Abstract
The hepatitis B virus X protein (pX) interacts directly with the bZip transactivator CREB and the bZip repressors ICERIIγ and ATF3, increasing their DNA-binding affinity in vitro and their transcriptional efficacy in vivo. However, the mechanism of bZip-pX interaction and of the pX-mediated increase in the bZip transcriptional efficacy remains to be understood. In this study with deletion mutants of pX, we delineated a 67-amino-acid region spanning residues 49 to 115 required for direct CREB, ATF3, and ICER IIγ interaction in vitro and in vivo and increased bZip/CRE binding in vitro. Transient transfections of the pX deletion mutants in AML12 hepatocytes demonstrate that pX49–115 is as effective as the full-length pX in enhancing the ATF3- and ICERIIγ-mediated transrepression. However, this pX region is inactive in increasing the transactivation efficacy of CREB; additional amino acid residues present in pX49–140 are required to mediate the increased transactivation efficacy of CREB in vivo. This requirement for different regions of pX in affecting CREB transactivation suggests that amino acid residues 115 to 140 integrate additional events in effecting pX-mediated transactivation, such as concomitant interactions with select components of the basal transcriptional apparatus.
The 16.5-kDa X protein, pX, encoded by the hepatitis B virus (HBV), is expressed during viral infection (17, 28) and is implicated in HBV-mediated hepatocarcinogenesis by an unknown mechanism. In addition to its role in the viral life cycle, pX exhibits several reported activities affecting cellular transcription (35), cell growth (4, 20), and apoptotic cell death (19, 40). Importantly, the transcriptional role of pX is implicated as a mechanism by which pX deregulates cellular gene expression, resulting in hepatocyte transformation (4, 41).
Although pX does not directly bind double-stranded DNA, pX activates transcription from diverse cis-acting elements, including the AP-1 (18, 31, 36, 42), AP-2 (36), NF-κB (23, 25, 37, 39, 43), and CRE sites (3, 26, 44). This transcriptional promiscuity of pX is attributed to its dual mechanism of transcriptional activation. Specifically, pX activates the mitogenic ras-raf-MAPK (5, 11) and JNK (6) pathways in the cytoplasm, leading to the activation of transcription from AP-1 and NF-κB sites. pX also interacts directly with specific components of the basal transcription apparatus, including the RPB5 subunit of RNA polymerase II (7, 24), TFIIB (15, 16, 24), and TFIIH (15, 33), and with cellular bZip transcription factors (3, 26, 44). Importantly, distinct regions of pX, namely, amino acids (aa) 51 to 136 and 102 to 136, are required for the interaction of pX with RPB5 and TFIIB, respectively (24). In addition, overexpression of RPB5 in the presence of pX stimulates expression of pX-responsive promoters, supporting the idea that pX transactivation occurs via direct interaction with components of the basal transcriptional apparatus (7). Likewise, pX rescues the transcriptional inhibitory effect of the dominant-negative TFIIB mutant C34,37S, a mutant which maintains its interacting potential with pX (15). Accordingly, these data favor the model that concurrent interactions occur between pX and several components of the basal transcriptional apparatus to effect pX-mediated transactivation. However, it is not yet clear whether the direct interaction of pX with the basal transcriptional apparatus effects a specific or general enhancement of gene expression. In order for pX to effect increased transcription from specific cis-acting elements, one has to envision the recruitment by pX of the basal transcriptional apparatus to those cis-acting elements via its interaction with sequence-specific cellular transcription factors. The only known class of cellular transcription factors which interacts directly with pX and displays functional interactions in vitro and in vivo is the bZip family of transcription factors (3, 26, 44). Our previous studies (3, 44) demonstrated that the direct interaction of pX with CREB/bZip family members increases transcription in a specific manner from CRE and C/EBPβ binding sites. Importantly, the interaction of pX with the bZip transcription factor C/EBPα was shown to synergistically activate the HBV enhancer II/pregenomic promoter (8), supporting the idea that the interaction of pX with bZip transcription factors is relevant for the expression of the viral genome.
Our previous studies (3, 44) have shown that pX interacts directly with CREB via the bZip domain, increases the affinity of CREB for either cellular or viral CRE sites by 1 order of magnitude, and increases its transcriptional efficacy in vivo in the presence of cyclic AMP. Likewise, pX interacts directly and increases the DNA-binding potential and transcriptional efficacy of inducible bZip transcription factors (C/EBPβ, ATF3, and ICERIIγ), activators or repressors of transcription, all of which play important roles in hepatocyte physiology (3). However, the increased DNA-binding potential of bZip proteins (3, 44) does not explain how pX effects their increased transcriptional efficacy.
To further understand the mechanism of bZip-pX interactions and the mechanism mediating their increased transcriptional efficacy by interaction with pX, we report here, first, the delineation of a pX region required for direct CREB/bZip binding in vitro and, second, the differential requirement of pX regions in effecting bZip-mediated transactivation versus transrepression.
MATERIALS AND METHODS
Construction of pX deletion mutants.
GST-X79–154, GST-X49–140, GST- X49–115, and GST-X49–90 were constructed as protein fusions with glutathione S-transferase (GST) by PCR. The respective pX DNA fragments were cloned into the EcoRI-HindIII sites of the pGEX-KG plasmid (14). GST-X1–154 and GST-X49–154 were cloned as previously described (3). The GST-X fusion proteins were expressed in Escherichia coli and purified on glutathione-Sepharose 4B resin (3). The protein concentration of the GST-X fusions was estimated by densitometric analysis by using the OPTIMAS 6.1 program and employing as a standard known amounts of bovine serum albumin (BSA), similarly analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and densitometric analysis. In the in vitro analyses, equivalent amounts of the GST-X deletion proteins were used.
Protein-protein interaction assays.
In vitro protein-protein interaction assays of pX deletion mutants in fusion with GST were carried out as previously described (3) by using recombinant, CRE-affinity-purified CREB (47), 32P radiolabeled at the PKA (Ser133) site (9), or in vitro-translated 35S-radiolabeled ATF3 and ICERIIγ, prepared as described earlier (3).
In vitro DNA-protein binding assays.
DNA-protein binding assays were carried out by employing the somatostatin CRE as the radiolabeled probe (1, 3) and recombinant CREB (9).
Transient transfections.
AML12 cells (45) were transfected by employing the calcium phosphate coprecipitation method or the FuGENE transfection protocol (Boehringer Mannheim) in the presence of 10 μM forskolin and 100 μM 3-isobutyl-1-methylxanthine (IBMX). Transfections with the luciferase reporter were performed in triplicate in 30-mm dishes. Chloramphenicol acetyltransferase (CAT) assays and luciferase assays were performed as previously described (3, 44).
RESULTS
Identification of the region of pX that interacts directly with CREB in vitro.
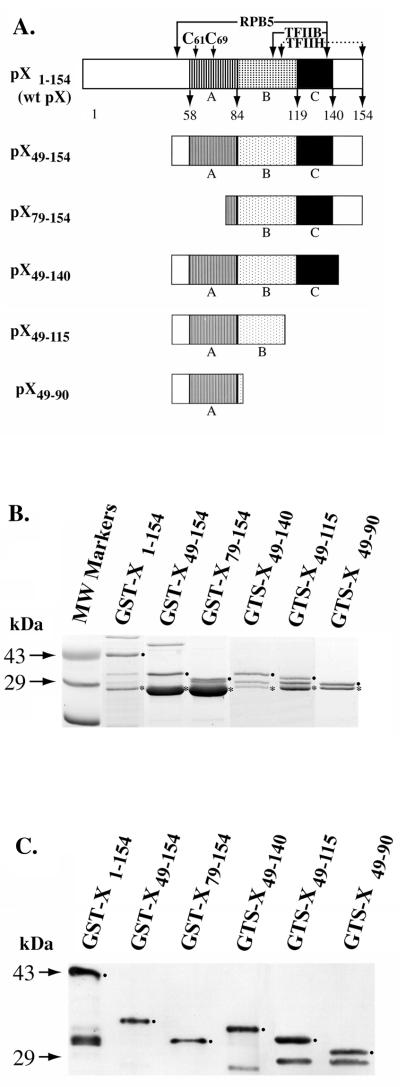
Earlier studies demonstrated that pX regions A, B, and C (Fig. 1A), which are conserved to various degrees in mammalian hepadnaviruses, are required for the pX-mediated transactivation of the simian virus 40 (SV40) promoter (2, 34) and Rous sarcoma virus long terminal repeat (RSV LTR) (21). However, considering that pX mediates transactivation of pX-responsive promoters via a dual mechanism (11), in this study we investigated whether the same pX region is required for the increased transcriptional efficacy of CREB/bZip proteins by pX. Accordingly, we constructed a series of deletion mutants of pX (Fig. 1A) by PCR-based cloning of the respective fragments into the pGEX-KG vector (14). The rationale for constructing these mutants is that pX49–154 lacks the first 48 aa of pX known to repress pX-mediated transactivation (29) and pX79–154 starts at an internal Met residue (22). pX49–140 contains regions A, B, and C (Fig. 1A) required for pX-mediated transactivation of the SV40 promoter (2, 34) and RSV LTR (21). pX49–115 contains regions A and B, and pX49–90 contains only region A (Fig. 1A).
FIG. 1.
(A) Diagram of constructed pX deletion mutants. pX regions A, B, and C are conserved to various degrees in hepadnaviruses and are essential for transactivation. C61 and C69 are conserved cysteines. The polymerase II, RPB5 subunit, TFIIH, and TFIIB interacting regions are indicated. (B) Coomassie blue-stained SDS gel (14%) of purified (4), recombinant GST-X and the indicated pX deletions. •, GST-X fusion protein; ∗, endogenously cleaved GST portion. (C) Western blot of recombinant wt pX, pX49–154, pX79–154, pX49–140, pX49–115, and pX49–90 (GST fusions) detected with a polyclonal pX-specific antibody. •, GST-X fusion protein. Protein bands smaller than the GST-X fusion proteins are degradation products.
These pX deletion mutants (Fig. 1A) were expressed as GST fusion proteins, purified (Fig. 1B) as previously described (3), and analyzed by Western blotting (Fig. 1C). All pX mutants express soluble GST-X fusion protein. To determine the potential of these pX deletion mutants to interact directly with CREB, we carried out in vitro protein-protein interaction assays by using the GST-X fusion proteins and CREB as the interacting protein (3). Recombinant CREB was purified by CRE-affinity chromatography (47) and radiolabeled with protein kinase A at Ser133 as previously described (9).
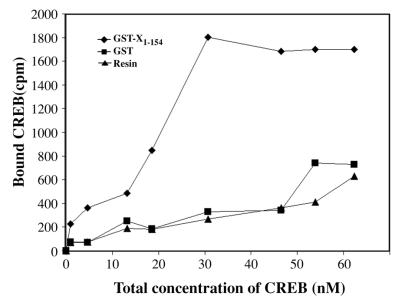
In order to ensure that the protein-protein interaction assays were performed at concentrations of CREB corresponding to the linear range for pX binding, we quantitated the relative Kd of CREB-pX interactions. Kd quantitations were performed by employing the previously established in vitro protein-protein interaction assay, thereby detecting specific interaction between CREB and pX (3). Increasing amounts of CRE affinity-purified (47), 32P-radiolabeled CREB (9), ranging in concentration from 0.2 to 62.5 nM, was incubated with a constant amount of GST-X1–154. After binding and electrophoretic analysis, the bound fraction was quantitated by scintillation counting of the SDS-PAGE-excised bands. Control in these assays included the binding of 32P-CREB to an equivalent amount of GST-bound resin. We estimated the relative Kd to be in the range of 1.8 × 10−8 M (Fig. 2).
FIG. 2.
Kd determination of CREB-pX interaction. GST-X1–154 resin (⧫) (5 μg) was incubated with increasing amounts of 32P-CREB (0.2 to 62.5 nM) for 3 h at 4°C in buffer containing 25 mM HEPES (pH 7.5), 100 mM KCl, 0.1% Triton X-100, 5 mM dithiothreitol, 5 mM phenylmethylsulfonyl fluoride, and 50 μg of BSA per ml. The beads were washed six times and eluted by boiling in SDS-PAGE loading buffer. Analysis was done on SDS–12% PAGE gels. Equivalent amounts of GST-bound resin (■) or equal amounts of glutathione beads (▴) were used as the control. Bound CREB was plotted against the total concentration of CREB. The Kd value was calculated as the concentration of CREB at which half-maximal binding to GST-X was observed. The experiment was repeated three times and shows a Kd value of approximately 1.8 × 10−8 M. A representative experiment from three independent experiments is shown above.
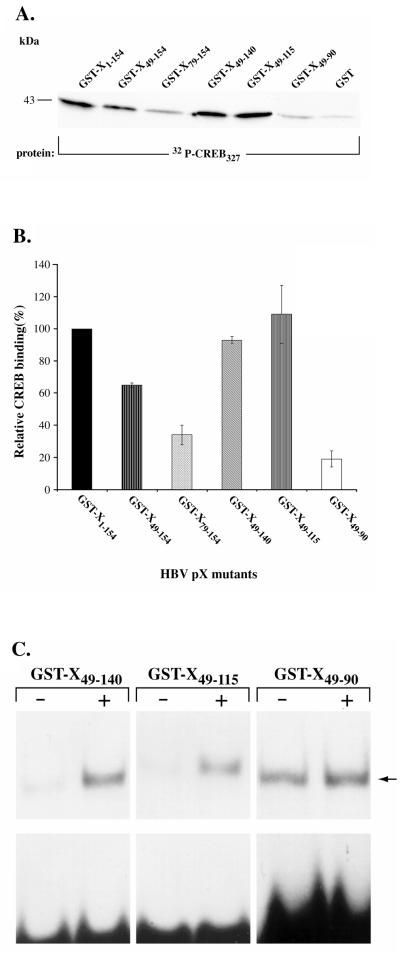
Employing 32P-CREB in the concentration corresponding to the linear range for pX binding, we assessed the potential of the pX deletion mutants to interact with CREB (Fig. 3A and B). Equivalent amounts of protein for each GST-X deletion mutant were used in these in vitro assays and assessed by densitometric analysis, using as a standard known amounts of BSA. GST-X49–154, GST-X49–140, and GST-X49–115 display binding comparable to wild-type (wt) pX. GST-X79–154 and GST-X49–90, in comparison to full-length pX, display only 30 and 20% binding to CREB, respectively (Fig. 3A and B). From the analysis of these pX deletion mutants, we conclude that the smallest region of pX required for direct CREB binding is the 67-aa region spanning residues 49 to 115. This region is rich in Cys residues and contains regions A and B, which are essential for pX-mediated transactivation of the SV40 early promoter (2, 34) and the RSV LTR (21).
FIG. 3.
Activity of pX deletions in vitro. (A) Protein-protein interaction assay with equivalent amounts of GST-X deletion mutants (3 μg) incubated with 32P-CREB (5 to 10 ng) for 3 h at 4°C in buffer containing 25 mM HEPES (pH 7.5), 100 mM KCl, 0.1% Triton X-100, 5 mM dithiothreitol, 5 mM phenylmethylsulfonyl fluoride, and 50 μg of BSA per ml. The beads were washed six times and eluted by boiling in SDS-PAGE loading buffer. Analysis was done by SDS-PAGE on 12% gels. A total of 12 to 15 μg of GST bound to resin was used as a control. (B) Quantitation of relative CREB binding to each GST-X deletion mutant compared to wt pX carried out by scintillation counting of the gel excised bands. Nonspecific binding of CREB to the GST-resin control is subtracted from all of the plotted values. The data shown are from four independent experiments. (C) DNA-protein binding assays of recombinant CREB in the presence of pX mutants were carried out as described previously (3) with 10 ng of recombinant CREB and 10,000 cpm of radiolabeled CRE, with an equivalent amount of the following: + lanes, GST-X49–140, GST-X49–115, and GST-X49–90; − lanes, recombinant GST protein, as indicated. Lanes containing GST-X49–90 were exposed for 2 days at −80°C during autoradiography, as opposed to 18 h of exposure for the other lanes.
Identification of the minimal functional region of pX that enhances the DNA-binding potential of CREB in vitro.
To determine the functional significance of the interaction of pX49–140 and pX49–115 with CREB, we tested the potential of these pX deletion mutants to enhance the CRE-binding potential of CREB in vitro. Gel retardation assays were performed by employing radiolabeled CRE as the probe (3) and recombinant CREB (9) in the presence or absence of the pX deletion mutants pX49–140, pX49–115, and pX49–90. Addition of equivalent amounts of either pX49–140 or pX49–115 enhanced the CRE-binding potential of CREB (Fig. 3C). By contrast, addition of equivalent amounts of pX49–90, which displays 80% reduced binding to CREB in vitro (Fig. 3B), failed to enhance the CRE-binding potential of CREB (Fig. 3C). Accordingly, we have delineated a minimal functional region of pX containing 67 aa residues, pX49–115, that is required for direct CREB interaction and enhanced CREB/CRE binding in vitro.
In vivo activity of the pX mutants in AML12 hepatocytes.
To confirm the in vitro results by in vivo analyses, we performed two types of assays in AML12 hepatocytes. First, we examined whether pX49–140 and pX49–115 interact directly with CREB in vivo by employing the mammalian two-hybrid assay. Second, we examined by transient-transfection assays in AML12 cells whether these pX deletion mutants are active in enhancing the transcriptional efficacy of CREB in vivo.
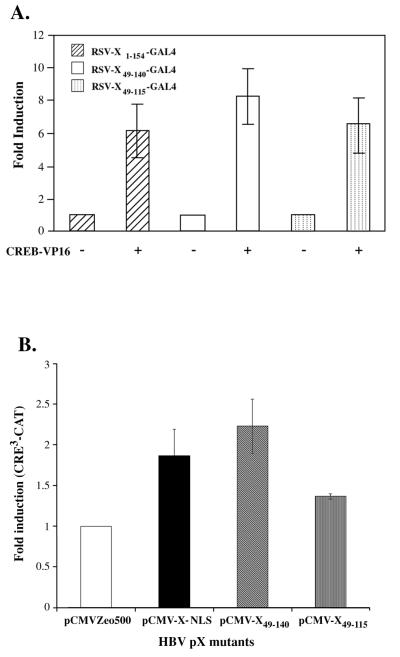
pX49–140 and pX49–115 were cloned in frame with the DNA-binding domain of Gal41–147 in the RSV LTR-driven expressor vector (3). The interacting CREB protein is in fusion with the activation domain of VP16 as described earlier (3). Transient transfections in AML12 hepatocytes were carried out by using the Gal4UAS-luciferase reporter in the presence of CREB-VP16 encoding plasmid, as a function of the RSV-X-Gal4 expression vectors (Fig. 4A). In agreement with the in vitro results (Fig. 3), the mammalian two-hybrid assays demonstrate that both pX49–140 and pX49–115, like wt pX, interact directly with CREB in vivo. However, it is important to note that this assay does not allow quantitative comparison of these interactions among the three pX constructs tested.
FIG. 4.
Activity of pX deletions in vivo. (A) Mammalian two-hybrid assay of pX deletion mutants in AML12 cells. A total of 500 ng of Gal4UAS-luciferase reporter was cotransfected by the FuGENE transfection protocol, with 700 ng each of RSV-CREB-VP16 and RSV-X-Gal4 expression vectors per 30-mm culture dish, performed in triplicate. Cells were harvested 18 h after transfection, and luciferase activity was quantitated and expressed per microgram of protein extract as previously described (3). Results are from three independent experiments. (B) Transient transfection of HBV pX mutants in AML12 cells. A total of 3 to 5 μg of CRE3-CAT reporter plasmid was cotransfected with pCMV-X-NLS (20 ng), pCMV-X49–140 (100 ng), and pCMV-X49–115 (50 ng) expressor plasmid. Control transfections contained equivalent amounts of pCMV-empty vector. The optimal amounts of pCMV-X expression vectors shown (wt and mutants) were established by titration analyses. Transfected cells were treated with 10 μM forskolin and 100 μM IBMX and harvested at 24 h. CAT assays were performed as described earlier (3). Relative CRE3-CAT induction values per microgram of protein extract were quantitated from three independent experiments.
To examine the potential of these CREB interacting regions of pX to increase the transcriptional efficacy of CREB in vivo, mammalian expression vectors encoding pX49–140 and pX49–115 were cotransfected at low density with the CRE3-CAT reporter in the AML12 hepatocyte cell line (Fig. 4B). In agreement with similar observations by others (10, 35, 38), we observe that wt pX is a weak transactivator, displaying a twofold induction in CREB/CRE-mediated transcription in the AML12 hepatocyte cell line. Like wt pX, pX49–140 induces the transactivation of endogenous CREB by approximately two- to threefold. By contrast, pX49–115 shows a minimal 1.4-fold induction of the CRE3-CAT reporter (Fig. 4B). These results demonstrate that pX49–140 retains its ability to transactivate CRE-mediated transcription in a manner similar to that of wt pX. However, in contrast to pX49–140, pX49–115 which interacts directly with CREB both in vitro (Fig. 3) and in vivo (Fig. 4B) and enhances its CRE-binding potential in DNA-protein binding assays (Fig. 3C) displays minimal transactivation in vivo. These results suggest that the increased transcriptional efficacy of CREB effected by pX requires other pX-dependent events in addition to the increased CRE binding of CREB.
Interaction of pX49–140 and pX49–115 with bZip repressors ATF3 and ICERIIγ.
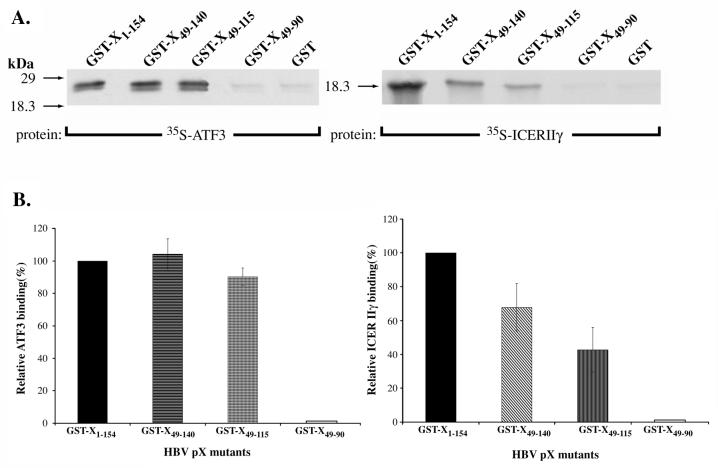
Since pX displays promiscuity in its interaction with bZip proteins (3), we examined whether these characterized pX deletion mutants display the same requirements in interacting with various bZip proteins. We selected the bZip proteins ATF3, ICERIIγ, and NF-IL6, which were shown previously to undergo functional interactions with pX (3). In vitro protein-protein interaction assays were carried out by using the GST fusion proteins of wt pX, pX49–140, pX49–115, and pX49–90 with in vitro-translated ICERIIγ, ATF3, and the bZip domain of NF-IL6 (data not shown). We observed (Fig. 5) that ATF3 interacts with both pX49–140 and pX49–115 in a way similar to wt pX. ICERIIγ also interacts with both pX49–140 and pX49–115 deletions, although in comparison to wt pX it displays reduced direct binding to these regions. Like CREB, neither ATF3 nor ICERIIγ displays appreciable direct binding to pX49–90 (Fig. 5). Therefore, all of the bZip proteins tested interact with the same minimal region of pX, spanning aa 49 to 115, which is also required for interaction with CREB in vitro (Fig. 3) and in vivo (Fig. 4A). These results support the idea that a common mechanism exists for the interaction of bZip transcription factors with viral pX.
FIG. 5.
Interaction of pX deletions with ATF3 and ICERIIγ. (A) Protein-protein interaction assays of GST-X, GST-X49–140, GST-X49–115, and GST-X49–90 with in vitro-translated, [35S]methionine-radiolabeled ATF3 (3 μl) and ICERIIγ (7 μl) as indicated. Experimental conditions were as described in Fig. 3A. (B) Quantitation of relative ATF3 and ICERIIγ binding to each GST-X deletion mutant compared to wt pX as determined by scintillation counting of the gel-excised bands. Nonspecific binding of ATF3 and ICERIIγ to the GST-bound resin is subtracted from all of the plotted values. The data shown are from three independent experiments.
In vivo activity of the pX mutants in mediating repression by ATF3 and ICERIIγ.
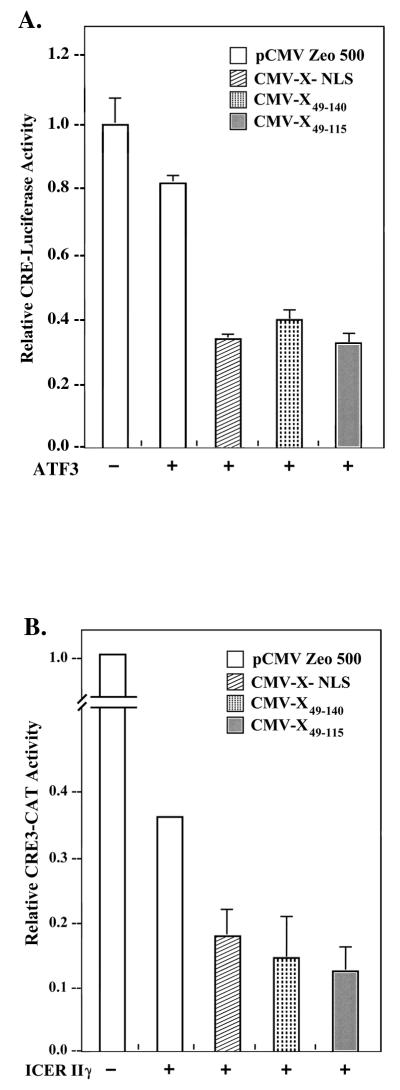
To assess the activity of deletions pX49–115 and pX49–140 in mediating enhanced transrepression by ATF3 and ICERIIγ, expression vectors encoding each of the pX deletion constructs were cotransfected with ATF3 or ICERIIγ in AML12 cells by employing the CRE-driven reporters CRE-luciferase (Fig. 6A) and CRE3-CAT (Fig. 6B), respectively. Earlier studies demonstrated that ATF3 and ICERIIγ repress CRE3-CAT expression, and pX coexpression further increases their transrepression efficacy (3). The results shown in Fig. 6 demonstrate that, like wt pX, both pX49–140 and pX49–115 further promote the repression efficacy of ATF3 and ICERIIγ. Therefore, while the pX-mediated enhancement of CREB/CRE transcription requires the pX region spanning aa 49 to 140 (Fig. 4B), the pX-mediated increase in the repression efficacy of ATF3 and ICERIIγ occurs as efficiently with pX49–115 (Fig. 6). Importantly, the functional activity of pX49–115 in promoting ATF3- and ICERIIγ-mediated repression excludes the possibility that this deletion mutant is unstable in vivo.
FIG. 6.
Transient transfection of HBV pX mutants in AML12 cells in the presence of bZip repressors ATF3 and ICERIIγ. (A) A total of 600 ng of CRE-luciferase reporter plasmid was cotransfected by the FuGENE protocol (Boehringer Mannheim) with 100 ng of pCMV-X-NLS, pCMV-X49–140, and pCMV-X49–115 expressor plasmid in the presence of 50 ng of ATF3 expression vector. (B) CRE3-CAT reporter plasmid (5 μg) cotransfected by the calcium phosphate protocol (BRL) with pCMV-X-NLS (20 ng), pCMV-X49–140 (50 ng), and pCMV-X49–115 (50 ng) expressor plasmid in the presence of 0.5 μg of ICERIIγ. Optimal amounts of the indicated expression vectors transfected in AML12 cells were determined by titration analyses. Relative CRE3-CAT activities per microgram of protein extract were quantitated from three independent experiments.
Since pX49–115 is the minimal, functional region required for CREB/bZip interaction in vitro, the observed increased repression efficacy of ATF3 and ICERIIγ effected by pX49–115 in vivo (Fig. 6) is a direct demonstration of the functional importance of the interaction of pX with bZip transcription factors. Specifically, our results identify the increased DNA-binding potential of bZip proteins effected by pX as the initial pX-dependent event, leading to increased transactivation or transrepression by bZip transcription factors.
DISCUSSION
Our earlier studies have demonstrated that the interaction of pX with bZip transcription factors CREB, ICERIIγ, ATF3, and NF-IL6 increases their DNA-binding affinity in vitro and their transcriptional efficacy in vivo (3, 44). In the present study we have employed the CREB-pX model system to examine the mechanism by which pX increases the transcriptional efficacy of bZip transcription factors. Our approach was to define a minimal, functional region of pX required for CREB interaction and activity. The rationale for undertaking these studies was that the definition of a minimal region of pX will provide convincing evidence to demonstrate the specificity of CREB (bZip)-pX interactions and will also provide a useful tool for biophysical analyses of pX and the bZip-pX complex.
We report the delineation of a 67-aa region of pX, spanning aa 49 to 115, as the minimal region required for direct CREB binding (Fig. 3 and 4A) and for the enhancement of the CRE-binding potential of CREB (Fig. 3C). Interestingly, although this minimal region of pX enhances the CRE-binding potential of CREB in vitro, it only minimally increases the transcriptional efficacy of CREB in vivo (Fig. 4B). By contrast, pX49–140 enhances both the CRE-binding potential of CREB (Fig. 3C) and its transcriptional efficacy in vivo (Fig. 4B). The transcriptionally active deletion pX49–140, tested by CREB/CRE-driven transcriptional induction, contains pX regions A, B, and C, all of which are required for transactivation of both the SV40 early promoter (34) and the RSV LTR (21). Importantly, this pX region has been shown to contain binding sites for both the RPB5 subunit of RNA polymerase II and TFIIB, mapped at amino acid residues 51 to 136 and 102 to 136, respectively (24). By contrast pX49–115, which results in a minimal increase in the transcriptional efficacy of CREB (Fig. 4B), lacks region C; this region contains a portion of the binding sites for RPB5 and TFIIB (25) and nine conserved amino acids (132 to 140), which were shown to be required for pX-mediated transactivation (2, 34).
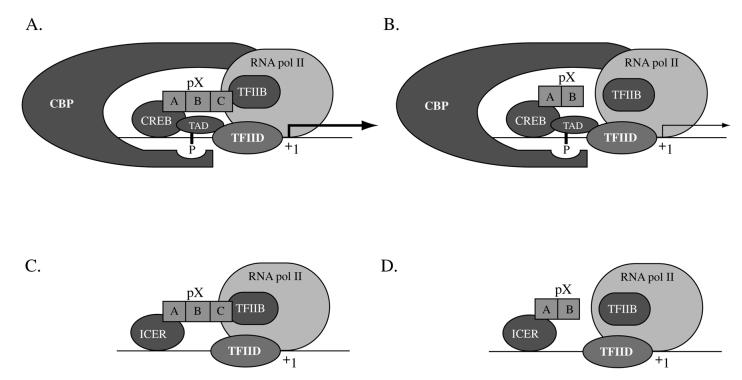
Therefore, it is interesting to speculate that concomitant interactions of pX with the bZip transcription factors and with one or more components of the basal transcriptional apparatus account for the pX-mediated enhancement of CRE transcription in vivo (Fig. 7). Since both pX49–140 and pX49–115 are functional in vitro (Fig. 3C), whereas only pX49–140 is functional in vivo (Fig. 4B), these data suggest that the interaction of pX with CREB may bridge specific components of the transcriptional apparatus to effect the observed increased transcriptional efficacy of CREB.
FIG. 7.
Model depicting concomitant interactions of pX with bZip transcription factors and the basal transcriptional apparatus. (A and B) pX-mediated CREB transactivation effected by pX49–140 (containing regions A, B, and C) and pX49–115 (containing regions A and B), respectively. (C and D) pX-mediated ICERIIγ transrepression effected by pX49–140 and pX49–115, respectively.
Based on in vitro transcription analyses and subcellular fractionation studies (30), it has been demonstrated that activated CREB interacts with the basal transcriptional apparatus at two distinct levels: (i) phospho(Ser133)-CREB recruits RNA polymerase II via interaction with CBP and (ii) the Q2 region of CREB associates with TFIID via interaction with hTAFII130. Accordingly, we propose that pX49–140, containing the conserved regions A, B, and C, tethers or stabilizes the transcriptional initiation complex via interaction with TFIIB or the RPB5 subunit of RNA polymerase II, as shown in Fig. 7A. According to our model, deletion of pX region C (Fig. 7B) only minimally promotes the interaction with the basal transcriptional apparatus, resulting in minimal induction of CREB-driven transcription. The significance of our observations and our proposed model is that CREB and the various bZip proteins (3) represent nonartificial, physiological cellular targets of pX (3, 26, 44). Furthermore, since bZip cis-acting elements are present within the HBV enhancers I and II (8, 12, 26), our model is relevant not only for cellular gene expression but also for the expression of the viral genome. Further studies are required to demonstrate directly the role of pX in bridging specific components of the basal transcriptional apparatus to CREB/bZip transcription factors.
Regarding the mechanism of the increased repression efficacy displayed by bZip repressors in the presence of pX (3), our results (Fig. 6) demonstrate that the minimal pX49–115 is as effective as the wt pX in increasing the repression efficacy of ATF3 and ICERIIγ in vivo. Since pX49–115 interacts directly with bZip proteins in vitro (Fig. 3 and 5) and in vivo (Fig. 4A) and also increases the CRE-binding potential of CREB in vitro (Fig. 3C), we interpret these observations as direct evidence in support of the importance of the increased DNA-binding potential of bZip proteins by pX in mediating enhanced bZip-driven transactivation or transrepression. Moreover, these results lend further support to the importance of the interaction of pX with the basal transcriptional apparatus (7, 15, 16, 24, 33). ICERIIγ represses CRE-mediated transcription due to lack of an activation domain (27). Accordingly, ICERIIγ is an example of a transcriptional repressor not engaging the basal transcriptional apparatus. The increased DNA-binding potential effected by pX49–115 is sufficient to mediate repression by ICERIIγ (Fig. 7C and D). We conclude that while pX-dependent coactivation is crucial in mediating CREB/CRE-driven transactivation, the interaction of pX with the basal transcriptional apparatus is not required for ICERIIγ- or ATF3-driven transcriptional repression (Fig. 6).
Since the minimal, functional pX region required for CREB interaction is also required for direct interaction with other bZip proteins (ATF3, ICERIIγ, and NF-IL6), this observation (Fig. 5) supports the idea that a common mechanism exists for the interaction of pX with bZip family members. Accordingly, this minimal region of pX is a powerful tool for biophysical studies, in order to determine the structural features of the CREB (bZip) interacting region of pX and its interaction with CREB/bZip family members. Importantly, no structural information is yet available for pX. Both pX mutants, spanning amino acid residues 49 to 140 and 49 to 115, express 1 to 2 mg of soluble protein per liter of bacterial culture (data not shown). Thus, these pX deletion mutants are amenable to biophysical analyses. The information derived from the biophysical studies should provide an important contribution toward understanding pX and, by examining naturally occurring pX variants, will provide a new approach to address the clinical relevance of CREB (bZip)-pX interactions. Very little is known of the sequence variations of pX, mainly because the pX gene overlaps the viral polymerase gene (46) and the precore gene (32) and carries several signals critical to the replicative viral lifecycle (13). It will be interesting to examine these naturally occurring sequence variations from the perspective of CREB-bZip interactions, employing our well-defined in vitro and in vivo functional assays (3, 41, 44).
ACKNOWLEDGMENTS
We thank R. L. Hullinger and Chi Tarn for critical reviews of the manuscript and for help with illustrations.
This work was supported by National Institute of Health grant DK44533 and American Cancer Society grant CN82450 to O.M.A.
REFERENCES
- 1.Andrisani O M, Pot D A, Zhu Z, Dixon J E. Three sequence-specific DNA-protein complexes are formed with the same promoter element essential for expression of the rat somatostatin gene. Mol Cell Biol. 1988;8:1947–1956. doi: 10.1128/mcb.8.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arii M, Takada K, Koike K. Identification of three essential regions of hepatitis B virus X protein for trans-activation function. Oncogene. 1992;7:397–403. [PubMed] [Google Scholar]
- 3.Barnabas S, Hai T, Andrisani O M. The hepatitis B virus X protein enhances the DNA-binding potential and transcription efficacy of bZip transcription factors. J Biol Chem. 1997;272:20684–20690. doi: 10.1074/jbc.272.33.20684. [DOI] [PubMed] [Google Scholar]
- 4.Benn J, Schneider R J. Hepatitis B virus HBx protein deregulates cell cycle checkpoint controls. Proc Natl Acad Sci USA. 1995;92:11215–11219. doi: 10.1073/pnas.92.24.11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benn J, Schneider R J. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc Natl Acad Sci USA. 1994;91:10350–10354. doi: 10.1073/pnas.91.22.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benn J, Su F, Doria M, Schneider R J. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol. 1996;70:4978–4985. doi: 10.1128/jvi.70.8.4978-4985.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheong J H, Yi M, Lin Y, Murakami S. Human RPB5, a subunit shared by eukaryotic nuclear RNA polymerases, binds human hepatitis B virus X protein and may play a role in X transactivation. EMBO J. 1995;14:143–150. doi: 10.1002/j.1460-2075.1995.tb06984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi B H, Park G T, Rho H M. Interaction of hepatitis B viral X protein and CCAAT/enhancer-binding protein α synergistically activates the hepatitis B viral enhancer II/pregenomic promoter. J Biol Chem. 1999;274:2858–2865. doi: 10.1074/jbc.274.5.2858. [DOI] [PubMed] [Google Scholar]
- 9.Colbran J L, Roach P J, Fiol C J, Dixon J E, Andrisani O M, Corbin J D. cAMP-dependent protein kinase, but not the cGMP-dependent enzyme, rapidly phosphorylates Δ-CREB, and a synthetic Δ-CREB peptide. Biochem Cell Biol. 1992;70:1277–1282. doi: 10.1139/o92-174. [DOI] [PubMed] [Google Scholar]
- 10.Colgrove R, Simon G, Ganem D. Transcriptional activation of homologous and heterologous genes by the hepatitis B virus X gene product in cells permissive for viral replication. J Virol. 1989;63:4019–4026. doi: 10.1128/jvi.63.9.4019-4026.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doria M, Klein N, Lucito R, Schneider R J. The hepatitis B virus HBx protein is a dual specificity cytoplasmic activator of Ras and nuclear activator of transcription factors. EMBO J. 1995;14:4747–4757. doi: 10.1002/j.1460-2075.1995.tb00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faktor O, Budlovsky S, Ben-Levy R, Shaul Y. A single element within the hepatitis B virus enhancer binds multiple proteins and responds to multiple stimuli. J Virol. 1990;64:1861–1863. doi: 10.1128/jvi.64.4.1861-1863.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganem D, Pollack J R, Tavis J. Hepatitis B virus reverse transcriptase and its many roles in hepadnaviral genomic replication. Infect Agents Dis. 1994;3:85–93. [PubMed] [Google Scholar]
- 14.Guan K, Dixon J E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 15.Haviv I, Shamay M, Doitsch G, Shaul Y. Hepatitis B virus pX targets TFIIB in transcription coactivation. Mol Cell Biol. 1998;18:1562–1569. doi: 10.1128/mcb.18.3.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haviv I, Vaizel D, Shaul Y. pX, the HBV-encoded transactivator, interacts with components of the transcription machinery and stimulates transcription in a TAF-independent manner. EMBO J. 1996;15:3413–3420. [PMC free article] [PubMed] [Google Scholar]
- 17.Kay A, Mandart E, Trepo C, Galibert F. The HBV HBx gene expressed in E. coli is recognized by sera from hepatitis patients. EMBO J. 1985;4:1287–1292. doi: 10.1002/j.1460-2075.1985.tb03774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kekule A S, Lauer U, Weiss L, Luber B, Hofschneider P H. Hepatitis B virus transactivator HBx uses a tumor promoter signalling pathway. Nature. 1993;361:742–745. doi: 10.1038/361742a0. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Lee H, Yun Y. X-gene product of hepatitis B virus induces apoptosis in liver cells. J Biol Chem. 1998;273:382–385. doi: 10.1074/jbc.273.1.381. [DOI] [PubMed] [Google Scholar]
- 20.Koike K, Moriya K, Yotsuyanagi H, Iino S, Kurokawa K. Induction of cell cycle progression by hepatitis B virus HBx gene expression in quiescent mouse fibroblasts. J Clin Investig. 1994;94:44–49. doi: 10.1172/JCI117343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar V, Jayasuryan N, Kumar R. A truncated mutant (residues 58–140) of the hepatitis B virus X protein retains transactivation function. Proc Natl Acad Sci USA. 1996;93:5647–5652. doi: 10.1073/pnas.93.11.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwee L, Lucito R, Aufiero B, Schneider R J. Alternate translation initiation on hepatitis B virus X mRNA produces multiple polypeptides that differentially trans-activate class II and III promoters. J Virol. 1992;66:4382–4389. doi: 10.1128/jvi.66.7.4382-4389.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levrero M, Balsano C, Natoli G, Avantaggiati M L, Elfassi E. Hepatitis B virus X protein transactivates the long terminal repeats of human virus types 1 and 2. J Virol. 1990;64:3082–3086. doi: 10.1128/jvi.64.6.3082-3086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Nomura T, Cheong J, Dorjsuren D, Iida K, Murakami S. Hepatitis B virus X protein is a transcriptional modulator that communicates with transcription factor IIB and the RNA polymerase II subunit 5. J Biol Chem. 1997;272:7132–7139. doi: 10.1074/jbc.272.11.7132. [DOI] [PubMed] [Google Scholar]
- 25.Lucito R, Schneider R J. Hepatitis B virus X protein activates transcription factor NF-κB without a requirement for protein kinase C. J Virol. 1992;66:983–991. doi: 10.1128/jvi.66.2.983-991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maguire H F, Hoeffler J P, Siddiqui A. HBV X protein alters the DNA binding specificity of CREB and ATF2 by protein-protein interactions. Science. 1991;252:842–844. doi: 10.1126/science.1827531. [DOI] [PubMed] [Google Scholar]
- 27.Molina C A, Foulkes N S, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 28.Moriarity A M, Alexander H, Lerner R A, Thornton G B. Antibodies to peptide detect new hepatitis B antigen: serological correlation with hepatocellular carcinoma. Science. 1985;227:429–433. doi: 10.1126/science.2981434. [DOI] [PubMed] [Google Scholar]
- 29.Murakami S, Cheong J H, Kaneko S. Human hepatitis virus X gene encodes a regulatory domain that represses transactivation of X protein. J Biol Chem. 1994;269:15118–15123. [PubMed] [Google Scholar]
- 30.Nakajima T, Uchida C, Anderson S, Parvin J, Montminy M. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 1997;11:738–747. doi: 10.1101/gad.11.6.738. [DOI] [PubMed] [Google Scholar]
- 31.Natoli G, Avantaggiati M L, Chirillo P, Costanzo A, Artini M, Balsano C, Levrero M. Induction of the DNA-binding activity of c-jun/c-fos heterodimers by the hepatitis B virus transactivator pX. Mol Cell Biol. 1994;14:989–998. doi: 10.1128/mcb.14.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto H, Tsuda F, Akahane Y, Sugai Y, Yoshiba M, Moriyama K, Tanaka T, Miyakawa Y, Mayumi M. Hepatitis B virus with mutations in the core promoter for an e-antigen phenotype in carriers with antibody to e antigen. J Virol. 1994;68:8102–8110. doi: 10.1128/jvi.68.12.8102-8110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qadri I, Conaway J W, Conaway R C, Schaack J, Siddiqui A. Hepatitis B virus transactivator protein, HBx, associates with the components of TFIIH and stimulates the DNA helicase activity of TFIIH. Proc Natl Acad Sci USA. 1996;93:10578–10583. doi: 10.1073/pnas.93.20.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renner M, Haniel A, Burgelt E, Hofschneider P H, Koch W. Transactivating function and expression of the X gene of the hepatitis B virus. J Hepatol. 1995;23:53–65. doi: 10.1016/0168-8278(95)80311-4. [DOI] [PubMed] [Google Scholar]
- 35.Rosner M T. Hepatitis B virus X-gene product: a promiscuous transcriptional activator. J Med Virol. 1992;36:101–117. doi: 10.1002/jmv.1890360207. [DOI] [PubMed] [Google Scholar]
- 36.Seto E, Mitchell P J, Yen T S B. Transactivation of the hepatitis B virus X protein depends on AP-2 and other transcription factors. Nature. 1990;344:72–74. doi: 10.1038/344072a0. [DOI] [PubMed] [Google Scholar]
- 37.Seto E, Yen T, Peterlin B, Ou J. Transactivation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci USA. 1988;85:8286–8290. doi: 10.1073/pnas.85.21.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seto E, Zhou D, Peterlin M, Yen B. Transactivation by the hepatitis B virus X protein shows cell-type specificity. Virology. 1989;173:764–766. doi: 10.1016/0042-6822(89)90594-1. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqui A, Gaynor R, Srinivasan A, Mapoles J, Farr R W. Transactivation of viral enhancers including long terminal repeat of the human immunodeficiency virus by the hepatitis B virus X protein. Virology. 1989;16:479–484. doi: 10.1016/0042-6822(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 40.Su F, Schneider R J. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor α. Proc Natl Acad Sci USA. 1997;94:8744–8749. doi: 10.1073/pnas.94.16.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarn C, Bilodeau M, Hullinger R, Andrisani O M. Differential immediate early gene expression in conditional hepatitis B virus pX-transforming versus nontransforming hepatocyte cell lines. J Biol Chem. 1999;274:2327–2336. doi: 10.1074/jbc.274.4.2327. [DOI] [PubMed] [Google Scholar]
- 42.Twu J S, Lai M Y, Chen D S, Robinson W S. Activation of protooncogene c-jun by the X protein of the hepatitis B virus. Virology. 1993;192:346–350. doi: 10.1006/viro.1993.1041. [DOI] [PubMed] [Google Scholar]
- 43.Twu J S, Wu J, Robinson W S. Transcriptional activation of the human immunodeficiency virus type 1 long terminal repeat by hepatitis B virus X-protein requires de novo protein synthesis. Virology. 1990;177:406–410. doi: 10.1016/0042-6822(90)90501-h. [DOI] [PubMed] [Google Scholar]
- 44.Williams J S, Andrisani O M. The hepatitis B virus X protein targets the basic region-leucine zipper domain of CREB. Proc Natl Acad Sci USA. 1995;92:3819–3823. doi: 10.1073/pnas.92.9.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J C, Merlino G, Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor α. Proc Natl Acad Sci USA. 1994;91:674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuh C H, Chang Y L, Ting L P. Transcriptional regulation of precore and pregenomic RNAs of hepatitis B virus. J Virol. 1992;66:4073–4084. doi: 10.1128/jvi.66.7.4073-4084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Z, Andrisani O M, Pot D A, Dixon J E. Purification and characterization of a 43-kDa transcription factor required for rat somatostatin gene expression. J Biol Chem. 1989;264:6550–6556. [PubMed] [Google Scholar]