Abstract
Objective:
Evaluate reduction in progressively motile sperm per high power field (HPF) in midcycle cervical mucus after intercourse with Ovaprene: an investigational monthly non-hormonal vaginal contraceptive consisting of a vaginal ring and mechanical barrier, releasing spermiostatic ferrous gluconate.
Study design:
Open-label, multicenter study enrolling heterosexually-active women with previous permanent contraception. Participants underwent a baseline postcoital test cycle with no device to confirm the presence of sperm, followed by one diaphragm postcoital test cycle, one Ovaprene safety cycle, and two Ovaprene postcoital test cycles. In each postcoital test cycle, participants underwent a midcycle cervical mucus evaluation to confirm an Insler score > 10 and absence of sperm, and then returned two to four hours after vaginal intercourse for repeat cervical mucus evaluation. We considered < 5 progressively motile sperm/HPF indicative of preliminary contraceptive effectiveness.
Results:
We enrolled 38 participants; 23 completed the study. All participants had ≥ 5 progressively motile sperm/HPF in the baseline cycle and < 5 progressively motile sperm/HPF in all 49 Ovaprene cycles and all 35 diaphragm cycles, meeting the definition of a successful postcoital test. This was true regardless of examiner blinding, prior vaginal delivery or vaginal ring use, body mass index, or dislodgements noted by the participant or investigator. The mean of 27.2 (± 17.9) progressively motile sperm/HPF in baseline postcoital test cycles was reduced to 0.5 (± 1.1) and 0.5 (± 1.3) progressively motile sperm/HPF in the first and second Ovaprene cycles, respectively. Ovaprene fit all participants and all could insert, position, and remove it.
Conclusion:
Use of Ovaprene resulted in meeting the prespecified criterion for contraceptive effect by all participants during all postcoital test cycles.
Implications:
The finding that use of Ovaprene, an investigational monthly non-hormonal vaginal contraceptive, resulted in postcoital testing of cervical mucus that met the pre-specified definition of success (< 5 progressively motile sperm/HPF) supports further evaluation of contraceptive efficacy of the device in users at risk for pregnancy.
Keywords: Contraception, Contraceptive, Ovaprene, Postcoital test, Vaginal
1. Introduction
Ovaprene (Poly-Med, Inc., Anderson, SC), an investigational monthly non-hormonal vaginal contraceptive, consists of a 55 mm silicone ring with a central permeable barrier (Fig. 1). The barrier’s pore size inhibits movement of sperm while allowing passage of fluids. The ring releases ferrous gluconate and ascorbic acid. Ferrous gluconate causes oxidative damage to the lipid bilayer of the sperm tail, leading to spermiostasis [1]. Ascorbic acid maintains ferrous gluconate in its ferrous state. Unlike other vaginal barrier methods, Ovaprene is inserted at the end of one menstrual period and left until the beginning of next, requiring no action at intercourse. It requires no clinician fitting, and a new product is used each month.
Fig. 1.
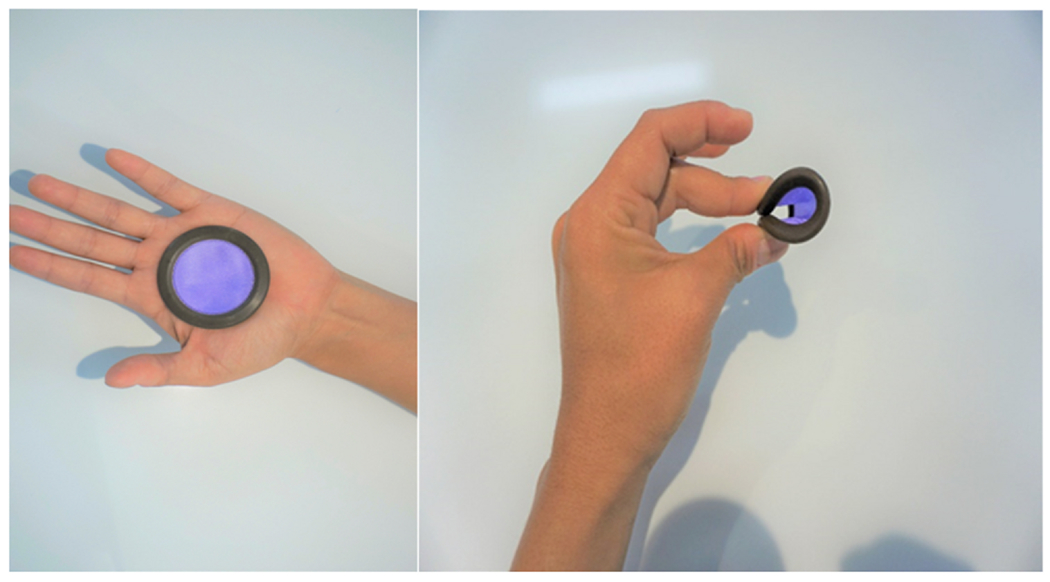
Ovaprene, an investigational vaginal contraceptive evaluated in a 2019 United States multicenter study. Ovaprene consists of a 55 mm silicone ring with a central permeable barrier. The barrier’s pore size inhibits movement of sperm while allowing passage of fluids. Ferrous gluconate released from the ring causes oxidative damage to the lipid bilayer of the sperm tail, leading to spermiostasis. Ascorbic acid is released to maintain ferrous gluconate in its ferrous state. Unlike other vaginal barrier methods, the Ovaprene is inserted at the end of one menstrual period and left until the beginning of the next, requiring no action at intercourse. It requires no clinician fitting, and a new product is used each month.
The postcoital test provides an objective evaluation of sperm entry into cervical mucus, a requisite step for natural fertility [2]. To evaluate a vaginal contraceptive, women relying on permanent contraception undergo midcycle postcoital testing in one cycle without the vaginal contraceptive and another with it [2]. In valid baseline cycles, a pre-specified minimum number of progressively motile sperm per high power field (HPF) averaged over nine HPFs must be present. In a test cycle indicative of efficacy, no more than a pre-specified maximum number of progressively motile sperm/HPF averaged over nine HPFs can be observed. In a 2009 postcoital test study evaluating Ovaprene, 20 sexually active participants used the device for one cycle [3]. No motile sperm were seen in the cervix in any subject. The device stayed over the cervix for up to 29 days. No mucosal changes were seen, wet mount examinations were normal, and semi-quantitative vaginal cultures showed no significant changes. Subjects reported no pain, bleeding, or discharge.
This paper describes our recent postcoital test study. While there have been no formulation changes in the product, our study was designed to provide more robust data prior to initiating a Phase 3 trial in that it included a baseline postcoital test cycle and two investigational product postcoital test cycles, as well as a diaphragm postcoital test cycle.
2. Methods
2.1. Design
We conducted a multi-center, open-label study to assess Ovaprene’s ability to prevent sperm from penetrating midcycle cervical mucus. We also assessed fit and ease of placement (reported here), and safety, release of ferrous gluconate, and acceptability (reported elsewhere).
We initiated the study at six sites: Eastern Virginia Medical School, Norfolk, VA; Oregon Health and Science University, Portland, OR; University of Pennsylvania, Philadelphia, PA; Clinical Research Prime, Idaho Falls, ID; University of California Davis, Sacramento, CA; and Segal Institute for Clinical Research Inc., Miami, FL. We consented and screened participants, but did not enroll at the last two sites. The study followed principles in the Helsinki Declaration of 1975, as revised in 2013. It was approved by the Advarra Institutional Review Board (Columbia, Maryland) before screening began. The ClinicalTrials.gov Identifier is NCT03598088.
2.2. Eligibility criteria
The trial was to recruit approximately 45 healthy, sexually active women who were not at risk for pregnancy due to previous permanent contraception and who reported regular menstrual cycles of 24–35 days, and their male partners, with the goal of approximately 25 couples completing the study (Appendix 2 – eligibility criteria, Supplementary Material). We did not use statistical considerations to determine the sample size; rather, we determined sample size by the maximum number of subjects who could be enrolled and complete this rigorous protocol in a reasonable time frame [2].
2.3. Study visits
We saw each woman in 21 visits during five menstrual cycles (Fig. 2 – Study visits and cycles): one baseline postcoital test cycle (no device) to collect baseline information on participants and demonstrate a postcoital test result consistent with unprotected intercourse at ovulation (> 5 progressively motile sperm/HPF); one diaphragm postcoital test cycle using the United States Food and Drug Administration-approved Caya diaphragm (HPSRx Enterprises, Inc., Salem, VA, known as the “SILCS diaphragm” during development) [4–6] with 3% nonxynol-9 (Gynol II Vaginal Contraceptive Gel, Revive Personal Products Company, Madison, NJ), used to demonstrate that our postcoital test, done with a marketed product, showed the expected contraceptive surrogate effect (< 5 progressively motile sperm/HPF) with an approved product); one Ovaprene safety, ferrous gluconate release, and acceptability assessment cycle with no acts of intercourse (abbreviated as “Ovaprene safety cycle”); and two Ovaprene postcoital test cycles evaluating one act of intercourse at the time of ovulation.
Fig. 2.
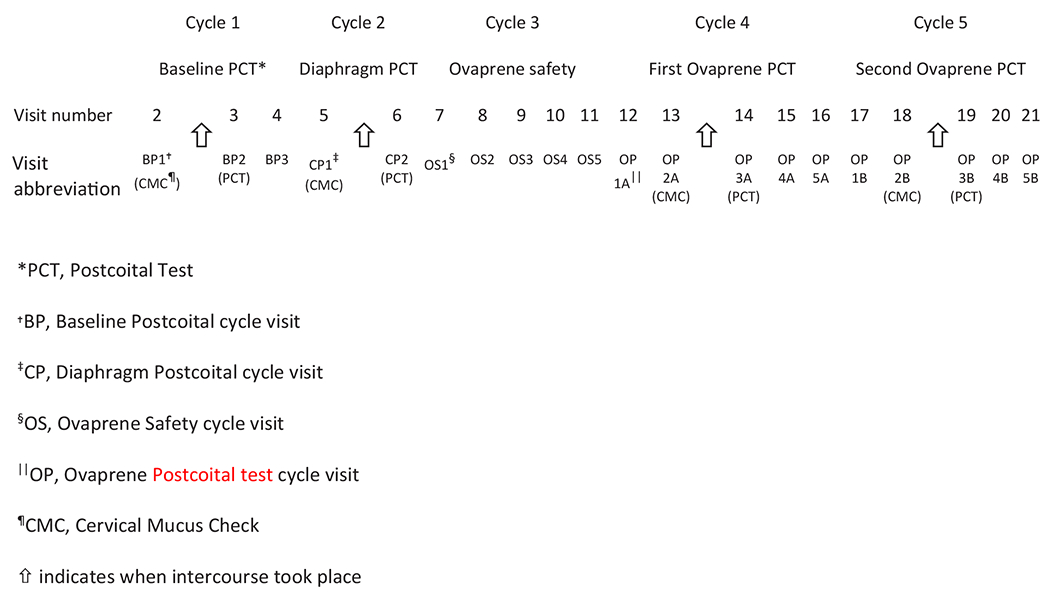
Study visits and cycles (following Visit 1 – Screening) in a 2019 United States multicenter study evaluating Ovaprene, an investigational vaginal contraceptive.
2.4. Objectives
The study’s objectives and endpoints are shown in Appendix 1, Supplementary Material. This paper describes the first objective (changes in postcoital test results due to device use) and one of the tertiary objectives (fit/placement). The procedures for and results of the remaining objectives will be described in detail elsewhere.
2.5. Study procedures – evaluation of cervical mucus
Informed consent and screening took place at Visit 1. We instructed participants about a web-based electronic diary (Trials.ai, San Diego, CA) through which they were prompted daily to report menses, intercourse, use of intravaginal products, adverse events (AEs), medications, and any device issues. Enrollment occurred at the fourth visit (the third visit in the baseline postcoital test cycle, or BP3 – Fig. 2) after all screening criteria, including a satisfactory baseline postcoital test cycle, had been met.
We carefully controlled intercourse timing and sample collection. At the beginning of the baseline, diaphragm, and Ovaprene postcoital test cycles, the participant contacted the site at the onset of her menses (cycle day 1). On cycle day 10, she began daily urine testing using an ovulation predictor kit (Clearblue Advanced Digital Ovulation Test) and contacted the site when the test yielded a “high” or “peak” result, indicating impending ovulation. The site scheduled the Cervical Mucus Check visit on that day or the next. We asked participants to use condoms on cycle days 1–10. From day 10 until after the Cervical Mucus Check visit, the participant abstained from intercourse and other vaginal activity and the male partner abstained from ejaculation.
In the two Ovaprene postcoital test cycles, we also saw the participant on the day following the end of menses (OP1, approximately cycle day 6) at which time the participant inserted Ovaprene and left it in place until the onset of the next menses, per product instructions. We sampled cervical mucus with Ovaprene in place by pulling Ovaprene’s anterior lip toward the posterior vaginal wall. Since the diaphragm is a pericoital contraceptive, the participant inserted it just before intercourse and the investigator removed it at the postcoital test visit.
At the Cervical Mucus Check visit (BP1, CP1, and both OP2 visits), we evaluated cervical mucus for midcycle characteristics and presence of sperm according to procedures adapted from the WHO Laboratory Manual for the Examination and Processing of Human Semen [7] and calculated a cervical mucus score (Insler score) per Figure 2. If we detected no sperm and, for the baseline and diaphragm cycles, the score was ≥10, we instructed the participant to have vaginal intercourse two to three hours before the postcoital test visit, scheduled for the same or following day. Because the ascorbic acid released from the ring may cause thickening of cervical mucus [8], we modified interpretation of Ovaprene cervical mucus checks such that a successful cervical mucus check was predefined to be the absence of sperm, regardless of score.
At the postcoital test visit (BP2, CP2, and both OP3 visits), we interpreted the results of vaginal and cervical mucus testing according to the most recently published postcoital test studies [2] (Appendix 3, Supplementary Material). A successful baseline postcoital test averaged ≥ 5 progressively motile sperm/HPF. A test cycle indicative of preliminary contraceptive effectiveness averaged < 5 progressively motile sperm/HPF.
2.6. Study procedures – evaluation of fit and placement
One tertiary objective was evaluating fit and placement of Ovaprene as follows; the investigator inserted the device and assessed fit by digital and speculum exam, according to pre-specified criteria: covering the cervix, not protruding outside the introitus, not easily dislodged, and not causing discomfort. The investigator removed the device and the participant attempted to insert and remove it using written product instructions. The investigator assisted as needed. If Ovaprene did not fit, or the participant could not insert, position, and remove it, even with assistance, the participant did not continue. For enrolled participants, the investigator assessed device position at every visit when Ovaprene was in place, a total of 15 times per participant, first via digital exam and then visually with a speculum.
2.7. Statistics
The primary statistical method for evaluating changes in the postcoital test due to device use (primary objective) for each product condition (baseline, diaphragm, Ovaprene) was the proportion of cycles (and 95% confidence interval) with an average (across nine HPFs) of < 5 progressively motile sperm/HPF, using SAS Version 9.4. We calculated the mean, median, standard deviation, and interquartile range (i.e., 25th, 75th percentiles) of each woman’s and cycle’s average number (across nine HPFs) of progressively motile sperm/HPF separately for baseline and each test postcoital test. We based qualitative assessments of change from baseline, if any, on the median and interquartile range, because of expected non-normality of data. We calculated these descriptive statistics by site and pooled across sites. There were no tests of statistical significance between the diaphragm and Ovaprene postcoital test cycle results. We expected that diaphragm postcoital test results in this study would be similar to published Caya postcoital test results [5,6], lending confidence to Ovaprene postcoital test results.
Although individuals examining cervical mucus are not typically blinded in postcoital test studies due to logistical difficulties, the examiner at Eastern Virginia Medical School was blinded to visit type and whether a barrier was used. We analyzed results by all sites combined, and by Eastern Virginia Medical School vs. the other sites combined to see if there was an effect of blinding.
We calculated the proportion of participants in whom the device fit correctly, the proportion who could correctly insert, position, and remove the device, and the proportion in whom the device was over the cervix at each visit.
3. Results
3.1. Enrollment, subject disposition, demographics
We consented the first participant on May 23, 2018; we made the last follow-up contact on November 15, 2019. We screened 135 participants and enrolled 38 (Fig. 3). Most screen fails (90.7%) were due to failure to meet eligibility criteria, usually failure to achieve target midcycle cervical mucus and/or an adequate number of progressively motile sperm/HPF at baseline. Thirty-five participants completed the diaphragm cycle, 26 completed at least one Ovaprene cycle, and 23 completed the study (five cycles). The most common discontinuation reasons were non-severe AEs (bacterial vaginosis) or withdrawing consent (four participants each). There were no serious AEs.
Fig. 3.
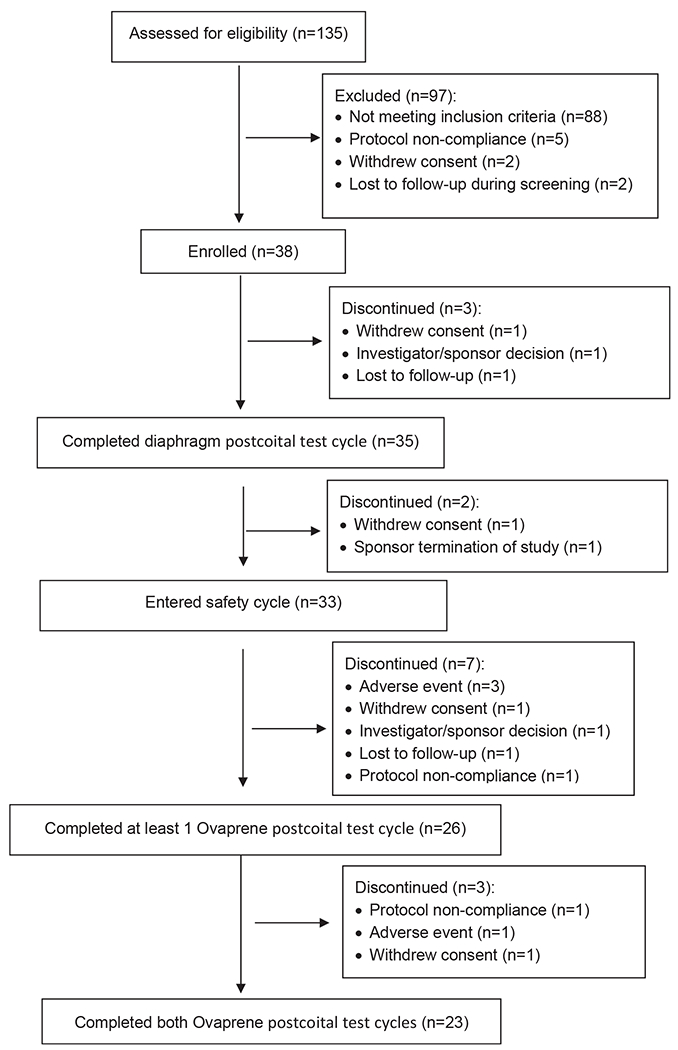
Participant disposition flow diagram in a 2019 United States multicenter study evaluating Ovaprene, an investigational vaginal contraceptive.
Demographics are shown in Table 1. Most (17/26 [65.4%]) participants completing an Ovaprene cycle had experienced at least one vaginal delivery and 11/26 (42.3%) had used a vaginal contraceptive ring.
Table 1.
Demographics of participants enrolled in a 2019 United States multicenter study evaluating Ovaprene, an investigational vaginal contraceptive
| Parameter category | Statistic | All enrolled participants (N = 38) | Ovaprene Populationa (N = 33) |
|---|---|---|---|
| Age Category, n (%) | 18–35 | 23 (60.5) | 19 (57.6) |
| 35–49 | 15 (39.5) | 14 (42.4) | |
| Ethnicity, n (%) | Hispanic/Latino | 3 (7.9) | 3 (9.1) |
| Not Hispanic/Latino | 34 (89.5) | 29 (87.9) | |
| Not Reported | 1 (2.6) | 1 (3.0) | |
| Raceb, n (%) | American Indian/Alaska Native | 0 | 0 |
| Asian | 2 (5.3) | 2 (6.1) | |
| Black | 6 (15.8) | 6 (18.2) | |
| Native Hawaiian/Other Pacific Islander | 0 | 0 | |
| White | 30 (78.9) | 26 (78.8) | |
| Otherc | 1 (2.6) | 0 | |
| Pt does not identify with any | 1 | 0 | |
| Body Mass Index Category, n (%) | Underweight (< 18.5) | 0 | 0 |
| Normal (18.5–24.9) | 13 (34.2) | 12 (36.4) | |
| Overweight (25.0–29.9) | 8 (21.1) | 7 (21.2) | |
| Obese (≥30.0) | 17 (44.7) | 14 (42.4) |
All participants who used Ovaprene in the study.
Race was a “Check All that Apply” question – a participant could check multiple races.
“Other” was a prespecified formal category in the database.
3.2. Cervical mucus evaluation
As expected [5,6], participants had fewer than five progressively motile sperm/HPF in all diaphragm cycles (data not shown). Participants had fewer than five progressively motile sperm/HPF in all 49 Ovaprene cycles, meeting the definition of a successful test postcoital test. This was true regardless of examiner blinding, history of vaginal delivery or vaginal ring use, body mass index (BMI), and dislodgements. Table 2 shows the mean, median, standard deviation, and interquartile range of progressively motile sperm/HPF for the baseline and Ovaprene cycles. The mean of 27.2 progressively motile sperm/HPF in the baseline cycle was reduced to 0.5 progressively motile sperm/HPF in the first and second Ovaprene cycles, respectively. When women were grouped by Eastern Virginia Medical School (blinded) vs. non-Eastern Virginia Medical School (not blinded) sites, the mean progressively motile sperm/HPF during Ovaprene cycles in both groups was less than one progressively motile sperm/HPF (0.08 and 0.00 in the first Ovaprene and second Ovaprene cycles, respectively, at Eastern Virginia Medical School, and 0.69 and 0.71 in the first Ovaprene and second Ovaprene cycles, respectively, at non-Eastern Virginia Medical School sites).
Table 2.
Analysis of progressively motile sperm per high power field across all cycles among participants enrolled in a 2019 United States multicenter study evaluating Ovaprene, an investigational vaginal contraceptive
| Baseline postcoital test cycle | First Ovaprene postcoital test cycle – Visit OP3Aa | Second Ovaprene postcoital test cycle – Visit OP3Bb | |
|---|---|---|---|
| n | 26 | 26 | 23 |
| Mean ± SDc | 27.2 ± 17.9 | 0.5 ± 1.1 | 0.5 ± 1.3 |
| Median | 23.2 | 0.0 | 0.0 |
| 25th, 75th percentiles | 16.1, 40.9 | 0.0, 0.2 | 0.0, 0.0 |
| Min, Max | 5.0, 74.2 | 0.0, 4.1 | 0.0, 4.7 |
OP3A, first Ovaprene postcoital test cycle, Visit 3.
OP3B, second Ovaprene postcoital test cycle, Visit 3.
SD, standard deviation.
We evaluated the effect of modifying the required mucus score in Ovaprene cycles via post-hoc analysis (Appendix 4, Supplementary Material). We predefined a successful cervical mucus check to be the absence of sperm regardless of score, following an ovulation predictor kit reading of “high” or “peak.” Compared with Insler scores in the baseline and Caya cycles combined, (cervical mucus score > 10 in 82.9%), we found a score of > 10 in 63.6% of Ovaprene cycles.
3.3. Fit and placement
Ovaprene fit all participants. The investigators were to allow the participants to attempt to insert, position, and remove Ovaprene first using written instructions only, without any verbal assistance. However, in the beginning of the study, the investigators misunderstood this and gave both written and verbal instructions first. We addressed this at approximately the same time that we revised the protocol to have Ovaprene fitting occur at BP3, after enrollment, rather than at Visit 1, to conserve Ovaprenes by not fitting participants who might be ineligible. At BP3, 13/15 (86.7%) were able to insert it using written instructions only, 12/15 (80%) could position it using written instructions only, and 11/15 (73.3%) could remove it using written instructions only. At subsequent visits, all participants were able to insert, position, and remove the device with written instructions only, except for one who needed verbal assistance to properly position the device. At no time in the study was physical assistance needed. Ovaprene was over the cervical os in 409 out of 421 (97.1%) of examinations.
4. Discussion
In comprehensive rigorous evaluation, use of Ovaprene resulted in a reduction in progressively motile sperm reaching midcycle cervical mucus consistent with preliminary evidence of contraceptive efficacy. Compared to baseline postcoital test results documenting motile sperm, use of both Ovaprene and the Caya diaphragm met the criterion for presumptive contraceptive efficacy (< 5 motile sperm/HPF), regardless of examiner blinding, history of vaginal delivery or vaginal ring use, BMI, and dislodgements.
Ovaprene is unique among vaginal contraceptives. Its primary mode of action is being a physical barrier to sperm, but, unlike diaphragms, it is not pericoital. It is a ring that is left in place for about 21 days, but, unlike the approved vaginal rings NuvaRing and Annovera, it does not contain hormones. Instead, its barrier function is augmented by release of the spermiostatic ferrous gluconate.
While a limitation of this type of study is that it provides only preliminary evidence of effectiveness, this postcoital test study was unusually rigorous with five objectives and 21 associated endpoints. Most postcoital test studies base results on 10 or fewer test cycles; this study with 49 completed cycles was unusually large [2]. Timing within the menstrual cycle and between intercourse and mucus testing were tightly controlled. It could be argued that allowing participants to proceed to Ovaprene postcoital test visits with a mucus score of < 10 at the Ovaprene Cervical Mucus Check visit could have created bias: because the cervical mucus could be thicker than it would be with a score of > 10, there could be fewer progressively motile sperm/HPF than there might have been if the mucus were thinner, biasing results toward success. However, to the extent that the effect of the scoring modification could be evaluated, it does not appear to have resulted in significant bias.
Ovaprene fit all participants and all were able to insert, position, and remove it, in most cases using only written instructions. The device was found to be over the cervix at almost every examination. Postcoital test cycles were successful even when the device was found to be out of place, probably reflecting the spermiostatic effect of ferrous gluconate.
A pivotal study is underway in which participants at risk of pregnancy will use the device for 13 cycles and actual contraceptive effectiveness will be assessed.
Supplementary Material
Acknowledgments
Drs. Thurman, Jensen, Schreiber, Baker, Hou, and Chavoustie were study investigators and are or were employed by institutions which received financial compensation from Daré Bioscience, Inc. Clint Dart and Dr. Wu were employed by Health Decisions, engaged by Daré Bioscience, Inc. to assist in the conduct of the study.
Funding:
Daré Bioscience, Inc. (Daré) was responsible for funding the study. Daré received non-dilutive grant funding from Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH to support the clinical development of Ovaprene and this study. Drs. Mauck, Thurman, and Friend, and Ms. Zack and Hatheway are or were employees of Daré Bioscience, Inc. and hold stock options or stock.
Footnotes
Conflicts of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Appendix A. Supporting material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.contraception.2024.110373.
References
- [1].Hong C, Lee MF, Lai LJ, Wang CP. Effect of lipid peroxidation on beating frequency of human sperm tail. Andrologia 1994;26:61–5. 10.1111/j.1439-0272.1994.tb00757.x [DOI] [PubMed] [Google Scholar]
- [2].Mauck CK, Vincent KL. The postcoital test in the development of new vaginal contraceptives. Biol Reprod 2020;103(2):437–44. 10.1093/biolre/ioaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Del Priore G, Malanowska-Stega J, Shalaby SW, Richman S. A pilot safety and tolerability study of a nonhormonal vaginal contraceptive ring. J Reprod Med 2009;54(11-12):685–90. [PubMed] [Google Scholar]
- [4].Schwartz JL, Weiner DH, Lai JJ, Frezieres RG, Creinin MD, Archer DF, et al. Contraceptive efficacy, safety, fit, and acceptability of a single-size diaphragm developed with end-user input. Obstet Gynecol 2015;125(4):895–903. 10.1097/AOG.0000000000000721 [DOI] [PubMed] [Google Scholar]
- [5].Mauck CK, Brache V, Kimble T, Thurman A, Cochon L, Littlefield S, et al. A phase I randomized postcoital testing and safety study of the Caya diaphragm used with 3% Nonoxynol-9 gel, ContraGel or no gel. Contraception 2017;96(2):124–30. 10.1016/j.contraception.2017.05.016 [DOI] [PubMed] [Google Scholar]
- [6].Schwartz JL, Ballagh SA, Creinin MD, Rountree RW, Kilbourne-Brook M, Mauck CK, et al. SILCS diaphragm: postcoital testing of a new single-size contraceptive device. Contraception 2008;78(3):237–44. 10.1016/jxontraception.2008.04.118 [DOI] [PubMed] [Google Scholar]
- [7].WHO. Laboratory manual for the examination and processing of human semen. fifth edition., Geneva: World Health Organization,; 2010. [Google Scholar]
- [8].Saxena BB, Singh M, Gospin RM, Chu CC, Ledger WJ. Efficacy of nonhormonal vaginal contraceptives from a hydrogel delivery system. Contraception 2004;70(3):213–9. 10.1016/jxontraception.2004.02.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


