Abstract
Oxidative stress can be associated with hyperoxia and hypoxia and is characterized by an increase in reactive oxygen (ROS) and nitrogen (RNS) species generated by an underlying disease process or by supplemental oxygen that exceeds the neutralization capacity of the organ system. ROS and RNS acting as free radicals can inactive several enzymes and vasodilators in the nitric oxide pathway promoting pulmonary vasoconstriction resulting in persistent pulmonary hypertension of the newborn (PPHN). Studies in animal models of PPHN have shown high ROS/RNS that is further increased by hyperoxic ventilation. In addition, antioxidant therapy increased PaO2 in these models, but clinical trials are lacking. We recommend targeting preductal SpO2 between 90 and 97%, PaO2 between 55 and 80 mmHg and avoiding FiO2 > 0.6–0.8 if possible during PPHN management. This review highlights the role of oxidative and nitrosative stress markers on PPHN and potential therapeutic interventions that may alleviate the consequences of increased oxidant stress during ventilation with supplemental oxygen.
Keywords: Persistent pulmonary hypertension, Oxidative stress, Free radical, Antioxidant therapy, Nitric oxide, Superoxide anions, Peroxynitrite
1. Introduction
Persistent pulmonary hypertension of the newborn (PPHN) is a clinical syndrome that is characterized by failure to achieve or sustain the decline in pulmonary vascular resistance (PVR) at birth, leading to extrapulmonary right-to-left shunting of blood across the patent foramen ovale (PFO) or patent ductus arteriosus (PDA) and severe hypoxemia [1]. Mechanisms that increase PVR in PPHN include high pulmonary vascular tone, abnormal vasoreactivity, remodeled vascular wall structure, and reduced vascular growth [2].
While neonatal lung injury is often a result of a multitude of etiologic factors, oxidative stress has been recognized as a critical factor in the pathophysiology of several neonatal lung diseases associated with PPHN. Oxidative stress is also a common endpoint for multiple events, including inflammation, hypoxia, hyperoxia, and mechanical ventilation, that contribute to sustained lung injury and result in impaired pulmonary hemodynamics and gas exchange [3].
Significance of oxidative stress in various diseases in the neonatal period was first recognized in the 1980’s and the term “oxygen radical disease in the newborn” was coined in 1988 [4]. Oxidative stress from various sources, combined with immature antioxidant defenses, may lead to oxidative lung injury in a vulnerable neonate [5].
In this review, we discuss the abnormal fetal-to-newborn transition at birth and the role of oxidative and nitrosative stress in the pathophysiology of PPHN. We briefly discuss the contribution of therapeutic interventions, such as increased FiO2, inhaled nitric oxide (iNO) and mechanical ventilation, on oxidative stress. We finally identify potential therapeutic interventions that may alleviate the consequences of increased oxidant stress during supplemental oxygen ventilation and further discuss the optimal oxygen targets in PPHN.
2. Birth and pulmonary vascular transition
At birth the organ of gas exchange changes from the placenta to the lung resulting in major circulatory adjustments. With initial crying, alveolar oxygen tension (PAO2) increases from fetal levels (~18 mmHg) to postnatal levels (~80–100 mmHg) [6] (Fig. 1). Under normal circumstances, this increase in PAO2 triggers a progressive fall in PVR, accompanied by a simultaneous rise in systemic vascular resistance (SVR) once umbilical blood flow ceases. Pulmonary vasodilation and reversal of ductal shunting leads to an 8–10 fold increase in pulmonary blood flow, which is regulated by complex physiologic and biochemical processes with a central role for nitric oxide (NO) [7]. Pulmonary endothelial NO production increases markedly at the time of birth in response to oxygen and exerts its action through soluble guanylate cyclase (sGC) and cyclic guanosine monophosphate (cGMP) [8,9]. Phosphodiesterase type 5 (PDE5) impairs this vasorelaxation by degrading cGMP. In addition, endothelial nitric oxide synthase (eNOS) dysfunction, induced through increased levels of asymmetric dimethyl arginine (ADMA), a competitive endogenous inhibitor of NOS, or by decreased synthesis of the NOS substrate L-arginine results in pulmonary vasoconstriction [10–12]. These enzymes are sensitive to oxidative stress (Fig. 2).
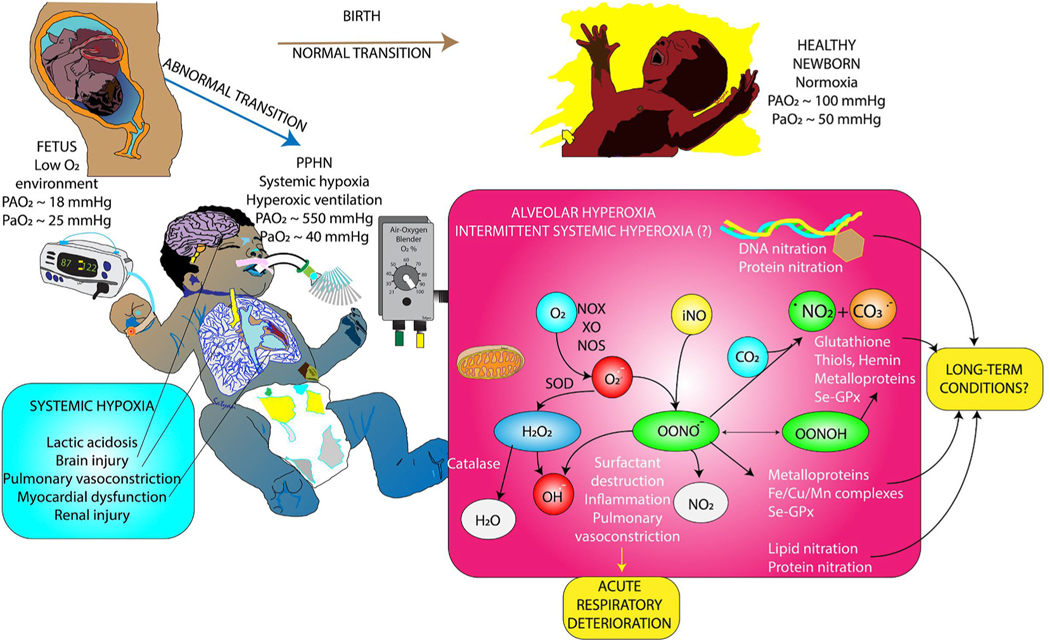
Fig. 1.
Normal and abormal transition at birth and role of oxidative stress in persistent pulmonary hypertension of the newborn (PPHN). The fetus lives in a state of hypoxemia with low arterial (PaO2) and alveolar oxygen (PAO2) tension. Normal transition at birth results in room air ventilation and a modest increase in PaO2 and PAO2 leading to pulmonary vasodilation and establishment of lungs as the site of gas exchange. When transition is abnormal, pulmonary vasodilation does not occur resulting in persistence of high pulmonary arterial pressures and right-to-left extrapulmonary shunts leading to systemic hypoxemia despite alveolar hyperoxia. Alveolar hyperoxia leads to oxidative stress and release of reactive oxygen species. See text for details. Copyright Satyan Lakshminrusimha.
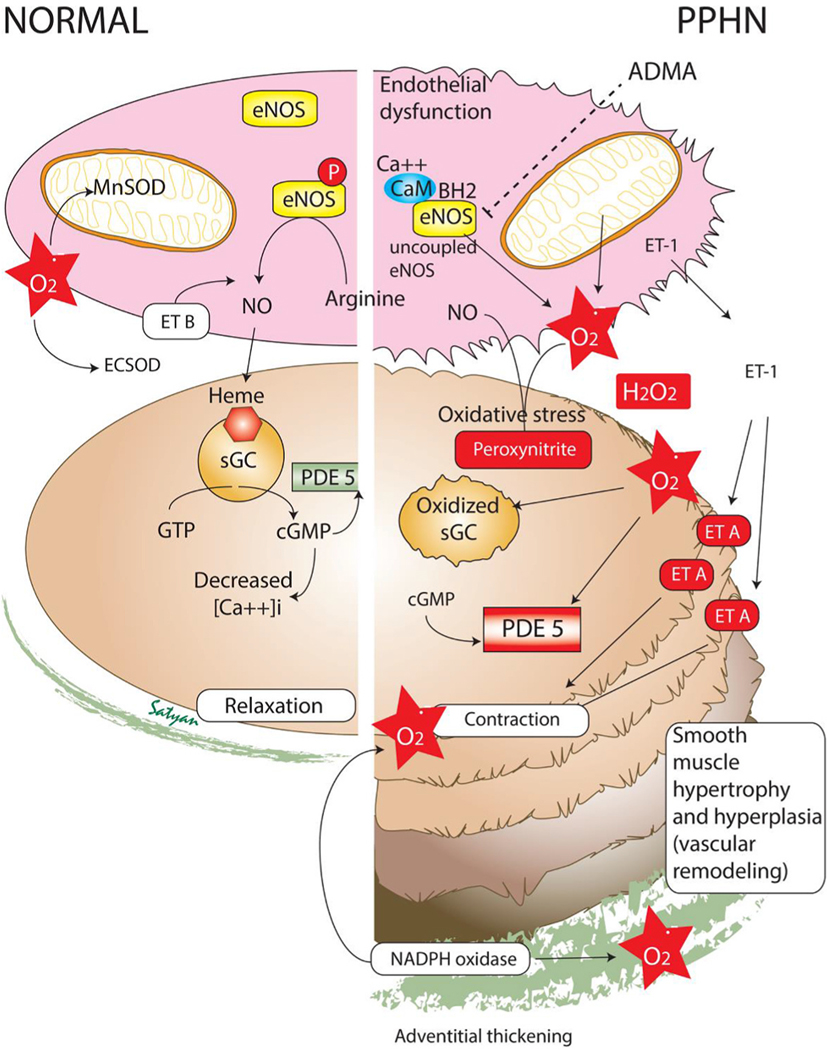
Fig. 2.
Alterations in biochemical pathways in pulmonary vascular endothelial and smooth muscle cells in normal and persistent pulmonary hypertension of the newborn (PPHN). Endothelial dysfunction, smooth muscle hyperplasia and hypertrophy and adventitial thickening are common in PPHN. Increased oxidative stress with high levels of superoxide anions () alters several enzymes in the nitric oxide (NO) pathway increasing the risk of vasoconstriction. ADMA - asymmetric dimethyl arginine; eNOS – endothelial nitric oxide synthase; ET – endothelin; SOD – superoxide dismutase; MnSOD – Manganese, mitochondrial superoxide dismutase; EC-SOD – extracellular superoxide dismutase; sGC – soluble guanylate cyclase; PDE 5 – phosphodiesterase 5; GTP – guanosine triphosphate; cGMP – cyclic guanosine monophosphate; NO – nitric oxide; CaM – calmodulin; Modified from Polin and Fox Fetal and Neonatal Physiology, 6th edition, copyright Satyan Lakshminrusimha (with permission).
The transition from prenatal to postnatal life causes a significant increase in arterial oxygen tension and the activation of metabolic pathways enabling the newborn’s adaptation to the extra-uterine environment. Cellular redox status represents the balance between reductive and oxidant metabolites and is indispensable for cellular growth, differentiation, and preservation of biological functions [13]. Oxidative stress has been defined as the imbalance of pro-and-antioxidants in favor of the former and is the consequence of an excessive free radical production or inability of the antioxidant defense system to neutralize them [14] (Fig. 3). Oxidative stress contributes to cellular and tissue damage and/or dysfunction and interferes with the normal postnatal decline in the PVR/SVR ratio causing the transitional circulation to persist leading to PPHN.
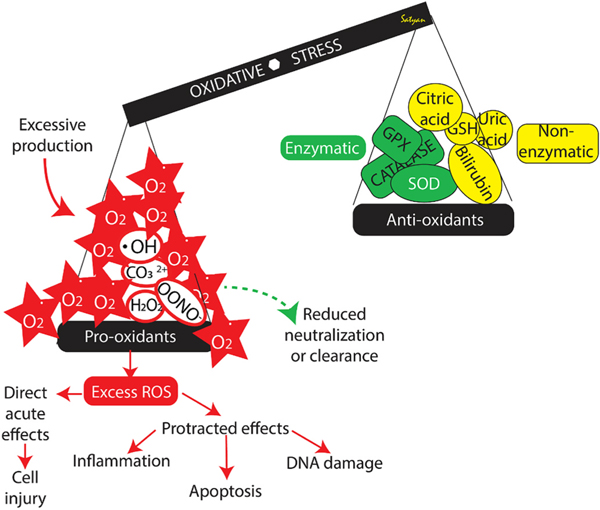
Fig. 3.
Definition of oxidative stress and the imbalance between pro-oxidants and antioxidants. Excess reactive oxygen species (ROS) can have acute and chronic effects. Please see text for details. Copyright Satyan Lakshminrusimha.
3. Oxidative stress and PPHN
ROS is a generic term for an ample variety of oxidants derived from molecular oxygen. They are part of a family of reactive species, including among others reactive nitrogen, sulfur, carbon, and selenium species that undergo reduction and oxidation reactions. Consequent to these reactions, biological macromolecules such as proteins, DNA, or lipids withstand oxidative modifications that contribute to redox signaling and biological function. However, excessive concentration of ROS reacts non-specifically with macromolecules, further generating other reactive species with potentially toxic effects [15].
The pulmonary vasculature undergoes morphological changes in PPHN that can be mediated by oxidative stress [9]. Past research has shown the expression of various oxidative stress markers changes in the lungs and pulmonary vasculature of animals and humans with PPHN.
3.1. Oxidative and nitrosative stress (Figs. 2–4)
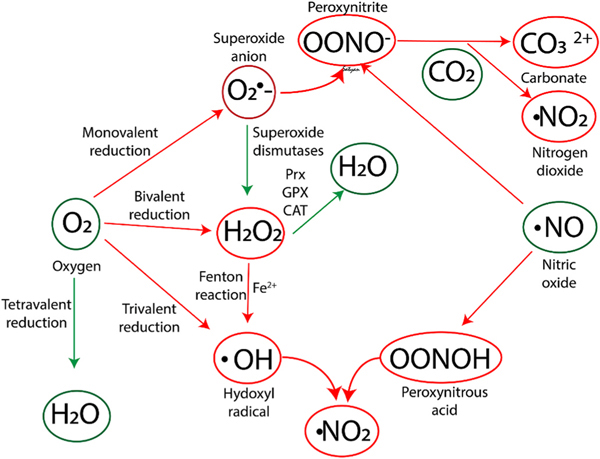
Fig. 4.
Biochemical reactions involved in oxidative and nitrosative stress. Prx - peroxiredoxins, GPX - glutathione peroxidases (GPX) and CAT - catalases. Please see text for details. Copyright Satyan Lakshminrusimha.
ROS are produced within different cellular substructures: plasma membrane, cytosol, peroxisomes, mitochondria, lysosomes, and endoplasmic reticulum [16]. The extracellular face of the plasma membrane is a major cellular site of the anion superoxide () and hydrogen peroxide (H2O2) generation via NADPH oxidases (NOXs) under physiological conditions [17]. Moreover, ROS are also generated from metabolic redox reactions mostly by the mitochondrial oxidative phosphorylation process in the electron transport chain, but also by the microsomal cytochrome P450 system and by the immune response [18]. Under physiologic circumstances, dioxygen undergoes a tetravalent reduction to water. However, under pro-oxidant conditions, oxygen is only partially reduced to reactive species (Fig. 4). Superoxide anion (), hydrogen peroxide (H2O2), and hydroxyl radical (⋅OH) result from monovalent, divalent, and trivalent reduction of oxygen, respectively [19,20]. The family of superoxide dismutases (SODs) in the cytosol, mitochondria, or extracellular fluid contain transition metals such as Cu, Mn, Zn, or Fe and catalyze the dismutation of the anion superoxide into H2O2 and O2. H2O2 can be directly reduced to water by peroxiredoxins (Prx), glutathione peroxidases (GPX), or catalases (CAT). Alternatively, in the presence of reduced transition metals, especially Fe2+, H2O2 generates highly reactive hydroxyl (OH•) radicals (Fenton chemistry). Of note, no effective antioxidant defenses against hydroxyl radicals are present in biological systems [21].
Nitric oxide (NO) is generated during the breakdown of arginine to citrulline by the NADPH-dependent NO synthase (NOS). •NO is a relatively stable and highly diffusible free radical. •NO-mediated pathogenicity depends upon the formation of highly reactive and toxic byproducts, such as peroxynitrite anion (ONOO− ), peroxynitrous acid (ONOOH), and nitrogen dioxide (•NO2). These are extremely cytotoxic compounds that oxidize membrane lipoproteins, enzymatic and structural proteins, and DNA [21]. The formation of RNS requires the interaction of •NO with oxidants, such as superoxide radicals, hydrogen peroxide, and transition metals like iron or copper. Of note, the rate constant of the reaction of the anion superoxide () with •NO is significantly higher than that of the SOD-catalyzed dismutation, and therefore, •NO kinetically outcompetes SOD for the anion superoxide [22]. In addition, in the presence of carbon dioxide (CO2), peroxynitrite forms highly reactive nitrogen dioxide and carbonate radicals, which directly react with DNA and proteins. In addition, peroxynitrous acid in the presence of hydroxyl radicals (•OH) generated through Fenton reaction leads to the formation of nitrogen dioxide, which directly attacks lipids and proteins [23]. Under stressful conditions, an excessive production of ROS and RNS will not only cause direct damage to cellular components, but it also lead to aberrant signaling that results in dysfunction and disease [15].
H2O2 is a redox signaling agent with no free radical characteristics. Under physiologic conditions, H2O2 is produced at a controlled steady-state level and acts as a second messenger amplifying its biological signal through kinase cascades or can be transmitted over long distances by conversion to more stable species, such as lipid peroxides or hydroxynonenal. Signal transduction by H2O2 is mediated by a selective and efficient oxidation of specific thiols on specific signaling proteins [24]. However, mechanisms and rates of mitochondrial H2O2 release required to exert biological effects are still unknown. Acute and intense bursts of mitochondrial H2O2 production, such as during post-ischemia reperfusion, do not appear to be sufficient to reach the cytosol. However, protracted generation of H2O2, such as during persistent hyperoxemia, inflammatory processes with persistent activation of macrophages, can enhance cytosolic oxidant levels, perhaps reflecting an eventual overriding of matrix antioxidant defense systems [25].
3.2. Analytical biomarkers of oxidative and nitrosative stress damage
The functional and structural damage caused to cellular components because of their interaction with free radicals is highly dynamic and difficult to assess. To date, no gold standard of oxidative/nitrosative stress damage in preterm infants has been established. A comprehensive approach to the pathophysiology of free radicals would include the assessment of the redox steady state and the damage to biomolecules promoted by oxidative stress. The latter is the most widely employed approach to assess oxidative damage and includes metabolites derived from damage to proteins, DNA, RNA, lipids, and carbohydrates. The analytical methods employed have evolved in recent years and include spectroscopic, colorimetric, fluorometric, enzymatic, immunoassay and most recently hyphenated separation techniques such as liquid or gas chromatography or capillary electrophoresis coupled to tandem mass spectrometry, which are at present the most reliable and applicable to most biofluids (including whole blood, plasma, serum, urine, amniotic fluid, or cerebral spinal fluid) [5]. Among the most widely employed and reliable oxidative damage biomarkers are the byproducts of lipid peroxidation, especially the polyunsaturated fatty acids (PUFA), which are essential components of biological membranes. Free radicals acting upon PUFA generate a burst of hydroperoxides in a radical chain reaction yielding to very labile endoperoxides that subsequently decompose in an ample array of compounds, such as isoprostanes/isofurans, neuroprostanes/neurofurans and dihomo-isoprostanes/dihomo-isofurans), which are derived from arachidonic acid, docosahexanoic acid and adrenic acid, respectively. Isoprostanoids and Isofuranoids are excellent biomarkers because of their stability when compared to other frequently employed biomarkers such as malondialdehyde. Moreover, it has also been reported that isofuranoids’ generation is closely dependent upon the oxygen tension, which is of utmost interest given the relevance of oxygen as a causative agent of severe conditions in the perinatal period [26,27].
Preterm infants’ DNA repair mechanisms are ineffective, and therefore, preterm infants are predisposed to long-lasting DNA structural alterations. During fetal-to-neonatal transition and thereafter, oxidative stress has been linked to oxidative alterations of DNA consisting of oxidative modifications of the guanine bases. The guanine base from the deoxyribonucleoside deoxyguanosine (2 dG) is the most susceptible to oxidative modifications yielding to the formation of 8-oxo-2′-deoxyguanosine (8-oxo-dG) also known as 8-hydroxy-2′-deoxyguanosine (8-OHdG). The quotient 8-OHdG/2 dG has been widely employed as a reliable marker of oxidative damage to DNA in preterm infants [28–30].
Free radicals induce modifications in the amino acid side chains causing an alteration of the structure and/or the function of proteins. The formation of protein carbonyl derivatives, which is produced by the direct action of free radicals or by the reaction with oxidized compounds, has been widely employed to assess oxidative stress in preterm infants [31–33]. In addition, the determination of specific byproducts of amino acid oxidation is a valid and widely employed alternative approach. Under physiologic conditions, phenylalanine is metabolized to para-tyrosine (P-tyr) but through non-enzymatic hydroxylation caused by oxygen and nitrogen free radicals, phenylalanine is metabolized to meta-tyrosine (m-Tyr), ortho-tyrosine (o-Tyr), 3-chlorotyrosine (3-Cl-Tyr), 3-nitrotyrosine (3-NO2-Tyr) and 3-nitro-4-hydroxyphenylacetic acid (NHPA). m-Tyr and o-Tyr reflect the direct action of hydroxyl radicals usually generated by the Fenton reaction in the presence of transition metals upon proteins. On the other hand, 3-Cl-Tyr reflects is a byproduct of the reaction of hypochlorous acid originated from myeloperoxidase with p-Tyr in neutrophils and monocytes. 3-NO2-Tyr is the byproduct of the reaction of p-Tyr with nitrogen free radicals, especially peroxynitrite; furthermore, NHPA is also employed as a marker of protein nitration [5]. Phenylalanine oxidation byproducts have been widely explored in urine samples of preterm infants. This non-invasive approach has allowed performance of serial determinations in different pathologic conditions [29]. Moreover, recently, a validated analytical method has been employed for the determination of protein oxidation byproducts in amniotic fluid to predict fetal outcome [34].
3.3. The relevance of ROS/RNS in neonatal lung disease
The importance of ROS in neonatal lung disease has been clearly demonstrated in animal models of PPHN. Studies in our laboratory utilizing the prenatally ligated ductus arteriosus lamb model have linked mitochondrial oxidative stress to maladaptive changes in NO signaling pathways and the pathogenesis of PPHN [35,36]. Furthermore, activation of phosphodiesterase-5 (PDE5), decreased cGMP-responsiveness to exogenous NO, and decreased expression of eNOS, may promote additional injury through increased vasoconstriction and impaired responsiveness to vasodilators (Fig. 2) [37].
Increased H2O2 in pulmonary arteries is associated with decreased expression of soluble guanylate cyclase and reductions in cGMP levels contributing to pulmonary vasoconstriction and decreased activity of antioxidant - extracellular superoxide dismutase (ecSOD) [35,38].
Reaction of with NO forms ONOO− which depletes bioavailable NO and impairs NO-mediated vasorelaxation. ONOO− is a potent oxidant that causes vascular dysfunction by non-specific nitration of proteins and results in a significant alteration in their functions. Impaired regulation of free radicals potentially induces vascular injury secondary to their interaction with proteins, DNA, RNA and lipids [39].
3.4. Cellular and biochemical changes from oxidative stress in PPHN (Fig. 2)
Normal pulmonary vascular tone is regulated by the interaction of NO and endothelin-1 (ET-1) produced by the vascular endothelium [40, 41]. NO is an endothelium-derived relaxing factor synthesized by the oxidation of l-arginine after activation of eNOS [42]. On the other hand, ET-1 has potent vasoactive properties and is mitogenic for pulmonary vascular smooth muscle cells (SMC) [43]. The pulmonary vasoactive effects of ET-1 are mediated by ETA receptors, located on vascular SMC (vasoconstriction) and ETB receptors, located on vascular EC (vasodilation) [44].
In PPHN, abnormal regulation of the ET-1 and NO signaling cascades occur where ET-1 levels are elevated and eNOS expression is decreased. Oxidative stress enhances expression of ET-1 resulting in vasoconstriction [45]. In addition, there is an increase in the expression of the genes that induce pulmonary vasoconstriction and a reduction in those that induce vasodilation [46]. Overall ET-1-mediated vasoconstriction with reduced NO-mediated vasodilation associated with oxidative stress contributes to pulmonary hypertension.
4. Evidence from animal studies
4.1. Hyperoxic resuscitation
Ventilation of lambs with 100% oxygen at birth and in the immediate postnatal period is associated with increased contractility of pulmonary arteries [47–49]. Such increased contractility of pulmonary arteries following resuscitation of asphyxiated lambs with 100% oxygen can be reversed by treatment of the vessels with antioxidants, suggesting that ROS is probably the mediator of such increased contractility [50]. In lambs with PPHN, resuscitation with 100% oxygen impairs the subsequent relaxation response to iNO [51]. These results suggest that although resuscitation with 100% oxygen may transiently enhance pulmonary vasodilation, subsequent response to pulmonary vasodilators is impaired and pulmonary vasoreactivity is enhanced, further contributing to PPHN.
4.2. Antenatal ductal ligation lamb model of PPHN
Our laboratory has shown that in fetal lambs, ligation or compression of the ductus arteriosus rapidly induces fetal and neonatal pulmonary hypertension. Similar to autopsy findings in newborns with PPHN [52], these lambs mimic the physiopathologic characteristics including medial hypertrophy within the small pulmonary arteries, complete muscularization of pulmonary arteries, and extension of muscle to non-muscularized arteries leading to persistently increased PVR, (pulmonary artery pressure (PAP), and right ventricular (RV) hypertrophy.
Evidence from animal models of PPHN supports the theory that oxidative stress plays an important role in the pathogenesis of PPHN. Lambs with PPHN demonstrate both antenatal and postnatal evidence of increased oxidative stress compared to control animals, including increased levels of and H2O2 in the pulmonary vasculature and exaggerated ROS production in response to hyperoxia after birth [38,53, 54]. In addition, increased oxidative stress in PPHN lambs is linked to negative effects on NO signaling pathways and aberrant pulmonary vasodilatory responses, which again supports the idea that ROS play an important role in the pathogenesis of PPHN [55]. These lambs exhibit alterations in major components of NO-mediated pulmonary vasodilation, including decreased NOS expression and activity, decreased expression of soluble guanylate cyclase, increased PDE5 expression and activity and increased ET-1 levels [46,56,57]. These alterations may be secondary to increased pulmonary artery H2O2, which decreases eNOS expression and impairs cGMP production [54,58]. Ventilation with 100% oxygen and iNO for 24 h resulted in significantly increased levels of peroxynitrite (3-NT) in pulmonary vasculature, and this signal was quenched by treatment with recombinant human superoxide dismutase (rhSOD) or by ventilation with lower concentrations of oxygen [9].
4.3. Ovine model of aortopulmonary shunt
Pulmonary hypertension can be created in lambs by antenatal placement of an aortopulmonary shunt to increase blood flow resulting in mechanical shear stress [59], oxidative stress, and vascular remodeling [60]. In this model, the predominant cause of superoxide anions appears to be NADPH oxidase [60].
4.4. Rodent model of PPHN
A study by Xu et al. found that increased oxidative stress contributes to pulmonary hypertension development in rodents [61]. In normal conditions, extracellular SOD is expressed in high concentrations in the lungs and is responsible for removing extracellular superoxide anions. This study showed that absence of SOD resulted in significantly worse pulmonary hypertension in hypoxic SOD knockout mice and MCT rats with a SOD loss-of-function gene mutation.
In another study in rats, Wang et al. examined the effects of 17β-estradiol and 2-methoxyestradiol on the oxidative stress-hypoxia inducible factor-1 (OS–HIF-1) pathway with hypoxia-induced pulmonary hypertension [62]. Hypoxic rats had a significant increase in oxidative stress levels as indicated by increased serum ROS levels, decreased serum SOD, and decreased manganese SOD (MnSOD) levels. Furthermore, MnSOD mRNA and protein levels were decreased in the lung tissue.
In a separate study, rats treated with a high dose of iron dextran demonstrated increased vasoconstriction and vascular hyper-reactivity of pulmonary arteries and reduced NO, which were reversed by antioxidant therapy [63].
4.5. In vitro studies
Pulmonary artery smooth muscle cells (PASMC) isolated from the ductal ligation PPHN lamb model had elevated cytosolic ROS, increased Nox4 expression (NADPH oxidase isoform), and decreased extracellular superoxide dismutase (ecSOD) activity relative to control PASMC. Nox4 RNA knockdown attenuated cytosolic ROS levels and elevated ecSOD activity in these cells [64].
In addition, PASMCs from this PPHN lamb model had increased basal PDE5 activity, decreased cGMP-responsiveness to NO, and increased mitochondrial matrix oxidant stress compared to control PASMC. Mitochondrially targeted catalase decreased PDE5 activity at baseline and after hyperoxia in PPHN PASMC. Similarly, catalase treatment of PPHN lambs ventilated with 100% O2 decreased PDE5 activity and increased cGMP in the PA [36]. We speculate that PPHN-induced mitochondrial superoxide is converted to H2O2 by MnSOD and crosses mitochondrial membranes thereby contributing to elevated cytosolic ROS.
Rao et al. have shown that ROS generated from using xanthine/xanthine oxidase stimulates vascular smooth muscle DNA synthesis and increases cell number, suggesting that altered ROS generation and scavenging also contribute to pulmonary vascular remodeling in PPHN [65]. Increased vascular remodeling results from disturbed development and enhanced proliferation of PASMC, leading to hyperplasia and hypertrophy of the vascular smooth muscle layer, narrowing vascular lumen, and increasing PVR.
5. Antioxidant defense and antioxidant therapy
The antioxidant defense system in newborns, especially premature infants, is underdeveloped [5]. At term, the antioxidant system starts maturing to help transition from the relatively hypoxic fetal environment to the high oxygen tension extrauterine environment after birth [66].
PPHN and hyperoxia elevate pulmonary ROS via distinct mechanisms, suggesting that ROS scavengers may be effective in limiting the oxidant stress in PPHN infants ventilated with oxygen. In the past, the attempts to therapeutically target these antioxidant systems have yielded inconsistent results [67]. Antioxidant therapy developments have been limited by biochemical properties like compound half-life, poor cell penetrance, and difficulty targeting intracellular organelles [68].
5.1. Therapeutic interventions against oxidative stress in PPHN
Oxygen free radicals act on the NO pathway reducing cGMP and promoting pulmonary vasoconstriction. Use of antioxidant therapy may improve systemic oxygenation. Antioxidants regulate vascular signaling pathways by scavenging free radicals. Enzyme systems donate an electron to molecular oxygen to generate superoxide anion (), and SODs generate the non-radical ROS H2O2 through dismutation of superoxide. H2O2 is diffusible across membranes and is scavenged by enzymes including catalase and glutathione peroxidase.
Previously, our laboratory has shown that intratracheal administration of antioxidants, such as the NADPH oxidase inhibitor apocynin and recombinant human SOD (rhSOD), decreases ROS, increases eNOS expression, and normalizes tetrahydrobiopterin levels after ventilation with 100% O2 for 24 h in neonatal lamb models of PPHN [69,70]. Intratracheal recombinant human SOD (rhSOD) also reduces ONOO− mediated protein nitration, decreases PDE5 activity, and increases cGMP in the pulmonary arteries of ventilated PPHN lambs [71,72]. Recombinant human SOD mitigated the increased PA contractility and lung isoprostanes levels and decreased the enhanced lung 3-nitrotyrosine fluorescence observed with iNO therapy [71]. Intratracheal catalase has shown to improve oxygenation by improving NO-mediated vasodilation, increasing lung ecSOD activity, and decreasing PA superoxide levels and PA PDE5 activity in the ductal ligation model of PPHN [36].
Glucocorticoids are commonly used in the management of PPHN. In studies on the PPHN lamb model, antenatal betamethasone and post-natal hydrocortisone are associated with improved oxygenation by increasing superoxide dismutase activity and reducing oxidant stress [73,74].
Clinically, antioxidant therapies have not been thoroughly evaluated. We speculate that the antioxidant therapies will need to be precisely targeted at a cellular and subcellular level to be most effective in the treatment of ROS-induced neonatal pulmonary hypertension. Clinical trials of antioxidant therapies in iNO-resistant PPHN are warranted.
6. Oxygen therapy, optimal oxygenation, and oxidative stress markers in PPHN
The hallmark of PPHN is labile hypoxemia, and providing adequate oxygenation forms the mainstay of PPHN therapy. Oxygen is a double-edged sword, as hypoxia (<45 mm Hg) increases PVR by hypoxic pulmonary vasoconstriction and contributes to the pathophysiology of PPHN (Fig. 5). On the other hand, hyperoxia (>80–100 mmHg) does not further decrease PVR and instead results in free radical injury [75]. Among term infants with PPHN, avoiding hypoxia and hyperoxia by titrating supplemental oxygen to maintain saturations in the low to mid 90%s (with alarm limits at 90 and 97%) seems to be a reasonable approach [76]. Kapadia et al. have demonstrated that the incidence of hypoxic-ischemic encephalopathy (HIE) increases if neonates with perinatal asphyxia had PaO2 > 100 mmHg in the first postnatal hour [77]. In the ovine ductal ligation model of PPHN, maintaining oxygen saturation in the 90–97% range results in low PVR [51]. In the same model, as mentioned previously, resuscitation with 100% O2 does not enhance pulmonary vasodilation compared to 21% and 50% O2 but impairs the subsequent response to iNO [51]. We speculate that even brief exposure to 100% oxygen at birth increases free radical generation that can impair effectiveness of iNO. Similar increases in oxidative stress have been observed in term human neonates after resuscitation with 100% oxygen [78].
Fig. 5.
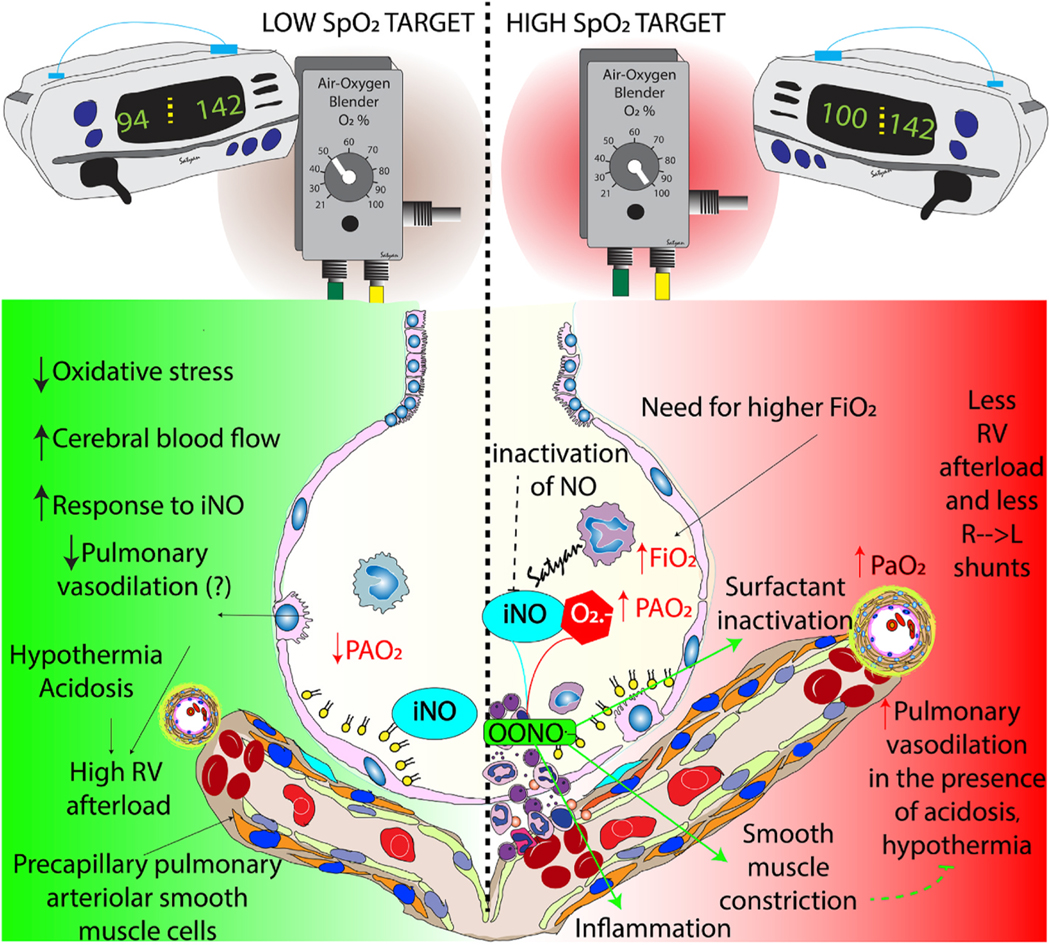
Benefits and risks of low and high oxygen saturation by pulse oximeter (SpO2) targets. Preductal SpO2 in the mid-90s (90–97% - green zone) results in lower oxidative stress, increased cerebral blood flow and better response to inhaled nitric oxide (iNO). However, in the presence of hypothermia and acidosis, low SpO2 target may not be adequate to promote optimal pulmonary vasodilation resulting in high right ventricular afterload. High SpO2 targets (~100%) require higher inspired (FiO2) and alveolar (PAO2) increasing the risk of oxidative stress. Such targets may transiently improve pulmonary vasodilation especially in the presence of hypothermia or acidosis but there is a higher risk of reactive oxygen species formation leading to inactivation of inhaled nitric oxide (iNO), surfactant inactivation and inflammation. Copyright Satyan Lakshminrusimha.
In a lamb model of perinatal asphyxia with meconium aspiration syndrome (MAS) and PPHN, initiation of resuscitation with 21% oxygen followed by titration to achieve the target SpO2 recommended by the Neonatal Resuscitation Program resulted in higher pulmonary blood flow compared to 21% oxygen ventilation without titration [79]. In the same model of MAS and PPHN, pulmonary blood flow and O2 delivery to the brain were higher with SpO2 targeted in the 95–99% range. However, the 90–94% target range is associated with significantly lower FiO2 and lower lung oxidative stress [80]. Based on these results, we recommend maintaining preductal oxygen saturation in the low to mid-90%s during management of PPHN, with preductal PaO2 levels between 55 and 80 mmHg.
7. Future directions
The role of oxidative stress in PPHN is complex. Though the supplemental oxygen is life-saving therapy for infants with PPHN, it increases oxidant stress through production of free radicals. Further high-quality evidence generated from randomized trials is required to guide oxygen therapy and target levels. Antioxidant therapies have been successfully studied in transitional animal models, but there are limited and inconsistent data in clinical practice. There is an urgent need to conduct randomized clinical studies targeting the oxidative stress signaling systems in infants with PPHN.
Practice Points.
Oxidative stress is defined as the imbalance of pro-and-antioxidants in favor of the former, and can be associated with hyperoxia and hypoxia.
Oxygen-derived free radicals compete with NOS for nitric oxide thus promoting pulmonary vasoconstriction and contributing to persistent pulmonary hypertension of the newborn
To date no gold standard test of oxidative/nitrosative stress damage in preterm infants has been established; However, isoprostanoids and isofuranoids are highly reputed biomarkers of oxidative stress due to their stability and correlation with oxygenation levels.
A reliable marker of oxidative damage to DNA in preterm infants is ratio of 8-hydroxy-2′-deoxyguanosine/deoxyribonucleoside deoxyguanosine (8OHdG/2 dG).
Based on animal studies, we recommend a target preductal SpO2 between 90 and 97%, PaO2 between 55 and 80 mmHg and avoid FiO2 > 0.6–0.8 (if possible) during PPHN management.
Research gaps.
Clinical trials using antioxidants in PPHN
Randomized trials evaluating optimal oxygen targets in PPHN
Acknowledgements
Supported by R01 HD072929 (SL) and PI20/00964 granted (MV) by the Instituto de Investigacion Sanitaria Carlos III (Spanish Ministry of ´ Science and Innovation).
Abbreviations
- ADMA
asymmetric dimethyl arginine
- PPHN
Persistent pulmonary hypertension of the newborn
- PVR
Pulmonary vascular resistance
- NO
Nitric Oxide
- cGMP
Cyclic guanosine monophosphate
- PDE5
Phosphodiesterase type 5
- eNOS
Endothelial nitric oxide synthase
- ROS
Reactive oxygen species
- NOX
NADPH oxidase isoforms
Superoxide anion
- H2O2
Hydrogen peroxide
- OH
Hydroxyl radical
- OONO−
Peroxynitrite
- SOD
Superoxide dismutase
- PASMC
Pulmonary artery smooth muscle cells
Footnotes
Declaration of competing interest
The authors declare no conflict of interest.
References
- [1].Mandell E, Kinsella JP, Abman SH. Persistent pulmonary hypertension of the newborn. Pediatr Pulmonol 2021;56(3):661–9. [DOI] [PubMed] [Google Scholar]
- [2].Murphy JD, Rabinovitch M, Goldstein JD, Reid LM. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr 1981;98(6): 962–7. [DOI] [PubMed] [Google Scholar]
- [3].Perez M, Robbins ME, Revhaug C, Saugstad OD. Oxygen radical disease in the newborn, revisited: oxidative stress and disease in the newborn period. Free Radic Biol Med 2019;142:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Frank L, Groseclose EE. Preparation for birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res 1984;18(3):240–4. [DOI] [PubMed] [Google Scholar]
- [5].Sanchez-Illana A, Pineiro-Ramos JD, Ramos-Garcia V, Ten-Domenech I, Vento M, Kuligowski J. Oxidative stress biomarkers in the preterm infant. Adv Clin Chem 2021;102:127–89. [DOI] [PubMed] [Google Scholar]
- [6].Rudolph AM. Aortopulmonary transposition in the fetus: speculation on pathophysiology and therapy. Pediatr Res 2007;61(3):375–80. [DOI] [PubMed] [Google Scholar]
- [7].Mathew B, Lakshminrusimha S. Persistent pulmonary hypertension in the newborn. Children 2017;4(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lakshminrusimha S, Steinhorn RH. Pulmonary vascular biology during neonatal transition. Clin Perinatol 1999;26(3):601–19. [PubMed] [Google Scholar]
- [9].Wedgwood S, Steinhorn RH, Lakshminrusimha S. Optimal oxygenation and role of free radicals in PPHN. Free Radic Biol Med 2019;142:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Konduri GG, Theodorou AA, Mukhopadhyay A, Deshmukh DR. Adenosine triphosphate and adenosine increase the pulmonary blood flow to postnatal levels in fetal lambs. Pediatr Res 1992;31(5):451–7. [DOI] [PubMed] [Google Scholar]
- [11].Pierce CM, Krywawych S, Petros AJ. Asymmetric dimethyl arginine and symmetric dimethyl arginine levels in infants with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med : J Soc Critic Care Med World Fed Pediatr Intens Critic Care Soc 2004;5(6):517–20. [DOI] [PubMed] [Google Scholar]
- [12].Steinhorn RH, Morin FC 3rd, Van Wylen DG, Gugino SF, Giese EC, Russell JA. Endothelium-dependent relaxations to adenosine in juvenile rabbit pulmonary arteries and veins. Am J Physiol 1994;266(5 Pt 2):H2001–6. [DOI] [PubMed] [Google Scholar]
- [13].Jones DP, Sies H. The redox code. Antioxidants Redox Signal 2015;23(9):734–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem 2017;86:715–48. [DOI] [PubMed] [Google Scholar]
- [15].Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol 2022. 10.1038/s41580-022-00456-z. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- [16].Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev 2016;2016: 1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nordzieke DE, Medrano-Fernandez I. The plasma membrane: a platform for intra- and intercellular redox signaling. Antioxidants 2018;7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Panfoli I, Candiano G, Malova M, De Angelis L, Cardiello V, Buonocore G, et al. Oxidative stress as a primary risk factor for brain damage in preterm newborns. Front Pediatr 2018;6:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res 1986;59(6): 612–9. [DOI] [PubMed] [Google Scholar]
- [20].Lyle AN, Griendling KK. Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology 2006;21:269–80. [DOI] [PubMed] [Google Scholar]
- [21].Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol 2013;1:304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Radi R. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc Natl Acad Sci U S A 2018;115(23):5839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vento M, Sanchez-Illana A. Nitric oxide and preterm resuscitation: some words of caution. Pediatr Res 2020;87(3):438–40. [DOI] [PubMed] [Google Scholar]
- [24].Stocker S, Van Laer K, Mijuskovic A, Dick TP. The conundrum of hydrogen peroxide signaling and the emerging role of peroxiredoxins as redox relay hubs. Antioxidants Redox Signal 2018;28(7):558–73. [DOI] [PubMed] [Google Scholar]
- [25].Malinouski M, Zhou Y, Belousov VV, Hatfield DL, Gladyshev VN. Hydrogen peroxide probes directed to different cellular compartments. PLoS One 2011;6(1): e14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kuligowski J, Aguar M, Rook D, Lliso I, Torres-Cuevas I, Escobar J, et al. Urinary lipid peroxidation byproducts: are they relevant for predicting neonatal morbidity in preterm infants? Antioxidants Redox Signal 2015;23(2):178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Milne GL, Dai Q, Roberts 2nd LJ. The isoprostanes–25 years later. Biochim Biophys Acta 2015;1851(4):433–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009;124(3):e439–49. [DOI] [PubMed] [Google Scholar]
- [29].Kuligowski J, Torres-Cuevas I, Quintas G, Rook D, van Goudoever JB, Cubells E, et al. Assessment of oxidative damage to proteins and DNA in urine of newborn infants by a validated UPLC-MS/MS approach. PLoS One 2014;9(4):e93703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chafer-Pericas C, Stefanovic V, Sanchez-Illana A, Escobar J, Cernada M, Cubells E, et al. Novel biomarkers in amniotic fluid for early assessment of intraamniotic infection. Free Radic Biol Med 2015;89:734–40. [DOI] [PubMed] [Google Scholar]
- [31].Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol Med 2003;9(4):169–76. [DOI] [PubMed] [Google Scholar]
- [32].Perrone S, Laschi E, Buonocore G. Biomarkers of oxidative stress in the fetus and in the newborn. Free Radic Biol Med 2019;142:23–31. [DOI] [PubMed] [Google Scholar]
- [33].Ahmed AE, Abd-Elmawgood EA, Hassan MH. Circulating protein carbonyls, antioxidant enzymes and related trace minerals among preterms with respiratory distress syndrome. J Clin Diagn Res 2017;11(7):BC17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cascant-Vilaplana MM, Albiach-Delgado A, Camprubi-Camprubi M, Perez-Cruz M, Gomez O, Arraez M, et al. A UPLC-MS/MS method for the determination of oxidative stress biomarkers in amniotic fluid. Free Radic Biol Med 2022;179: 164–9. [DOI] [PubMed] [Google Scholar]
- [35].Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, Steinhorn RH. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxidants Redox Signal 2011;15(6):1497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Farrow KN, Wedgwood S, Lee KJ, Czech L, Gugino SF, Lakshminrusimha S, et al. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol 2010;174(3):272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Farrow KN, Lee KJ, Perez M, Schriewer JM, Wedgwood S, Lakshminrusimha S, et al. Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells. Antioxidants Redox Signal 2012;17(3):460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, et al. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 2005;289(4):L660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rhoades RA, Packer CS, Roepke DA, Jin N, Meiss RA. Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can J Physiol Pharmacol 1990;68(12):1581–9. [DOI] [PubMed] [Google Scholar]
- [40].Fineman JR, Soifer SJ, Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol 1995;57:115–34. [DOI] [PubMed] [Google Scholar]
- [41].Wiklund NP, Persson MG, Gustafsson LE, Moncada S, Hedqvist P. Modulatory role of endogenous nitric oxide in pulmonary circulation in vivo. Eur J Pharmacol 1990;185(1):123–4. [DOI] [PubMed] [Google Scholar]
- [42].Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 1988;333(6174):664–6. [DOI] [PubMed] [Google Scholar]
- [43].Wedgwood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 2001;281(5):L1058–67. [DOI] [PubMed] [Google Scholar]
- [44].Shetty SS, Okada T, Webb RL, DelGrande D, Lappe RW. Functionally distinct endothelin B receptors in vascular endothelium and smooth muscle. Biochem Biophys Res Commun 1993;191(2):459–64. [DOI] [PubMed] [Google Scholar]
- [45].Kähler J, Ewert A, Weckmüller J, Stobbe S, Mittmann C, Koster R, et al. Oxidativë stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. J Cardiovasc Pharmacol 2001;38(1):49–57. [DOI] [PubMed] [Google Scholar]
- [46].Black SM, Johengen MJ, Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 1998;44(6):821–30. [DOI] [PubMed] [Google Scholar]
- [47].Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC 3rd, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res 2006;59(1):137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lakshminrusimha S, Russell JA, Gugino SF, Ryan RM, Mathew B, Nielsen LC, et al. Adjacent bronchus attenuates pulmonary arterial contractility. Am J Physiol Lung Cell Mol Physiol 2006;291(3):L473–8. [DOI] [PubMed] [Google Scholar]
- [49].Lakshminrusimha S, Morin FC 3rd, Steinhorn RH, Gugino SF, Ryan RM, Kumar VH, et al. Ovine bronchial-derived relaxing factor: changes with development and hyperoxic ventilation. J Appl Physiol 2006;101(1):135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lakshminrusimha S, Steinhorn RH, Wedgwood S, Savorgnan F, Nair J, Mathew B, et al. Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21 and 100% oxygen. J Appl Physiol 2011;111(5):1441–7. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM, et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res 2009;66(5):539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chandrasekharan P, Kozielski R, Kumar VH, Rawat M, Manja V, Ma C, et al. Early use of inhaled nitric oxide in preterm infants: is there a rationale for selective approach? Am J Perinatol 2017;34(5):428–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Konduri GG, Bakhutashvili I, Eis A, Pritchard K Jr. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 2007;292(4):H1812–20. [DOI] [PubMed] [Google Scholar]
- [54].Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, et al. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res 2008;102(2):226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin 3rd FC. Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol 1997;272(5 Pt 1):L1005–12. [DOI] [PubMed] [Google Scholar]
- [56].Steinhorn RH, Russell JA, Morin 3rd FC. Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol 1995;268(4 Pt 2):H1483–9. [DOI] [PubMed] [Google Scholar]
- [57].Ivy DD, Ziegler JW, Dubus MF, Fox JJ, Kinsella JP, Abman SH. Chronic intrauterine pulmonary hypertension alters endothelin receptor activity in the ovine fetal lung. Pediatr Res 1996;39(3):435–42. [DOI] [PubMed] [Google Scholar]
- [58].Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 2005;288(3):L480–7. [DOI] [PubMed] [Google Scholar]
- [59].Zemskov EA, Lu Q, Ornatowski W, Klinger CN, Desai AA, Maltepe E, et al. Biomechanical forces and oxidative stress: implications for pulmonary vascular disease. Antioxidants Redox Signal 2019;31(12):819–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, et al. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 2006;290(6):L1069–77. [DOI] [PubMed] [Google Scholar]
- [61].Xu D, Guo H, Xu X, Lu Z, Fassett J, Hu X, et al. Exacerbated pulmonary arterial hypertension and right ventricular hypertrophy in animals with loss of function of extracellular superoxide dismutase. Hypertension 2011;58(2):303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang L, Zheng Q, Yuan Y, Li Y, Gong X. Effects of 17beta-estradiol and 2-methoxyestradiol on the oxidative stress-hypoxia inducible factor-1 pathway in hypoxic pulmonary hypertensive rats. Exp Ther Med 2017;13(5):2537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bertoli SR, Marques VB, Rossi EM, Krause M, Carneiro M, Simoes MR, et al. Chronic iron overload induces vascular dysfunction in resistance pulmonary arteries associated with right ventricular remodeling in rats. Toxicol Lett 2018;295: 296–306. [DOI] [PubMed] [Google Scholar]
- [64].Wedgwood S, Lakshminrusimha S, Czech L, Schumacker PT, Steinhorn RH. Increased p22(phox)/Nox4 expression is involved in remodeling through hydrogen peroxide signaling in experimental persistent pulmonary hypertension of the newborn. Antioxidants Redox Signal 2013;18(14):1765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res 1992;70(3):593–9. [DOI] [PubMed] [Google Scholar]
- [66].Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med 2010;15(4):191–5. [DOI] [PubMed] [Google Scholar]
- [67].Berkelhamer SK, Farrow KN. Developmental regulation of antioxidant enzymes and their impact on neonatal lung disease. Antioxidants Redox Signal 2014;21(13): 1837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lavoie JC, Chessex P. Development of glutathione synthesis and gamma-glutamyltranspeptidase activities in tissues from newborn infants. Free Radic Biol Med 1998;24(6):994–1001. [DOI] [PubMed] [Google Scholar]
- [69].Wedgwood S, Lakshminrusimha S, Farrow KN, Czech L, Gugino SF, Soares F, et al. Apocynin improves oxygenation and increases eNOS in persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 2012;302(6): L616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, et al. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2008;295(6):L979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med 2006;174(12): 1370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Davis JM, et al. SOD and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2010;299(1):L109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Konduri GG, Bakhutashvili I, Eis A, Afolayan A. Antenatal betamethasone improves postnatal transition in late preterm lambs with persistent pulmonary hypertension of the newborn. Pediatr Res 2013;73(5):621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Perez M, Wedgwood S, Lakshminrusimha S, Farrow KN, Steinhorn RH. Hydrocortisone normalizes phosphodiesterase-5 activity in pulmonary artery smooth muscle cells from lambs with persistent pulmonary hypertension of the newborn. Pulm Circ 2014;4(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chandrasekharan P, Rawat M, Lakshminrusimha S. How do we monitor oxygenation during the management of PPHN? Alveolar, arterial, mixed venous oxygen tension or peripheral saturation? Children 2020;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chandrasekharan P, Lakshminrusimha S. Oxygen therapy in preterm infants with pulmonary hypertension. Semin Fetal Neonatal Med 2020;25(2):101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kapadia VS, Chalak LF, DuPont TL, Rollins NK, Brion LP, Wyckoff MH. Perinatal asphyxia with hyperoxemia within the first hour of life is associated with moderate to severe hypoxic-ischemic encephalopathy. J Pediatr 2013;163(4):949–54. [DOI] [PubMed] [Google Scholar]
- [78].Vento M, Asensi M, Sastre J, Lloret A, Garcia-Sala F, Vina J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr 2003;142(3): 240–6. [DOI] [PubMed] [Google Scholar]
- [79].Rawat M, Chandrasekharan PK, Swartz DD, Mathew B, Nair J, Gugino SF, et al. Neonatal resuscitation adhering to oxygen saturation guidelines in asphyxiated lambs with meconium aspiration. Pediatr Res 2016;79(4):583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rawat M, Chandrasekharan P, Gugino SF, Koenigsknecht C, Nielsen L, Wedgwood S, et al. Optimal oxygen targets in term lambs with meconium aspiration syndrome and pulmonary hypertension. Am J Respir Cell Mol Biol 2020; 63(4):510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]