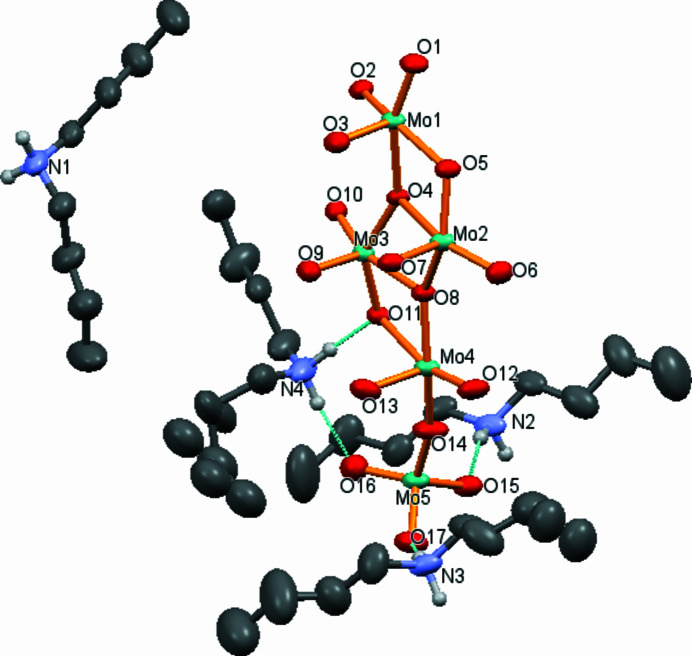
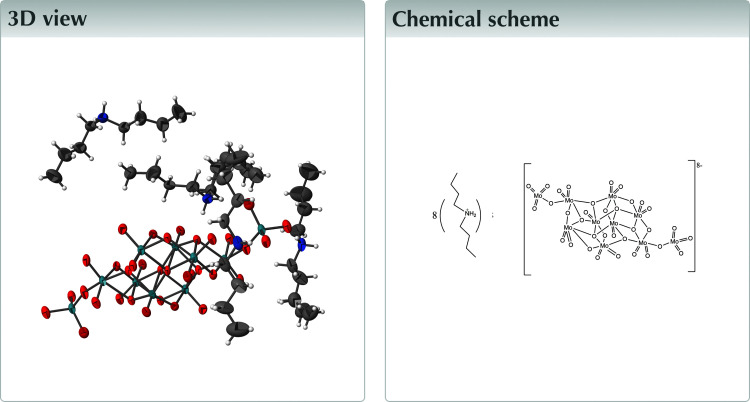
(C8H20N)8[Mo10O34] comprises a centrosymmetric decamolybdate polyanion linked through N—H⋯O hydrogen bonds to dibutylammonium counter-cations.
Keywords: crystal structure, polyoxidometalate, β-octamolybdate, hybrid compound
Abstract
In the title salt, (C8H20N)8[Mo10O34], the [Mo10O34]8− polyanion is located about an inversion centre and can be considered as a β-type octamolybdate anion to which two additional MoO4 tetrahedra are linked via common corners. The [Mo10O34]8− polyanions are packed in rows extending parallel to [001] and are connected to the dibutylammonium counter-cations through N—H⋯O hydrogen-bonding interactions.
Structure description
Polyoxometalates (POMs) are obtained by self-assembly of transition-metal oxide units [MO n ] p− in acidic media (M = metal; n = 3, 4, 6, ⋯; p = 0, 1, 2, 3, ⋯). POMs and their derivatives are an important group of materials that have attracted considerable interest in areas such as electrochemistry (Zhang et al., 2021 ▸), materials science (Hao et al., 2007 ▸; Li et al., 2007 ▸), and medicine (Cronin et al., 2002 ▸; Müller et al., 1999 ▸). In recent years, research on organic–inorganic hybrid POMs has experienced significant growth, supported by possible modifications and/or functionalizations of the oxide surface of the POM with preselected organic moieties (Xu et al., 2003 ▸). The structural diversity of the corresponding isopolyoxomolybdates is due to characteristic large polyanionic units and organic ammonium cations, which consolidate the crystal structures through non-covalent supramolecular interactions. In this regard, several octamolybdate polyanions [Mo8O26]4–, charge-balanced by organic counter-ions, have been synthesized and structurally characterized (Allis et al., 2004 ▸; Harchani & Haddad, 2015 ▸). For the current study, we used diisobutylammonium as a counter-cation and obtained the hybrid organic–inorganic decamolybdate (C8H20N)8[Mo10O34].
The asymmetric unit of (C8H20N)8[Mo10O34] is shown in Fig. 1 ▸. The [Mo10O34]8– anion is located about an inversion centre and is displayed in Fig. 2 ▸. Such kind of decamolybdate anion is known from other ammonium salts and has been reported for the first time for (NH4)8[Mo10O34] (Fuchs et al., 1975 ▸). The [Mo10O34]8– anion can be considered as a β-type octamolydate to which two additional MoO4 tetrahedra are added via vertex-sharing. Two types of β-octamolybdate anions can be distinguished, type A with the general formula [Mo8O26]4– and type B with the general formula [H x Mo8O28](8–x) (Pavani et al., 2007 ▸). Thus, the [Mo10O34]8– anion of the title compound can be considered as of the β-octamolybdate B type (Du et al. 2011 ▸; Isobe et al. 1978 ▸).
Figure 1.
The asymmetric unit of the title compound. Displacement ellipsoids are drawn at the 50% probability level. Dotted lines indicate N—H⋯O hydrogen-bonding interactions. The C-bound H atoms are omitted for clarity.
Figure 2.
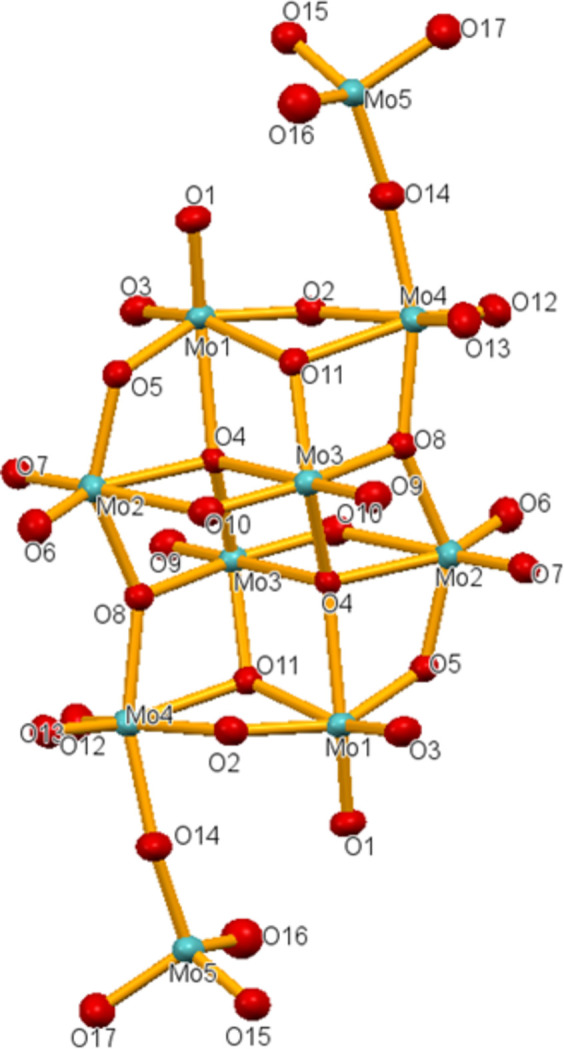
The centrosymmetric [Mo10O34]8– polyanion in the title compound. Displacement ellipsoids are drawn at the 50% probability level.
The [Mo10O34]8– polyanion is made up of eight MoO6 octahedra linked to each other by edge and/or vertex sharing, building up an octamolybdate anion. Similar POMs with an Mo8 core linked to the ends by Mo x O y groups are found in the crystal structures of [NH3(CH2)2NH2(CH2)2NH3]2[Mo9O30] and [NH3(CH2)2NH2(CH2)3NH3]2[Mo10O33] (Chakrabarti & Natarajan, 2002 ▸). In (C8H20N)8[Mo10O34], the β-octamolybdate polyanion is linked with two additional MoO4 tetrahedra, which can be expressed by the formula [(MoO3)2 β-Mo8O28]8–. Bond-valence calculations show that the five crystallographically unique Mo atoms are in the +VI oxidation state. According to the role of the oxygen ligands (terminal or bridging) in the β-octamolybdate moiety, the corresponding Mo—O bond lengths for Mo1–Mo4 range from 1.703 (3) to 2.451 (3) Å and the O—Mo—O bond angles from 71.01 (12) to 179.57 (14)°. These values are in the range expected for octahedrally coordinated MoVI atoms and in agreement with those in the previously reported octamolybdate structure. (Pavani & Ramanan, 2005 ▸; Wu et al., 2002 ▸). The Mo5 site is tetrahedrally surrounded by three terminal oxygen atoms (O15, O16, O17) with bond lengths between 1.738 (4) and 1.767 (4) Å and a bridging oxygen atom O14 to the β-octamolybdate anion with 1.804 (4) Ā. The angles of the tetrahedron range from 107.5 (2) to 112.0 (2)°.
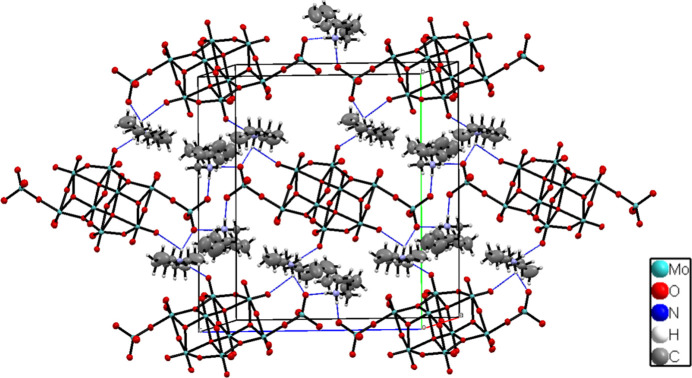
In the crystal, the [Mo10O34]8– polyanions are stacked into rows parallel to [001] and surrounded by dibutylammonium counter-cations. Next to Coulombic interactions, cations and anions are linked through rather strong N—H⋯O hydrogen bonds between the ammonium cations and the terminal oxygen atoms of the MoO4 tetrahedra (O15⋯H3A—N3 and O17⋯H3B—N3; Table 1 ▸, Fig. 3 ▸). The other ammonium groups are involved in hydrogen-bonding interactions with the terminal O atoms of the β-octamolybdate moiety (Table 1 ▸).
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1A⋯O2i | 0.91 | 1.72 | 2.627 (6) | 172 |
| N1—H1B⋯O10i | 0.91 | 1.97 | 2.755 (6) | 143 |
| N2—H2C⋯O15 | 0.91 | 1.91 | 2.778 (6) | 160 |
| N2—H2D⋯O7ii | 0.91 | 1.90 | 2.812 (5) | 176 |
| N3—H3A⋯O15iii | 0.91 | 1.87 | 2.770 (6) | 172 |
| N3—H3B⋯O17 | 0.91 | 1.88 | 2.786 (6) | 176 |
| N4—H4A⋯O11 | 0.91 | 1.80 | 2.695 (6) | 166 |
| N4—H4B⋯O16 | 0.91 | 1.86 | 2.762 (7) | 172 |
Symmetry codes: (i)
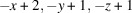 ; (ii)
; (ii)
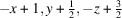 ; (iii)
; (iii)
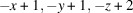 .
.
Figure 3.
The unit-cell packing viewed down [001] with hydrogen bonds indicated by blue dashed lines.
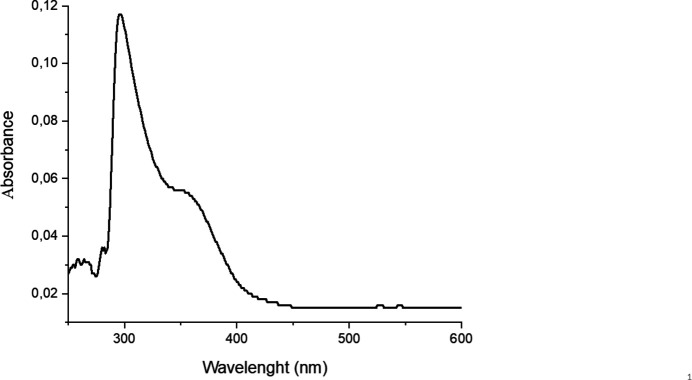
The UV-vis absorption spectrum of the title compound was recorded in the range 250–700 nm in aqueous solution (0.1 N) and is shown in Fig. 4 ▸. It shows two absorption bands at 297 nm and 353 nm. The strongest band at 297 nm is attributed to a charge-transfer transition of the type Ot —Mo and the shoulder peak at 353 nm to a charge-transfer transition of the type Mo—O—Mo (Gong et al., 2006 ▸; Zhang et al., 1997 ▸)
Figure 4.
UV/Vis spectrum of the title compound.
Synthesis and crystallization
Ammonium heptamolybdate, (NH4)6[Mo7O24]·4H2O (4.943 g), and dibutylamine, C8H19N (1.559 g), were dissolved in 40 ml of hot water. The mixture was heated for 2 h at 473 K under reflux and then filtered. The filtrate was kept for three months at ambient conditions, affording colourless crystals in about 8% yield (based on Mo).
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | (C8H20N)8[Mo10O34] |
| M r | 2545.39 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 130 |
| a, b, c (Å) | 14.21628 (18), 20.7477 (2), 18.2210 (2) |
| β (°) | 110.0785 (15) |
| V (Å3) | 5047.74 (12) |
| Z | 2 |
| Radiation type | Cu Kα |
| μ (mm−1) | 10.44 |
| Crystal size (mm) | 0.15 × 0.03 × 0.02 |
| Data collection | |
| Diffractometer | XtaLAB Synergy, Dualflex, HyPix |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2023 ▸) |
| T min, T max | 0.500, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 42024, 9853, 8753 |
| R int | 0.043 |
| (sin θ/λ)max (Å−1) | 0.625 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.045, 0.119, 1.02 |
| No. of reflections | 9853 |
| No. of parameters | 541 |
| No. of restraints | 18 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 1.65, −1.09 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624004632/wm4210sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624004632/wm4210Isup4.hkl
CCDC reference: 2332866
Additional supporting information: crystallographic information; 3D view; checkCIF report
full crystallographic data
Crystal data
| (C8H20N)8[Mo10O34] | F(000) = 2584 |
| Mr = 2545.39 | Dx = 1.675 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54184 Å |
| a = 14.21628 (18) Å | Cell parameters from 25536 reflections |
| b = 20.7477 (2) Å | θ = 3.3–76.7° |
| c = 18.2210 (2) Å | µ = 10.44 mm−1 |
| β = 110.0785 (15)° | T = 130 K |
| V = 5047.74 (12) Å3 | Needle, clear colourless |
| Z = 2 | 0.15 × 0.03 × 0.02 mm |
Data collection
| XtaLAB Synergy, Dualflex, HyPix diffractometer | 9853 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, PhotonJet (Cu) X-ray Source | 8753 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.043 |
| Detector resolution: 10.0000 pixels mm-1 | θmax = 74.5°, θmin = 3.3° |
| ω scans | h = −17→14 |
| Absorption correction: multi-scan (CrysAlisPro; Rigaku OD, 2023) | k = −24→24 |
| Tmin = 0.500, Tmax = 1.000 | l = −22→22 |
| 42024 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H-atom parameters constrained |
| wR(F2) = 0.119 | w = 1/[σ2(Fo2) + (0.0685P)2 + 16.9839P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max = 0.001 |
| 9853 reflections | Δρmax = 1.65 e Å−3 |
| 541 parameters | Δρmin = −1.09 e Å−3 |
| 18 restraints |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Mo1 | 0.46738 (3) | 0.39489 (2) | 0.35188 (2) | 0.02715 (10) | |
| Mo2 | 0.39496 (3) | 0.38023 (2) | 0.50285 (2) | 0.02920 (10) | |
| Mo3 | 0.60253 (3) | 0.46487 (2) | 0.57062 (2) | 0.02504 (10) | |
| Mo4 | 0.50325 (3) | 0.45478 (2) | 0.70777 (2) | 0.02984 (10) | |
| Mo5 | 0.60971 (3) | 0.54579 (2) | 0.89668 (2) | 0.03279 (11) | |
| O1 | 0.3980 (3) | 0.37372 (16) | 0.25734 (19) | 0.0367 (8) | |
| O2 | 0.5564 (3) | 0.45633 (15) | 0.33548 (19) | 0.0323 (7) | |
| O3 | 0.5479 (3) | 0.33261 (16) | 0.3883 (2) | 0.0358 (7) | |
| O4 | 0.5043 (2) | 0.43977 (14) | 0.47119 (17) | 0.0248 (6) | |
| O5 | 0.3602 (3) | 0.37118 (15) | 0.39495 (18) | 0.0298 (7) | |
| O6 | 0.2865 (3) | 0.37049 (17) | 0.5231 (2) | 0.0386 (8) | |
| O7 | 0.4603 (3) | 0.30910 (16) | 0.53384 (19) | 0.0343 (7) | |
| O8 | 0.4764 (2) | 0.43107 (15) | 0.59639 (18) | 0.0284 (6) | |
| O9 | 0.6765 (3) | 0.39936 (16) | 0.60011 (19) | 0.0345 (7) | |
| O10 | 0.6746 (2) | 0.51676 (16) | 0.53399 (19) | 0.0305 (7) | |
| O11 | 0.6152 (2) | 0.50842 (14) | 0.66534 (18) | 0.0284 (6) | |
| O12 | 0.3977 (3) | 0.41825 (18) | 0.7121 (2) | 0.0425 (9) | |
| O13 | 0.5989 (3) | 0.39987 (17) | 0.7488 (2) | 0.0419 (8) | |
| O14 | 0.5422 (4) | 0.50320 (18) | 0.8079 (2) | 0.0483 (10) | |
| O15 | 0.5518 (3) | 0.61967 (17) | 0.9041 (2) | 0.0406 (8) | |
| O16 | 0.7336 (3) | 0.5584 (2) | 0.9045 (2) | 0.0486 (9) | |
| O17 | 0.6081 (3) | 0.49797 (18) | 0.9747 (2) | 0.0473 (9) | |
| N1 | 1.2542 (4) | 0.5261 (3) | 0.5803 (3) | 0.0528 (13) | |
| H1A | 1.317838 | 0.533197 | 0.613310 | 0.063* | |
| H1B | 1.257981 | 0.498154 | 0.542852 | 0.063* | |
| C1 | 1.0075 (8) | 0.6326 (6) | 0.3483 (6) | 0.103 (4) | |
| H1C | 1.003411 | 0.670808 | 0.315617 | 0.155* | |
| H1D | 0.944901 | 0.627889 | 0.358998 | 0.155* | |
| H1E | 1.018427 | 0.594290 | 0.320836 | 0.155* | |
| C2 | 1.0942 (7) | 0.6403 (5) | 0.4248 (5) | 0.087 (3) | |
| H2A | 1.152069 | 0.659291 | 0.414106 | 0.104* | |
| H2B | 1.074403 | 0.670627 | 0.458930 | 0.104* | |
| C3 | 1.1249 (5) | 0.5784 (4) | 0.4665 (4) | 0.068 (2) | |
| H3C | 1.143849 | 0.547696 | 0.432336 | 0.081* | |
| H3D | 1.067847 | 0.559794 | 0.478613 | 0.081* | |
| C4 | 1.2132 (5) | 0.5878 (4) | 0.5420 (4) | 0.0581 (16) | |
| H4C | 1.266756 | 0.611691 | 0.530394 | 0.070* | |
| H4D | 1.191445 | 0.614235 | 0.578467 | 0.070* | |
| C5 | 1.1975 (5) | 0.4946 (4) | 0.6249 (4) | 0.0613 (17) | |
| H5A | 1.217167 | 0.448646 | 0.633571 | 0.074* | |
| H5B | 1.124920 | 0.496506 | 0.594592 | 0.074* | |
| C6 | 1.2188 (7) | 0.5285 (5) | 0.7041 (5) | 0.078 (2) | |
| H6A | 1.292066 | 0.533544 | 0.729856 | 0.093* | |
| H6B | 1.188595 | 0.572041 | 0.695217 | 0.093* | |
| C7 | 1.1781 (8) | 0.4919 (6) | 0.7566 (6) | 0.097 (3) | |
| H7A | 1.204890 | 0.447427 | 0.763302 | 0.116* | |
| H7B | 1.104202 | 0.489454 | 0.733008 | 0.116* | |
| C8 | 1.2075 (11) | 0.5256 (7) | 0.8371 (6) | 0.132 (5) | |
| H8A | 1.164148 | 0.563035 | 0.833396 | 0.197* | |
| H8B | 1.277455 | 0.539697 | 0.853090 | 0.197* | |
| H8C | 1.199623 | 0.495291 | 0.875880 | 0.197* | |
| N2 | 0.5610 (4) | 0.7400 (2) | 0.8400 (3) | 0.0435 (11) | |
| H2C | 0.558726 | 0.697106 | 0.849875 | 0.052* | |
| H2D | 0.551162 | 0.761770 | 0.880137 | 0.052* | |
| C9 | 0.9281 (8) | 0.7162 (6) | 0.9733 (10) | 0.136 (5) | |
| H9A | 0.925536 | 0.730830 | 1.023715 | 0.204* | |
| H9B | 0.993739 | 0.726632 | 0.969919 | 0.204* | |
| H9C | 0.917704 | 0.669441 | 0.968751 | 0.204* | |
| C10 | 0.8498 (7) | 0.7484 (4) | 0.9101 (6) | 0.083 (3) | |
| H10A | 0.856978 | 0.736603 | 0.859627 | 0.099* | |
| H10B | 0.858736 | 0.795591 | 0.916640 | 0.099* | |
| C11 | 0.7436 (5) | 0.7304 (3) | 0.9077 (4) | 0.0569 (16) | |
| H11A | 0.737917 | 0.682872 | 0.908117 | 0.068* | |
| H11B | 0.733897 | 0.747021 | 0.955539 | 0.068* | |
| C12 | 0.6631 (5) | 0.7559 (3) | 0.8388 (4) | 0.0496 (14) | |
| H12A | 0.670511 | 0.737780 | 0.790857 | 0.059* | |
| H12B | 0.669932 | 0.803313 | 0.837023 | 0.059* | |
| C13 | 0.4791 (5) | 0.7553 (3) | 0.7679 (3) | 0.0521 (15) | |
| H13A | 0.487501 | 0.730037 | 0.724489 | 0.063* | |
| H13B | 0.482772 | 0.801585 | 0.755759 | 0.063* | |
| C14 | 0.3794 (6) | 0.7416 (4) | 0.7727 (5) | 0.071 (2) | |
| H14A | 0.369298 | 0.768421 | 0.814244 | 0.085* | |
| H14B | 0.376330 | 0.695772 | 0.786885 | 0.085* | |
| C15 | 0.2959 (6) | 0.7553 (4) | 0.6953 (6) | 0.085 (3) | |
| H15A | 0.301443 | 0.800569 | 0.680112 | 0.102* | |
| H15B | 0.305073 | 0.727183 | 0.654378 | 0.102* | |
| C16 | 0.1939 (9) | 0.7446 (7) | 0.6986 (9) | 0.138 (5) | |
| H16A | 0.187367 | 0.767691 | 0.743506 | 0.207* | |
| H16B | 0.183356 | 0.698427 | 0.703895 | 0.207* | |
| H16C | 0.143702 | 0.760723 | 0.650403 | 0.207* | |
| N3 | 0.5165 (4) | 0.3786 (2) | 0.9706 (3) | 0.0484 (12) | |
| H3A | 0.498144 | 0.376434 | 1.013761 | 0.058* | |
| H3B | 0.548926 | 0.416775 | 0.972730 | 0.058* | |
| C18 | 0.2616 (9) | 0.3213 (6) | 0.8363 (6) | 0.105 (3) | |
| H18A | 0.261341 | 0.352706 | 0.795295 | 0.126* | 0.333 (15) |
| H18B | 0.244014 | 0.278560 | 0.811063 | 0.126* | 0.333 (15) |
| H18C | 0.227094 | 0.359599 | 0.847226 | 0.126* | 0.667 (15) |
| H18D | 0.267273 | 0.327310 | 0.784033 | 0.126* | 0.667 (15) |
| C19 | 0.3625 (8) | 0.3181 (4) | 0.8945 (6) | 0.091 (3) | |
| H19A | 0.398549 | 0.281599 | 0.881310 | 0.110* | |
| H19B | 0.356831 | 0.309039 | 0.946131 | 0.110* | |
| C20 | 0.4248 (7) | 0.3793 (4) | 0.9009 (4) | 0.0668 (19) | |
| H20A | 0.443553 | 0.383491 | 0.853603 | 0.080* | |
| H20B | 0.383793 | 0.417232 | 0.903352 | 0.080* | |
| C21 | 0.5880 (6) | 0.3257 (4) | 0.9748 (6) | 0.080 (2) | |
| H21A | 0.558598 | 0.284238 | 0.983043 | 0.096* | |
| H21B | 0.600695 | 0.322988 | 0.924752 | 0.096* | |
| C22 | 0.6830 (8) | 0.3368 (6) | 1.0391 (7) | 0.102 (3) | |
| H22A | 0.668767 | 0.339739 | 1.088525 | 0.123* | |
| H22B | 0.710307 | 0.378925 | 1.030655 | 0.123* | |
| C23 | 0.7605 (11) | 0.2871 (8) | 1.0484 (10) | 0.156 (6) | |
| H23A | 0.775827 | 0.284082 | 0.999411 | 0.187* | |
| H23B | 0.734182 | 0.244800 | 1.057416 | 0.187* | |
| C24 | 0.8592 (12) | 0.3022 (9) | 1.1178 (9) | 0.163 (7) | |
| H24A | 0.891581 | 0.340187 | 1.105114 | 0.245* | |
| H24B | 0.904591 | 0.265168 | 1.126657 | 0.245* | |
| H24C | 0.843257 | 0.310613 | 1.165148 | 0.245* | |
| C17X | 0.189 (2) | 0.3390 (16) | 0.868 (2) | 0.106 (5) | 0.333 (15) |
| H17A | 0.124953 | 0.344533 | 0.825605 | 0.159* | 0.333 (15) |
| H17B | 0.208992 | 0.379747 | 0.896216 | 0.159* | 0.333 (15) |
| H17C | 0.183507 | 0.305427 | 0.903659 | 0.159* | 0.333 (15) |
| C17 | 0.2031 (13) | 0.2674 (8) | 0.8340 (12) | 0.106 (5) | 0.667 (15) |
| H17D | 0.234106 | 0.229559 | 0.819533 | 0.159* | 0.667 (15) |
| H17E | 0.136177 | 0.273997 | 0.795289 | 0.159* | 0.667 (15) |
| H17F | 0.197592 | 0.260561 | 0.885594 | 0.159* | 0.667 (15) |
| N4 | 0.7926 (4) | 0.5318 (3) | 0.7782 (3) | 0.0639 (17) | |
| H4A | 0.733073 | 0.530722 | 0.737974 | 0.077* | |
| H4B | 0.779178 | 0.542145 | 0.822093 | 0.077* | |
| C25 | 0.9612 (7) | 0.6165 (5) | 0.5961 (6) | 0.088 (3) | |
| H25A | 0.897792 | 0.615423 | 0.552036 | 0.132* | |
| H25B | 0.997253 | 0.575933 | 0.598168 | 0.132* | |
| H25C | 1.002122 | 0.652488 | 0.589347 | 0.132* | |
| C26 | 0.9400 (8) | 0.6254 (5) | 0.6717 (7) | 0.097 (3) | |
| H26A | 1.004535 | 0.628966 | 0.715395 | 0.116* | |
| H26B | 0.903308 | 0.666367 | 0.668837 | 0.116* | |
| C27 | 0.8807 (5) | 0.5722 (4) | 0.6890 (4) | 0.0587 (17) | |
| H27A | 0.919594 | 0.531675 | 0.695964 | 0.070* | |
| H27B | 0.818304 | 0.566349 | 0.643671 | 0.070* | |
| C28 | 0.8530 (6) | 0.5842 (5) | 0.7627 (5) | 0.076 (2) | |
| H28A | 0.915332 | 0.588959 | 0.808361 | 0.091* | |
| H28B | 0.815198 | 0.625050 | 0.756318 | 0.091* | |
| C29 | 0.8358 (6) | 0.4650 (5) | 0.7886 (5) | 0.084 (3) | |
| H29C | 0.851243 | 0.451059 | 0.742069 | 0.100* | 0.354 (12) |
| H29D | 0.789472 | 0.433582 | 0.798999 | 0.100* | 0.354 (12) |
| H29A | 0.787341 | 0.437231 | 0.801932 | 0.100* | 0.646 (12) |
| H29B | 0.834884 | 0.450959 | 0.736475 | 0.100* | 0.646 (12) |
| C30 | 0.9371 (9) | 0.4479 (14) | 0.8450 (7) | 0.116 (9) | 0.646 (12) |
| H30A | 0.951345 | 0.402023 | 0.838008 | 0.140* | 0.646 (12) |
| H30B | 0.988524 | 0.474333 | 0.833678 | 0.140* | 0.646 (12) |
| C31 | 0.9435 (14) | 0.4588 (10) | 0.9272 (9) | 0.118 (4) | 0.646 (12) |
| H31A | 0.928863 | 0.504727 | 0.933306 | 0.142* | 0.646 (12) |
| H31B | 1.013140 | 0.450351 | 0.961847 | 0.142* | 0.646 (12) |
| C32 | 0.8742 (14) | 0.4181 (10) | 0.9538 (11) | 0.118 (4) | 0.646 (12) |
| H32A | 0.811153 | 0.411778 | 0.910258 | 0.178* | 0.646 (12) |
| H32B | 0.860589 | 0.439740 | 0.996901 | 0.178* | 0.646 (12) |
| H32C | 0.905380 | 0.376152 | 0.971561 | 0.178* | 0.646 (12) |
| C30X | 0.930 (2) | 0.4727 (16) | 0.8584 (18) | 0.116 (9) | 0.354 (12) |
| H30C | 0.914077 | 0.496308 | 0.900096 | 0.140* | 0.354 (12) |
| H30D | 0.979275 | 0.498075 | 0.843703 | 0.140* | 0.354 (12) |
| C31X | 0.973 (2) | 0.4078 (16) | 0.888 (2) | 0.118 (4) | 0.354 (12) |
| H31C | 1.040851 | 0.413603 | 0.926996 | 0.142* | 0.354 (12) |
| H31D | 0.978545 | 0.382011 | 0.844473 | 0.142* | 0.354 (12) |
| C32X | 0.910 (2) | 0.3715 (18) | 0.926 (2) | 0.118 (4) | 0.354 (12) |
| H32D | 0.846812 | 0.358446 | 0.886039 | 0.178* | 0.354 (12) |
| H32E | 0.895563 | 0.399259 | 0.964503 | 0.178* | 0.354 (12) |
| H32F | 0.946241 | 0.333117 | 0.952337 | 0.178* | 0.354 (12) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Mo1 | 0.0428 (2) | 0.01922 (17) | 0.02010 (17) | 0.00043 (13) | 0.01172 (15) | −0.00267 (12) |
| Mo2 | 0.0424 (2) | 0.02309 (19) | 0.02335 (18) | −0.00671 (14) | 0.01292 (15) | −0.00140 (13) |
| Mo3 | 0.03428 (19) | 0.01990 (17) | 0.02015 (17) | 0.00191 (13) | 0.00831 (14) | −0.00127 (12) |
| Mo4 | 0.0462 (2) | 0.02416 (19) | 0.01977 (18) | −0.00317 (14) | 0.01209 (15) | −0.00028 (13) |
| Mo5 | 0.0532 (2) | 0.02420 (19) | 0.02242 (19) | −0.00053 (15) | 0.01481 (17) | −0.00126 (13) |
| O1 | 0.056 (2) | 0.0296 (17) | 0.0240 (16) | −0.0013 (15) | 0.0126 (15) | −0.0059 (13) |
| O2 | 0.0432 (19) | 0.0299 (17) | 0.0275 (16) | 0.0011 (13) | 0.0169 (14) | −0.0018 (13) |
| O3 | 0.052 (2) | 0.0268 (17) | 0.0303 (17) | 0.0063 (14) | 0.0166 (15) | −0.0031 (13) |
| O4 | 0.0383 (16) | 0.0193 (14) | 0.0198 (14) | −0.0021 (12) | 0.0137 (12) | 0.0006 (11) |
| O5 | 0.0451 (18) | 0.0216 (15) | 0.0237 (15) | −0.0047 (13) | 0.0130 (14) | −0.0026 (12) |
| O6 | 0.047 (2) | 0.0343 (19) | 0.0380 (19) | −0.0090 (15) | 0.0195 (16) | −0.0030 (15) |
| O7 | 0.052 (2) | 0.0274 (17) | 0.0259 (16) | −0.0035 (14) | 0.0164 (15) | 0.0004 (13) |
| O8 | 0.0424 (18) | 0.0222 (15) | 0.0220 (15) | −0.0007 (13) | 0.0130 (13) | 0.0001 (12) |
| O9 | 0.0438 (19) | 0.0296 (17) | 0.0290 (17) | 0.0063 (14) | 0.0110 (14) | −0.0011 (13) |
| O10 | 0.0340 (17) | 0.0302 (17) | 0.0277 (16) | 0.0014 (13) | 0.0111 (13) | −0.0039 (13) |
| O11 | 0.0402 (17) | 0.0216 (15) | 0.0224 (15) | −0.0015 (12) | 0.0097 (13) | −0.0010 (12) |
| O12 | 0.065 (2) | 0.037 (2) | 0.0317 (18) | −0.0111 (17) | 0.0241 (17) | −0.0015 (15) |
| O13 | 0.061 (2) | 0.0318 (18) | 0.0295 (18) | −0.0034 (16) | 0.0103 (16) | 0.0049 (14) |
| O14 | 0.086 (3) | 0.0331 (19) | 0.0272 (18) | −0.0084 (19) | 0.0214 (19) | −0.0041 (15) |
| O15 | 0.062 (2) | 0.0308 (18) | 0.0322 (18) | 0.0013 (16) | 0.0202 (17) | −0.0009 (14) |
| O16 | 0.057 (2) | 0.044 (2) | 0.045 (2) | 0.0032 (18) | 0.0185 (19) | −0.0007 (18) |
| O17 | 0.076 (3) | 0.0330 (19) | 0.0311 (19) | −0.0027 (18) | 0.0162 (18) | 0.0021 (15) |
| N1 | 0.042 (3) | 0.078 (4) | 0.041 (3) | 0.005 (2) | 0.017 (2) | −0.016 (3) |
| C1 | 0.089 (7) | 0.137 (9) | 0.067 (6) | 0.042 (6) | 0.005 (5) | −0.010 (6) |
| C2 | 0.082 (6) | 0.102 (7) | 0.070 (5) | 0.033 (5) | 0.018 (4) | −0.002 (5) |
| C3 | 0.054 (4) | 0.088 (6) | 0.060 (4) | 0.018 (4) | 0.017 (3) | −0.008 (4) |
| C4 | 0.046 (3) | 0.082 (5) | 0.048 (3) | 0.009 (3) | 0.018 (3) | −0.009 (3) |
| C5 | 0.052 (4) | 0.080 (5) | 0.054 (4) | 0.003 (3) | 0.021 (3) | −0.007 (3) |
| C6 | 0.091 (6) | 0.087 (6) | 0.068 (5) | 0.018 (5) | 0.043 (5) | −0.006 (4) |
| C7 | 0.088 (6) | 0.138 (9) | 0.069 (6) | 0.022 (6) | 0.034 (5) | 0.010 (6) |
| C8 | 0.148 (11) | 0.186 (13) | 0.058 (6) | 0.072 (10) | 0.031 (6) | −0.005 (7) |
| N2 | 0.075 (3) | 0.024 (2) | 0.035 (2) | 0.003 (2) | 0.022 (2) | −0.0035 (17) |
| C9 | 0.067 (6) | 0.115 (10) | 0.221 (17) | −0.008 (6) | 0.044 (8) | 0.018 (10) |
| C10 | 0.080 (5) | 0.057 (5) | 0.125 (8) | −0.010 (4) | 0.051 (6) | 0.006 (5) |
| C11 | 0.071 (4) | 0.034 (3) | 0.075 (5) | −0.003 (3) | 0.037 (4) | 0.000 (3) |
| C12 | 0.082 (4) | 0.028 (3) | 0.051 (3) | −0.007 (3) | 0.039 (3) | −0.008 (2) |
| C13 | 0.082 (4) | 0.033 (3) | 0.039 (3) | 0.007 (3) | 0.018 (3) | −0.007 (2) |
| C14 | 0.089 (5) | 0.044 (4) | 0.064 (5) | −0.003 (3) | 0.005 (4) | −0.002 (3) |
| C15 | 0.070 (5) | 0.064 (5) | 0.095 (7) | 0.000 (4) | −0.005 (4) | −0.012 (4) |
| C16 | 0.086 (8) | 0.120 (10) | 0.179 (15) | −0.008 (7) | 0.010 (8) | 0.010 (10) |
| N3 | 0.076 (3) | 0.040 (3) | 0.042 (3) | −0.004 (2) | 0.035 (3) | −0.001 (2) |
| C18 | 0.103 (8) | 0.138 (10) | 0.080 (7) | 0.005 (7) | 0.038 (6) | 0.004 (6) |
| C19 | 0.108 (7) | 0.066 (5) | 0.074 (6) | −0.014 (5) | −0.002 (5) | 0.000 (4) |
| C20 | 0.103 (6) | 0.054 (4) | 0.042 (4) | 0.008 (4) | 0.023 (4) | 0.000 (3) |
| C21 | 0.071 (5) | 0.064 (5) | 0.109 (7) | 0.000 (4) | 0.035 (5) | −0.018 (5) |
| C22 | 0.090 (7) | 0.112 (8) | 0.100 (8) | 0.013 (6) | 0.026 (6) | 0.019 (6) |
| C23 | 0.120 (10) | 0.151 (13) | 0.176 (15) | 0.045 (9) | 0.025 (10) | −0.044 (11) |
| C24 | 0.143 (13) | 0.201 (18) | 0.128 (12) | 0.051 (12) | 0.023 (10) | −0.022 (12) |
| C17X | 0.094 (10) | 0.100 (10) | 0.135 (14) | −0.002 (8) | 0.053 (9) | 0.015 (9) |
| C17 | 0.094 (10) | 0.100 (10) | 0.135 (14) | −0.002 (8) | 0.053 (9) | 0.015 (9) |
| N4 | 0.040 (3) | 0.114 (5) | 0.038 (3) | −0.007 (3) | 0.013 (2) | −0.019 (3) |
| C25 | 0.063 (5) | 0.129 (8) | 0.076 (6) | −0.003 (5) | 0.029 (4) | 0.022 (5) |
| C26 | 0.090 (6) | 0.107 (8) | 0.106 (8) | −0.035 (6) | 0.051 (6) | −0.028 (6) |
| C27 | 0.039 (3) | 0.085 (5) | 0.047 (3) | 0.001 (3) | 0.009 (3) | −0.007 (3) |
| C28 | 0.055 (4) | 0.116 (7) | 0.057 (4) | −0.021 (4) | 0.019 (3) | −0.034 (4) |
| C29 | 0.058 (4) | 0.131 (8) | 0.065 (5) | 0.012 (5) | 0.025 (4) | 0.019 (5) |
| C30 | 0.058 (6) | 0.20 (2) | 0.088 (10) | 0.030 (9) | 0.021 (6) | 0.058 (12) |
| C31 | 0.110 (9) | 0.138 (12) | 0.098 (9) | 0.018 (7) | 0.023 (6) | 0.028 (8) |
| C32 | 0.110 (9) | 0.138 (12) | 0.098 (9) | 0.018 (7) | 0.023 (6) | 0.028 (8) |
| C30X | 0.058 (6) | 0.20 (2) | 0.088 (10) | 0.030 (9) | 0.021 (6) | 0.058 (12) |
| C31X | 0.110 (9) | 0.138 (12) | 0.098 (9) | 0.018 (7) | 0.023 (6) | 0.028 (8) |
| C32X | 0.110 (9) | 0.138 (12) | 0.098 (9) | 0.018 (7) | 0.023 (6) | 0.028 (8) |
Geometric parameters (Å, º)
| Mo1—O1 | 1.723 (3) | C15—H15B | 0.9900 |
| Mo1—O2 | 1.891 (3) | C15—C16 | 1.489 (16) |
| Mo1—O3 | 1.703 (3) | C16—H16A | 0.9800 |
| Mo1—O4 | 2.255 (3) | C16—H16B | 0.9800 |
| Mo1—O5 | 2.001 (3) | C16—H16C | 0.9800 |
| Mo1—O11i | 2.291 (3) | N3—H3A | 0.9100 |
| Mo2—O4 | 2.211 (3) | N3—H3B | 0.9100 |
| Mo2—O5 | 1.865 (3) | N3—C20 | 1.475 (10) |
| Mo2—O6 | 1.717 (4) | N3—C21 | 1.481 (9) |
| Mo2—O7 | 1.732 (3) | C18—H18A | 0.9900 |
| Mo2—O8 | 2.001 (3) | C18—H18B | 0.9900 |
| Mo2—O10i | 2.354 (3) | C18—H18C | 0.9900 |
| Mo3—O4 | 1.941 (3) | C18—H18D | 0.9900 |
| Mo3—O4i | 2.451 (3) | C18—C19 | 1.463 (14) |
| Mo3—O8 | 2.122 (3) | C18—C17X | 1.386 (18) |
| Mo3—O9 | 1.690 (3) | C18—C17 | 1.386 (16) |
| Mo3—O10 | 1.767 (3) | C19—H19A | 0.9900 |
| Mo3—O11 | 1.901 (3) | C19—H19B | 0.9900 |
| Mo4—O2i | 2.070 (3) | C19—C20 | 1.529 (12) |
| Mo4—O8 | 1.994 (3) | C20—H20A | 0.9900 |
| Mo4—O11 | 2.284 (3) | C20—H20B | 0.9900 |
| Mo4—O12 | 1.706 (4) | C21—H21A | 0.9900 |
| Mo4—O13 | 1.734 (4) | C21—H21B | 0.9900 |
| Mo4—O14 | 1.987 (4) | C21—C22 | 1.471 (14) |
| Mo5—O14 | 1.804 (4) | C22—H22A | 0.9900 |
| Mo5—O15 | 1.767 (4) | C22—H22B | 0.9900 |
| Mo5—O16 | 1.738 (4) | C22—C23 | 1.476 (16) |
| Mo5—O17 | 1.741 (4) | C23—H23A | 0.9900 |
| N1—H1A | 0.9100 | C23—H23B | 0.9900 |
| N1—H1B | 0.9100 | C23—C24 | 1.56 (2) |
| N1—C4 | 1.479 (9) | C24—H24A | 0.9800 |
| N1—C5 | 1.480 (9) | C24—H24B | 0.9800 |
| C1—H1C | 0.9800 | C24—H24C | 0.9800 |
| C1—H1D | 0.9800 | C17X—H17A | 0.9800 |
| C1—H1E | 0.9800 | C17X—H17B | 0.9800 |
| C1—C2 | 1.520 (12) | C17X—H17C | 0.9800 |
| C2—H2A | 0.9900 | C17—H17D | 0.9800 |
| C2—H2B | 0.9900 | C17—H17E | 0.9800 |
| C2—C3 | 1.480 (13) | C17—H17F | 0.9800 |
| C3—H3C | 0.9900 | N4—H4A | 0.9100 |
| C3—H3D | 0.9900 | N4—H4B | 0.9100 |
| C3—C4 | 1.523 (10) | N4—C28 | 1.471 (11) |
| C4—H4C | 0.9900 | N4—C29 | 1.500 (12) |
| C4—H4D | 0.9900 | C25—H25A | 0.9800 |
| C5—H5A | 0.9900 | C25—H25B | 0.9800 |
| C5—H5B | 0.9900 | C25—H25C | 0.9800 |
| C5—C6 | 1.540 (10) | C25—C26 | 1.518 (13) |
| C6—H6A | 0.9900 | C26—H26A | 0.9900 |
| C6—H6B | 0.9900 | C26—H26B | 0.9900 |
| C6—C7 | 1.485 (13) | C26—C27 | 1.488 (12) |
| C7—H7A | 0.9900 | C27—H27A | 0.9900 |
| C7—H7B | 0.9900 | C27—H27B | 0.9900 |
| C7—C8 | 1.547 (14) | C27—C28 | 1.543 (10) |
| C8—H8A | 0.9800 | C28—H28A | 0.9900 |
| C8—H8B | 0.9800 | C28—H28B | 0.9900 |
| C8—H8C | 0.9800 | C29—H29C | 0.9900 |
| N2—H2C | 0.9100 | C29—H29D | 0.9900 |
| N2—H2D | 0.9100 | C29—H29A | 0.9900 |
| N2—C12 | 1.496 (8) | C29—H29B | 0.9900 |
| N2—C13 | 1.461 (8) | C29—C30 | 1.497 (11) |
| C9—H9A | 0.9800 | C29—C30X | 1.502 (12) |
| C9—H9B | 0.9800 | C30—H30A | 0.9900 |
| C9—H9C | 0.9800 | C30—H30B | 0.9900 |
| C9—C10 | 1.460 (16) | C30—C31 | 1.486 (12) |
| C10—H10A | 0.9900 | C31—H31A | 0.9900 |
| C10—H10B | 0.9900 | C31—H31B | 0.9900 |
| C10—C11 | 1.541 (10) | C31—C32 | 1.500 (12) |
| C11—H11A | 0.9900 | C32—H32A | 0.9800 |
| C11—H11B | 0.9900 | C32—H32B | 0.9800 |
| C11—C12 | 1.476 (10) | C32—H32C | 0.9800 |
| C12—H12A | 0.9900 | C30X—H30C | 0.9900 |
| C12—H12B | 0.9900 | C30X—H30D | 0.9900 |
| C13—H13A | 0.9900 | C30X—C31X | 1.502 (13) |
| C13—H13B | 0.9900 | C31X—H31C | 0.9900 |
| C13—C14 | 1.477 (11) | C31X—H31D | 0.9900 |
| C14—H14A | 0.9900 | C31X—C32X | 1.502 (12) |
| C14—H14B | 0.9900 | C32X—H32D | 0.9800 |
| C14—C15 | 1.526 (11) | C32X—H32E | 0.9800 |
| C15—H15A | 0.9900 | C32X—H32F | 0.9800 |
| O1—Mo1—O2 | 101.45 (16) | N2—C13—H13B | 109.0 |
| O1—Mo1—O4 | 158.94 (15) | N2—C13—C14 | 112.9 (6) |
| O1—Mo1—O5 | 94.45 (16) | H13A—C13—H13B | 107.8 |
| O1—Mo1—O11i | 89.75 (14) | C14—C13—H13A | 109.0 |
| O2—Mo1—O4 | 85.83 (12) | C14—C13—H13B | 109.0 |
| O2—Mo1—O5 | 150.37 (13) | C13—C14—H14A | 109.3 |
| O2—Mo1—O11i | 74.19 (13) | C13—C14—H14B | 109.3 |
| O3—Mo1—O1 | 105.88 (17) | C13—C14—C15 | 111.6 (7) |
| O3—Mo1—O2 | 100.13 (16) | H14A—C14—H14B | 108.0 |
| O3—Mo1—O4 | 92.02 (14) | C15—C14—H14A | 109.3 |
| O3—Mo1—O5 | 99.31 (15) | C15—C14—H14B | 109.3 |
| O3—Mo1—O11i | 164.24 (14) | C14—C15—H15A | 108.9 |
| O4—Mo1—O11i | 73.09 (11) | C14—C15—H15B | 108.9 |
| O5—Mo1—O4 | 71.33 (12) | H15A—C15—H15B | 107.7 |
| O5—Mo1—O11i | 81.10 (12) | C16—C15—C14 | 113.3 (10) |
| O4—Mo2—O10i | 71.49 (11) | C16—C15—H15A | 108.9 |
| O5—Mo2—O4 | 74.75 (12) | C16—C15—H15B | 108.9 |
| O5—Mo2—O8 | 144.85 (13) | C15—C16—H16A | 109.5 |
| O5—Mo2—O10i | 81.80 (12) | C15—C16—H16B | 109.5 |
| O6—Mo2—O4 | 152.33 (15) | C15—C16—H16C | 109.5 |
| O6—Mo2—O5 | 106.39 (17) | H16A—C16—H16B | 109.5 |
| O6—Mo2—O7 | 104.51 (17) | H16A—C16—H16C | 109.5 |
| O6—Mo2—O8 | 100.31 (15) | H16B—C16—H16C | 109.5 |
| O6—Mo2—O10i | 81.24 (15) | H3A—N3—H3B | 107.4 |
| O7—Mo2—O4 | 102.40 (14) | C20—N3—H3A | 108.3 |
| O7—Mo2—O5 | 100.16 (15) | C20—N3—H3B | 108.3 |
| O7—Mo2—O8 | 94.73 (15) | C20—N3—C21 | 116.0 (6) |
| O7—Mo2—O10i | 172.96 (14) | C21—N3—H3A | 108.3 |
| O8—Mo2—O4 | 71.01 (12) | C21—N3—H3B | 108.3 |
| O8—Mo2—O10i | 80.09 (12) | H18A—C18—H18B | 107.8 |
| O4—Mo3—O4i | 75.88 (12) | H18C—C18—H18D | 107.6 |
| O4—Mo3—O8 | 74.10 (12) | C19—C18—H18A | 109.0 |
| O8—Mo3—O4i | 81.68 (11) | C19—C18—H18B | 109.0 |
| O9—Mo3—O4i | 179.57 (15) | C19—C18—H18C | 108.6 |
| O9—Mo3—O4 | 104.54 (15) | C19—C18—H18D | 108.6 |
| O9—Mo3—O8 | 98.32 (15) | C17X—C18—H18A | 109.0 |
| O9—Mo3—O10 | 103.39 (16) | C17X—C18—H18B | 109.0 |
| O9—Mo3—O11 | 103.82 (15) | C17X—C18—C19 | 113.1 (19) |
| O10—Mo3—O4 | 97.80 (14) | C17—C18—H18C | 108.6 |
| O10—Mo3—O4i | 76.62 (12) | C17—C18—H18D | 108.6 |
| O10—Mo3—O8 | 158.14 (14) | C17—C18—C19 | 114.5 (12) |
| O10—Mo3—O11 | 99.60 (14) | C18—C19—H19A | 108.6 |
| O11—Mo3—O4i | 75.77 (12) | C18—C19—H19B | 108.6 |
| O11—Mo3—O4 | 142.06 (14) | C18—C19—C20 | 114.7 (9) |
| O11—Mo3—O8 | 77.31 (13) | H19A—C19—H19B | 107.6 |
| O2i—Mo4—O11 | 71.28 (12) | C20—C19—H19A | 108.6 |
| O8—Mo4—O2i | 85.53 (13) | C20—C19—H19B | 108.6 |
| O8—Mo4—O11 | 71.79 (12) | N3—C20—C19 | 112.1 (6) |
| O12—Mo4—O2i | 99.29 (17) | N3—C20—H20A | 109.2 |
| O12—Mo4—O8 | 94.19 (15) | N3—C20—H20B | 109.2 |
| O12—Mo4—O11 | 163.32 (15) | C19—C20—H20A | 109.2 |
| O12—Mo4—O13 | 105.07 (19) | C19—C20—H20B | 109.2 |
| O12—Mo4—O14 | 98.93 (17) | H20A—C20—H20B | 107.9 |
| O13—Mo4—O2i | 155.20 (16) | N3—C21—H21A | 109.5 |
| O13—Mo4—O8 | 97.15 (15) | N3—C21—H21B | 109.5 |
| O13—Mo4—O11 | 86.08 (15) | H21A—C21—H21B | 108.1 |
| O13—Mo4—O14 | 90.34 (18) | C22—C21—N3 | 110.5 (8) |
| O14—Mo4—O2i | 81.24 (15) | C22—C21—H21A | 109.5 |
| O14—Mo4—O8 | 162.67 (14) | C22—C21—H21B | 109.5 |
| O14—Mo4—O11 | 93.27 (14) | C21—C22—H22A | 108.5 |
| O15—Mo5—O14 | 112.07 (19) | C21—C22—H22B | 108.5 |
| O16—Mo5—O14 | 111.0 (2) | C21—C22—C23 | 115.3 (11) |
| O16—Mo5—O15 | 110.43 (19) | H22A—C22—H22B | 107.5 |
| O16—Mo5—O17 | 108.5 (2) | C23—C22—H22A | 108.5 |
| O17—Mo5—O14 | 107.55 (18) | C23—C22—H22B | 108.5 |
| O17—Mo5—O15 | 107.13 (18) | C22—C23—H23A | 109.1 |
| Mo1—O2—Mo4i | 116.86 (17) | C22—C23—H23B | 109.1 |
| Mo1—O4—Mo3i | 96.86 (11) | C22—C23—C24 | 112.3 (12) |
| Mo2—O4—Mo1 | 93.97 (11) | H23A—C23—H23B | 107.9 |
| Mo2—O4—Mo3i | 96.67 (11) | C24—C23—H23A | 109.1 |
| Mo3—O4—Mo1 | 149.92 (16) | C24—C23—H23B | 109.1 |
| Mo3—O4—Mo2 | 104.56 (12) | C23—C24—H24A | 109.5 |
| Mo3—O4—Mo3i | 104.12 (12) | C23—C24—H24B | 109.5 |
| Mo2—O5—Mo1 | 115.25 (16) | C23—C24—H24C | 109.5 |
| Mo2—O8—Mo3 | 105.76 (13) | H24A—C24—H24B | 109.5 |
| Mo4—O8—Mo2 | 148.17 (17) | H24A—C24—H24C | 109.5 |
| Mo4—O8—Mo3 | 105.24 (14) | H24B—C24—H24C | 109.5 |
| Mo3—O10—Mo2i | 114.80 (15) | C18—C17X—H17A | 109.5 |
| Mo3—O11—Mo1i | 114.04 (14) | C18—C17X—H17B | 109.5 |
| Mo3—O11—Mo4 | 102.46 (13) | C18—C17X—H17C | 109.5 |
| Mo4—O11—Mo1i | 95.11 (12) | H17A—C17X—H17B | 109.5 |
| Mo5—O14—Mo4 | 165.0 (3) | H17A—C17X—H17C | 109.5 |
| H1A—N1—H1B | 107.4 | H17B—C17X—H17C | 109.5 |
| C4—N1—H1A | 108.2 | C18—C17—H17D | 109.5 |
| C4—N1—H1B | 108.2 | C18—C17—H17E | 109.5 |
| C4—N1—C5 | 116.2 (5) | C18—C17—H17F | 109.5 |
| C5—N1—H1A | 108.2 | H17D—C17—H17E | 109.5 |
| C5—N1—H1B | 108.2 | H17D—C17—H17F | 109.5 |
| H1C—C1—H1D | 109.5 | H17E—C17—H17F | 109.5 |
| H1C—C1—H1E | 109.5 | H4A—N4—H4B | 107.2 |
| H1D—C1—H1E | 109.5 | C28—N4—H4A | 107.9 |
| C2—C1—H1C | 109.5 | C28—N4—H4B | 107.9 |
| C2—C1—H1D | 109.5 | C28—N4—C29 | 117.8 (6) |
| C2—C1—H1E | 109.5 | C29—N4—H4A | 107.9 |
| C1—C2—H2A | 109.1 | C29—N4—H4B | 107.9 |
| C1—C2—H2B | 109.1 | H25A—C25—H25B | 109.5 |
| H2A—C2—H2B | 107.8 | H25A—C25—H25C | 109.5 |
| C3—C2—C1 | 112.5 (9) | H25B—C25—H25C | 109.5 |
| C3—C2—H2A | 109.1 | C26—C25—H25A | 109.5 |
| C3—C2—H2B | 109.1 | C26—C25—H25B | 109.5 |
| C2—C3—H3C | 109.4 | C26—C25—H25C | 109.5 |
| C2—C3—H3D | 109.4 | C25—C26—H26A | 108.8 |
| C2—C3—C4 | 111.0 (7) | C25—C26—H26B | 108.8 |
| H3C—C3—H3D | 108.0 | H26A—C26—H26B | 107.7 |
| C4—C3—H3C | 109.4 | C27—C26—C25 | 114.0 (8) |
| C4—C3—H3D | 109.4 | C27—C26—H26A | 108.8 |
| N1—C4—C3 | 112.6 (6) | C27—C26—H26B | 108.8 |
| N1—C4—H4C | 109.1 | C26—C27—H27A | 108.9 |
| N1—C4—H4D | 109.1 | C26—C27—H27B | 108.9 |
| C3—C4—H4C | 109.1 | C26—C27—C28 | 113.4 (7) |
| C3—C4—H4D | 109.1 | H27A—C27—H27B | 107.7 |
| H4C—C4—H4D | 107.8 | C28—C27—H27A | 108.9 |
| N1—C5—H5A | 109.6 | C28—C27—H27B | 108.9 |
| N1—C5—H5B | 109.6 | N4—C28—C27 | 112.8 (6) |
| N1—C5—C6 | 110.1 (6) | N4—C28—H28A | 109.0 |
| H5A—C5—H5B | 108.1 | N4—C28—H28B | 109.0 |
| C6—C5—H5A | 109.6 | C27—C28—H28A | 109.0 |
| C6—C5—H5B | 109.6 | C27—C28—H28B | 109.0 |
| C5—C6—H6A | 109.2 | H28A—C28—H28B | 107.8 |
| C5—C6—H6B | 109.2 | N4—C29—H29C | 111.3 |
| H6A—C6—H6B | 107.9 | N4—C29—H29D | 111.3 |
| C7—C6—C5 | 112.1 (8) | N4—C29—H29A | 106.3 |
| C7—C6—H6A | 109.2 | N4—C29—H29B | 106.3 |
| C7—C6—H6B | 109.2 | N4—C29—C30X | 102.4 (16) |
| C6—C7—H7A | 109.7 | H29C—C29—H29D | 109.2 |
| C6—C7—H7B | 109.7 | H29A—C29—H29B | 106.4 |
| C6—C7—C8 | 110.0 (11) | C30—C29—N4 | 124.2 (13) |
| H7A—C7—H7B | 108.2 | C30—C29—H29A | 106.3 |
| C8—C7—H7A | 109.7 | C30—C29—H29B | 106.3 |
| C8—C7—H7B | 109.7 | C30X—C29—H29C | 111.3 |
| C7—C8—H8A | 109.5 | C30X—C29—H29D | 111.3 |
| C7—C8—H8B | 109.5 | C29—C30—H30A | 109.3 |
| C7—C8—H8C | 109.5 | C29—C30—H30B | 109.3 |
| H8A—C8—H8B | 109.5 | H30A—C30—H30B | 108.0 |
| H8A—C8—H8C | 109.5 | C31—C30—C29 | 111.5 (13) |
| H8B—C8—H8C | 109.5 | C31—C30—H30A | 109.3 |
| H2C—N2—H2D | 107.6 | C31—C30—H30B | 109.3 |
| C12—N2—H2C | 108.6 | C30—C31—H31A | 108.5 |
| C12—N2—H2D | 108.6 | C30—C31—H31B | 108.5 |
| C13—N2—H2C | 108.6 | C30—C31—C32 | 114.9 (13) |
| C13—N2—H2D | 108.6 | H31A—C31—H31B | 107.5 |
| C13—N2—C12 | 114.6 (5) | C32—C31—H31A | 108.5 |
| H9A—C9—H9B | 109.5 | C32—C31—H31B | 108.5 |
| H9A—C9—H9C | 109.5 | C31—C32—H32A | 109.5 |
| H9B—C9—H9C | 109.5 | C31—C32—H32B | 109.5 |
| C10—C9—H9A | 109.5 | C31—C32—H32C | 109.5 |
| C10—C9—H9B | 109.5 | H32A—C32—H32B | 109.5 |
| C10—C9—H9C | 109.5 | H32A—C32—H32C | 109.5 |
| C9—C10—H10A | 109.0 | H32B—C32—H32C | 109.5 |
| C9—C10—H10B | 109.0 | C29—C30X—H30C | 109.6 |
| C9—C10—C11 | 112.7 (8) | C29—C30X—H30D | 109.6 |
| H10A—C10—H10B | 107.8 | C29—C30X—C31X | 110 (2) |
| C11—C10—H10A | 109.0 | H30C—C30X—H30D | 108.1 |
| C11—C10—H10B | 109.0 | C31X—C30X—H30C | 109.6 |
| C10—C11—H11A | 108.8 | C31X—C30X—H30D | 109.6 |
| C10—C11—H11B | 108.8 | C30X—C31X—H31C | 109.1 |
| H11A—C11—H11B | 107.7 | C30X—C31X—H31D | 109.1 |
| C12—C11—C10 | 113.8 (6) | C30X—C31X—C32X | 112.5 (14) |
| C12—C11—H11A | 108.8 | H31C—C31X—H31D | 107.8 |
| C12—C11—H11B | 108.8 | C32X—C31X—H31C | 109.1 |
| N2—C12—H12A | 109.1 | C32X—C31X—H31D | 109.1 |
| N2—C12—H12B | 109.1 | C31X—C32X—H32D | 109.5 |
| C11—C12—N2 | 112.4 (5) | C31X—C32X—H32E | 109.5 |
| C11—C12—H12A | 109.1 | C31X—C32X—H32F | 109.5 |
| C11—C12—H12B | 109.1 | H32D—C32X—H32E | 109.5 |
| H12A—C12—H12B | 107.9 | H32D—C32X—H32F | 109.5 |
| N2—C13—H13A | 109.0 | H32E—C32X—H32F | 109.5 |
| O1—Mo1—O2—Mo4i | 72.5 (2) | C5—C6—C7—C8 | 176.2 (8) |
| O3—Mo1—O2—Mo4i | −178.82 (17) | N2—C13—C14—C15 | −177.5 (6) |
| O4—Mo1—O2—Mo4i | −87.51 (17) | C9—C10—C11—C12 | −172.9 (9) |
| O4—Mo2—O5—Mo1 | 18.83 (15) | C10—C11—C12—N2 | −177.6 (6) |
| O4—Mo3—O10—Mo2i | 78.67 (16) | C12—N2—C13—C14 | −177.5 (5) |
| O4i—Mo3—O10—Mo2i | 5.27 (13) | C13—N2—C12—C11 | −171.1 (5) |
| O5—Mo1—O2—Mo4i | −48.5 (4) | C13—C14—C15—C16 | −177.6 (8) |
| O6—Mo2—O5—Mo1 | 170.08 (17) | N3—C21—C22—C23 | −179.5 (12) |
| O7—Mo2—O5—Mo1 | −81.38 (19) | C18—C19—C20—N3 | 168.1 (8) |
| O8—Mo2—O5—Mo1 | 32.2 (3) | C20—N3—C21—C22 | 170.1 (8) |
| O8—Mo3—O10—Mo2i | 12.3 (4) | C21—N3—C20—C19 | 60.7 (9) |
| O9—Mo3—O10—Mo2i | −174.29 (16) | C21—C22—C23—C24 | 180.0 (14) |
| O10i—Mo2—O5—Mo1 | 91.78 (17) | C17X—C18—C19—C20 | −95.2 (19) |
| O11i—Mo1—O2—Mo4i | −13.91 (15) | C17—C18—C19—C20 | −176.1 (12) |
| O11—Mo3—O10—Mo2i | −67.48 (17) | N4—C29—C30—C31 | 64 (2) |
| O15—Mo5—O14—Mo4 | 130.5 (8) | N4—C29—C30X—C31X | 168 (2) |
| O16—Mo5—O14—Mo4 | 6.5 (9) | C25—C26—C27—C28 | −175.7 (8) |
| O17—Mo5—O14—Mo4 | −112.0 (9) | C26—C27—C28—N4 | 178.8 (8) |
| N1—C5—C6—C7 | −170.0 (7) | C28—N4—C29—C30 | 52.8 (12) |
| C1—C2—C3—C4 | 178.8 (7) | C28—N4—C29—C30X | 60.7 (19) |
| C2—C3—C4—N1 | −172.8 (6) | C29—N4—C28—C27 | 57.9 (9) |
| C4—N1—C5—C6 | −77.2 (8) | C29—C30—C31—C32 | 63 (3) |
| C5—N1—C4—C3 | −78.5 (7) | C29—C30X—C31X—C32X | −70 (4) |
Symmetry code: (i) −x+1, −y+1, −z+1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1A···O2ii | 0.91 | 1.72 | 2.627 (6) | 172 |
| N1—H1B···O10ii | 0.91 | 1.97 | 2.755 (6) | 143 |
| N2—H2C···O15 | 0.91 | 1.91 | 2.778 (6) | 160 |
| N2—H2D···O7iii | 0.91 | 1.90 | 2.812 (5) | 176 |
| N3—H3A···O15iv | 0.91 | 1.87 | 2.770 (6) | 172 |
| N3—H3B···O17 | 0.91 | 1.88 | 2.786 (6) | 176 |
| N4—H4A···O11 | 0.91 | 1.80 | 2.695 (6) | 166 |
| N4—H4B···O16 | 0.91 | 1.86 | 2.762 (7) | 172 |
Symmetry codes: (ii) −x+2, −y+1, −z+1; (iii) −x+1, y+1/2, −z+3/2; (iv) −x+1, −y+1, −z+2.
References
- Allis, D. G., Burkholder, E. & Zubieta, J. A. (2004). Polyhedron, 23, 1145–1152.
- Chakrabarti, S. & Natarajan, S. (2002). Cryst. Growth Des. 2, 333–335.
- Cronin, L., Beugholt, C., Krickemeyer, E., Schmidtmann, M., Bögge, H., Kögerler, P., Luong, T. K. K. & Müller, A. (2002). Angew. Chem. Int. Ed. 41, 2805–2808. [DOI] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Du, J., Yu, J., Tang, J., Wang, J., Zhang, W., Thiel, W. R. & Jia, M. (2011). Eur. J. Inorg. Chem. pp. 2361–2365.
- Fuchs, J., Hartl, H., Hunnius, W.-D. & Mahjour, S. (1975). Angew. Chem. 87, 634–635.
- Gong, Y., Hu, C., Li, H., Tang, W., Huang, K. & Hou, W. (2006). J. Mol. Struct. 784, 228–238.
- Hao, J., Ruhlmann, L., Zhu, Y., Li, Q. & Wei, Y. (2007). Inorg. Chem. 46, 4960–4967. [DOI] [PubMed]
- Harchani, A. & Haddad, A. (2015). J. Clust Sci. 26, 1773–1785.
- Isobe, M., Marumo, F., Yamase, T. & Ikawa, T. (1978). Acta Cryst. B34, 2728–2731.
- Li, T., Lü, J., Gao, S., Li, F. & Cao, R. (2007). Chem. Lett. 36, 356–357.
- Müller, A., Shah, S. Q. N., Bögge, H. & Schmidtmann, M. (1999). Nature, 397, 48–50.
- Pavani, K., Lofland, S. E., Ramanujachary, K. V. & Ramanan, A. (2007). Eur. J. Inorg. Chem. pp. 568–578.
- Pavani, K. & Ramanan, A. (2005). Eur. J. Inorg. Chem. pp. 3080–3087.
- Rigaju OD (2021). CrysAlis PRO. Rigaku Oxford Diffraction, Yarnton, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Wu, C.-D., Lu, C.-Z., Zhuang, H.-H. & Huang, J.-S. (2002). Inorg. Chem. 41, 5636–5637. [DOI] [PubMed]
- Xu, L., Qin, C., Wang, X., Wei, Y. & Wang, E. (2003). Inorg. Chem. 42, 7342–7344. [DOI] [PubMed]
- Zhang, J. Y., Chang, Z. H., Wang, X. L., Wang, X. & Lin, H. Y. (2021). New J. Chem. 45, 3328–3334.
- Zhang, X.-M., Shan, B.-Z., You, X.-Z. & Fun, H.-K. (1997). Polyhedron, 16, 95–102.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2414314624004632/wm4210sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2414314624004632/wm4210Isup4.hkl
CCDC reference: 2332866
Additional supporting information: crystallographic information; 3D view; checkCIF report