Abstract
Introduction
S-1 has been shown to be an effective adjuvant treatment option for East Asian patients who underwent gastrectomy for stage II/III gastric cancer. We conducted a phase I/II study to evaluate the feasibility, tolerability, and efficacy of administering S-1 in the adjuvant setting after R0-resection of adenocarcinoma of the stomach and esophagogastric junction (EGJ) in Caucasian patients.
Methods
In this single-cohort, open-label, phase I/II trial, we enrolled patients with locally advanced adenocarcinoma of the stomach or EGJ having undergone R0-resection with or without neoadjuvant treatment. One treatment cycle consisted of oral S-1 (30 mg/m2 bid) for 14 days. Cycles were repeated every 3 weeks for 18 cycles (54 weeks). Primary endpoint was feasibility and tolerability. Safety was evaluated according to the Common Toxicity Criteria Adverse Events (CTCAE) version 4.0. Secondary endpoints were 1-year relapse-free survival (RFS) rate, RFS, and overall survival (OS).
Results
Between October 2015 and February 2018, 32 patients were enrolled in 12 German centers, and 30 started adjuvant study treatment. Seventeen patients completed all 18 cycles. Two patients terminated study treatment early due to adverse events (AEs), 7 due to patient’s or investigator’s decision, and 4 due to recurrence or distant metastasis during adjuvant therapy. Dose levels were reduced to 25 mg/m2 in 9 patients and to 20 mg/m2 in 1 patient. Of patients completing all 18 cycles, 5 did so with reduced dosage of S-1. Documented grade ≥3 AEs were neutropenia, diarrhea, vomiting, polyneuropathy, palmar-plantar erythrodysaesthesia, and rash. Serious AEs were observed in 7 patients. Median RFS was 32.2 months. One-year RFS rate was 77%. Data on OS were still premature at the end of the study.
Conclusion
Adjuvant treatment with S-1 for 1 year is a feasible and safe treatment option for Caucasian patients diagnosed with gastric adenocarcinoma or cancer of the EGJ after R0-resection.
Keywords: Gastric cancer, Adjuvant treatment, S-1, Esophagogastric junction cancer, Gastrointestinal cancer
Introduction
Gastric cancer is among the most common causes of tumor-related death worldwide [1]. Outcome is primarily determined by the stage of the disease at its initial presentation.
Surgical resection is the established standard treatment for localized gastric cancer of the stomach and esophagogastric junction (EGJ), offering the only possibility for curative treatment. Depending on tumor location, different types of resections, usually involving D2-lymphadenectomy (compartments I and II), have to be performed [2]. Despite successful R0-resection, 5-year survival rates after surgery alone are poor due to local recurrence and distant metastases. To improve outcome, several trials investigated the perioperative treatment for patients with locally advanced cancer of the stomach or the EGJ [3, 4]. In the MAGIC trial, perioperative combination treatment with epirubicin, cisplatin, and fluorouracil (ECF) led to improved overall survival (OS) and progression-free survival (PFS) when compared to surgery alone [4]. In the FLOT-4 trial, perioperative treatment with fluorouracil, oxaliplatin, and docetaxel (FLOT) was associated with significantly longer OS than perioperative ECF (50 vs. 35 months) and has, thus, become the standard of care in Western Europe [2, 3].
Current guidelines do not recommend adjuvant treatment in totally resected cancer of the stomach or the EGJ without preceding preoperative chemotherapy [2]. In patients with certain risk factors, application of adjuvant treatment without neoadjuvant chemotherapy should, however, be discussed [2]. Especially for patients who underwent primary surgery due to the understaging of the primary tumor, adjuvant treatment is the only chance to improve the outcome. A meta-analysis from 2010 comprising 17 randomized, controlled trials in 3,838 patients (recruitment before 2004) compared adjuvant chemotherapy (excluding anthracycline-containing regimens) with surgery alone and showed a statistically significant benefit for surgery plus adjuvant chemotherapy versus surgery alone (5-year OS rate of 55.3% vs. 49.6%) [5].
S-1 is an oral fixed-dose combination of tegafur, a 5-fluorouracil (5-FU) prodrug, the reversible dihydropyrimidine dehydrogenase (DPD) inhibitor gimeracil, and the orotate phosphoribosyltransferase (OPRT) inhibitor oteracil. Gimeracil leads to suppression of rapid degradation of 5-FU via DPD inhibition, while oteracil reduces activation of 5-FU in the gastrointestinal mucosa through inhibition of OPRT and thus reduces gastrointestinal toxicity [6]. Since the pharmacokinetics of S-1 are expected to differ between Asian and Caucasian patients, different doses of S-1 need to be used and treatment results need to be viewed in relation to ethnicity.
S-1 has been shown to be an effective adjuvant treatment for Japanese patients who underwent gastrectomy with a D2-lymphadenectomy for stage II or stage III gastric cancer [7, 8]. The 5-year survival rate was 71.7% in patients who received adjuvant chemotherapy with S-1 after surgery versus 61.1% in the surgery-only group [7, 8]. Administration of S-1 was started within 6 weeks after surgery and was continued for 1 year [7, 8]. The regimen applied in these patients consisted of 6-week cycles with S-1 given for 4 weeks followed by 2 weeks with no chemotherapy given at a dose of 2 × 40 mg/day, 2 × 50 mg/day, or 2 × 60 mg/day based on body surface area (BSA) [7, 8].
A global phase III study performed in patients with untreated diffuse gastric cancer reported similar outcome results when S-1 plus cisplatin was compared to infusional 5-FU plus cisplatin [9]. Significant safety advantages were observed in the S-1 plus cisplatin arm compared with the infusional 5-FU plus cisplatin arm [9]. S-1 is currently approved by the European Medicines Agency (EMA) in combination with cisplatin for the treatment of patients suffering from advanced gastric cancer (dose 25 mg/m2). It is also approved for the treatment of patients with metastatic colorectal cancer who do not tolerate treatment with another fluoropyrimidine due to hand-foot syndrome or cardiovascular side effects (dose for single-agent therapy: 30 mg/m2, dose for combination therapy: 25 mg/m2).
So far, no studies on adjuvant treatment with S-1 have been performed in Caucasian patients. Additionally, the studies performed to date in Caucasian patients did not investigate prolonged use of S-1 for the duration of 1 year.
The present study aimed to evaluate the safety and feasibility of adjuvant application of orally applied S-1 in Caucasian patients with gastric or EGJ cancer who underwent R0-resection, taking into account the high rates of patients that did not continue postoperative chemotherapy after surgery [3, 4]. The trial did not only focus on determining the tolerable dose of S-1 but also evaluated patient adherence to a treatment duration of 1 year. Furthermore, feasibility of an oral fluoropyrimidine application in patients with resected or partially resected stomach was tested.
Methods
Patients
Main inclusion criteria included Caucasian ethnicity, age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status 0–1, adequate organ function including calculated creatinine clearance (CrCl) according to MDRD equation ≥50 mL/min, histologically confirmed adenocarcinoma with a pathological stage of pT2-4, any pN category, M0 or any uT, uN+, M0 of the stomach or EGJ, R0-resection with or without neoadjuvant treatment, and D2 lymph node dissection. Main exclusion criteria were metastatic disease, polyneuropathy CTCAE grade >1, ascites, liver cirrhosis, major surgical procedure within 28 days prior to the study, and pre-existing cardiac conditions.
Design of the Trial and Endpoints
The trial was designed by V.H. and C.S. Primary objective of the study was to assess the feasibility and tolerability of an adjuvant treatment schedule for S-1 by determining the number of patients with discontinuation of S-1 due to intolerable adverse reactions or due to the patient’s decision as well as the dose intensity and treatment duration in the absence of relapse. Recommended dose for S-1 should be the dose level, at which less than 10 of the 30 patients required dose reduction to the next lower dose level or discontinuation of S-1 due to hematological or non-hematological toxicities during the first 3 cycles. Secondary endpoints were 1-year relapse-free survival (RFS), RFS, 1-year survival rate, OS and type, incidence, and severity of adverse events (AEs) according to CTCAE version 4.0.
The initial trial design included two consecutive cohorts (cohort 1 with 30 patients who receive S-1 twice daily for 18 cycles (d1-14 q3w) and cohort 2 with 30 patients who receive S-1 twice daily for 9 cycles (d1-28 q6w)). Due to slow patient enrollment, the trial protocol was later amended, and the final trial included a single cohort 1.
Trial Conduct
The trial recruited patients in 12 centers in Germany in accordance with the protocol and in compliance with the Declaration of Helsinki. The protocol was approved by the responsible ethics committees of the participating centers. Patients provided written informed consent before trial entry. Sponsor of the trial is the AIO-Studien-gGmbH. A contract research organization (ClinAssess GmbH, Leverkusen, Germany) was responsible for data management, monitoring, and primary data analysis. The trial is registered with EudraCT (No.: 2014-004116-11). The trial was conducted with financial support by TAIHO Pharmaceutical Co., Ltd.
Statistical Analysis
The trial was planned to include 30 patients in a single cohort and to be performed in an exploratory way without formal statistics. Results would be analyzed in a descriptive manner.
OS was defined as the time from day 1 of the first adjuvant treatment cycle to the date of death from any cause. One-year survival rate was defined as the rate of patients, who were alive 1 year after the first day of the first adjuvant treatment cycle. RFS was defined as the time from the beginning of the first adjuvant treatment cycle to the date of confirmation of relapse or death without confirmed relapse, whichever occurred first. The rate of patients, who were alive and free of recurrence at 1 year after the start of adjuvant therapy, was also determined.
To evaluate the safety of adjuvant treatment with S-1, the following variables were analyzed: exposure to study drug S-1 (duration and dose intensity), dose reduction, temporary and permanent discontinuation of study drug S-1 due to AEs, type, incidence, and severity of AEs, and reasons for premature discontinuation of S-1.
Treatment
Based on data from phase I studies performed in Caucasian patients treated for metastatic disease [10], treatment in the present study started at a daily dose of 2 × 30 mg/m2 applied for 2 weeks, followed by 1 week of rest (single cohort). Duration of treatment was planned for 18 cycles of 3 weeks (54 weeks, 378 days). Adjuvant treatment was scheduled to start within 6–8 weeks after surgery.
Assessments
Baseline imaging after surgery was to be performed within 3 weeks before the first dose of the study drug with either CT or MRI. Routine CT or MRI examinations of the abdomen and chest were to be performed every 6 months during adjuvant treatment.
Results
Patients
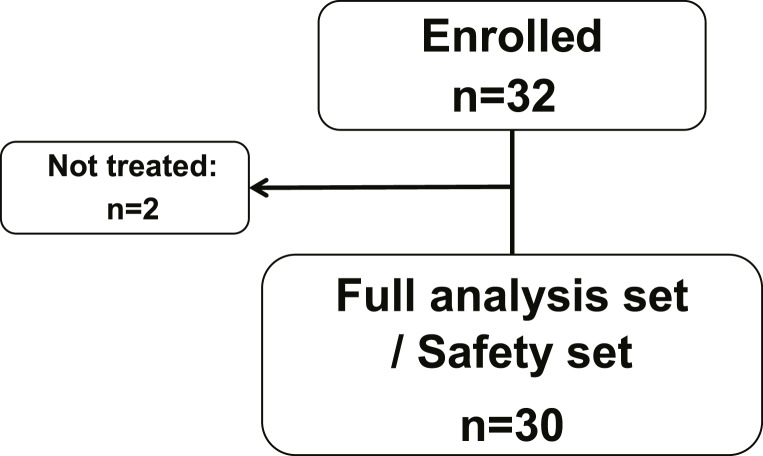
Thirty-two patients were enrolled in the trial between October 2015 and February 2018. Two patients did not receive study-related medication; 30 patients received at least one dose of S-1 (see Fig. 1). This population served as full analysis set (FAS) for the analysis of baseline and efficacy parameters and as safety set for the analysis of safety parameters.
Fig. 1.
Consort diagram of study population.
Of 30 patients, 17 (57%) were male and 13 (43%) were female. Median age was 61.5 years, and most patients presented with ECOG 1 (63%). Included patients were diagnosed with EGJ cancer in 67% (n = 20; n = 3 EGJ Siewert Type I, n = 6 EGJ Siewert Type II, and n = 11 EGJ Siewert Type III) and with gastric cancer in 33% (n = 19) of the cases. For further details, refer to Table 1.
Table 1.
Baseline characteristics
| Full analysis set, N = 30 | |
|---|---|
| Age, years | |
| Median | 61.5 |
| Range | 39–77 |
| Sex, n (%) | |
| Male | 17 (56.7) |
| Female | 13 (43.3) |
| ECOG performance status, n (%) | |
| 0 | 11 (36.7) |
| 1 | 19 (63.3) |
| Type of disease, n (%) | |
| Esophagus junction cancer | 20 (66.7) |
| EGJ Siewert Type I | 3 (10) |
| EGJ Siewert Type II | 6 (20) |
| EGJ Siewert Type III | 11 (55) |
| Gastric cancer | 10 (33.3) |
| Laurén classification, n (%) | |
| Intestinal type | 13 (43.3) |
| Diffuse type | 9 (30) |
| Indeterminate type | 3 (10.0) |
| Unknown | 5 (16.7) |
| Previous neoadjuvant chemotherapy, n (%) | |
| Esophagus junction cancer | 9 (45.0) |
| Gastric cancer | 3 (30.0) |
| Becker score, n (%) | |
| 1a | 1 (3.3) |
| 1b | 3 (10) |
| 2 | 3 (10) |
| 3 | 5 (16.7) |
| Not applicable | 15 (50) |
| Unknown | 3 (10) |
| Type of surgery, n (%) | |
| Total gastrectomy | 20 (66.7) |
| Partial/subtotal gastrectomy | 7 (23.3) |
| Esophagectomy | 2 (6.7) |
| Unknown | 1 (3.3) |
| Postoperative TNM classification, n (%) | |
| pT-stadium | |
| pT0 | 1 (3.3) |
| pT1 | 3 (10.0) |
| pT2 | 5 (16.7) |
| pT3 | 19 (63.3) |
| pT4 | 2 (6.7) |
| pN-stadium | |
| pN0 | 10 (33.3) |
| pN1 | 7 (23.3) |
| pN2 | 4 (13.3) |
| pN3 | 9 (30.0) |
Only 12 patients (40%) were treated with neoadjuvant chemotherapy. Reasons for no previous neoadjuvant treatment were understaging (n = 9, 30%), medical reasons (n = 1, 3%), patient’s wish (n = 1, 3%), other (n = 3, 10%), and unknown (n = 4, 13%). If treated with neoadjuvant chemotherapy, most patients were treated with combination chemotherapy containing 5-fluorouracil (5-FU), a platin-derivative (oxaliplatin or cisplatin), and a taxane (paclitaxel or docetaxel) (n = 10, 33%). One patient was treated with epirubicin, oxaliplatin, and capecitabine, and 2 patients received 5-FU, folinic acid, and oxaliplatin (n = 2, 7%). One patient changed treatment regimen during neoadjuvant chemotherapy and was documented with two different treatment regimens. Most patients received 4 cycles of neoadjuvant treatment (n = 7, 23%). Four patients (13%) were treated with neoadjuvant radiochemotherapy. Of the patients treated with neoadjuvant therapy, the majority (n = 5, 39%) showed histopathological tumor regression of Becker score 3, and only 31% (n = 4) reached Becker score 1a/b [11]. All patients underwent R0-resection. Most patients included in the trial underwent total gastrectomy (n = 20, 67%) or subtotal gastrectomy (n = 7, 23%). Postoperative tumor stage was mostly IIA/B (n = 15, 50%). One patient showed complete pathological remission after neoadjuvant (radio-)chemotherapy. For more details, see Table 1. Postoperative tumor stage and, where applicable, Becker regression score for the subgroup of patients with and without prior neoadjuvant therapy are presented in online supplementary Table 3 (for all online suppl. material, see https://doi.org/10.1159/000538143).
Treatment
Median time from surgery to the start of adjuvant treatment was 7.7 weeks in the FAS. Median treatment duration was 371 days (range 36–426 days). Reasons for longer treatment duration were, among others, the patient’s wish, AEs, and suspected recurrence. Seventeen patients completed all 18 planned cycles of S-1. Reasons for early termination of study treatment were AEs not resulting in death (n = 2), local or distant relapse (n = 4), patient’s decision (n = 5), and lack of compliance (n = 2).
In 21 patients (70%), treatment cycles had to be interrupted or delayed due to side effects. A dose reduction had to be performed in 10 patients (see Table 2a/b).
Table 2.
a Dose reductions or discontinuation in the first 3 cycles of treatment, full analysis set, n = 30; b Dose reductions or discontinuation in the overall duration of the study, full analysis set, n = 30
| n (%) | |
|---|---|
| a | |
| No dose reduction or discontinuation of treatment | 25 (83.3) |
| First dose reduction to 2 × 25 mg/m2 | 4 (13.3) |
| Second dose reduction to 2 × 20 mg/m2 | – |
| Discontinuation of treatment | 1 (3.3) |
| b | |
| No dose reduction | 20 (66.7) |
| Dose reduction due to AEs, overall | 10 (33.3) |
| Lowest dose level received: 2 × 25 mg/m2 (DL-1) | 8 (26.7) |
| Lowest dose level received: 2 × 20 mg/m2 (DL-2) | 1 (3.3) |
| Lowest dose level received: 2 × 15 mg/m2 (DL-3) | 1 (3.3) |
| Treatment interruption or delay due to AEs | 21 (70.0) |
| Treatment discontinuation due to AEs (investigator’s decision) | 2 (6.7) |
| Treatment discontinuation due AEs (patient’s decision) | 4 (13.3) |
Regular termination on schedule after 18 cycles: 17 (56.7%).
One patient started treatment at a daily dose of 2 × 15 mg/m2 due to a site error and continued with that dose up to cycle 5. The other 29 patients started treatment with the planned dose of daily 2 × 30 mg/m2. Five patients required dose reduction to the next lower dose level or discontinuation of S-1 due to hematological or non-hematological toxicities during the first 3 cycles. In 12 patients (40%), treatment could be continued with a daily dose of 2 × 30 mg/m2 until the last cycle. In 8 patients (27%), treatment had to be reduced to dose level −1 (2 × 25 mg/m2), and 5 patients (17%) finished the 18 cycles on dose level −1. In 1 patient, treatment had to be reduced to dose level −2 (2 × 20 mg/m2) but had to be discontinued in cycle 16.
Relapse and Survival
Median follow-up was 28.6 months (range 6–52.6 months). During that time, 12 patients (40%) experienced a recurrence of cancer disease. Relapse was local in 17%, distant in 42%, and combined in 33% of patients. In 1 patient, localization of relapse was unknown.
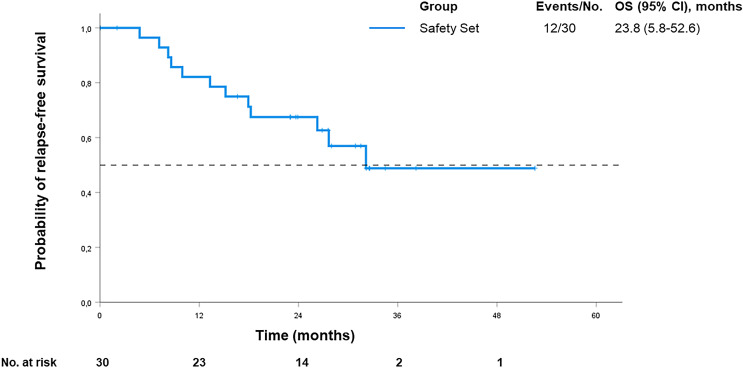
Median RFS was 32.2 months in the full analysis set (see Fig. 2). One-year RFS rate was 77%. RFS was not significantly affected by age (p = 0.07), sex (p = 0.16), or neoadjuvant treatment (p = 0.07). No effect on RFS was seen with regard to tumor site (p = 0.84), Laurén classification (p = 0.84), total versus partial gastrectomy (p = 0.62), and Becker score (p = 0.36). Further details on all subgroups are displayed in online supplementary Tables 1 and 2. It should be noted that the high variability in neoadjuvant treatment regimens and modalities may restrict the interpretation of subgroup analyses based on Becker regression score. Primary tumor stage appeared to be significantly associated with RFS. Patients diagnosed with a tumor stage ≤ IIIA had a significantly better RFS (HR 0.08; 95% CI: 0.02–0.31; p < 0.001) compared to patients who initially presented with more advanced disease (stage >IIIB, IIIC, or IVA).
Fig. 2.
Relapse-free survival.
Nine patients died during study treatment (n = 1) or follow-up (n = 8); all deaths were tumor related. Data on OS were still premature at the time of the data cut-off since only 9 patients died during follow-up.
Safety
All patients (n = 30) experienced at least one AE; 90% of patients (n = 27) had only one AE related to S-1. In 23.3% of patients, S-1 dose had to be reduced due to an AE. In 14 patients, AEs led to temporary interruption of S-1; in 2 patients, S-1 had to be permanently discontinued due to AEs. Three patients decided to discontinue treatment after AEs. Serious AEs (SAEs) were observed in 23% (n = 7) of patients. No fatal AEs were observed.
The most frequent AEs were gastrointestinal side effects such as nausea, vomiting, stomatitis, or diarrhea (see Table 3). Most side effects were low grade; only a small percentage of patients experienced grade 3 or higher AEs (see Table 3). AEs leading to dose reduction were grade 4 neutropenia (n = 1), Gilbert’s syndrome (n = 1), diarrhea (n = 2), nausea (n = 1), fatigue (n = 2), decreased creatinine clearance (n = 1), decreased weight (n = 1), decreased appetite (n = 1), and rash (n = 1). Reasons for permanent discontinuation of S-1 were polyserositis (n = 1) and grade 3 polyneuropathy (n = 1). The type of gastrectomy (total vs. partial) had no impact on the occurrence of AEs.
Table 3.
Most frequent AEs by maximum NCI-CTCAE grade Population: Safety set, n = 30
| AE terma | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Grade ≥3, n (%) | Any grade, n (%) |
|---|---|---|---|---|---|---|
| Nausea | 10 (33.3) | 7 (23.3) | – | – | – | 17 (56.7) |
| Vomiting | 5 (16.7) | 2 (6.7) | 1 (3.3) | – | 1 (3.3) | 8 (26.7) |
| Diarrhea | 8 (26.7) | 6 (20.0) | 2 (6.7) | – | 2 (6.7) | 16 (53.3) |
| Stomatitis | 5 (16.7) | 1 (3.3) | – | – | – | 6 (20.0) |
| Fatigue/asthenia | 12 (40.0) | 4 (13.3) | – | – | – | 16 (53.3) |
| Decreased appetite | 5 (16.7) | 1 (3.3) | – | – | – | 6 (20.0) |
| Leuko-/neutropenia | 1 (3.3) | 1 (3.3) | 1 (3.3) | 1 (3.3) | 2 (10.0) | 4 (13.3) |
| Anemia | 1 (3.3) | 1 (3.3) | – | – | – | 2 (6.7) |
| Peripheral neuropathy | 3 (10.0) | 2 (6.7) | 1 (3.3) | – | – | 6 (20.0) |
| Dysgeusia | 5 (16.7) | – | – | – | – | 5 (16.7) |
| Dizziness | 7 (23.3) | 1 (3.3) | – | – | – | 8 (26.7) |
| Vertigo | 2 (6.7) | – | – | – | – | 2 (6.7) |
| Headache | 5 (16.7) | – | – | – | – | 5 (16.7) |
| Palmar-plantar erythrodysesthesia syndrome | 2 (6.7) | 1 (3.3) | 1 (3.3) | – | – | 4 (13.3) |
| Rash | 3 (10.0) | 1 (3.3) | 1 (3.3) | – | 1 (3.3) | 5 (16.7) |
| Alopecia | 5 (16.7) | – | – | – | – | 5 (16.7) |
| Eye disordersb | 8 (26.7) | – | – | – | – | 8 (26.7) |
| Psychiatric disordersc | 4 (13.3) | – | – | – | – | 4 (13.3) |
aAEs starting after the first administration of S-1 until 28 days after the last administration of S-1 are considered TE(S)AEs. Grade according to the National Cancer Institute Common Terminology Criteria Adverse Event (NCI-CTCAE), Version 4.0.
bEye disorders included dry eye, increased lacrimation, ocular discomfort, blurred vision, and visual impairment.
cPsychiatric disorders included anxiety, depression, insomnia, nervousness, and sleep disorder.
Discussion
Despite an EMA approval of S-1 combined with cisplatin for treatment of patients with advanced gastric cancer, the use of S-1 lags behind 5-FU and its prodrug, capecitabine. The present study investigated the feasibility and tolerability of 18 cycles of S-1 in the adjuvant setting after R0-resection of gastric or EGJ.
Metabolism of S-1 is different in Caucasian than in Asian patients. This is most likely due to interethnic variability of pharmacokinetics and dynamics of S-1 caused by various mechanisms, including different polymorphisms of cytochrome P-450 2A6 which affect tegafur metabolism mainly in the liver. Higher conversion of tegafur to 5-FU, but at the same time also higher conversion of 5-FU to its catabolite fluoro-beta-alanine through DPD, has been shown in Caucasian compared to Asian patients receiving similar doses of S-1 [12].
In a Dutch phase I study, the recommended dose of S-1 for patients with solid tumors was determined to be 2 × 40 mg/m2 in chemotherapy-naïve patients and 2 × 35 mg/m2 for pretreated patients if S-1 was applied for 4 weeks followed by 1 week of rest [13]. The dose-limiting toxicity was diarrhea, whereas other toxicities were mild [13]. The high rates of diarrhea were confirmed in two phase II trials investigating S-1 in patients with advanced or metastatic colorectal or gastric cancer [14, 15]. In both trials, dosage had to be reduced from initially 2 × 40 mg/m2 to 2 × 35 mg/m2 due to severe non-hematological adverse reactions, mainly diarrhea [14, 15].
In a phase I study conducted in the USA, Hoff et al. [10] investigated the pharmacokinetics of S-1 in 16 patients with solid tumors. Having analyzed 3 dose levels (2 × 30 mg/m2, 2 × 35 mg/m2, and 2 × 40 mg/m2), a S-1 dose of 2 × 30 mg/m2, applied for 4 weeks followed by 1 week of rest, was recommended. Most frequent side effects were gastrointestinal AEs such as diarrhea, nausea, and vomiting [10].
In a single-agent application, the maximum tolerated dose of S-1 in Caucasian patients is 30 mg/m2 twice daily for 14 days of a 21-day cycle. This treatment regimen has also been used in the present trial [13, 14]. Available pharmacokinetic data suggest no bioavailability concerns in patients receiving oral S-1 who have undergone prior gastrectomy [16, 17].
In the present study, treatment was well tolerated. Only 2 of 30 patients discontinued treatment due to AEs; 17 patients completed all 18 cycles. Most documented side effects were low grade, and only a few patients experienced AEs grade ≥3. Hence, the trial confirms other early clinical trials suggesting that a daily dose of 2 × 30 mg/m2 is tolerable in Caucasian patients and will be defined as the recommended dose according to protocol [10, 13–15]. The present trial indicates that application of S-1 is feasible in patients who underwent gastric surgery without resulting in excessive toxicity. The type of gastrectomy (partial vs. total) had no impact on feasibility.
Median RFS in this trial was 32.2 months, which is in line with other clinical trials conducted around the same time [3], although comparison is of course limited by different study populations in terms of prior treatment, chemotherapeutic drugs, stage, and recruitment periods [18]. In a large randomized phase III trial comparing adjuvant therapy with S-1 and surgery alone in Asian patients who underwent gastrectomy with a D2-lymphadenectomy, the RFS rate at 5 years was 65.4% in the S-1 group and 53.1% in the surgery-only group. Here, it should be noted that, apart from an Asian population in the ACTS-GC study, the treatment schedule was different, using the more common Asian treatment schedule of a 4-week treatment with 2 weeks off [7, 8]. Trials in a larger Caucasian population are currently missing to adequately compare results.
During follow-up in the present study, 9 patients died. Data on OS are therefore still premature at the time of study completion and can hardly be compared to the literature. In general, comparability with trials conducted in Western Europe is only possible with major restrictions. Almost exclusively, patients were treated perioperatively or primarily neoadjuvant due to limited application of adjuvant chemotherapy in about half of the patients [4, 19]. According to German treatment guidelines, adjuvant treatment without prior neoadjuvant treatment can be offered in case of insufficient surgery and in the presence of risk factors as in a case-by-case decision [20]. This adds to the difficulty of defining the impact of adjuvant treatment.
A limitation of the study is the heterogeneity of an already small patient cohort. Inclusion of gastric cancer and cancer of EGJ resulted in various surgical approaches. The same is true for preoperative treatment. While some patients were treated with neoadjuvant (radio-)chemotherapy, other patients were primarily operated on due to understaging or reduced general condition, thus following current treatment recommendations [2].
Another limitation is the change in clinical practice in the treatment of locally advanced gastric and the EGJ cancer at the time of study recruitment. The analysis of the phase II part of the FLOT-4 trial was published in 2016 [21], changing clinical practice to the use of the FLOT-regimen in the perioperative setting. This made recruitment for the trial even more difficult as patients fit enough to receive FLOT after surgery were treated accordingly. An interesting approach for further studies would be the implementation of a prolonged oral adjuvant S-1 treatment into the current standard intensive perioperative treatment with FLOT, for instance, as adjuvant treatment in case of the intolerable toxicity of neoadjuvant treatment.
In conclusion, the present study demonstrated that prolonged treatment with S-1 in Caucasian patients is feasible and can be tolerated at a dose of 2 × 30 mg/m2 daily in most patients. It is, to our knowledge, the first trial showing that treatment with S-1 over 1 year is tolerable and feasible in this setting. Adjuvant treatment with S-1 could be an option for patients with gastric or EGJ cancer who are ineligible or not willing to receive postoperative combination chemotherapy.
Acknowledgments
The authors thank all patients and families as well as all participating study centers.
Statement of Ethics
The trial recruited patients in 12 centers in Germany in accordance with the protocol and in compliance with the Declaration of Helsinki. The protocol was approved by the responsible ethics committees of the participating centers (leading ethics committee: Ethics Committee of the LMU Munich, No. 195-15 fed). Patients provided written informed consent before trial entry.
Conflict of Interest Statement
Kathrin Heinrich: honoraria: Roche, Taiho, BMS, Merck, and streamedup!; consulting or advisory role: Servier, MSD (Institutional), Merck, and Janssen; and travel support/expenses: Amgen, Merck, and Servier. Volker Heinemann: honoraria: Roche, Celgene, Amgen, Sanofi, Merck, Sirtex Medical, Baxalta, Lilly, Boehringer Ingelheim, Taiho Pharmaceutical, and Servier; consulting or advisory role: Merck, Amgen, Roche, Sanofi, Boehringer Ingelheim, Celgene, Sirtex Medical, Baxalta, Servier, Halozyme, MSD, Bristol Myers Squibb, and MSD Oncology; research funding: Merck, Amgen, Roche, Celgene, Boehringer Ingelheim, Sirtex Medical, Shire, Servier; and travel, accommodations, and expenses: Merck, Roche, Sirtex Medical, Amgen, Servier, Shire, MSD, and Bristol Myers Squibb. Sebastian Stintzing: honoraria: Merck KGaA, Roche, Amgen, Bayer, Sanofi, Lilly, Pierre Fabre, Takeda, Taiho Pharmaceutical, Servier, and MSD; consulting or advisory role: Merck KGaA, Roche, Sanofi, Bayer, Amgen, Boehringer Ingelheim, Lilly, Takeda, MSD, Servier, and Pierre Fabre; and research funding: Pierre Fabre, Roche Molecular Diagnostics, Merck Serono Travel, Accommodations, Expenses: Merck KGaA, Roche, Sanofi, Bayer, Sirtex Medical, Amgen, Lilly, Takeda, and Pierre Fabre. Lothar Müller: honoraria: Roche. Thomas J. Ettrich grants and personal fees from AstraZeneca, Bayer, BMS, Daiichi Sankyo, Eisai, Incyte, Ipsen, Lilly, Merck Serono, Merck Sharp & Dome (MSD), Pierre Fabre, Roche, Sanofi, and Servier. Jörg Trojan: Anstellungsverhältnis oder Führungsposition: Goethe-Universität Frankfurt; consulting or advisory role/honoraria: Amgen, AstraZeneca, Bristol Myers Squibb, Eisai, Ipsen, Merck Serono, Merck Sharp & Dome, Lilly Imclone, Onkowissen TV, Roche, and Servier; and honoraria: Amgen, AstraZeneca, Bioprojet, Bristol Myers Squibb, Eisai, Ipsen, Medac, Merck Serono, Merck Sharp & Dome, Lilly Imclone, Roche, Servier, and streammedup!. Petra Büchner-Steudel, Michael Geißler, Nicolas Moosmann, Gunnar Folprecht, Johannes Schmidt, Stephan Kanzler, Frank Kullmann, Jean-Charles Moulin, Jens Werner, Martin K. Angele, Victoria Probst, Swantje Held, and Christoph Schulz: none declared. Myrto Boukovala: employment: Servier Germany (start after initial manuscript submission).
Funding Sources
The trial was conducted with financial support by Taiho Pharmaceutical Co., Ltd.
Author Contributions
Conception and design of the trial: Volker Heinemann, Christoph Schulz, and Sebastian Stintzing. Administrative support: Kathrin Heinrich, Christoph Schulz, and Sebastian Stintzing. Provision of study materials or patients: Kathrin Heinrich, Volker Heinemann, Sebastian Stintzing, Lothar Müller, Thomas J. Ettrich, Petra Büchner-Steudel, Michael Geißler, Jörg Trojan, Nicolas Moosmann, Gunnar Folprecht, Johannes Schmidt, Stephan Kanzler, Frank Kullmann, Jean-Charles Moulin, Jens Werner, Martin K. Angele, Christoph Schulz, Collection and assembly of data: Kathrin Heinrich, Volker Heinemann, Sebastian Stintzing, Lothar Müller, Thomas J. Ettrich, Petra Büchner-Steudel, Michael Geißler, Jörg Trojan, Nicolas Moosmann, Gunnar Folprecht, Johannes Schmidt, Stephan Kanzler, Frank Kullmann, Jean-Charles Moulin, Jens Werner, Martin K. Angele, Christoph Schulz, Myrto Boukovala, and Victoria Probst. Data analysis and interpretation: Kathrin Heinrich, Myrto Boukovala, Sebastian Stintzing, Christoph Schulz, Volker Heinemann, Victoria Probst, and Swantje Held. Manuscript writing, final approval of the manuscript, and accountablity for all aspects of the work: all authors.
Funding Statement
The trial was conducted with financial support by Taiho Pharmaceutical Co., Ltd.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF) . S3-Leitlinie Magenkarzinom – Diagnostik und Therapie der Adenokarzinome des Magens und des ösophagogastralen Übergangs – Langversion 2.0 – August 2019. AWMF-Registernummer: 032/009OL; 2019. [Google Scholar]
- 3. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57. [DOI] [PubMed] [Google Scholar]
- 4. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. [DOI] [PubMed] [Google Scholar]
- 5. GASTRIC Global Advanced/Adjuvant Stomach Tumor Research Internation, al Collaboration Group; Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303(17):1729–37. [DOI] [PubMed] [Google Scholar]
- 6. Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7(5):548–57. [DOI] [PubMed] [Google Scholar]
- 7. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20. [DOI] [PubMed] [Google Scholar]
- 8. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93. [DOI] [PubMed] [Google Scholar]
- 9. Ajani JA, Abramov M, Bondarenko I, Shparyk Y, Gorbunova V, Hontsa A, et al. A phase III trial comparing oral S-1/cisplatin and intravenous 5-fluorouracil/cisplatin in patients with untreated diffuse gastric cancer. Ann Oncol. 2017;28(9):2142–8. [DOI] [PubMed] [Google Scholar]
- 10. Hoff PM, Saad ED, Ajani JA, Lassere Y, Wenske C, Medgyesy D, et al. Phase I study with pharmacokinetics of S-1 on an oral daily schedule for 28 days in patients with solid tumors. Clin Cancer Res. 2003;9(1):134–42. [PubMed] [Google Scholar]
- 11. Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98(7):1521–30. [DOI] [PubMed] [Google Scholar]
- 12. Chuah B, Goh BC, Lee SC, Soong R, Lau F, Mulay M, et al. Comparison of the pharmacokinetics and pharmacodynamics of S-1 between caucasian and East Asian patients. Cancer Sci. 2011;102(2):478–83. [DOI] [PubMed] [Google Scholar]
- 13. van Groeningen CJ, Peters GJ, Schornagel JH, Gall H, Noordhuis P, de Vries MJ, et al. Phase I clinical and pharmacokinetic study of oral S-1 in patients with advanced solid tumors. J Clin Oncol. 2000;18(14):2772–9. [DOI] [PubMed] [Google Scholar]
- 14. Van den Brande J, Schoffski P, Schellens JH, Roth AD, Duffaud F, Weigang-Kohler K, et al. EORTC Early Clinical Studies Group early phase II trial of S-1 in patients with advanced or metastatic colorectal cancer. Br J Cancer. 2003;88(5):648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chollet P, Schoffski P, Weigang-Kohler K, Schellens JH, Cure H, Pavlidis N, et al. Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer. 2003;39(9):1264–70. [DOI] [PubMed] [Google Scholar]
- 16. Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28(9):1547–53. [DOI] [PubMed] [Google Scholar]
- 17. EMA . Assessment report for teysuno (tegafur/gimeracil/oteracil) procedure No.: EMEA/H/C/0001242; 2010. [Google Scholar]
- 18. Group G, Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303(17):1729–37. [DOI] [PubMed] [Google Scholar]
- 19. Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715–21. [DOI] [PubMed] [Google Scholar]
- 20. Moehler M, Al-Batran SE, Andus T, Arends J, Arnold D, Baretton G, et al. [Not available]. Z Gastroenterol. 2019;57(12):1517–632. [DOI] [PubMed] [Google Scholar]
- 21. Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17(12):1697–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.