbon O, Savale L, Montani D, Godinas L, et al. Criteria for diagnosis of exercise pulmonary hypertension. Eur Respir J. 2015;46:728–37. 10.1183/09031936.00021915. [DOI] [PubMed] [Google Scholar]
- 107.Mullin CJ, Hsu S, Amancherla K, Wand A, Rhodes P, Leary PJ, et al. Evaluation of criteria for exercise-induced pulmonary hypertension in patients with resting pulmonary hypertension. Eur Respir J. 2017;50(3). 10.1183/13993003.00784-2017. [DOI] [PubMed] [Google Scholar]
- 108.Keusch S, Bucher A, Müller-Mottet S, Hasler E, Maggiorini M, Speich R, et al. Experience with exercise right heart catheterization in the diagnosis of pulmonary hypertension: a retrospective study. Multidiscip Respir Med. 2014;9:51. 10.1186/2049-6958-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stamm A, Saxer S, Lichtblau M, Hasler ED, Jordan S, Huber LC, et al. Exercise pulmonary haemodynamics predict outcome in patients with systemic sclerosis. Eur Respir J. 2016;48:1658–67. 10.1183/13993003.00990-2016. [DOI] [PubMed] [Google Scholar]
- 110.Hager WD, Collins I, Tate JP, Azrin M, Foley R, Lakshminarayanan S, et al. Exercise during cardiac catheterization distinguishes between pulmonary and left ventricular causes of dyspnea in systemic sclerosis patients. Clin Respir J. 2013;7:227–36. 10.1111/j.1752-699X.2012.00310.x. [DOI] [PubMed] [Google Scholar]
- 111.Lau EM, Humbert M, Celermajer DS. Early detection of pulmonary arterial hypertension. Nat Rev Cardiol. 2015;12:143–55. 10.1038/nrcardio.2014.191. [DOI] [PubMed] [Google Scholar]
- 112.Avouac J, Huscher D, Furst DE, Opitz CF, Distler O, Allanore Y, et al. Expert consensus for performing right heart catheterisation for suspected pulmonary arterial hypertension in systemic sclerosis: a Delphi consensus study with cluster analysis. Ann Rheum Dis. 2014;73:191–7. 10.1136/annrheumdis-2012-202567. [DOI] [PubMed] [Google Scholar]
- 113. Tedford RJ, Mudd JO, Girgis RE, Mathai SC, Zaiman AL, Housten-Harris T, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail. 2013;6:953–63. 10.1161/CIRCHEARTFAILURE.112.000008 •• This is the first study that demonstrated by means of invasive hemodynamics the presence of alterations in intrinsic RV contractile function in patients with SSc-PAH.
- 114.Mok MY, Lau CS, Chiu SS, Tso AW, Lo Y, Law LS, et al. Systemic sclerosis is an independent risk factor for increased coronary artery calcium deposition. Arthritis Rheum. 2011;63:1387–95. 10.1002/art.30283. [DOI] [PubMed] [Google Scholar]
- 115.Ngian GS, Sahhar J, Wicks IP, Van Doornum S. Cardiovascular disease in systemic sclerosis–an emerging association? Arthritis Res Ther. 2011;13:237. 10.1186/ar3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mueller KA, Mueller II, Eppler D, Zuern CS, Seizer P, Kramer U, et al. Clinical and histopathological features of patients with systemic sclerosis undergoing endomyocardial biopsy. PLoS One. 2015;10:e0126707. 10.1371/journal.pone.0126707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2014;130(19):1749–67. 10.1161/CIR.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 118.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136(6): e137–61. 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 119.McMahan ZH, Hummers LK. Systemic sclerosis–challenges for clinical practice. Nat Rev Rheumatol. 2013;9:90–100. 10.1038/nrrheum.2012.191. [DOI] [PubMed] [Google Scholar]
- 120.Sato T, Ambale-Venkatesh B, Lima JAC, Zimmerman SL, Tedford RJ, Fujii T, et al. The impact of ambrisentan and tadalafil upfront combination therapy on cardiac function in scleroderma associated pulmonary arterial hypertension patients: cardiac magnetic resonance feature tracking study. Pulm Circ. 2018;8:2045893217748307. 10.1177/2045893217748307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mercurio V, Mukherjee M, Tedford RJ, Zamanian RT, Khair RM, Sato T, et al. Improvement in right ventricular strain with ambrisentan and tadalafil upfront therapy in scleroderma-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2018;197:388–91. 10.1164/rccm.201704-0789LE • This study highlights the beneficial effects of initial combination treatment with ambrisentan and tadalafil on right ventricular function in patients with SSc-PAH.
- 122.Lammi MR, Mathai SC, Saketkoo LA, Domsic RT, Bojanowski C, Furst DE, et al. Association between initial oral therapy and outcomes in systemic sclerosis-related pulmonary arterial hypertension. Arthritis Rheum. 2016;68:740–8. 10.1002/art.39478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sandner P From molecules to patients: exploring the therapeutic role of soluble guanylate cyclase stimulators. Biol Chem. 2018;399:679–90. 10.1515/hsz-2018-0155. [DOI] [PubMed] [Google Scholar]
- 124.Humbert M, Coghlan JG, Ghofrani HA, Grimminger F, He JG, Riemekasten G, et al. Riociguat for the treatment of pulmonary arterial hypertension associated with connective tissue disease: results from PATENT-1 and PATENT-2. Ann Rheum Dis. 2017;76:422–6. 10.1136/annrheumdis-2015-209087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Distler O, Pope J, Denton C, Allanore Y, Matucci-Cerinic M, de Oliveira PJ, et al. RISE-SSc: riociguat in diffuse cutaneous systemic sclerosis. Respir Med. 2017;122(Suppl 1):S14–7. 10.1016/j.rmed.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 126.Bae S, Saggar R, Bolster MB, Chung L, Csuka ME, Derk C, et al. Baseline characteristics and follow-up in patients with normal haemodynamics versus borderline mean pulmonary arterial pressure in systemic sclerosis: results from the PHAROS registry. Ann Rheum Dis. 2012;71:1335–42. 10.1136/annrheumdis-2011-200546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hsu VM, Chung L, Hummers LK, Wigley F, Simms R, Bolster M, et al. Development of pulmonary hypertension in a high-risk population with systemic sclerosis in the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) cohort study. Semin Arthritis Rheum. 2014;44:55–62. 10.1016/j.semarthrit.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 128.Chung L, Domsic RT, Lingala B, Alkassab F, Bolster M, Csuka ME, et al. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res (Hoboken). 2014;66:489–95. 10.1002/acr.22121. [DOI] [PubMed] [Google Scholar]
- 129.Kolstad KD, Li S, Steen V, Chung L, Investigators P. Long-term outcomes in systemic sclerosis-associated pulmonary arterial hypertension from the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma Registry (PHAROS). Chest. 2018;154:862–71. 10.1016/j.chest.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barsotti S, Bruni C, Orlandi M, Della Rossa A, Marasco E, Codullo V, et al. One year in review 2017: systemic sclerosis. Clin Exp Rheumatol. 2017;35(Suppl 106):3–20. [PubMed] [Google Scholar]
- 131.Hsu VM, Chung L, Hummers LK, Shah A, Simms R, Bolster M, et al. Risk factors for mortality and cardiopulmonary hospitalization in systemic sclerosis patients at risk for pulmonary hypertension, in the PHAROS Registry. J Rheumatol. 2019;46:176–83. 10.3899/jrheum.180018. [DOI] [PubMed] [Google Scholar]
- 132.Imazio M, Bobbio M, Cecchi E, Demarie D, Pomari F, Moratti M, et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch Intern Med. 2005;165:1987–91. 10.1001/archinte.165.17.1987. [DOI] [PubMed] [Google Scholar]
- 133.Paik JJ, Hirpara R, Heller JA, Hummers LK, Wigley FM, Shah AA. Thrombotic complications after radial arterial line placement in systemic sclerosis: a case series. Semin Arthritis Rheum. 2016;46:196–9. 10.1016/j.semarthrit.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Farge D, Burt RK, Oliveira MC, Mousseaux E, Rovira M, Marjanovic Z, et al. Cardiopulmonary assessment of patients with systemic sclerosis for hematopoietic stem cell transplantation: recommendations from the European Society for Blood and Marrow Transplantation Autoimmune Diseases Working Party and collaborating partners. Bone Marrow Transplant. 2017;52:1495–503. 10.1038/bmt.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Valentini G, Huscher D, Riccardi A, Fasano S, Irace R, Messiniti V, et al. Vasodilators and low-dose acetylsalicylic acid are associated with a lower incidence of distinct primary myocardial disease manifestations in systemic sclerosis: results of the DeSScipher inception cohort study. Ann Rheum Dis. 2019. 10.1136/annrheumdis-2019-215486. [DOI] [PubMed] [Google Scholar]
- 136.Benatti FB, Pedersen BK. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat Rev Rheumatol. 2015;11:86–97. 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 137.Wermuth PJ, Piera-Velazquez S, Rosenbloom J, Jimenez SA. Existing and novel biomarkers for precision medicine in systemic sclerosis. Nat Rev Rheumatol. 2018;14:421–32. 10.1038/s41584-018-0021-9. [DOI] [PubMed] [Google Scholar]
- 138.Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol. 2017;69:1718–34. 10.1016/j.jacc.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 139.Mercurio V, Bianco A, Campi G, Cuomo A, Diab N, Mancini A. New drugs, therapeutic strategies, and future direction for the treatment of pulmonary arterial hypertension. Curr Med Chem. 2019;26:2844–64. 10.2174/0929867325666180201095743. [DOI] [PubMed] [Google Scholar]
- 140.Tampakakis E, Shah SJ, Borlaug BA, Leary PJ, Patel HH, Miller WL, et al. Pulmonary effective arterial elastance as a measure of right ventricular afterload and its prognostic value in pulmonary hypertension due to left heart disease. Circ Heart Fail. 2018;11:e004436. 10.1161/CIRCHEARTFAILURE.117.004436. [DOI] [PMC free article] [PubMed] [Google Scholar]


