Abstract
Activated by retinoids, metabolites of vitamin A, the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs) play important roles in a wide variety of biological processes, including embryo development, homeostasis, cell proliferation, differentiation and death. In this review, we summarized the functional roles of nuclear receptor RAR/RXR heterodimers in liver physiology. Specifically, RAR/RXR modulate the synthesis and metabolism of lipids and bile acids in hepatocytes, regulate cholesterol transport in macrophages, and repress fibrogenesis in hepatic stellate cells. We have also listed the specific genes that carry these functions and how RAR/RXR regulate their expression in liver cells, providing a mechanistic view of their roles in liver physiology. Meanwhile, we pointed out many questions regarding the detailed signaling of RAR/RXR in regulating the expression of liver genes, and hope future studies will address these issues.
Keywords: Retinoic acid receptors, Retinoid X receptors, Gene regulation, Liver physiology, Lipid metabolism, Bile acid hemostasis, Liver fibrogenesis, Nuclear receptors
1. Introduction
Retinoic acids (RAs), the major active metabolites of vitamin A, play a pivotal role in many essential biological processes, including embryogenesis, organogenesis, cell growth, differentiation and apoptosis. The biologic effects of RAs are mainly mediated through nuclear receptors, the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs). Both RARs and RXRs have three isotypes, −α, −β, and −γ, that are encoded by different genes in humans and rodents, i.e. NR1B1–3 and NR2B1–3, respectively. In addition, each isotype also has multiple variants due to alternative promoter and splicing. Despite these differences, RAR isotypes share over 90% identity in their DNA binding domain (DBD), followed by ~85% identity in the ligand binding domain (LBD) whereas the N- and C- terminal regions (also call A and F domains) vary markedly. Fig. 1 illustrates the modular structure of these domains, designated A-F. Similarly, RXR isotypes also share high sequence identity in their DBD and LBD domains. In contrast, the sequence identity in DBD and LBD between RAR and RXR are 60% and 27%, respectively despite both being activated by RAs and their metabolites [1].
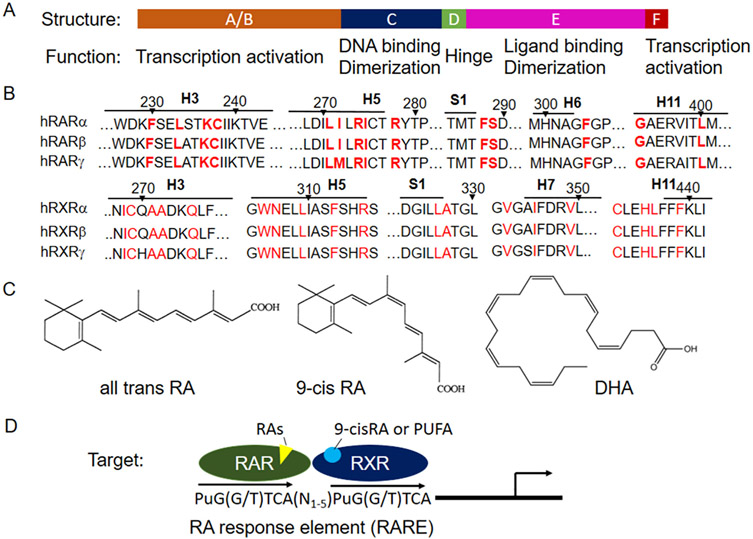
Fig. 1.
A structural model of retinoid receptors in gene regulation. A) structural modules of RAR and RXR. B) Residues that directly interact with atRA in RARs and 9-cis RA in RXRs are well conserved among isotypes within the family. C) Structure of atRA, 9-cis RA and docosahexaenoic acid (DHA, 22:6 omega-3). D) Transcription activation of target genes by RAR/RXR.
All-trans retinoic acid (atRA) is believed to be the endogenous ligand for RARs as it activates RARs at nanomolar levels, a physiologically relevant concentration, although the EC50 for each isotype differs slightly [2]. Other stereoisomers of RA also activate RARs, including 9-cis RA and 13-cis RA [3]. Fig. 1B demonstrates that the residues that directly interact with atRA in RAR are well conserved among the isotypes [4]. However, the endogenous ligand for RXRs remains debatable. Although 9-cis RA was initially identified as a ligand for RXRs by in vitro assays [5], its concentration in plasma and tissues is either undetectable or below the levels used in in vitro assays [6]; nevertheless, atRA can isomerize to 9-cis RA in vivo. In contrast, polyunsaturated fatty acids (PUFA) have been shown binding also activating RXRα both in tissue extracts and gene reporter assay [7-9], including docosahexaenoic acid and arachidonic acid. These observations indicate that PUFA are endogenous ligands for RXRs. This speculation is further supported by a recent report where deficiency of vitamin A has limited effects in RXRα activation in vivo. In contrast, deficiency of PUFA greatly repressed RXRα activation [10].
As type II nuclear receptors, RARs are obligated to form heterodimers with RXRs and bind to retinoic acid response elements (RAREs), a sequence in the promoter of the target genes that regulates their expression, whereas RXRs can form homodimers and regulate gene expression. In vitro assays indicate that a given RAR isotype is able to heterodimerize with any of the RXR isotypes and bind to a RARE [11]. The consensus RARE favors a direct repeat (DR) of AGGTCA with a gap of 1–5 nucleotides (DR1–5) (Fig. 1). At resting state, RAR/RXR may function as a repressor of gene transcription because of the associated corepressors. Upon ligand binding, its LBD undergoes a conformation change, resulting in the release of corepressors and recruitment of coactivators, leading to activation of gene transcription. Because RXR is the obligated partner for RAR, agonists of RXR can permissively activate RAR and regulate its target’s expression. This is also true for a few other members of class II nuclear receptors, including PPARs, LXRs, and FXRs [12-15]. As these receptors are the subject of other reviews, we will not discuss RA’s role in the activation of these receptors here.
Both RAR and RXR are broadly expressed in almost all tissues, but the relative abundance of each isotype differs. Table 1 lists the expression pattern of these isotypes and the phenotype of the gene knockout in mouse. Numerous studies in the past few decades, summarized in many excellent reviews, have demonstrated the functional significance of RA mediated RAR/RXR activation in a wide variety of biological processes, including embryo development, homeostasis, cell proliferation, differentiation and death. In this review, we focus on RAR/RXR’s role in liver physiology where all of their isotypes are expressed (Table 1).
Table 1.
Tissue distributions of RARs and RXRs in mammals, and phenotypes of isotype-specific knockout in mouse.
| Receptor (gene symbol) |
Expression pattern | Knockout phenotype | Ref | ||
|---|---|---|---|---|---|
| Most abundant | Liver | General | Liver specific | ||
| RARα (NR1B1) | Widely expressed | Parenchyma, endothelia, kupffer and stellate cell | Postnatal lethality and some abnormalities including testis degeneration | [16-21] | |
| RARβ (NR1B2) | Lung, intestine, genitalia, epithelia, limbs, brain. | Parenchyma, endothelial, Kupffer and stellate cell | No obvious external and internal abnormalities | [19,22-24] | |
| PARγ (NR1B3) | Cartilage, embryo, epidermis, skeleton | Parenchyma, endothelial, Kupffer and stellate cell | Postpartum lethality and growth deficiency. (male sterility; Agenesis of the ocular Harderian gland and homeotic transformations of the axial skeleton) | [19,25-27] | |
| RXRα (NR2B1) | Liver, kidney, spleen, placenta, epidermis and a variety of visceral tissues | Parenchyma, endothelial, kupffer and stellate cell | Died in utero and displayed myocardial and ocular malformations | Disturbed lipid metabolism; a deficiency of Epo expression in the fetal liver; impaired regenerative capacity | [19,28-30] |
| RXRβ (NR2B2) | Widely expressed | Parenchyma, endothelial, Kupffer and stellate cell | Normal. Except male infertility. | [19,30-32] | |
| RXRγ (NR2B3) | Muscle, brain | Parenchyma, endothelial, and Kupffer cell | Postnatal lethality and some abnormalities including homeotic transformation | [19,27,32,33] | |
Note: The expression levels of all RXR subtypes is higher than all RAR subtypes in all liver cells.
2. RAR/RXR in the regulation of lipid metabolism
The liver is the major organ involved in lipid metabolism. Both RARα and RXRα, which are abundantly expressed, play an important role in regulating the expression of genes involved in hepatic lipid metabolism and homeostasis. Although deficiency of specific RAR isotypes in mice does not show any liver-specific phenotype, likely due to functional compensation from other RAR isotypes [21], mice with liver-specific knockout of RXRα, the most abundantly expressed isoform in the liver, exhibit increased hepatic levels of triglyceride and cholesterol [34]. Table 2 lists RAR/RXR target genes involved in lipid metabolism, which are also depicted in Fig. 2.
Table 2.
RAR/RXR target genes involved in hepatic lipid metabolism.
| Target gene | Regulation | Function | Species | Cell type | References |
|---|---|---|---|---|---|
| FGF21 (RAR/RAR) | Stimulation | The hepatokine for promotion of fatty acid oxidation and suppression of lipid synthesis | Human | Hepatoma (hepG2) | [44] |
| Mice | Hepatocytes | [44] | |||
| FGF19 (Fgf15 in rodent) (RAR/RXR) | Stimulation | The gut-derived hormone for suppression of lipogenesis and induction of fatty acid β-oxidation | Human | Ileum (HT-29 cell, caco-2 cell) | [45,46] |
| Apolipoprotein C-III (RAR/RXR, RXR/RXR) | Stimulation | The protein for inhibition of hepatic uptake of triglyceride-rich particles | Human | Hepatoma (Hep3B cells) | [48] |
| Mice | Preadipocyte | [95] | |||
| Hes6 (RAR/RXR) | Stimulation | Transcriptional repressor for inhibition of HNF-4α transcriptional activity, subsequently repress HNF-4α-activated PPAR-γ2 expression | Mice | Hepatocytes | [49] |
| Apolipoprotein A-I (RAR/RXR) | Stimulation | The plasma protein for cholesterol and other lipids transportation in the plasma | Human | Hepatoma (HepG2) | [50] |
| ABCA1 (RAR/RXR) | Stimulation | The membrane protein for the efflux of cholesterol and phospholipids to lipidpoor apolipoproteins, generating nascent HDL particles. | Human | Macrophages (THP-1) | [53] |
| Mice | Macrophages | [52] | |||
| ABCG1 (RAR/RXR) | Stimulation | The membrane protein for promoting cholesterol efflux from macrophages to HDL particles | Human | Macrophages (THP-1) | [53] |
| CYP27A1 (RAR/RXR) | Stimulation | The enzyme for degradation of cholesterol to bile acids | Human | Macrophages (THP-1) | [54-57] |
| CPT1 (RAR/RXR) | Stimulation | The rate-limiting enzyme in long chain fatty acyl-CoA uptake and oxidation in mitochondria | Mice | Hepatocytes | [96] |
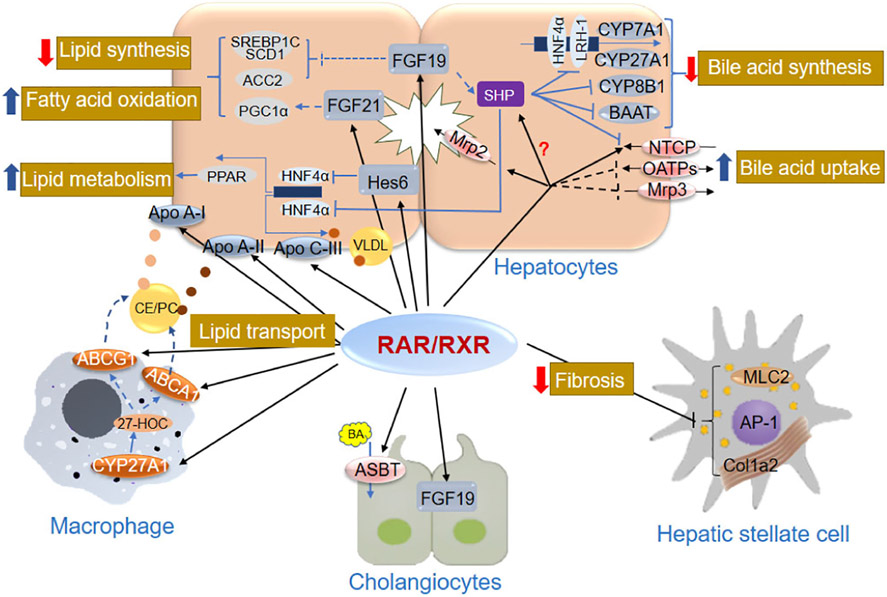
Fig. 2.
Functional role of RAR/RXR in liver physiology. The black arrows or bar-headed lines show key genes involved in lipid metabolism and fibrogenesis that are directly regulated by RAR/RXR signaling pathways in liver cells, including hepatocytes and cholangiocytes Kupffer cells, and stellate cells. Dashed lines indicate that genes are directly or indirectly regulated by RAR/RXR signaling pathways, the detail of which has not been defined. Thick red arrows mean inhibition, and thick blue arrows mean stimulation. Yellow, brown, and red dots represent apo A-I, apo A-II, and apo C-III, respectively. Abbreviations can be found separately in the “list of abbreviations.” More information about the regulations and function of the individual genes can be found in Tables 2-4.
2.1. Regulation of fatty acids and triglycerides by RAR/RXR
Several independent studies have indicated that the retinoid signaling modulates energy expenditure and lipid metabolism in rodents as the administration of RA ameliorates obesity and glucose intolerance and suppresses adipose lipid stores in mouse models of obesity and diabetes [35-37]. Impaired hepatic retinoid signaling is also linked with non-alcoholic fatty liver disease in humans [38], further supporting the functional significance of RA in normal liver physiology. Subsequent studies indicate that this signaling is mediated through fibroblast growth factor 21 (FGF21), a hormone secreted by the liver, involved in gluco-neogenesis, lipid metabolism, and ketogenesis. As a hormone, FGF21 regulates gene expression through activation of its membrane receptors, FGF receptor (FGFR) 1 and β-Klotho. The target genes in FGF21/FGFR1-β-Klotho signaling pathway include glucose-6-phosphatase, phospho-enolpyruvate carboxykinase, carnitine palmitoyl transferase 1α, and 3-hydroxybutyrate dehydrogenase type 1. Pharmacological administration of FGF21 improves insulin sensitivity, normalizes plasma lipids levels, causes weight loss, and increases whole-body energy expenditure in obese rodents or monkeys [39-43].
Hepatic expression of FGF21 is directly regulated by RAR/RXR in both human and mouse. Li et al. reported that both RARα and β but not γ stimulated FGF21 expression in HepG2 cells [44]. A DR1 RARE is identified in human FGF21 promoter region at −644 to −632 nt upstream of the translation initiation site in gene reporter assays. Mutation of this RARE abolishes RA induction of this promoter. The functional role of RAR in FGF21 expression regulation is further confirmed in vivo in mouse liver, where over-expression of RARβ with a viral vector significantly elevated hepatic levels of Fgf21, leading to increased energy expenditure by promoting hepatic fatty acid oxidation and ketogenesis. Two putative DR5 RAREs are identified in the mouse Fgf21 promoter at −602 to −586 and −537 to −521 nt, by Chromatin immunoprecipitation (ChIP)-PCR analysis [44].
Like FGF21, FGF19 (Fgf15 in rodents) is another growth factor that plays an important role in modulating hepatic lipid, bile acid, and carbohydrate metabolism. Human hepatocytes and cholangiocytes express FGF19 [45], and RA stimulates FGF19 mRNA expression in both human hepatocytes and HepG2 cells [45]. A DR5 RARE (+3423 to +3439 bp in intron 2) is identified in the human FGF19 gene. Mutation of this RARE significantly reduced 9-cis RA-induction of its promoter activity in a gene reporter assay [46]. However, this RARE is not conserved in its mouse ortholog, suggesting that RARs do not directly regulate Fgf15 expression in this species.
The liver is one of the major organs that synthesizes Apolipoprotein C-III (Apo C-III), which facilitates hepatic very-low-density lipoprotein (VLDL) particle formation and secretion, thus affecting the levels of hepatic and plasma triglycerides. Vu-Dac et al. [47] demonstrated that retinoids increase human Apo C-III mRNA expression by activating RXR, thereby contributing to the hypertriglyceridemic effect of retinoids. Takahashi et al. [95] further identified a DR1 RARE in the human Apo C-III promoter. Interestingly, this DR1 RARE only binds RXR/RXR homodimers. Mutation of this DR1 RARE abolishes 9-cis RA stimulation of RXR-mediated Apo C-III transcription. In contrast, AM580, a RARα specific agonist, repressed the expression of Apo C-III and HNF4α in HepG2 and Hep3B cells, in association with increased expression of the small heterodimer partner (SHP/NR0B2) [48]. Decreased hepatic expression of Apo C-III and HNF4α was also found in vivo in mice fed a high-fat diet when administrated with AM580, where lower plasma levels of triglycerides (TG) and cholesterol were also seen. Because HNF4α is a positive transcription factor for hepatic Apo C-III expression, and because SHP blocks HNF4α transcriptional activity, the authors speculated that AM580 reduces Apo C-III expression by activating RARα, leading to up-regulation of SHP that then results in repressing HNF4α. However, it remains unclear how RARα stimulates SHP expression, an important player in modulating the expression of genes involved in maintaining lipid and bile acid homeostasis.
In addition, RAR also plays a role in de novo lipogenesis (DNL), the first step in lipid metabolism, mediated through the hairy and enhancer of split 6 (Hes6), a transcriptional repressor of HNF4α. This leads to reduced expression and activity of peroxisome proliferator-activated receptor-γ (PPARγ), a potent lipogenic transcription factor [49]. Three putative RAREs were identified in the proximal promoter of mouse Hes6 gene using a gene reporter assay and ChIP-PCR. Most importantly, over-expression of RARα or treatment with atRA in vivo significantly reduced hepatic fat accumulation in obese mouse models, demonstrating the physiological significance of this regulation. However, it is not known whether this HES6-HNF4α-PPARγ-mediated pathway is conserved in humans.
2.2. Regulation of cholesterol metabolism
The liver is also the principal site for cholesterol metabolism. Both the ATP-binding cassette (ABC) transporter A1 (ABCA1) and G1 (ABCG1) mediate the efflux of cellular cholesterol to apolipoproteins (Apo) A-I and A-II, two plasma proteins that are the major components of high-density lipoprotein (HDL). These four proteins are predominantly synthesized in the liver, where their expression is directly regulated by RAR and/or RXR. Rottman et al. [50] first reported that retinoids stimulate Apo A-I expression in HepG2 cells. At least three RAREs have been identified in the apoA-I promoter. One of the RARE (located from −214 to −192 bp upstream of transcription start) is mostly activated by RXR alone, whereas the other two (located from −169 to −146 bp, and −134 to −119 bp) are responsible for the stimulatory effects of RARα and β [50]. In the case of apo A-II, only one RARE is identified in its promoter region and it only binds RXR [51]. In addition, retinoids also induce the expression of the cholesterol efflux transporters ABCA1 and ABCG1 in macrophages. Costet et al. found that atRA and TTNPB, a synthetic RAR agonist, increased ABCA1 mRNA and protein expression in both human and mouse macrophages. This up-regulation is mediated through a DR4 RARE in the human ABCA1 promoter, a site that LXR/RXR also binds to. ChIP analysis in macrophages revealed that RARγ/RXR bind to this DR4 element in the presence of atRA, where weaker binding of RARα/RXR was also found. In contrast, RARβ/RXR did not show any binding to this site [52]. The RAREs in the human ABCG1 promoter were characterized by Ayaori et al. There are two RAREs localized in the ABCG1 gene upstream of exon 1 in promoter A and upstream of exon 5 in promoter B. While promoter A only responds to atRA with minor transcription activity, promoter B can be activated by both atRA and other RAR agonists, TTNPB, and AM580, which generates the major transcript. Gene reporter assays have confirmed that ABCG1 level is regulated more by promoter B than promoter A, where the identified a DR4 RARE in the promoter B overlaps with a liver X receptor-responsive element (LXRE). This RARE was further confirmed by ChIP assay and overexpression of RAR isoforms in reporter assays [53].
Sterol 27-hydroxylase (CYP27A1) catalyzes the degradation of cholesterol, an important pathway in sterol elimination. It is also the initiating enzyme in the acidic pathway that converts cholesterol to bile acids. CYP27A1 is highly expressed in human macrophage. Several studies have found that retinoids and synthetic RAR agonists (e.g. AM580, TTNPB) induce CYP27A1 expression and increase 27-hydroxy-cholesterol production in human macrophages, including primary cells and THP-1 cell lines [54-56]. At least one RARE, which is also shared with PPAR/RXR is identified in the proximal promoter of this human gene, localized between −853 and −217 bp upstream of its transcription start site. Both ChIP assays and reporter assays indicate that a DR1 RARE plays the dominant role in response to retinoid stimulation of RAR/RXR [54]. A recent study indicates that the activity of carboxylesterase 1 also modulates CYP27A1 expression in macrophages via the nuclear receptors PPARγ, RAR, and/or RXR [57].
3. RAR/RXR regulation of bile acid homeostasis
Bile formation is one of the most important functions of the liver. RAR/RXR regulates the expression of a number of genes involved in bile formation, including bile acid synthesis and transport (Table 3) (Fig. 2).
Table 3.
RAR/RXR target genes involved in bile acid metabolism.
| Target gene | Regulation | Function | Species | Cell type | References |
|---|---|---|---|---|---|
| CYP7A1\–/RAR/RXR) | Repression | The rate-limiting enzyme in the synthesis of bile acid from cholesterol by catalyzing the formation of 7α-hydroxycholesterol | Human | HepG2 | [45,97] |
| Hepatocytes | [45] | ||||
| FGF19 (RXR/RAR) | Stimulation | The gut-derived hormone for inhibition of CYP7A1 expression via the FGFR4/Klotho-β receptor complexes in the liver | Human | Ileum (HT-29 cell, caco-2 cell) | [46] |
| CYP27A1 (RAR/RXR) | Repression | The enzyme for degradation of cholesterol to bile acids | Human | Hepatocyte | [45,59] |
| NTCP (RAR/RXR) | Repression | The major basolateral bile acid uptake transporter in hepatocytes | Human | HepG2 | [62,63,69,98] |
| Rat | Hepatocytes Stellate cell. Kupffer cell | [99,100] | |||
| Mice | Hepatocytes | [65] | |||
| MRP2 (RAR/RXR) | Stimulation | The hepatic apical transporter for bilirubin, glutathione, bile acids and other organic anions and their conjugates | Human | HepG2 | [63,69] |
| Rat | Hepatocytes | [66] | |||
| MRP3 (RAR/RXR) | Repression | The hepatic basolateral pump for extrude bilirubin, bile acids and other organic anions and their conjugates | Human | HepG2 | [71] |
| OATP1B1,1B3 (RAR/RXR) | Repression | The basolateral uptake transporter for organic anions | Human | Hepatocytes | [70] |
| OCT1 (RAR/RXR) | Repression | The basolateral uptake transporter for organic cations | |||
| OAT2 (RAR/RXR) | Repression | The basolateral uptake of organic anions/dicarboxylate exchanger | |||
| ASBT (RAR/RXR) | Stimulation | The apical bile acid uptake transporter in bile duct and ileum | Human | CT-26, Caco-2, HIBEC | [72-76] |
3.1. Regulation of bile acid synthesis
The classic pathway for bile acid biosynthesis occurs primarily in the liver, which contributes around 90% of bile acid production. Cholesterol 7a-hydroxylase (CYP7A1) is the rate-limiting enzyme in bile acid synthesis. The expression of CYP7A1 is transcriptionally regulated and tightly controlled. While HNF4α and LRH-1 activate CYP7A1 gene transcription, there are two separate signaling pathways that repress this transcription. The major one is mediated through FGF19 (Fgf15 in mouse) /FGFR4/β-Klotho signaling. In humans, both hepatocytes and gallbladder epithelia express FGF19, in addition to the ileal enterocytes. Although details of this regulatory mechanism remain unclear, when FGF19 activates its receptor FGFR4/β-Klotho on the plasma membrane of hepatocytes, this initiates a signaling cascade that transactivates MAPKs, resulting in repression of CYP7A1 expression [58]. A second pathway is mediated through the nuclear receptor SHP (NR0B2). SHP is not a usual nuclear receptor as it does not have a DNA binding domain. However, when SHP interacts with other transcription factors, it blocks gene transcription, including HNF4α and LRH-1, two transcription factors that normally activate CYP7A1 expression. We have found that RA represses CYP7A1 expression in human hepatocytes and HepG2 cells by stimulating the expression of both FGF19 and SHP [45]. This repression is mediated through both FXR-dependent and -independent pathways. While Jahn’s study [46] identified a novel DR5 RARE in human FGF19 gene as described earlier, it remains to be determined how a RAR agonist that stimulated SHP expression also can repress CYP7A1 expression.
The alternative acidic pathway of bile acid synthesis, accounting for around 10% of bile acid production, is initiated by CYP27A1, described in the previous section. In contrast to macrophages, we found that both bile acids and retinoids repressed CYP27A1 expression in human hepatocytes [45]. Chen and Chiang identified the bile acid response element in human CYP27A1 gene, localized at −147 bp of its promoter, a site that also binds HNF4α [59]. Because both bile acids and retinoids induce SHP expression in hepatocytes, it is likely that SHP represses HNF4α activity, leading to reduced CYP27A1 expression in hepatocytes. The different effects of retinoids on CYP27A1 expression in human hepatocytes and macrophages are likely due to cell-specific transcriptional regulatory mechanisms.
Similar to CYP7A1 and CYP27A1, retinoids also have inhibitory effects on several other genes involved in bile acid synthesis, including BAAT, CYP8B1, and AKR1D1 [60,61], where RAR or RXR may also play a role. This repressive regulation is likely mediated through RA-stimulated expression of SHP in hepatic parenchymal cells, although details remain elusive.
3.2. Regulation of hepatic bile acid transporters
The sodium taurocholate co-transporting polypeptide (NTCP, SLC10A1) and the multidrug resistance-associated protein 2 (MRP2, ABCC2), represent basolateral and apical membrane transporters respectively that are responsible for the uptake and efflux of bile acids, drugs and other organic anions from hepatocytes. Hepatic expression of NTCP and Mrp2 are induced by retinoids through activation of RAR/RXR heterodimer in vitro [62]. A DR5 RARE has been identified in the promoter of both rat Ntcp and Mrp2 genes [63]. RAR’s role in NTCP expression is also seen in human cells. A DR5 RARE in human NTCP is localized to nucleotides −112 to −96 of its promoter. When a synthetic chemical Ro41-5253 antagonizes RAR transactivation, the expression of NTCP, which also functions as the receptor for human hepatitis B virus entry into the hepatocyte, is reduced in cultured human hepatic cells, leading to decreased infection of hepatitis B virus infection [64].
Moreover, in inflammatory conditions, NTCP is down regulated, mainly by IL-1β-induced suppression of RXR/RAR nuclear binding activity as shown in vitro. The retinoid response element of the NTCP promoter was identified at −158 to −36 nt upstream of the translation initiation site, which contains a putative DR2 RARE sequence [63]. Mutations of this DR2 RARE reduced the NTCP promoter activity and NTCP transcription. Besides direct induction by retinoids, transcription of NTCP is also suppressed by the FXR/RXR heterodimer via its up-regulation of SHP [65]. Recent studies have suggested that SHP can suppress RXR/RAR activity through a direct protein-protein interaction [66]. Thus, it means that NTCP is also regulated by cross-talk between a retinoid-RAR-dependent direct pathway and a bile acid-FXR-mediated indirect pathway. However, the expression of NTCP is maximally regulated by bile acid-activation compared to retinoid-activation when both retinoids and bile acids are present [62].
Several lines of evidence indicate that decreased hepatic Mrp2 expression in cholestatic livers is also associated with the reduction of nuclear RARα/RXRα levels as seen with NTCP expression. The proinflammatory cytokine IL-1 β was identified as the responsible inducer of this down-regulation [67-69]. However, this regulatory mechanism is only seen in cholestatic livers, as down-regulation of NTCP and Mrp2 in CCl4-treated rats is independent of nuclear RAR/RXR but regulated by HNF1 [69].
Organic anion transporting polypeptides (OATPs), OATP1B1 and 1B3, organic cation transporter 1 (OCT1) and organic anion transporter 2 (OAT2), are organic anion and cation transporters expressed on the basolateral membrane of the hepatocyte, which are also thought to be regulated in an RXR/RAR-dependent manner, as atRA treatment decreases mRNA expression of these transporters in both primary human hepatocytes and hepatoma HepaRG cells. Knockdown of RARα or RXRα in HepaRG cells using siRNA transfection diminishes atRA repression of the expression of these genes [70], providing corroborating evidence of involvement of these nuclear receptors. However, how atRA and RAR/RXR repress the expression of these genes has yet to be defined. We have also found that RAR/RXR represses human MRP3 (ABCC3) expression in gene reporter assays [71]. MRP3 is expressed on the basolateral membrane of hepatocytes. It effluxes intracellular organic anions and bile acid glucuronides. Its expression is increased in cholestatic livers as an adaptive response to protect the liver from toxic chemicals. Under normal physiologic conditions, RAR/RXR binds to Sp1, a transcription activator of MRP3. This protein-protein interaction blocks Sp1 transcription activity and represses MRP3 expression. However, in cholestasis, hepatic expression of RAR/RXR is reduced. This results in increased transcription activity of Sp1 with the MRP3 promoter, providing a mechanistic explanation for RAR/RXR’s role in regulating MRP3 expression [71].
The apical sodium-dependent bile acid transporter (ASBT, SLC10A2), located on the luminal membrane of cholangiocytes as well as the terminal ileum, functions to actively reabsorb bile acids, thereby facilitating the cholehepatic and enterohepatic circulations of bile acids. Gene promoter reporter assays indicate that RAR/RXR positively regulates human ASBT mRNA expression. A DR2 RARE is identified from +118 to +131 nt downstream of the transcription initiation site in the human ASBT gene. RA stimulates this promoter activity by four-fold, while site-mutations of this DR2 RARE attenuate the basal activity of the promoter by 50% [62]. Further studies indicate that this DR2 RARE is also responsible for bile acid repression of ASBT expression as the mutation of this DR-2 RARE eliminated bile acid repression. This negative feedback regulation is mediated through the FXR signaling pathway. When FXR is activated, SHP and FGF19/Fgf15 expression is stimulated. Increased SHP in turn represses RAR/RXR activity in ASBT transcription, leading to this down-regulation [73-75]. In addition, elevated FGF19/Fgf15 represses ASBT expression in human cholangiocarcinoma Mz-ChA-1 cells and colon cancer Caco2 cells through FGFR4/β-Klotho receptors as mentioned earlier, although details of this signaling pathway still remain unclear [76].
The bile salt export pump (BSEP, ABCB11) is the major apical transporter in the hepatocyte for the efflux of bile salts. FXR/RXR heterodimers activate transcription of human BSEP through binding to the IR-1 site [77]. Although FXR/RXR is thought to be the permissive RXR heterodimer, Kassam A et al. reported that RXR agonists didn’t activate the FXR/RXR heterodimer, but prevented the binding of FXR/RXR heterodimers to the BSEP promoter, suggesting RXR-mediated antagonism for ligand-bound FXR-induced expression of BSEP in both rodent and human [78]. During the transactivation of the IR-1 element, the presence of ligand for the FXR/RXR complex requires the recruitment of steroid receptor coactivators, raising the possibility that RXR ligands may have an antagonistic effect on FXR activation [78].
In summary, RAR-, RXR- mediated pathways are involved in suppressing bile acid synthesis and stimulating bile acid export by directly or indirectly regulating enzymes (CYP7A1, CYP27A1, CYP8B1, BAAT) and transporters (NTCP, MRP2, MRP3, OATPs, and others), and thus can be interpreted as having anti-cholestatic effects.
4. RAR/RXR in the regulation of liver fibrogenesis
Lipid droplets in hepatic stellate cells (HSC) are the central site for the storage of retinoids in the body. Activated HSCs are characterized by loss of retinoids and production of extracellular matrix (ECM), and play an important pathogenic role in liver fibrosis. RXRs and RARs are expressed in quiescent HSCs from rodents and humans, where their alpha isoforms are most highly expressed. However, their expression is reduced when HSCs are activated [79].
When HSC are activated in vivo in the livers of patients with cirrhosis or carcinoma or in bile duct ligated cholestatic rats, the mRNA expression levels of RARβ and RXRα are down-regulated, suggesting that these nuclear receptors play a role in HSC activation and liver fibrogenesis [80,81]. Cortes et al. demonstrate that atRA promotes human HSC deactivation via RARβ-dependent transcriptional down-regulation of myosin light chain 2 (MLC-2) expression (Table 4). MLC-2 plays a major role in cytoskeletal tension, force generation, mechanosensing, and ECM deposition. Elevated hepatic expression of MLC-2 is associated with liver fibrosis in both humans and mice [81]. Consistent with these observations, reduced liver fibrosis is also seen in cholestatic rodent models after administration of atRA [82-84], where atRA demonstrates inhibitory effects on the expression of profibrotic genes, including TGF-β1, COL1A1, MMP2, and α-SMA both in vivo in rodent livers and in vitro in primary human hepatic stellate cells and LX-2 cells. Reduced mRNA expression of all three RAR isotypes is also found in freshly isolated rat HSC where treatment with retinoids decreased markers of activation [85,86]. In further support of RAR’s role in HSC activation, Mezaki et al. found that RARα and β proteins formed insoluble aggregated speckled droplets in the cytosol of retinol activated rat HSC [87], suggesting that RARs have lost their function as transcription factors. Indeed, the expression of RARα, RARβ and RARγ are undetectable in chronically activated rat HSC [86].
Table 4.
RAR/RXR target genes involved in liver fibrosis.
| Target gene |
Regulation | Function | Species | Cell type |
References |
|---|---|---|---|---|---|
| AP-1 (RAR/RXR) | Repression | The transcription factor for regulating expression of collagenase, stromelysin, TGF-β1 and TNF-α. promoting fibrogenesis. | Rat | HSC | [80] |
| MLC-2 (RAR/RXR) | Repression | The sarcomeric protein for increasing HSC contractility (the RAR-β/MLC-2 axis) | Human | HSC | [81] |
| Mice | HSC | ||||
| Col1a2 (RAR/ RXR) | Repression | A protein for the major component of extracellular matrix | Rat | HSC | [91] |
More direct evidence for RXR’s role in HSC activation comes from over-expression of RXRα in transfected rat HSC cultures, which induces a quiescent phenotype [88]. RXR specific agonists are also able to inhibit cell proliferation in HSC. Similarly, treatment of activated rat HSC with atRA or RAR agonists both reduced the expression of profibrotic genes, including collagen I, collagen III, and fibronectin. AtRA also reduced HSC proliferation, whereas RAR agonists did not. In contrast, RAR specific antagonists enhance HSC proliferation, indicating that RARs do play a role in HSC proliferation [89]. Similarly, the combined treatment of ligands for PPAR, RAR, and RXR results in an anti-proliferative effect by inducing cell cycle arrest at the G0/G1 phase [90].
Two RAREs in mouse Collagen I alpha-2 chain (Col1a2) promoter have been identified. They are localized at −879 to −874 bp (site 1) and −930 to −911 bp (site 2). When the reporter construct was cotransfected with RARβ and RXRα expression vectors into stellate cells or the transfected cells were treated with RA, the promoter activity was suppressed. Conversely, mutation of these RAREs enhanced promoter activity, demonstrating the direct role of RAR/RXR in this gene expression regulation [91]. However, these two RAREs are not typical RAREs. Site 1 appears to be half of the regular RARE, whereas site 2 is an everted repeat of the conserved sequence AGGTCA with a gap of 8 base-pairs. Also, a liganded RAR/RXR normally transactivates gene expression rather than represses it. It is not known whether this repressive effect is due to these two atypical RAREs. Alternatively, the RAR/RXR repression of mouse Col1a2 is mediated through transrepression of another transcription factor, such as AP1 (Fig. 2). Several studies have indicated that AP1 positively regulates the expression of many genes involved in liver fibrosis, including TGF-β1, collagenase, stromelysin, and TNF-α [77,89-92], where retinoids repress the expression of these genes. This transrepression is thought to be mediated through RAR/RXR. In this case, liganded RAR does not directly bind to a traditional RARE in the promoter but rather associates with AP1 through protein-protein interactions [92-94]. When RAR binds to AP1, it blocks AP1 transcription activity, leading to reduced expression of the target genes. As a previously mentioned example, RAR/RXR counteracts Sp1 transactivation of MRP3 expression [71]. These regulatory mechanisms may also provide an explanation for atRA and RARβ repression of MLC-2 expression in HSC [81]. In summary, it is clear that RARs and RXRs play a major role in HSC activation by modulating cell proliferation and the expression of profibrotic genes, although many mechanistic details still remain to be determined.
5. RAR/RXR in the regulation of liver inflammation
Supplementation of RA has been shown to reduce liver inflammation in several animal models of liver disease, suggesting a potential role for RAR/RXR in regulating the immune response [101-103]. Many studies have indicated that RA plays a very important role in immune cells differentiation and activation [104,105]. In particular, RA represses the production of proinflammatory cytokines in immune cells, including dendritic cells, monocytes, macrophages, T-cells [3,105-108]. The anti-inflammatory effects of RA are partially RAR/RXR dependent. Dawson et al. showed that repression of IFN-gamma and TNF-alpha by RAs is mediated through activation of RARα, but not RARβ or RARγ in human T cells because the RARα-selective agonist AM580, but not the RARβ/γ ligand 4-hydroxyphenylretinamide, recapitulates RA’s effect [3]. Dzhagalov et al. revealed that RARγ is a positive regulator of inflammatory cytokine production in CD8+ T-cells and macrophages using RARγ-deficient mice [109]. Together, these observations indicate that individual retinoid receptors play specific roles in the differential regulation of immune responses. Several studies have also revealed that RXR alone or in association with RAR block NF-κB or AP-1 activation in immune cells [102,106-108,110], where both NF-κB and AP-1 are transactivators of proinflammatory cytokine expression. (Table 5).
Table 5.
RAR/RXR target genes involved in liver Inflammation.
| Target gene | Regulation | Function | Species | Cell type | References |
|---|---|---|---|---|---|
| IFN-γ (RAR/RXR) | Repression | The cytokine of the immune system related to the T helper type 1 (Th1) response to infection | Human | T lymphoblastoid cell | [106] |
| NF-κB (RAR/RXR) | Repression | The transcription factor for regulating expression of proinflammatory genes, including cytokines, chemokines, and adhesion molecules | Human | THP-1 cells | [108] |
| AP-1 (RAR/RXR) | Repression | The transcription factor for regulating expression of proinflammatory genes, | Human | THP-1 cells | [108] |
The anti-inflammatory effects of RA are also found in microglia and astrocytes [111-113]. Reduced production of metalloproteases (MMP), cyclooxygenase-2 (COX-2), and prostaglandin I synthase (PGIS) were also found in other cells [110,114-116]. However, we were not able to find any reports in the literature regarding RAR/RXR’s role in proinflammatory cytokine production in hepatic cells. Instead, several studies indicate that hepatic cells respond to proinflammatory cytokines by altering gene expression where RAR/RXR is involved as mentioned earlier in the regulation of NTCP, MRP2 and MRP3 expression. Furthermore, Aguirre and Karpen indicate that interleukin-1β and TNFα cause RXRα SUMOylation in hepatocytes, resulting in decreased expression of its target genes [117]. Clearly, more studies are needed to examine RAR/RXR’s role in hepatic inflammation.
6. Conclusions and prospective
RAR and RXR are significant therapeutic targets for various clinical disorders, i.e., atopic dermatitis, breast cancer, acute promyelocytic leukemia (APL), and diabetic cardiomyopathy [118-121]. Emerging findings have shown that vitamin A homeostasis is implicated in the prevention of hepatic fibrosis, regulation of hepatic immunological response to cholestasis, and reduction of liver injury [83,84,122-124]. Given the crucial role of Vitamin A deficiency in chronic cholestasis, RAR and RXR may hold therapeutic potential for the treatment of cholestatic liver diseases. Although UDCA and Obeticholic acid have been approved by the FDA for early-stage and UDCA-refractory primary biliary cholangitis [125,126], not all patients respond, and beneficial effects on clinical endpoints in primary sclerosing cholangitis (PSC) patients remains controversial [127]. Thus there is a need for alternative therapies. Several lines of evidence indicate that atRA has potentially positive effects on liver injury in several rodent models of chronic cholestasis, including bile duct ligation and alpha-naphthyl isothiocyanate in the rat and in Mdr2 null mice, a model of PSC [84,128]. The most positive findings were seen in rats treated with atRA with and without UDCA, which demonstrated marked reductions in necrosis and hepatic fibrosis [83]. In addition, markers of inflammation and significant decreases in the bile acid pool and bile duct proliferation were also seen, with the combination of atRA and UDCA having the greatest effect. The regulatory mechanisms may involve suppressing the activity of cytochrome CYP7A1 [83,84], which led to reductions in the bile acid pool size and reduced bile duct proliferation. In addition, RA repressed collagen 1A1 expression in stellate cells suggesting an anti-fibrotic effect [83]. These encouraging findings led to a small clinical trial that found that combination therapy of UDCA and atRA for 12 weeks in patients with PSC had an inhibitory effect on bile acid synthesis (C4) and significantly reduced serum aminotransferases, thus potentially reducing hepatic inflammation [129]. However, retinoids can also exhibit serious side effects such as teratogenicity, hypervitaminosis, and resistance can develop [130]. Therefore, several synthetic RAR- and RXR- ligands (Rexinoids) have been developed in recent years that show potential as anti-tumor agents in clinical and pre-clinical studies, including AM80 (dual RARα/β-selective agonist) for APL [131] and bexarotene (RXR ligands) for persistent cutaneous T-cell lymphoma [132]. It is possible that these specific ligands could also be promising new therapeutic agents in the therapy of chronic cholestatic disorders.
As summarized in this review, RAR and RXR play critical roles in many aspects of hepatic physiology and responsiveness to disease (Fig. 2). Here we have focused on those aspects that relate to hepatic lipid metabolism, bile acid metabolism and the pathogenesis of hepatic fibrosis and inflammation, as well as the possibility that ligands of these nuclear receptors may have potential therapeutic anti-inflammatory and anti-fibrotic benefits in liver disease. While many details of these interactions have been described, there is still much to be learned about these pathways and particularly where novel therapeutic interventions might be targeted. Given the widespread roles of RAR and RXR in the regulation of these hepatic functions, future studies should continue to focus on this important area.
Acknowledgments
This study was supported by National Institutes of Health Grants DK34989 (Yale Liver Center), DK25636 (to J.L.B.) and Gilead Pharmaceuticals (Foster City, CA).
Abbreviations:
- atRA
all-trans retinoic acid
- ASBT/SLC10A2
apical sodium-dependent bile salt transporter
- ABC
ATP-binding cassette transporter
- Apo
apolipoprotein
- AKR1D1
aldo-keto reductase family 1 member D1
- AP-1
activator protein 1
- BAAT
bile acid coenzyme A: amino acid N-acyltransferase
- BSEP/ABCB11
bile salt export pump
- CYP7A1
cholesterol 7-alpha-hydroxylase
- CD36
cluster determinant 36
- COL1A1
collagen type I alpha 1 chain
- COL1A2
collagen type I alpha 2 chain
- CE
cholesterol ester
- DBD
DNA-binding domain
- DR
direct repeat
- DNL
de novo lipogenesis
- ECM
extracellular matrix
- FXR
farnesoid X receptor
- FGF15/19
fibroblast growth factor 15/19
- FGFR
fibroblast growth factor receptor
- IR
inverted repeat
- Hes6
hairy and enhancer of split 6
- HDL
high-density lipoprotein
- HNF4A
hepatocyte nuclear factor 4 alpha
- HSC
hepatic stellate cells
- IL
interleukin
- LBD
ligand binding domain
- LDLR
low-density lipoprotein receptor
- LRH-1
liver receptor homolog-1
- NTCP/SLC10A1
sodium/taurocholate cotransporting polypeptide
- MRP
multidrug resistance-associated protein
- MLC-2
myosin light chain 2
- MMP-2
matrix metalloproteinase-2
- OATP
organic anion transporting polypeptide
- NF-κB
nuclear factor-kappa B
- PPARγ
peroxisome proliferator-activated receptor gamma
- PC
phosphatidylcholine
- RA
retinoic acid
- RARE
retinoic acid response element
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- 9cRA
9-cis retinoic acid
- SHP/NR0B2
small heterodimer partner
- CYP8B1
sterol 12-alpha-hydroxylase
- CYP27A1
sterol 27-hydroxylase
- α-SMA
alpha-smooth muscle actin
- TNFα
tumor necrosis factor-alpha
- Sp1
specificity protein 1
- TGF-β1
transforming growth factor-beta 1
- TG
triglycerides
- VLDL
very-low-density lipoprotein.
Footnotes
Declaration of competing interest
The authors accept the invitation to submit an invited article for BBA on nuclear receptors RAR/RXR.
References
- [1].Dawson MI, Xia Z, The retinoid X receptors and their ligands, Biochim. Biophys. Acta 1821 (1) (Jan 2012) 21–56, 10.1016/j.bbalip.2011.09.014 (Epub 2011 Oct 1.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Idres N, Marill J, Flexor MA, Chabot GG, Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers, J. Biol. Chem 277 (35) (Aug 30 2002) 31491–31498, 10.1074/jbc.M205016200 (Epub 2002 Jun 17.). [DOI] [PubMed] [Google Scholar]
- [3].Dawson HD, Collins G, Pyle R, Key M, Taub DD, The Retinoic Acid Receptor-alpha mediates human T-cell activation and Th2 cytokine and chemokine production, BMC Immunol. 9 (Apr 16 2008) 16, 10.1186/1471-2172-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Renaud JP, Rochel N, Ruff M, Vivat V, Chambon P, Gronemeyer H, Moras D, Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid, Nature 378 (6558) (Dec 14 1995) 681–689, 10.1038/378681a0. [DOI] [PubMed] [Google Scholar]
- [5].Toporova L, Macejova D, Brtko J, Radioligand binding assay for accurate determination of nuclear retinoid X receptors: a case of triorganotin endocrine disrupting ligands, Toxicol. Lett 254 (Jul 8 2016) 32–36, 10.1016/j.toxlet.2016.05.005 (Epub 2016 May 3.). [DOI] [PubMed] [Google Scholar]
- [6].Jones JW, Pierzchalski K, Yu J, Kane MA, Use of fast HPLC multiple reaction monitoring cubed for endogenous retinoic acid quantification in complex matrices, Anal. Chem 87 (6) (Mar 17 2015) 3222–3230, 10.1021/ac504597q (Epub 2015 Mar 9.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de Urquiza AM, Liu S, Sjöberg M, Zetterström RH, Griffiths W, Sjövall J, Perlmann T, Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain, Science 290 (5499) (Dec 15 2000) 2140–2144, 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- [8].Goldstein JT, Dobrzyn A, Clagett-Dame M, Pike JW, DeLuca HF, Isolation and characterization of unsaturated fatty acids as natural ligands for the retinoid-X receptor, Arch. Biochem. Biophys 420 (1) (Dec 1 2003) 185–193, 10.1016/j.abb.2003.09.034. [DOI] [PubMed] [Google Scholar]
- [9].Lengqvist J, Mata De Urquiza A, Bergman AC, Willson TM, Sjövall J, Perlmann T, Griffiths WJ, Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain, Mol. Cell. Proteomics 3 (7) (Jul 2004) 692–703, 10.1074/mcp.M400003-MCP200 (Epub 2004 Apr 8.). [DOI] [PubMed] [Google Scholar]
- [10].Niu H, Fujiwara H, di Martino O, Hadwiger G, Frederick TE, Menéndez-Gutiérrez MP, Ricote M, Bowman GR, Welch JS, Endogenous retinoid X receptor ligands in mouse hematopoietic cells, Sci. Signal 10 (503) (Oct 31 2017), eaan1011, 10.1126/scisignal.aan1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leid M, Kastner P, Lyons R, Nakshatri H, Saunders M, Zacharewski T, Chen JY, Staub A, Garnier JM, Mader S, et al. , Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently, Cell 68 (2) (Jan 24 1992) 377–395, 10.1016/0092-8674(92)90478-u (Erratum in: Cell. 1992 Nov 27;71(5):887.). [DOI] [PubMed] [Google Scholar]
- [12].Willy PJ, Mangelsdorf DJ, Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR, Genes Dev. 11 (3) (Feb 1 1997) 289–298, 10.1101/gad.11.3.289. [DOI] [PubMed] [Google Scholar]
- [13].Schulman IG, Li C, Schwabe JW, Evans RM, The phantom ligand effect: allosteric control of transcription by the retinoid X receptor, Genes Dev. 11 (3) (Feb 1 1997) 299–308, 10.1101/gad.11.3.299. [DOI] [PubMed] [Google Scholar]
- [14].Shulman AI, Larson C, Mangelsdorf DJ, Ranganathan R, Structural determinants of allosteric ligand activation in RXR heterodimers, Cell 116 (3) (Feb 6 2004) 417–429, 10.1016/s0092-8674(04)00119-9. [DOI] [PubMed] [Google Scholar]
- [15].Kojetin DJ, Matta-Camacho E, Hughes TS, Srinivasan S, Nwachukwu JC, Cavett V, Nowak J, Chalmers MJ, Marciano DP, Kamenecka TM, Shulman AI, Rance M, Griffin PR, Bruning JB, Nettles KW, Structural mechanism for signal transduction in RXR nuclear receptor heterodimers, Nat. Commun 6 (Aug 20 2015) 8013, 10.1038/ncomms9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de The H, Marchio A, Tiollais P, Dejean A, Differential expression and ligand regulation of the retinoic acid receptor alpha and beta genes, EMBO J. 8 (2) (Feb 1989) 429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Giguere V, Ong ES, Segui P, Evans RM, Identification of a receptor for the morphogen retinoic acid, Nature 330 (6149) (Dec 17–23 1987) 624–629, 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- [18].Leroy P, Krust A, Zelent A, Mendelsohn C, Garnier JM, Kastner P, Dierich A, Chambon P, Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid, EMBO J. 10 (1) (Jan 1991) 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ulven SM, Natarajan V, Holven KB, Løvdal T, Berg T, Blomhoff R, Expression of retinoic acid receptor and retinoid X receptor subtypes in rat liver cells: implications for retinoid signalling in parenchymal, endothelial, Kupffer and stellate cells, Eur. J. Cell Biol 77 (2) (Oct 1998) 111–116, 10.1016/S0171-9335(98)80078-2. [DOI] [PubMed] [Google Scholar]
- [20].Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P, Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants, Development 120 (10) (Oct 1994) 2723–2748. [DOI] [PubMed] [Google Scholar]
- [21].Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P, High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice, Proc. Natl. Acad. Sci. U. S. A 90 (15) (Aug 1 1993) 7225–7229, 10.1073/pnas.90c.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dollé P, Ruberte E, Leroy P, Morriss-Kay G, Chambon P, Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis, Development 110 (4) (Dec 1990) 1133–1151. [DOI] [PubMed] [Google Scholar]
- [23].Mendelsohn C, Larkin S, Mark M, LeMeur M, Clifford J, Zelent A, Chambon P, RAR beta isoforms: distinct transcriptional control by retinoic acid and specific spatial patterns of promoter activity during mouse embryonic development, Mech. Dev 45 (3) (Mar 1994) 227–241, 10.1016/0925-4773(94)90010-8. [DOI] [PubMed] [Google Scholar]
- [24].Luo J, Pasceri P, Conlon RA, Rossant J, Giguère V, Mice lacking all isoforms of retinoic acid receptor beta develop normally and are susceptible to the teratogenic effects of retinoic acid, Mech. Dev 53 (1) (Sep 1995) 61–71, 10.1016/0925-4773(95)00424-6. [DOI] [PubMed] [Google Scholar]
- [25].Ruberte E, Dolle P, Krust A, Zelent A, Morriss-Kay G, Chambon P, Specific spatial and temporal distribution of retinoic acid receptor gamma transcripts during mouse embryogenesis, Development 108 (2) (Feb 1990) 213–222. [DOI] [PubMed] [Google Scholar]
- [26].Ruberte E, Friederich V, Chambon P, Morriss-Kay G, Retinoic acid receptors and cellular retinoid binding proteins. III. Their differential transcript distribution during mouse nervous system development, Development 118 (1) (May 1993) 267–282. [DOI] [PubMed] [Google Scholar]
- [27].Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P, Function of retinoic acid receptor gamma in the mouse, Cell 73 (4) (May 21 1993) 643–658, 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- [28].Wan YJ, An D, Cai Y, Repa JJ, Hung-Po Chen T, Flores M, Postic C, Magnuson MA, Chen J, Chien KR, French S, Mangelsdorf DJ, Sucov HM, Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver, Mol. Cell. Biol 20 (12) (Jun 2000) 4436–4444, 10.1128/mcb.20.12.4436-4444.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM, RXR alpha mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis, Genes Dev. 8 (9) (May 1 1994) 1007–1018, 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- [30].Mangelsdorf DJ, Borgmeyer U, Heyman RA, Zhou JY, Ong ES, Oro AE, Kakizuka A, Evans RM, Characterization of three RXR genes that mediate the action of 9-cis retinoic acid, Genes Dev. 6 (3) (Mar 1992) 329–344, 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- [31].Kastner P, Mark M, Ghyselinck N, Krezel W, Dupé V, Grondona JM, Chambon P, Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development, Development 124 (2) (Jan 1997) 313–326. [DOI] [PubMed] [Google Scholar]
- [32].Krezel W, Dupé V, Mark M, Dierich A, Kastner P, Chambon P, RXR gamma null mice are apparently normal and compound RXR alpha +/−/RXR beta −/−/RXR gamma −/− mutant mice are viable, Proc. Natl. Acad. Sci. U. S. A 93 (17) (Aug 20 1996) 9010–9014, 10.1073/pnas.93.17.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brown NS, Smart A, Sharma V, Brinkmeier ML, Greenlee L, Camper SA, Jensen DR, Eckel RH, Krezel W, Chambon P, Haugen BR, Thyroid hormone resistance and increased metabolic rate in the RXR-gamma-deficient mouse, J. Clin. Invest 106 (1) (Jul 2000) 73–79, 10.1172/JCI9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].He Y, Gong L, Fang Y, Zhan Q, Liu HX, Lu Y, Guo GL, Lehman-McKeeman L, Fang J, Wan YJ, The role of retinoic acid in hepatic lipid homeostasis defined by genomic binding and transcriptome profiling, BMC Genomics 14 (Aug 28 2013) 575, 10.1186/1471-2164-14-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Berry DC, Noy N, All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor, Mol. Cell. Biol 29 (12) (Jun 2009) 3286–3296, 10.1128/MCB.01742-08 (Epub 2009 Apr 13.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Berry DC, DeSantis D, Soltanian H, Croniger CM, Noy N, Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity, Diabetes 61 (5) (May 2012) 1112–1121, 10.2337/db111620 (Epub 2012 Mar 6.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tsuchiya H, Ikeda Y, Ebata Y, Kojima C, Katsuma R, Tsuruyama T, Sakabe T, Shomori K, Komeda N, Oshiro S, Okamoto H, Takubo K, Hama S, Shudo K, Kogure K, Shiota G, Retinoids ameliorate insulin resistance in a leptin-dependent manner in mice, Hepatology 56 (4) (Oct 2012) 1319–1330, 10.1002/hep.25798. [DOI] [PubMed] [Google Scholar]
- [38].Ashla AA, Hoshikawa Y, Tsuchiya H, Hashiguchi K, Enjoji M, Nakamuta M, Taketomi A, Maehara Y, Shomori K, Kurimasa A, Hisatome I, Ito H, Shiota G, Genetic analysis of expression profile involved in retinoid metabolism in non-alcoholic fatty liver disease, Hepatol. Res 40 (6) (Jun 2010) 594–604, 10.1111/j.1872-034X.2010.00646.x. [DOI] [PubMed] [Google Scholar]
- [39].Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB, FGF-21 as a novel metabolic regulator, J. Clin. Invest 115 (6) (Jun 2005) 1627–1635, 10.1172/JCI23606 (Epub 2005 May 2.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, Vonderfecht S, Hecht R, Li YS, Lindberg RA, Chen JL, Jung DY, Zhang Z, Ko HJ, Kim JK, Véniant MM, Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice, Diabetes 58 (1) (Jan 2009) 250–259, 10.2337/db08-0392 (Epub 2008 Oct 7.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A, Fibroblast growth factor 21 corrects obesity in mice, Endocrinology 149 (12) (Dec 2008) 6018–6027, 10.1210/en.2008-0816 (Epub 2008 Aug 7.). [DOI] [PubMed] [Google Scholar]
- [42].Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ, The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21, Endocrinology 148 (2) (Feb 2007) 774–781, 10.1210/en.2006-1168 (Epub 2006 Oct 26). [DOI] [PubMed] [Google Scholar]
- [43].Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, Michael MD, Adams AC, Kharitonenkov A, Kahn CR, Interplay between FGF21 and insulin action in the liver regulates metabolism, J. Clin. Invest 124 (2) (Feb 2014) 515–527, 10.1172/JCI67353 (Epub 2014 Jan 9. Erratum in: J Clin Invest. 2015 Jan;125(1):458). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li Y, Wong K, Walsh K, Gao B, Zang M, Retinoic acid receptor β stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice, J. Biol. Chem 288 (15) (Apr 12 2013) 10490–10504, 10.1074/jbc.M112.429852 (Epub 2013 Feb 19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cai SY, He H, Nguyen T, Mennone A, Boyer JL, Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms, J. Lipid Res 51 (8) (Aug 2010) 2265–2274, 10.1194/jlr.M005546 (Epub 2010 Mar 25). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jahn D, Sutor D, Dorbath D, Weiß J, Götze O, Schmitt J, Hermanns HM, Geier A, Farnesoid X receptor-dependent and -independent pathways mediate the transcriptional control of human fibroblast growth factor 19 by vitamin A, Biochim. Biophys. Acta 1859 (2) (Feb 2016) 381–392, 10.1016/j.bbagrm.2015.12.007 (Epub 2015 Dec 23). [DOI] [PubMed] [Google Scholar]
- [47].Vu-Dac N, Gervois P, Torra IP, Fruchart JC, Kosykh V, Kooistra T, Princen HM, Dallongeville J, Staels B, Retinoids increase human apo C-III expression at the transcriptional level via the retinoid X receptor. Contribution to the hypertriglyceridemic action of retinoids, J. Clin. Invest 102 (3) (Aug 1 1998) 625–632, 10.1172/JCI1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lee SJ, Mahankali M, Bitar A, Zou H, Chao E, Nguyen H, Gonzalez J, Caballero D, Hull M, Wang D, Schultz PG, Shen W, A novel role for RARα agonists as apolipoprotein CIII inhibitors identified from high throughput screening, Sci. Rep 7 (1) (Jul 19 2017) 5824, 10.1038/s41598-017-05163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kim SC, Kim CK, Axe D, Cook A, Lee M, Li T, Smallwood N, Chiang JY, Hardwick JP, Moore DD, Lee YK, All-trans-retinoic acid ameliorates hepatic steatosis in mice by a novel transcriptional cascade, Hepatology 59 (5) (May 2014) 1750–1760, 10.1002/hep.26699 (Epub 2014 Mar 26). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rottman JN, Widom RL, Nadal-Ginard B, Mahdavi V, Karathanasis SK, A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways, Mol. Cell. Biol 11 (7) (Jul 1991) 3814–3820, 10.1128/mcb.11.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vu-Dac N, Schoonjans K, Kosykh V, Dallongeville J, Heyman RA, Staels B, Auwerx J, Retinoids increase human apolipoprotein A-11 expression through activation of the retinoid X receptor but not the retinoic acid receptor, Mol. Cell. Biol 16 (7) (Jul 1996) 3350–3360, 10.1128/mcb.16.7.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Costet P, Lalanne F, Gerbod-Giannone MC, Molina JR, Fu X, Lund EG, Gudas LJ, Tall AR, Retinoic acid receptor-mediated induction of ABCA1 in macrophages, Mol. Cell. Biol 23 (21) (Nov 2003) 7756–7766, 10.1128/mcb.23.21.7756-7766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ayaori M, Yakushiji E, Ogura M, Nakaya K, Hisada T, Uto-Kondo H, Takiguchi S, Terao Y, Sasaki M, Komatsu T, Iizuka M, Yogo M, Uehara Y, Kagechika H, Nakanishi T, Ikewaki K, Retinoic acid receptor agonists regulate expression of ATP-binding cassette transporter G1 in macrophages, Biochim. Biophys. Acta 1821 (4) (Apr 2012) 561–572, 10.1016/j.bbalip.2012.02.004 (Epub 2012 Feb 14). [DOI] [PubMed] [Google Scholar]
- [54].Szanto A, Benko S, Szatmari I, Balint BL, Furtos I, Rühl R, Molnar S, Csiba L, Garuti R, Calandra S, Larsson H, Diczfalusy U, Nagy L, Transcriptional regulation of human CYP27 integrates retinoid, peroxisome proliferator-activated receptor, and liver X receptor signaling in macrophages, Mol. Cell. Biol 24 (18) (Sep 2004) 8154–8166, 10.1128/MCB.24.18.8154-8166.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Langmann T, Liebisch G, Moehle C, Schifferer R, Dayoub R, Heiduczek S, Grandl M, Dada A, Schmitz G, Gene expression profiling identifies retinoids as potent inducers of macrophage lipid efflux, Biochim. Biophys. Acta 1740 (2) (May 30 2005) 155–161, 10.1016/j.bbadis.2004.11.016 (Epub 2004 Dec 9). [DOI] [PubMed] [Google Scholar]
- [56].Quinn CM, Jessup W, Wong J, Kritharides L, Brown AJ, Expression and regulation of sterol 27-hydroxylase (CYP27A1) in human macrophages: a role for RXR and PPARgamma ligands, Biochem. J 385 (Pt 3) (Feb 1 2005) 823–830, 10.1042/BJ20041776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mangum LC, Hou X, Borazjani A, Lee JH, Ross MK, Crow JA, Silencing carboxylesterase 1 in human THP-1 macrophages perturbs genes regulated by PPARγ/RXR and RAR/RXR: down-regulation of CYP27A1-LXRα signaling, Biochem. J 475 (3) (Feb 9 2018) 621–642, 10.1042/BCJ20180008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, Donahee M, Wang DY, Mansfield TA, Kliewer SA, Goodwin B, Jones SA, Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis, Genes Dev. 17 (13) (Jul 1 2003) 1581–1591, 10.1101/gad.1083503 (Epub 2003 Jun 18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Chen W, Chiang JY, Regulation of human sterol 27-hydroxylase gene (CYP27A1) by bile acids and hepatocyte nuclear factor 4alpha (HNF4alpha), Gene 313 (Aug 14 2003) 71–82, 10.1016/s0378-1119(03)00631-0. [DOI] [PubMed] [Google Scholar]
- [60].Mamoon A, Subauste A, Subauste MC, Subauste J, Retinoic acid regulates several genes in bile acid and lipid metabolism via upregulation of small heterodimer partner in hepatocytes, Gene 550 (2) (Oct 25 2014) 165–170, 10.1016/j.gene.2014.07.017 (Epub 2014 Jul 9). [DOI] [PubMed] [Google Scholar]
- [61].Yang F, He Y, Liu HX, Tsuei J, Jiang X, Yang L, Wang ZT, Wan YJ, All-trans retinoic acid regulates hepatic bile acid homeostasis, Biochem. Pharmacol 91 (4) (Oct 15 2014) 483–489, 10.1016/j.bcp.2014.08.018 (Epub 2014 Aug 28). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Denson LA, Sturm E, Echevarria W, Zimmerman TL, Makishima M, Mangelsdorf DJ, Karpen SJ, The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp, Gastroenterology 121 (1) (Jul 2001) 140–147, 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- [63].Denson LA, Auld KL, Schiek DS, McClure MH, Mangelsdorf DJ, Karpen SJ, Interleukin-1beta suppresses retinoid transactivation of two hepatic transporter genes involved in bile formation, J. Biol. Chem 275 (12) (Mar 24 2000) 8835–8843, 10.1074/jbc.275.12.8835. [DOI] [PubMed] [Google Scholar]
- [64].Tsukuda S, Watashi K, Iwamoto M, Suzuki R, Aizaki H, Okada M, Sugiyama M, Kojima S, Tanaka Y, Mizokami M, Li J, Tong S, Wakita T, Dysregulation of retinoic acid receptor diminishes hepatocyte permissiveness to hepatitis B virus infection through modulation of sodium taurocholate cotransporting polypeptide (NTCP) expression, J. Biol. Chem 290 (9) (Feb 27 2015) 5673–5684, 10.1074/jbc.M114.602540 (Epub 2014 Dec 30). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zollner G, Wagner M, Fickert P, Geier A, Fuchsbichler A, Silbert D, Gumhold J, Zatloukal K, Kaser A, Tilg H, Denk H, Trauner M, Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver, Am. J. Physiol. Gastrointest. Liver Physiol 289 (5) (Nov 2005) G798–G805, 10.1152/ajpgi.00319.2004 (Epub 2005 Jul 7). [DOI] [PubMed] [Google Scholar]
- [66].Lee YK, Dell H, Dowhan DH, Hadzopoulou-Cladaras M, Moore DD, The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression, Mol. Cell. Biol 20 (1) (Jan 2000) 187–195, 10.1128/MCB.20.1.187-195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Denson LA, Bohan A, Held MA, Boyer JL, Organ-specific alterations in RAR alpha:RXR alpha abundance regulate rat Mrp2 (Abcc2) expression in obstructive cholestasis, Gastroenterology 123 (2) (Aug 2002) 599–607, 10.1053/gast.2002.34758. [DOI] [PubMed] [Google Scholar]
- [68].Dietrich CG, Geier A, Salein N, Lammert F, Roeb E, Oude Elferink RP, Matern S, Gartung C, Consequences of bile duct obstruction on intestinal expression and function of multidrug resistance-associated protein 2, Gastroenterology 126 (4) (Apr 2004) 1044–1053, 10.1053/j.gastro.2003.12.046. [DOI] [PubMed] [Google Scholar]
- [69].Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, Lorenzen J, Matern S, Gartung C, Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis, Hepatology 38 (2) (Aug 2003) 345–354, 10.1053/jhep.2003.50317. [DOI] [PubMed] [Google Scholar]
- [70].Le Vee M, Jouan E, Stieger B, Fardel O, Differential regulation of drug transporter expression by all-trans retinoic acid in hepatoma HepaRG cells and human hepatocytes, Eur. J. Pharm. Sci 48 (4–5) (Mar 12 2013) 767–774, 10.1016/j.ejps.2013.01.005 (Epub 2013 Jan 23). [DOI] [PubMed] [Google Scholar]
- [71].Chen W, Cai SY, Xu S, Denson LA, Soroka CJ, Boyer JL, Nuclear receptors RXRalpha:RARalpha are repressors for human MRP3 expression, Am. J. Physiol. Gastrointest. Liver Physiol 292 (5) (May 2007), G1221–7, 10.1152/ajpgi.00191.2006 (Epub 2007 Feb 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Neimark E, Chen F, Li X, Shneider BL, Bile acid-induced negative feedback regulation of the human ileal bile acid transporter, Hepatology 40 (1) (Jul 2004) 149–156, 10.1002/hep.20295. [DOI] [PubMed] [Google Scholar]
- [73].Duane WC, Xiong W, Wolvers J, Effects of bile acids on expression of the human apical sodium dependent bile acid transporter gene, Biochim. Biophys. Acta 1771 (11) (Nov 2007) 1380–1388, 10.1016/j.bbalip.2007.09.003 (Epub 2007 Sep 26). [DOI] [PubMed] [Google Scholar]
- [74].Duane WC, Xiong W, Lofgren J, Transactivation of the human apical sodium-dependent bile acid transporter gene by human serum, J. Steroid Biochem. Mol. Biol 108 (1–2) (Jan 2008) 137–148, 10.1016/j.jsbmb.2007.07.005 (Epub 2007 Sep 11). [DOI] [PubMed] [Google Scholar]
- [75].Pan DH, Chen F, Neimark E, Li X, Shneider BL, FTF and LRH-1, two related but different transcription factors in human Caco-2 cells: their different roles in the regulation of bile acid transport, Biochim. Biophys. Acta 1732 (1–3) (Dec 30 2005) 31–37, 10.1016/j.bbaexp.2006.01.003. Epub 2006 Jan 26. [DOI] [PubMed] [Google Scholar]
- [76].Sinha J, Chen F, Miloh T, Burns RC, Yu Z, Shneider BL, beta-Klotho and FGF15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes, Am. J. Physiol. Gastrointest. Liver Physiol 295 (5) (Nov 2008) G996–G1003, 10.1152/ajpgi.90343.2008 (Epub 2008 Sep 4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ, Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor, J. Biol. Chem 276 (31) (Aug 3 2001) 28857–28865, 10.1074/jbc.M011610200 (Epub 2001 May 31). [DOI] [PubMed] [Google Scholar]
- [78].Kassam A, Miao B, Young PR, Mukherjee R, Retinoid X receptor (RXR) agonist-induced antagonism of farnesoid X receptor (FXR) activity due to absence of coactivator recruitment and decreased DNA binding, J. Biol. Chem 278 (12) (Mar 21 2003) 10028–10032, 10.1074/jbc.M208312200 (Epub 2003 Jan 7). [DOI] [PubMed] [Google Scholar]
- [79].Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, Blaner WS, An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro, J. Lipid Res 41 (6) (Jun 2000) 882–893. [PubMed] [Google Scholar]
- [80].Ohata M, Lin M, Satre M, Tsukamoto H, Diminished retinoic acid signaling in hepatic stellate cells in cholestatic liver fibrosis, Am. J. Phys 272 (3 Pt 1) (Mar 1997) G589–G596, 10.1152/ajpgi.1997.272.3.G589. [DOI] [PubMed] [Google Scholar]
- [81].Cortes E, Lachowski D, Rice A, Chronopoulos A, Robinson B, Thorpe S, Lee DA, Possamai LA, Wang H, Pinato DJ, Del Río Hernández AE, Retinoic acid receptor-β is downregulated in hepatocellular carcinoma and cirrhosis and its expression inhibits myosin-driven activation and durotaxis in hepatic stellate cells, Hepatology 69 (2) (Feb 2019) 785–802, 10.1002/hep.30193 (Epub 2018 Dec 17). [DOI] [PubMed] [Google Scholar]
- [82].Wang H, Dan Z, Jiang H, Effect of all-trans retinoic acid on liver fibrosis induced by common bile duct ligation in rats, J. Huazhong Univ. Sci. Technol. Med. Sci 28 (5) (Oct 2008) 553–557, 10.1007/s11596-008-0514-x (Epub 2008 Oct 10). [DOI] [PubMed] [Google Scholar]
- [83].He H, Mennone A, Boyer JL, Cai SY, Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells, Hepatology 53 (2) (Feb 2011) 548–557, 10.1002/hep.24047 (Epub 2010 Dec 10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cai SY, Mennone A, Soroka CJ, Boyer JL, All-trans-retinoic acid improves cholestasis in α-naphthylisothiocyanate-treated rats and Mdr2−/− mice, J. Pharmacol. Exp. Ther 349 (1) (Apr 2014) 94–98, 10.1124/jpet.113.209353 (Epub 2014 Feb 3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Mezaki Y, Yoshikawa K, Yamaguchi N, Miura M, Imai K, Kato S, Senoo H, Rat hepatic stellate cells acquire retinoid responsiveness after activation in vitro by post-transcriptional regulation of retinoic acid receptor alpha gene expression, Arch. Biochem. Biophys 465 (2) (Sep 15 2007) 370–379, 10.1016/j.abb.2007.06.024 (Epub 2007 Jul 14). [DOI] [PubMed] [Google Scholar]
- [86].Milliano MT, Luxon BA, Rat hepatic stellate cells become retinoid unresponsive during activation, Hepatol. Res 33 (3) (Nov 2005) 225–233, 10.1016/j.hepres.2005.08.007 (Epub 2005 Oct 25). [DOI] [PubMed] [Google Scholar]
- [87].Mezaki Y, Yamaguchi N, Yoshikawa K, Miura M, Imai K, Itoh H, Senoo H, Insoluble, speckled cytosolic distribution of retinoic acid receptor alpha protein as a marker of hepatic stellate cell activation in vitro, J. Histochem. Cytochem 57 (7) (Jul 2009) 687–699, 10.1369/jhc.2009.953208 (Epub 2009 Mar 30). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Li H, Zhang J, Huang G, Zhang N, Chen Q, Zhang X, Effect of retinoid kappa receptor alpha (RXRalpha) transfection on the proliferation and phenotype of rat hepatic stellate cells in vitro, Chin. Med. J 115 (6) (Jun 2002) 928–932. [PubMed] [Google Scholar]
- [89].Hellemans K, Verbuyst P, Quartier E, Schuit F, Rombouts K, Chandraratna RA, Schuppan D, Geerts A, Differential modulation of rat hepatic stellate phenotype by natural and synthetic retinoids, Hepatology 39 (1) (Jan 2004) 97–108, 10.1002/hep.20015. [DOI] [PubMed] [Google Scholar]
- [90].Sharvit E, Abramovitch S, Reif S, Bruck R, Amplified inhibition of stellate cell activation pathways by PPAR-γ, RAR and RXR agonists, PLoS One 8 (10) (Oct 1 2013), e76541, 10.1371/journal.pone.0076541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wang L, Tankersley LR, Tang M, Potter JJ, Mezey E, Regulation of the murine alpha(2)(I) collagen promoter by retinoic acid and retinoid X receptors, Arch. Biochem. Biophys 401 (2) (May 15 2002) 262–270, 10.1016/S0003-9861(02)00058-9. [DOI] [PubMed] [Google Scholar]
- [92].Pan L, Eckhoff C, Brinckerhoff CE, Suppression of collagenase gene expression by all-trans and 9-cis retinoic acid is ligand dependent and requires both RARs and RXRs, J. Cell. Biochem 57 (4) (Apr 1995) 575–589, 10.1002/jcb.240570402. [DOI] [PubMed] [Google Scholar]
- [93].Salbert G, Fanjul A, Piedrafita FJ, Lu XP, Kim SJ, Tran P, Pfahl M, Retinoic acid receptors and retinoid X receptor-alpha down-regulate the transforming growth factor-beta 1 promoter by antagonizing AP-1 activity, Mol. Endocrinol 7 (10) (Oct 1993) 1347–1356, 10.1210/mend.7.10.8264664. [DOI] [PubMed] [Google Scholar]
- [94].Nicholson RC, Mader S, Nagpal S, Leid M, Rochette-Egly C, Chambon P, Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site, EMBO J. 9 (13) (Dec 1990) 4443–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Takahashi Y, Inoue J, Kagechika H, Sato R, ApoC-III gene expression is sharply increased during adipogenesis and is augmented by retinoid X receptor (RXR) agonists, FEBS Lett. 583 (2) (Jan 22 2009) 493–497, 10.1016/j.febslet.2008.12.050 (Epub 2008 Dec 31). [DOI] [PubMed] [Google Scholar]
- [96].Trasino SE, Tang XH, Jessurun J, Gudas LJ, Retinoic acid receptor β2 agonists restore glycaemic control in diabetes and reduce steatosis, Diabetes Obes. Metab 18 (2) (Feb 2016) 142–151, 10.1111/dom.12590 (Epub 2015 Dec 23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Crestani M, Sadeghpour A, Stroup D, Galli G, Chiang JY, The opposing effects of retinoic acid and phorbol esters converge to a common response element in the promoter of the rat cholesterol 7 alpha-hydroxylase gene (CYP7A), Biochem. Biophys. Res. Commun 225 (2) (Aug 14 1996) 585–592, 10.1006/bbrc.1996.1215. [DOI] [PubMed] [Google Scholar]
- [98].Li D, Zimmerman TL, Thevananther S, Lee HY, Kurie JM, Karpen SJ, Interleukin-1 beta-mediated suppression of RXR:RAR transactivation of the Ntcp promoter is JNK-dependent, J. Biol. Chem 277 (35) (Aug 30 2002) 31416–31422, 10.1074/jbc.M204818200 (Epub 2002 Jun 24). [DOI] [PubMed] [Google Scholar]
- [99].Arrese M, Trauner M, Ananthanarayanan M, Pizarro M, Solís N, Accatino L, Soroka C, Boyer JL, Karpen SJ, Miquel JF, Suchy FJ, Down-regulation of the Na+/taurocholate cotransporting polypeptide during pregnancy in the rat, J. Hepatol 38 (2) (Feb 2003) 148–155, 10.1016/s0168-8278(02)00379-3. [DOI] [PubMed] [Google Scholar]
- [100].Sturm E, Havinga R, Baller JF, Wolters H, van Rooijen N, Kamps JA, Verkade HJ, Karpen SJ, Kuipers F, Kupffer cell depletion with liposomal clodronate prevents suppression of Ntcp expression in endotoxin-treated rats, J. Hepatol 42 (1) (Jan 2005) 102–109, 10.1016/j.jhep.2004.09.019. [DOI] [PubMed] [Google Scholar]
- [101].Yu D, Cai SY, Mennone A, Vig P, Boyer JL, Cenicriviroc, a cytokine receptor antagonist, potentiates all-trans retinoic acid in reducing liver injury in cholestatic rodents, Liver Int. 38 (6) (Jun 2018) 1128–1138, 10.1111/liv.13698 (Epub 2018 Feb 13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Jie Z, Liang Y, Yi P, Tang H, Soong L, Cong Y, Zhang K, Sun J, Retinoic acid regulates immune responses by promoting IL-22 and modulating S100 proteins in viral hepatitis, J. Immunol 198 (9) (May 1 2017) 3448–3460, 10.4049/jimmunol.1601891 (Epub 2017 Mar 31). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Elshal M, Abu-Elsaad N, El-Karef A, Ibrahim T, Retinoic acid modulates IL-4, IL-10 and MCP-1 pathways in immune mediated hepatitis and interrupts CD4+ T cells infiltration, Int. Immunopharmacol 75 (Oct 2019) 105808, 10.1016/j.intimp.2019.105808 (Epub 2019 Aug 13). [DOI] [PubMed] [Google Scholar]
- [104].Kim CH, Retinoic acid, immunity, and inflammation, Vitam. Horm 86 (2011) 83–101, 10.1016/B978-0-12-386960-9.00004-6. [DOI] [PubMed] [Google Scholar]
- [105].Erkelens MN, Mebius RE, Retinoic acid and immune homeostasis: a balancing act, Trends Immunol. 38 (3) (Mar 2017) 168–180, 10.1016/j.it.2016.12.006 (Epub 2017 Jan 13). [DOI] [PubMed] [Google Scholar]
- [106].Cippitelli M, Ye J, Viggiano V, Sica A, Ghosh P, Gulino A, Santoni A, Young HA, Retinoic acid-induced transcriptional modulation of the human interferon-gamma promoter, J. Biol. Chem 271 (43) (Oct 25 1996) 26783–26793, 10.1074/jbc.271.43.26783. [DOI] [PubMed] [Google Scholar]
- [107].Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G, Im SY, Lee JW, Kim TS, Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB, J. Biol. Chem 274 (12) (Mar 19 1999) 7674–7680, 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- [108].Hoang TX, Jung JH, Kim JY, All-trans retinoic acid enhances bacterial flagellin-stimulated proinflammatory responses in human monocyte THP-1 cells by upregulating CD14, Biomed. Res. Int 2019 (Dec 26 2019), 8059312, 10.1155/2019/8059312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Dzhagalov I, Chambon P, He YW, Regulation of CD8+ T lymphocyte effector function and macrophage inflammatory cytokine production by retinoic acid receptor gamma, J. Immunol 178 (4) (Feb 15 2007) 2113–2121, 10.4049/jimmunol.178.4.2113. [DOI] [PubMed] [Google Scholar]
- [110].Schüle R, Rangarajan P, Yang N, Kliewer S, Ransone LJ, Bolado J, Verma IM, Evans RM, Retinoic acid is a negative regulator of AP-1-responsive genes, Proc. Natl. Acad. Sci. U. S. A 88 (14) (Jul 15 1991) 6092–6096, 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Xu J, Drew PD, 9-Cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes, J. Neuroimmunol 171 (1–2) (Feb 2006) 135–144, 10.1016/j.jneuroim.2005.10.004 (Epub 2005 Nov 21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dheen ST, Jun Y, Yan Z, Tay SS, Ling EA, Retinoic acid inhibits expression of TNF-alpha and iNOS in activated rat microglia, Glia 50 (1) (Apr 1 2005) 21–31, 10.1002/glia.20153. [DOI] [PubMed] [Google Scholar]
- [113].van Neerven S, Regen T, Wolf D, Nemes A, Johann S, Beyer C, Hanisch UK, Mey J, Inflammatory chemokine release of astrocytes in vitro is reduced by all-trans retinoic acid, J. Neurochem 114 (5) (Sep 1 2010) 1511–1526, 10.1111/j.1471-4159.2010.06867.x (Epub 2010 Jun 16). [DOI] [PubMed] [Google Scholar]
- [114].Nagpal S, Athanikar J, Chandraratna RA, Separation of transactivation and AP1 antagonism functions of retinoic acid receptor alpha, J. Biol. Chem 270 (2) (Jan 13 1995) 923–927, 10.1074/jbc.270.2.923. [DOI] [PubMed] [Google Scholar]
- [115].Alique M, Lucio FJ, Herrero JF, Vitamin A active metabolite, all-trans retinoic acid, induces spinal cord sensitization. II. Effects after intrathecal administration, Br. J. Pharmacol 149 (1) (Sep 2006) 65–72, 10.1038/sj.bjp.0706826 (Epub 2006 Jul 17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Camacho M, Rodríguez C, Salazar J, Martínez-González J, Ribalta J, Escudero JR, Masana L, Vila L, Retinoic acid induces PGI synthase expression in human endothelial cells, J. Lipid Res 49 (8) (Aug 2008) 1707–1714, 10.1194/jlr.M700559-JLR200 (Epub 2008 May 5). [DOI] [PubMed] [Google Scholar]
- [117].Schneider Aguirre R, Karpen SJ, Inflammatory mediators increase SUMOylation of retinoid X receptor α in a c-Jun N-terminal kinase-dependent manner in human hepatocellular carcinoma cells, Mol. Pharmacol 84 (2) (Aug 2013) 218–226, 10.1124/mol.113.085555 (Epub 2013 May 20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Mihály J, Gericke J, Aydemir G, Weiss K, Carlsen H, Blomhoff R, Garcia J, Rühl R, Reduced retinoid signaling in the skin after systemic retinoid-X receptor ligand treatment in mice with potential relevance for skin disorders, Dermatology 225 (4) (2012) 304–311, 10.1159/000345496 (Epub 2012 Dec 22). [DOI] [PubMed] [Google Scholar]
- [119].Wu Q, Dawson MI, Zheng Y, Hobbs PD, Agadir A, Jong L, Li Y, Liu R, Lin B, Zhang XK, Inhibition of trans-retinoic acid-resistant human breast cancer cell growth by retinoid X receptor-selective retinoids, Mol. Cell. Biol 17 (11) (Nov 1997) 6598–6608, 10.1128/mcb.17.11.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Altucci L, Rossin A, Raffelsberger W, Reitmair A, Chomienne C, Gronemeyer H, Retinoic acid-induced apoptosis in leukemia cells is mediated by paracrine action of tumor-selective death ligand TRAIL, Nat. Med 7 (6) (Jun 2001) 680–686, 10.1038/89050. [DOI] [PubMed] [Google Scholar]
- [121].Saeed A, Bartuzi P, Heegsma J, Dekker D, Kloosterhuis N, de Bruin A, Jonker JW, van de Sluis B, Faber KN, Impaired hepatic vitamin A metabolism in NAFLD mice leading to vitamin A accumulation in hepatocytes, Cell. Mol. Gastroenterol. Hepatol 11 (1) (2021), 10.1016/j.jcmgh.2020.07.006 (309–325.e3. Epub 2020 Jul 19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Murakami K, Kaji T, Shimono R, Hayashida Y, Matsufuji H, Tsuyama S, Maezono R, Kosai K, Takamatsu H, Therapeutic effects of vitamin A on experimental cholestatic rats with hepatic fibrosis, Pediatr. Surg. Int 27 (8) (Aug 2011) 863–870, 10.1007/s00383-011-2853-0 (Epub 2011 Feb 3). [DOI] [PubMed] [Google Scholar]
- [123].Erickson JM, Mawson AR, Possible role of endogenous retinoid (Vitamin A) toxicity in the pathophysiology of primary biliary cirrhosis, J. Theor. Biol 206 (1) (Sep 7 2000) 47–54, 10.1006/jtbi.2000.2102. [DOI] [PubMed] [Google Scholar]
- [124].Fallon MB, Boyer JL, Hepatic toxicity of vitamin A and synthetic retinoids, J. Gastroenterol. Hepatol 5 (3) (May–Jun 1990) 334–342, 10.1111/j.1440-1746.1990.tb01635.x. [DOI] [PubMed] [Google Scholar]
- [125].Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R, The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis, Hepatology 32 (6) (Dec 2000) 1196–1199, 10.1053/jhep.2000.20240. [DOI] [PubMed] [Google Scholar]
- [126].Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ, REGENERATE Study Investigators, Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial, Lancet 394 (10215) (Dec 14 2019) 2184–2196, 10.1016/S0140-6736(19)33041-7 (Epub 2019 Dec 5. Erratum in: Lancet. 2020 Aug 1;396(10247):312). [DOI] [PubMed] [Google Scholar]
- [127].Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F, High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis, Hepatology 50 (3) (Sep 2009) 808–814, 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Roth RA, Hewett JA, The cholestatic agent, alpha-naphthylisothiocyanate, stimulates superoxide release by rat neutrophils in vitro, Lab. Investig 62 (6) (Jun 1990) 736–741. [PubMed] [Google Scholar]
- [129].Assis DN, Abdelghany O, Cai SY, Gossard AA, Eaton JE, Keach JC, Deng Y, Setchell KD, Ciarleglio M, Lindor KD, Boyer JL, Combination therapy of all-trans retinoic acid with ursodeoxycholic acid in patients with primary sclerosing cholangitis: a human pilot study, J. Clin. Gastroenterol 51 (2) (Feb 2017) e11–e16, 10.1097/MCG.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].le Maire A, Alvarez S, Shankaranarayanan P, Lera AR, Bourguet W, Gronemeyer H, Retinoid receptors and therapeutic applications of RAR/RXR modulators, Curr. Top. Med. Chem 12 (6) (2012) 505–527, 10.2174/156802612799436687. [DOI] [PubMed] [Google Scholar]
- [131].Kagechika H, Kawachi E, Hashimoto Y, Himi T, Shudo K, Retinobenzoic acids. 1. Structure-activity relationships of aromatic amides with retinoidal activity, J. Med. Chem 31 (11) (Nov 1988) 2182–2192, 10.1021/jm00119a021 (Erratum in: J Med Chem 1989 Dec;32(12):2583). [DOI] [PubMed] [Google Scholar]
- [132].Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, Wagoner M, Mais DE, Suto CM, Davies JA, Heyman RA, et al. , Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells, J. Med. Chem 38 (16) (Aug 4 1995) 3146–3155, 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]