Abstract
It has been suggested that hepatitis B virus (HBV) binds to a receptor on the plasma membrane of human hepatocytes via the pre-S1 domain of the large envelope protein as an initial step in HBV infection. However, the nature of the receptor remains controversial. In an attempt to identify a cell surface receptor for HBV, purified recombinant fusion protein of the pre-S1 domain of HBV with glutathione S-transferase (GST), expressed in Escherichia coli, was used as a ligand. The surface of human hepatocytes or HepG2 cells was biotinylated, and the cell lysate (precleared lysate) which did not bind to GST and glutathione-Sepharose beads was used as a source of receptor molecules. The precleared lysate of the biotinylated cells was incubated with the GST–pre-S1 fusion protein, and the bound proteins were visualized by Western blotting and enhanced chemiluminescence. An approximately 80-kDa protein (p80) was shown to bind specifically to the pre-S1 domain of the fusion protein. The receptor binding assay using serially or internally deleted segments of pre-S1 showed that amino acid residues 12 to 20 and 82 to 90 are essential for the binding of pre-S1 to p80. p80 also bound specifically to the pre-S1 of native HBV particles. Analysis of the tissue and species specificity of p80 expression in several available human primary cultures and cell lines of different tissue origin showed that p80 expression is not restricted to human hepatocytes. Taken together the results suggest that p80 may be a component of the viral entry machinery.
Hepatitis B virus (HBV) causes acute and chronic hepatitis and is an etiological agent for hepatocellular carcinoma (6). HBV infection is highly species specific; only humans and closely related species are known to be susceptible to HBV infection (32). The tissue and species specificity of HBV infection suggests the preferential attachment and entry of HBV into hepatocytes as an initial step in HBV infection and/or the existence of host- and liver-specific regulatory elements for viral replication. However, little is known about the molecular mechanism of the initiation of HBV infection.
The HBV envelope proteins have been considered crucial molecules in recognizing a possible receptor on the plasma membrane of human hepatocytes (22). The envelope consists of three distinct related proteins, designated small (S), middle (M), and large (L) (24). All of these proteins have a common carboxyl-terminal 226-amino-acid sequence (S protein). M protein has an additional 55-amino-acid (pre-S2) sequence located at the amino terminus; beyond this segment, L protein contains an additional 108- or 119-amino-acid (pre-S1) sequence (depending on subtype ay or ad, respectively). Neurath et al. (23) postulated that HBV particles bind to human hepatocytes and hepatoma HepG2 cells via pre-S1(21-47) (a peptide consisting of amino acids [aa] 21 to 47 of pre-S1). Pontisso et al. (28) also showed that L protein bound to human liver membrane and that a monoclonal antibody specific to pre-S1(21-47) inhibited this binding. However, the identity of the putative receptor that binds to pre-S1(21-47) remains controversial. Human immunoglobulin A (IgA) receptor (29), glyceraldehyde-3-phosphate dehydrogenase (27), interleukin-6 (25), asialoglycoprotein receptor (34), a 31-kDa protein (4), and a serum glycoprotein of 50 kDa (3) have been proposed as possible receptors, but the nature of the interaction of HBV with the putative receptors in HBV infection has not been confirmed.
In this study, in an attempt to identify an HBV receptor on human hepatocytes, a recombinant fusion protein of the pre-S1 or pre-S (preS1/preS2) domain with glutathione S-transferase (GST) was produced from Escherichia coli and used as a ligand, the surface of human hepatocytes or hepatoma HepG2 cells was biotinylated, and the cell lysate was used as a source of receptor molecules. Through the ligand-receptor binding experiments, we have identified an 80-kDa protein (p80) on the cell surface that specifically binds to the pre-S1 domain of HBV. The p80 binding sites on the pre-S1 domain were mapped, and the tissue and species specificity of p80 expression was analyzed.
MATERIALS AND METHODS
Cell culture.
Adult human hepatocytes were prepared by enzymatic dissociation of a noncancerous liver fragment (10). All experimental procedures were in compliance with French laws and regulations and were approved by the National Ethics Committee. Rat hepatocytes were prepared by the same method. Freshly isolated human and rat hepatocytes were cultured in medium for human hepatocytes (H medium) (75% minimal essential medium [Gibco], 25% medium 199 [Gibco], insulin [5 μg/ml], bovine serum albumin [BSA; 1 mg/ml], antibiotic-antimycotic [Gibco]) supplemented with 5 × 10−6 M hydrocortisone hemisuccinate, 2% dimethyl sulfoxide, and 10% porcine serum (9). HepG2 cells were cultured in H medium supplemented with 5 × 10−7 M hydrocortisone hemisuccinate and 10% fetal bovine serum. Human oral epidermal and dermal fibroblasts primary cultures were prepared and maintained as described previously (5, 8). Human peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Hypaque density gradient centrifugation from healthy human plasma. Human embryonic kidney (HEK) and HeLa cell lines were grown in Dulbecco modified Eagle medium (Gibco) supplemented with 10% fetal bovine serum.
Construction and expression of GST fusion proteins containing pre-S1, pre-S, or partial pre-S1.
The coding sequence of pre-S, pre-S1, or partial pre-S1 was synthesized from pHBV315 (adr subtype [15]) or ayw subtype plasmid (14) by PCR using 5′ and 3′ primers and subcloned into the BamHI or BamHI-EcoRI site of plasmid pGEX-2T (Pharmacia), respectively. To construct the pre-S1 mutants with internal deletion, the 5′- and 3′-end fragments, which do not contain the internally deleted segment, were synthesized by PCR from their wild-type plasmid counterparts and recombined by subsequent recombinant PCR (20). The 5′ primer contained a BamHI site, and the 3′ primer contained a BamHI or EcoRI site. The GST fusion proteins were expressed and purified according to the protocol provided by the supplier (Pharmacia). The fusion proteins were expressed in E. coli DH5α by induction with 0.1 mM isopropyl-β-d-thiogalactopyranoside for 3 h. The intracellular soluble proteins were applied to glutathione-Sepharose beads, and the bound proteins were eluted with 5 mM reduced glutathione and subsequently dialyzed against phosphate-buffered saline (PBS) buffer.
Cell surface biotinylation.
Human and rat hepatocytes, HepG2, HEK, and HeLa cells, human oral epidermal and dermal fibroblast primary cultures, and PBMC were biotinylated by using an ECL (enhanced chemiluminescence) protein biotinylation system (Amersham). The cultured cells in a 75-cm2 flask were washed twice with ice-cold PBS, mixed with 4 ml of bicarbonate buffer containing 160 μl of biotinylation reagent (biotinamidocaproate N-hydroxysuccinamide ester in dimethylformamide) at 4°C for 30 min, and then washed again twice with ice-cold PBS. The cells were treated with 4 ml of lysis buffer (25 mM Tris-HCl, [pH 7.5], 250 mM NaCl, 5 mM EDTA [pH 8.0], 1% Nonidet P-40, aprotinin [2 μg/ml], phenylmethylsulfonyl fluoride [100 μg/ml], leupeptin [5 μg/ml]) at 4°C for 20 min. Nuclei were removed by centrifugation, and the cell lysates were stored at −70°C before use. The protein concentration in the cell lysates was quantitated by using a bicinchoninic acid protein assay kit (Pierce).
Detection of pre-S1-binding protein.
To remove the cellular proteins which nonspecifically bind to GST protein and glutathione-Sepharose beads, 1 ml of the biotinylated cell lysate was incubated with 20 μg of GST protein at 4°C for 5 h, which was then added to 20 μl of 50% glutathione-Sepharose beads. After incubation overnight, the precleared lysate was recovered and the beads were extensively washed with lysis buffer for use as negative controls for the binding experiment. The precleared lysate was incubated with 20 μg of GST–pre-S, GST–pre-S1, or mutant GST–pre-S1 fusion protein at 4°C for 5 h and further incubated with the glutathione-Sepharose beads as described above. To inhibit the interaction between the pre-S1 and cellular proteins, various amounts of purified pre-S1 (13), pre-S1 peptide, or pre-S1-specific monoclonal antibodies (KR127 and KR359) (C. J. Ryu, Y. K. Kim, H. S. Kim, H. Hur, Y. J. Kang, and H. J. Hong, submitted for publication) were individually added to the mixture before incubation with the beads. The beads were extensively washed with lysis buffer, and the bound proteins were eluted from the beads by heating to 100°C for 5 min. The precleared lysate or eluted proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% polyacrylamide gel under denaturing conditions and transferred to a nitrocellulose membrane. The membrane was immersed in PBST (PBS containing 0.1% [vol/vol] Tween 20)–5% skim milk at room temperature for 1 h. After two rinses with PBST, the membrane was incubated with streptavidin-horseradish peroxidase (HRP) conjugate (1:1,500 [vol/vol]; Amersham) at room temperature for 1 h. After washing, the biotinylated proteins were visualized by ECL Western blotting detection reagent (Amersham).
Preparation of HBV particles.
HBV particles were purified from HBsAg- and HBeAg-positive human sera as described by Robinson and Greenman (31), with some modifications. Briefly, the sera were layered onto a 10 to 30% discontinuous sucrose gradient, prepared in TS-BSA buffer (0.01 M Tris, 0.5 M NaCl, 0.1% NaN3, 1% BSA [pH 8.0]), and centrifuged in a Beckman SW41 rotor at 36,000 rpm for 4 h. The pellet was suspended in TS-BSA buffer and centrifuged again under the same conditions. The new pellet was resuspended in one-half of the starting volume in TS-BSA buffer and stored at −70°C before use. Viral genomic equivalents (vge) of the prepared viral particles were quantitated by DNA dot blot assay with HBV genome (15) as the standard.
Binding of p80 to HBV particles.
One milliliter of the biotinylated HepG2 cell lysate was incubated with 30 μl of 10% protein A-Sepharose (Amersham) at 4°C overnight. The precleared lysate was recovered and incubated with 100 μl of mock preparation (TS-BSA) or HBV particles (1011 vge) at room temperature for 2 h. In a separate experiment, the HepG2 cell lysate was preincubated with 100 μg of pre-S1 peptides (aa 12 to 20 and 73 to 90) at room temperature for 20 min prior to HBV addition. The mixture was immunoprecipitated with 10 μg of anti-pre-S1 antibody KR127 and 30 μl of 10% protein A-Sepharose. After extensive washing with PBS, the immunocomplex was subjected to SDS-PAGE (10% gel) and Western blotting. The HBV-binding protein was visualized by ECL.
RESULTS
Identification of a protein (p80) on human hepatocytes that binds specifically to the pre-S1 domain of HBV.
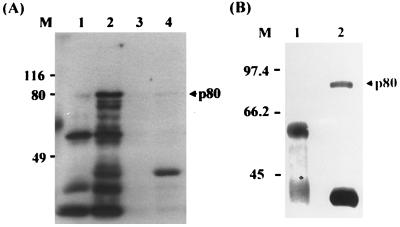
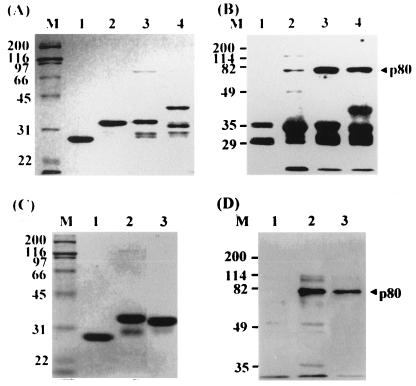
GST–pre-S1 (adr subtype, 119 aa) and GST as a negative control were expressed, purified from E. coli, and used as a ligand to search for HBV cell receptors. As a source of receptor, the surface of human hepatocytes was biotinylated and the cell lysate was prepared. To identify the pre-S1-binding protein, the purified GST protein was incubated with the biotinylated cell lysate and further incubated with glutathione-Sepharose beads. The unbound proteins (precleared lysate) were then incubated with GST–pre-S1 followed by incubation with glutathione-Sepharose beads. Subsequently, the bound proteins were eluted from the beads by heating to 100°C and subjected to SDS-PAGE (10% gel) followed by Western blot analysis using streptavidin-HRP conjugate. The pre-S1-binding protein was visualized by ECL. As shown in Fig. 1A, an approximately 80-kDa protein (p80) bound to the GST–pre-S1 beads (lane 2), but not to the GST-glutathione-Sepharose beads (lane 1). In the same binding experiment, the lysate of unbiotinylated human hepatocytes was used as a negative control. As expected, p80 was not detected in the eluent of the GST (lane 3)- or GST–pre-S1 (lane 4)-immobilized beads. The GST–pre-S1 protein used as a ligand was detected (lanes 2 and 4), possibly due to the nonspecific binding of streptavidin-HRP conjugate to the pre-S1 of the GST–pre-S1 protein on the membrane.
FIG. 1.
An 80-kDa cell surface protein binds to the pre-S1 domain of the GST–pre-S1 fusion protein. Cultured human hepatocytes (A) and HepG2 cells (B) were biotinylated. The cell lysates were incubated with GST and further incubated with glutathione-Sepharose beads. After the Sepharose beads were extensively washed with lysis buffer, the bound proteins were eluted by heating to 100°C for 5 min, subjected to SDS-PAGE (10% gel) and Western analysis using streptavidin-HRP conjugate, and visualized by ECL (lane 1). The unbound proteins (precleared lysate) were incubated with GST–pre-S1 fusion protein followed by glutathione-Sepharose beads. The bound proteins were then analyzed as described above (lane 2). As a negative control, cell lysates of unbiotinylated human hepatocyte were incubated with GST (lane 3) or GST–pre-S1 (lane 4) and then analyzed as described above. Molecular size markers (M; in kilodaltons) are shown on the left, and the position of p80 is indicated on the right.
Since human hepatocytes are not easily available, human hepatoma cell line HepG2, which was shown to have the biosynthetic capabilities of normal liver parenchymal cells (1, 16) and binds to HBV (23), was used for subsequent binding study. HepG2 cells were grown in H medium to support the maintenance of hepatocyte-specific differentiated function because these cells tend to be dedifferentiated with extended time in culture in ordinary culture medium (30); the biotinylated cell lysate was incubated with GST or GST–pre-S1 protein. As shown in Fig. 1B, HepG2 cells also expressed p80 that bound to the pre-S1 domain (lane 2) but not to GST (lane 1).
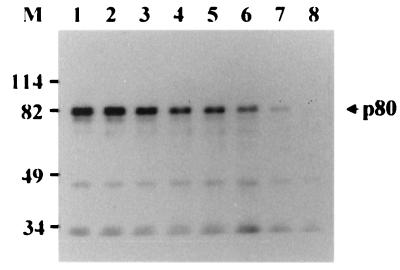
To further confirm the specificity of the binding of p80 to pre-S1, the precleared lysate of HepG2 cells was incubated with GST–pre-S in the presence of increasing amounts of free pre-S1. The result showed that the interaction between GST–pre-S and p80 was competitively inhibited by pre-S1 in a dose-dependent manner and completely inhibited by 100 μg of pre-S1 (Fig. 2). This result demonstrates that p80 binds to the pre-S1 domain specifically but not to the pre-S2 domain.
FIG. 2.
Competitive inhibition of p80 binding of GST–pre-S1 by pre-S1. The precleared lysate of biotinylated HepG2 cells was incubated with GST–pre-S fusion protein in the presence of 0 (lane 1), 1 (lane 2), 2 (lane 3), 5 (lane 4), 10 (lane 5), 25 (lane 6), 50 (lane 7), or 100 (lane 8) μg of purified pre-S1 polypeptide, and the binding experiment was done as described for Fig. 1. Molecular size markers (M; in kilodaltons) are shown on the left, and the position of p80 is indicated on the right.
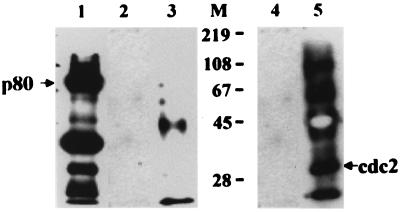
The biotinylation reagent used in this study is reported to be too bulky to penetrate the surface of viable cells (19). Since we biotinylated the hepatocytes attached to dishes after removal of dead cells by extensive washes, p80 is likely to be expressed on the cell surface. Nevertheless, specificity of the cell surface biotinylation was assessed by using a known intracellular protein, human Cdc2 (34 kDa), as an internal control. The biotinylated HepG2 cell lysate was immunoprecipitated with rabbit anti-human Cdc2 antibody and protein A-Sepharose and subjected to Western analysis using streptavidin-HRP (Fig. 3, lane 3) or rabbit anti-human Cdc2 antibody and anti-rabbit IgG-HRP (lane 5). Cdc2 was immunoprecipitated with anti-Cdc2 antibody (lane 5) but not with streptavidin-HRP (lane 3), while p80 was detected with streptavidin-HRP (lane 1). The result indicates that Cdc2 was not accessible to the cell surface biotinylation reagent, providing indirect evidence that the cell surface biotinylation is potentially cell surface specific.
FIG. 3.
Cell surface location of p80. Biotinylated HepG2 cell lysates were divided into three fractions; one was subjected to the pre-S1 binding assay (lane 1), and the other two were incubated with protein A-Sepharose. The precleared lysate was immunoprecipitated with rabbit anti-human Cdc2 antibody (Santa Cruz Biotechnology) and protein A-Sepharose. The precleared supernatant (lane 2 and 4) and immunoprecipitates (lane 3 and 5) were subjected to Western analysis using streptavidin-HRP (lanes 1 to 3) or rabbit anti-human Cdc2 antibody and anti-rabbit IgG-HRP (lanes 4 and 5). The bands corresponding to 50 and 25 kDa are likely the heavy and light chains, respectively, of rabbit anti-human Cdc2 antibody (lane 5). Molecular size markers (M; in kilodaltons) are shown in the middle, and the positions of p80 and Cdc2 are indicated.
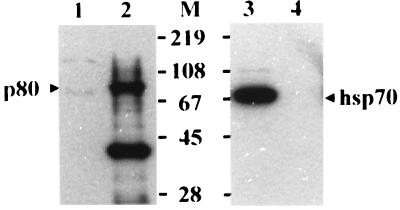
Recently, it was shown that cytosolic heat shock protein Hsc70 binds to the pre-S1 region (aa 70 to 94 of the ayw subtype), which is likely to be responsible for the repression of cotranslational translocation of the pre-S domain (21). To test whether p80 is Hsc70, the biotinylated proteins bound to the GST–pre-S1 were eluted and subjected to Western blot analysis using streptavidin-HRP or mouse anti-Hsp/Hsc70 monoclonal antibody and anti-mouse IgG-HRP. As shown in Fig. 4, p80 did not react with anti-Hsp/Hsc70 antibody (lane 4), while a 70-kDa protein from HepG2 cell lysates reacted with the antibody (lane 3). This result demonstrates that p80 is not Hsp/Hsc70.
FIG. 4.
p80 is not Hsc70. Twenty-microliter aliquots of unbiotinylated HepG2 lysates (lanes 1 and 3) and the biotinylated proteins bound to GST–pre-S1 (lanes 2 and 4) were subjected to Western analysis using streptavidin-HRP (lanes 1 and 2) or mouse anti-Hsp/Hsc70 antibody (Santa Cruz Biotechnology) and anti-mouse IgG-HRP (lanes 3 and 4). Molecular size markers (M; in kilodaltons) are shown in the middle, and the positions of p80 and Hsc70 are indicated.
To rule out the possibility that p80 is a serum protein that bound tightly to the surface of cultured hepatocytes, biotinylated fetal bovine serum was incubated with GST–pre-S1, and the bound protein was eluted and visualized by Western blot analysis. p80 was not detected (data not shown). Also, the possibility of p80 being the serum protein lactoferrin, whose molecular mass is approximately 80 kDa, was checked. Bovine lactoferrin that was biotinylated and subjected to the GST–pre-S1 binding experiment failed to bind to pre-S1 (data not shown).
Binding of p80 to the two sites of pre-S1.
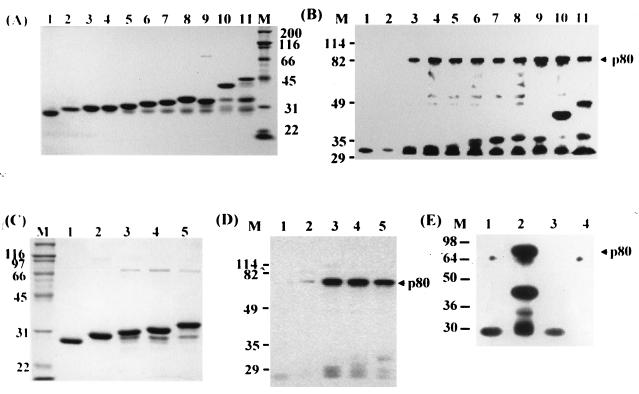
To locate the binding sites for p80 on the pre-S1 domain, pre-S1 (adr subtype) was cleaved into two fragments and the N-terminal fragment was serially deleted from the C terminus. Each fragment was expressed as a fusion protein with GST in E. coli, purified (Fig. 5A), and then incubated with the precleared lysate of the biotinylated HepG2 cells to test its ability to bind to p80. As shown in Fig. 5B, GST and GST–pre-S1(1-11) did not bind to p80 (lanes 1 and 2), while GST–pre-S1(1-20, 1-28, 1-35, 1-42, 1-49, 1-56, 57-119, or 1-119) and GST–pre-S bound to p80 (lanes 3 to 11). The results indicate that pre-S1(12-20) is essential for the binding and that pre-S1(57-119) also has the binding site for p80. To map the binding site more precisely, GST–pre-S1(57-72, 57-90, 57-104, and 57-119) were expressed and purified (Fig. 5C), and the receptor binding experiment was performed. As shown in Fig. 5D, p80 did not bind to GST–pre-S1(57-72) (lane 2) but did bind to GST–pre-S1(57-90, 57-104, and 57-119) (lanes 3 to 5), indicating that pre-S1(73-90) is involved in the binding of pre-S1 to p80. To further dissect this p80 binding site, GST–pre-S1(57-81) was additionally prepared and subjected to the receptor binding assay, which showed that p80 did not bind to pre-S1(57-81) (Fig. 5E, lane 4). Taken together, the results led to the conclusion that pre-S1(12-20) and pre-S1(82-90) are essential for p80 binding.
FIG. 5.
Mapping of the p80 binding site on the pre-S1 domain. The pre-S1 domain and serially truncated segments thereof were expressed as fusion proteins with GST in E. coli and purified (A and C). These proteins were separately incubated with the precleared lysates of biotinylated HepG2 cells, and the receptor binding assay was carried out (B, D, and E). (A and B) Lane 1, intact GST; lane 2, pre-S1(1-11); lane 3, pre-S1(1-20); lane 4, pre-S1(1-28); lane 5, pre-S1(1-35); lane 6, pre-S1(1-42); lane 7, pre-S1(1-49); lane 8, pre-S1(1-56); lane 9, pre-S1(57-119); lane 10, pre-S1(1-119); lane 11, pre-S. (C and D) Lane 1, GST; lane 2, pre-S1(57-72); lane 3, pre-S1(57-90); lane 4, pre-S1(57-104); lane 5, pre-S1(57-119). (E) Lanes 1 and 3, GST; lane 2, pre-S1(1-119); lane 4, pre-S1(57-81). Positions of molecular size markers (lanes M; in kilodaltons) and p80 are indicated.
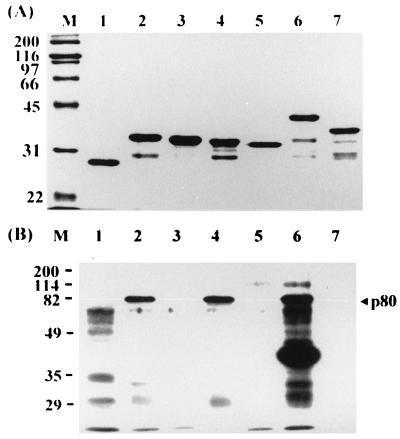
To further confirm the two p80 binding sites, pre-S1(12-20), pre-S1(73-90), or both were deleted from GST–pre-S1(1-56), GST–pre-S1(57-119), or GST–pre-S1(1-119), respectively. These mutant proteins were prepared (Fig. 6A) and tested for p80 binding. As shown in Fig. 6B, deletion of aa 12 to 20 from pre-S1(1-56) (lane 3), aa 73 to 90 from pre-S1(57-119) (lane 5), or both segments from pre-S1(1-119) (lane 7) completely abolished the ability of pre-S1 to bind to p80, while the intact pre-S1, pre-S1(1-56), and pre-S1(57-119) bound to p80 (lanes 2, 4, and 6). These results clearly demonstrate that p80 binds to the two sites of pre-S1.
FIG. 6.
p80 binding of pre-S1 mutants with internally deleted segments. (A) Amino acid residues 12 to 20 (lane 3), 73 to 90 (lane 5), or both (lane 7) were deleted from GST–pre-S1(1-56) (lane 2), GST–pre-S1(57-119) (lane 4), or GST–pre-S1(1-119) (lane 6), respectively, and these wild-type and mutant proteins as well as GST (lane 1) were expressed and purified. (B) Each protein was analyzed for p80 binding. Molecular size markers (in kilodaltons) are shown in lane M, and the position of p80 is indicated on the right.
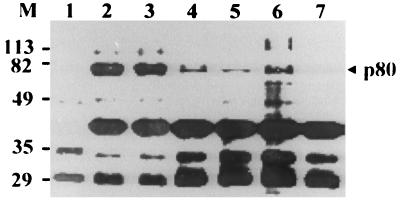
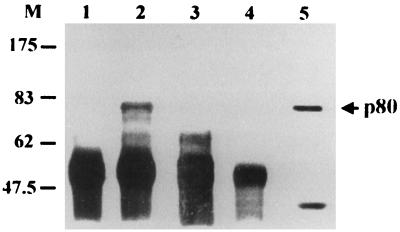
Our data are not consistent with the previous postulate that HBV binds to human hepatocyte via pre-S1(21-47) (23). To explore this issue further, we examined the binding of p80 to GST–pre-S1 in the presence of different pre-S1 peptides (Fig. 7). The binding of GST–pre-S1 to p80 was strongly inhibited by either pre-S1(12-20) or pre-S1(73-90) or both peptides but not by pre-S1(21-47). The inhibitory effect of anti-pre-S1 monoclonal antibodies on the binding of pre-S1 to p80 was also examined in assays using two monoclonal antibodies, KR359 (epitope, aa 20 to 28) and KR127 (epitope, aa 42 to 49) (Ryu et al., submitted). The binding of GST–pre-S to p80 was partially inhibited by KR359 in a dose-dependent manner (Fig. 8A) but was not inhibited by KR127 (Fig. 8B). The difference may be due to the fact that the epitope of KR359 is immediately adjacent to the first binding site (aa 12 to 20), while that of KR127 is distant from the both binding sites. These results clearly shows that p80 binds to two sites other than pre-S1(21-47).
FIG. 7.
Effect of pre-S1 peptides on the binding of p80 to pre-S1. Biotinylated HepG2 lysates were incubated with GST (lane 1), and precleared lysates were incubated with GST–pre-S1 fusion protein in the presence of PBS (lane 2), 100 μg of peptide pre-S1(21-47) (lane 3), 100 μg of peptide pre-S1(12-20) (lane 4), 100 μg of peptide pre-S1(73-90) (lane 5), 50 μg of peptide pre-S1(12-20) plus 50 μg of peptide pre-S1(73-90) (lane 6), or 100 μg of peptide pre-S1(12-20) plus 100 μg of peptide pre-S1(73-90) (lane 7), and the binding experiment was done as described for Fig. 1. Molecular size markers (M; in kilodaltons) are shown on the left, and the position of p80 is indicated on the right.
FIG. 8.
Effect of anti-pre-S1 monoclonal antibodies KR359 (A) and KR127 (B) on the p80 binding of pre-S1. Precleared lysates of biotinylated HepG2 cells were incubated with GST–pre-S1 fusion protein in the absence (lane 1) or presence of 5 (lane 2), 10 (lane 3), 50 (lane 4), 100 (lane 5), or 200 (lane 6) μg of KR359 or KR127. Positions of molecular size markers (M; in kilodaltons) and p80 are indicated.
The pre-S1 sequence of the ay subtype of HBV is shorter than that of the ad subtype in that the first 11 aa of the ad subtype are not present in the ay subtype. To examine whether the pre-S1 domain of the ay subtype also binds to p80, a GST fusion protein with pre-S1(1-45), pre-S1(46-108), or pre-S1(1-108) of the ayw subtype was prepared (Fig. 9A) and tested for p80 binding. As shown in Fig. 9B, p80 bound to pre-S1(1-45) (lane 2), pre-S1(46-108) (lane 3), and pre-S1(1-108) (lane 4) of the ayw subtype, but binding of pre-S1(1-45) to p80 was weaker than that of pre-S1(46-108). Comparison of the strength of p80 binding between pre-S1(1-56) of the adr subtype (lane 2) and pre-S1(1-45) of the ayw subtype (lane 3) showed that pre-S1(1-45) was weaker than pre-S1(1-56). Taken together, these results indicate that p80 may bind to all subtypes of HBV.
FIG. 9.
p80 binding of pre-S1 of the ayw subtype of HBV. GST (lane 1) and GST fusion proteins with pre-S1(1-45) (lane 2), pre-S1(46-108) (lane 3), or pre-S1(1-108) (lane 4) of the ayw subtype were expressed and purified (A). The biotinylated HepG2 cell lysates were incubated with GST (lane 1), and the precleared lysates were individually incubated with each of the GST–pre-S1 fusions for p80 binding (B). For comparison, GST (lane 1), GST–pre-S1(1-56, adr subtype) (lane 2), and GST–pre-S1(1-45, ayw subtype) (lane 3) were expressed (C), and the binding experiment was carried out (D). Positions of molecular size markers (M; in kilodaltons) and p80 are indicated.
Binding of p80 to HBV particles.
To examine whether p80 binds to native HBV particles, the biotinylated HepG2 cell lysate was incubated with 1011 vge of HBV particles (adr subtype) purified from patient sera, and the mixture was immunoprecipitated with anti-pre-S1 monoclonal antibody KR127. As shown in Fig. 10, p80 indeed bound to HBV particles (lane 2) as well as GST–pre-S1 (lane 5), while p80 was not detected from the immunoprecipitation without HBV particles (lane 1) or with an irrelevant antibody with specificity for 4-1BB of human T cells (lane 3). In addition, the binding of p80 to HBV particles was completely inhibited by peptides pre-S1(12-20) and pre-S1(73-90) (lane 4). The results indicate that p80 binds specifically to the pre-S1 domain of native HBV particles.
FIG. 10.
Binding of p80 to HBV particles. Biotinylated HepG2 lysates were incubated with a mock preparation (lane 1) or HBV particles (1011 vge) in the absence (lane 2) or presence (lane 4) of 100 μg of peptides pre-S1(12-20) and pre-S1(73-90) at room temperature for 2 h and then immunoprecipitated with anti-pre-S1 antibody KR127 and protein A-Sepharose. As a negative control, an irrelevant antibody (anti-human 4-1BB monoclonal antibody) instead of KR127 was used (lane 3). The immunoprecipitates were subjected to Western analysis using streptavidin-HRP. The biotinylated HepG2 cell lysates were subjected to the GST–pre-S1 binding assay in parallel (lane 5). Molecular size markers (M; in kilodaltons) are shown on the left, and the position of p80 is indicated on the right.
Tissue and species specificity of p80 expression.
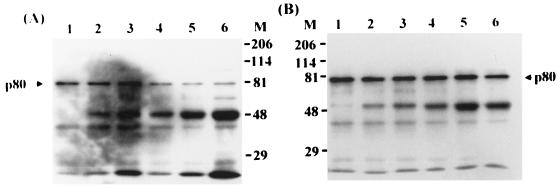
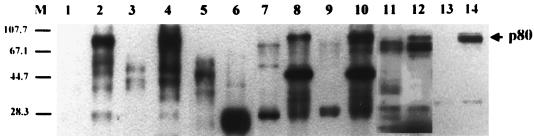
To address the tissue specificity of p80 expression, several available human primary cultures and cell lines of different tissue origin, including extrahepatic tissues such as skin, kidney, and PBMC that are susceptible to HBV infection (7), were separately biotinylated, and the same amounts of total cellular proteins of the cells were used to carry out the GST–pre-S1 binding assay. As shown in Fig. 11, p80 was detected in HepG2 cells (lane 2), human oral epidermal primary culture (lane 4), PBMC (lane 8), HEK cells (lane 10), and HeLa (cervix adenocarcinoma) cells (lane 12) but not human dermal fibroblast primary culture (lane 6). Thus, p80 was found in all human tissues and cell lines examined except fibroblasts. In an attempt to check the species specificity of p80 expression, rat hepatocyte primary culture was biotinylated and subjected to the pre-S1 binding assay. The result showed that rat hepatocytes also expressed p80 (lane 14).
FIG. 11.
Tissue and species specificity of p80 expression. HepG2 (lanes 1 and 2), human oral epidermal primary culture (lanes 3 and 4), human dermal fibroblast primary culture (lanes 5 and 6), PBMC (lanes 7 and 8), HEK cells (lanes 9 and 10), HeLa cells (lanes 11 and 12), and rat hepatocyte primary culture (lanes 13 and 14) were biotinylated and precleared with GST and glutathione-Sepharose beads (lanes 1, 3, 5, 7, 9, 11, and 13). The precleared lysates were then incubated with GST–pre-S1 fusion protein (lanes 2, 4, 6, 8, 10, 12, and 14), and the binding assay was performed. Molecular size markers (M; in kilodaltons) are shown on the left, and the position of p80 is indicated on the right.
DISCUSSION
The molecular mechanism of the attachment and penetration of HBV into human hepatocytes is not known. This study shows that an approximately 80-kDa protein (p80) on human hepatocytes binds specifically to the pre-S1 domain of HBV of both adr and ayw subtypes. The p80 binding sites on pre-S1 are located at aa 12 to 20 and 82 to 90. Tissue and species specificity of p80 expression was not observed, however. Taken together, the data suggest that p80 may be involved in HBV entry.
Since Neurath et al. (23) proposed that the attachment of HBV to HepG2 cells is mediated via pre-S1(21-47), the putative receptors that bind to pre-S1(21-47) had been identified. Neurath et al. (25) reported that interleukin-6 contained recognition sites for pre-S1(21-47). Petit et al. (27) showed that 35-kDa glyceraldehyde-3-phosphate dehydrogenase strongly bound to the anti-idiotypic antibody against pre-S1(21-47). Dash et al. (4) showed that a 31-kDa protein in the lysate of HepG2 cells bound to peptide 21-47. Also, human IgA receptor (29), asialoglycoprotein receptor (34), and a serum glycoprotein of 50 kDa (3) have been proposed as HBV receptors. However, the nature of the interaction of HBV with the putative receptors has not been confirmed. On comparing the assay systems to identify an HBV receptor, we noted that the previous studies used synthetic peptide 21-47 or HBsAg (or HBV) as a ligand and the lysate of HepG2 cells or liver membrane proteins as a source of receptor. In contrast, in this study, purified pre-S1 domain was used as a ligand and cell surface proteins of human hepatocytes or HepG2 cells were used as a source of receptor. Our assay system may have several advantages. The whole pre-S1 domain may exhibit more specific interaction with the receptor molecule than the synthetic peptides because its three-dimensional structure is closer in configuration to that of the native protein. In addition, the purified pre-S1 protein is less likely to react nonspecifically with cellular proteins compared to HBsAg, which contains a small percentage of the L protein and consists mostly of major S protein. Regarding the source of receptor, human hepatocytes and HepG2 cells, grown under conditions designed to maintain the hepatocyte-specific differentiated function, are thought to express HBV receptor at maximum levels. In addition, biotinylation of only the cell surface proteins will render undetectable intracellular proteins that could bind to the pre-S1 domain. Using the assay system, we indeed identified a 80-kDa protein (p80) that binds specifically to the pre-S1 domain (Fig. 1 and 2). So far, no other 80-kDa protein that binds to HBV has been found.
Although we detected p80 by cell surface biotinylation, it might be also located within the cell, as is the case of the cellular receptor gp180 for duck HBV (DHBV), which is a Golgi-resident protein (2). p80 was not detected on Coomassie blue- or silver-stained gels after the total lysates of unbiotinylated HepG2 cells were subjected to the receptor-ligand binding assay (data not shown), suggesting that it is a relatively minor cellular component. More detailed analysis is needed to determine the subcellular distribution of p80.
Initial mapping of the p80 binding site on the pre-S1 domain revealed that p80 binds to aa 12 to 20 and 73 to 90 (Fig. 5). We further confirmed the p80 binding sites by using the pre-S1 mutants lacking the segments (Fig. 6) or synthetic peptides of the segments (Fig. 7). In addition, these peptides were used to confirm the specificity of the binding of p80 to HBV particles (Fig. 10). Recently, however, Loffler-Mary et al. reported that Hsc70 bound to aa 70 to 94 of pre-S1 (ayw subtype), corresponding to aa 81 to 105 of pre-S1 of the adr subtype (21). To examine whether the p80 binding site overlaps the Hsc70 binding site, GST–pre-S1(57-81) was additionally produced and subjected to the receptor binding assay (Fig. 5E). The result showed that p80 did not bind to pre-S1(57-81), indicating that aa 82 to 90 of pre-S1 are essential for p80 binding. Since the p80 binding site overlapped the Hsc70 binding site, we tested whether p80 is Hsc70. The result showed that p80 did not react with an anti-Hsp/Hsc70 monoclonal antibody, demonstrating that p80 is other than Hsp/Hsc70 (Fig. 4).
Our data showed that aa 12 to 20 and 82 to 90 of pre-S1 are essential for p80 binding and that each site is responsible for the binding (Fig. 5, 6, and 9). These two sites correspond to aa 1 to 9 and 71 to 79 of the ayw subtype. However, p80 binding by the first binding site (aa 1 to 9) of the ayw subtype was weaker than that of the adr subtype, while the second binding sites of the two subtypes showed equally strong binding (Fig. 9). Comparison of amino acid sequences of the first binding sites between these two subtypes showed that the sequence of the adr subtype is MGTNLSVPN, while that of the ayw subtype is MGTNLSTSN. The adjacent sequences (aa 21 to 34) of these two subtypes are identical. Therefore, the difference in binding strength between these two subtypes may be due to the two divergent residues (VP of the adr subtype and TS of the ayw subtype). However, the first binding site of viral particles of the ay subtype may not be functional in p80 binding because it is myristylated and anchored to the membrane (26). In the case of the second binding sites, the two subtypes have homologous sequences (adr subtype, GLLGWSPQAQGILTTVPA; ayw subtype, underlined T and V residues were changed to Q and L, respectively). The mode of interaction between p80 and the pre-S1 domain of the viral particle remains to be elucidated.
It has been suggested that tissue and species specificity of HBV infection may be due to the preferential attachment and penetration of HBV into hepatocytes. We observed that p80 expression is not restricted to human hepatocytes (Fig. 11); it was also detected in human oral epidermal cells, PBMC, HEK cells, HeLa cells, and rat hepatocytes but not human dermal fibroblasts. This result suggests that p80 may be expressed in most human tissues and also other mammals, probably in epithelial cells. With respect to its role in viral infection, p80 might be a component of the viral entry machinery, and some other protein(s) which is specifically expressed on human hepatocytes may also play a roles in these processes. For example, Hertogs et al. (11, 12) found that endonexin II, present on human but not rat liver plasma membranes, specifically binds to the phospholipid of small HBsAg, and they postulated that endonexin II may play an important role in the initiation of HBV infection, likely by inducing membrane fusion. Therefore, it is conceivable that initiation of HBV infection of hepatocytes is a multistep process involving interactions of viral surface proteins with two or more cellular components.
Recently, gp180 (carboxypeptidase D), a transmembrane protein, was reported to be a cellular receptor for DHBV (17, 18, 33). gp180 interacts with a distinct pre-S subdomain of DHBV (35). However, gp180 is found not only in duck liver but also in other tissues which are not susceptible to DHBV infection (17). Also, its expression did not render transfected heterologous cells permissive for productive infection (2). The observations led the authors to conclude that gp180 possibly functions as a primary virus attachment site and that a species-specific coreceptor is needed to complete viral entry. Taken together, the available data suggest that p80 may be analogous to gp180.
ACKNOWLEDGMENTS
We gratefully acknowledge Young Sook Son for providing human epidermal and dermal fibroblast primary cultures.
This work was supported by grants FG1110 and FG1220 from the Ministry of Science and Technology of Korea and by INSERM and the Association pour la Recherche contre le Cancer, France.
REFERENCES
- 1.Aden D P, Fogel A, Plotkin S, Damjanov I, Knowles B B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979;282:615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- 2.Breiner K M, Urban S, Schaller H. Carboxypeptidase D (gp180), a Golgi-resident protein, functions in the attachment and entry of avian hepatitis B viruses. J Virol. 1998;72:8098–8104. doi: 10.1128/jvi.72.10.8098-8104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budkowska A, Quan C, Groh F, Bedossa P, Dubreuil P, Bouvet J P, Pillot J. Hepatis B virus binding factor in human serum: candidate for a soluble form hepatocyte HBV receptor. J Virol. 1993;67:4316–4322. doi: 10.1128/jvi.67.7.4316-4322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dash S, Rao K V S, Panda S K. Receptor for pre-S1(21-47) component of hepatitis B virus on the liver cell: role in virus cell interaction. J Med Virol. 1992;37:116–121. doi: 10.1002/jmv.1890370208. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs E, Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981;25:617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- 6.Ganem D, Varmus H E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–694. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- 7.Ganem D. Hepadnaviridae and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2703–2737. [Google Scholar]
- 8.Genever P G, Wood E J, Cunliffe W J. The wound dermal equivalent offers a simplified model for studying wound repair. Exp Dermatol. 1993;2:266–273. doi: 10.1111/j.1600-0625.1993.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 9.Gripon P, Diot C, Guguen-Guillouzo C. Reproducible high level infection of cultured adult human hepatocytes by hepatitis B virus: effect of polyethylene glycol on adsorption and penetration. Virology. 1993;192:534–540. doi: 10.1006/viro.1993.1069. [DOI] [PubMed] [Google Scholar]
- 10.Guguen-Guillouzo C, Guillouzo A. Methods of preparation of adult and fetal hepatocytes. In: Guillouzo A, Guguen-Guillouzo C, editors. Isolated and cultured hepatocytes. Les Editions INSERM Paris; 1986. pp. 1–12. , Libbey, London, England. [Google Scholar]
- 11.Hertogs K, Leenders W, De Bruin W, Depla E, Meheus L, Raymackers J, Moshage H, Yap S H. Endonexin II, present on human liver plasma membranes, is a specific binding protein of small hepatitis B virus envelope protein. Virology. 1993;197:549–557. doi: 10.1006/viro.1993.1628. [DOI] [PubMed] [Google Scholar]
- 12.Hertogs K, Depla E, Crabbe T, Bruin W D, Leenders W, Moshage H, Yap S H. Spontaneous development of anti-hepatitis B virus envelope (anti-idiotypic) antibodies in animals immunized with human liver endotoxin II or with the F(ab′)2 fragment of anti-human liver endonexin II immunoglobulin G: evidence for a receptor-ligand-like relationship between small hepatitis B surface antigen and endonexin II. J Virol. 1994;68:1516–1521. doi: 10.1128/jvi.68.3.1516-1521.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H S, Hong H J. Efficient expression, purification and characterization of hepatitis B virus preS1 protein from Escherichia coli. Biotechnol Lett. 1995;17:871–876. [Google Scholar]
- 14.Kim H S, Hong H J. Hepatitis B virus preS1 functions as a transcriptional activation domain. J Gen Virol. 1997;78:1083–1086. doi: 10.1099/0022-1317-78-5-1083. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y S, Kang H S. Cloning and expression of hepatitis B virus surface antigen gene. Korean Biochem J. 1984;17:70–79. [Google Scholar]
- 16.Knowles B B, Howe C C, Aden D P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–596. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 17.Kuroki K, Cheung R, Marion P L, Ganem D. A cell surface protein that binds avian hepatitis B virus particles. J Virol. 1994;68:2091–2096. doi: 10.1128/jvi.68.4.2091-2096.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroki K, Eng F, Ishikawa T, Turck C, Harada F, Ganem D. gp180, a host cell glycoprotein that binds duck hepatitis B virus particles, is encoded by a member of the carboxypeptidase gene family. J Biol Chem. 1995;270:15022–15028. doi: 10.1074/jbc.270.25.15022. [DOI] [PubMed] [Google Scholar]
- 19.Lantz L M, Holmes K L. Improved nonradioactive cell surface labelling technique for immunoprecipitation. BioTechniques. 1995;18:58–60. [PubMed] [Google Scholar]
- 20.Lewis A P, Crowe J S. Immunoglobulin complementarity-determining region grafting by recombinant polymerase chain reaction to generate humanized monoclonal antibodies. Gene. 1991;101:297–302. doi: 10.1016/0378-1119(91)90427-d. [DOI] [PubMed] [Google Scholar]
- 21.Loffler-Mary H, Werr M, Prange R. Sequence-specific repression of cotranslational translocation of the hepatitis B virus envelope proteins coincides with binding of heat shock protein Hsc70. Virology. 1997;235:144–152. doi: 10.1006/viro.1997.8689. [DOI] [PubMed] [Google Scholar]
- 22.Meyer S D, Gong Z J, Suwandhi W, van Pelt J, Soumillion A, Yap S H. Organ and species specificity of hepatitis B virus (HBV) infection: a review of literature with a special reference to preferential attachment of HBV to human hepatocytes. J Viral Hepat. 1997;4:145–153. doi: 10.1046/j.1365-2893.1997.00126.x. [DOI] [PubMed] [Google Scholar]
- 23.Neurath A R, Kent S B H, Strick N, Parker K. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell. 1986;46:429–436. doi: 10.1016/0092-8674(86)90663-x. [DOI] [PubMed] [Google Scholar]
- 24.Neurath A R, Kent S B H. The preS region of hepadnavirus envelope proteins. Adv Virus Res. 1988;34:65–142. doi: 10.1016/s0065-3527(08)60516-3. [DOI] [PubMed] [Google Scholar]
- 25.Neurath A R, Strich N, Li Y. Cells transfected with human IL6 cDNA acquire binding sites for the hepatitis B virus envelope protein. J Exp Med. 1992;176:1561–1569. doi: 10.1084/jem.176.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Persing D, Varmus H, Ganem D. The preS1 protein of hepatitis B virus is acylated at its amino terminus with myristic acid. J Virol. 1987;61:1672–1677. doi: 10.1128/jvi.61.5.1672-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petit M A, Capel F, Dubanchet S, Mabit H. PreS1-specific binding protein as a potential receptors for hepatitis B virus in human hepatocytes. Virology. 1992;187:211–222. doi: 10.1016/0042-6822(92)90309-d. [DOI] [PubMed] [Google Scholar]
- 28.Pontisso P, Ruvoletto M G, Gerlich W H, Heerman K H, Bardini R, Alberti A. Identification of an attachment site for human liver plasma membranes on hepatitis B virus particles. Virology. 1989;173:522–530. doi: 10.1016/0042-6822(89)90564-3. [DOI] [PubMed] [Google Scholar]
- 29.Pontisso P, Ruvoletto M G, Tiribelli C, Gerlich W H, Ruol A, Alberti A. The preS1 domain of hepatitis B virus and IgA cross-react in their binding to hepatocyte surface. J Gen Virol. 1992;73:2041–2045. doi: 10.1099/0022-1317-73-8-2041. [DOI] [PubMed] [Google Scholar]
- 30.Raney A K, Milich D R, Easton A J, McLachlan A. Differentiation-specific transcriptional regulation of the hepatitis B virus large surface antigen gene in human hepatoma cell lines. J Virol. 1990;64:2360–2368. doi: 10.1128/jvi.64.5.2360-2368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson W S, Greenman R I. DNA polymerase in the core of the human hepatitis B virus candidate. J Virol. 1974;13:1231–1236. doi: 10.1128/jvi.13.6.1231-1236.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson W S, Marion P L, Feitelson M, Siddiqui A. The hepadna virus group: hepatitis B and related viruses. In: Szmuness W, et al., editors. Viral hepatitis. Philadelphia, Pa: Franklin Institute Press; 1982. pp. 57–68. [Google Scholar]
- 33.Song L, Fricker L D. Purification and characterization of carboxypeptidase D, a novel carboxypeptidase E-like enzyme, from bovine pituitary. J Biol Chem. 1996;270:25007–25013. doi: 10.1074/jbc.270.42.25007. [DOI] [PubMed] [Google Scholar]
- 34.Treichel U, zum Buschenfelde K-H M, Stockert R J, Poralla T, Gerken G. The asialoglycoprotein receptor mediates hepatic binding and uptake of natural hepatitis B virus particles derived from viraemic carriers. J Gen Virol. 1994;75:3021–3029. doi: 10.1099/0022-1317-75-11-3021. [DOI] [PubMed] [Google Scholar]
- 35.Urban S, Breiner K M, Fehler F, Klingmuller U, Schaller H. Avian hepatitis B virus infection is initiated by the interaction of a distinct pre-S subdomain with the cellular receptor gp180. J Virol. 1998;72:8089–8097. doi: 10.1128/jvi.72.10.8089-8097.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]