Abstract
It has recently been shown that rapid and profound CD4+ T-cell depletion occurs almost exclusively within the intestinal tract of simian immunodeficiency virus (SIV)-infected macaques within days of infection. Here we demonstrate (by three- and four-color flow cytometry) that this depletion is specific to a definable subset of CD4+ T cells, namely, those having both a highly and/or acutely activated (CD69+ CD38+ HLA-DR+) and memory (CD45RA− Leu8−) phenotype. Moreover, we demonstrate that this subset of helper T cells is found primarily within the intestinal lamina propria. Viral tropism for this particular cell type (which has been previously suggested by various studies in vitro) could explain why profound CD4+ T-cell depletion occurs in the intestine and not in peripheral lymphoid tissues in early SIV infection. Furthermore, we demonstrate that an acute loss of this specific subset of activated memory CD4+ T cells may also be detected in peripheral blood and lymph nodes in early SIV infection. However, since this particular cell type is present in such small numbers in circulation, its loss does not significantly affect total CD4+ T cell counts. This finding suggests that SIV and, presumably, human immunodeficiency virus specifically infect, replicate in, and eliminate definable subsets of CD4+ T cells in vivo.
Although human immunodeficiency virus (HIV) infection is generally associated with a prolonged period of clinical latency, increasing evidence points to the importance of early events for determining the outcome of HIV infection (25, 34). Unfortunately, early (first month) events in the pathogenesis of HIV infection are poorly characterized, mainly due to the difficulty in obtaining appropriate samples from humans with primary infection. Furthermore, examination of peripheral lymph nodes and blood provides a limited view of the immunopathologic processes that are occurring in early infection. For example, it has recently been shown that simian immunodeficiency virus (SIV) infection causes profound loss of CD4+ T cells from within the intestinal immune system (which is the largest immunologic organ in the body) long before any significant T-cell alterations occur in peripheral lymph nodes or blood (24, 48, 53). Although mounting evidence also implicates the intestinal tract as a major target organ in early HIV infection (12, 27, 46, 53, 54), it is difficult to adequately examine the intestinal tracts of humans with early HIV infection. Fortunately, observations of viral loads and distribution of SIV in lymphoid tissues of acutely infected macaques have been validated as an excellent model of the effects of HIV in human tissues (38). Moreover, it has been demonstrated that the intestinal T-cell compartments of rhesus macaques are more similar to those of humans than any other laboratory animal (55). Thus, characterizing the effects of SIV infection on gut-associated lymphoid tissues in the SIV-rhesus model is highly relevant to HIV infection in humans.
To explain the increased tissue viral loads and the rapidity of CD4+ T-cell losses in the intestine, we hypothesized that SIV optimally replicates within CD4+ T cells having an activated, memory immunophenotype, which are present in abundance in the intestinal tract. In vitro experiments with HIV have demonstrated that peripheral blood CD4+ T cells may remain latently infected with provirus indefinitely. However, appropriate activation of these cells consistently results in productive viral replication and cell lysis (16, 19). Several other in vitro studies have confirmed that optimal viral replication occurs only in CD4+ T cells having an activated and/or memory phenotype (8, 10, 11, 31, 33, 36, 47, 49, 52, 58). Moreover, activated memory CD4+ T cells have been shown to express high levels of CCR5, which is a major coreceptor for both SIV and HIV (7, 17). Therefore, it was reasonable to hypothesize that the reason intestinal lamina propria CD4+ T cells are eliminated first is that they are predominantly activated and/or memory cells. To test this hypothesis, four-color flow cytometry was performed on lymphocytes isolated from both the intestinal epithelium and lamina propria of the jejunum, ileum, and colon and compared to results for lymphocytes from peripheral and mesenteric lymph nodes, spleen, and blood. Lymphocyte phenotypes were compared by using a panel of monoclonal antibodies designed to distinguish naive from memory T cells, as well as to categorize the expression of an array of markers associated with cellular activation on well-defined T-cell subsets (Table 1). Since intestinal lamina propria CD4+ T cells are essentially depleted within the first 2 weeks of SIV infection (24, 48, 53), emphasis was placed on phenotyping percentages and numbers of intestinal lamina propria CD4+ T cells in uninfected, normal rhesus macaques and in those with very early SIV infection (up to 2 weeks postinoculation [p.i.]). SIV-infected animals in the early stages of infection were also compared to those in the terminal stage of disease (AIDS).
TABLE 1.
Cell surface marker expression examined on lymphocytes by four-color flow cytometry
| Marker expressed
|
Cell type defined | |||
|---|---|---|---|---|
| FL-1 | FL-2 | FL-3 | FL-4 | |
| CD45RA | CD62L | CD3 | CD4 | Naive or memory CD4+ T cells |
| CD45RA | CD62L | CD3 | CD8 | Naive or memory CD8+ T cells |
| γδ TCR | CD8 | CD3 | CD4 | γδ T cells |
| CD20 | CD2 | CD3 | CD4 | B cells, T cells |
| CD25 | CD69 | CD3 | CD4 | Activated T cells |
| CD38 | HLA-DR | CD3 | CD8 | Activated MHC II+ CD8+ T cells |
| CD38 | HLA-DR | CD3 | CD4 | Activated MHC II+ CD4+ T cells |
| CD8 | CD28 | CD3 | CD4 | Costimulatory T cells |
| CD38 | CD56 | CD3 | CD8 | NK cells (CD3− CD8+ CD56+) |
| CD4 | HLA-DR | CD34 | CD8 | Pluripotential stem cells |
MATERIALS AND METHODS
Examination of T-cell subsets and activation state by flow cytometry.
To assess lymphocyte activation and distinguish naive from memory T cells, a variety of antibodies against cell surface markers were used in various combinations, based on current knowledge of T-cell activation and memory (Table 1). Activation markers included CD69, a cell surface antigen expressed very early in T-cell activation (59), CD28, a costimulatory molecule important in T-cell activation and cytokine regulation (6, 15, 26, 28, 51), CD38, a transmembrane glycoprotein thought to be involved in T-cell signaling, activation, and adhesion (2, 32, 42), and HLA-DR (a major histocompatibility complex type II [MHC II] molecule), which is also upregulated on activated lymphocytes. To distinguish naive from memory CD4+ T cells, expression of CD45RA and CD62L (L-selectin) was examined. Although the concept of naive and memory T cells is continuously being revised (reviewed by Bell et al. [3]), it is currently thought that CD4+ T lymphocytes that have not previously (or at least not recently) encountered their T-cell receptor (TCR)-specific antigen express the CD45RA isoform of the common leukocyte antigen, CD45. Lymphocytes that have previously encountered antigen are known to express the CD45RO isoform (3). Unfortunately, existing anti-human CD45RO monoclonal antibodies do not cross-react in the rhesus macaque, and so memory and naive CD4+ T lymphocytes are defined in this report as either CD45RA+ (naive) or CD45RA− (memory). Since there is a transient switching period in which cells may express both isoforms of CD45, antibodies against CD62L were simultaneously used to help distinguish truly naive (CD45RA+ CD62L+) from memory (CD45RA− CD62L+/−) lymphocytes in peripheral blood and lymph nodes, using previously described methodology (40, 43). However, it is important to note that CD62L (a peripheral lymph node homing receptor) is not usually expressed on intestinal lamina propria lymphocytes (LPL) (4). Since CD62L is shed rapidly upon lymphocyte activation (51), this may be another reflection of the highly activated state of intestinal LPL. Although CD62L plays a minor role in trafficking of naive lymphocytes to the intestinal inductive lymphoid tissues (Peyer's patches and lymphoid follicles), other homing molecules (such as α4β7 and αEβ7) primarily govern the homing of lymphocytes to the diffuse effector lymphoid tissues (lamina propria) of the intestine (9). Therefore, while CD62L is particularly useful in defining resting naive lymphocytes (CD62L+ CD45RA+) in peripheral lymphoid tissues, it may not be as useful in discriminating naive and memory cells in the intestine. For these reasons, naive and memory CD4+ T cells in the intestine were defined primarily on the basis of CD45RA expression. With this rationale, activated memory CD4+ T cells should be both CD45RA and CD62L negative, regardless of the tissue examined.
Animals and virus.
A total of 39 rhesus macaques were examined in this study. Of these, 29 were sacrificed by barbiturate overdose, and lymphoid tissues were harvested at necropsy. An additional 10 animals were followed prospectively, with sequential intestinal lymphoid tissues collected by biopsy in three of these animals. Of the 29 animals from which tissues were collected at necropsy, 10 were uninfected controls, 15 were juvenile (1- to 2-year-old) males in the acute stage of infection, and 4 were adults with terminal AIDS. Of the sacrificed controls, seven were adult (5- to 20-year-old) females, two were adult males, and one was a 2-year-old juvenile. The 15 animals in the acute stage of infection were juveniles infected with equal doses (50 ng of p27) of SIVmac239 or SIVmac251 intravenously. We have previously shown that these two viruses have identical effects on intestinal lymphocytes (53) and virtually identical courses of disease in macaques (56). Therefore animals infected with these viruses were grouped together. Of the 15 acutely infected animals, 5 were sacrificed and examined at 7 days p.i., 4 were sacrificed and examined at 14 days p.i., and an additional 6 animals in groups of two were sacrificed and examined at 3, 21, and 50 days p.i. Data on the intestinal CD4 depletion from some of the acutely infected animals have previously been reported (53). The four animals sacrificed in the terminal stages of infection (AIDS) had been inoculated with similar doses of either SIVmac239 or SIVmac251 at least 1 year prior to sacrifice.
To determine whether age differences could account for the differences observed between the control and uninfected animals, a prospective analysis was performed on an additional 10 juvenile macaques intravenously infected with SIVmac251 (50 ng of p27). From these, peripheral blood was examined before infection and at 7, 14, 21, and 30 days following infection. In addition, sequential endoscope-guided intestinal pinch biopsies of duodenum and colon were obtained from three of these animals prior to infection and at 7, 14, 21, and 35 days p.i. to assess longitudinal changes in intestinal lymphocyte subsets.
Isolation of lymphocytes and flow cytometry.
Segments of intestine 6 to 8 cm long from jejunum, ileum, and colon were collected from the 29 animals that were killed, and lymphocytes were separately isolated from the intestinal epithelium (intraepithelial lymphocytes [IEL]) and lamina propria (LPL) from each intestinal segment as previously described (53, 55). Sections of jejunum were consistently taken between 30 and 40 cm distal to the pylorus (proximal jejunum) to obtain representative LPL and IEL (which comprise the effector arm of the intestinal immune system) with minimal contamination from organized lymphoid tissues (the inductive arm of the intestinal immune system). In contrast, cells obtained from sections of ileum and colon were more likely to contain lymphocytes from both effector sites (LPL and IEL) and inductive sites (Peyer's patches and solitary lymphoid follicles), the latter of which contain higher proportions of naive resting lymphocytes.
Endoscope-guided pinch biopsies from the proximal jejunum and distal colon were collected from three juvenile macaques at multiple time points. These biopsies were processed and examined separately, but no attempt was made to separate IEL from LPL in these samples. Since IEL are essentially all CD8+ T cells, this resulted in slightly lower percentages of CD4+ T cells in these samples, but since CD4+ T cells are mostly found in the lamina propria (55), no differences in phenotypes of the CD4+ T cells obtained by this method would be expected. Histologic examinations of adjacent tissues were routinely performed in all cases to ensure the quality of the samples taken.
Briefly, IEL were isolated from intestinal segments by using EDTA and mechanical agitation, and LPL were isolated from remaining intestinal pieces by using collagenase. Biopsy specimens were similarly treated with EDTA and collagenase, but cells derived from these samples were pooled. Lymphocytes from all regions were enriched by Percoll density gradient centrifugation (55). Intestinal cell viability was always greater than 90%, as determined by trypan blue dye exclusion. In all cases, cells were stained the day of sampling and cell suspensions were kept on ice between each incubation so that no changes in cell surface expression could occur after the tissues were harvested. Previous studies have shown that these procedures do not affect the expression of cell surface markers, including those associated with cell activation (60). Lymphocytes were also obtained from the spleen and axillary, inguinal, and mesenteric lymph nodes (from sacrificed animals only) by gently cutting and pressing tissues through nylon mesh screens. Peripheral blood from all animals was stained by a whole blood lysis technique as described below.
Cells were stained for four-color flow cytometric analysis using monoclonal antibodies to the panel of markers listed in Table 1. Cells were stained by incubating 106 cells from each of the above-described samples with excess amounts of monoclonal antibodies at 4°C for 30 min, followed by a wash (400 × g, 7 min) and fixation in 2% paraformaldehyde. Blood was stained by incubating 100 μl of whole blood with monoclonal antibodies for 30 min at 4°C, followed by a 7-min lyse with FACS (fluorescence-activated cell sorting) lysing solution (Becton Dickinson, San Jose, Calif.). Cells were then washed (400 × g, 7 min) and resuspended in 2% paraformaldehyde. All antibodies were directly conjugated to either fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll protein (PerCP), or allophycocyanin (APC) or (for CD3) were biotinylated and stained in a second step with streptavidin 613 (Gibco). Monoclonal antibodies used were Leu-5b (CD2-PE), Leu-18 (CD45RA-FITC), Leu-8 (L-selectin or CD62L-PE), Leu-16 (CD20-FITC), Leu19 (CD56-PE), Leu-2b (CD8-FITC, CD8-PE), anti-HLA-DR-PE, Leu-28 (CD28-PE), Leu3a (CD4-APC), and anti-interleukin-2 receptor (CD25-FITC), all obtained from Becton Dickinson. OKT4 (CD4-FITC) and OKT10 (CD38-FITC) were obtained from Ortho Diagnostics (Raritan, N.J.), 5A6.E9 (pan-γδ TCR-FITC) was obtained from Endogen (Woburn, Mass.), QBEND-10 (CD34-biotin) was obtained from Immunotech (Westbrook, Maine), 3B5 (CD8-APC) and BL-Ac/p26 (CD69-PE) were obtained from Caltag Laboratories (San Francisco, Calif.), and 6G12 (CD3-biotin) was kindly provided by J. Wong, Massachusetts General Hospital (22). Controls consisted of appropriate unstained and irrelevant isotype-stained samples as well as single-color-stained samples to verify the staining specificity of experimental antibodies. Data were acquired by using a Vantage or FacsCalibur flow cytometer (Becton Dickinson) and analyzed with Cell Quest software (Becton Dickinson).
Statistical analyses.
Differences in percentages and numbers of lymphocytes were compared between groups of animals at different time points, using a Student pairwise t test and commercial statistical software (SigmaPlot; SPSS, Chicago, Ill.).
Immunohistochemistry and quantitative analysis.
For determination of numbers of T cells per square millimeter of lamina propria, immunohistochemistry for CD3 was performed on 6-μm-thick sections of paraffin-embedded jejunum from 4 uninfected and 10 acutely infected macaques as previously described (55). Diaminobenzidine was used as the chromagen, and slides were lightly counterstained with hematoxylin. Thus, all CD3+ cells were dark brown and negative cells were light blue. Positive (brown) cells were then counted using an Olympus Vanox-S microscope interfaced to a Quantimet 570C image analysis system (Leica, Cambridge, Mass.). First, a detection threshold that would detect all brown cells and discard blue cells for all sections was determined. Then, a two-dimensional, irregularly shaped field was carefully drawn around the lamina propria (from the basal villi to the lamina muscularis [excluding crypts and solitary lymphoid follicles]), and the total area in square millimeters was determined. The positive cells within this field were then counted using a computer program designed by one of us to exclude artifacts and distinguish adjacent cells. The number of CD3+ T cells per square millimeter was then determined by dividing the total positive cells in a particular field by the area in square millimeters of the field in which they were counted. At least five fields (0.5 to 1.0 mm2, total area) were counted for each section. The mean number of T cells per square millimeter was determined by averaging all fields counted in each section.
RESULTS
Normal intestinal lamina propria CD4+ T cells have an activated memory phenotype.
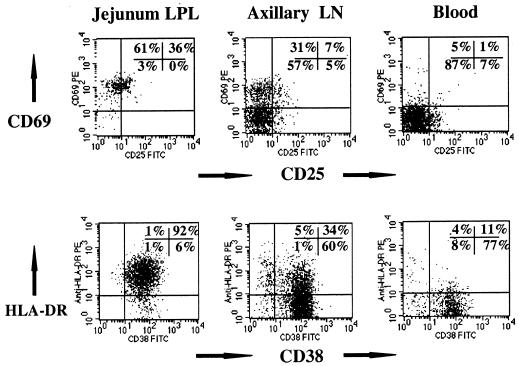
As previously described, the proportions of the major intestinal T-cell subsets of normal macaques are similar, if not identical, to those found in humans (55). Macaque IEL are predominantly CD3+ CD8+, whereas LPL consist of approximately equal percentages of CD4+ and CD8+ T cells (55). In this study, three- and four-color flow cytometry of lymphocytes from uninfected adult macaques revealed that the vast majority (mean, 93%) of the CD3+ CD4+ double-positive (DP) T cells in the lamina propria of the jejunum and to a slightly lesser extent (mean, 78 to 79%) ileum and colon consistently express high levels of CD69, an early and reliable marker of lymphocyte activation (59) (Fig. 1; Table 2). In contrast, CD69 expression was significantly lower (P < 0.01) on CD3+ CD4+ cells obtained from peripheral lymph nodes (mean, 27.2%) and rare (mean, 3.2%) on peripheral blood CD3+ CD4+ T cells of normal macaques (Fig. 1; Table 2). Interleukin-2 receptor (CD25) expression was also consistently and significantly (P < 0.01) higher on intestinal lymphocytes than on peripheral lymph nodes, spleen, or blood (Fig. 1; Table 2). As with CD69, CD25 expression was consistently highest on lamina propria CD4+ T cells from the jejunum (mean, 25%; range, 11 to 37%) compared to peripheral blood (mean, 5.8%; range, 2 to 11%) or lymph nodes (mean, 8.4%; range, 4 to 13%) (Fig. 1; Table 2).
FIG. 1.
Comparison of lymphocyte activation (CD69, CD25, CD38, and HLA-DR expression) on CD4+ T cells from the intestinal lamina propria (left) to CD4+ T cells obtained from the axillary lymph node (LN; center) and blood (right) from an uninfected normal rhesus macaque. Note that essentially all intestinal CD4+ T cells are CD69+ HLA-DR+ CD38+. Plots were generated by gating first through lymphocytes and then through CD4+ cells.
TABLE 2.
Summary of results of three- and four-color immunophenotyping of CD4+ T cells in normal macaque tissues
| Gate | No. of animals examined | Lymphocyte subset | % T cell expressing marker (mean ± SD)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LPL
|
IEL
|
Lymph nodes
|
Spleen | Blood | ||||||||
| Jejunum | Ileum | Colon | Jejunum | Ileum | Colon | Axilliary | Mesenteric | |||||
| CD3+ CD4+ | 6 | CD45RA+ | 4.9 ± 2.1 | 13.0 ± 1.9 | 14.2 ± 7.3 | 7.5 ± 5.5 | 20.4 ± 16.5 | 27.6 ± 17.8 | 52.0 ± 13.4 | 55.3 ± 12.4 | 47.6 ± 9.9 | 61.3 ± 3.1 |
| CD3+ CD4+ | 6 | L-selectin+ | 5.5 ± 4.3 | 6.2 ± 2.0 | 9.2 ± 3.9 | 9.2 ± 4.3 | 14.4 ± 10.6 | 18.0 ± 13.0 | 38.6 ± 15.2 | 28.0 ± 7.4 | 20.8 ± 6.8 | 62.7 ± 8.1 |
| CD3+ CD4+ | 6 | DP 45RA+ L-selectin+ | 2.2 ± 1.3 | 3.4 ± 0.7 | 4.1 ± 1.0 | 4.1 ± 3.6 | 9.6 ± 11.0 | 12.6 ± 11.2 | 28.6 ± 14.5 | 20.8 ± 7.3 | 16.2 ± 6.5 | 50.7 ± 6.1 |
| CD4+ | 4 | CD69+ | 93.0 ± 4.8 | 79.3 ± 6.1 | 77.5 ± 3.7 | 97.5 ± 1.7 | 93.8 ± 3.3 | 74.0 ± 25.9 | 27.2 ± 7.7 | 36.0 ± 11.5 | 24.0 ± 0.0 | 3.2 ± 2.7 |
| CD4+ | 8 | CD25+ | 24.9 ± 12.4 | 14.3 ± 8.9 | 17.0 ± 7.7 | 18.4 ± 10.7 | 19.8 ± 16.9 | 16.8 ± 11.2 | 8.4 ± 3.3 | 10.3 ± 3.4 | 8.0 ± 4.7 | 5.8 ± 3.8 |
| CD3+ CD4+ | 8 | HLA-DR+ | 48.3 ± 36.5 | 42.9 ± 32.6 | 33.7 ± 29.1 | 35.8 ± 34.0 | 36.8 ± 27.4 | 33.7 ± 29.1 | 26.3 ± 18.5 | 21.0 ± 15.0 | 36.6 ± 24.7 | 19.4 ± 13.6 |
| CD3+ CD4+ | 8 | CD38+ | 82.0 ± 12.8 | 84.9 ± 6.8 | 77.9 ± 10.1 | 83.3 ± 10.1 | 82.3 ± 9.8 | 83.8 ± 7.3 | 85.7 ± 6.2 | 96.2 ± 1.5 | 80.2 ± 7.4 | 85.8 ± 10.0 |
| CD4+ | 8 | CD28+ | 82.3 ± 15.6 | 88.6 ± 13.5 | 95.0 ± 4.2 | 52.1 ± 24.1 | 69.8 ± 27.9 | 76.2 ± 25.9 | 96.6 ± 2.9 | 95.4 ± 4.5 | 89.2 ± 5.1 | 91.6 ± 2.4 |
HLA-DR expression was also consistently higher on intestinal lamina propria CD4+ T cells than on peripheral CD4+ T cells in individual animals (Fig. 1; Table 2). However, marked variation in the expression of HLA-DR was noted between individual macaques. Although some animals had very high HLA-DR expression (up to 95% of CD3+ CD4+ intestinal lymphocytes), others had very little HLA-DR expression on CD4+ T cells. On average, 48% of lamina propria CD3+ CD4+ cells expressed HLA-DR, whereas 26% of axillary lymph node and 19% of peripheral blood CD3+ CD4+ T cells were HLA-DR+ (Fig. 1; Table 2). However, the wide variation in HLA-DR expression between individuals resulted in no statistically significant differences between tissues.
Intestinal lamina propria CD4+ T cells from uninfected adult macaques were essentially all memory (CD45RA−) cells (Fig. 2; Table 2). Over 95% of jejunum LPLs and 90% of ileum and colon LPLs were CD45RA negative, indicating a memory phenotype. Duodenal CD4+ T lymphocytes obtained from uninfected juvenile macaques had slightly higher CD45RA expression (mean, 15%) (Fig. 3). In contrast, 68% (range, 43 to 86%) of the peripheral blood and 50% (range, 36 to 75%) of peripheral lymph node CD3+ CD4+ lymphocytes coexpress CD45RA, indicating that they are naive or resting lymphocytes (Table 2). Moreover, a large percentage of the CD3+ CD4+ CD45RA+ cells in peripheral tissues were L-selectin+, which is also consistent with naive cells. In contrast, less than 10% of the intestinal CD3+ CD4+ T cells (both LPL and IEL) expressed L-selectin (Table 2).
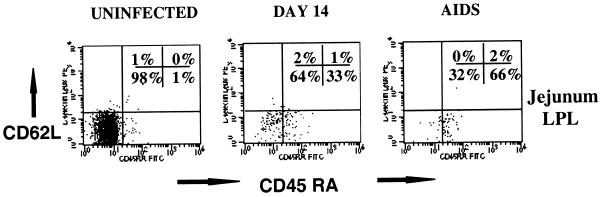
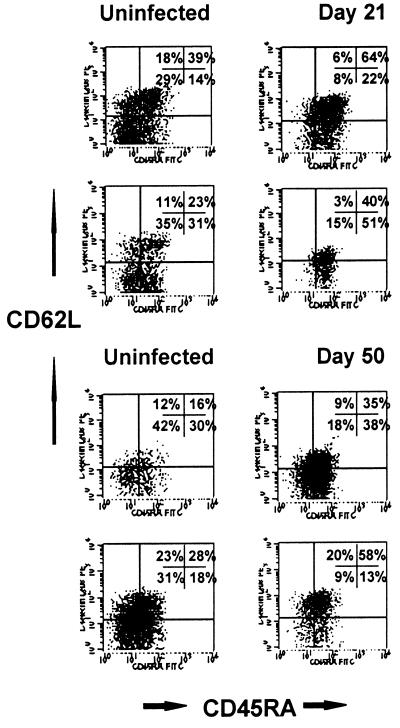
FIG. 2.
Flow cytometry dot plots of CD45RA and L-selectin expression on intestinal lamina propria CD3+ CD4+ T cells from an uninfected macaque (left), a macaque infected for 14 days (center), and an animal with AIDS (right). Note that the vast majority of intestinal memory (CD45RA−) cells are eliminated in early SIV infection, resulting in an increased proportion of naive (CD45RA+) cells remaining. Plots were generated by gating first through lymphocytes and then through CD3+ CD4+ DP T cells (four-color flow cytometry).
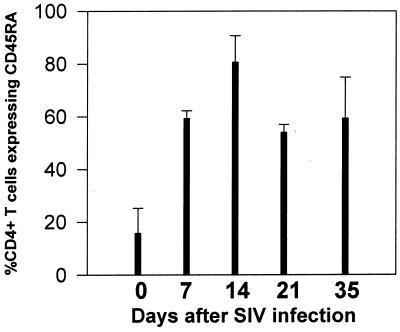
FIG. 3.
Sequential changes in CD45RA expression on duodenal CD4+ T cells in juvenile macaques infected with SIV. Intestinal biopsies were taken from the same three macaques before infection (day 0) and in the first few weeks after SIVmac251 infection. Bars represent the proportion of the total remaining CD4+ T cells that express CD45RA. Note that most intestinal CD4+ T cells are memory (CD45RA−) cells before infection, whereas increased proportions of naive (CD45RA+) CD4+ T cells are detected in the intestine of the same animals within weeks of SIV infection. Each bar represents the mean of the three animals examined ± standard deviation.
Acute SIV infection specifically results in the elimination of activated memory CD4+ T lymphocytes.
As previously described, intestinal lamina propria CD4+ T cells were selectively and profoundly depleted within 14 days of SIV infection (24, 48, 53). Gating on the remaining CD3+ CD4+ T cells in the lamina propria at all stages of SIV infection revealed a dramatic increase in the proportion of naive (CD45RA+) CD4+ T cells remaining in the intestinal lamina propria (Fig. 2 and 3). Although CD45RA expression in the jejunum LPL was consistently low in uninfected animals, a marked increase in the proportion of the remaining CD3+ CD4+ T cells after 14 days of infection expressed CD45RA (Fig. 2 and 3). This was particularly evident in animals followed prospectively by examining serial intestinal biopsies (Fig. 3). However, since intestinal CD4+ T cells are profoundly depleted after 14 days of infection, the latter time points are usually based on a small number of cells (often fewer than 100 events per 20,000 collected). Therefore, it could not be determined whether this proportional increase in naive CD4+ T cells was due to recruitment (or expansion) of small numbers of naive T cells in the intestine, or if they simply represented a preexisting population which remained after the memory cells were depleted. Moreover, CD4+ T cell loss was consistently (yet only slightly) more profound in the jejunum than in the ileum or colon, which also correlates with the findings of slightly higher percentages of memory CD4+ T cells in the jejunum. This finding further supports the hypothesis that a selective depletion of activated memory CD4+ T cells was occurring in the intestinal tract.
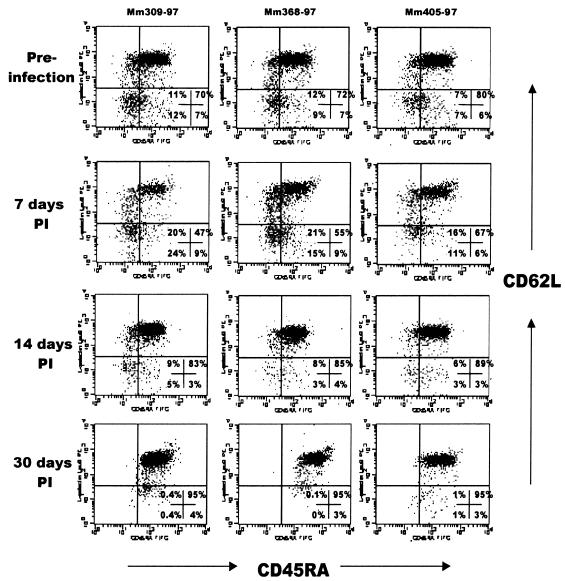
A relative increase in CD45RA expression was also detected in peripheral lymph nodes and blood, despite the absence of significant changes in overall CD4+ T-cell percentages or number. In fact, with gating through CD3+ CD4+ T cells, a remarkably consistent (11 of 11 animals examined) elimination of CD45RA− Leu8− (memory) CD4+ T cells occurred in peripheral blood when the same animals were compared at different time points in early infection (Fig. 4). However, since the starting percentages of memory CD4+ T cells in peripheral blood are substantially lower than those in the LPL, no significant changes in absolute numbers of CD4+ T cells in the blood were observed during these early time points, as previously described (24, 48, 53). A similar increase in the proportion of naive cells was noted in the lymph nodes in early SIV infection, indicating that a loss of memory cells occurs in peripheral lymphoid tissues as well (Fig. 5).
FIG. 4.
Flow cytometry dot plots demonstrating a selective loss of memory CD4+ T cells in the peripheral blood of three macaques with early SIV infection. Each column shows the data from a single animal (animal numbers are listed above each column) examined before (top) and at 7, 14, and 30 days after SIV infection. Plots were generated by gating first through lymphocytes and then through CD4+ cells (three-color flow cytometry). Note that in each animal, a consistent loss of cells occurs in the upper left, lower left, and lower right quadrants by 14 days p.i., leaving only naive CD4+ (CD45RA+ CD62L+) T cells by 30 days p.i. (upper right quadrant). These results were representative of three separate experiments (n = 11).
FIG. 5.
Flow cytometry dot plots demonstrating a specific loss of memory CD4+ T cells in lymph nodes in early SIV infection. Plots on the left are from uninfected macaques, and those on the right are from different animals (cross-sectional study) infected with SIVmac239 for 21 or 50 days. Plots were generated by gating first through lymphocytes and then through CD3+ CD4+ cells (four-color flow cytometry). As in the blood, significantly fewer memory (CD45RA−) cells are detected in lymph nodes from animals in early SIV infection than in those from uninfected animals. Note the decreased proportion of cells in the upper and lower left quadrants (memory cells) in the infected animals.
Regional variations exist in intestinal lymphocyte compartments.
Since samples from the jejunum, ileum, and colon were processed and analyzed separately, distinct regional differences in lymphocyte subsets were detected between these regions. Importantly, lymphocytes from the ileum and colon consistently had slightly (<10% difference) higher percentages of both naive (CD45RA+) and resting (CD69− CD25−) CD4+ T cells (Table 2). As previously mentioned, these findings are consistent with the presence of organized lymphoid tissues (Peyer's patches and organized lymphoid follicles) which are more frequent in the ileum and colon than in the jejunum. While these regional differences were detectable, they were insignificant compared to the marked differences between LPL from any region and peripheral lymphocytes. As previously described, intestinal IEL were predominantly CD3+ CD8+, yet some (up to 20%) were CD4+ (and usually CD4+ CD8+ DP), particularly in samples from the ileum and/or colon. The intestinal epithelium that overlies human Peyer's patches (follicle-associated epithelium) is known to contain more CD4+ T cells (5), and thus these regional differences in the IEL are consistent with the presence of Peyer's patches in the ileum and colon.
Increased numbers of CD8+ T cells appear in intestinal tissues following SIV infection.
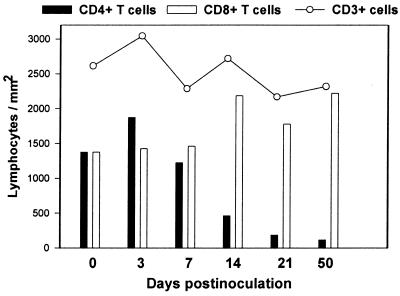
To examine the possibility that changes in T-cell subsets could be due to recruitment or expansion of other cell subsets, overall percentages of CD3+ T cells within the lymphocyte gate were compared. No significant differences in the percentages of CD3+ T cells were detected in any of the compartments examined (data not shown). To quantitate T-cell numbers in the intestinal lamina propria, immunohistochemistry for CD3 was done on sections of jejunum, and total T-cell numbers per square millimeter were then determined in the lamina propria of uninfected and acutely infected animals. Although there was a trend for an overall decrease, no significant changes in absolute numbers of CD3+ T cells per square millimeter were detected on immunohistochemically stained intestinal sections after early SIV infection (Fig. 6). To estimate total numbers of CD4+ and CD8+ T cells per square millimeter of intestine, the number of total T cells (as determined by immunohistochemistry and image analysis) was multiplied by the percentage of T cells that were either CD4+ or CD8+ (as determined by electronically gating through CD3+ T cells) for each corresponding jejunal segment. Using this method, we determined that a marked loss in absolute numbers of CD4+ T cells was occurring concurrently with an increase in numbers of CD8+ T cells in the intestinal lamina propria (Fig. 6).
FIG. 6.
Estimation of the changes in the total numbers of T cells per square millimeter in the intestinal lamina propria in early SIV infection. Each time point represents the mean of two (infected) or four (uninfected control) animals. Morphometric analysis of CD3+ T cells in immunohistochemically stained jejunum sections was used to determine the numbers of CD3+ T cells per square millimeter of lamina propria (see text), and CD4+ and CD8+ T cells per square millimeter were determined by multiplying the number of CD3+ cells per square millimeter by the percentage of gated CD3+ lymphocytes coexpressing CD4 or CD8 in corresponding jejunum lamina propria as determined by flow cytometry (DP cells would be included in both bars). Note that a profound loss in CD4+ T cells per square millimeter occurs by 21 days after SIV infection, corresponding with an increase in absolute numbers of CD8+ T cells. Overall, this results in minimal changes in absolute numbers of CD3+ T cells in the lamina propria.
Other findings.
Although γδ T cells represented 5 to 25% of all IEL in the macaques examined, no significant changes in percentages or numbers of γδ T cells were detected in response to SIV infection. Moreover, no significant change in CD20 expression (resting B cells) was detected in early SIV infection compared to controls. A considerable (yet variable) proportion of macaque intestinal T cells express both CD4 and CD8 (i.e., are DP cells). Since the only other organ that harbors large numbers of these unique T cells is the thymus, our staining panel was designed to further characterize these cells. However, by three- and four-color analysis, DP cells were determined to share most of the phenotypic traits of the single-positive CD4+ T cells in the intestine: they have an activated (CD69+) memory (CD45RA− L-selectin−) phenotype and are also rapidly eliminated in early SIV infection. In fact, both the speed and the degree of intestinal DP cell depletion was greater than those of CD4+ single-positive T cells (data not shown). Moreover, these cells lacked CD34 expression (unlike thymocytes) and expressed CD69 similarly to single-positive CD4+ T cells (data not shown). This finding indicates that intestinal DP cells are very different from thymus DP cells and are probably highly activated effector cells rather than immature precursor cells.
Approximately 80% of intestinal lamina propria CD4+ T cells expressed CD38, but these percentages were not significantly different from those of CD4+ T cells from the lymph nodes, spleen, and blood (Table 2). In addition, the vast majority of CD3+ CD4+ T cells in the intestinal lamina propria coexpress CD28, which was similar to percentages of CD28 expression by peripheral lymph node and blood CD4+ T cells (Table 2). Interestingly, CD28 expression was lower on jejunum LPL than LPL from the ileum, colon, or peripheral tissues (Table 2).
DISCUSSION
Combined, these data clearly show that the vast majority of activated (CD69+ HLA-DR+ CD38+) memory (CD45RA− Leu8−) CD3+ CD4+ T lymphocytes in normal primates reside within the intestinal mucosa and that it is this specific T-cell subset that is preferentially eliminated in primary SIV infection. Moreover, the intestinal CD4 depletion is preceded by infection of large numbers of intestinal lymphocytes in early SIV infection (20, 53). Combined, these data indicate that the primary target cell for SIV replication is a T cell positive for CD2, CD3, CD4, CD69, and CD38 and variably but consistently higher than other CD4+ T cells in its expression of HLA-DR and CD25. In addition, these cells may have slightly lower expression of CD28. Decreased expression of CD28 has also been described as a feature of activated CD4+ T cells (51). Combined, the pattern of cell surface molecule expression on intestinal CD4+ T cells is consistent with a high degree of, and/or recent, cellular activation. Moreover, these cells are consistently negative for both CD45RA and L-selectin, which indicates that they have been antigen primed (i.e., are memory cells). Thus, the target cell for primary SIV infection may be defined as an activated memory CD4+ T cell. Furthermore, we have shown that loss of this specific subset of CD4+ T cells can consistently be detected in the blood and peripheral lymph nodes as well as in the intestinal mucosa in the first few weeks of SIV infection.
Activated mucosal memory T cells are by definition actively engaged in protecting mucosal surfaces from invasion by pathogens, as well as in regulating local immune responses to the large quantities of dietary antigens that are consumed. These cells are abundant throughout the intestinal tract, which is the largest immunologic organ of the body (30, 35). The size of the gastrointestinal tract combined with the high density of activated memory CD4+ T cells in the intestinal lamina propria may make this the most plentiful T-cell subset in the body of uninfected healthy individuals. By combining flow cytometry with quantitative assessments of T-cell numbers in the intestine, we have confirmed that SIV infection results in massive CD4+ T-cell loss per unit area of intestinal lamina propria. This represents an extensive loss of total CD4+ T cells in primary SIV infection since the gut is such a large reservoir of CD4+ T cells. Moreover, as demonstrated in this report, the intestine is the main reservoir for activated memory CD4+ T cells.
Combined, these data strongly suggest that there is a preference for SIV replication and amplification within a specific subset of CD4+ T cells. If primary infection and optimal viral replication depend on the presence of activated memory CD4+ T cells (which are found primarily in the intestine and are rapidly eliminated), this could contribute to the decline in viral loads observed following primary infection, as well as the establishment of a viral set point that signals the onset of clinical latency. A similar in vivo model for HIV infection whereby the reduction in viral loads in acute infection is hypothesized to be independent of an HIV-specific immune response has been proposed (39). If this model is correct, then possibly HIV replication in clinical latency is limited by the rate of T-cell production and conversion from naive to memory cells, which would be very slow in healthy individuals unless there was a specific stimulus for CD4+ T cell proliferation. Establishment of an opportunistic mucosal infection would provide this stimulus. Indeed, opportunistic infections as well as immune challenge and vaccination have been associated with increased viral loads in HIV infection (18, 37, 50). Alternatively, the virus itself may be involved in converting resting CD4+ T cells to appropriately activated memory T cells that promote viral replication. The nef genes of both HIV and SIV have been demonstrated to play a role in T-cell activation and viral replication in vitro (1, 13, 14, 23, 29, 57) and, for SIV, in vivo (14, 44). The biochemical pathways by which nef induces lymphocyte activation have not been completely elucidated, but studies have shown that nef interacts with a series of cellular partners including CD4, components of the adapter complexes AP-1 and AP-2, and several protein kinases (41).
Viral dynamics are also clearly influenced by the presence of virus specific CD8+ T cells, as shown by recent CD8+ T-cell depletion studies (21, 45). It is likely that both the depletion of optimal target cells and the development of SIV-specific immune responses contribute to the decline in viral loads and the establishment of viral set points in early SIV or HIV infection. In the future, it will be important to examine viral load, anti-SIV-specific CD8+ T-cell responses, and CD4+ T cells in the intestinal mucosa to address this issue. It is possible that intestinal CD4+ T-cell depletion is a result of direct viral lysis as well as CTL-mediated cell destruction. Thus, the depletion of CD8 cells may decrease the rate at which infected CD4+ T cells are lost, which could account for the increased viral loads.
In conclusion, we have defined the major target cell of primary SIV infection as an activated memory CD4+ T cell. The normal intestinal tract contains large numbers of this cell type, making this the preferred site of SIV and HIV replication, at least in primary infection. These data strongly suggest that inducing an appropriate anti-HIV immune response specifically within mucosal sites may be of paramount importance in producing an effective HIV vaccine.
ACKNOWLEDGMENTS
We thank Michael O'Connell for coordinating these studies and the animal care staff at the New England Regional Primate Research Center for their excellent care of the macaques.
This work was supported by NIH grants DK50550, RR00168, HD36310, and HL59787. A. A. Lackner is the recipient of an Elizabeth Glaser Scientist Award.
REFERENCES
- 1.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akari H, Mori K, Otani I, Terao K, Ono F, Adachi A, Yoshikawa Y. Induction of MHC-IIDR expression on circulating CD8+ lymphocytes in macaques infected with SIVmac239 nef-open but not with its nef-deletion mutant. AIDS Res Hum Retroviruses. 1998;14:619–625. doi: 10.1089/aid.1998.14.619. [DOI] [PubMed] [Google Scholar]
- 3.Bell E B, Sparshott S M, Bunce C. CD4+ T-cell memory, CD45R subsets, and the persistence of antigen—a unifying concept. Immunol Today. 1998;19:60–64. doi: 10.1016/s0167-5699(97)01211-5. [DOI] [PubMed] [Google Scholar]
- 4.Berg M, Murakawa Y, Camerini D, James S P. Lamina propria lymphocytes are derived from circulating cells that lack the Leu-8 lymph node homing receptor. Gastroenterology. 1991;101:90–99. doi: 10.1016/0016-5085(91)90464-v. [DOI] [PubMed] [Google Scholar]
- 5.Bjerke K, Brandtzaeg P, Fausa O. T cell distribution is different in follicle-associated epithelium of human Peyer's patches and villus epithelium. Clin Exp Immunol. 1988;74:270–275. [PMC free article] [PubMed] [Google Scholar]
- 6.Blair P J, Riley J L, Carroll R G, St. Louis D C, Levine B L, Saha B, Lee K P, Perrin P J, Harlan D M, June C H. CD28 co-receptor signal transduction in T-cell activation. Biochem Soc Trans. 1997;25:651–657. doi: 10.1042/bst0250651. [DOI] [PubMed] [Google Scholar]
- 7.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borvak J, Chou C-S, Bell K, Van Dyke G, Zola H, Ramilo O, Vitetta E S. Expression of CD25 defines peripheral blood mononuclear cells with productive versus latent HIV infection. J Immunol. 1995;155:3196–3204. [PubMed] [Google Scholar]
- 9.Butcher E C, Picker L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 10.Chou C-S, Ramilo O, Vitetta E S. Highly purified CD25− resting T cells cannot be infected de novo with HIV-1. Proc Natl Acad Sci USA. 1997;94:1361–1365. doi: 10.1073/pnas.94.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun T-W, Engel D, Mizell S B, Ehler L A, Fauci A S. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188:83–91. doi: 10.1084/jem.188.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton F, Snow G, Reka S, Kotler D P. Selective depletion of rectal lamina propria rather than lymphoid aggregate CD4 lymphocytes in HIV infection. Clin Exp Immunol. 1997;107:288–292. doi: 10.1111/j.1365-2249.1997.236-ce1111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Z, Ilyinskii P O, Sasseville V G, Newstein M, Daniel M D, Lackner A A, Desrosiers R C. Requirements for lymphocyte activation by unusual strains of simian immunodeficiency virus. J Virol. 1996;70:4157–4161. doi: 10.1128/jvi.70.6.4157-4161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Z, Lang S M, Sasseville V G, Lackner A A, Ilyinskii P O, Daniel M D, Jung J U, Desrosiers R C. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 15.Edmead C E, Lamb J R, Hoyne G F. The T cell surface protein, CD28. Int J Biochem Cell Biol. 1997;29:1053–1057. doi: 10.1016/s1357-2725(97)00012-5. [DOI] [PubMed] [Google Scholar]
- 16.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 17.Finzi D, Siliciano R F. Viral dynamics in HIV-1 infection. Cell. 1998;93:665–671. doi: 10.1016/s0092-8674(00)81427-0. [DOI] [PubMed] [Google Scholar]
- 18.Grossman Z, Feinberg M B, Paul W E. Multiple modes of cellular activation and virus transmission in HIV infection: a role for chronically and latently infected cells in sustaining viral replication. Proc Natl Acad Sci USA. 1998;95:6314–6319. doi: 10.1073/pnas.95.11.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haseltine W A, Wong-Staal F. The molecular biology of the AIDS virus. Sci Am. 1988;259:52–62. doi: 10.1038/scientificamerican1088-52. [DOI] [PubMed] [Google Scholar]
- 20.Heise C, Vogel P, Miller C J, Lackner A, Dandekar S. Distribution of SIV infection in the gastrointestinal tract of rhesus macaques at early and terminal stages of AIDS. J Med Primatol. 1993;22:187–193. [PubMed] [Google Scholar]
- 21.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai T, Wong J, MacLean J, Cosimi A B, Wee S. Characterization of a monoclonal antibody (6G12) recognizing the cynomolgus monkey CD3 antigen. Transplant Proc. 1994;26:1845. [PubMed] [Google Scholar]
- 23.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high viral loads and for the development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 24.Kewenig S, Schneider T, Hohloch K, Lampe-Dreyer K, Ullrich R, Stolte N, Stahl-Hennig C, Kaup F J, Stallmach A, Zeitz M. Rapid mucosal CD4+ T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115–1123. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 25.Lefrere J J, Roudot-Thoraval F, Mariotti M, Thauvin M, Lerable J, Salpetrier J, Morand-Joubert L. The risk of disease progression is determined during the first year of human immunodeficiency virus type 1 infection. J Infect Dis. 1998;177:1541–1548. doi: 10.1086/515308. [DOI] [PubMed] [Google Scholar]
- 26.Lenschow D J, Walunas T L, Bluestone J A. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 27.Lim S G, Condez A, Lee C A, Johnson M A, Elia C, Poulter L W. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin Exp Immunol. 1993;92:448–454. doi: 10.1111/j.1365-2249.1993.tb03419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lloyd T E, Yang L, Tang D N, Bennett T, Schober W, Lewis D E. Regulation of CD28 costimulation in human CD8+ T cells. J Immunol. 1997;158:1551–1558. [PubMed] [Google Scholar]
- 29.Luo T, Downing J R, Garcia J V. Induction of phosphorylation of human immunodeficiency virus type 1 nef and enhancement of CD4 downregulation by phorbol myristate acetate. J Virol. 1997;71:2535–2539. doi: 10.1128/jvi.71.3.2535-2539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald T T, Spencer J. Lymphoid cells and tissues of the gastrointestinal tract. In: Heatley R H, editor. Gastrointestinal and hepatic immunology. Cambridge, England: Cambridge University Press; 1994. pp. 394–421. [Google Scholar]
- 31.Mahalingham M, Peakman M, Davies E T, Pozniak A, McManus T J, Vergani D. T cell activation and disease severity in HIV infection. Clin Exp Immunol. 1993;93:337–343. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malavasi F, Funaro A, Roggero S, Horenstein A L, Calosso L, Mehta K. Human CD38: one molecule in search of a function. Immunol Today. 1994;15:95–97. doi: 10.1016/0167-5699(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 33.McDougal J S, Mawle A, Cort S P, Nicholson J K A, Cross G D, Schleppler-Campbell J A, Hicks D, Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985;135:3151–3162. [PubMed] [Google Scholar]
- 34.Mellors J W, Rinaldo C R, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 35.Mowat A M, Viney J L. The anatomical basis of intestinal immunity. Immunol Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 36.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 37.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 38.Pantaleo G, Graziosi C, Fauci A S. Virologic and immunologic events in primary HIV infection. Springer Semin Immunopathol. 1997;18:257–266. doi: 10.1007/BF00813497. [DOI] [PubMed] [Google Scholar]
- 39.Phillips A N. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science. 1996;271:497–498. doi: 10.1126/science.271.5248.497. [DOI] [PubMed] [Google Scholar]
- 40.Picker L J, Treer J R, Ferguson-Darnell B, Collins P A, Buck D, Terstappen L W M M. Control of lymphocyte recirculation in man. 1. Differential regulation of the peripheral lymph node homing receptor L-selectin on T cells during the virgin to memory cell transition. J Immunol. 1993;150:1105–1121. [PubMed] [Google Scholar]
- 41.Piguet V, Trono D. The nef protein of primate lentiviruses. Rev Med Virol. 1999;9:111–120. doi: 10.1002/(sici)1099-1654(199904/06)9:2<111::aid-rmv245>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 42.Reinis M, Morra M, Funaro A, Di Primio R, Malavasi F. Functional associations of CD38 with CD3 on the T cell membrane. J Biol Regul Homeost Agents. 1997;11:137–142. [PubMed] [Google Scholar]
- 43.Roederer M, Dubs J G, Anderson M T, Raju P A, Herzenberg L A. CD8 naive T cell counts decrease progressively in HIV-infected patients. J Clin Investig. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasseville V G, Du Z, Chalifoux L V, Pauley D R, Young H Y, Sehgal P K, Desrosiers R C, Lackner A A. Induction of lymphocyte proliferation and severe gastrointestinal disease in macaques by a nef gene variant of SIVmac239. Am J Pathol. 1996;149:163–175. [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 46.Schneider T, Jahn H-U, Schmidt W, Reicken E-O, Zeitz M, Ulrich R. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Gut. 1995;37:524–529. doi: 10.1136/gut.37.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnittman S M, Lane H C, Greenhouse J, Justement J S, Baseler M, Fauci A S. Preferential infection of CD4+ memory cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smit-McBride Z, Mattapallil J J, McChesney M, Ferrick D, Dandekar S. Gastrointestinal T lymphocytes retain high potential for cytokine responses but have severe CD4+ T-cell depletion at all stages of simian immunodeficiency virus infection compared to peripheral lymphocytes. J Virol. 1998;72:6646–6656. doi: 10.1128/jvi.72.8.6646-6656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spina C A, Prince H E, Richman D D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Investig. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staprans S I, Hamilton B L, Follansbee S E, Elbeik T, Barbosa P, Grant R M, Feinberg M B. Activation of virus replication after vaccination of HIV-1 infected individuals. J Exp Med. 1995;182:1727–1737. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Hove L E, Vandenberghe P, Van Gool S W, Ceuppens J L, Demuynck H, Verhoef G E, Boogaerts M A. Peripheral blood lymphocyte subset shifts in patients with untreated hematological tumors: evidence for systemic activation of the T cell compartment. Leuk Res. 1998;22:175–184. doi: 10.1016/s0145-2126(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 52.van Noesel C J, Gruters R A, Terpstra F G, Schellekens P T, van Lier R A, Miedema F. Functional and phenotypic evidence for a selective loss of memory T cells in asymptomatic human immunodeficiency virus-infected men. J Clin Investig. 1990;86:293–299. doi: 10.1172/JCI114698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson R P, Desrosiers R C, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 54.Veazey R S, Lackner A A. The gastrointestinal tract and the pathogenesis of AIDS. AIDS. 1998;12:S35–S42. [PubMed] [Google Scholar]
- 55.Veazey R S, Rosenzweig M, Shvetz D E, Pauley D R, DeMaria M, Chalifoux L V, Johnson R P, Lackner A A. Characterization of gut-associated lymphoid tissues (GALT) of normal rhesus macaques. Clin Immunol Immunopathol. 1997;82:230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- 56.Westmoreland S V, Halpern E, Lackner A A. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J Neurovirol. 1998;4:260–268. doi: 10.3109/13550289809114527. [DOI] [PubMed] [Google Scholar]
- 57.Whetter L, Novembre F J, Saucier M, Gummurulu S, Dewhurst S. Costimulatory pathways in lymphocyte proliferation induced by the simian immunodeficiency virus SIVsmmPBj14. J Virol. 1998;72:6155–6158. doi: 10.1128/jvi.72.7.6155-6158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willerford D M, Gale M J, Jr, Benveniste R E, Clark E A, Gallatin W M. Simian immunodeficiency virus is restricted to a subset of blood CD4+ T lymphocytes that includes memory cells. J Immunol. 1990;144:3779–3783. [PubMed] [Google Scholar]
- 59.Zeigler S F, Ramsdell F, Alderson M R. The activation antigen CD69. Stem Cells. 1994;12:456–465. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
- 60.Zeitz M, Greene W C, Peffer N J, James S P. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T cell activation. Gastroenterology. 1988;94:647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]