Abstract
Antigens derived from host cells are detectable in the envelope of human immunodeficiency virus type 1 (HIV-1) and result in a distinctive viral phenotype reflecting that of the host cell. An immunomagnetic capture assay targeting discriminatory host proteins was developed to differentiate between HIV-1 derived from macrophages and lymphocytes. HIV-1 propagated in macrophages or lymphocytes in vitro was selectively captured by monoclonal antibodies directed against the virally incorporated cell-type-specific host markers CD36 (macrophages) and CD26 (lymphocytes). Furthermore, by targeting these markers, virus of defined cellular origin was selectively captured from a mixed pool of in vitro-propagated viruses. This technique was further refined in order to determine the impact of opportunistic infection on HIV-1 expression from these cellular compartments in vivo. Analysis of cell-free virus purified from plasma of patients with HIV-1 infection suggested that in those with an opportunistic infection, viral replication occurred in activated lymphocytes. Interestingly, there was also significant replication in activated macrophages in those patients with untreated pulmonary tuberculosis. Thus, in addition to lymphocytes, the macrophage cellular pool may serve as an important source of cell-free HIV-1 in patients with opportunistic infections that lead to marked macrophage activation. This novel viral capture technique may allow researchers to address a wide range of important questions regarding virus-host dynamics.
Both viral and cellular proteins are incorporated into the human immunodeficiency virus type 1 (HIV-1) envelope during viral maturation and release from host cells (1, 7, 18). Numerous cellular proteins have been identified in the HIV-1 envelope (reviewed in reference 30), including human lymphocyte antigen (HLA) classes I and II (22), CD44 (18), complement control proteins CD55 and CD59 (16), and the adhesion molecules LFA-1 and ICAM (10, 18). Although a number of such host-derived proteins retain functionality when incorporated into the viral envelope, the involvement of many of these host molecules in AIDS pathogenesis is not entirely clear. For example, CD44 in the HIV-1 envelope maintains hyaluronate-binding activity similar to that of the cell surface CD44 (10). Antibody neutralization of adhesion molecules in the viral envelope blocks HIV-1 infection, suggesting that these molecules may function as docking proteins for the virus (6). HLA class II molecules are detectable within the envelope of HIV-1 purified from patient plasma (23) and have been shown to function in superantigen presentation and to enhance viral infectivity in vitro (4, 22). Furthermore, the complement-regulatory proteins CD55 and CD59, which are incorporated in the HIV-1 envelope, block the formation of membrane attack complexes, suggesting a mechanism to evade complement-mediated lysis similar to that employed by normal human cells (24, 25, 28).
Incorporation of host proteins into the HIV-1 envelope leads to the acquisition of an antigenic phenotype that reflects that of the host cell (2), and we have previously demonstrated that this aspect of viral maturation is conserved among diverse HIV-1 subtypes (21). While many studies have focused on functionality of virion-associated host proteins, we previously suggested that cell-type-specific antigens might serve as markers of the cellular origin of HIV-1 replication (21). Indeed, HIV-1 derived in vitro from T cells and dendritic cells has recently been shown to incorporate discriminatory host antigens into the viral envelope (8). In this study, we describe an immunomagnetic viral capture assay that is able to distinguish between lymphocyte-derived and macrophage-derived viruses propagated in vitro based upon the detection of defined host antigens in the HIV-1 envelope. Furthermore, we demonstrate that this technique can be applied to clinical samples, yielding insights into the impact of opportunistic infection on HIV-1 replication in these cellular pools. Further refinement of this technique may provide a novel approach for addressing many issues related to virus-host dynamics and AIDS pathogenesis.
MATERIALS AND METHODS
Generation of in vitro HIV-1 stocks.
Stocks of macrophage-derived HIV-1Ba-L (HIV-1Ba-L-MΦ) were prepared by propagation of virus in purified normal human monocytes and were commercially obtained (Advanced Biotechnologies, Inc., Columbia, Md.). CD4+ T lymphocytes, purified (>95% pure) by affinity column exclusion (R&D Systems, Inc., Minneapolis, Minn.), were phytohemagglutinin activated and infected with either a syncytium-inducing field strain (f/s.8) or HIV-1Ba-L to generate two lymphocyte-derived stocks (HIV-1f/s.8 and HIV-1Ba-L-CD4, respectively). All HIV-1 stocks were further purified by standard sucrose density gradient centrifugation (32). Banded viral stocks were quantified by HIV-1 p24 antigen enzyme immunoassay (Coulter/Immunotech, Inc., Westbrook, Maine), and virion counts were estimated based on 100 pg of p24 antigen being equivalent to 106 virus particles (3).
Capture and detection of in vitro HIV-1 stocks.
Murine antibodies to human cellular antigens were selected based on the T-lymphocyte and monocyte cell surface density, as described by the Leukocyte Differentiation Antigen Database, and were obtained from commercial sources. Sheep anti-mouse immunoglobulin G magnetic beads (Dynal, Inc., Great Neck, N.Y.) were first conjugated with the murine anti-human antibodies (0.5 μg of antibody per 2 × 107 beads) by rotation at 4°C for 1.5 h. Conjugated beads were washed twice with standard phosphate-buffered saline containing 2% fetal bovine serum (PBS–2% FBS). Viral stocks were added at a concentration of 5 × 106 virions per 0.5 μg of capture antibody in PBS–2% FBS and rotated at 4°C for 1.5 h to allow virus immunocapture. Nonspecific viral binding was assessed by adding virus to unconjugated or anti-CD19-conjugated magnetic beads (negative control). After viral capture, magnetic beads were washed five times with PBS. Bound virions were lysed with 0.5% NP-40 in PBS for 30 min at 4°C, and the amount of captured virus was determined by using an HIV-1 p24 antigen enzyme immunoassay (Coulter).
Plasma interference with HIV-1 capture from serum samples.
To identify serum components potentially inhibitory to the capture of HIV-1 from clinical samples, HIV-1Ba-L-MΦ (5 × 106 virions) was first incubated with 10 μl of either PBS or various characterized patient sera at room temperature for 1 h prior to capture. Sera tested in this way were from uninfected healthy subjects, HIV-seropositive patients with an undetectable HIV-1 RNA load (to assess the effect of anti-HIV antibodies), and HIV-seronegative patients with acute hepatitis A (to assess the effect of acute-phase proteins). The relative extent of capture of HIV-1Ba-L-MΦ incubated in PBS or the different sera was subsequently determined by using anti-CD36, anti-HLA-DR, and anti-CD44 antibodies.
To overcome capture inhibition by serum components, a virus purification algorithm was devised. Briefly, HIV-1 was pelleted by ultracentrifugation at 87,000 × g for 1 h at 4°C and resuspended in 0.5 ml of PBS. Virus was then salt treated by being mixed with NaCl (final concentration, 0.5 M) at 37°C for 1 h and subsequently purified by passage through Microspin S-400 HR Sephacryl columns (Pharmacia Biotech, Piscataway, N.J.). The effectiveness of this algorithm was validated by capturing HIV-1Ba-L-MΦ that had been preincubated with defined inhibitory sera.
Patients.
With consent, plasma samples were obtained from patients attending the Komfo Anokye Teaching Hospital, Kumasi, Ghana. Patients with untreated pulmonary tuberculosis (TB) and HIV-1 coinfection (TB/HIV.1 to TB/HIV.3) had radiographic evidence of pulmonary consolidation and two sputum smears positive by conventional Ziehl-Neelsen microscopy. Plasma samples from patients with HIV-1 infection but no opportunistic infection (HIV.1 to HIV.4) and from one patient with a microbiologically undefined opportunistic infection (OI.HIV.4) were also obtained. HIV-1 infection in patients with positive HIV-1–HIV-2 serology was confirmed by using the Multispot assay (Sanofi Diagnostics Pasteur S.A., Marnes la Coquette, France). Plasma HIV-1 load was quantified by the Amplicor HIV-1 Monitor Test (Roche Diagnostic Systems, Inc., Branchburg, N.J.). Patients were divided into two groups according to the presence or absence of opportunistic infection, and the two groups of patients were matched for CD4+ lymphocyte count (all <200 × 106/liter) and for plasma HIV-1 RNA load (range, 2.9 × 105 to 2.7 × 106 versus 1.7 × 105 to 1.7 × 106 copies/ml). No patients had received antiretroviral drug treatment.
HIV-1 capture from clinical samples.
The standard capture protocol described above was optimized for analysis of virus purified from clinical samples as follows. A standardized input of 2 × 104 to 5 × 104 virions per capture was incubated with each of the conjugated beads for 4 h in the presence of PBS–5% normal human serum (NHS), and anti-interleukin-6 (anti-IL-6)-conjugated beads were used as the negative control. Bound virus was lysed and, in view of the input of virus per capture, HIV-1 was quantified by using the Amplicor HIV-1 Monitor Test (Roche Diagnostic Systems, Inc.) rather than by p24 antigen enzyme-linked immunosorbent assay (ELISA).
Markers of immune activation and acute-phase response.
Total tumor necrosis factor alpha (TNF-α), tumor necrosis factor receptor type 1 (TNF-R1, 55 kDa), and IL-6 were measured in patient plasma samples by using Quantikine ELISAs (R&D Systems). C-reactive protein, an acute-phase protein, and soluble serum CD14 (sCD14) were measured by enzyme immunoassays by using the Virgo CRP 150 Kit (Hemagen Diagnostics, Inc., Waltham, Mass.) and the sCD14 EASIA kit (Medgenix, Fleurus, Belgium), respectively.
RESULTS
Characterization of host molecules incorporated in the HIV-1 envelope in vitro.
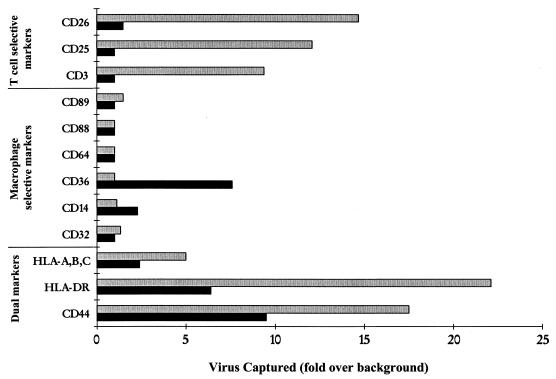
We examined whether HIV-1 propagated in different primary cell types in vitro could be distinguished based on incorporation of host-cell-specific antigens. A panel of antibodies against various cell surface CD antigens that might distinguish between macrophage-derived (HIV-1Ba-L-MΦ) and lymphocyte-derived (HIV-1f/s.8) viral stocks was examined (Fig. 1). Among antibodies to macrophage-specific markers, only anti-CD36 captured HIV-1Ba-L-MΦ to an appreciable extent. Anti-CD14 also captured HIV-1Ba-L-MΦ above the background level, but to a much lesser extent than anti-CD36. Antibodies to other antigens expressed at a high level by macrophages (CD32, CD64, CD88, and CD89) did not capture virus. Anti-CD3, anti-CD25, and anti-CD26 antibodies all selectively captured the T-cell-derived virus stock, HIV-1f/s.8. Both HIV-1Ba-L-MΦ and HIV-1f/s.8 were captured by anti-HLA-A/B/C-, anti-HLA-DR-, and anti-CD44-conjugated beads. Further experiments with antibodies to CD44, CD36, and CD26 determined the efficiency of capture of both lymphocyte- and macrophage-derived stocks in vitro (Table 1).
FIG. 1.
HIV-1 capture by antibodies targeted against highly expressed cell surface antigens selective for macrophages or CD4+ T lymphocytes. Antibodies against the macrophage-specific marker, CD36, but not the highly expressed complement receptors, CD32 and CD64, selectively captured HIV-1Ba-L-MΦ (■). T-cell-derived HIV-1f/s.8 ( ) was captured selectively by use of anti-CD3, anti-CD25, and anti-CD26. Antibodies to antigens common to both T lymphocytes and macrophages (HLA-A/B/C, HLA-DR, and CD44) captured both viral stocks. Data are representative of three independent experiments with <20% variability in the magnitude of capture.
) was captured selectively by use of anti-CD3, anti-CD25, and anti-CD26. Antibodies to antigens common to both T lymphocytes and macrophages (HLA-A/B/C, HLA-DR, and CD44) captured both viral stocks. Data are representative of three independent experiments with <20% variability in the magnitude of capture.
TABLE 1.
Percent capture of input macrophage- and lymphocyte-derived in vitro HIV-1 stocks (HIV-1Ba-L-MΦ and HIV-1Ba-L-CD4, respectively) with antibodies to CD44, CD36, CD26, and IL-6a
| Antibody | % Virus captured (mean ± SE)
|
|
|---|---|---|
| HIV-1Ba-L-MΦ | HIV-1Ba-L-CD4 | |
| CD44 | 42.1 ± 3.1 | 53.2 ± 3.6 |
| CD36 | 20.5 ± 1.7 | NS |
| CD26 | NS | 12.0 ± 1.9 |
| IL-6b | 1.9 ± 0.5 | 1.5 ± 0.4 |
Data represent three independent runs. NS, no significant capture above background.
Negative control antibody.
Change in HIV-1 envelope phenotype after acute infection of CD4+ T cells with macrophage-derived HIV-1Ba-L.
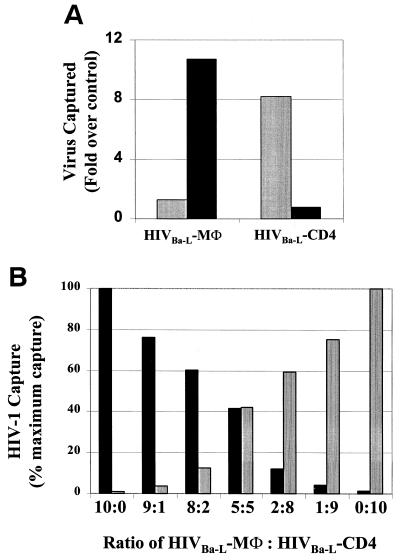
To confirm the ability of anti-CD26 and anti-CD36 antibodies to distinguish the cellular origin of HIV-1 replication, we examined phenotypic changes of the viral envelope after propagation of the same virus stock (HIV-1Ba-L) through either CD4+ T-cell or macrophage cultures (Fig. 2A). Virus propagated in macrophages (HIV-1Ba-L-MΦ) was captured by anti-CD36. In contrast, the same virus expanded in T-cell cultures (HIV-1Ba-L-CD4) acquired a switch in envelope phenotype, resulting from the incorporation of CD26 and the absence of CD36 (Fig. 2A). Both HIV-1Ba-L-MΦ and HIV-1Ba-L-CD4 incorporated HLA-DR in the envelope and were captured to similar extents (data not shown).
FIG. 2.
(A) Comparison of viral capture of macrophage- and lymphocyte-derived HIV-1. The HIV-1Ba-L-MΦ isolate was selectively captured by anti-CD36 (■). When HIV-1Ba-L-MΦ was propagated in T lymphocytes, the virus obtained (HIV-1Ba-L-CD4) was selectively captured by anti-CD26 ( ) and not by anti-CD36, indicating a discriminating phenotype for identifying the cellular origin of viral replication. (B) HIV-1Ba-L-MΦ and HIV-1Ba-L-CD4 isolates were mixed at various ratios and then captured with both anti-CD36 (■) and anti-CD26 (
) and not by anti-CD36, indicating a discriminating phenotype for identifying the cellular origin of viral replication. (B) HIV-1Ba-L-MΦ and HIV-1Ba-L-CD4 isolates were mixed at various ratios and then captured with both anti-CD36 (■) and anti-CD26 ( ). The amount of virus captured by each antibody was proportional to the input of each type of virus, further illustrating the selective capture of virus derived from diverse cell types. Data are representative of three independent experiments.
). The amount of virus captured by each antibody was proportional to the input of each type of virus, further illustrating the selective capture of virus derived from diverse cell types. Data are representative of three independent experiments.
We then examined the ability of anti-CD26 and anti-CD36 antibodies to differentially capture macrophage- and lymphocyte-derived viruses from a mixed viral pool (Fig. 2B). HIV-1Ba-L-CD4 and HIV-1Ba-L-MΦ stocks were mixed in various ratios. Capture of virus with either anti-CD26 or anti-CD36 antibody was proportional to the input of HIV-1Ba-L-CD4 and HIV-1Ba-L-MΦ, respectively (Fig. 2B), indicating that viral capture by these antibodies was selective for the host cell of origin.
Inhibition of capture by serum components.
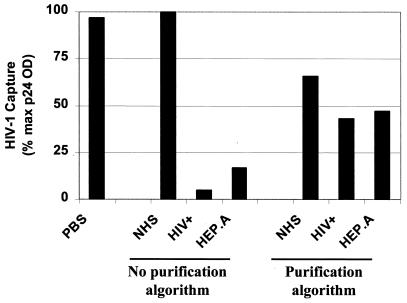
Initial attempts at HIV-1 capture from patient serum samples revealed substantial inhibition of capture. To delineate components within the HIV-infected serum that were responsible for this inhibition, in vitro-derived HIV-1Ba-L-MΦ was captured after preincubation in PBS, NHS, or sera containing anti-HIV antibodies or acute-phase proteins. In comparison to PBS, NHS had no inhibitory effect on capture of HIV-1Ba-L-MΦ (Fig. 3). However, HIV antibody-positive serum with an undetectable virus load (HIV+) and serum from the patient with acute hepatitis A infection (HEP.A) both inhibited anti-CD36 capture by >80%. The magnitude of this inhibition was similar with anti-HLA-DR and anti-CD44 antibodies (data not shown).
FIG. 3.
To investigate the potential inhibitory role of normal serum proteins, HIV antibodies, and acute-phase proteins in HIV-1 capture, HIV-1Ba-L-MΦ was first incubated in PBS, NHS, HIV antibody-positive serum (HIV+), and serum from a patient with acute hepatitis A infection (HEP.A). Virus was subsequently captured by using anti-CD36 antibody, and the experiment showed that both HIV+ and HEP.A sera were markedly inhibitory to virus capture. However, use of the virus purification algorithm substantially overcame the inhibitory effects of the HIV+ and HEP.A sera on capture. Data are representative of two independent experiments with <15% variability in the magnitude of capture.
To overcome the inhibition of the capture by patient serum proteins, a purification algorithm was developed. By using anti-CD36 antibody, capture of in vitro-derived HIV-1Ba-L-MΦ purified from NHS yielded >65% of the capturable virus (Fig. 3). More importantly, the algorithm substantially restored the capture of virus spiked into the defined HIV+ and HEP.A inhibitory sera.
Capture of HIV-1 from patient plasma reflects activation of specific cellular compartments by opportunistic infections.
We proceeded to analyze samples from HIV-infected patients who did or did not have an opportunistic infection in order to define the contributions of macrophage and lymphocyte cellular pools to the plasma viral load. Surprisingly, antibodies to the cell-type-specific antigens (CD26, CD36, and CD14) did not significantly capture HIV-1 from those patients who did not have an opportunistic infection (Fig. 4A). In contrast, however, virus from all four individuals with opportunistic infections was captured by the anti-CD26 antibody. Furthermore, antibodies to both macrophage-specific antigens (CD36 and CD14) captured virus from the three patients with active TB. Subsequent attempts to capture virus from three patients (TB/HIV.1, OI/HIV.4, and HIV.1) by using antibodies to other cell-type-specific antigens (CD3, CD45Ro, CD25, CD32, CD64, CD88, and CD89) did not demonstrate significant virus capture above background levels (data not shown). Anti-CD44 was used as the positive control antibody since it captured both lymphocyte- and macrophage-derived HIV-1 in vitro stocks (Fig. 1). Correspondingly, anti-CD44 antibody also successfully captured HIV-1 purified from the plasma of all eight patients (Fig. 4A).
FIG. 4.
(A) Capture of HIV-1 purified from plasma samples of HIV-infected patients by using antibodies to lymphocyte- and macrophage-specific markers. Three patients had untreated smear-positive pulmonary TB (TB/HIV.1 to TB/HIV.3), another had a microbiologically undefined opportunistic infection (OI/HIV.4), and four other patients (HIV.1 to HIV.4) had no opportunistic infection. A signal-to-background ratio of ≥0.5 log10 was taken as significant, and the data are representative of three independent experiments. HIV-1 from all samples was captured by anti-CD44 antibody, serving as a positive control. Virus from all four patients with an opportunistic infection was captured by antibody to the lymphocyte-specific marker (CD26), but only virus from those with TB was captured by antibodies to macrophage-specific markers (CD36 and CD14). Antibodies to CD26, CD14, and CD36 did not capture HIV-1 from patients with no opportunistic infection. (B) Plasma levels of acute-phase and immune activation markers expressed as a percentage of the maximum level of each seen in any patient. The maximum levels of TNF-α, TNF-R1, C-reactive protein, sCD14, and IL-6 were 22 pg/ml, 6,010 pg/ml, 220 μg/ml, 17.2 μg/ml, and 45 pg/ml, respectively.
Reproducible data regarding the efficiency of viral capture was obtained for two patients (Table 2). The high efficiency of HIV-1 capture with anti-CD44 antibody was similar to the efficiency of capture of in vitro stocks (Tables 1 and 2), and background nonspecific binding was low. In addition, >20% of plasma virus of patient TB/HIV.1 (untreated TB and HIV-1 coinfection) was captured by macrophage- and lymphocyte-specific antibodies (Table 2).
TABLE 2.
Percentage of purified HIV-1 captured from plasma of patient TB/HIV.1 (TB and HIV-1 coinfection) and patient HIV.1 (HIV-1 and no opportunistic infection) with a panel of antibodiesa
| Antibody | % Virus captured (mean ± SE)
|
|
|---|---|---|
| Patient TB/HIV.1 | Patient HIV.1 | |
| CD44 | 43.6 ± 9.8 | 53.0 ± 2.5 |
| CD36 | 7.0 ± 2.8 | NS |
| CD14 | 14.4 ± 4.2 | NS |
| CD26 | 6.4 ± 3.1 | NS |
| IL-6b | 1.2 ± 0.5 | 0.8 ± 0.3 |
Data represents three independent runs. NS, no significant capture above background.
Negative control antibody.
Cellular activation regulates the cell surface expression of CD36, CD14, and CD26. We therefore hypothesized that immune activation caused by opportunistic infection led to upregulation of these molecules on cells supporting viral replication and thereby facilitated the capture of virus incorporating high levels of these host antigens. To establish a correlation between immune activation and the ability to capture HIV-1 with antibodies to CD36, CD14, and CD26, soluble indicators of immune status were measured in the plasma samples from all eight patients. C-reactive protein was increased specifically in the four patients with opportunistic infection (Fig. 4B). More importantly, elevated plasma concentrations of TNF-α, TNF-R1, IL-6, and sCD14 confirmed a heightened state of systemic immune activation in patients with opportunistic infections. Of note, markedly elevated levels of the macrophage-derived cytokines (TNF-α and IL-6) were detected in the plasma of the three patients with active TB (TB/HIV.1 to TB/HIV.3) and this correlated with elevated plasma levels of sCD14, a soluble marker of macrophage activation. Correspondingly, HIV-1 was captured from plasma of the patients with TB by using antibodies directed against the macrophage-specific markers, i.e., CD14 and CD36.
DISCUSSION
Using an immunomagnetic capture technique, we demonstrated the ability to distinguish between macrophage-derived and lymphocyte-derived HIV-1, based upon the detection of cell-type-specific host antigens incorporated in the viral envelope. We also demonstrated that the envelope of macrophage-derived HIV-1 changes when the virus is propagated in T lymphocytes and acquires a new phenotype, reflecting that of the new host cell (Fig. 2A). Furthermore, we were able to selectively capture HIV-1 from a mixed pool of macrophage-derived and lymphocyte-derived viruses (Fig. 2B). These key observations enabled us to develop an assay to detect cell-free virus derived from macrophage and lymphocyte compartments in vivo. Results from the analysis of patient plasma samples suggest that the pattern of HIV-1 capture reflects the replication of virus in specific cellular pools that have been activated as part of the host response to opportunistic infection.
In developing an assay to distinguish HIV-1 derived from T lymphocytes or macrophages based on viral envelope phenotype, we restricted our choice of host protein targets to those that were specifically expressed at a high level on one cell type or the other. Selective exclusion of different host proteins from the HIV-1 envelope (18, 25; reviewed in reference 5) may account for the failure to capture in vitro-propagated HIV-1Ba-L-MΦ with antibodies to a number of macrophage markers known to be highly expressed by host cells (Fig. 1). Only anti-CD36 and, to a much lesser extent, anti-CD14 antibodies captured macrophage-derived HIV-1 stocks propagated in vitro. Although the significance to HIV-1 pathogenesis of the presence of many host molecules within the viral envelope is still largely speculative, CD36 functions as an adhesive receptor for defined cellular components (29) and potentially could serve an accessory docking role for HIV-1 attachment.
Among the markers to identify T-cell-derived HIV-1, antibodies against CD25 and CD26 captured HIV-1 with approximately equal efficiency, and antibody to CD3 captured virus to a lesser extent (Fig. 1). Since CD25 expression is not strictly specific to lymphocytes (26) and is substantially downregulated during progression of HIV-1 infection in vivo (11), we examined CD26 as the distinguishing T-lymphocyte marker in this assay. In support of this choice, we previously demonstrated that CD26 is readily detected in the viral envelope of lymphocyte-derived viruses representing diverse HIV-1 subtypes (21). The use of CD26 and CD36 as selective markers in this assay was further validated by demonstrating that a switch in envelope phenotype occurred when macrophage-derived HIV-1 was propagated in lymphocytes and also by demonstrating selective capture of macrophage- and lymphocyte-derived viruses from a mixed pool (Fig. 2).
Examination of the inhibitory effects of various patient sera on HIV-1 capture suggested that a combination of anti-HIV antibodies and acute-phase proteins was potentially responsible for the suppression of capture of virus from clinical plasma samples (Fig. 3). Steric hindrance and masking of host antigens within the viral envelope via specific or nonspecific protein coating could cause this inhibition. Salt treatment during the virus purification algorithm was an important step in dissociating these inhibitory proteins from the virion surface and permitted analysis of clinical material. In part, the purification algorithm used in this study may account for the greater success of capture of HIV-1 from plasma samples than has previously been reported (23).
Application of the capture assay to HIV-1 purified from plasma of patients resulted in capture of HIV-1 from all eight samples (Fig. 4A). Substantial capture of virus with anti-CD44 antibody suggested that CD44 is incorporated at a high level in the HIV-1 envelope. CD44 is expressed on many cell types, including both lymphocytes and macrophages, and is involved in lymphocyte homing (27). Since this molecule is functional when incorporated into the HIV-1 envelope (10), we speculate that the acquisition of this molecule by HIV-1 might assist in virus trafficking to lymphoid tissue.
The failure of antibodies to CD36, CD14, and CD26 to capture HIV-1 from the plasma of the patients with no opportunistic infection was surprising and contrasted with the successful capture of virus from those who did have opportunistic infections (Fig. 4A). This difference was even more striking since the opportunistic infections were associated with a marked acute-phase response (as indicated by increased levels of CRP [Fig. 4B]), which would have tended to inhibit capture of virus from these patients. It is likely that a threshold density of each of the host antigens in the viral envelope is required to enable successful antibody capture of HIV-1. Viral envelope antigen density may affect both the ability to capture HIV-1 and the efficiency of capture with different antibodies (Table 1). It is therefore notable that CD36, CD14, and CD26 are all inducibly expressed (13, 14, 20), and cellular upregulation of these antigens in the presence of marked systemic immune activation associated with opportunistic infection (Fig. 4A) could have resulted in the acquisition of higher antigen densities in the HIV-1 envelope. Indeed, elevated plasma concentrations of plasma immune markers confirmed a heightened state of systemic immune activation in patients with opportunistic infections (Fig. 4B). Levels of cellular activation may also be responsible for the differences in capture between in vitro-derived HIV-1 stocks and virus purified from patients HIV.1 to HIV.4 (Fig. 1 and 4A).
Both macrophages and lymphocytes are highly activated in the host response to TB. The successful capture of HIV-1 purified from the plasma of patients with active TB by using antibodies to CD26, CD36, and CD14 (Fig. 4A) is consistent with viral replication occurring in activated macrophage and lymphocyte pools in these patients. Interestingly, anti-CD14 antibody captured HIV-1 purified from patients with TB (Fig. 4A) to a greater extent than macrophage-derived in vitro HIV-1 stocks (Fig. 1). This difference possibly reflects the pivotal role of CD14 in the host response to TB. CD14 serves as the receptor for the mycobacterial cell wall antigen lipoarabinomannan (20, 33), and CD14 expression is upregulated in patients with mycobacterial disease (12, 15). The finding that the highest plasma concentrations of soluble CD14 were observed in the patients with TB (Fig. 4B) suggests that CD14 was indeed more highly upregulated in those patients. Furthermore, the greatly elevated plasma concentrations of macrophage-derived cytokines (TNF-α and IL-6) in the patients with TB also provide additional evidence of marked activation of macrophages.
Previous studies have speculated that <1% of cell-free HIV-1 is derived from macrophages and that the vast majority of virus is of lymphocytic origin (19). However, our data demonstrate that under certain circumstances of generalized immune activation (i.e., active TB) macrophages contribute significantly to the plasma HIV-1 pool. Approximately 10% of the virus from patient TB/HIV.1 was found to be macrophage derived (Table 2), and in view of the modest efficiency of viral capture (Table 1), it is conceivable that substantially more than 10% of the plasma HIV-1 in this patient was, in fact, derived from this cellular pool. Thus, macrophages may contribute significantly to the increase in HIV-1 plasma load reported to occur in patients who develop active TB (9). Our results corroborate a previous report that macrophages within lymph nodes coinfected with Mycobacterium avium and HIV-1 have high levels of cell-associated replicating virus (17) and further support the theory that macrophages serve as an important source of HIV replication in those with opportunistic infections (31).
As the HIV-1 capture technique is refined, we may be able to target antigens incorporated in the viral envelope at lower concentrations. With enhanced sensitivity, this technique may enable us to address important questions regarding virus-host dynamics and increase our understanding of HIV-1 disease progression, transmission, and pathogenesis. For example, determining the ratio of virus derived from these cellular origins during the early viremic state and how that ratio changes during progression to AIDS might provide new insight into the disease process. Furthermore, if appropriate selective markers were identified, virus from specialized tissues such as neural or thymic tissue could be studied.
ACKNOWLEDGMENTS
Stephen D. Lawn is funded by the Wellcome Trust, London, United Kingdom. The Committee on Human Research, Publications, and Ethics of the School of Medical Sciences, Kumasi, Ghana, approved the collection of field samples by S.D.L. within the Department of Medicine.
Paul Sandstrom is acknowledged for giving advice regarding the salt-dissociation step of the HIV-1 purification algorithm.
REFERENCES
- 1.Arthur L O, Bess J W, Jr, Sowder R C, Benveniste R E, Mann D L, Chermann J C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implication for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 2.Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourinbaiar A S. The ratio of defective HIV-1 particles to replication-competent infectious virions. Acta Virol. 1994;38:59–61. [PubMed] [Google Scholar]
- 4.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The acquisition of host-derived major histocompatibility complex class II glycoproteins by human immunodeficiency virus type 1 accelerates the process of virus entry and infection in human T-lymphoid cells. Blood. 1997;90:1091–1100. [PubMed] [Google Scholar]
- 5.Dierich M P, Frank I, Stoiber A, Clivio M, Spruth M, Katinger H W. The envelope of HIV. Immunol Lett. 1996;54:205–206. doi: 10.1016/s0165-2478(96)02674-0. [DOI] [PubMed] [Google Scholar]
- 6.Fortin J F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank I, Stoiber H, Godar S, Stockinger H, Steindl F, Katinger H W, Dierich M P. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS. 1996;10:1611–1620. doi: 10.1097/00002030-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Frank I, Kacani L, Stoiber H, Stüssel H, Spruth M, Steindl F, Romani N, Dierich M P. Human immunodeficiency virus type 1 derived from cocultures of immature dendritic cells with autologous T cells carries T-cell-specific molecules on its surface and is highly infectious. J Virol. 1999;73:3449–3454. doi: 10.1128/jvi.73.4.3449-3454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goletti D, Weissman D, Jackson R W, Graham N M H, Vlahov D, Klein R S, Munsiff S S, Ortona L, Cauda R, Fauci A S. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J Immunol. 1996;157:1271–1278. [PubMed] [Google Scholar]
- 10.Guo M M L, Hildreth J E K. HIV acquires functional adhesion receptors from host cells. AIDS Res Hum Retroviruses. 1995;11:1007–1013. doi: 10.1089/aid.1995.11.1007. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann B, Nishanian P, Fahey J L, Esmail I, Jackson A L, Detels R, Cumberland W. Serum increases and lymphoid cell surface losses of IL-2 receptor CD25 in HIV infection: distinctive parameters of HIV-induced change. Clin Immunol Immunopathol. 1991;61:212–224. doi: 10.1016/s0090-1229(05)80025-x. [DOI] [PubMed] [Google Scholar]
- 12.Hoheisel G, Zheng L, Teschler H, Striz I, Costabel U. Increased soluble CD14 levels in BAL fluid in pulmonary tuberculosis. Chest. 1995;108:1614–1616. doi: 10.1378/chest.108.6.1614. [DOI] [PubMed] [Google Scholar]
- 13.Huh H Y, Pearce S F, Yesner L M, Schindler J L, Silverstein R L. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- 14.Kahne T, Kroning H, Thiel U, Ulmer A J, Flad H D, Ansorge S. Alterations in structure and cellular localization of DPIV/CD26 during T cell activation. Cell Immunol. 1996;170:63–70. doi: 10.1006/cimm.1996.0134. [DOI] [PubMed] [Google Scholar]
- 15.Lien E, Aukrust P, Sundan A, Muller F, Froland S S, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 infection: correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- 16.Montefiori D C, Cornell R J, Zhou J T, Hirsch V M, Johnson P R. Complement control proteins, CD46, CD55, and CD59, as common surface constituents of human and simian immunodeficiency viruses and possible targets for vaccine protection. Virology. 1994;205:82–92. doi: 10.1006/viro.1994.1622. [DOI] [PubMed] [Google Scholar]
- 17.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 18.Orentas R J, Hildreth J E. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 19.Perelson A S, Neumann A, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 20.Pugin J, Heumann I D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 21.Roberts B D, Butera S T. Host protein incorporation is conserved among diverse HIV-1 subtypes. AIDS. 1999;13:425–427. doi: 10.1097/00002030-199902250-00020. [DOI] [PubMed] [Google Scholar]
- 22.Rossio J L, Bess J, Henderson L E, Cresswell P, Arthur L O. HLA class II on HIV particles is functional in superantigen presentation to human T-cells: implications for HIV pathogenesis. AIDS Res Hum Retroviruses. 1995;11:1433–1439. doi: 10.1089/aid.1995.11.1433. [DOI] [PubMed] [Google Scholar]
- 23.Saarloos M-N, Sullivan B L, Czerniewski M A, Parameswar K D, Spear G T. Detection of HLA-DR associated with monocytotropic, primary, and plasma isolates of human immunodeficiency virus type 1. J Virol. 1997;71:1640–1643. doi: 10.1128/jvi.71.2.1640-1643.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saifuddin M, Parker C J, Peeples M E, Gorny M K, Zolla-Pazner S, Ghassemi M, Rooney I A, Aikinson J P, Spear G T. Role of virion-associated glycosylphosphatidylinositol-linked proteins CD55 and CD59 in complement resistance of cell line-derived and primary isolates of HIV-1. J Exp Med. 1995;182:501–509. doi: 10.1084/jem.182.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saifuddin M, Hedayati T, Atkinson J P, Holguin M H, Parker C T, Spear G T. Human immunodeficiency virus type 1 incorporates both glycosylphosphatidylinositol-anchored CD55 and CD59 and integral membrane CD46 at levels that protect from complement-mediated destruction. J Gen Virol. 1997;78:1907–1911. doi: 10.1099/0022-1317-78-8-1907. [DOI] [PubMed] [Google Scholar]
- 26.Scheibenbogen C, Keilholz U, Richter M, Andreesen R, Huntstein W. The interleukin-2 receptor in human monocytes and macrophages: regulation of expression and release of the alpha and beta chains (p55 and p75) Res Immunol. 1992;143:33–37. doi: 10.1016/0923-2494(92)80077-x. [DOI] [PubMed] [Google Scholar]
- 27.Stemenkovic I, Aruffo A, Amiot M, Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989;56:1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan B L, Knopoff E J, Saifuddin M, Takefman D M, Saarloos M N, Sha B E, Spear G T. Susceptibility of HIV-1 plasma virus to complement-mediated lysis. Evidence for a role in clearance of virus in vivo. J Immunol. 1996;157:1791–1798. [PubMed] [Google Scholar]
- 29.Tandon N N, Kralisz U, Jamieson G A. Identification of glycoprotein IV (CD36) as a primary receptor of platelet-collagen adhesion. J Biol Chem. 1989;264:7576–7583. [PubMed] [Google Scholar]
- 30.Tremblay M J, Fortin J-F, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1993;19:346–351. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 31.Wahl S M, Green-Wild T, Peng G, Hale-Donze H, Orenstein J M. Coinfection with opportunistic pathogens promotes human immunodeficiency virus type 1 infection in macrophages. J Infect Dis. 1999;179:S457–S460. doi: 10.1086/314814. [DOI] [PubMed] [Google Scholar]
- 32.Wiley R L, Smith D H, Lasky L A, Theodore T S, Earl P L, Moss B, Capon D J, Martin M A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu W, Soprana E, Cosetino G, Volta M, Lichenstein H S, Viale G, Vercelli D. Soluble CD14 confers responsiveness to both lipoarabinomannan and lipopolysaccharide in a novel HL-60 cell bioassay. J Immunol. 1998;161:4244–4251. [PubMed] [Google Scholar]