Abstract
Introduction
Orthotopic heart transplantation is the gold standard for the treatment of advanced heart failure in the absence of contraindications. Infective endocarditis is a rare complication in patients after heart transplantation. The treatment of endocarditis after heart transplantation is challenging since there is a need for ongoing immunosuppression.
Case description
We present the case of a 51-year-old orthotopic heart transplant recipient enrolled in a chronic dialysis program, in whom we diagnosed and successfully treated recurrent infective endocarditis of the mitral valve caused by Enterococcus and Enterobacter species. Despite the complicated course of the disease, the treatment was successful.
Conclusions
Recurrent infective endocarditis after heart transplantation can be treated successfully with a multidisciplinary approach and robust antimicrobial therapy.
LEARNING POINTS
There is a high risk of bacteraemia and subsequent endocarditis in patients with recurrent catheter-related sepsis.
The spectrum of bacteria causing endocarditis in patients after heart transplantation differs from that in the general population.
Scrupulous targeted antibiotic treatment is warranted for the treatment of immunosuppressed patients with endocarditis.
Keywords: Immunosuppression, haemodialysis, antibiotics, bacteraemia, catheter sepsis
INTRODUCTION
Infective endocarditis after heart transplantation is a rare complication. In registries, the average time that endocarditis develops after transplantation is 8.4 months[1]. The mitral valve is most frequently affected, and Staphylococcus aureus is the most common pathogen causing endocarditis. The second most common pathogen is Aspergillus fumigatus, followed by Enterococcus faecalis. The prevalence of viridans Streptococci is substantially lower[2].
CASE DESCRIPTION
The patient was a 51-year-old male orthotopic heart transplant recipient enrolled in a chronic dialysis program, in whom we diagnosed and successfully treated a recurrent infective endocarditis of the mitral valve. The patient underwent orthotopic heart transplantation in 2012 for ischemic cardiomyopathy. The post-transplant period was complicated by repeated graft rejections which were treated successfully with preserved graft function. The patient was included in a chronic haemodialysis program five years later due to worsening chronic kidney disease caused by vascular nephropathy and calcineurin toxicity. Repeated attempts to establish a functional arteriovenous fistula were unsuccessful, and the patient had a permanent dialysis catheter inserted. He overcame repeated catheter-related infections and needed three catheter replacements over four years. Another episode prompted an echocardiographic examination, which revealed a 13 × 9 mm fluttering vegetation on the anterior leaflet of the mitral valve with severe mitral regurgitation (Fig. 1). We treated the patient with a combination of vancomycin and gentamycin. Enterococcus faecalis was detected in blood cultures. As it was resistant to gentamycin, we switched the patient to piperacillin-tazobactam. The course of the disease was complicated by an embolism to the splenic artery (Fig. 2), which presented itself with sharp pain in the left hypochondrium. After a four-week course of antibiotic therapy, the vegetation on the mitral valve was not recognizable on echocardiography. The extraction of the dialysis catheter was carefully considered but the catheter was left in place as it did not show signs of inflammation. The patient had absolved more catheter exchanges before, and sparing of venous accesses was another reason. After four months, the patient was readmitted for fever and suspected endocarditis. A transthoracic echocardiography showed no signs of endocarditis. However, a transoesophageal examination found a vegetation on the posterior leaflet of the mitral valve (Fig. 3A and B). Again, we started an empirical treatment with vancomycin and gentamycin. Enterobacter cloacae was identified as the cause of infection. Antibiotic therapy was switched from vancomycin to ceftazidime with good results. The rest of the patient’s hospital stay was uneventful. The dialysis catheter was replaced. Also, an arteriovenous fistula was successfully constructed. Transoesophageal echocardiography found no signs of valve vegetation (Fig. 3C and D), and the patient was discharged home. During the following months, the patient stayed in good health without any recurrence of endocarditis.
Figure 1.
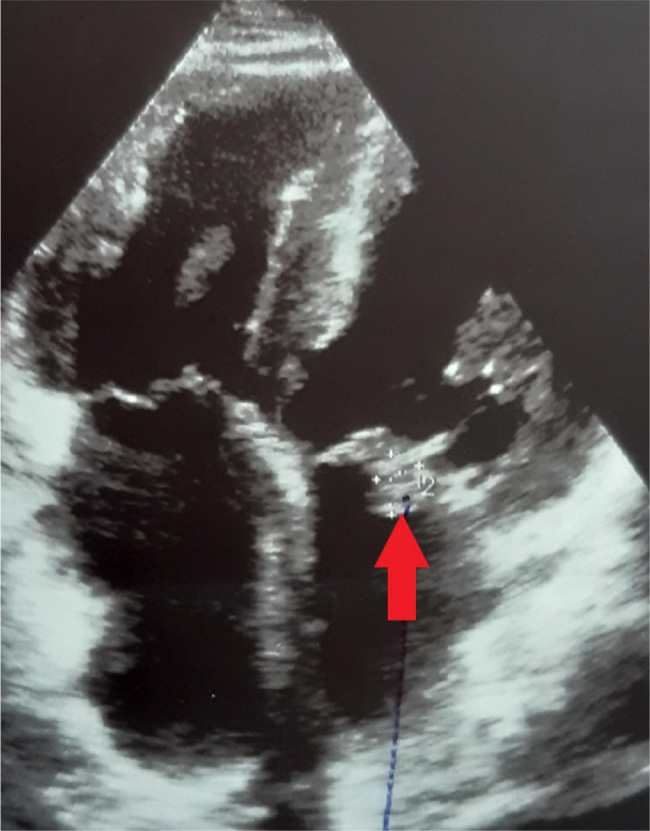
Apical four-chamber view showing a vegetation on the anterior leaflet of the mitral valve.
Table 1.
The timeline of the case report.
| April 2012 | Orthotopic heart transplantation |
| 2012–2013 | Repeated transplant rejections |
| August 2017 | Start of dialysis treatment |
| 2018–2021 | Repeated catheter-associated sepsis |
| May 2022 | Endocarditis of the anterior leaflet of the mitral valve |
| June 2022 | Embolization to the splenic artery |
| October 2022 | Endocarditis of the posterior leaflet of the mitral valve |
| November 2022 | Replacement of the dialysis catheter and AV fistula creation |
Abbreviations: AV, arteriovenous
Figure 2.
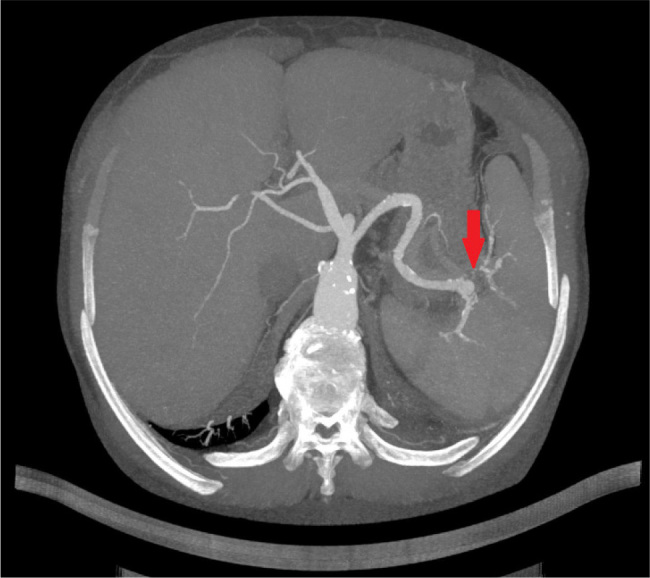
Transversal plane of a CT angiography of the aorta showing a septic embolization of the spleen.
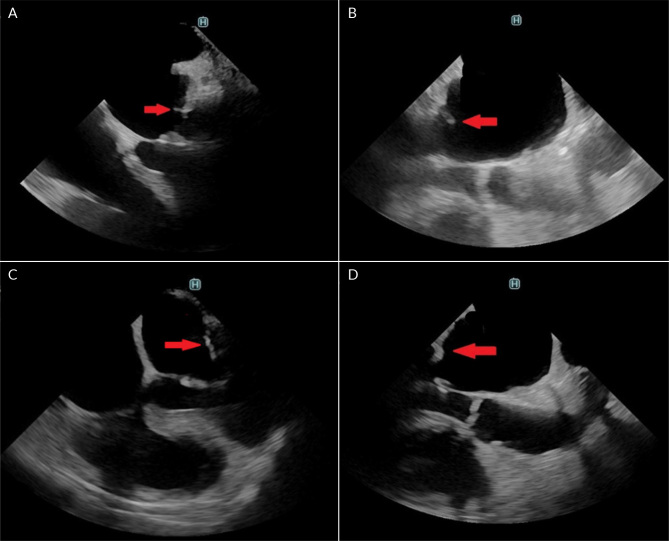
Figure 3.
Transoesophageal echocardiography. A) a vegetation of the posterior leaflet of the mitral valve; B) long axis view of the left ventricle showing a vegetation of the posterior leaflet of the mitral valve; C) four-chamber view with no vegetation of posterior leaflet of the mitral valve after treatment; D) long-axis view of the left ventricle with no vegetation of posterior leaflet of the mitral valve after treatment.
DISCUSSION
Chronic haemodialysis is recognized as a risk factor for developing infectious complications, including infective endocarditis. The incidence is 50 – 60 times higher in dialysis patients than non-dialysis patients and is higher in patients dialyzed through a catheter than through an arteriovenous fistula[3]. The finding of E. faecalis in the first episode of the endocarditis is in concordance with the literature, as was mentioned in the introduction. E. cloacae is a very rare aetiology of endocarditis. A recent systematic review identified 20 cases of endocarditis caused by this agent. The mitral valve was the most frequently affected[4]. A report of a similar case described Enterobacter a patient with a chronic dialysis catheter left on site after an episode of previous bacteraemia who developed Enterobacter endocarditis[5]. In our opinion, in our patient a combination of more risk factors, such as immunosuppression, repeated bacteraemia, and underlying heart disease, led to recurrent endocarditis caused by more sporadic microbial agents.
Acknowledgements
We kindly thank the microbiological and pharmacological teams of the Faculty Hospital in Nitra for their constant and passionate help with the treatment of severe infectious diseases, such as the one described.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interests.
Patient Consent: The patient gave written informed consent to process the healthcare data and publish a case report regarding his disease.
REFERENCES
- 1.Martínez-Sellés M, Tattevin P, Valerio-Minero M, de Alarcón A, Fariñas MC, Mirabet-Pérez S, et al. Infective endocarditis in patients with heart transplantation. Int J Cardiol. 2021;328:158–162. doi: 10.1016/j.ijcard.2020.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Jordan AM, Tatum R, Ahmad D, Patel SV, Maynes EJ, Weber MP, et al. Infective endocarditis following heart transplantation: A systematic review. Transplant Rev. 2022;36:100672. doi: 10.1016/j.trre.2021.100672. [DOI] [PubMed] [Google Scholar]
- 3.Pericàs JM, Llopis J, Jiménez-Exposito MJ, Kourany WM, Almirante B, Carosi G, et al. Infective Endocarditis in Patients on Chronic Hemodialysis. J Am Coll Cardiol. 2021;77:1629–1640. doi: 10.1016/j.jacc.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Ioannou P, Vamvoukaki R, Kofteridis DP. Infective endocarditis by Enterobacter cloacae: a systematic review and meta-analysis. J Chemother. 2022;34:1–8. doi: 10.1080/1120009X.2021.1959786. [DOI] [PubMed] [Google Scholar]
- 5.Karasahin O, Yildiz Z, Unal O, Arslan U. A rare cause of healthcare-associated infective endocarditis: Enterobacter cloacae. IDCases. 2018;12:18–20. doi: 10.1016/j.idcr.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]