Abstract
The herpes simplex virus type 1 UL34 gene encodes a protein that is conserved in all human herpesviruses. The association of the UL34 protein with membranes in the infected cell and its expression as a gamma-1 gene suggest a role in maturation or egress of the virus particle from the cell. To determine the function of this gene product, we have constructed a recombinant virus that fails to express the UL34 protein. This recombinant virus, in which the UL34 protein coding sequence has been replaced by green fluorescent protein, forms minute plaques and replicates in single-step growth experiments to titers 3 to 5 log orders of magnitude lower than wild-type or repair viruses. On Vero cells, the deletion virus synthesizes proteins of all kinetic classes in normal amounts. Electron microscopic and biochemical analyses show that morphogenesis of the deletion virus proceeds normally to the point of formation of DNA-containing nuclear capsids, but electron micrographs show no enveloped virus particles in the cytoplasm or at the surface of infected cells, suggesting that the UL34 protein is essential for efficient envelopment of capsids.
Infectious herpes simplex virus (HSV) particles consist of three complex components: a DNA-containing nucleocapsid, an amorphous tegument layer composed of viral proteins, and a lipid bilayer envelope containing specific viral proteins. The nucleocapsid is produced in the nucleus of the infected cell and must then assemble with the other two components and egress from the cell. The process by which these components come together and leave the cell is poorly understood, but is generally accepted that the nucleocapsid first acquires an envelope by budding into the space between the inner and outer nuclear membranes (14).
Several requirements for efficient envelopment at the inner nuclear membrane have been defined. This process apparently requires prior completion of the DNA packaging process. In wild-type virus-infected cells, empty capsids are only rarely enveloped (35), and a variety of viral mutants that synthesize capsids but fail to package DNA do not efficiently envelop the empty capsids produced (2, 9, 11, 28, 29, 39). A deletion in the gene encoding the virus-encoded alkaline nuclease results in failure to efficiently envelop DNA-containing capsids, suggesting that the packaging reaction may be incomplete and that completion is required in order to generate a nucleocapsid that is competent for envelopment (42). Several other viral gene products have been reported to contribute to the process of primary envelopment. A recombinant HSV carrying a deletion in the UL11 gene produces reduced numbers of enveloped virus particles, and an unusually high number of partial envelopment events are observed at the inner nuclear membrane, suggesting that UL11 is required for efficient completion of the envelopment process (4). Glycoprotein K (gK) plays a critical role in trafficking of enveloped virus particles through the cytoplasm in HSV-infected cells (22, 24) and may also play a role in primary envelopment, since complete deletion of gK results in overaccumulation of intranuclear nucleocapsids at very late times after infection (24). Brown et al. have reported that a deletion in the gene encoding ICP34.5 results in failure to accumulate cytoplasmic and extracellular virions in a cell-type- and growth-state-dependent manner (7). This region of the viral genome is complex, encoding multiple overlapping genes with regulatory functions, so it is not clear that ICP34.5 itself is required for envelopment (1, 8, 34). Also, the ICP34.5 gene product is required for efficient late protein synthesis in some cells, raising the possibility that the envelopment defect observed may be secondary to a defect in synthesis of other viral late proteins (10, 21).
Purves et al. (32, 33) have reported that the UL34 gene product of HSV type 1 (HSV-1) is a membrane-associated phosphoprotein with an Mr of ≈30,000. Analysis of the predicted sequence of the UL34 protein suggests that the protein has no cleavable signal sequence but does contain a potential transmembrane domain located near the C terminus. The distribution of charges in the vicinity of the putative transmembrane domain further suggests that UL34 is likely to be a type II integral membrane protein, with a long, cytoplasmic N-terminal domain and a very short, luminal or extracellular C-terminal domain. These properties suggest that UL34 may participate in events mediated at the infected cell membranes, such as envelopment of the tegument and nucleocapsid, viral release, expression or activity of the viral envelope glycoproteins, or modification of the activity of cellular membrane proteins. The UL34 protein coding sequence is well conserved in the alphaherpesviruses for which sequence data are available (15, 16, 43). Significant sequence similarity is also evident between HSV-1 UL34 and the human cytomegalovirus (HCMV) UL50 protein (6). The HCMV UL50 gene is also a positional homolog of UL34; that is, it is found between the UL49 and UL51 genes of HCMV, which are homologous to the HSV-1 UL33 and UL35 genes, respectively. The UL50 gene and its relatives in human herpesvirus 6 (HHV-6), HHV-7, HHV-8, and Epstein-Barr virus all encode putative type II transmembrane proteins. It seems likely, therefore, that the UL34 function is widely conserved among herpesviruses.
The function of this protein is of interest from several other points of view. The UL34 gene product is the substrate of multiple HSV regulatory events. UL34 is a substrate of the protein kinase encoded by the HSV-1 US3 gene (32). The function of the phosphorylation is presently unknown, but it may be responsible for regulating the function of cellular proteins. It has been shown that the phosphorylation of a set of cellular proteins that associate with UL34 is dependent on the phosphorylation state of UL34 (32, 33). The UL34 transcription unit is also regulated by the HSV-1 US11 protein (36). In the absence of the US11 protein, a truncated transcript accumulates from the UL34 locus. The function of this regulation and of the truncated transcript is unknown. Purves et al. reported that repeated attempts to delete the UL34 gene in the absence of a complementing cell line were unsuccessful, suggesting that the product of the gene may be essential for viral replication in cell culture. To begin addressing the function of UL34 and of the regulatory events that impinge upon it, we have constructed a virus in which most of the UL34 coding sequence is deleted and which expresses no detectable UL34 protein. Here we report that this deletion virus has defects in plaque formation and single-step growth in cell culture. Although the virus assembles DNA-containing nucleocapsids, it fails to envelop them, suggesting that the UL34 protein is required for primary envelopment.
MATERIALS AND METHODS
Cells and viruses.
HEp-2, 143B, HEL299, and Vero cells (all from the American Type Culture Collection) were maintained in Dulbecco's modified Eagle's medium (high glucose) supplemented with 5% fetal bovine serum. The properties of HSV-1(F), the thymidine kinase (TK) deletion recombinant Δ305, and the TK insertion recombinant R7309 (kindly provided by Bernard Roizman) have been previously described (30, 32).
Plasmid constructions.
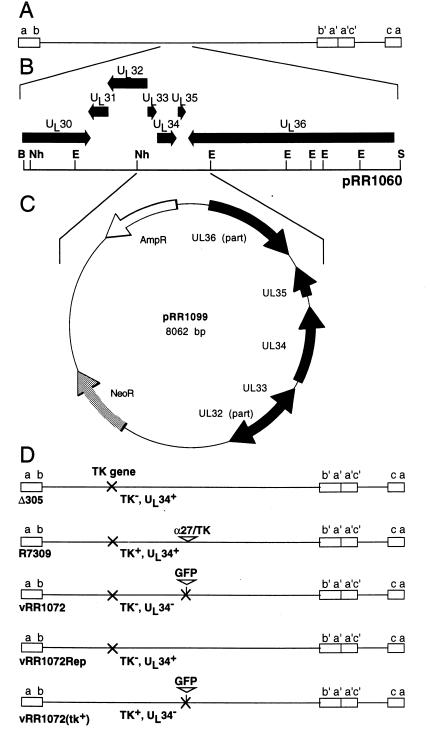
The structures of the plasmids used for construction of a UL34-complementing cell line and for construction of a UL34-null virus are shown in Fig. 1. pRR1060 (Fig. 1B) was constructed by ligating the 17.3-kb BglII-SpeI fragment of the HSV-1(F) genome containing the UL34 gene into BamHI- and XbaI-digested pGEM-3Z(f+). pRR1099 (Fig. 1C) was generated by insertion of a 3.4-kb UL34 gene-containing fragment of pRR1060 into pcDNA3. The insert fragment was obtained by sequential treatment of pRR1060 with restriction enzyme EcoNI, Klenow enzyme and deoxynucleoside triphosphates to generate blunt ends, and restriction enzyme NheI. This insert was ligated to the large NruI-XbaI restriction fragment of pcDNA3. pRR1072 was generated by replacement of UL34 sequences in pRB4177 (33) with the coding sequence for a mutant form of green fluorescent protein (GFP). The insert fragment was obtained by sequential treatment of phGFPS65T (Clontech) with restriction enzyme XbaI, Klenow enzyme and deoxynucleoside triphosphates to generate blunt ends, and restriction enzyme NcoI. This insert was ligated to the large NcoI-HpaI restriction fragment of pRB4177. Plasmid pRB166, containing the BamHI Q fragment of HSV-1(F) in pGEM-3Z, used for repairing the TK locus of the virus, was obtained from Bernard Roizman.
FIG. 1.
Sequence arrangement of plasmids and viruses used in this study. (A) Schematic diagram of the HSV-1 genome in prototype arrangement, showing the unique sequences (lines) flanked by inverted repeats (boxes). (B) Expansion of the region of the HSV genome cloned in pRR1060, showing the positions of viral open reading frames (arrows) and selected restriction enzyme cleavage sites. (C) Schematic diagram of the plasmid pRR1099, produced by ligating the NheI-EcoNI fragment of pRR1060 between the NruI and XbaI sites of pcDNA3. (D) Schematic diagrams of the genomes of HSV-1 recombinants used in this study. The names and properties of these viruses as they relate to TK and UL34 expression are indicated beneath each diagram. Restriction enzyme abbreviations: B, BglII; E, EcoNI; Nh, NheI; S, SpeI.
Construction of a stably transfected clonal UL34-complementing cell line.
143/1099 complementing cells were obtained by transfecting 10-cm2 cultures of 143B cells with 1 μg of BglII-linearized pRR1099, using 4 μl of LipofectAmine (Gibco/BRL) in Dulbecco's modified Eagle's medium without serum or antibiotics according to the manufacturer's suggested protocol. Two days after transfection, cells were seeded into medium containing 400 μg of Geneticin (Gibco/BRL) per ml. After passage for 2 weeks in selective medium, the cell population was assayed for ability to support plaque formation in the presence of bromodeoxyuridine (BUdR) by a virus stock derived from cotransfection of R7309 viral genomic DNA and pRR1072 plasmid DNA. Ability to complement the UL34 deletion was indicated by ability to support formation of BUdR-resistant, GFP-positive plaques. Clonal cell lines were derived from the stably transfected population by limiting dilution. Forty-two such lines were assayed for ability to complement UL34 deletion, and one of these (143/1099E) was chosen, based on number and size of plaques formed, for isolation and growth of UL34-negative virus.
Recombinant viruses.
The genotypes of recombinant viruses used in this study with respect to the TK and UL34 loci are indicated schematically in Fig. 1D. The UL34 deletion virus HSV-1(vRR1072) was obtained by cotransfection of 5-cm2 cultures of Vero cells with 250 ng of HSV-1 R7309 viral genomic DNA and 100 ng of EcoRI-linearized pRR1072 plasmid DNA, using 5 μl of LipofectAmine according to the manufacturer's suggested protocol. Viral stock was prepared from the culture after the culture exhibited 100% cytopathic effect. Serial dilutions of this stock were plated on 143/1099E cells in the presence of BUdR to select for TK-negative viruses, and GFP-positive plaques were subjected to three cycles of plaque purification. The homologous UL34 repair virus vRR1072Rep was obtained by cotransfection of vRR1072 viral genomic DNA and pRR1099 plasmid DNA. GFP-negative plaques were subjected to three cycles of plaque purification. The UL34-negative TK-positive virus vRR1072(TK+) for electron microscopic (EM) studies was obtained by cotransfection of vRR1072 viral genomic DNA and pRB166 plasmid DNA. TK-positive viruses were selected in hypoxanthine-aminopterin-thymidine medium as described elsewhere (30) and subjected to three cycles of plaque purification.
Fractionation of nuclear lysates for capsid analysis.
Nuclear lysates were prepared and fractionated by a modification of the method described by Lamberti and Weller (26). Confluent monolayer cultures of Vero cells (25 cm2) that had been infected at a multiplicity of infection of 10 for 20 h were washed twice with phosphate-buffered saline (PBS), scraped into 1 ml of PBS, and pelleted at low speed in a microcentrifuge. The cell pellet was resuspended in 0.4 ml of PBS containing 0.5% NP-40 and incubated on ice for 1 min to lyse the cells. Nuclei were pelleted from the suspension by centrifugation at 2,000 rpm for 5 min. Nuclei were resuspended in 0.25 ml of PBS containing 0.5% NP-40 and then lysed by a single cycle of freezing and thawing followed by sonication three times for 10 s each. Debris was pelleted for 2 min at top speed in the microcentrifuge, and then nonencapsidated DNA was digested by adding MgCl2 to 2 mM and 5 U of RQ1 DNase (Promega) and allowing the reaction to proceed at 37°C for 30 min. The nuclear extract was fractionated by centrifugation on a 10-ml 15 to 45% sucrose gradient made up in PBS at 35,000 rpm for 30 min. Gradients were separated into 20 0.5-ml fractions taken by dripping from the bottom of the tube. For analysis of DNA, 0.2-ml aliquots of each fraction were mixed with an equal volume of 10 mM EDTA–1% sodium dodecyl sulfate (SDS)–50 μg of glycogen per ml and extracted with an equal volume of 1:1 phenol-chloroform. DNA was precipitated from the aqueous phase with ethanol, resuspended in Tris-EDTA, and applied to an agarose gel. For analysis of capsid protein, 0.2-ml aliquots of each fraction were adjusted to 12% trichloroacetic acid (TCA) after addition of 10 μg of bovine serum albumin as carrier, allowed to react on ice for 30 min, and then centrifuged in a microcentrifuge for 5 min to pellet precipitated protein. Pellets were washed once in 12% TCA, three times with cold acetone to remove residual TCA, dried, and then resuspended in SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer.
Isolation and analysis of RNA.
Total RNA was isolated and analyzed by Northern blotting as previously described (36).
SDS-PAGE and immunoblotting.
Proteins were separated by SDS-PAGE, electrically transferred to a nitrocellulose sheet, and probed with antibodies as previously described (38). Anti-UL34 rabbit antiserum and anti-US11 monoclonal antibody have been previously described (33, 38). Anti-gD and anti-gE antibodies were obtained from the Goodwin Institute. Anti-VP5 monoclonal antibody was obtained from Biodesign International.
Measurement of virus replication in single-step growth.
Cultures of Vero, 143B, 143/1099E, HEL299, and HEp-2 cells were exposed to virus at a multiplicity of infection of 10 for 90 min at 4°C to allow attachment of virus. The inoculum was then aspirated, and cells were washed three times in 37°C PBS and placed at 37°C in growth medium. This was designated time zero of infection. After incubation for 90 min to allow virus entry and initiation of infection, cells were washed once with citrate buffer (50 mM sodium citrate, 4 mM KCl, adjusted to pH 3.0 with HCl) and then incubated in a second wash of citrate buffer for 1 min to inactivate most of the residual virus. Monolayers were then washed twice in PBS and incubated in growth medium for the remainder of the infection. At various times, cultures were frozen at −80°C and then thawed to lyse the cells, diluted 1:1 with autoclaved skim milk, and sonicated with a Fisher Sonic Dismembrator at power level 0 for 20 s to fully disrupt cells and release virus particles. The virus stocks were then titrated on 143/1099E cell monolayers as described below.
Plaque assays.
Cultures of 143/1099E, 143B, Vero, or HEL299 cells that were roughly 70% confluent were exposed to virus at 37°C for 90 min and then incubated in growth medium containing 0.01% pooled human immune globulin (Gammar, Armour Pharmaceutical) for 48 h to permit virus plaque formation. Plaques were visualized either by GFP fluorescence or by immunoassay using a monoclonal antibody directed against HSV-1 gD (Goodwin Cancer Research Laboratories) as previously described (37).
Transmission EM of infected cells.
Confluent Vero cell monolayers were infected at a multiplicity of infection of 10 for 20 h and then fixed by incubation in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 2 h. Cells were postfixed in 1% osmium tetroxide, washed in cacodylate buffer, dehydrated, embedded in Spurr's resin, and cut into 95-nm sections. Sections were mounted on grids, stained with uranyl acetate and lead citrate, and examined with a Hitachi 7000 model transmission electron microscope.
RESULTS
Construction of a recombinant UL34 deletion virus.
To create a UL34-null recombinant virus, we created a plasmid (pRR1072) in which UL34 coding sequences between the NcoI site (which includes the UL34 initiator methionine) and an HpaI site 682 nucleotides 3′ to the initiator methionine were replaced with the coding sequence for GFP, including a stop codon (Fig. 2B). This substitution removes the sequences coding for amino acids 1 to 228 of UL34 but does not disturb the coding sequence for UL33 or UL35. The UL34 coding sequences remaining have no methionine codons that might support initiation and expression of a fragment of UL34. HSV-1 R7309 (Fig. 1D) is derived from the TK-negative recombinant Δ305 by insertion of an α27-TK fusion immediately upstream of the UL34 protein coding sequence (32). Cotransfection of pRR1072 with HSV-1 R7309 DNA results in production of progeny in which the inserted α27-TK fusion and the wild-type UL34 sequences are replaced by sequences with the GFP substitution. These progeny are then TK negative (and therefore BUdR resistant) and GFP positive. Cotransfection of R7309 and pRR1072 gave rise to no BUdR-resistant, GFP-positive plaques, suggesting that expression of UL34 is essential for efficient propagation of the virus. A complementing cell line was constructed by stable transfection of 143B cells with pRR1099 and screening of clonal cell lines for the ability to support formation of BUdR-resistant, GFP-positive virus following infection with the R7309-pRR1072 cotransfection stock. Progeny having the desired phenotypes were plaque purified on the complementing cell line, amplified, and characterized with respect to genome structure and UL34 protein expression. One of these isolates, designated HSV-1 vRR1072, was chosen for further study. A homologous repair virus for vRR1072, designated vRR1072Rep, was constructed by cotransfection of recombinant viral DNA with pRR1099 and purification of GFP-negative plaques.
FIG. 2.
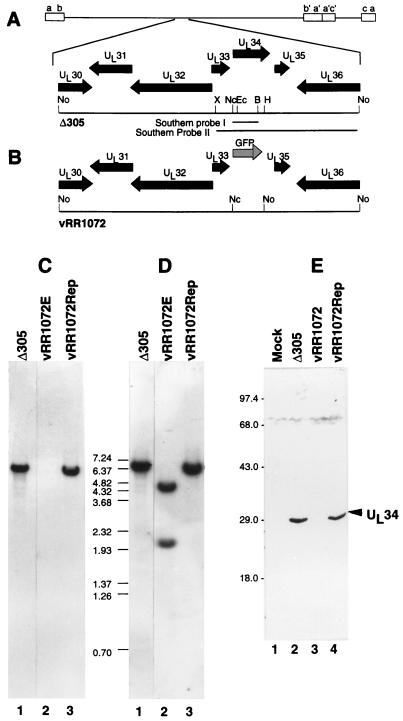
Structures of recombinant virus genomes and UL34 expression in recombinant viruses. (A and B) Sequence arrangement of viral genomes of Δ305 (A) and vRR1072 (B) around the UL34 locus, showing the positions of viral open reading frames (filled arrows), GFP insertion (shaded arrow), and probes used for Southern analysis of recombinant virus genome structure. Restriction enzyme abbreviations: B, BamHI; Ec, EcoRI; H, HpaI; Nc, NcoI; No, NotI; X, XbaI. (C) Autoradiographic image of a Southern blot of NotI-digested viral DNAs from cells infected with Δ305 (lane 1), the UL34 deletion virus vRR1072 (lane 2), or homologous repair virus vRR1072Rep probed with Southern probe I. (D) Same blot as in panel C but stripped and reprobed with Southern probe II. The sizes of migration standards (in kilobase pairs) are indicated between the panels. All lanes were from the same blot, but lane 1 was not adjacent to lanes 2 and 3 in the original autoradiogram. (E) Photographic image of a Western blot of SDS-PAGE-separated proteins from cells either mock infected (lane 1) or infected with Δ305 (lane 2), vRR1072 (lane 3), or vRR1072Rep (lane 4). The migration positions of size standards are indicated at the left. The position of the UL34 signal is indicated by the arrowhead.
The genome structures of the UL34 deletion and repair viruses were assessed by Southern blotting. The sequence of the UL34 gene is contained within a 6.7-kb NotI restriction fragment in wild-type virus. This fragment is subdivided into 4.6- and 2.1-kb fragments in the UL34 deletion due to the introduction of a NotI site in the GFP sequences. Viral DNA was digested with restriction endonuclease NotI, electrophoretically separated, and probed first with Southern probe I, which detects sequences that are deleted in the UL34-negative recombinant (Fig. 1C). As expected, a band of 6.7 kb is detected in Δ305 and vRR1072Rep, but no signal is observed in DNA from vRR1072, suggesting that the UL34 coding sequences are missing in the recombinant and that the recombinant is not contaminated by significant amounts of wild-type virus. Southern probe II spans the sequences deleted in vRR1072 and was expected to detect the full-length 6.7-kb NotI fragment in wild-type virus and the two subfragments produced in the UL34 recombinant. After the blot shown in Fig. 1C was stripped and reprobed with Southern probe II, the 4.6- and 2.1-kb fragments were observed in the UL34-negative recombinant but not in Δ305 or vRR1072Rep. The virus genome thus has the expected structure.
To test for UL34 protein expression from the viral genome, Vero cells were either mock infected or infected for 18 h with Δ305, vRR1072, and vRR1072Rep, and cellular lysates were prepared, separated on an SDS-polyacrylamide gel, blotted to nitrocellulose, and probed with anti-UL34 antiserum (Fig. 2E). Equivalent amounts of total protein were loaded, as shown by equivalent Coomassie blue staining of identical samples run on the same gel (not shown). A strong signal is observed at Mr ∼30,000 in Δ305 and the repair virus, but no signal is detectable in proteins from cells infected with the UL34-negative recombinant.
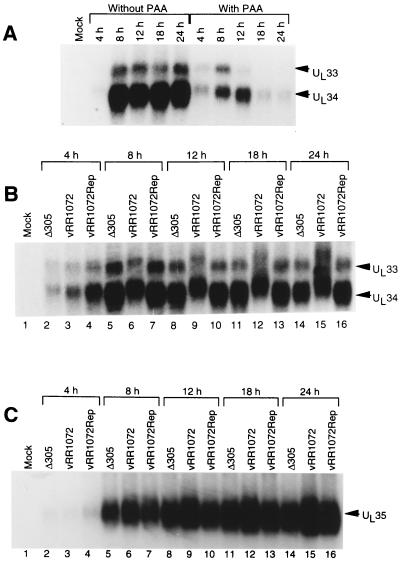
Expression of the UL33, UL34, and UL35 mRNAs was examined in wild-type and deletion viruses by Northern blotting. The normal kinetics of expression for UL33 and UL34 were examined in a time course experiment with and without the DNA replication inhibitor phosphonoacetic acid (PAA). Total RNA was harvested from Vero cells infected with 10 PFU of HSV-1(F) per cell and treated or not treated with 300 μg of PAA per ml throughout the infection. At various times after infection, total RNA was purified, electrophoretically separated, blotted to a nylon membrane, and probed with a 32P-labeled RNA antisense to UL33 and UL34 (Fig. 3A). Equal A260 units of total RNA were loaded into each lane, and equivalence of sample loading was confirmed by examination of rRNA staining intensity in each lane (not shown). In the absence of PAA, UL33 and UL34 RNAs are expressed with identical kinetics. Maximal expression of both RNAs is achieved by 8 h after infection and is maintained until late in the infectious cycle. Expression of both RNAs is partially sensitive to PAA. Early expression occurs in the presence of PAA, but late expression is not maintained, suggesting that these RNAs are expressed with leaky-late, or gamma-1, kinetics. To examine RNA expression in the deletion virus, total RNA was harvested from Vero cells infected for various times with Δ305, vRR1072, or vRR1072Rep, and equal amounts of total RNA were electrophoretically separated, blotted, and probed with the probe used for Fig. 3A (Fig. 3B and C). RNA samples for detection of UL33 and UL34 were separated on 1.3% agarose gels; those for UL35 detection were separated on 1.8% agarose gels. In the deletion virus, as in Δ305 and the repair virus, UL33 and UL34 mRNAs (Fig. 3B) are expressed in parallel and are first detectable at the same time, but both RNAs accumulate more slowly in the deletion virus-infected cells. Maximal amounts of UL33 and UL34 mRNAs are not achieved until 24 h after infection. In contrast, UL35 mRNA accumulates in deletion virus-infected cells at levels similar to those in cells infected with wild-type and repair viruses at all time points tested, suggesting that the deletion in UL34 has no significant effect on transcription of UL35.
FIG. 3.
Expression of UL33, UL34, and UL35 mRNAs. Shown are autoradiographic images of Northern blots of formaldehyde agarose gel-separated RNAs purified from infected Vero cells and probed with RNA probe antisense to the UL34 gene. (A) RNAs purified at various times after infection in the presence or absence of PAA. (B) RNAs purified at various times after infection with Δ305 (lanes 2, 5, 8, 11, and 14), the UL34 deletion virus vRR1072 (lanes 3, 6, 9, 12, and 15), or the homologous repair virus vRR1072Rep (lanes 4, 7, 10, 13, and 16), separated on a 1.3% agarose gel and probed with labeled RNA antisense to sequences between the BspEI and XbaI sites that flank the UL34 gene. Arrowheads indicate the migration positions of wild-type UL33 and UL34 mRNAs. These mRNAs migrate more slowly in the deletion virus due to the insertion of GFP sequences. (C) Same as panel B but run on a 1.8% agarose gel and probed with labeled RNA antisense to sequences between the BstBI and BspEI restriction sites that flank the UL35 gene. The region of the gel containing UL35 mRNA is shown, and an arrowhead indicates the migration position of UL35 mRNA.
UL34 expression is required for production of normal viral plaques.
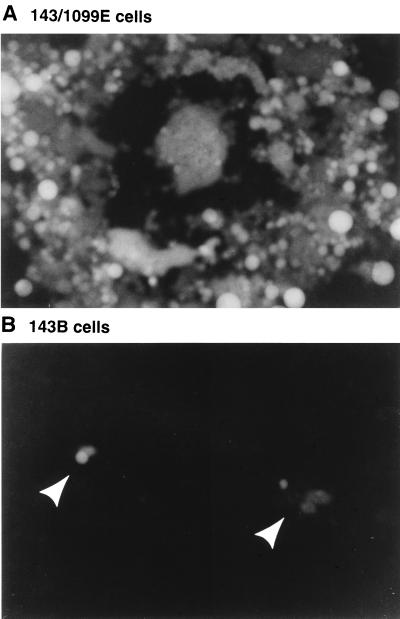
To determine the importance of UL34 expression in plaque formation, noncomplementing 143B cells and complementing 143/1099E cells were infected at low multiplicity and plaques were allowed to develop for 48 h. Monolayers were then examined for formation of fluorescent plaques with a fluorescence microscope. On complementing cells, the UL34 deletion virus made robust plaques containing hundreds of infected cells (Fig. 4A). These plaques were syncytial, since the recombinant Δ305, from which vRR1072 is derived, is syncytial due to deletion of a part of the UL24 gene (23, 30, 40). On noncomplementing cells, the virus forms only minute plaques, containing two to fewer than ten cells (Fig. 4B). Plating efficiency of the UL34 deletion virus on Vero and HEL299 cells was compared to that of Δ305 and vRR1072Rep viruses (Table 1). The plating efficiency was found to depend on the cell type. On HEL299 cells, the UL34 deletion virus formed as many plaques as Δ305 and the repair virus, whereas on Vero cells many fewer plaques formed. On all cell lines tested, the plaques formed by the UL34 deletion were minute, consisting of only a few infected cells (not shown). On Vero cells, numerous individual infected cells were observed but were not counted as plaques.
FIG. 4.
Plaque formation by the UL34 deletion virus on complementing and noncomplementing cells. Photographic images show 143/1099E cells (A) and 143B cells (B) infected with vRR1072, viewed with UV illumination to excite GFP fluorescence. Plaques in panel B are indicated with arrowheads.
TABLE 1.
Plating efficiency of the UL34-negative recombinant vRR1072 on complementing and noncomplementing cells
| Virus | Titera
|
Relative efficiency:
|
|||
|---|---|---|---|---|---|
| 143/1099E | Vero | HEL299 | Verob | HEL299c | |
| Δ305 | (1.6 ± 0.4) × 108 | (9.9 ± 1.1) × 108 | (2.6 ± 0.3) × 108 | 6.2 | 1.6 |
| vRR1072 | (3.7 ± 0.3) × 107 | (1.8 ± 0.9) × 106 | (3.9 ± 0.5) × 107 | 0.05 | 1.1 |
| vRR1072Rep | (1.4 ± 0.7) × 107 | (5.9 ± 1.4) × 107 | (2.8 ± 0.2) × 107 | 4.1 | 1.9 |
Each value is the mean and standard deviation from three independent titrations of the same viral stock. Any grouping of two or more infected cells was counted as a plaque.
Calculated as titer on Vero cells divided by titer on complementing 143/1099E cells.
Calculated as titer on HEL299 cells divided by titer on complementing 143/1099E cells.
UL34 expression is required for efficient single-step growth of HSV-1.
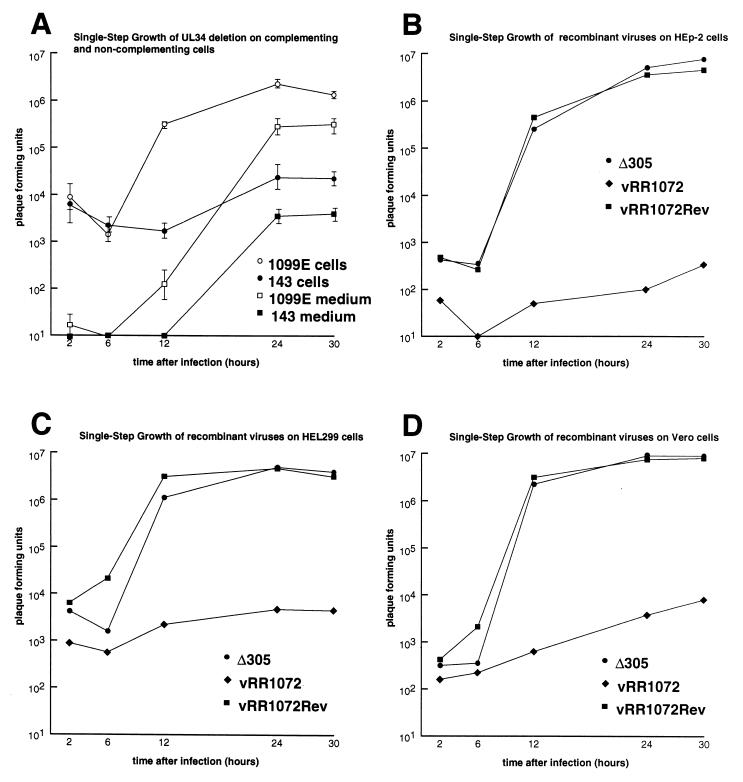
Plaque formation is a complex process whose success is determined by the efficiencies of single-step growth, viral egress, and cell-cell spread. To determine whether the deficiency in plaque formation might reflect a deficiency in infectious virus production, monolayer cultures of noncomplementing 143B and 143/1099E cells were infected with vRR1072 at a multiplicity of infection of 10, washed with PBS and treated with pH 3.0 buffer to remove and inactivate most of the residual virus, and incubated at 37°C to allow virus growth. At various times after infection, virus stocks were prepared from the total infected culture (cells and medium together) or from culture medium alone, and stocks were titered on complementing 143/1099E cells (Fig. 5A). Replication of vRR1072 on 143/1099E cells showed typical kinetics, with an increase of viral titer in the culture of several log orders of magnitude. The yield of virus on these cells was, however, about a log order of magnitude less than is typical of a wild-type virus on these cells (not shown), suggesting that complementation is incomplete. About 10% of the virus produced in complementing cell culture is released to the extracellular medium. On noncomplementing 143B cells, total viral yield in the culture is reduced more than 2 log orders of magnitude, suggesting that UL34 expression is necessary for efficient production of infectious virus. A similar fraction of the infectivity produced (about 10%), however, is released to the extracellular medium, suggesting that infectious particles produced can egress from the cell. To assess the cell type dependence of UL34 function, and to compare wild-type and deletion yields outside of a complementing cell system, single-step growth kinetics of the UL34-expressing Δ305 and vRR1072Rep and of the UL34 deletion virus were measured on HEp-2, Vero, and HEL299 cells (Fig. 5B to D). In all of these cell types, the deletion virus produced three to more than four log orders of magnitude less virus than Δ305. Replication of the repair virus was indistinguishable from that of Δ305, suggesting that the defect in single-step replication was due specifically to the deletion of UL34 sequences in vRR1072.
FIG. 5.
Single-step growth of UL34 deletion, wild-type, and repair viruses on different cell lines. Shown are plots of the logarithm of PFU of virus accumulated in total culture or in medium against time after infection. Cultures were infected, harvested, and titered on 143/1099E cells as described in Materials and Methods. (A) Growth and release of vRR1072 on noncomplementing 143B cells and complementing 143/1099E cells. Each point represents the mean of three independent experiments; error bars indicate the sample standard deviation. (B to D) Growth of Δ305, vRR1072, and vRR1072Rep on HEp-2 (B), HEL299 (C), and Vero (D) cells. Results of representative experiments are shown.
The defect in the UL34-negative virus is not due to a global failure of viral gene expression.
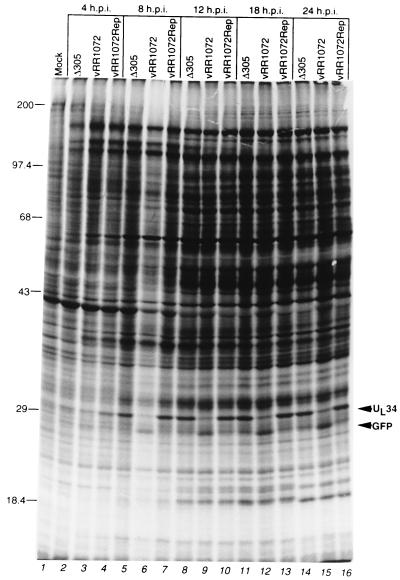
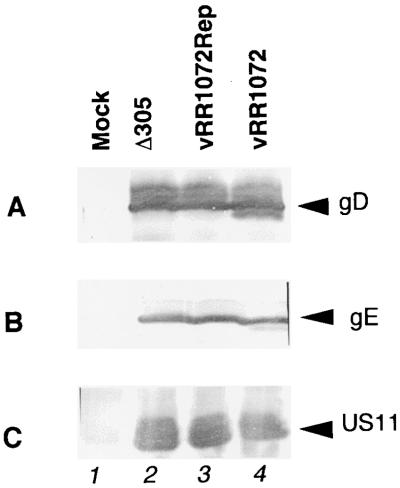
The kinetics and membrane association of UL34 protein suggest a role in virion maturation or egress, but the large defect in UL34-null virus replication is consistent with a variety of explanations, such as failure of steps prior to virion assembly, including DNA replication and gene expression. To test these possibilities Vero cells infected with Δ305, vRR1072, and vRR1072Rep were labeled with [35S]methionine for 2 h at various times after infection, and labeled proteins were separated by SDS-PAGE. Separated proteins were stained with Coomassie blue to assess loading (not shown) and visualized by autoradiography (Fig. 6). The patterns of protein synthesis in wild-type and UL34-negative viruses are highly similar, with two exceptions: (i) a band of the size expected for the UL34 protein is present in protein from cells infected with Δ305 and vRR1072Rep but not vRR1072, and (ii) a band of the size expected for GFP is present in protein from cells infected with vRR1072 but not Δ305 and vRR1072Rep. Some specific late proteins such as VP5 (at about 150,000) and VP16 (at about 68,000) are distinguishable in the patterns and are synthesized at the same level in cells infected with all viruses, suggesting that DNA replication and the replication-dependent late protein synthesis occur normally in cells infected with the deletion virus. To test specifically for late protein expression, SDS-PAGE-separated proteins from cells infected for 16 h with these same viruses were blotted to nitrocellulose and probed for gD (Fig. 7A), gE (Fig. 7B), and US11 (Fig. 7C). gD and gE are both gamma-1, or leaky-late, gene products; US11 is a gamma-2, or true-late, gene product (25, 41). Equivalent amounts of each of these proteins were accumulated in cells infected with all three viruses.
FIG. 6.
Protein synthesis in Vero cells infected with wild-type, UL34-negative, and repair viruses. Shown is an autoradiographic image of SDS-PAGE-separated proteins from Vero cells either mock infected (lane 1) or infected with the indicated virus for 4 h (lanes 2 to 5), 8 h (lanes 6 to 8), 12 h (lanes 9 to 11), 18 h (lanes 12 to 14), or 24 h (lanes 14 to 16) and labeled with [35S]methionine for 2 h prior to harvest. The migration positions of size standards (in kilodaltons) are indicated at the left. The migration positions of UL34 and GFP are indicated by arrowheads at the right. The overall lower level of label seen in lane 6 is due to a corresponding overall lower level of protein loaded in that lane. p.i., postinfection.
FIG. 7.
Accumulation of late gene products in wild-type, UL34-negative, and repair viruses. Photographic images show Western blots of proteins from Vero cells either mock infected (lane 1) or infected with the indicated virus for 16 h. (A) Blot probed with anti-gD monoclonal antibody; (B) blot probed with anti-gE monoclonal antibody; (C) blot probed with anti-US11 monoclonal antibody.
UL34 is not required for generation of DNA-containing capsids.
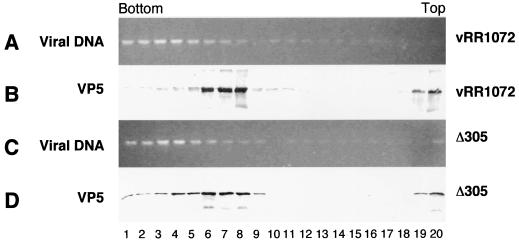
Nuclear lysates were made from cells infected for 16 h with Δ305 or vRR1072, digested with DNase, and fractionated on 15 to 45% sucrose gradients. To test for viral genomes, DNA was purified from aliquots of gradient fractions and separated on agarose gels (Fig. 8A and C). The sedimentation position of capsid structures was detected by Western blotting of TCA-precipitated aliquots of gradient fractions and probing with antibody directed against the major capsid protein VP5 (Fig. 8B and D). The amounts of DNase-resistant, rapidly sedimenting DNA are identical in wild-type and deletion mutant-derived gradients, suggesting that encapsidation of viral genomes occurs in cells infected with both viruses. Similarly, the amounts of rapidly sedimenting capsid protein are similar in nuclei of cells infected with both viruses. The greater amount of protein sedimenting as empty capsids in vRR1072-infected cells in this experiment was not a consistent feature of these experiments.
FIG. 8.
Nuclear capsids and encapsidated DNA in wild-type and UL34-negative virus. Photographic images show electrophoretically separated and ethidium bromide-stained DNA (A and C) and Western-blotted proteins (B and D) from sucrose gradient fractionation of DNase I-treated nuclear lysates from cells infected with either vRR1072 (A and B) or Δ305 (C and D).
Structural features of UL34 deletion virus-infected cells.
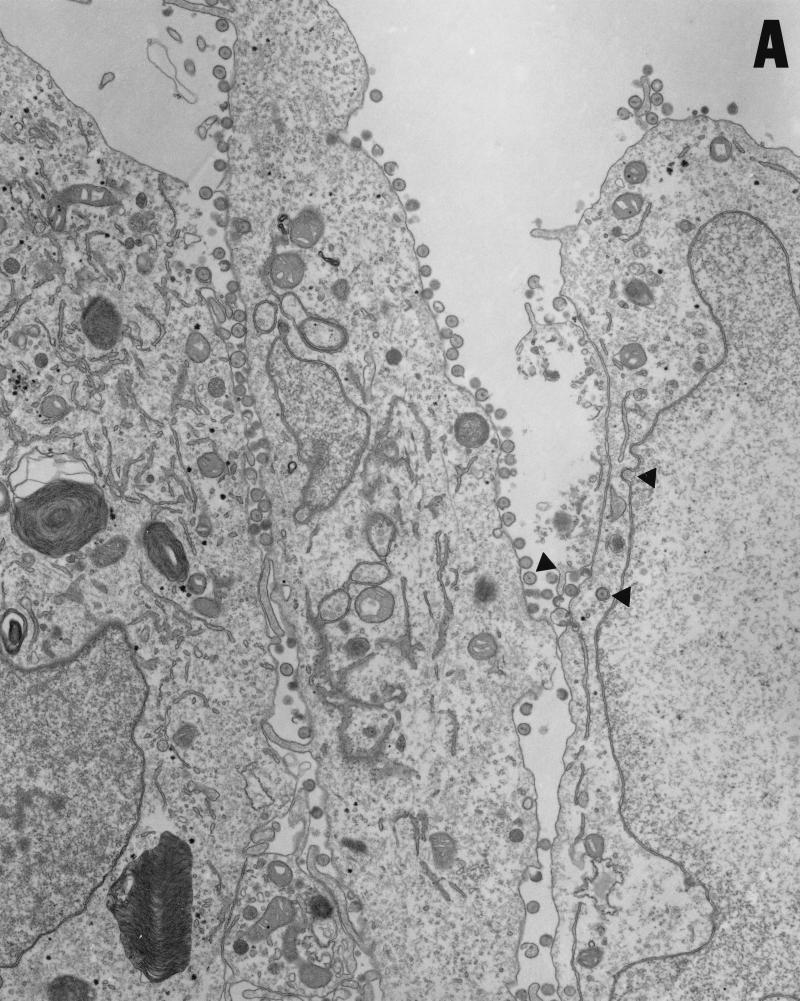
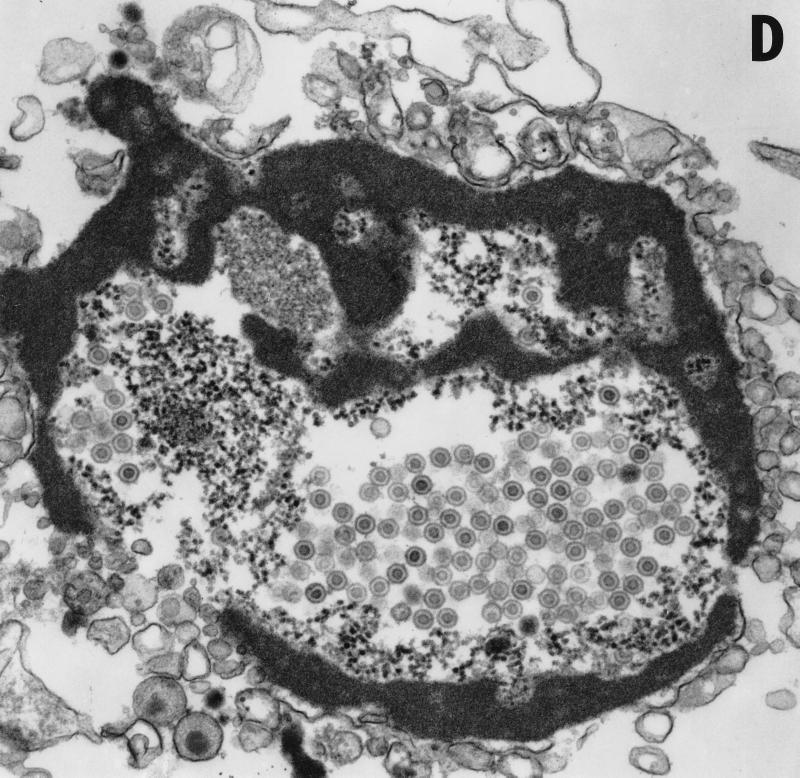
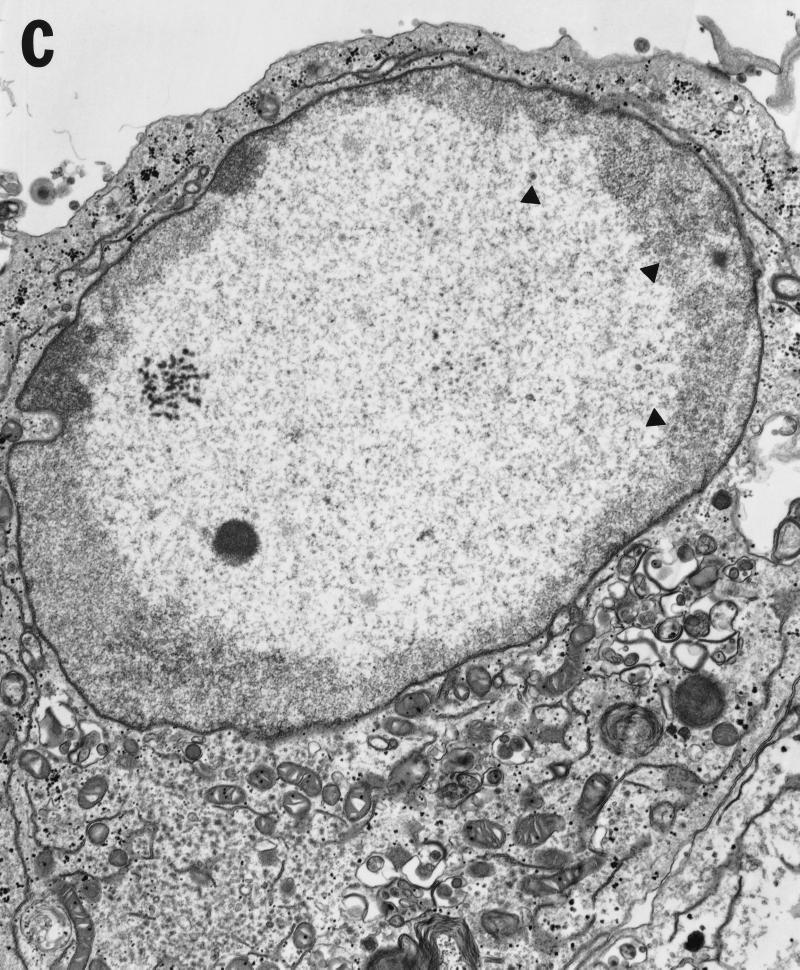
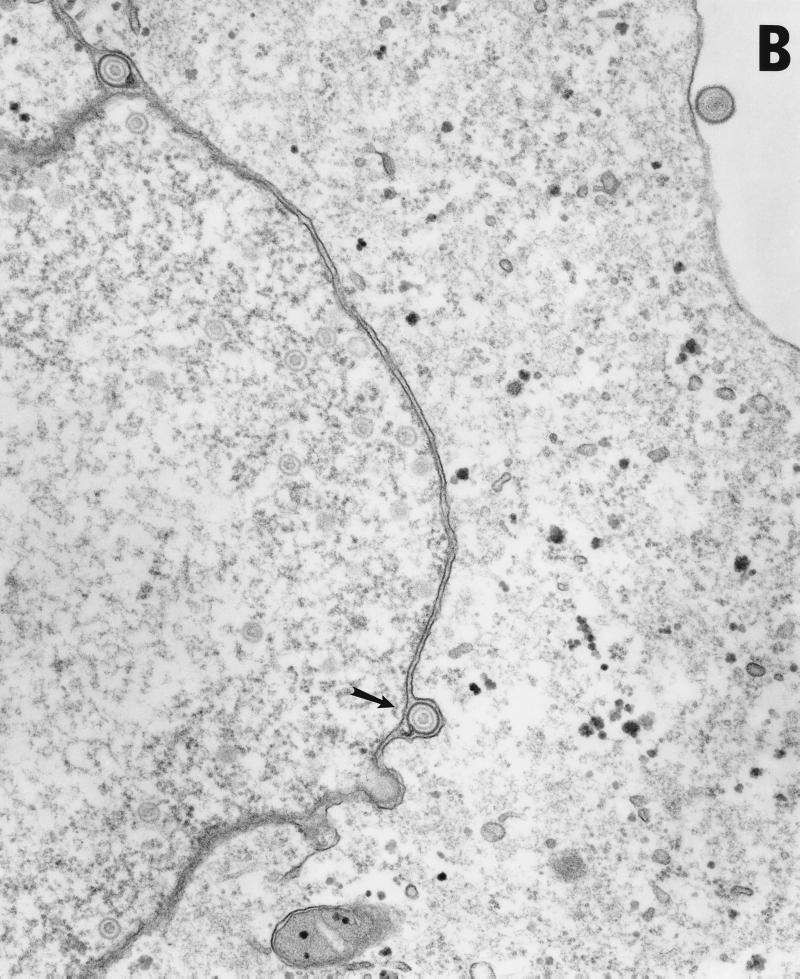
Vero cells were infected for 20 h with either HSV-1(F) or vRR1072(TK+), fixed with glutaraldehyde, and processed for transmission EM as described in Materials and Methods. The following features were noted. (i) In cells infected with both viruses, full and empty virus capsids are evident in the nucleus of the infected cell in comparable amounts (Fig. 9A to C). (ii) In cells infected with the wild-type virus, particles in a variety of stages of maturation are evident; these particle include capsids in the process of envelopment at the inner nuclear membrane (Fig. 9A), enveloped virus particles between the inner and outer nuclear membrane (Fig. 9B), enveloped virus particles in vesicles in the cytoplasm (Fig. 9A), and enveloped virus particles studding the surface of the cells and in the spaces between adjacent cells (Fig. 9A). Representative UL34 deletion-infected cells are shown in Fig. 9C. No enveloped virus particles were observed in any cellular compartment in the deletion virus-infected cells. (iii) In preparations of cells infected with UL34 deletion virus but not with wild-type virus, extracellular bodies that contain a large electron dense aggregate surrounding unenveloped capsids were observed (Fig. 9D). All of these were surrounded, but not completely bounded, by membranes.
FIG. 9.
Transmission EM analysis of cells infected with wild-type and UL34-negative virus. Micrographs show Vero cells infected with either HSV-1(F) (panels A and B) or vRR1072(TK+) (panels C to F) for 20 h. (A) Wild-type-infected Vero cells showing envelopment of capsid at the inner nuclear membrane, enveloped virus particles in vesicles in the cytoplasm, and enveloped virus at the surface of, and between cells. Examples of each are indicated by arrowheads. Final magnification is ×13,500. (B) Wild-type-infected Vero cell showing capsids in the nucleus and enveloped virus particles between the inner and outer nuclear membranes (one example indicated by arrow). Final magnification is ×40,500. (C) Deletion mutant-infected Vero cell showing capsids in the nucleus (a few examples indicated with arrowheads) but no cytoplasmic or cell-surface-associated enveloped virus particles. Final magnification is ×13,500. (D) Cell fragment from deletion mutant-infected Vero cells containing numerous viral capsids. Final magnification is ×40,500.
DISCUSSION
We have observed that deletion of coding sequences within the UL34 gene of HSV-1 results in defects in single-step growth and plaque formation in the deletion virus. The defect in plaque formation is likely to be caused by the profound defect in single-step viral replication. These phenotypes are specifically due to deletion of the UL34 sequences, since (i) Southern and Western blotting experiments show that UL34 protein coding sequences are missing in the deletion virus and that no detectable UL34 protein is expressed; (ii) the observed defects in plaque formation and single-step growth can be obviated either by complementation in trans or by homologous repair of the UL34 locus; and (iii) there is no evidence for a specific or general failure to express other viral proteins (Fig. 6). While the deletion virus accumulates UL33 mRNA somewhat more slowly than the wild-type virus, this is unlikely to be responsible for the phenotypes observed. The UL33 protein is essential for packaging of viral DNA into capsids in the nucleus (2, 13). Thus, any substantial defect in UL33 function should be reflected in failure to produce DNA-containing capsids, and its deletion is also associated with a profound defect in viral replication. The UL34 deletion virus, however, produces normal amounts of intranuclear DNA-containing capsids (Fig. 7), suggesting that the defect in single-step growth of vRR1072 is not due to an insufficiency of UL33 protein. The UL34 deletion virus also shows no impairment in accumulation of UL35 mRNA, suggesting that the sequences deleted in vRR1072 are not critical for UL35 promoter function. Although the single-step growth phenotype was measured with virus carrying mutations in the UL34, TK, and UL24 genes, the phenotype is unlikely to be due to a combination of mutations. The vRR1072(TK+) virus used for EM analysis, in which the TK and UL24 loci are repaired, shows the same defect in plaque formation as the TK− virus (not shown) and fails to produce enveloped virus particles (Fig. 9).
Several considerations suggest that the failure of single-step replication by the UL34 deletion virus is due to failure to envelop DNA-containing capsids at the inner nuclear envelope. (i) In noncomplementing cells, the virus successfully completes all of the events that precede envelopment during infection. All classes of viral protein are synthesized at normal levels, including late gene products, suggesting that there is no defect in overall gene expression or in replication of the viral DNA. Both EM and gradient fractionation of nuclear lysates provide evidence that the virus produces and packages viral DNA into capsids at normal levels. We cannot yet exclude the possibility that the DNA packaging observed in the absence of UL34 is not fully completed. Shao et al. observed that a virus unable to express the viral alkaline nuclease was also able to produce normal amounts of B and C capsids in the nucleus but was unable to envelop those capsids (42). They suggested that this failure might be due to a defect in a very late stage of DNA packaging. Because of the association of UL34 with cellular membranes, we favor the hypothesis that UL34 is responsible for recruiting or mediating interactions between some components of the envelopment complex. (ii) EM analysis of cells infected with the UL34 deletion virus shows no evidence of enveloped virus particles either in the cell cytoplasm or on the surface of the cells. Defects in maturation of the virus particles that occur later than primary envelopment should be evidenced by the presence of enveloped or unenveloped virus particles in the cytoplasm as seen in UL20 or gK deletion viruses (5, 22, 24). Though enveloped virus particles were not observed in EM analysis of deletion virus-infected cells, due to their rarity, the results of single-step growth analysis suggest that infectious particles are produced at low levels in all cells tested. At least in 143B cells, a normal fraction of these particles is released to the extracellular medium with kinetics similar to that seen for wild-type virus. It is not clear whether the virus released to the medium in the noncomplementing cells represents the product of normal egress or, possibly, the result of the disruption of infected cells that gives rise to the cellular remnants observed in deletion virus-infected cultures (Fig. 9F).
A second unusual feature of the deletion virus-infected cells is production of cellular remnants containing highly condensed material, possibly derived from the nucleus, surrounding an accumulation of capsids and surrounded by cellular membranes. These remnants are very similar to so-called apoptotic bodies (reviewed in reference 12), which also have shrunken nuclei containing highly condensed chromatin surrounded by a condensed cytoplasm. The UL34 protein is a substrate for the US3 protein kinase. This kinase has been reported to play a role in protection of infected cells from apoptosis (3, 20, 27). It is possible that UL34 protein participates in the antiapoptotic function of US3. In HSV-1, deletion of the US3 gene does not result in detectable impairment of single-step viral replication (31), suggesting that phosphorylation of UL34 by US3 is not required for the essential function of UL34 in viral maturation. However, it has been reported that in pseudorabies virus (PRV), deletion of the US3 homolog results in a defect in viral egress (44). While it is not yet known if a PRV UL34 homolog exists and is phosphorylated by the PRV US3 homolog, it is tempting to speculate that the egress defect observed in US3-negative PRV may be due to a failure to phosphorylate UL34.
The UL34 gene is conserved in the genomes of all human herpesviruses, suggesting that it may function similarly in these diverse viruses. The function may not, however, be identical in all species. It is clear that there are critical species-dependent differences in the pathways for egress, since the phenotypes of similar mutations are species specific. For example, the UL20 gene products of HSV and PRV are both required for trafficking of enveloped virus particles to the cell surface. Deletions that remove these genes result in accumulation of enveloped particles within intracellular membranes (5, 18). The sites of accumulation differ, however, with HSV accumulating in the perinuclear space and PRV accumulating in cytoplasmic vesicles. The UL3.5 genes of PRV and bovine herpesvirus 1 appear to function in reenvelopment of nucleocapsids in the cytoplasm of infected cells, but no homolog of this gene is found in HSV (17, 19). Whether the function of the HSV-1 UL34 gene product is identical to that of the counterpart gene products of other herpesviruses remains to be determined.
ACKNOWLEDGMENTS
This work was funded by the University of Iowa, by Research Project grant RPG-97-070-01-VM from the American Cancer Society, and by PHS award 1 RO1 A141478-01A2.
We are grateful to Bernard Roizman for providing viruses and plasmids. We are grateful to Jean Ross of the Central Microscopy Research Facility of the University of Iowa for excellent technical assistance and to other members of our laboratory for invaluable discussions and for critical reading of the manuscript.
REFERENCES
- 1.Ackermann M, Chou J, Sarmiento M, Lerner R A, Roizman B. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J Virol. 1986;58:843–850. doi: 10.1128/jvi.58.3.843-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.al-Kobaisi M F, Rixon F J, McDougall I, Preston V G. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology. 1991;180:380–388. doi: 10.1016/0042-6822(91)90043-b. [DOI] [PubMed] [Google Scholar]
- 3.Asano S, Honda T, Goshima F, Watanabe D, Miyake Y, Sugiura Y, Nishiyama Y. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J Gen Virol. 1999;80:51–56. doi: 10.1099/0022-1317-80-1-51. [DOI] [PubMed] [Google Scholar]
- 4.Baines J D, Roizman B. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J Virol. 1992;66:5168–5174. doi: 10.1128/jvi.66.8.5168-5174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines J D, Ward P L, Campadelli-Fiume G, Roizman B. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J Virol. 1991;65:6414–6424. doi: 10.1128/jvi.65.12.6414-6424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Chee M S, Hutchinson III C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. The DNA sequence of the human cytomegalovirus genome. DNA Seq. 1991;2:1–12. doi: 10.3109/10425179109008433. [DOI] [PubMed] [Google Scholar]
- 7.Brown M, MacLean A R, Aitken J D, Harland J. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J Gen Virol. 1994;75:3679–3686. doi: 10.1099/0022-1317-75-12-3679. [DOI] [PubMed] [Google Scholar]
- 8.Bruni R, Roizman B. Open reading frame P—a herpes simplex virus gene repressed during productive infection encodes a protein that binds a splicing factor and reduces synthesis of viral proteins made from spliced mRNA. Proc Natl Acad Sci USA. 1996;93:10423–10427. doi: 10.1073/pnas.93.19.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Y E, Van Sant C, Krug P W, Sears A E, Roizman B. The null mutant of the UL31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J Virol. 1997;71:8307–8315. doi: 10.1128/jvi.71.11.8307-8315.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou J, Poon A P, Johnson J, Roizman B. Differential response of human cells to deletions and stop codons in the γ134.5 gene of herpes simplex virus. J Virol. 1994;68:8304–8311. doi: 10.1128/jvi.68.12.8304-8311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Church G A, Wilson D W. Study of herpes simplex virus maturation during a synchronous wave of assembly. J Virol. 1997;71:3603–3612. doi: 10.1128/jvi.71.5.3603-3612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen J J. Apoptosis. Immunol Today. 1993;14:126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham C, Davison A J. A cosmid-based system for constructing mutants of herpes simplex virus type 1. Virology. 1993;197:116–124. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- 14.Darlington R W, Moss L H. Herpesvirus envelopment. J Virol. 1968;2:49–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 16.Dolan A, Jamieson F E, Cunningham C, Barnett B C, McGeoch D J. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs W, Granzow H, Mettenleiter T C. Functional complementation of UL3.5-negative pseudorabies virus by the bovine herpesvirus 1 UL3.5 homolog. J Virol. 1997;71:8886–8892. doi: 10.1128/jvi.71.11.8886-8892.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs W, Klupp B G, Granzow H, Mettenleiter T C. The UL20 gene product of pseudorabies virus functions in virus egress. J Virol. 1997;71:5639–5646. doi: 10.1128/jvi.71.7.5639-5646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs W, Klupp B G, Granzow H, Rziha H J, Mettenleiter T C. Identification and characterization of the pseudorabies virus UL3.5 protein, which is involved in virus egress. J Virol. 1996;70:3517–3527. doi: 10.1128/jvi.70.6.3517-3527.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvan V, Roizman B. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc Natl Acad Sci USA. 1998;95:3931–3936. doi: 10.1073/pnas.95.7.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchinson L, Johnson D C. Herpes simplex virus glycoprotein K promotes egress of virus particles. J Virol. 1995;69:5401–5413. doi: 10.1128/jvi.69.9.5401-5413.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson J G, Martin S L, Coen D M. A conserved open reading frame that overlaps the herpes simplex virus thymidine kinase gene is important for viral growth in cell culture. J Virol. 1989;63:1839–1843. doi: 10.1128/jvi.63.4.1839-1843.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayachandra S, Baghian A, Kousoulas K G. Herpes simplex virus type 1 glycoprotein K is not essential for infectious virus production in actively replicating cells but is required for efficient envelopment and translocation of infectious virions from the cytoplasm to the extracellular space. J Virol. 1997;71:5012–5024. doi: 10.1128/jvi.71.7.5012-5024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson P A, MacLean C, Marsden H S, Dalziel R G, Everett R D. The product of gene US11 of herpes simplex virus type 1 is expressed as a true late gene. J Gen Virol. 1986;67:871–883. doi: 10.1099/0022-1317-67-5-871. [DOI] [PubMed] [Google Scholar]
- 26.Lamberti C, Weller S K. The herpes simplex virus type 1 cleavage/packaging protein UL32 is involved in efficient localization of capsids to replication compartments. J Virol. 1998;72:2463–2473. doi: 10.1128/jvi.72.3.2463-2473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNab A R, Desai P, Person S, Roof L L, Thomsen D R, Newcomb W W, Brown J C, Homa F L. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J Virol. 1998;72:1060–1070. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poon A P, Roizman B. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J Virol. 1993;67:4497–5503. doi: 10.1128/jvi.67.8.4497-4503.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Post L E, Roizman B. A generalized technique for deletion of specific genes in large genomes: a gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 31.Purves F C, Longnecker R M, Leader D P, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purves F C, Spector D, Roizman B. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J Virol. 1991;65:5757–5764. doi: 10.1128/jvi.65.11.5757-5764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purves F C, Spector D, Roizman B. UL34, the target of the herpes simplex virus US3 protein kinase, is a membrane protein which in its unphosphorylated state associates with novel phosphoproteins. J Virol. 1992;66:4295–4303. doi: 10.1128/jvi.66.7.4295-4303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randall G, Lagunoff M, Roizman B. The product of ORF O located within the domain of herpes simplex virus 1 genome transcribed during latent infection binds to and inhibits in vitro binding of infected cell protein 4 to its cognate DNA site. Proc Natl Acad Sci USA. 1997;94:10379–10384. doi: 10.1073/pnas.94.19.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 36.Roller R, Roizman B. Herpes simplex virus 1 RNA binding protein US11 negatively regulates the accumulation of a truncated viral RNA. J Virol. 1991;65:5873–5879. doi: 10.1128/jvi.65.11.5873-5879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roller R, Roizman B. A herpes simplex virus 1 US11-expressing cell line is resistant to herpes simplex virus infection at a step in viral entry mediated by glycoprotein D. J Virol. 1994;68:2830–2839. doi: 10.1128/jvi.68.5.2830-2839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roller R J, Roizman B. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J Virol. 1992;66:3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmon B, Cunningham C, Davison A J, Harris W J, Baines J D. The herpes simplex virus type 1 UL17 gene encodes virion tegument proteins that are required for cleavage and packaging of viral DNA. J Virol. 1998;72:3779–3788. doi: 10.1128/jvi.72.5.3779-3788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanders P G, Wilkie N M, Davison A J. Thymidine kinase deletion mutants of herpes simplex virus type 1. J Gen Virol. 1982;63:277–295. doi: 10.1099/0022-1317-63-2-277. [DOI] [PubMed] [Google Scholar]
- 41.Sethna M, Weir J P. Mutational analysis of the herpes simplex virus type 1 glycoprotein E promoter. Virology. 1993;196:532–540. doi: 10.1006/viro.1993.1508. [DOI] [PubMed] [Google Scholar]
- 42.Shao L, Rapp L M, Weller S K. Herpes simplex virus 1 alkaline nuclease is required for efficient egress of capsids from the nucleus. Virology. 1993;196:146–162. doi: 10.1006/viro.1993.1463. [DOI] [PubMed] [Google Scholar]
- 43.Telford E A, Watson M S, McBride K, Davison A J. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 44.Wagenaar F, Pol J M, Peeters B, Gielkens A L, de Wind N, Kimman T G. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J Gen Virol. 1995;76:1851–1859. doi: 10.1099/0022-1317-76-7-1851. [DOI] [PubMed] [Google Scholar]