Abstract
Herpes simplex virus (HSV) immediate-early (IE) gene expression is initiated via the recruitment of the structural protein VP16 onto specific sites upstream of each IE gene promoter in a multicomponent complex (TRF.C) that also includes the cellular proteins Oct-1 and HCF. In vitro results have shown that HCF binds directly to VP16 and stabilizes TRF.C. Results from transfection assays have also indicated that HCF is involved in the nuclear import of VP16. However, there have been no reports on the role or the fate of HCF during HSV type 1 (HSV-1) infection. Here we show that the intracellular distribution of HCF is dramatically altered during HSV-1 infection and that the protein interacts with and colocalizes with VP16. Moreover, viral protein synthesis and replication were significantly reduced after infection of a BHK-21-derived temperature-sensitive cell line (tsBN67) which contains a mutant HCF unable to associate with VP16 at the nonpermissive temperature. Intracellular distribution of HCF and of newly synthesized VP16 in tsBN67-infected cells was similar to that observed in Vero cells, suggesting that late in infection the trafficking of both proteins was not dependent on their association. We constructed a stable cell line (tsBN67r) in which the temperature-sensitive phenotype was rescued by using an epitope-tagged wild-type HCF. In HSV-1-infected tsBN67r cells at the nonpermissive temperature, direct binding of HCF to VP16 was observed, but virus protein synthesis and replication were not restored to levels observed at the permissive temperature or in wild-type BHK cells. Together these results indicate that the factors involved in compartmentalization of VP16 alter during infection and that late in infection, VP16 and HCF may have additional roles reflected in their colocalization in replication compartments.
One of the earliest regulatory events after a productive infection by herpes simplex virus (HSV) is the induction of virus immediate-early (IE) gene expression by VP16, a structural component of the viral particle (33). Transcriptional activation is initiated by the recruitment of VP16 (3), together with two cellular proteins, Oct-1 (31, 34, 41) and HCF (15, 50, 52), into a multicomponent complex formed on the regulatory sites, TAATGARAT motifs, present within each of the IE promoters (for reviews, see references 30 and 48). VP16 also plays an essential role later in infection, in virion morphogenesis (45). Although its precise function(s) at this stage remains to be elucidated, VP16 has been shown to interact with at least two virus proteins, VP22 (7) and the virion host shutoff protein (20, 39), both of which are also assembled into the tegument.
With regard to its role in IE gene expression, previous results from several laboratories indicate that the first step in assembly of the multicomponent complex (called TRF.C in this work) is the formation of a binary complex between VP16 and HCF, which subsequently associates with Oct-1 already bound to the TAATGARAT motifs (8, 15, 46). While a precise mechanistic understanding of the role of HCF remains to be determined, several recent reports have helped elucidate its function in more detail. HCF is synthesized as a large, 2,035-residue precursor protein which is subsequently cleaved at specific reiterated sites located toward the middle of the protein, to yield a family of polypeptides (16, 50). After cleavage, the amino- and carboxyl-terminal portions of HCF remain stably bound together. We and others have shown recently that the region of HCF comprising the first 380 residues binds directly to VP16 (12, 19, 38, 49). This domain of HCF contains six reiterations of about 50 residues (the kelch repeats) and is thought to form a β-propeller structure of linked β-sheets (2, 6, 53). Residues predicted to be present in repeats 5 and 6 of the kelch domain have been shown to be important for VP16 binding and complex assembly (14).
A BHK-21-derived temperature-sensitive hamster cell line (tsBN67), which undergoes a G0/G1 cell cycle arrest at the nonpermissive temperature (29), was rescued by HCF (9). The endogenous HCF in the cell line contained a single amino acid substitution within the N-terminal kelch repeats, and this mutation was shown to be the basis of the block in cell cycle progression. However, the kelch repeat domain itself was not sufficient for rescue of the cell cycle phenotype, which required residues 1 to approximately 900 (49). In vitro analysis of tsBN67 cell extracts has shown that at the nonpermissive temperature, while the stability and processing of HCF were not affected by the mutation, TRF.C formation and VP16 transcriptional activity were abrogated (9). These results indicate that VP16 may interact with HCF determinants which are involved in its cell cycle function.
Although the importance of VP16 in stimulating IE gene expression during HSV type 1 (HSV-1) infection has been previously established from the analysis of viruses encoding mutant VP16 proteins (1, 32, 40), parallel studies of virus replication in cells deficient in cellular components of the VP16 pathway have not been performed. We therefore wished to examine the role and the fate of HCF during HSV infection. In this report, we show that the subcellular distribution of HCF is profoundly altered upon HSV infection and that the protein colocalizes with a proportion of newly synthesized VP16. We also examined the role of HCF in VP16 transcriptional activity by infecting tsBN67 cells at the nonpermissive temperature and showed that VP16-HCF binding was abrogated and that viral gene expression and viral replication were significantly reduced compared to infection of parental BHK-21 cells. In addition, we rescued the tsBN67 proliferation defect by establishing a stable cell line expressing simian virus 5 (SV5)-tagged wild-type (wt) HCF (tsBN67r). Infection of tsBN67r cells at the nonpermissive temperature resulted in the binding of newly synthesized VP16 to exogenous HCF but only partly rescued virus protein synthesis and replication.
MATERIALS AND METHODS
Virus and cells.
The BHK-21-derived temperature-sensitive cell line tsBN67 (29) was obtained from RIKEN Gene Bank (Tsukuba, Japan). Vero, wt BHK-21, and tsBN67 cells were maintained in Dulbecco's modified minimal essential medium supplemented with 10% newborn calf serum. Vero and BHK-21 cells were propagated at 37°C, whereas tsBN67 cells were propagated at 33.5°C (permissive temperature). Previous results have demonstrated that tsBN67 cells undergo a reversible G0/G1 block after incubation at 39.5°C (nonpermissive temperature). The rescued cell line (tsBN67r) was maintained at 39.5°C in the medium described below, supplemented with G418 (0.8 mg per ml). Virus infections were carried out with HSV-1 strain 17. Vero cells were infected at a multiplicity of infection (MOI) of 10 PFU/cell. BHK-21 and its derivatives tsBN67 and tsBN67r were seeded 2 days prior to infection (4 × 105 cells/dish) at the assay temperature (33.5 or 39.5°C). Separate cultures of each cell types were counted immediately prior to infection to ensure identical MOIs. Infected cells were harvested at the indicated times postinfection (p.i.), and samples were processed for either immunoprecipitation, Western blotting, or immunofluorescence. Total viral progeny was assayed by plaque assay on Vero cells.
Immunoprecipitations.
Mock-infected or infected cells were washed in cold phosphate-buffered saline, and high-salt extracts were prepared as previously described (11, 51). VP16 was immunoprecipitated with the anti-VP16 monoclonal antibody (MAb) LP1 (1:100) in a buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, and 1% NP-40. Immune complexes were recovered by adding protein A-Sepharose. Beads were washed five times in the incubation buffer and then lysed in 2× sodium dodecyl sulfate (SDS) loading buffer.
SDS-PAGE and Western blotting.
Mock-infected or infected cells were washed in cold phosphate-buffered saline, and total lysates were prepared by adding 250 μl of 1× SDS loading buffer. Samples were briefly sonicated prior to electrophoresis. Equal amounts of total proteins were fractionated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto Hybond-C membranes. Primary antibodies for immunodetection were diluted as follow: anti-IE110k (MAb 11060, 1:10,000), anti-IE175k (MAb 10176, 1:5,000), anti-HSV thymidine kinase (1:2,000), anti-VP16 (MAb LP1 [1:4,000] or polyclonal rabbit antibody POS1 [1:2,000]), anti-VP22 (polyclonal rabbit antibody AGV30, 1:50,000), antiactin (MAb AC-40, 1:5,000; Sigma), and anti-HCF (rabbit polyclonal antibody, 1:10,000). Reactive proteins were visualized by enhanced chemiluminescence (Pierce).
Immunofluorescence.
Cells grown on coverslips were mock infected or infected with HSV at an MOI of 10 PFU/cell and were fixed in 100% methanol for 15 min at the indicated times p.i. Immunofluorescence reactions were carried out as described previously (7). Primary antibodies were diluted as follows: LP1, 1:400; anti-HCF, 1:200; anti-RNA polymerase II (Pol II) reacting against the carboxy-terminal tail, 1:200; and anti-SV5, a mouse MAb against a short peptide region of SV5 M protein used as an epitope tag in HCF constructs, 1:2,000. Secondary antibodies were fluorescein-conjugated anti-mouse immunoglobulin G (1:100) and tetramethyl rhodamine isocyanate-conjugated anti-rabbit immunoglobulin G (1:200). Cells were examined in a Zeiss LSM410 or Bio-Rad MRC600 confocal microscope with an ×40 or ×63 objective lens. Images were collected by scanning each channel separately, and control assays demonstrated no significant cross-channel leak-over. After collection, the images were processed and annotated with Adobe Photoshop software.
Rescue of the tsBN67 temperature-sensitive defect.
A stable cell line expressing SV5-tagged HCF was constructed as follow. tsBN67 cells grown for 2 days at 39.5°C were transfected with 2 μg of pSL25, a plasmid expressing SV5-tagged full-length HCF (18), together with 2 μg of pSV2Neo, a plasmid expressing the selectable marker for neomycin resistance. Cells were transfected by the calcium phosphate method modified by the inclusion of N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid-buffered saline as previously described (10). Two days after transfection, cells were washed and incubated in the presence of G418 (0.8 mg/ml) for 2 weeks. Individual colonies were selected and examined for expression of exogenous HCF by Western immunoblotting and immunofluorescence using the anti-SV5 and anti-HCF antibodies. A suitable clone (tsBN67r) was selected and expanded for analysis.
RESULTS
The intracellular distribution of HCF is altered in HSV-infected cells.
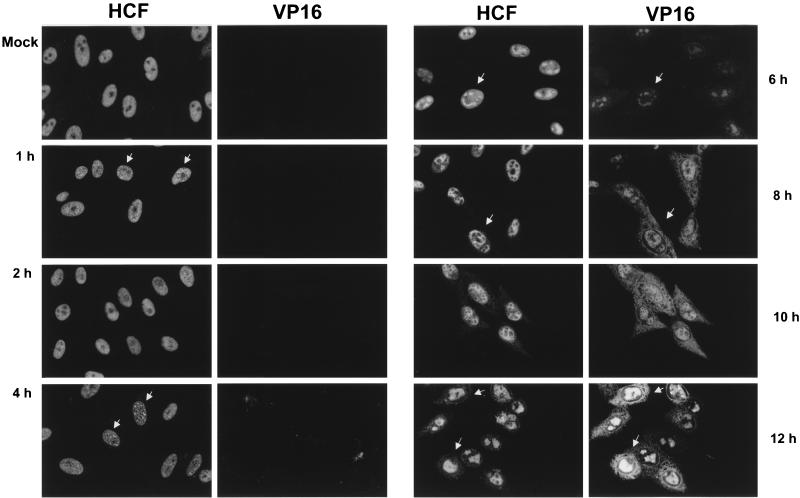
To determine the effect of HSV infection on HCF localization, a time course of infection was carried out with Vero cells. Cells were mock infected or infected at an MOI of 10, fixed at various times p.i., and stained for HCF and VP16 (Fig. 1). In mock-infected cells (Fig. 1, Mock), HCF exhibited a diffuse nuclear pattern with the exclusion of nucleoli, in agreement with previous results for other cell types (16, 18). At 1 h p.i. we observed subtle but distinct changes in HCF localization, with the appearance of speckled foci, which became more prominent by 4 h p.i. (Fig. 1, 4 h, arrows). By 6 h p.i. significant reorganization of HCF distribution was readily apparent (Fig. 1, 6 h, arrow, left panel), with a substantial amount of the protein accumulating in large intranuclear domains reminiscent of HSV replication compartments. Finally, at later times of infection (8 to 12 h), HCF localization progressed to nuclear honeycomb-like structures, with a minor but reproducible component in the cytoplasm (Fig. 1, 8 to 12 h, arrows). The very early alterations in HCF localization after infection are illustrated in a separate experiment with higher-magnification images which show more clearly the appearance of the protein in speckled foci between 1 and 2 h p.i., small bright foci by 4 h p.i., and the start of the coalescence into larger globular domains at 6 h p.i. (Fig. 2a).
FIG. 1.
Intracellular compartmentalization of HCF and VP16 in HSV-1-infected Vero cells. Vero cells were mock infected (Mock) or infected with HSV-1 (strain 17) at an MOI of 10 and fixed at the various times indicated. Double immunofluorescence was carried out with anti-HCF and anti-VP16 (LP1) antibodies. The panel for each time point shows the pattern of detection of HCF or VP16 in the same field of cells. VP16 was first detected between 4 and 6 h p.i., while concentration of HCF into speckled foci could be detected by 2 h p.i. The later nuclear colocalization of HCF and VP16 is readily apparent.
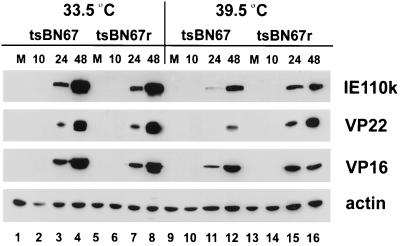
FIG. 8.
HSV-1 protein synthesis in infected tsBN67 and tsBN67r cells. tsBN67 and tsBN67r cells were grown for 2 days at 33.5 or 39.5°C exactly and then mock infected or infected with HSV at an MOI of 0.1; protein synthesis analyzed as for Fig. 5.
FIG. 2.
HCF reorganization and colocalization with RNA Pol II. (a) Vero cells were mock infected (Mock) or infected with HSV-1 (strain 17) at an MOI of 10 and fixed at the various times indicated. Cells were stained with anti-HCF antibody alone and analyzed by confocal microscopy using an ×63 objective combined with an ×2.5 digital zoom. (b) Mock-infected or HSV-infected cells (6 h p.i.) were double labeled for RNA Pol II and HCF. For the mock-infected cells, only RNA Pol II is shown; for the infected cells, the panels show the same field of cells stained for RNA Pol II or HCF, with clear colocalization of the two proteins in globular or coalesced foci (arrows).
We have recently shown in transient transfection assays that HCF colocalizes with VP16 (18). To determine whether such colocalization would also be observed in the context of viral infection, the infected cells shown in Fig. 1 were costained for VP16 (right panels). Newly synthesized VP16 was first observed between 4 and 6 h p.i. and was primarily detected in the nucleus in diffuse or globular structures, with prominent colocalization with HCF. At later times of infection (right panels, 8 to 12 h p.i.), most of the intranuclear VP16 remained localized with HCF, and a distinct perinuclear accumulation could also be observed. In addition, significant amounts of diffuse cytoplasmic VP16 were observed.
Earlier studies have shown that the major DNA replication proteins of HSV are recruited into subnuclear compartments termed replication compartments, which progressively accumulate into large globular domains (35). A number of cellular proteins, (e.g., RNA Pol II, topoisomerase II, Rb, and p53) are also recruited into these sites (36, 47). Since the pattern of relocalized HCF at later times of infection, from 6 to 8 h onwards, was reminiscent of replication compartments, we examined HCF location in dual-staining experiments in comparison to RNA Pol II. The results (Fig. 2b, arrowed cells) indicate that HCF did indeed colocalize with RNA Pol II late in infection. Together, these data demonstrate that there is a profound reorganization of HCF during virus infection, that HCF colocalizes with VP16, and that both proteins are present in viral replication compartments, with additional cytoplasmic accumulation of VP16.
HCF interacts with VP16 in HSV-infected cells.
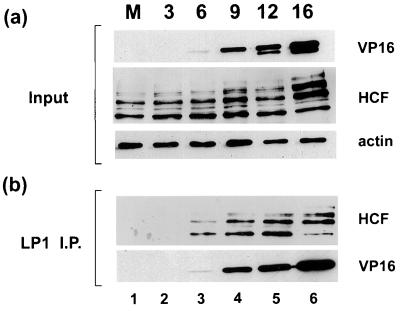
Biochemical assays have indicated that VP16 interacts with HCF in the absence of the other components of TRF.C, but to date there are no reports which specifically address HCF interaction with VP16 during HSV infection. Since we observed that HCF colocalizes with VP16, we next wished to determine whether the two proteins directly interact with one another during infection. Vero cells were mock infected or infected with HSV-1 at an MOI of 10 PFU/cell, and soluble extracts were prepared at 3, 6, 9, 12, and 16 h p.i. (Fig. 3). Samples were first analyzed directly by Western blotting to evaluate the relative amounts of proteins present in each extract (Fig. 3a). Reaction with an antiactin antibody demonstrated that approximately equivalent protein levels were loaded for each sample (Fig. 3a, actin). VP16 was first detected at 6 h p.i., increasing in abundance as infection progressed (Fig. 3a, VP16). Analysis of HCF with a polyclonal antibody raised against the C terminus of the protein showed that its steady-state level remained relatively constant throughout the course of infection. Note that the slight change in ratio of the different C-terminal species seen at 16 h p.i. was not significant and was not reproducibly observed (see, e.g., Fig. 4). The samples were next immunoprecipitated with a MAb against VP16, and VP16 was then visualized with a rabbit polyclonal antibody (Fig. 3b, VP16). Coprecipitated HCF was detected by Western blotting of the immunoprecipitates with the anti-HCF antibody (Fig. 3b, HCF). While no HCF was observed from the mock- or early-infected samples, as infection progressed HCF was coprecipitated in increasing amounts, corresponding to the amounts of precipitated VP16. While it is reasonable to expect from previous biochemical analyses that HCF interacts with VP16 immediately after infection, the amounts of VP16 present at that time were below our levels of detection in either total or immunoprecipitated extracts (Fig. 3). More sensitive assays will have to be developed to examine the HCF-VP16 interaction immediately after infection. Nevertheless, these results demonstrate that HCF associates with newly synthesized VP16 in HSV-1-infected cells, and it is likely that the interaction occurs in replication compartments where the two proteins accumulate throughout infection.
FIG. 3.
Direct interaction between HCF and VP16 in HSV-1-infected Vero cells. Vero cells were mock infected (lane M) or infected with HSV-1 at an MOI of 10, and soluble cell extracts were prepared at 3, 6, 9, 12, and 16 h p.i. VP16 was immunoprecipitated with LP1 (1:100), immune complexes were resolved in an SDS–10% polyacrylamide gel, and proteins were transferred onto a Hybond-C membrane. HCF and VP16 products were detected with anti-HCF and POS1 antibodies, respectively, before (a) and after (b) immunoprecipitation (I.P.). An antiactin antibody was used to ensure that similar amounts of proteins were present in each extract prior to immunoprecipitation.
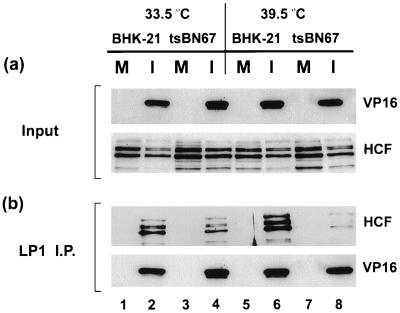
FIG. 4.
Interaction between HCF and VP16 in HSV-1-infected BHK-21 and tsBN67 cells. BHK-21 and tsBN67 cells grown for 2 days at 33.5 or 39.5°C were mock infected (lanes M) or infected (lanes I) at an MOI of 10. Soluble extracts were prepared 16 h p.i., and VP16 was immunoprecipitated with LP1. Detection of the input and immunoprecipitated proteins was as for Fig. 3.
Reduced association of VP16 with HCF in infected tsBN67 cells.
In transfection assays, VP16 fails to activate IE transcription in tsBN67 cells at the nonpermissive temperature, with the defect being attributed to reduced TRF.C formation (9, 49). We wished to examine the consequences of the defect in HCF on VP16 function in virus-infected cells and specifically to determine if VP16 binds to HCF in infected tsBN67 cells at the nonpermissive temperature. Parental BHK-21 and tsBN67 cells grown for 2 days at 33.5 and 39.5°C were infected at an MOI of 10 PFU/cell or were mock infected, and soluble cell extracts were prepared at 16 h p.i. (Fig. 4). As before, controls with the antiactin antibody confirmed that similar levels of proteins were present in all cell extracts (data not shown). Levels of HCF and VP16 were first assessed prior to immunoprecipitation (Fig. 4a). HCF was present in similar amounts in mock-infected and infected cells, both BHK-21 and tsBN67, with little difference in relative abundance of individual species (Fig. 4a, HCF). In infected cells, VP16 was expressed at similar levels in each of the cell types; in particular for tsBN67, there was little reduction in expression at the elevated temperature (Fig. 4a, VP16).
VP16 was next immunoprecipitated with LP1, and the presence of coprecipitated HCF was examined by Western blotting. As a control, the membrane was also probed with a separate anti-VP16 antibody (POS1) to confirm that similar levels of VP16 had been immunoprecipitated relative to the inputs (Fig. 3b, VP16). As expected, HCF was not recovered in any of the mock-infected cells. For infected BHK-21 cells, HCF coprecipitation was observed at both temperatures, with if anything slightly more HCF being detected at the elevated temperature (lanes 2 and 6). By comparison, in tsBN67 cells significantly less HCF was observed at 39.5°C than at 33.5°C (lanes 4 and 8; see also Fig. 7). This result demonstrates that after a high-multiplicity infection of tsBN67 cells at the restrictive temperature, VP16 was expressed at similar levels late in infection, while there was a clear deficiency in its ability to bind HCF.
FIG. 7.
Characterization of a stable tsBN67 cell line expressing SV5-tagged HCF. (a) Parental tsBN67 cells (top) and cells rescued with the SV5-tagged wt HCF (tsBN67r cells; bottom) were grown on coverslips at 39.5°C for 2 days before fixation. Indirect immunofluorescence was performed with either the anti-SV5 MAb (panels 1 and 3) or the anti-HCF polyclonal antibody (panels 2 and 4). (b) tsBN67 or tsBN67r cells grown for 2 days at 33.5°C or 39.5°C were mock infected (lanes M) or infected (lanes I) with HSV at an MOI of 10. Soluble extracts were prepared at 16 h p.i., and VP16 was immunoprecipitated (I.P.) with LP1 and detected by Western blotting of the immunoprecipitates with POS1. Coprecipitated HCF was detected with the anti-HCF antibody. The increased amount of HCF in the rescued line is demonstrated in the top panel, showing a Western blot of the total amount of HCF in each sample prior to the immunoprecipitation (input). The difference in efficiency of HCF coprecipitation at 39.5°C between the two lines can be seen by comparing the longer (for tsBN67 cells; see also Fig. 4) and shorter (tsBN67r cells) exposures (exp.). An antiactin antibody was used as a control to assess the relative amounts of proteins present in each extract.
HSV protein synthesis is severely impaired in tsBN67-infected cells at the nonpermissive temperature.
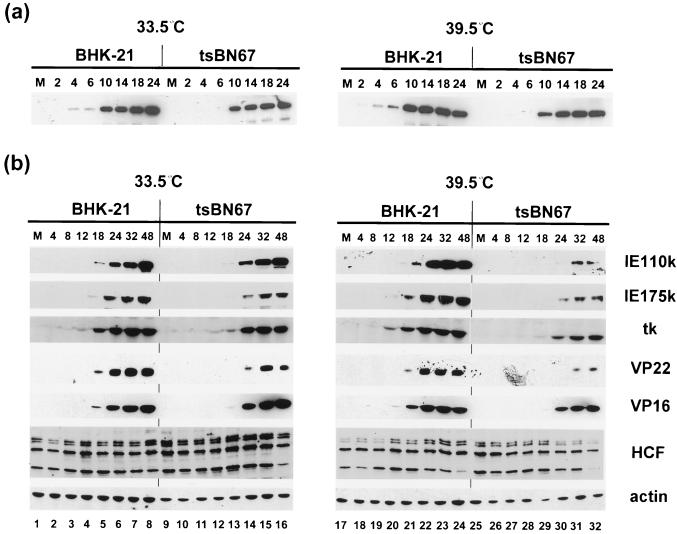
Previous analyses have shown that the effects of mutations in certain viral proteins, including VP16 (1), may be more distinct at low MOIs. Therefore, it was possible that any consequences of reduced binding of HCF to VP16 in the tsBN67 cells would be manifest at low MOI. To address this question, BHK-21 and tsBN67 cells were grown for 2 days at 33.5 or 39.5°C and then infected at high (10 PFU/cell) and low (0.1 PFU/cell) MOIs. Total cell lysates prepared at various times p.i. were analyzed by Western blotting using a variety of antibodies to different virus-encoded proteins (Fig. 5). We first analyzed the appearance of an IE protein, IE110k (ICP0), at the high MOI (Fig. 5a). At both temperatures, IE110k appeared slightly earlier in BHK-21 cells than in tsBN67-infected cells. (Note that despite its optimal rates of synthesis at IE times, accumulation of IE110k to maximal amounts occurs late in infection.) There was, however, no significant difference in the expression of IE110k in tsBN67 cells infected at the permissive compared to the nonpermissive temperature. Similar results were obtained for the other virus proteins (data not shown). This result suggests that at a MOI, viral gene expression proceeds normally despite the defect in HCF.
FIG. 5.
HSV-1 protein synthesis in infected BHK-21 and tsBN67 cells. (a) BHK-21 and tsBN67 cells grown for 2 days at 33.5 or 39.5°C were infected with HSV-1 at an MOI of 10. Total cell lysates were prepared at 0 (lanes M), 2, 4, 6, 10, 14, 18, and 24 h p.i. Proteins were fractionated in an SDS–10% polyacrylamide gel and transferred onto a Hybond-C membrane, and IE110k was detected with a MAb. (b) BHK-21 and tsBN67 cells were infected with 0.1 PFU/cell, and total cell lysates were prepared at 0 (lanes M), 4, 8, 12, 18, 24, 32, and 48 h p.i. Proteins were fractionated in an SDS–10% polyacrylamide gel, transferred onto a Hybond-C membrane, and reacted with antibodies directed against IE (IE110k and IE175k), early (thymidine kinase [tk]), and late (VP16 and VP22) viral proteins, HCF, and actin, which served as a control for the relative amounts of proteins present in each sample. Note that threefold more lysates were used for the detection of IE175k.
By contrast, at low MOI there was a clear difference in the response to elevated temperature between the two cell lines (Fig. 5b). At 33.5°C, IE110k expression was slightly advanced in BHK-21 versus tsBN67, but there was only a marginal difference between the two lines (Fig. 5b, 33.5°C, IE110k); comparable levels of synthesis of IE175k (ICP4) and representative members of early and late classes of virus proteins were also observed (Fig. 5b, 33.5°C). However, at 39.5°C, while in BHK-21 cells IE110k expression appeared if anything slightly earlier and in greater amounts than at 33.5°C, in tsBN67 cells IE110k expression was both delayed and significantly reduced. This divergent response to the elevated temperature resulted in a very substantial difference in the expression levels of IE110k between the two cell lines. Similar reductions in expression of additional virus proteins were also observed (Fig. 5b, 39.5°C). As for the earlier experiments, total protein levels of HCF and actin remained relatively constant throughout the course of infection in both cell types (Fig. 5b, HCF and actin). Thus, from the results for the parental BHK-21 cells, the elevated temperature does not per se have a detrimental effect on infection or virus gene expression, and the profound delay in viral protein expression in tsBN67 cells infected at low MOI is consistent with the defect in HCF abrogating TRF.C formation and IE gene expression.
In parallel with the experiments described above, we measured total virus yields after infection at each MOI (Table 1). At an MOI of 0.1 PFU/cell, titers at 24 h p.i. show no significant difference in BHK-21 cells at 33.5°C compared to 39.5°C. In tsBN67 cells, yields were approximately 30-fold lower at 39.5°C than at 33.5°C, and these titers were 150-fold lower than in BHK-21 cells at 39.5°C. By 48 h p.i., the relative efficiency of replication in the tsBN67 cells had recovered somewhat, and there was only a 10-fold difference between titers obtained from BHK-21 and tsBN67 cells infected at 39.5°C. In addition, the titer of HSV propagated on tsBN67 cells for 14 h at an MOI of 10 PFU/cell at the nonpermissive temperature is 10- to 30-fold lower than titers obtained at 33.5°C or in BHK-21 cells at both temperatures. These results indicate that the defect in HCF results in reduced HSV replication particularly at low MOI but that (possibly due to secondary infections at higher MOIs) this effect is reduced at later times of infection.
TABLE 1.
Titration of HSV propagated on BHK-21 and tsBN67 cellsa
| MOI | Time (h) p.i. | Titer of virusb grown on indicated cell line
|
|||
|---|---|---|---|---|---|
| 33.5°C
|
39.5°C
|
||||
| BHK-21 | tsBN67 | BHK-21 | tsBN67 | ||
| 0.1 PFU/cell | 24 | 1.5 × 107 | 6 × 106 | 1.7 × 107 | 2.1 × 105 |
| 48 | 2.4 × 107 | 8.4 × 107 | 4 × 107 | 3 × 106 | |
| 10 PFU/cell | 14 | 3.6 × 106 | 2.4 × 106 | 6 × 106 | 1.4 × 105 |
BHK-21 or tsBN67 cells grown for 48 h at the permissive (33.5°C) or nonpermissive (39.5°C) temperature were infected with 0.1 or 10 PFU of HSV-1 per cell. Total progeny was harvested at 14 h p.i. (MOI of 10 PFU/cell) and at 24 and 48 h p.i. (MOI of 0.1 PFU/cell).
Determined by plaque assay titration on Vero cells of total intracellular plus extracellular virus.
Intracellular localization of HCF and VP16 in tsBN67-infected cells.
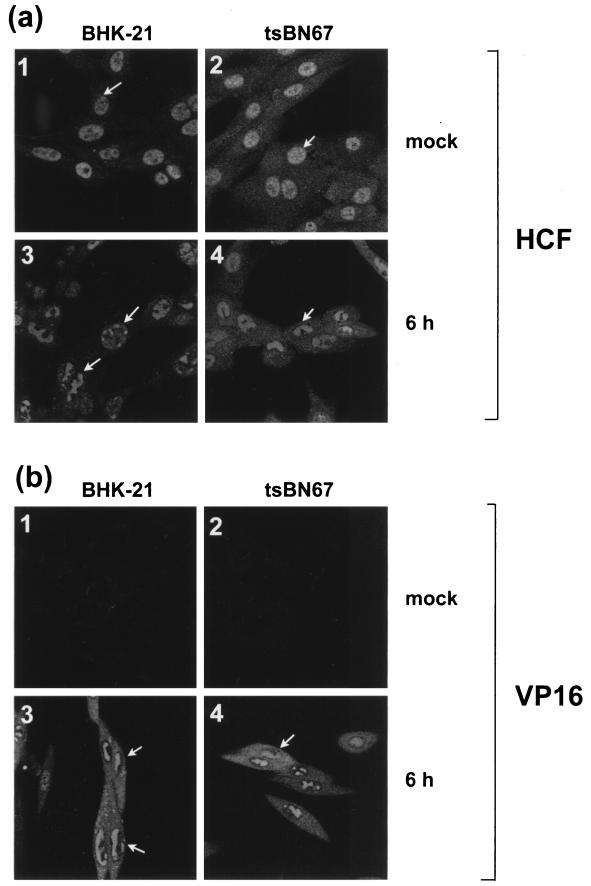
The biochemical analysis by coprecipitation indicated that at both MOIs, VP16 binding to HCF was defective in tsBN67 cells at the nonpermissive temperature. We next wished to investigate whether this defect would affect the intracellular localization of HCF and VP16 during HSV infection. BHK-21 and tsBN67 cells were mock infected or infected at 39.5°C with an MOI of 10 PFU/cell. Cells were fixed at 6 h p.i. and stained with the anti-HCF antibody (Fig. 6a). In mock-infected BHK-21 cells, HCF exhibited a diffuse nuclear localization pattern (Fig. 6a1) identical to that observed in Vero cells (see above). In tsBN67 cells however, while the distribution pattern of the protein was predominantly nuclear, somewhat more cytoplasmic HCF was also detected (Fig. 6a2). A similar pattern was observed at the permissive temperature (data not shown). In infected cells, however, HCF was relocalized in both BHK-21 and tsBN67 cells (Fig. 6a3 and -4), with patterns again very similar to those observed in infected Vero cells. Therefore, despite the defective association between HCF and VP16 observed in coprecipitation assays, the protein was still relocalized into replication compartments during HSV infection.
FIG. 6.
Intracellular distribution of HCF and VP16 in BHK-21 and tsBN67 cells infected at 39.5°C. BHK-21 and tsBN67 cells grown for 2 days at 39.5°C were mock infected or infected with HSV-1 at an MOI of 10 and fixed 6 h later. Indirect immunofluorescence was carried out with the anti-HCF polyclonal antibody (a) or LP1, the anti-VP16 MAb (b).
Parallel samples were also examined for VP16 localization (Fig. 6b). The results show that in both BHK-21 and tsBN67 cells infected at 39.5°C, VP16 was detected in the nuclear replication compartments and in the cytoplasm, and its pattern was essentially identical to that observed in Vero cells. Taken together, these results indicate that the trafficking of HCF and VP16 late during infection does not require a direct interaction between the two proteins.
Rescue of the tsBN67 cell defect by HCF restores VP16 interaction.
To confirm that the decrease of viral protein synthesis and replication in tsBN67 cells at the nonpermissive temperature was caused by the defect in HCF, we rescued the temperature-sensitive phenotype by establishing a stable cell line expressing full-length wild-type HCF. The tsBN67 cells were transfected with a vector expressing SV5-tagged HCF (18), individual colonies were selected for growth at the nonpermissive temperature, and a suitable clone (tsBN67r) was chosen for further experiments. Growth at 39.5°C was restored in these cells, with a doubling time similar to that of wild-type BHK cells (results not shown). Expression of exogenous HCF was confirmed by Western blotting (data not shown) and by immunofluorescence analyses using the anti-SV5 and anti-HCF antibodies (Fig. 7a). As expected, staining of tsBN67 and tsBN67r cells with the anti-HCF antibody revealed that HCF was present in higher amount in the nuclei of tsBN67r cells (compare Fig. 8a2 and -4). Using the anti-SV5 antibody to detect the N terminus of the introduced wt HCF, we observed that while HCF was also largely nuclear, there was also some cytoplasmic staining (Fig. 8a3). In virus-infected cells, the SV5-tagged HCF was also relocalized into nuclear replication compartments (data not shown). Although this result was not unexpected, it confirms the relocalization that was observed in the Vero and tsBN67 cells with an independent antibody and removes the possibility that this may have been due to the anti-HCF antibody cross-reacting with virus components in the replication compartments.
To analyze whether the presence of the exogenous HCF was sufficient to restore an association with VP16, tsBN67 and tsBN67r cells were mock infected or infected at 33.5 or 39.5°C with HSV at an MOI of 10 PFU/cell. Soluble cell extracts were prepared 16 h p.i., and VP16 was immunoprecipitated with LP1 as before (Fig. 7b).
Prior to immunoprecipitation, samples were analyzed by Western blotting to evaluate the relative amounts of HCF present in each cell extract (Fig. 7, upper panels). Note that as expected, HCF was present in higher amounts in tsBN67r cells than in tsBN67 cells (Fig. 7b, input, HCF). After VP16 immunoprecipitation, HCF was recovered in tsBN67 infected at 33.5°C (lane 2) but in much reduced amounts at 39.5°C (lane 6), consistent with the results in Fig. 4. By comparison, in the tsBN67r cells, HCF was efficiently coprecipitated with VP16 at 33.5°C, and similar amounts were coprecipitated at 39.5°C. While total HCF was more abundant in the tsBN67r cells, the relative efficiency of HCF coprecipitation with VP16 at 39.5°C in these cells can be readily seen by comparing the longer (for tsBN67 cells) and shorter (for tsBN67r cells) exposures of the blot. Controls show that similar amounts of VP16 were immunoprecipitated (Fig. 7b, VP16). These results demonstrate that the rescue of the temperature-sensitive defect in a tsBN67 stable cell line expressing SV5-tagged wt HCF restores the association of VP16 with HCF at 39.5°C.
HSV protein synthesis and replication in tsBN67r cells.
We next wished to determine whether expression of exogenous wt HCF would reduce the delay in virus protein synthesis and replication observed in tsBN67 cells at the nonpermissive temperature. In parallel, tsBN67 and tsBN67r cells were infected (MOI of 0.1 PFU/cell) at both temperatures, and total cell lysates were prepared at 10, 24, and 48 h p.i. Samples were analyzed for levels of IE110k, VP22, VP16, and actin, which again served as a control for the total protein load in each sample (Fig. 8). At 33.5°C, similar amounts of viral proteins were synthesized in both cell lines (compare lanes 3 and 4 with lanes 7 and 8), which could be expected since HCF is functional in the tsBN67 cells at this temperature. But it is of note that the higher levels of HCF in the rescued tsBN67r cells did not accelerate the progression of the HSV replicative cycle. At 39.5°C, IE110k, VP22, and VP16 were only marginally more abundant in tsBN67r cells than in tsBN67 cells (compare lanes 11 and 12 with lanes 15 and 16). Although this effect was modest, viral protein synthesis was detected earlier in tsBN67r cells. Nevertheless, perhaps the most salient feature of the results is that in both cell lines, and in contrast to what was found for the parental BHK-21 cells (Fig. 5b), lower amounts of viral proteins were detected at 39.5°C than at 33.5°C (compare lanes 3 and 4 with 11 and 12 and lanes 7 and 8 with 15 and 16). Even at later times of infection at 39.5°C, virus protein levels never accumulated to those observed at 33.5°C. Therefore, the delay in viral protein synthesis at 39.5°C is only partly rescued in tsBN67r cells, and generally levels of protein synthesis remained lower than those observed at 33.5°C or in BHK-21 cells infected 39.5°C.
The observation of limited rescue of virus protein synthesis was reflected in analysis of HSV replication in the tsBN67 and tsBN67r cells. The results (Table 2) show that at 24 and 48 h p.i., the viral titers obtained in tsBN67r cells at 39.5°C were approximately six- and threefold higher, respectively, than those obtained in tsBN67 cells. Nevertheless, the titers obtained at 39.5°C remained lower (10- to 20-fold) than those obtained at 33.5°C in tsBN67r cells, with a ratio which was not significantly different from that obtained for the tsBN67 cells. Taken together with the results in Fig. 5, these data indicate that the HCF-VP16 interaction is defective in tsBN67 cells and virus protein expression is significantly reduced at 39.5°C, but rescue of the ts defect by wt HCF in tsBN67r cells only partly rescues virus protein synthesis and replication.
TABLE 2.
Titration of HSV propagated on tsBN67 and tsBN67r cellsa
| Time (h) p.i. | Titer of virusb grown on indicated cell line
|
|||
|---|---|---|---|---|
| 33.5°C
|
39.5°C
|
|||
| tsBN67 | tsBN67r | tsBN67 | tsBN67r | |
| 24 | 1.5 × 106 | 1.3 × 106 | 1.0 × 105 | 6.5 × 105 |
| 48 | 6.8 × 107 | 1.0 × 108 | 3 × 106 | 8 × 106 |
tsBN67 or tsBN67r cells grown for 48 h at the permissive (33.5°C) or nonpermissive (39.5°C) temperature were infected with 0.1 PFU of HSV-1 per cell. Total progeny was harvested at 24 and 48 h p.i.
Determined by plaque assay titration on Vero cells of total intracellular plus extracellular virus.
DISCUSSION
HCF and VP16 interaction and compartmentalization in infected cells.
While biochemical aspects of mechanism of transcriptional activation by VP16 are understood in some detail, there are few reports on the interactions between VP16 and its associated cellular factors in vivo in virus-infected cells. In this study, we analyzed HCF compartmentalization during HSV infection in wt cells and in cells containing a mutant HCF defective in VP16 binding. We show that HCF undergoes a subtle but distinct recruitment into small speckled foci within 1 to 2 h p.i., and a pronounced recruitment into concentrated and merged large globular aggregates was observed beginning at 6 to 8 h p.i. It would be reasonable to predict that the speckled foci of HCF which are seen very early after infection may represent sites of VP16 recruitment and activity. However, using a variety of antibodies and conditions for immunofluorescence, we have been unable to follow the fate of input virion-associated VP16 immediately after infection.
By definition, activation of IE transcription by VP16 takes place on input viral genomes. Results of Maul and colleagues suggest that HSV IE transcription may take place at defined preexisting sites, overlapping or adjacent to cellular subnuclear compartments termed nuclear domain 10 (ND10) or PML oncogenic domains (13, 25, 26). We are currently examining whether early HCF foci exhibit any spatial relationship with ND10 regions, an observation which would provide mutually reinforcing evidence for a role in IE transcription.
The dramatic relocalization observed later in infection represents the recruitment of HCF into virus replication compartments. These structures originally defined by the progressive localization of the major single-stranded DNA binding protein (ICP8, UL29) from prereplicative sites into large nuclear domains (4, 22, 35) have been shown to contain each of the essential virus DNA replication proteins, and indeed the formation of similar compartments has been recapitulated in transfection experiments (24, 55). In addition, a number of cellular proteins, including RNA Pol II (36) and tumor suppressors p53 and Rb (47, 55), have been found to accumulate in replication compartments. Our results now indicate that HCF is also recruited into replication compartments and that within these compartments HCF is colocalized with VP16. The precise spatial relationships between replication proteins, cleavage/packaging proteins, and mature structural proteins remain somewhat controversial 5, 21, 42, 44; for a discussion, see reference 5). Furthermore, in relation to the points above on the possible association of the very early speckled foci of HCF with ND10 compartments, recent results have indicated that only a subset of virus prereplicative sites progress to form replication compartments, and this subset is associated with ND10 (23). It is not our point here to identify specific subcompartments within replication compartments but to indicate that HCF is relocalized early and that it appears to associate later with replication compartments, where it colocalizes with VP16. Refined analysis of HCF colocalization with ND10 and specific replication proteins should help inform the debate on the location of active templates early after infection and the relationship between these sites and later replication sites. Although there is no information to date on the relevance of the cellular proteins in replication compartments, it is clear that HCF may play an active role at stages other than IE gene expression (see below).
Notwithstanding the various reports of subnuclear organization in virus-infected cells, our results suggest that the mechanism responsible for the localization of HCF late in infected cells and in particular its presence in viral replication compartments may not require a direct association with VP16. From the immunofluorescence data, both proteins were found in replication compartments in tsBN67 cells infected at the nonpermissive temperature, while from the biochemical data, significantly reduced amounts of HCF were found to coprecipitate with VP16. Although it is possible that the two proteins can still interact at the nonpermissive temperature, but do so with a weaker affinity that is more readily manifest by coprecipitation assays than immunofluorescence assays, it is also possible that HCF can be actively recruited through interaction(s) with some other viral or cellular partner(s).
With regard to VP16, it was reported that at late times of infection the protein was predominantly localized near the periphery of the nucleus, adjacent or overlapping with nuclear subcompartments containing mature capsid proteins (44). In contrast to these results, Morrison et al. (27) observed that newly synthesized VP16 was localized in replication compartments with ICP8, and also in compartments with the scaffolding VP22a, suggesting that VP16 associated with sites of DNA replication and capsid maturation. Our results appear more compatible with the latter observations, but considering the recent higher-resolution analysis and subdivision of replication compartments by de Bruyn Kops et al. (5), more refined analysis of VP16 and HCF subnuclear localization will be required.
As pointed out previously, VP16 does not possess its own nuclear localization signal, is largely cytoplasmic in transfection assays, and may presumably rely on the binding to a nuclear localization signal-bearing protein for nuclear import. We have shown in cotransfection experiments that HCF promoted nuclear import of VP16, either directly acting as a chaperone promoting transport from the cytoplasm or as a binding factor acting to retain VP16 in the nucleus (18). However, our present results indicate that direct HCF binding does not appear necessary for VP16 nuclear compartmentalization late in infection. There are several not mutually exclusive possible explanations to reconcile these results. As stated above, it is possible that HCF interaction is involved in late VP16 compartmentalization but that the interaction is not detected by coimmunoprecipitation in the tsBN67 cells. However, in this report we are examining the location of de novo-synthesized VP16, and it is also possible that immediately after infection input VP16 translocates to the nucleus via an HCF-dependent pathway, whereas newly synthesized VP16 nuclear trafficking is HCF independent. We cannot currently test this hypothesis since we have been unable to detect input virion-associated VP16, even using conditions similar to those described in a recent report (27), and we are attempting to devise more sensitive techniques to address this question. However, as for capsid proteins (28, 37), it is clearly possible that VP16 late in infection is transported via an association with other tegument proteins, and nuclear import by an association with capsid components cannot be ruled out. Preliminary evidence indicates that VP16 is relocalized in the nucleus in cotransfection experiments with additional tegument proteins (G. Elliott, personal communication).
Importance of HCF in stimulating the HSV lytic cycle.
At low MOIs in the temperature-sensitive cell line encoding a mutant HCF, HSV-1 protein synthesis and replication were significantly reduced at the nonpermissive temperature. Our results are consistent with previous studies using HSV strains encoding mutant VP16 proteins which are defective in TRF.C formation or transcriptional activation (1, 40). However, previous reports have indicated that HSV replicates more efficiently in cycling than in growth-arrested temperature-sensitive cell lines (43, 54), and this could be an additional contributory factor in the reduced virus expression in the tsBN67 cells at 39.5°C. However, in the cell line rescued for HCF function in cell cycle progression, only partial rescue of HSV replication was observed. At low MOI, virus protein synthesis was only marginally higher in the rescued cells, and the ratio of virus yield at 39.5°C to that at 33.5°C was not substantially different from the parental temperature-sensitive line. While these results indicate that the reduced protein synthesis and replication were not simply due to defective cell cycle progression, they also prompt the question as to why the introduction of HCF did not restore virus replication to that observed in wt BHK cells. HCF may have additional roles in virus replication, but if anything the rescued line expressed greater amounts of HCF, and in any case (unless this was positively detrimental to replication) all relevant functions of HCF would have been expected to be restored. Perhaps one explanation could be that since the tsBN67 cells were generated by broad chemical mutagenesis, there was disruption of additional cellular functions which while not involved in the cell cycle block were nevertheless important for normal levels of virus replication. These considerations indicate that it is formally difficult to attribute the defect in virus expression to a defect in HCF function and illustrate the complexities in attempting to provide supporting genetic evidence for the biochemical data implicating HCF in VP16 activity and virus replication. However, recent biochemical results on the selective assembly of HCF into the VP16 complex, together with studies of HCF compartmentalization indicating that HCF is cytoplasmic in neurons but translocates to the nucleus following stimuli which reactivate latent HSV infections, indicate that the protein plays an important role in virus replication (14, 17). Our results on HCF colocalization in replication compartments, independently of direct VP16 binding, indicate that the protein may have as yet unidentified roles in HSV replication. Studies are now under way to relate the fate of input VP16 to early HCF localization and to examine late HCF compartmentalization with respect to possible subcompartments for replication or assembly.
ACKNOWLEDGMENTS
We thank T. Minson for antibody LP1, W. Herr for the polyclonal antibody against HCF, R. Everett for MAbs against IE110k and IE175, and R. Randall for antibody against the SV5 epitope.
This work was funded by the Marie Curie Cancer Care.
REFERENCES
- 1.Ace C I, McKee T A, Ryan J M, Cameron J M, Preston C M. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J Virol. 1989;63:2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bork P, Doolittle R F. Drosophila kelch motif is derived from a common enzyme fold. J Mol Biol. 1994;236:1277–1282. doi: 10.1016/0022-2836(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 3.Campbell M E, Palfreyman J W, Preston C M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984;180:1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- 4.de Bruyn Kops A, Knipe D M. Preexisting nuclear architecture defines the intranuclear location of herpesvirus DNA replication structures. J Virol. 1994;68:3512–3526. doi: 10.1128/jvi.68.6.3512-3526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bruyn Kops A, Uprichard S L, Chen M, Knipe D. Comparison of the intranuclear distributions of herpes simplex virus proteins involved in various viral functions. Virology. 1998;252:162–178. doi: 10.1006/viro.1998.9450. [DOI] [PubMed] [Google Scholar]
- 6.Eichinger L, Bomblies L, Vanderkerckhove J, Schleicher M, Gettemans J. A novel type of protein kinase phosphorylates actin in the actin-fragmin complex. EMBO J. 1996;15:5547–5556. [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott G, Mouzakitis G, O'Hare P. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J Virol. 1995;69:7932–7941. doi: 10.1128/jvi.69.12.7932-7941.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerster T, Roeder R G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci USA. 1988;85:6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto H, Motomura S, Wilson A C, Freiman R N, Nakabeppu Y, Fukushima K, Fujishima M, Herr W, Nishimoto T. A single-point mutation in HCF causes temperature-sensitive cell-cycle arrest and disrupts VP16 function. Genes Dev. 1997;11:726–737. doi: 10.1101/gad.11.6.726. [DOI] [PubMed] [Google Scholar]
- 10.Greaves R, O'Hare P. Separation of requirements for protein-DNA complex assembly from those for functional activity in the herpes simplex virus regulatory protein Vmw65. J Virol. 1989;63:1641–1650. doi: 10.1128/jvi.63.4.1641-1650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greaves R F, O'Hare P. Structural requirements in the herpes simplex virus type 1 transactivator Vmw65 for interaction with the cellular octamer-binding protein and target TAATGARAT sequences. J Virol. 1990;64:2716–2724. doi: 10.1128/jvi.64.6.2716-2724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes T A, LaBoissiere S, O'Hare P. Analysis of functional domains of the host cell factor involved in VP16 complex formation. J Biol Chem. 1999;274:16437–16443. doi: 10.1074/jbc.274.23.16437. [DOI] [PubMed] [Google Scholar]
- 13.Ishov A M, Maul G G. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J Cell Biol. 1996;134:815–826. doi: 10.1083/jcb.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson K M, Mahajan S S, Wilson A C. Herpes simplex virus transactivator VP16 discriminates between HCF-1 and a novel member, HCF-2. J Virol. 1999;73:3930–3940. doi: 10.1128/jvi.73.5.3930-3940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katan M, Haigh A, Verrijzer C P, van der Vliet P C, O'Hare P. Characterization of a cellular factor which interacts functionally with Oct-1 in the assembly of a multicomponent transcription complex. Nucleic Acids Res. 1990;18:6871–6880. doi: 10.1093/nar/18.23.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristie T M, Pomerantz J L, Twomey T C, Parent S A, Sharp P A. The cellular C1 factor of the herpes simplex virus enhancer complex is a family of polypeptides. J Biol Chem. 1995;270:4387–4394. doi: 10.1074/jbc.270.9.4387. [DOI] [PubMed] [Google Scholar]
- 17.Kristie T M, Vogel J L, Sears A E. Nuclear localisation of the C1 factor (host cell factor) in sensory neurons correlates with reactivation of herpes simplex virus from latency. Proc Natl Acad Sci USA. 1999;96:1229–1233. doi: 10.1073/pnas.96.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Boissiere S, Hughes T, O'Hare P. HCF-dependent nuclear import of VP16. EMBO J. 1999;18:480–490. doi: 10.1093/emboj/18.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaBoissiere S, Walker S, O'Hare P. Concerted action of host cell factor subregions in prommoting stable complex assembly and preventing interference by the acidic domain of VP16. Mol Cell Biol. 1997;17:7108–7118. doi: 10.1128/mcb.17.12.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam Q, Smibert C A, Koop K E, Lavery C, Capone J P, Weinheimer S P, Smiley J R. Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function. EMBO J. 1996;15:2575–2581. [PMC free article] [PubMed] [Google Scholar]
- 21.Lamberti C, Weller S K. The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments. J Virol. 1998;72:2463–2473. doi: 10.1128/jvi.72.3.2463-2473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liptak L M, Uprichard S L, Knipe D M. Functional order of assembly of herpes simplex virus DNA replication proteins into prereplicative site structures. J Virol. 1996;70:1759–1767. doi: 10.1128/jvi.70.3.1759-1767.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukonis C J, Burkham J, Weller S K. Herpes simplex virus type 1 prereplicative sites are a heterogeneous population: only a subset are likely to be precursors to replication compartments. J Virol. 1997;71:4771–4781. doi: 10.1128/jvi.71.6.4771-4781.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukonis C J, Weller S K. Formation of herpes simplex virus type 1 replication compartments by transfection: requirements and localization to nuclear domain 10. J Virol. 1997;71:2390–2399. doi: 10.1128/jvi.71.3.2390-2399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maul G. Nuclear domain 10, the site of DNA virus transcription and replication. Bioessays. 1998;20:660–667. doi: 10.1002/(SICI)1521-1878(199808)20:8<660::AID-BIES9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 26.Maul G G, Ishov A M, Everett R D. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology. 1996;217:67–75. doi: 10.1006/viro.1996.0094. [DOI] [PubMed] [Google Scholar]
- 27.Morrison E E, Stevenson A J, Wang Y F, Meredith D M. Differences in the intracellular localization and fate of herpes simplex virus tegument proteins early in the infection of Vero cells. J Gen Virol. 1998;79:2517–2528. doi: 10.1099/0022-1317-79-10-2517. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson P, Addison C, Cross A M, Kennard J, Preston V G, Rixon F J. Localization of the herpes simplex virus type 1 major capsid protein VP5 to the cell nucleus requires the abundant scaffolding protein VP22a. J Gen Virol. 1994;75:1091–1099. doi: 10.1099/0022-1317-75-5-1091. [DOI] [PubMed] [Google Scholar]
- 29.Nishimoto T, Basilico C. Analysis of a method for selecting temperature-sensitive mutants of BHK cells. Somatic Cell Genet. 1978;4:323–340. doi: 10.1007/BF01542846. [DOI] [PubMed] [Google Scholar]
- 30.O'Hare P. The virion transactivator of herpes simplex virus. Semin Virol. 1993;4:145–155. [Google Scholar]
- 31.O'Hare P, Goding C R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988;52:435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- 32.Poon A P, Roizman B. The phenotype in vitro and in infected cells of herpes simplex virus 1 alpha trans-inducing factor (VP16) carrying temperature-sensitive mutations introduced by substitution of cysteines. J Virol. 1995;69:7658–7667. doi: 10.1128/jvi.69.12.7658-7667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Post L E, Mackem S, Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981;24:555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- 34.Preston C M, Frame M C, Campbell M E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988;52:425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- 35.Quinlan M P, Chen L B, Knipe D M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984;36:857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- 36.Rice S A, Long M C, Lam V, Spencer C A. RNA polymerase II is aberrantly phosphorylated and localized to viral replication compartments following herpes simplex virus infection. J Virol. 1994;68:988–1001. doi: 10.1128/jvi.68.2.988-1001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rixon F J, Addison C, McGregor A, Macnab S J, Nicholson P, Preston V G, Tatman J D. Multiple interactions control the intracellular localization of the herpes simplex virus type 1 capsid proteins. J Gen Virol. 1996;77:2251–2260. doi: 10.1099/0022-1317-77-9-2251. [DOI] [PubMed] [Google Scholar]
- 38.Simmen K A, Newell A, Robinson M, Mills J S, Canning G, Handa R, Parkes K, Borkakoti N, Jupp R. Protein interactions in the herpes simplex virus type 1 VP16-induced complex: VP16 peptide inhibition and mutational analysis of host cell factor requirements. J Virol. 1997;71:3886–3894. doi: 10.1128/jvi.71.5.3886-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smibert C A, Popova B, Xiao P, Capone J P, Smiley J R. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein Vhs. J Virol. 1994;68:2339–2346. doi: 10.1128/jvi.68.4.2339-2346.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smiley J R, Duncan J. 1814 linker insertion mutation. J. Virol. 71:6191–6193. 1997. Truncation of the C-terminal acidic transcriptional activation domain of herpes simplex virus VP16 produces a phenotype similar to that of the. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stern S, Tanaka M, Herr W. The Oct-1 homoeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989;341:624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- 42.Taus N S, Salmon B, Baines J D. The herpes simplex virus 1 UL17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged. Virology. 1998;252:115–125. doi: 10.1006/viro.1998.9439. [DOI] [PubMed] [Google Scholar]
- 43.Umene K, Nishimoto T. Replication of herpes simplex virus type 1 DNA is inhibited in a temperature-sensitive mutant of BHK-21 cells lacking RCC1 (regulator of chromosome condensation) and virus DNA remains linear. J Gen Virol. 1996;77:2261–2270. doi: 10.1099/0022-1317-77-9-2261. [DOI] [PubMed] [Google Scholar]
- 44.Ward P L, Ogle W O, Roizman B. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J Virol. 1996;70:4623–4631. doi: 10.1128/jvi.70.7.4623-4631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinheimer S P, Boyd B A, Durham S K, Resnick J L, O'Boyle D R., II Deletion of the VP16 open reading frame of herpes simplex virus type 1. J Virol. 1992;66:258–269. doi: 10.1128/jvi.66.1.258-269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werstuck G H, Capone J P. An unusual cellular factor potentiates protein-DNA complex assembly between Oct-1 and Vmw65. J Biol Chem. 1993;268:1272–1278. [PubMed] [Google Scholar]
- 47.Wilcock D, Lane D P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991;349:429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- 48.Wilson A C, Cleary M A, Lai J S, LaMarco K, Peterson M G, Herr W. Combinatorial control of transcription: the herpes simplex virus VP16-induced complex. Cold Spring Harbor Symp Quant Biol. 1993;58:167–178. doi: 10.1101/sqb.1993.058.01.021. [DOI] [PubMed] [Google Scholar]
- 49.Wilson A C, Freiman R N, Goto H, Nishimoto T, Herr W. VP16 targets an amino-terminal domain of HCF involved in cell cycle progression. Mol Cell Biol. 1997;17:6139–6146. doi: 10.1128/mcb.17.10.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson A C, LaMarco K, Peterson M G, Herr W. The VP16 accessory protein HCF is a family of polypeptides processed from a large precursor protein. Cell. 1993;74:115–125. doi: 10.1016/0092-8674(93)90299-6. [DOI] [PubMed] [Google Scholar]
- 51.Wu C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature. 1984;309:229–234. doi: 10.1038/309229a0. [DOI] [PubMed] [Google Scholar]
- 52.Xiao P, Capone J P. A cellular factor binds to the herpes simplex virus type 1 transactivator Vmw65 and is required for Vmw65-dependent protein-DNA complex assembly with Oct-1. Mol Cell Biol. 1990;10:4974–4977. doi: 10.1128/mcb.10.9.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue F, Cooley L. kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell. 1993;72:681–693. doi: 10.1016/0092-8674(93)90397-9. [DOI] [PubMed] [Google Scholar]
- 54.Yanagi K, Talavera A, Nishimoto T, Rush M G. Inhibition of herpes simplex virus type 1 replication in temperature-sensitive cell cycle mutants. J Virol. 1978;25:42–50. doi: 10.1128/jvi.25.1.42-50.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhong L, Hayward G S. Assembly of complete, functionally active herpes simplex virus DNA replication compartments and recruitment of associated viral and cellular proteins in transient cotransfection assays. J Virol. 1997;71:3146–3160. doi: 10.1128/jvi.71.4.3146-3160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]