Abstract
Introduction
During the first COVID-19 outbreak in 2020 in the Netherlands, the incidence of pulmonary embolism (PE) appeared to be high in COVID-19 patients admitted to the intensive care unit (ICU). This study was performed to evaluate the incidence of PE during hospital stay in COVID-19 patients not admitted to the ICU.
Methods
Data were retrospectively collected from 8 hospitals in the Netherlands. Patients admitted between February 27, 2020, and July 31, 2020, were included. Data extracted comprised clinical characteristics, medication use, first onset of COVID-19-related symptoms, admission date due to COVID-19, and date of PE diagnosis. Only polymerase chain reaction (PCR)-positive patients were included. All PEs were diagnosed with computed tomography pulmonary angiography (CTPA).
Results
Data from 1,852 patients who were admitted to the hospital ward were collected. Forty patients (2.2%) were diagnosed with PE within 28 days following hospital admission. The median time to PE since admission was 4.5 days (IQR 0.0–9.0). In all 40 patients, PE was diagnosed within the first 2 weeks after hospital admission and for 22 (55%) patients within 2 weeks after onset of symptoms. Patient characteristics, pre-existing comorbidities, anticoagulant use, and laboratory parameters at admission were not related to the development of PE.
Conclusion
In this retrospective multicenter cohort study of 1,852 COVID-19 patients only admitted to the non-ICU wards, the incidence of CTPA-confirmed PE was 2.2% during the first 4 weeks after onset of symptoms and occurred exclusively within 2 weeks after hospital admission.
Keywords: COVID-19, Pulmonary embolism, Epidemiology, Hospital ward, Vascular research
Introduction
Already in the first wave of the SARS-CoV-2 pandemic, elevated biochemical markers for blood clotting activation, like D-dimers, were observed in COVID-19 patients [1–3]. Whether venous thromboembolism (VTE), including deep-vein thrombosis (DVT) and PE, occurred more frequently in these patients had not yet been clarified.
Many reports have been published since describing the incidence of PE in hospitalized patients with COVID-19. Nevertheless, large (multicenter) cohorts on non-ICU cohorts have been limited. In a systematic review of 57 studies (n = 18.110 patients), the incidence of PE was 7.1% in all hospitalized patients, 6% in patients admitted to non-ICU wards, and 13.7% in patients admitted to the ICU [4]. In this review study, the incidence was substantially lower (3%) in studies (n = 7) with 400 or more patients. In a recent meta-analysis by Mai et al. [5], an increased risk of VTE in COVID-19 patients was found for ICU cohorts (RR 3.10, 95% CI: 1.54–6.23) but not for non-ICU cohorts (0.95, 95% CI: 0.81–1.11) compared to non-COVID-19 cohorts [5, 6].
In the Netherlands, no multicenter cohort study has reported on the incidence of PE in hospitalized patients not admitted to the ICU. This large multicenter cohort study was performed to fill this gap and to evaluate the incidence of CTPA-proven PE in PCR-positive patients hospitalized due to COVID-19 during the first wave of infections.
Methods
Study Design
Eight hospitals in the Netherlands collaborated in this multicenter retrospective cohort study as part of the COVID-PREDICT consortium (https://covidpredict.nl/). We report on the patients of 18 years or older, within this data set, admitted due to COVID-19 during the first COVID wave, between February 27, 2020, and July 31, 2020. All patients who tested PCR positive by nasal or pharyngeal swab specimens for COVID-19 were classified as COVID-19 positive and included. Since there has been no general consensus on which criteria can be used in the absence of a PCR-positive result, we chose to exclude PCR-negative patients from this analysis, even though some patients may have been highly suggestive of having COVID-19 (due to clinical presentation, laboratory results, typical findings on pulmonary CT scans) and have been treated accordingly. Data were extracted from electronic health records, pseudonymized, and stored in an online database (Castor EDC, Amsterdam, the Netherlands) at hospital level. Data collection included clinical patient characteristics, such as age, sex, body mass index, medical history, medication use, and duration of COVID-19-related symptoms, as well as laboratory results. The case report from CRF used in data collection in this multicenter study was based on the WHO COVID-19 CRF but has had several iterations prospectively. Nevertheless, some variables related to PE diagnosis such as Padua score, PE severity, and CTPA in the non-PE group were not added to the CRF and therefore could not be retrieved in all participating centers retrospectively. When data were not available or erroneous, they were considered missing. Given the exceptional circumstances related to the COVID-19 crisis and in accordance with national guidelines and European privacy law, the need for active informed consent was waived and patients had the opportunity to opt out. More details of COVID-PREDICT are described elsewhere [7]. The STROBE statement checklist was used for reporting of findings [8].
Study Population
We included first admissions of patients that were not transferred to the ICU at all or not within 24 h after diagnosis of PE. Reason for admission in general was oxygen requirement and/or generalized weakness. PE diagnosis was confirmed by computed tomography angiography (CTPA). Indication for CTPA was based on Wells score, YEARS criteria, and/or on clinical judgment of the attending clinician. In all included patients, diagnosis and classification of PE was verified by clinicians from all participating hospitals individually because CTPA methods and protocols applied in the participating hospitals differed. At the start of the COVID-19 pandemic, the Padua score [9] was used for determining whether prophylactic anticoagulants with low molecular weight heparin (LMWH) should be started. Due to emerging evidence on the increased risk of VTE, the national guidelines advised from April 16, 2020, and onward for all hospitalized COVID-19 patients to be started on prophylactic anticoagulants [10]. During the first wave, only (hydroxy)chloroquine was used as unproven antiviral therapy and corticosteroids were not systematically prescribed.
Statistical Analysis
Descriptive statistics were used to compare patient characteristics, comorbidities, and anticoagulant use between patients with proven PE and without proven PE in the hospital ward cohort. χ2 tests were applied to compare distribution of categorical variables and t test, Fisher’s exact test for comparing mean scores of continuous variables between groups. When data were not normally distributed, the Mann-Whitney U test was applied. Kaplan-Meier curves were estimated for PE incidence since time of diagnosis and since onset of symptoms. Time to PE diagnosis within 4 weeks (28 days) was determined from admission date and from first onset of symptoms. We considered patients developing PE within 28 days after admission linked to COVID-19. Patients who developed PE more than 28 days after admission were censored and allocated to the non-PE group for all analyses. Patients initially admitted to a regular ward were considered at risk of PE at the ward until they were transferred to the ICU or another facility, were discharged home, or died; the data were censored at that timepoint. IBM SPSS version 26 was used for data analysis and p values below 0.05 were considered statistically significant.
Results
A total of 2,395 hospitalized patients due to COVID-19 were registered in the database of COVID-PREDICT during the first wave. In general, reasons for hospital admission were hypoxemic respiratory insufficiency, generalized weakness (mostly due to gastro-intestinal symptoms), delirium, or suspected severe course of disease based on patient characteristics. Of these, 1,852 (77.3%) were admitted to non-ICU wards. Reason for ICU admission in general was invasive ventilation (88%). In total, 40 of the 1,852 (2.2%) patients admitted to a regular, non-ICU hospital ward were diagnosed with PE within the first 28 days after admission compared to 75 of the 543 (13.8%) patients admitted to the ICU. Of these 40 patients, 3 were admitted to the ICU in a later stage at 7 (PE after 4 days), 10 (PE after 1 day), and 14 days (PE after 13 days) following their hospital admission. Furthermore, 9 of the 1,852 (0.5%) hospitalized patients developed PE later than 28 days after admission and were therefore censored. Mortality was not significantly different between both groups, 6/40 (15%) for the PE group and 340/1,812 (18.8%) for the non-PE group, 3 weeks after admission.
Table 1 describes the patients in our study population who were and were not diagnosed with PE. There was no relation in patient characteristics between patients with and without PE nor due to the home use of anticoagulants between patients who developed PE and those who did not (p = 0.806). The use of (hydroxy)chloroquine was not related to the development of PE; it was used by 629 patients, 16 (40%) patients in the PE group and 613 (33.8%) patients in the non-PE group. Twelve out of 40 cases were diagnosed after 16th of April, the date national policy changed to prescribing standard LMWH prophylaxis. Vital signs at admission were comparable between groups, expect for oxygen saturation, which was on average about 2 percent lower in the PE group compared to the no PE group (p = 0.004). Many laboratory values related to coagulation were missing at time of admission. Those that were known were not notably different between both groups. D-dimer, for example, was missing in 1,534 patients; it was registered in 15 of the 40 patients (37.5%) who developed PE and in 302 of the 1,812 patients (16.7%) who did not develop PE. D-dimer was above the conventional clinical cut-off point indicative of further PE diagnostics of 500 ng/mL for 14 of 15 PE cases (93.3%) as opposed to 241 of 302 non-PE cases (79.8%; p < 0.001). The case with a D-dimer below 500 ng/mL on admission was diagnosed with PE on day 9.
Table 1.
Patient characteristics, comorbidities, anticoagulant use, and laboratory parameters at admission of patients diagnosed with and without CTPA-confirmed PE within 28 days after hospital admission at the hospital ward
| Total (n = 1,852) | PE (n = 40) | No PE (n = 1,812) | p value | |
|---|---|---|---|---|
| Patient characteristics, n (%) | ||||
| Age (mean, SD), years | 67.4 (15.1) | 69.9 (11.8) | 67.3 (15.2) | 0.1801 |
| Sex (female) | 754 (40.7) | 17 (42.5) | 737 (40.7) | 0.8162 |
| BMI (mean, SD) (645 missing) | 27.8 (5.4) | 28.2 (5.5) | 27.8 (5.4) | 0.7021 |
| Smoking (464 missing), n (%) | 0.6842 | |||
| Never | 707 (50.9) | 14 (46.7) | 693 (51.0) | |
| Former | 593 (42.7) | 13 (43.3) | 580 (42.7) | |
| Current | 88 (6.3) | 3 (10.0) | 85 (6.3) | |
| Alcohol abuse (314 missing) | 44 (2.9) | 0 | 44 (2.9) | – |
| Previous VTE/thrombophlebitis (1,143 missing) | 38 (5.4) | 1 (4.3) | 37 (5.4) | 0.9993 |
| Vital signs at admission | ||||
| Body temperature | 37.8 (36.7–38.4) | 37.7 (36.7–38.4) | 37.8 (37.7–38.6) | 0.519 |
| Heart rate | 90.0 (79.0–102.0) | 92.0 (81.2–103.0) | 90.0 (79.0–102.0) | 0.363 |
| Respiratory rate | 21.0 (17.0–26.0) | 22.0 (16.0–25.0) | 21.0 (17.0–26.0) | 0.917 |
| Systolic blood pressure | 133.0 (120.0–149.0) | 131.0 (117.8–140.0) | 133.0 (120.0–150.0) | 0.276 |
| Diastolic blood pressure | 79.0 (70.0–88.0) | 77.5 (66.3–88.0) | 79.0 (70.0–88.0) | 0.536 |
| Oxygen saturation | 93.8 (93.6–94.0) | 91.6 (89.9–93.3) | 93.8 (93.6–94.1) | 0.004 |
| Comorbidities, n (%) | ||||
| Chronic cardiac disease (18 missing) | 606 (33.0) | 12 (31.6) | 594 (33.1) | 0.8442 |
| Hypertension (6 missing) | 868 (47.0) | 19 (48.7) | 849 (47.0) | 0.8302 |
| COPD (24 missing) | 380 (20.8) | 8 (20.5) | 372 (20.8) | 0.9993 |
| Chronic hematologic disease (25 missing) | 72 (3.9) | 4 (10.5) | 68 (3.8) | 0.0603 |
| Diabetes (35 missing) | 473 (26.0) | 4 (10.5) | 469 (26.4) | 0.0253 |
| Anticoagulant use, n (%) | 0.8062 | |||
| No medication use at all | 254 (13.7) | 7 (17.5) | 247 (13.6) | |
| Other home medication use | 884 (47.7) | 21 (52.5) | 863 (47.6) | |
| Antiplatelet drugs | 405 (21.9) | 7 (17.5) | 398 (22.0) | |
| DOAC/VKA | 271 (14.7) | 4 (10.0) | 267 (14.7) | |
| Combination of anticoagulation drugs | 38 (2.1) | 1 (2.5) | 37 (2.0) | |
| Laboratory parameters at admission (median and IQR) | ||||
| Platelet count (0 missing) | 230.9 (224.8–280.4) | 240.0 (199.5–280.4) | 230.7 (224.5–237.0) | 0.681 |
| Blood albumin (806 missing, 18+1,028 data present) | 36.6 (33.3–39.7) | 35.0 (30.5–37.4) | 36.6 (33.3–39.8) | 0.1024 |
| Creatine kinase (1,065 missing, 13+774 data present) | 115.0 (65.0–237.0) | 130.0 (72.0–168.0) | 114.0 (65.0–238.5) | 0.9804 |
| Lactate dehydrogenase (543 missing, 28+1,281 data present) | 318.0 (248.0–421.0) | 375.0 (283.8–487.8) | 316.0 (248.0–419.0) | 0.0524 |
| D-dimer (1,534 missing, 14+300 data present) | 964.0 (561.0–1,747.0) | 1,240.0 (868.0–2,625.0) | 955.0 (553.0–1,695.0) | 0.0764 |
| Ferritin (1,157 missing, 11+ 684 data present) | 618.0 (295.0–1,136.0) | 783.0 (206.0–2,340.0) | 616.5 (295.3–1,131.8) | 0.3344 |
| Fibrinogen (1,596 missing, 7+249 data present) | 5.7 (4.7–6.7) | 5.4 (4.3–6.0) | 5.7 (4.7–6.7) | 0.3314 |
Percentages are given as proportion of complete cases per variable. SD, standard deviation; BMI, body mass index; VTE, venous thromboembolism; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; VKA, vitamin K antagonist; IQR, interquartile range.
1 p value from t test. 2p value from χ2 test. 3p value from Fisher’s exact test. 4p value from Mann-Whitney U test.
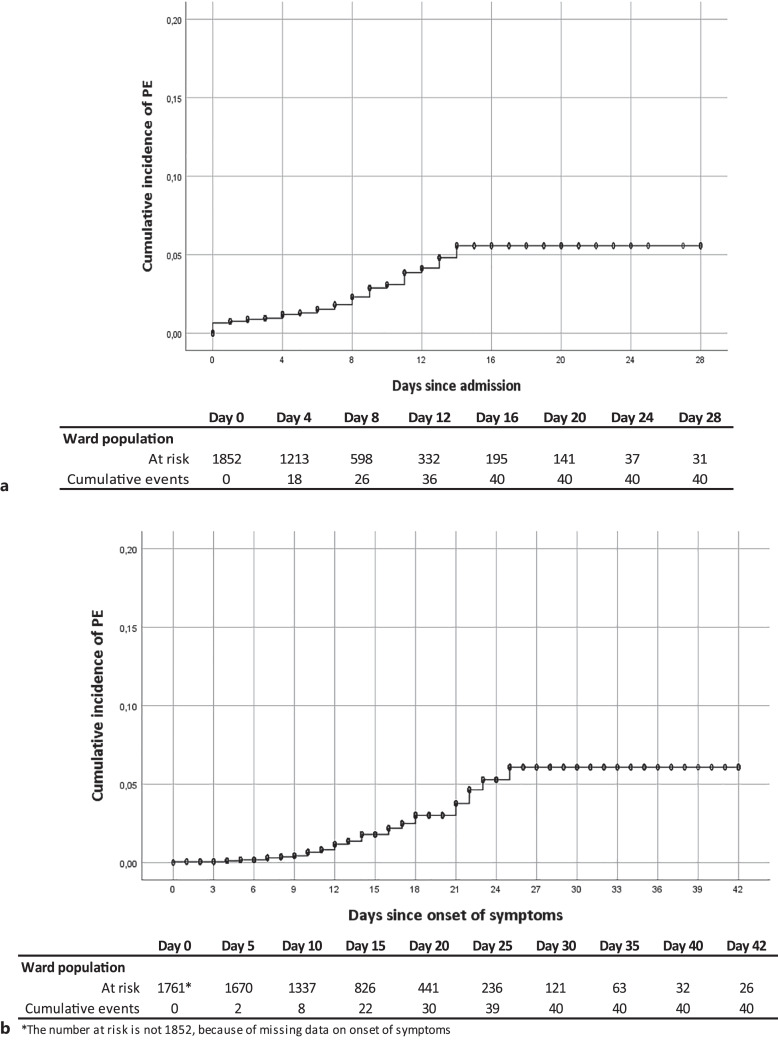
The Kaplan-Meier curve shows that all PEs were diagnosed within 2 weeks after hospital admission (Fig. 1a) and within 4 weeks from onset of symptoms (Fig. 1b). The number of patients at risk of PE since admission has decreased from 1852 at start to 195 at day 16. In 14 of the 40 (35%) patients, PE was diagnosed in the first 48 h after admission implying they were prevalent at time of admission. In 22 of the 40 patients (55%), PE was diagnosed within 2 weeks after onset of symptoms. The median time to PE since admission was 4.5 days (IQR 0.0–9.0). Median time from first onset of symptoms until hospital admission was 8.8 days (IQR 5.0–12.0), while median time from first onset of symptoms until diagnosis of PE was 14.0 days (IQR 10.3–20.3), respectively.
Fig. 1.
Kaplan-Meier curves with survival table for pulmonary embolism (PE) incidence for 28 days (a) since hospital admission and 42 days (b) since onset of symptoms in a non-ICU cohort of patients admitted to the hospital for COVID-19.
Discussion
We present a large multicenter cohort of patients, admitted due to COVID-19 in a non-ICU ward, in which the incidence of PE is 2.2% within the first 28 days after admission. This incidence of PE is lower than in some reported cohorts [11–13] but is in line with a systematic review that observed even lower incidences in larger cohorts [4]. Patient characteristics were comparable with other cohorts of COVID-19 patients [7, 14], although the percentage of current smokers is lower (6.3%) in this cohort compared to 11.6–14.9 in others [11, 15].
All cases of PE were diagnosed within 2 weeks of admission and no new cases were diagnosed in the 3rd or 4th week after admission in this large Dutch cohort, which is in line with a recent large retrospective observational study of 54,354 people who tested COVID-19 positive [16]. In the latter study, a 29-fold increase of VTE during the first week following COVID-19 diagnosis was reported compared to the pre-COVID timeframe and most VTE cases were identified within 3 weeks after testing positive for COVID-19. It is also in line with the large cohort study of Miro et al. [17] with a relatively low incidence of PE in cases presenting at emergency departments. Our findings support the observation that the risk of COVID-19-related PE is highest in the first 3 weeks. If confirmed in other studies, this validated a high index of suspicion in this 3-week time period and could therefore have consequences for the diagnostic strategy of PE. This could also limit the possible effectiveness of extended prophylactic treatment with LMWH, but this requires further investigation.
Severity of PE was not classified but only 3 patients of this non-ICU cohort with PE were transferred to the ICU more than 24 h after diagnosis. In a recent publication, the recurrence rate of VTE after subsegmental PE was higher than expected treatment [18], so it seems valid to also count subsegmental PE. It is also possible that due to the fact that different diagnostic strategies were used (Years criteria, Wells score, clinical suspicion) cases were found or missed in different centers. This observational study was not suitable for determining an optimal diagnostic strategy or evaluating diagnostic algorithms.
Limitations
Data on the use/dosage and indication for prophylactic anticoagulants during admission and reason for hospitalization were not available at individual level. We consider this a major limitation since it is possible there could be differences in adherence to the guidelines, which could potentially influence the results. Main reason for admission during the first wave generally was oxygen requirement or generalized weakness in elderly patients. In our study, we found 28 PE diagnosed before (+/−1.5 months) and 12 PE diagnosed after (+/−3.5 months) national protocols changed in favor of standard prophylactic LMWH. No data were collected on total CTPAs made during the study period, which limits the interpretation of the presented data. In the beginning of the outbreak on a study basis, patients were treated with (hydroxy)chloroquine. Although hydroxychloroquine is known to have some antithrombotic properties [19], it is not known if it protects against the development of PE in patients with COVID-19 [20].
There are several reasons why our findings could be an underestimation. First, in the first wave diagnostic possibilities using CTPA were limited due to isolation measures and due to shortage of personal protective equipment. It has been reported that the total number of CTPA performed was reduced in the first COVID-19 wave compared to the year before (pre-COVID) [21]. Primary data on how frequent this was a problem and the total number of CTPA made are lacking in the COVID-PREDICT cohort; this also makes it difficult comparing it to other cohorts. Second, in the case of a positive leg ultrasonography for DVT no additional CTPA will be made, especially in severely ill patients who were untransferable and who will be treated as having a VTE. It is plausible that some of these patients will have a PE as well, with an occurrence of 11.7% for patients with DVT and PE (16). Third, we only included patients who had a positive SARS-CoV-2 PCR. This means that the included patients were indeed suffering from COVID-19 and not from other diseases but that we missed false negatively tested patients [22, 23]. As a consequence, the results of this study will overall be valid, but the incidence is likely to be underestimated. Finally, selecting a population that did not go to the ICU could have resulted in an underestimation because they potentially did not undergo the workup for PE diagnosis and because disease progression toward ICU admission is a risk factor. Nevertheless, only 3 patients of the 40 with a PE diagnosis within this cohort went to the ICU at a later stage, so the risk of misclassification due to disease progression was relatively low.
Conclusion
In this retrospective multicenter cohort study, the incidence of PE was 2.2% in hospitalized patients due to COVID-19 not admitted to the ICU, which is a conservative estimate. PE has been mostly diagnosed from the start of the second week after onset of complaints related to COVID-19 and within the first 2 weeks of hospital admission.
Acknowledgments
We would like to thank dr. Kim Sigaloff for coordinating data collection for Amsterdam UMC and all others who have collected data in all participating centers in the Netherlands.
Statement of Ethics
The medical Ethics Committee of the Amsterdam University Medical Centers (Amsterdam UMC; 20.131) stated that no medical ethics approval was required and approved the design of the COVID-PREDICT consortium and thereby waived the need for informed consent.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research was supported by the VieCuri Corona Foundation and Regio Noord-Limburg.
Author Contributions
CRediT contributor role definitions are used for the roles below (https://casrai.org/credit/): (1) Conceptualization, (2) data curation, (3) formal analysis, (4) funding acquisition, (5) investigation, (6) methodology, (7) project administration, (8) resources, (9) software, (10) supervision, (11) validation, (12) visualization, (13) writing – original draft, and (14) writing – review and editing. A F.G.M. contributed to conceptualization, funding acquisition, data curation, data analysis, methodology, visualization, and writing – reviewing and editing of the draft and final manuscript. C.W. contributed to conceptualization, funding acquisition, data curation, methodology, and writing – reviewing and editing of the draft and final manuscript. A.D., D.G.B., V.E.M.K., and T.E.K. contributed to writing – review and editing. M.K., S.S., B.S., N.C.G., S.D., H.M., P.N., A.R., R.D., E.J.N., M.B., and P.E. contributed to gathering data and to writing – review and editing. T.H. and B.A. contributed to gathering data and writing – review and editing and data curation. S.M. contributed to gathering data, methodology, validation, and writing – review and editing. N.E. contributed to gathering data, methodology, supervision, and writing – review and editing. J.P.W.B. contributed to conceptualization, funding acquisition, data collection, methodology, project administration, supervision, validation, visualization, and writing – reviewing and editing of the draft and final manuscript. F.H.MO. contributed to conceptualization, data curation, data analysis, funding acquisition, data collection, methodology, project administration, supervision, validation, visualization, and writing – reviewing and editing of the draft and final manuscript.
Funding Statement
This research was supported by the VieCuri Corona Foundation and Regio Noord-Limburg.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Data and corresponding scripts are available upon request to the corresponding author. More information on the data collection process and availability can be read on the Castor EDC Website (https://www.castoredc.com/blog/covid-predict-study/) and the COVID-PREDICT Website (https://www.covidpredict.org/design/).
References
- 1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gallastegui N, Zhou JY, Drygalski AV, Barnes RFW, Fernandes TM, Morris TA. Pulmonary embolism does not have an unusually high incidence among hospitalized COVID19 patients. Clin Appl Thromb Hemost. 2021;27:1076029621996471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mai V, Tan BK, Mainbourg S, Potus F, Cucherat M, Lega JC, et al. Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: a systematic review with meta-analysis. Vascul Pharmacol. 2021;139:106882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miró Ò, Llorens P, Aguirre A, Lozano L, Beaune S, Roussel M, et al. Association between covid-19 and pulmonary embolism (AC-19-PE study). Thromb Res. 2020;196:322–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ottenhoff MC, Ramos LA, Potters W, Janssen MLF, Hubers D, Hu S, et al. Predicting mortality of individual patients with COVID-19: a multicentre Dutch cohort. BMJ Open. 2021;11(7):e047347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–24. [DOI] [PubMed] [Google Scholar]
- 9. Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–7. [DOI] [PubMed] [Google Scholar]
- 10. Huiskam M, Coppens M, Eikenboom J, et al. Leidraad COVID-19 coagulopathie - Diagnostiek en tromboprofylaxe bij diepe veneuze trombose en longembolie (version 6, 16-04-2020). Ned Internisten Ver (NIV), Ned Ver van Artsen voor Longziekten en Tuberc (NVALT), Ned Ver voor Intensive Care (NVIC), Ned Ver voor Klin Chemie (NVKC) [Internet]. 2020; Available from: doi: https://www.demedischspecialist.nl/sites/default/files/Leidraad-COVID-19-coagulopathie.pdf.
- 11. Fauvel C, Weizman O, Trimaille A, Mika D, Pace N, Douair A, et al. Pulmonary embolism in covid-19 patients: a French Multicentre Cohort study. Supplements. 2021;13(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trimaille A, Curtiaud A, Marchandot B, Matsushita K, Sato C, Leonard-Lorant I, et al. Venous thromboembolism in non-critically ill patients with COVID-19 infection. Supplements. 2021;13(1):103–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brüggemann RAG, Spaetgens B, Gietema HA, Brouns SHA, Stassen PM, Magdelijns FJ, et al. The prevalence of pulmonary embolism in patients with COVID-19 and respiratory decline: a three-setting comparison. Thromb Res. 2020;196:486–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–93. [DOI] [PubMed] [Google Scholar]
- 15. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasha AK, McBane RD, Chaudhary R, Padrnos LJ, Wysokinska E, Pruthi R, et al. Timing of venous thromboembolism diagnosis in hospitalized and non-hospitalized patients with COVID-19. Thromb Res. 2021;207:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miró Ò, Jiménez S, Mebazaa A, Freund Y, Burillo-Putze G, Martín A, et al. Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome, Eur Heart J. 2021;42(33):3127–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Gal G, Kovacs MJ, Bertoletti L, Couturaud F, Dennie C, Hirsch AM, et al. Risk for recurrent venous thromboembolism in patients with subsegmental pulmonary embolism managed without anticoagulation: a multicenter prospective cohort study. Ann Intern Med. 2022;175(1):29–35. [DOI] [PubMed] [Google Scholar]
- 19. Oscanoa TJ, Romero-Ortuno R, Carvajal A, Savarino A. A pharmacological perspective of chloroquine in SARS-CoV-2 infection: an old drug for the fight against a new coronavirus?. Int J Antimicrob Agents. 2020;56(3):106078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Devaux CA, Camoin-Jau L, Mege J-L, Raoult D. Can hydroxychloroquine be protective against COVID-19-associated thrombotic events? J Microbiol Immunol Infect. 2021;54(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tilliridou V, Kirkbride R, Dickinson R, Tiernan J, Yong GL, van Beek EJ, et al. Pulmonary embolism severity before and during the COVID-19 pandemic. Br J Radiol. 2021;94(1123):20210264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanji JN, Zelyas N, MacDonald C, Pabbaraju K, Khan MN, Prasad A, et al. False negative rate of COVID-19 PCR testing: a discordant testing analysis. Virol J. 2021;18(1):13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, Zambrano-Achig P, Del Campo R, Ciapponi A, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Data and corresponding scripts are available upon request to the corresponding author. More information on the data collection process and availability can be read on the Castor EDC Website (https://www.castoredc.com/blog/covid-predict-study/) and the COVID-PREDICT Website (https://www.covidpredict.org/design/).