Abstract
Introduction
Home parenteral nutrition (HPN) is the primary treatment modality for patients with chronic intestinal failure, one of the least common organ failures. This article provides a retrospective analysis of the data collected on HPN patients in the Czech Republic over the past 30 years.
Methods
National registry data were collected using a standardised online form based on the OASIS registry (Oley – A.S.P.E.N. Information System) across all centres providing HPN in the Czech Republic. Data collected prospectively from adult patients in the HPN program were analysed in the following categories: epidemiology, demographics, underlying syndrome, diagnosis, complications, and teduglutide therapy prevalence.
Results
The registry identified a total of 1,838 adult patient records, reflecting almost 1.5 million individual catheter days. The prevalence of HPN has risen considerably over the last few decades, currently reaching 5.5 per 100,000 population. The majority of patients have short bowel syndrome and GI obstruction, with cancer being the most prevalent underlying disease. Catheter-related bloodstream infections have been the most prevalent acute complication. However, the incidence in 2022 was only 0.15 per 1,000 catheter days. The study also observed an increase in the prevalence of patients on palliative HPN over the last decade.
Conclusion
This study presents a thorough analysis of data from the Czech REgistr Domaci NUtricni Podpory (REDNUP) registry. It shows an increasing prevalence of HPN, namely, in the palliative patient group. The sharing of national data can improve understanding of this rare condition and facilitate the development of international guidelines.
Keywords: Home parenteral nutrition, Chronic intestinal failure, National registry, Epidemiology
Introduction
In recent decades, home parenteral nutrition (HPN) has advanced considerably. It is used as a life-saving therapy for patients with chronic intestinal failure (CIF) [1]. By facilitating nutrient intake outside hospital settings, HPN improves patient survival rates and enhances their quality of life [2]. However, despite its critical importance, comprehensive, long-term studies of HPN usage and its impacts are scarce.
In the Czech Republic, HPN was first used as an experimental treatment in 1988 [3]. In 1993, the National Registry of Home Parenteral Nutrition (REgistr Domaci NUtricni Podpory [REDNUP]) was established, making it one of the earliest national registries worldwide dedicated solely to HPN patients. The purpose of the registry was to organise data collection, standardise HPN-related procedures, and promote the technique as an efficient and cost-effective option for healthcare providers and insurance companies.
National registries, such as REDNUP, provide unbiased insights into the epidemiology of CIF, despite the limitation of missing data. This information is invaluable for healthcare professionals and policy-makers as well as researchers. Although many other countries have already established their registries [4–8], there is still a noticeable lack of published data.
In the Czech Republic, HPN is only administered in specialised HPN centres. The HPN working group, which operates under the Czech Society for Clinical Nutrition and Intensive Metabolic Care (Společnost Klinické Výživy a Intenzivní Metabolické Péče, SKVIMP), is responsible for harmonising national protocols. At a European level, the European Society of Parenteral and Enteral Nutrition (ESPEN) oversees the process, publishes guidelines, collects data, and educates professionals [9]. The SKVIMP provides internal guidelines to centres and certifies home care agencies and other HPN care providers.
This study presents a detailed analysis of the REDNUP, which has not yet been widely published. It provides valuable insights into the evolution of HPN therapy in the Czech Republic over the past 30 years and the current status in 2022.
Methods
The aim of the retrospective analysis of data from REDNUP was to evaluate the care provided to adult patients receiving HPN and their outcomes in the Czech Republic, which has a population of roughly 10 million inhabitants. REDNUP has been collecting data on HPN for the last 30 years. It was founded in 1993, and its structure was inspired by the OASIS registry (Oley – A.S.P.E.N. Information System), a joint effort of the Oley Foundation and A.S.P.E.N., which operated from 1985 to 1992 [10]. Patient data are entered into the REDNUP online database by medical professionals on a voluntary basis. The responsibility for data input lies with each individual centre, although it is coordinated by the SKVIMP.
The database is located on the server of the University Hospital Královské Vinohrady (UHKV) in Prague, and patient data are entered pseudonymously. In the Czech Republic, there are currently 28 centres providing HPN, 21 of which care for adult patients and 7 for paediatric patients.
The REDNUP database requires annual data input, summarising the care provided over the previous year. The database is organised into several sections, including basic socio-demographic characteristics (such as age, sex, and economic activity), prognostic categories, underlying syndrome and diagnosis, and details about the parenteral support administered.
In REDNUP, CIF is classified into major categories based on diagnosis and syndrome. There are seven major diagnostic groups: Crohn’s disease, mesenteric infarction, non-cancer pancreatopathy, pseudo-obstruction, non-cancer surgical condition, radiation enteritis, and cancer. The syndrome categories include malabsorption, bowel obstruction, fistula, anorexia, and short bowel syndrome (SBS). They indicate the pathophysiological background of the disease. Additional data are collected for patients with SBS, including the type of resection and the length of the remaining bowel, which are crucial for treatment management and prognosis.
REDNUP also monitors other variables, such as the type of parenteral nutrition (PN) (commercial or individually prepared by a pharmacy) and the number of parenteral bags or hydromineral support bags provided per week, the type of catheter used for HPN administration, hospitalisations related to HPN, as well as any associated complications. These are divided into catheter-related (sepsis, catheter exit site infection, thrombosis, occlusion) and metabolic complications (incidence of hepatopathy, metabolic bone disease).
The patient’s file is closed when HPN treatment ends. This may happen after restoring intestinal autonomy (weaning off HPN) through conservative therapy, after surgical reconstruction of the digestive tract, or if the patient passes away.
Additionally, since 2019, REDNUP has been collecting data on patients prescribed the glucagon-like peptide-2 (GLP-2) analogue teduglutide, which is approved and reimbursed in the Czech Republic for SBS. Patients who receive this drug are monitored at 3-month intervals. During these check-ups, detailed data are collected, including body weight, drug dosage, parenteral support, the energy content of PN per week, and any drug complications. This allows for a more precise evaluation of the effectiveness of teduglutide therapy.
Statistics
Statistical data processing was carried out using the programme R version 4.3.1d [11] with the graphical interface RStudio version 2023.06.2 [12]. Data manipulation and visualisation were performed using the tidyverse version 2.0.0 [13] collection of libraries for data science. Survival analysis (Kaplan-Meier estimator) and achievement of weaning (cumulative hazard function) were performed using the survival library version 3.5–7 [14].
Exploratory data analysis was conducted for all parameters. Mean and standard deviation are used to present continuous data, while counts and percentages are used for categorical data.
The duration of parenteral support received by a patient in the HPN programme is expressed in catheter days. The prevalence of HPN-associated events is then expressed as the number of events per 1,000 catheter days for the total population.
Results
Epidemiology and Demography
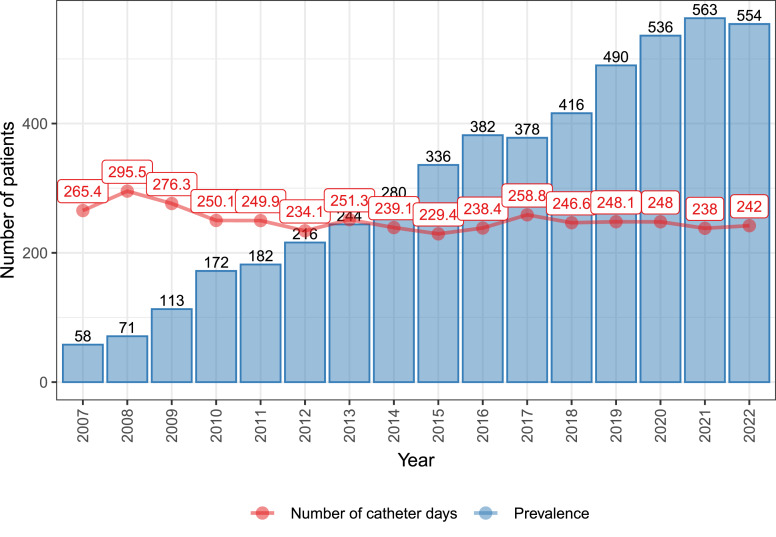
Since it was established, REDNUP has recorded data from 1,838 adult patients, with a total of 1,430,959 catheter days recorded since 2007. The point prevalence of HPN in 2022 was 5.5 per 100,000 citizens, as shown in Figure 1, which also displays the average number of catheter days per patient per year. The figure presents data starting from 2007 as there were only 59 patients in the register before this year, with important missing data.
Fig. 1.
Per-year prevalence of HPN patients and the average number of catheter days per patient during the years 2007–2022.
The prevalence of HPN has been steadily increasing, while the average number of catheter days per patient has remained stable. In 2022, 198 new patients were initiated on HPN, corresponding to an incidence rate of 1.98 per 100,000 in the Czech Republic. The adult patients have an average age of 57.5 ± 15.4 years, with the majority of them being female (58.1%).
Prognostic Classification of Patients on HPN
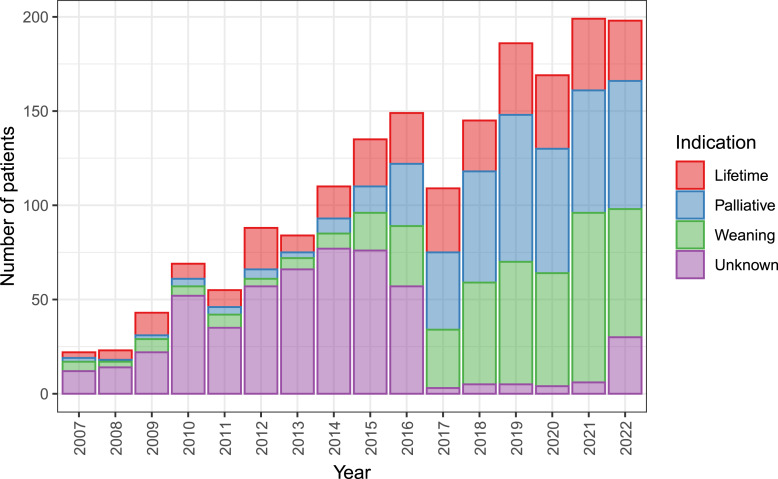
The distribution of patients across prognostic categories is shown in Figure 2. At the start of HPN, patients are classified into one of 3 groups based on the expected HPN prognosis and duration. This not only denotes the expected outcomes of HPN but also helps with care management and future treatment planning. The first group, which makes up approximately 20% of the cohort, consists of patients for whom all options to restore bowel function have been exhausted, and therefore, lifetime dependence is anticipated. The second group, accounting for approximately 40% of indicated patients, has considerable potential for further bowel adaptation or planned surgical reconstruction and weaning from HPN and is therefore expected to be only temporarily on parenteral support. The final 40% of the patients are receiving HPN in a palliative regimen. The figure also includes a group of patients with an unknown prognosis due to incomplete data entry. Recently, there has been a decline in patients who require lifetime dependence on parenteral support and an increase in patients indicated for primary weaning or palliative regimens.
Fig. 2.
Annual incidence of patients, i.e., new patients per year, according to the prognostic classification at the HPN initiation. Patients are expected to be lifetime-dependent, to be weaned off HPN and palliative HPN. Note: The category was newly introduced to the registry in 2017, and the preceding years were collected retrospectively.
Indications for HPN
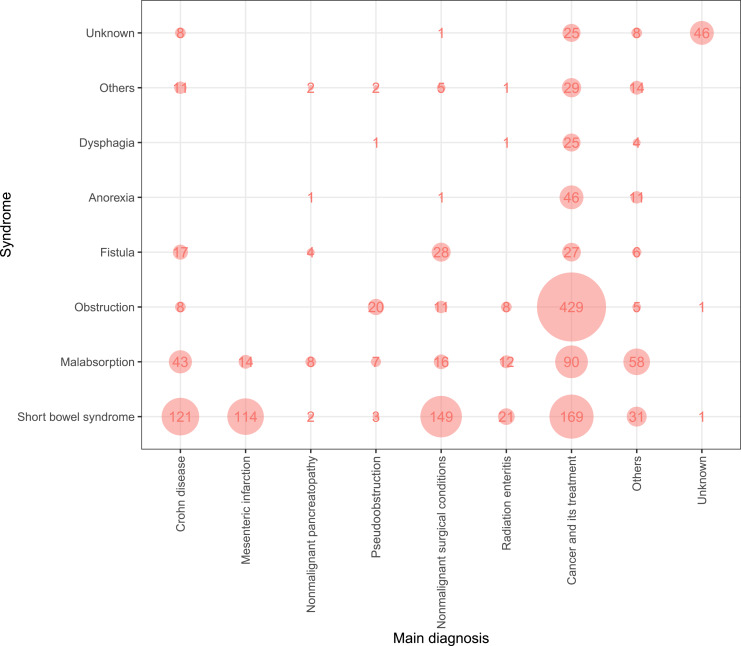
Figure 3 shows the main diagnoses and syndromes that characterise CIF. The most common syndrome underlying CIF in HPN patients is SBS, accounting for 36.6% of the cases, followed by GI obstruction at 28.9% and malabsorption at 14.9%. The most frequent diagnosis in the REDNUP cohort is cancer, accounting for 50.4%, followed by non-malignant surgical conditions and Crohn’s disease with 12.6% and 12.5%, respectively. However, to obtain a more comprehensive overview of HPN patients, it is useful to combine the diagnosis and syndrome categories. The largest group of patients (25.7%) suffer from GI tract obstruction due to cancer. This is followed by patients with SBS resulting from tumour-related bowel surgery (10.1%), non-malignant surgical conditions (complication of intra-abdominal nontraumatic disorders or abdominal injuries, 8.9%), Crohn’s disease (7.3%), and mesenteric infarction (6.8%).
Fig. 3.
Prevalence of syndromes over diagnosis across all patients in the REDNUP (n = 1,838).
The most common type of SBS is jejunostomy (type 1, 57.8%), followed by other surgical placements (18.3%), jejuno-coloanastomosis (type 2, 16.3%) and jejuno-ileoanastomosis (type 3, 4.5%), with 3.1% remaining unknown. On average, the remnant length of ileum is 104.3 ± 68.4 cm, with longer remnants in type 1 SBS and shorter remnants in types 2 and 3 (data not shown).
Catheters and Parenteral Support
Most of the patients have a Broviac-type tunnelled central venous catheter (49.1%), followed by PICC (24.1%) and venous port (19.8%), with the remaining catheter types unspecified in the reports. The most commonly used parenteral infusion type is commercially prepared (69.7%), whereas 24.7% are individually prepared pharmacy admixtures. Patients receive PN daily (7/7) in 66.9%, 5–6 times a week in 11.1%, 3–4 times in 12.7%, and 2 times or less in 6.3%.
Complications
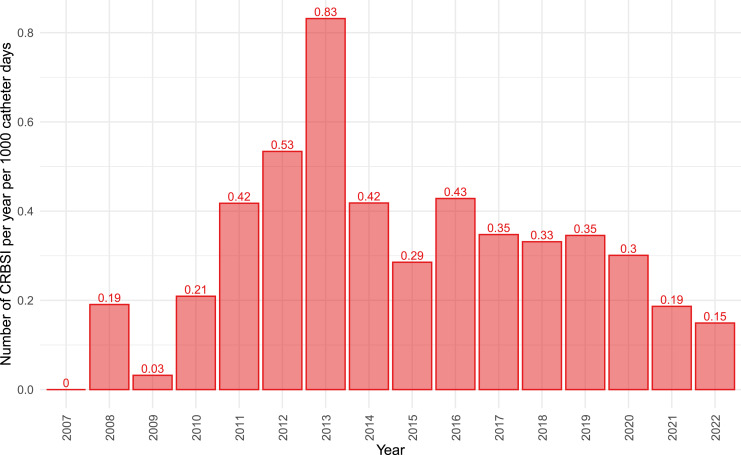
Complications are a significant concern when providing HPN as they can worsen health outcomes and reduce patients’ quality of life. Additionally, managing complications inevitably leads to increased costs. The most severe catheter-related complication is catheter-related bloodstream infection, which decreased to 0.15 events per 1,000 catheter days in 2022 (Fig. 4). Other significant catheter-related complications include catheter occlusion or thrombosis. The incidence rates for the year 2022 are 0.15 and 0.04 events per 1,000 catheter days, respectively.
Fig. 4.
Catheter-related bloodstream infection incidence in years, in events per 1,000 catheter days.
Long-Term Metabolic Complications
Intestinal failure-associated liver disease is a severe complication of PN, diagnosed in 22.3% of REDNUP patients. The most frequent manifestation is chronic cholestasis (65.2%), while steatosis and fibrosis occur less frequently, occurring in 35.3% and 14% of the patients with intestinal failure-associated liver disease, respectively.
Metabolic bone disease is another long-term complication, while diagnosis is based on a decline in bone density. This syndrome was diagnosed in 15.6% of patients, most of them having the diagnosis of radiation enteritis.
Duration of Administration of HPN
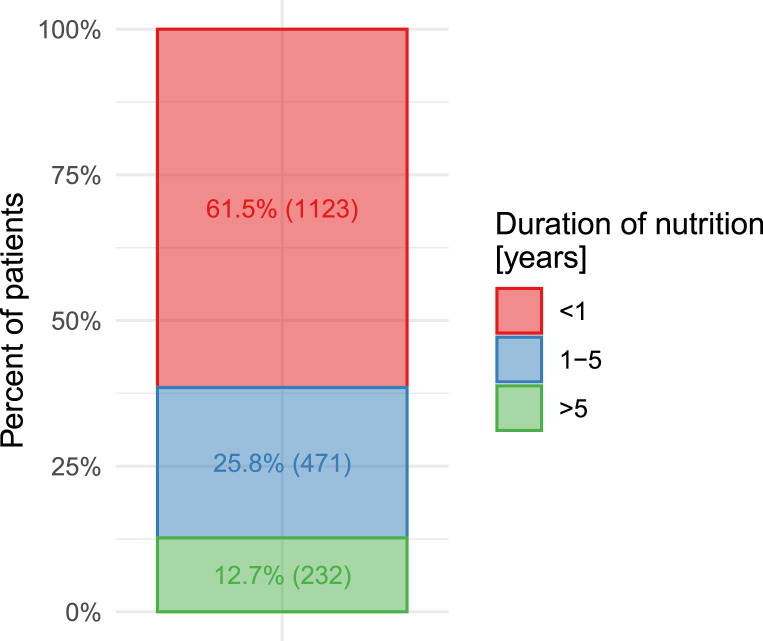
The duration of HPN administration in patients recorded in REDNUP is approximately 2 years on average. However, this average does not provide an accurate representation of the patients’ status. To obtain a more representative overview, patients can be categorised according to time periods of less than 1 year, 1–5 years, and more than 5 years, as shown in Figure 5. The majority of patients fall into the category of less than 1 year, with only a small percentage receiving HPN for more than 5 years.
Fig. 5.
Duration of HPN administration (n = 1,826).
Prognosis of Weaning and Survival of Patients on HPN
Weaning is the successful transfer of patients onto a form of enteral nutrition, signifying the end of their dependence on infusion therapy. In the first year, approximately 28% of patients achieve intestinal autonomy, increasing to about 45% after 5 years from the whole sample.
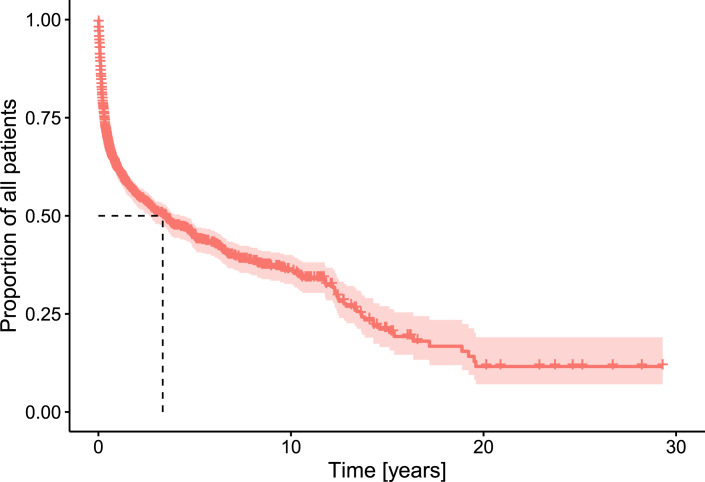
Patient survival rates are around 62% in the first year, 44% at 5 years, and 35% at the 10-year mark, as shown in Figure 6. The mortality rate is highest during the first 2 years and mainly comprises of palliative HPN.
Fig. 6.
Survival of patients on HPN. Kaplan-Meier analysis (n = 1,838).
Teduglutide Treatment Prevalence
In 2019, teduglutide, a GLP-2 receptor agonist treatment, was approved in the Czech Republic. It became more widely available after insurance companies agreed to provide its reimbursement. Since then, 22 adult patients have started this treatment. After 12 months, the required PV volume reduced to 60.5% of baseline values.
Discussion
The HPN programme in the Czech Republic has undergone a significant evolution since it was put in place 30 years ago. As the number of patients increased, so did the organisation of care. Specialised teams in HPN centres now provide care that is accessible to patients throughout the country. This increase is also evident in publications from other countries [15–19]. Reports worldwide have described considerable variability in the prevalence of HPN, ranging from 0.6 to 7.5 per 100,000 inhabitants in more recent reports [4, 20]. The Czech Republic has one of the highest prevalence rates, which may be attributed to the comprehensive reimbursement provided by health insurance companies, resulting in high patient numbers and greater access to HPN. Furthermore, the extensive network of HPN centres ensures nationwide accessibility for patients. This phenomenon may also be attributed to the presence of HPN-certified home care agencies. The standard of care is maintained through the implementation of uniform care guidelines by the SKVIMP. In the past decade, the most significant increase has been observed in the palliative HPN setting, which has co-evolved with improved survival rates for cancer patients.
Indication
Over the years, there has been an observed change in the spectrum of patients indicated for treatment. With the increased options for bowel reconstruction and adaptation, more patients are being scheduled primarily for early weaning. Palliative indications are also on the rise, as reported in other countries [15, 16, 21]. The most common diagnosis for patients with CIF in the Czech Republic is cancer, which is consistent with other countries [6, 15, 21–24]. In the international ESPEN study [25], Crohn’s disease was the most frequently reported diagnosis. It is important to note that diagnostic categories may not be uniform across countries, which could have an impact on the results. SBS is the most common underlying syndrome of intestinal failure in all reports, with a range of 30–65.3% [6, 16, 21, 23, 25].
Complications
The incidence of catheter-related bloodstream infection, the most severe acute complication, has been reported in the literature to range from 0.38 to 4.58 per 1,000 catheter days [22, 26, 27]. The Czech registry data show a gradual decline since 2013 when antiseptic catheter luminal flushes with taurolidine were endorsed by national guidelines and were widely introduced into clinical practice. The prevalence of long-term complications increases with the duration of HPN administration. Hepatobiliary complications have been reported in 0–50% of patients on HPN [28, 29]. The wide range is due to inconsistent diagnostic criteria, making comparisons between countries difficult [30]. Metabolic bone disease is typically diagnosed using dual X-ray absorptiometry according to the WHO criteria for osteoporosis. A study examining its prevalence found it in 41% of the patients studied [31], which is higher than in REDNUP. However, many patients with this complication go undetected because bone densitometry screening is typically performed 1 year after initiating the HPN programme, thus excluding short-term HPN admissions.
Survival and Weaning
The survival rates of patients with HPN worldwide are 85–91.8% and 53–69.3% in the first and fifth year, respectively. However, these studies excluded patients with malignant disease or those in palliative care [16, 21]. In the REDNUP analysis, this group was not excluded, and survival in the first year appears to be significantly lower (75%). Data on the development of PN dependence are available from a study conducted at the Glasgow Centre. The study found that 73% of patients developed dependence in the first year, or 56% in 5 years, which is comparable to the situation in the Czech Republic [16].
Treatment with GLP-2 Analogues
PN can cause serious complications, particularly over the long term. Patients’ quality of life improves with reduced days spent on parenteral support. Therefore, efforts are underway to reduce PN dependence. The current drug approved for enhancing intestinal adaptation in SBS patients is the GLP-2 analogue teduglutide. In the Czech Republic, it has been approved for non-palliative SBS patients who still require parenteral support after spontaneous bowel adaptation. Patients who receive this drug are treated at specialised SBS treatment centres. According to Czech reimbursement guidelines, drug administration should be discontinued if a 20% reduction in PV volume is not achieved after 52 weeks of use. In the REDNUP analysis, 73% of treated patients met the criteria. Further data are required to evaluate the long-term treatment efficacy of this drug. The database created by ESPEN serves this purpose [32].
Limitations
While REDNUP provides a detailed 30-year insight into HPN treatment in the Czech Republic, it is important to acknowledge certain limitations. The most significant limitation is the underreported and missing clinical data, which constrains the analytical scope. This is largely due to the voluntary nature of data entry and past interface conversions. The process of completing patient charts, performed voluntarily by medical staff in individual centres, has become time-consuming and can lead to errors and incomplete records, a common issue in other analyses as well [33]. This can result in an overestimation of the number of active patients and catheter days. Therefore, incidence data provide a more accurate overview of the current status. However, the strength of REDNUP lies in its prospective longitudinal data collection, which enables analyses over time and represents state-of-the-art HPN programmes across the Czech Republic. These data are crucial for negotiating with third-party payers for HPN care reimbursement and is presented biannually at national meetings of HPN providers. On an international level, it can be used to inform and compare experiences across countries.
Conclusion
In conclusion, this study presents the first comprehensive analysis of adult patient data from the national REDNUP registry. Over the past 3 decades, the prevalence of patients requiring HPN has increased in the Czech Republic, as well as in other countries. The number of patients with HPN is comparable to those in other countries, with SBS being the most common pathophysiological syndrome and increasing palliative indication for PN. In recent years, there have been improvements in therapeutic approaches and treatment options for patients with CIF. This contributes to the higher survival rate and quality of life for patients who require HPN.
Acknowledgment
The members of the HPN Working Group of the Czech Society for Clinical Nutrition and Intensive Metabolic Care were as follows: Barnetová Dagmar, Bezděk Kamil, Bronský Jiří, Charvát Jiří, Čermáková Dagmar, Čubová Eva, Čulíková Jitka, Daniš Lukáš, Dastych Milan, Jirka Adam, Hrabovský Vladimír, Karásková Eva, Konopásková Kateřina, Kotrlíková Eva, Kovářová Kateřina, Krauzová Eva, Křížová Jarmila, Melek Jan, Pajerek Jan, Pavlíková Jitka, Pěkná Jana, Polák Martin, Pöschl Zdeněk, Pozler Oldřich, Rušavý Zdeněk, Satinský Igor, Schwarz Jan, Solař Svatopluk, Staněk Ivo, Stýblová Jitka, Svobodová Jana, Szitányi Peter, Šuláková Astrid, Tejnická Jana, Tichý Michal, Tláskal Petr, Tuček Štěpán, and Víšek Jakub.
Statement of Ethics
The study protocol was reviewed and approved by the Ethical Committee of Královské Vinohrady University Hospital (EK-VP 54/0/2023), and the need for informed consent was waived under no. EK-VP 54/0/2023.
Conflict of Interest Statement
K.K., J.M., and P.W2.: Takeda (honoraria); M.Š.: Takeda, USA, BBraun, Germany, Fresenius-Kabi, Nestlé, France, and Danone, Switzerland (honoraria); P.B.: Takeda (honoraria and consulting fees); P.K.: Takeda, USA (honoraria), Nestlé, France (honoraria), and Danone, Switzerland (honoraria); J.V.: Takeda, USA (honoraria); J.M.: VectivBio, Switzerland (study contract); P.T. and J.G.: Takeda, USA (honoraria), and VectivBio, Switzerland (study contract); F.N., P.W1., E.M., and F.F.: none.
Funding Sources
REDNUP was supported by Baxter Czech Ltd. and Takeda Pharmaceuticals Czech Republic Ltd. J.G. was supported by the EFSD Mentorship Programme supported by AstraZeneca and EU LX22NPO5104, EXCELES projects.
Author Contributions
Kateřina Koudelková drafted the manuscript, performed data curation, and collected data; Petr Waldauf performed the statistical analysis; Petr Wohl, Michal Šenkyřík, Petr Beneš, Pavel Kohout, Jiří Vejmelka, Jan Maňák, Pavel Těšínský, František Novák, Eva Meisnerová, and Filip Fencl collected and cleared data and revised the manuscript. Jan Gojda was responsible for conceptualization, methodology, data collection/curation, and manuscript revision. All the authors have read and approved the final version of the manuscript and bear full responsibility for their respective work.
Funding Statement
REDNUP was supported by Baxter Czech Ltd. and Takeda Pharmaceuticals Czech Republic Ltd. J.G. was supported by the EFSD Mentorship Programme supported by AstraZeneca and EU LX22NPO5104, EXCELES projects.
Data Availability Statement
Data are not available due to ethical reasons. Pseudonymised data could be acquired from the corresponding author upon a reasonable request.
References
- 1. Pironi L, Boeykens K, Bozzetti F, Joly F, Klek S, Lal S, et al. ESPEN guideline on home parenteral nutrition. Clin Nutr. 2020;39(6):1645–66. [DOI] [PubMed] [Google Scholar]
- 2. Baxter JP, Fayers PM, Bozzetti F, Kelly D, Joly F, Wanten G, et al. An international study of the quality of life of adult patients treated with home parenteral nutrition. Clin Nutr. 2019;38(4):1788–96. [DOI] [PubMed] [Google Scholar]
- 3. Anděl M, Filip K, Brůček P. Long-term home parenteral nutrition using a totally implanted cannulation system. Cas Lek Cesk. 1992;131(13):395–8. [PubMed] [Google Scholar]
- 4. Wanden-Berghe C, Virgili Casas N, Cuerda Compés C, Ramos Boluda E, Pereira Cunill JL, Maíz Jiménez MI, et al. [Home and ambulatory artificial nutrition (NADYA) group report: home parenteral nutrition in Spain, 2019]. Nutr Hosp. 2021;38(6):1304–9. [DOI] [PubMed] [Google Scholar]
- 5. Neelis EG, Roskott AM, Dijkstra G, Wanten GJ, Serlie MJ, Tabbers MM, et al. Presentation of a nationwide multicenter registry of intestinal failure and intestinal transplantation. Clin Nutr. 2016;35(1):225–9. [DOI] [PubMed] [Google Scholar]
- 6. Smith T, Naghibi M. Artificial nutrition support in the UK on behalf of the BANS committee; 2005. [Google Scholar]
- 7. D’Eusebio C, dario MF, Ossola M, Bioletto F, Ippolito M, Locatelli M, et al. Mortality and parenteral nutrition weaning in patients with chronic intestinal failure on home parenteral nutrition: a 30-year retrospective cohort study. Nutrition. 2023:107. [DOI] [PubMed] [Google Scholar]
- 8. Gondolesi GE, Ortega ML, Doeyo M, Buncuga M, Pérez C, Mauriño E, et al. First registry of adult patients with chronic intestinal failure due to short bowel syndrome in Argentina: the RESTORE project. JPEN J Parenter Enteral Nutr. 2022;46(7):1623–31. doi: [DOI] [PubMed] [Google Scholar]
- 9.ESPEN n.d. https://www.espen.org/(accessed April 29, 2022).
- 10. Howard L, Heaphey L, Fleming CR, Lininger L, Steiger E. Four years of North American registry home parenteral nutrition outcome data and their implications for patient management. JPEN J Parenter Enteral Nutr. 1991;15(4):384–93. [DOI] [PubMed] [Google Scholar]
- 11. R Core Team. R . A language and environment for statistical computing 2023.
- 12. Posit team . RStudio Integrated Development Environment for R; 2023.
- 13. Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4(43):1686. [Google Scholar]
- 14. Therneau TM. A package for survival analysis in R; 2023. [Google Scholar]
- 15. Hortencio TDR, Arendt BM, Teterina A, Jeejeebhoy KN, Gramlich LM, Whittaker JS, et al. Changes in home parenteral nutrition practice based on the Canadian home parenteral nutrition patient registry. JPEN J Parenter Enteral Nutr. 2017;41(5):830–6. [DOI] [PubMed] [Google Scholar]
- 16. McKee RF, Knight K, Leitch EF, Stevens P. Changes in adult home parenteral nutrition practice over 25 years. Clin Nutr ESPEN. 2021;45:170–6. doi: [DOI] [PubMed] [Google Scholar]
- 17. Wanden-Berghe Lozano C, Virgili Casas N, Ramos Boluda E, Cuerda Compes C, Moreno Villares JM, Pereira Cunill JL, et al. [Home and ambulatory artificial nutrition (NADYA) group report - home parenteral nutrition in Spain, 2016]. Nutr Hosp. 2017;34(5):1497–501. [DOI] [PubMed] [Google Scholar]
- 18. Wanden-Berghe C, Cuerda Compes JC, Burgos Peláez R, Gómez Candela C, Virgili Casas N, Pérez de la Cruz A, et al. A home and ambulatory artificial nutrition (NADYA) group report, home parenteral nutrition in Spain, 2013. Nutr Hosp. 2015;31(6):2533–8. [DOI] [PubMed] [Google Scholar]
- 19. Wanden-Berghe Lozano C, Pereira Cunill JL, Cuerda Compes C, Ramos Boluda E, Maiz Jiménez MI, Gómez Candela C, et al. Nutrición parenteral domiciliaria en España 2017. Informe del Grupo de Nutrición Artificial Domiciliaria y Ambulatoria NADYA. Nutr Hosp. 2018;35:1491–6. [DOI] [PubMed] [Google Scholar]
- 20. Mundi MS, Mercer DF, Iyer K, Pfeffer D, Zimmermann LB, Berner-Hansen M, et al. Characteristics of chronic intestinal failure in the USA based on analysis of claims data. JPEN J Parenter Enteral Nutr. 2022;46(7):1614–22. [DOI] [PubMed] [Google Scholar]
- 21. Kopczynska M, Hvas CL, Jepsen P, Teubner A, Abraham A, Burden ST, et al. Standardised survival and excess Life Years Lost in patients with type 3 intestinal failure. Clin Nutr. 2022;41(11):2446–54. [DOI] [PubMed] [Google Scholar]
- 22. Reber E, Staub K, Schönenberger KA, Stanga A, Leuenberger M, Pichard C, et al. management of home parenteral nutrition: complications and survival. Ann Nutr Metab. 2021;77(1):46–55. [DOI] [PubMed] [Google Scholar]
- 23. Brandt CF, Hvistendahl M, Naimi RM, Tribler S, Staun M, Brøbech P, et al. Home parenteral nutrition in adult patients with chronic intestinal failure: the evolution over 4 decades in a tertiary referral center. JPEN J Parenter Enteral Nutr. 2017;41(7):1178–87. [DOI] [PubMed] [Google Scholar]
- 24. Wanden-Berghe C, Cuerda C, Maíz Jiménez MI, Pereira Cunill JL, Ramos Boluda E, Gómez Candela C, et al. Grupo de Trabajo SENPE. Nutrición parenteral domiciliaria en España 2018. Informe del Grupo de Nutrición Artificial Domiciliaria y Ambulatoria NADYA. Nutr Hosp. 2020;37:403–7. [DOI] [PubMed] [Google Scholar]
- 25. Pironi L, Konrad D, Brandt C, Joly F, Wanten G, Agostini F, et al. Clinical classification of adult patients with chronic intestinal failure due to benign disease: an international multicenter cross-sectional survey. Clin Nutr. 2018;37(2):728–38. [DOI] [PubMed] [Google Scholar]
- 26. Dreesen M, Foulon V, Spriet I, Goossens GA, Hiele M, De Pourcq L, et al. Epidemiology of catheter-related infections in adult patients receiving home parenteral nutrition: a systematic review. Clin Nutr. 2013;32(1):16–26. [DOI] [PubMed] [Google Scholar]
- 27. Saqui O, Fernandes G, Allard J. CE article: central venous catheter infection in Canadian home parenteral nutrition patients: a 5-year multicenter retrospective study. J Assoc Vasc Access. 2020;25(1):16–26. [DOI] [PubMed] [Google Scholar]
- 28. Bond A, Huijbers A, Pironi L, Schneider SM, Wanten G, Lal S. Review article: diagnosis and management of intestinal failure-associated liver disease in adults. Aliment Pharmacol Ther. 2019;50(6):640–53. [DOI] [PubMed] [Google Scholar]
- 29. Pironi L, Sasdelli AS. Intestinal failure-associated liver disease. Clin Liver Dis. 2019;23(2):279–91. [DOI] [PubMed] [Google Scholar]
- 30. Sasdelli AS, Agostini F, Pazzeschi C, Guidetti M, Lal S, Pironi L. Assessment of intestinal failure associated liver disease according to different diagnostic criteria. Clin Nutr. 2019;38(3):1198–205. [DOI] [PubMed] [Google Scholar]
- 31. Pironi L, Labate AMM, Pertkiewicz M, Przedlacki J, Tjellesen L, Staun M, et al. Prevalence of bone disease in patients on home parenteral nutrition. Clin Nutr. 2002;21(4):289–96. [DOI] [PubMed] [Google Scholar]
- 32. Pironi L, Raschi E, Sasdelli AS. The safety of available treatment options for short bowel syndrome and unmet needs. Expert Opin Drug Saf. 2021;20(12):1501–13. [DOI] [PubMed] [Google Scholar]
- 33. Baxter JP, Gillanders L, Angstmann K, Staun M, O’Hanlon C, Smith T, et al. Home parenteral nutrition: an international benchmarking exercise. ESPEN J. 2012;7(5):e211–e214. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not available due to ethical reasons. Pseudonymised data could be acquired from the corresponding author upon a reasonable request.