Abstract
Background:
Few studies have evaluated the role of cytoreductive surgery in patients with recurrent adult granulosa cell tumors of the ovary. Despite a multitude of treatment modalities in the recurrent setting, the optimal management strategy is not known. Cytoreductive surgery offers an attractive option for disease confined to the abdomen/pelvis. However, few studies have evaluated the role of surgery compared to systemic therapy alone following the first recurrence and subsequent disease progressions.
Objective:
To determine the impact of secondary, tertiary, and quaternary cytoreductive surgery on survival outcomes in recurrent adult granulosa cell tumors of the ovary.
Study Design:
This is a multicenter, retrospective cohort study evaluating patients with recurrent adult granulosa cell tumor of the ovary enrolled in the MD Anderson Rare Gynecologic Malignancy Registry from 1970 to 2022. Study inclusion criteria consisted of histology-proven recurrent disease, at least one documented recurrence, and treatment/treatment planning at MD Anderson Cancer Center or Lyndon B. Johnson General Hospital. The primary exposure was cytoreductive surgery and the outcomes of interest were progression-free survival and overall survival. Survival analyses were restricted to eligible patients with resectable disease without medical barriers to surgery at each progression episode. Demographic and Clinicopathologic characteristics were summarized using descriptive statistics. Progression-free survival (after first, second, and third progression) and overall survival were estimated with methods of Kaplan and Meier and were modeled via cox proportional hazards regression. Multivariable analyses were performed for progression-free survival after first progression and overall survival.
Results:
Among the 369 patients with adult granulosa cell tumors of the ovary in the registry, there were 149 patients who met the study inclusion criteria. Secondary cytoreductive surgery was associated with a significant improvement in progression-free survival on univariable (HR 0.37, 95% CI 0.17 – 0.81, p = 0.01) and multivariable analyses (HR 0.42, 95% CI 0.19 – 0.92, p = 0.03). Those who underwent secondary cytoreductive surgery had a significantly improved median overall survival compared to those who did not undergo cytoreductive surgery (181.92 months vs 61.56 months, respectively; p = 0.002). Overall survival benefit remained statistically significant on multivariable analysis (HR 0.28, 95% CI 0.11 – 0.67, p = 0.004). Tertiary cytoreductive surgery was similarly associated with a significant improvement in progression-free survival (HR 0.43, 95% CI 0.26 – 0.70, p = 0.001). Despite a similar trend, quaternary cytoreductive surgery was not associated with a significant improvement in progression-free survival (HR 0.74, 95% CI 0.42 – 1.26, p = 0.27).
Conclusions:
Among those with resectable disease and no medical contraindications to surgery, cytoreductive surgery may offer a beneficial impact on progression-free survival and overall survival in patients with recurrent adult granulosa cell tumor of the ovary.
Keywords: granulosa cell tumor of the ovary, ovarian neoplasms, cohort studies, surgery, tumor cytoreduction, survival, gynecologic oncology
Introduction
Adult-type granulosa cell tumors of the ovary (aGCT) are rare tumors that represent 3–5% of all ovarian malignancies but comprise the majority (70%) of sex-cord stromal tumors.1,2 Afflicted patients will typically present with early-stage disease and are treated with upfront surgery with or without adjuvant therapy.1,3 Outcomes of frontline management for early-stage aGCT are quite favorable with 5-year overall survival rates over 90%.1,3,4 Despite many patients achieving long-term, disease-free survival, aGCT requires continued surveillance as the disease process follows an indolent course and relapses have been detected more than a decade after clinical remission.4,5 The median time to recurrence for patients with aGCT is 4 to 6 years following initial diagnosis.6 In the recurrent setting, there are multiple treatment modalities that have been reported in the literature.1,7 However, the optimal treatment strategy remains unknown.7
Given the indolent nature and recurrence patterns of aGCT, tumor cytoreductive surgery (CRS) presents an appealing management approach for recurrent aGCT. Sites of recurrent disease are generally limited to the pelvis and abdomen.6,8,9 In a multicenter, retrospective study (MITO-9), investigators reported no cases (0 of 35) of recurrent aGCT with distant metastases; 94% of these patients had CRS for their first recurrence.10 Similarly, other studies have demonstrated recurrent disease confined to the abdominopelvic cavity with optimal CRS rates of >80%.8,9 Despite expert opinion support for CRS in many cases of first recurrence of aGCT, there are few studies that have evaluated the clinical benefit of secondary CRS compared to systemic treatment alone.7,11 Importantly, prior studies that have evaluated the role of CRS in recurrent aGCT have been subject to selection bias (e.g. patients with poor performance status or comorbidities who did not undergo CRS were included into the cohort), thus significantly confounding the impact of CRS on survival outcomes.7,12–15 Furthermore, data regarding the impact of additional lines of CRS (e.g. tertiary or quaternary) on survival outcomes is limited.
The study objectives were to determine the impact of secondary, tertiary, and quaternary CRS on survival outcomes compared to systemic therapy alone. We hypothesized that patients who underwent CRS would have greater survival compared to those who received systemic therapy without CRS.
Materials and Methods
Patient population
In this retrospective cohort study, we reviewed all patients with aGCT who were enrolled in an institutional review board (IRB) – approved Rare Gynecologic Malignancy Registry (PA17–0586). In brief, this tumor registry was established at MD Anderson Cancer Center with the purpose of cataloging information regarding patients with rare tumors of the female reproductive system who were treated or received treatment planning at MD Anderson. This registry contains information related to their diagnosis, treatment course, surveillance and recurrence patterns, and disease outcomes. Among the rare ovarian tumors, this registry includes patients diagnosed with malignant germ cell, sex cord-stromal, rare epithelial (carcinosarcoma, clear cell, mucinous), and neuroendocrine tumors. Each patient’s tumor histology was reviewed by two expert gynecologic pathologists. For this registry, patients were accrued retrospectively from January 1970 and will be prospectively accrued through January 2027. The specific analyses reported here were IRB-approved (2020–1156). To evaluate the study objectives, the inclusion criteria were as follows: patients who had histology-proven diagnosis of aGCT or granulosa cell tumor not otherwise specified (GCT NOS), at least one documented recurrence, and received treatment or treatment planning at MD Anderson or Lyndon B. Johnson General Hospital, an affiliated county hospital with MD Anderson Gynecologic Oncology Faculty. Patients with mixed ovarian histology were included if there was an aGCT component as the driving histology in the recurrence episodes (biopsy-proven). Study exclusion criteria were as follows: disease refractory to frontline treatment, mixed histologies with other non-aGCT histology driving disease progression, and no documented follow-up after the first consultation visit.
Data Collection
Study data were collected and managed using REDCap electronic data capture tools hosted at MD Anderson.16,17 REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing 1) an interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources. The data collection cutoff date for the present study was October 1, 2022. The following clinical and demographic data were extracted from the registry: age, stage, race/ethnicity, treatment center, tumor histology, cancer treatment history (surgical and medical management in the frontline and recurrent setting), recurrence patterns/history, and vital status. This study was conducted according to the guidelines of the Declaration of Helsinki and received IRB-approval (Protocol 2020–1156). All patients provided written informed consent for the tumor registry or had a waiver of informed consent if they had not been seen at MD Anderson or Lyndon B. Johnson Hospital for at least three years or were deceased.
Statistical analysis
Descriptive statistics were used to summarize the demographic and clinical characteristics of the study population. We estimated progression-free survival (PFS) beyond the initial recurrence. Progression-free survival after the first recurrence (PFS2) was defined as date from first recurrence to progression or death, whichever came first. Patients who were alive and known to not have progression were censored at the last clinic visit. Progression-free survival following second (PFS3) and third (PFS4) progression were similarly defined. PFS2, PFS3, and PFS4 were estimated with the methods of Kaplan and Meier and modeled via Cox proportional hazards regression. Overall survival (OS) was defined from date of first progression to death. Patients who were still alive were censored at the date of last contact. To evaluate effect of CRS at time of progression on survival outcomes, univariable analyses were performed for PFS2, PFS3, PFS4, and OS. Multivariable analyses were performed for PFS2 and OS adjusting for age (<60 years vs ≥60 years), administration of chemotherapy for the first progression (no vs yes), and prior adjuvant therapy in frontline management (no vs yes). Univariable and multivariable analyses were performed among those with resectable disease at time of disease progression. The definition of resectable disease was the absence of metastases to the lung, brain/central nervous system, bone and significant liver parenchymal involvement at time of the respective progression. Furthermore, those who were deemed medically unfit for surgery (e.g. medical comorbidities or poor performance status) or had unknown resectability status prior to surgery were excluded from the respective PFS and OS analyses. These patients were identified, and their surgical eligibility status was evaluated through a detailed medical chart review by two independent reviewers (J.A.H and A.F.L). Non-concordant results were arbitrated by a third reviewer (R.T.H). Per survival analysis, the remaining study population consisted of only patients without medical or surgical contraindications to CRS, thereby minimizing selection bias. Among patients who underwent CRS, the impact of residual disease at CRS on PFS and OS was evaluated. All p-values were two-sided and considered statistically significant if p<0.05; 95% confidence intervals were calculated. All statistical analyses were performed using Stata/MP v17.0 (College Station, TX).
Results
Patient population
Figure 1 demonstrates the study flow diagram. There were 369 patients who had a diagnosis of aGCT or GCT NOS in the rare tumor registry from January 1, 1970 to October 1, 2022. Among these patients, 220 patients were excluded from the analysis (198 had no documented recurrence, 6 had disease refractory to frontline treatment, and 16 had missing follow-up data) with the remaining 149 patients who met the inclusion criteria for the study analysis. Among the 149 patients, the first documented progression ranged from September 1986 to December 2021. Demographic and clinical characteristics are demonstrated in Table 1. At the first documented progression, the median age was 52.62 years (interquartile range 40 – 60.9) and median time to first progression from diagnosis was 50.20 months (95% CI 45.17 – 63.70). The median follow-up time for all patients was 71.04 months (interquartile range 41.04 – 159.60).
Figure 1:
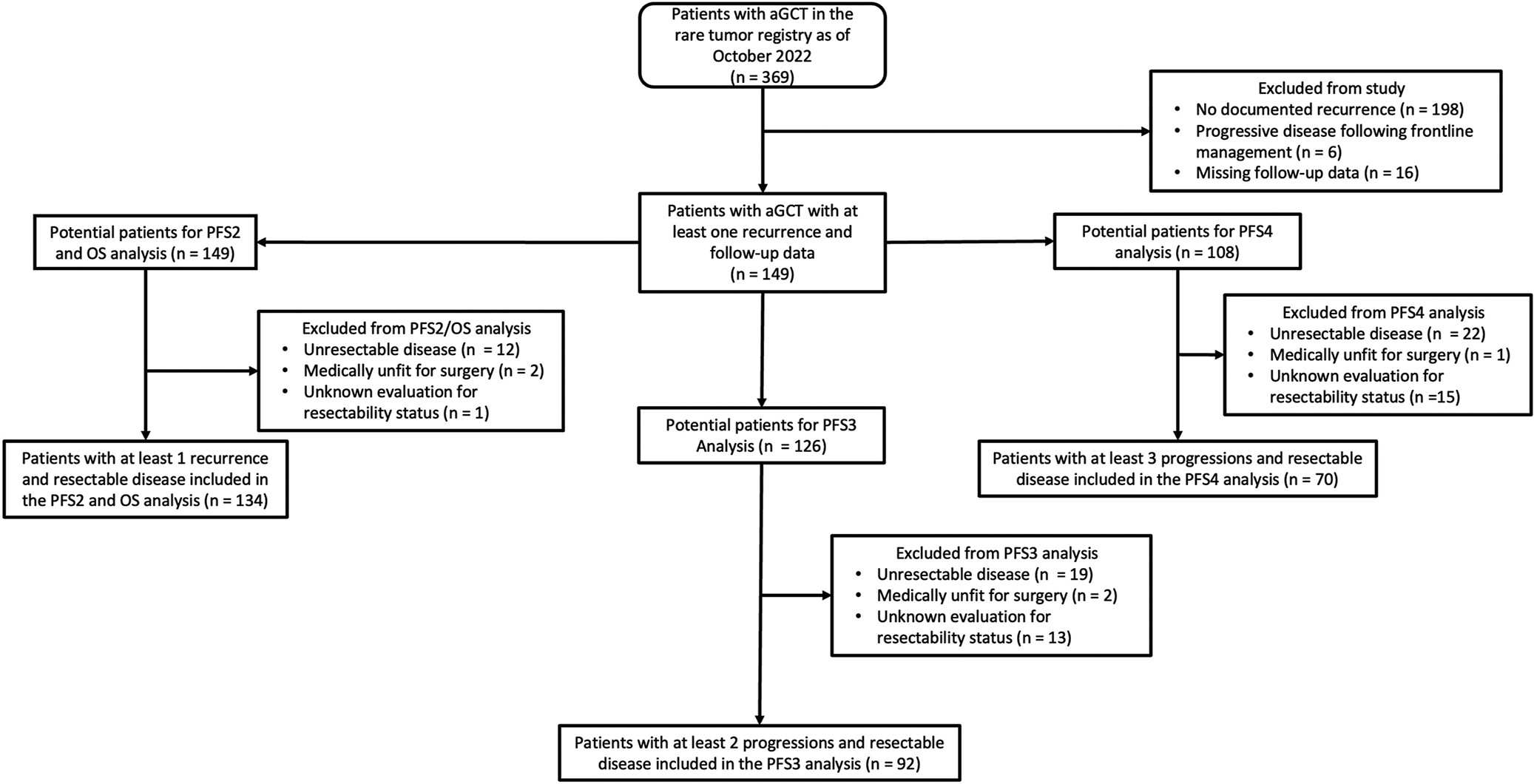
aGCT = adult-type granulosa cell tumor of the ovary. OS = overall survival. PFS2 = progression-free survival after first recurrence. PFS3 = progression-free survival after second recurrence/progression. PFS4 = progression-free survival after third recurrence/progression.
Table 1:
Demographic and clinical characteristics of the study population (n = 149)
| Characteristic | n (%) |
|---|---|
| Age at the time of diagnosis (years) | |
| Mean (SD) | 44.36 (12) |
| Median (IQR) | 45.00 (45 – 54) |
| Age at first documented progression (years) | |
| Mean (SD) | 50.98 (13) |
| Median (range) | 52.62 (40 – 60.9) |
| Stage | |
| I | 81 (74) |
| II | 16 (15) |
| III | 12 (11) |
| Unknown | 40 (NA) |
| Histology | |
| Adult-type granulosa cell tumora | 135 (91) |
| Mixedb | 14 (9) |
| Frontline adjuvant treatment | |
| None | 103 (70) |
| Chemotherapy alone | 39 (26) |
| Hormonal therapy alone | 5 (3) |
| Chemotherapy and hormonal therapy | 2 (1) |
| Race | |
| White | 108 (74) |
| Black/African American | 24 (17) |
| Asian/Native Hawaiian or other Pacific Islander | 6 (4) |
| Other | 7 (5) |
| Unknown | 4 (NA) |
| Primary institution | |
| MD Anderson Cancer Center | 147 (99) |
| LBJ | 2 (1) |
| Treatment modalities through disease course | |
| Cytoreductive surgeriesc | 2 (1 – 3) |
| Lines of chemotherapyc | 2 (1 – 3) |
| Lines of hormonal therapyc | 2 (1 – 3) |
| Lines of targeted therapyc | 0 (0 – 1) |
| Lines of radiotherapyc | 0 (0 – 1) |
International Federation of Gynecology and Obstetrics (FIGO) 2009 staging at time of diagnosis. IQR = interquartile range. NA = not applicable. SD = standard deviation.
Includes adult granulosa cell tumor and granulosa cell tumor not otherwise specified.
Juvenile granulosa cell tumor (n = 6), sertoli-leydig tumor (n = 4), juvenile granulosa cell tumor/sertoli-leydig tumor (n = 1), and sex cord tumor with annular tubules (n = 3).
Represented as median (interquartile range).
Survival outcomes among the overall study population
Following the first progression, there were 126 (85%) patients who had a 2nd progression, 108 (72%) who had a 3rd progression, and 89 (60%) who had a 4th progression. Among all 149 patients, median PFS2 was 25.68 months (95% CI 17.76 – 31.92). Among those with a 2nd progression, median PFS3 was 13.92 months (95% CI 10.56 – 16.44). Among those with a 3rd progression, median PFS4 was 12.48 months (95% CI 10.80 – 17.88). Overall, median OS was 169.8 months (95% CI 146.52 – 208.32). Supplemental Figure 1 demonstrates the Kaplan-Meier curves stratified by CRS status among all patients.
Survival outcomes among patients with resectable disease
Figure 1 demonstrates the flow diagram for selection of patients for the survival analyses. Univariable analyses and respective Kaplan-Meier curves for PFS2, PFS3, and PFS4 are demonstrated in Table 2 and Figure 2. Among the 149 patients who had a 1st progression, 134 patients had resectable disease. Secondary CRS was performed in 127 patients, and seven patients received medical recommendation for systemic therapy. Treatment regimens for patients with resectable disease at the time of progression or after CRS are shown in Supplemental Table 1. Surgical approaches/procedures performed for those who underwent CRS are shown in Supplemental Table 2. Those who underwent secondary CRS had a significant improvement in median PFS2 compared to those who did not undergo secondary CRS (31.80 vs 13.56 months, respectively) (Figure 2A) with HR 0.37 (95% CI 0.17 – 0.81; p = 0.01). Those with no gross residual at time of secondary CRS had the highest median PFS2 followed by gross residual disease ≤1 cm and gross residual disease >1 cm (39.24 vs 15.00 vs 10.32 months; p = 0.007).
Table 2:
Univariable model for progression-free and overall survival among patients with resectable disease
| PFS2 | PFS3 | PFS4 | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristica | n | HR (95% CI) | p | n | HR (95% CI) | p | n | HR (95% CI) | p | n | HR (95% CI) | p |
| Age | ||||||||||||
| <60 years | 97 | Ref | 62 | Ref | 45 | Ref | 97 | Ref | ||||
| ≥60 years | 37 | 1.34 (0.90 – 2.01) | 0.15 | 30 | 0.90 (0.56 – 1.44) | 0.66 | 25 | 0.72 (0.42 – 1.23) | 0.23 | 37 | 1.94 (1.11 – 3.39) | 0.02 |
| CRS | ||||||||||||
| No | 7 | Ref | 28 | Ref | 23 | Ref | 7 | Ref | ||||
| Yes | 127 | 0.37 (0.17 – 0.81) | 0.01 | 64 | 0.43 (0.26 – 0.70) | 0.001 | 47 | 0.74 (0.44 – 1.26) | 0.27 | 127 | 0.29 (0.12 – 0.68) | 0.004 |
| Residual disease | ||||||||||||
| R0 | 51 | Ref | 28 | Ref | 18 | Ref | 51 | Ref | ||||
| R≤1 cm | 19 | 2.57 (1.36 – 4.85 | 0.003 | 9 | 0.97 (0.43 – 2.19) | 0.94 | 7 | 1.10 (0.42 – 2.90) | 0.84 | 19 | 2.40 (0.78 – 7.38) | 0.13 |
| R>1 cm | 5 | 2.43 (0.72 – 5.85) | 0.18 | 2 | 0.93 (0.44 – 1.98) | 0.86 | 2 | 41.05 (3.57 – 472) | 0.003 | 5 | 4 (0.82 – 19.46) | 0.09 |
| Unknown | 52 | NA | NA | 25 | NA | NA | 20 | NA | NA | 52 | NA | NA |
| Chemotherapy | ||||||||||||
| No | 53 | Ref | 52 | Ref | 53 | Ref | 53 | Ref | ||||
| Yes | 81 | 1.18 (0.80 – 1.75) | 0.39 | 40 | 0.88 (0.57 – 1.36) | 0.56 | 17 | 0.99 (0.55 – 1.78) | 0.98 | 81 | 1.62 (0.94 – 2.81) | 0.08 |
| Targeted therapy | ||||||||||||
| No | 131 | Ref | 111 | Ref | 60 | Ref | 131 | Ref | ||||
| Yes | 3 | 2.22 (0.69 – 7.11) | 0.18 | 5 | 1.55 (0.62 – 3.89) | 0.35 | 10 | 1.41 (0.69 – 2.89) | 0.35 | 3 | 17.94 (3.58 – 89.9) | <0.001 |
| Radiotherapy | ||||||||||||
| No | 124 | Ref | 115 | Ref | 66 | Ref | 124 | Ref | ||||
| Yes | 10 | 0.69 (0.33 – 1.41) | 0.31 | 4 | 1.51 (0.55 – 4.17) | 0.42 | 4 | 1.23 (0.38 – 3.94) | 0.73 | 10 | 0.76 (0.30 – 1.92) | 0.57 |
| Hormonal therapy | ||||||||||||
| No | 100 | Ref | 55 | Ref | 31 | Ref | 100 | Ref | ||||
| Yes | 34 | 0.80 (0.51 – 1.26) | 0.34 | 37 | 0.92 (0.59 – 1.44) | 0.72 | 39 | 0.51 (0.30 – 0.86) | 0.01 | 34 | 0.87 (0.42 – 1.77) | 0.70 |
CRS = cytoreductive surgery. HR = Hazard ratio. NA = not applicable. OS = overall survival. PFS2 = progression-free survival after first recurrence. PFS3 = progression-free survival after second recurrence/progression. PFS4 = progression-free survival after third recurrence/progression. Ref = reference. R0 = no gross residual present at end of cytoreduction. R≤1 = gross residual disease ≤1 cm present at end of cytoreduction. R>1 cm = gross residual >1 cm present at end of cytoreduction. 95% CI = 95% confidence interval. p = p-value.
Characteristic at time of respective disease progression.
Figure 2:
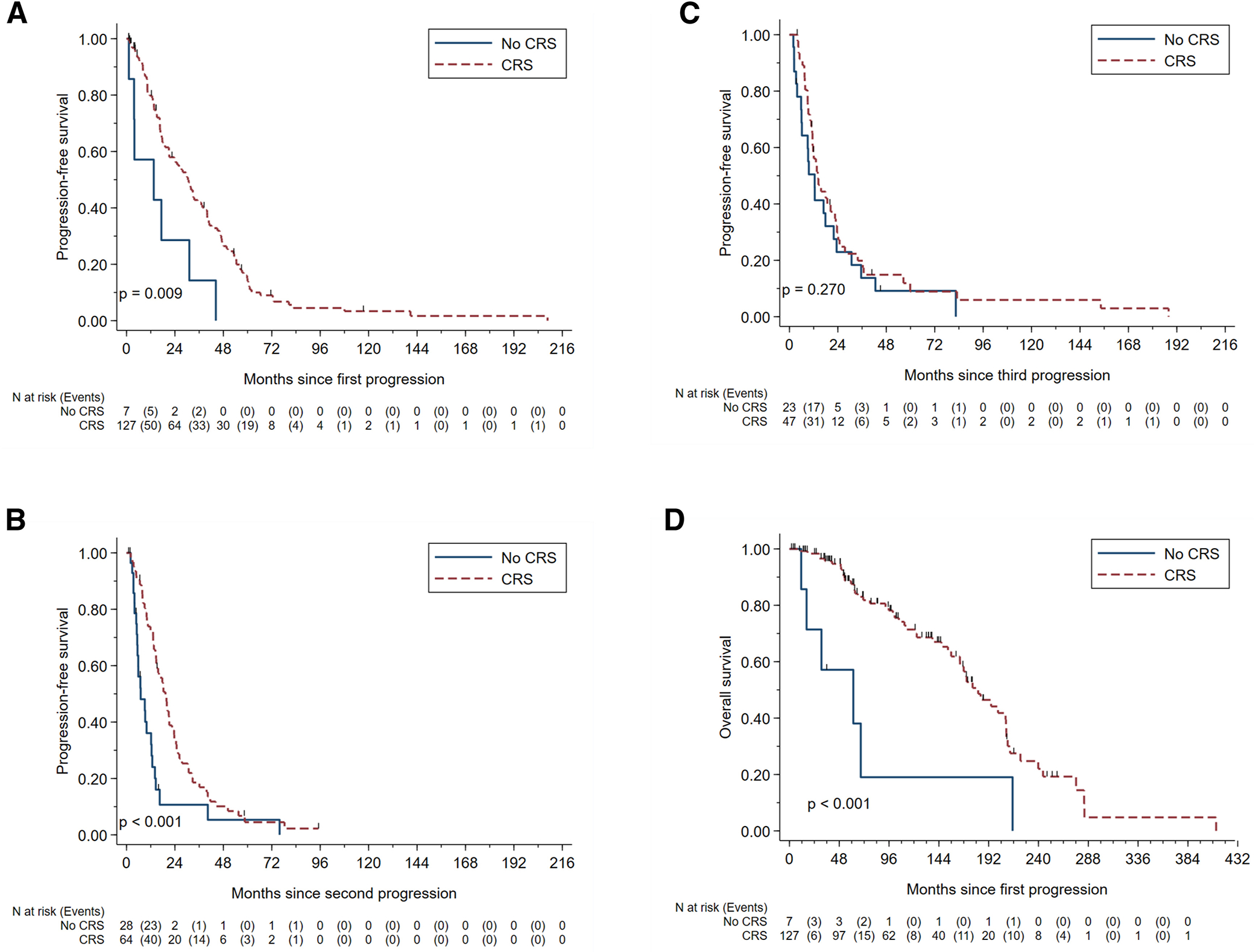
Kaplan-Meier survival curves for progression-free survival and overall survival among patients with resectable disease. (a) PFS2, progression-free survival following 1st recurrence (b) PFS3, progression-free survival following 2nd progression (c) PFS4, progression-free survival following 3rd progression (d) OS, overall survival.
Among the 126 patients with a 2nd progression, there were 92 patients with resectable disease. Tertiary CRS was performed in 64 patients and 28 received systemic therapy due to medical recommendation (n = 26) or patient choice (n = 2). Among the 64 patients with tertiary CRS, 59 previously had secondary CRS (92%). Those who underwent tertiary CRS had a significant improvement in median PFS3 compared to those who did not undergo tertiary CRS (19.32 vs 7.08 months) (Figure 2B) with HR 0.43 (95% CI 0.26 – 0.70, p = 0.001) (Table 2). There was no difference in median PFS3 based on gross residual disease status (p = 0.96).
Among the 108 patients with a 3rd progression, there were 70 patients with resectable disease. Quaternary CRS was performed in 47 patients, and 23 received systemic therapy due to medical recommendation (n = 21) or patient choice (n = 2). Among the 47 patients, four had no prior CRS (9%), 27 had at least a prior tertiary CRS (54%), and 24 had prior secondary and tertiary CRS (51%). There was no significant difference in median PFS4 between those who underwent quaternary CRS compared to those who did not undergo quaternary CRS (14.04 vs 12.48 months, respectively) (Figure 2C) with an HR 0.74 (95% CI 0.44 – 1.26, p = 0.27) (Table 2). There was a significant difference in median PFS4 based on residual disease status. Those who had no gross residual had the highest median PFS4 followed by those with residual disease ≤1 cm then by those with residual disease >1 cm (18.84 vs 14.04 vs 3.96 months, respectively; p <0.001).
Univariable analysis and Kaplan-Meier curves for OS are shown in Table 2 and Figure 2D, respectively. On univariable analyses, age less than 60 years old (p = 0.02) and CRS at first progression (p <0.001) were associated with significant improvement in survival. Targeted therapy at first progression was significantly associated with worse survival (p<0.001). Of note, only one of three patients underwent any CRS (performed as first progression) and her OS was 41.04 months and still alive at the time of analysis. Despite having resectable disease and being eligible surgical candidates, the remaining two patients received systemic therapy only and their OS was 16.56 and 30.84 months.
The median OS was significantly higher in those who underwent secondary CRS compared to those who did not undergo secondary CRS (181.92 vs 61.56 months, respectively; p = 0.002) (Figure 2D). Although not statistically significant (p = 0.10), by residual disease status at secondary CRS, median OS was highest in those without gross residual disease (194.28 months) followed by residual disease ≤1 cm (155.88 months) then residual disease >1 cm (77.64 months).
Multivariable analyses were performed for PFS2 and OS and shown in Table 3. When adjusting for age, adjuvant therapy, and chemotherapy, CRS at first progression was associated with significant improvement in PFS2 (HR 0.42, 95% CI 0.19 – 0.92, p = 0.03) and OS (HR 0.28, 95% 0.11 – 0.67, p = 0.004).
Table 3:
Multivariable analysis for progression-free survival 2 and overall survival among patients with resectable disease
| PFS2 | OS | |||||
|---|---|---|---|---|---|---|
| Characteristic | n | HR (95% CI) | p | HR (95% CI) | p | |
| Age | ||||||
| <60 years | 97 | Ref | Ref | |||
| ≥60 years | 37 | 1.35 (0.89 – 2.03) | 0.16 | 2.25 (1.26 – 4.04) | 0.006 | |
| CRS | ||||||
| No | 7 | Ref | Ref | |||
| Yes | 127 | 0.42 (0.19 – 0.92) | 0.03 | 0.28 (0.11 – 0.67) | 0.004 | |
| Chemotherapy | ||||||
| No | 53 | Ref | Ref | |||
| Yes | 81 | 1.21 (0.81 – 1.82) | 0.35 | 1.49 (0.84 – 2.62) | 0.17 | |
| History of adjuvant therapy | ||||||
| No | 94 | Ref | Ref | |||
| Yes | 40 | 1.17 (0.78 – 1.76) | 0.45 | 1.08 (0.60 – 1.94) | 0.81 | |
CRS = cytoreductive surgery. HR = hazard ratio. OS = overall survival. PFS2 = progression-free survival following 1st recurrence. Ref = reference. 95% CI = 95% confidence interval. p = p-value
Locations of residual disease after CRS are shown in Supplemental Table 3. There were no predictive factors associated with achieving no gross residual following secondary CRS based on age (p = 0.13), prior chemotherapy (p = 0.20), and number of metastatic sites (p = 0.84) or presence of upper abdominal disease (p = 0.24) on preoperative imaging.
Comment
Principal Findings
Patients who underwent secondary CRS were observed to have a significant improvement in median PFS by 18.24 months, and the benefit of secondary CRS on PFS continued to be statistically significant when adjusting for confounders on multivariable analysis. Tertiary CRS was similarly found to be associated with a significant improvement in median PFS by 12.24 months. Although it was not statistically significant, quaternary CRS demonstrated a similar trend in improvement in PFS. The benefit of CRS on OS is quite striking as those who underwent secondary CRS had a greater median OS benefit by 120.36 months. The benefit on OS remained statistically significant while controlling for confounders on multivariable analyses.
Results in the Context of What is Known
Multiple series have reported the feasibility of performing tumor cytoreduction for the management of recurrent aGCT.14,15 Despite the feasibility of performing CRS in the recurrent setting, there are few studies that have evaluated its associated clinical benefit and most of the studies have had small sample sizes.10 MITO-9 reported 33 of 35 patients with recurrent aGCT who underwent secondary CRS and did not observe any difference in relapse rate by adding chemotherapy.10 Other retrospective studies have reported improved outcomes of CRS in the recurrent setting but do not account for selection bias (e.g. poor performance status or significant medical comorbidities) that preclude patients from surgical intervention and likely have worse prognoses, thus resulting in significant confounders and limiting generalizability of study findings.12,13
Clinical Implications
With a paucity of evidence delineating the role of surgical interventions in the management of aGCT in the recurrent setting, these study results support the role of secondary and tertiary CRS (when surgically feasible) to improve oncologic outcomes. There was a similar trend of improvement of PFS among patients who underwent quaternary CRS but this was not statistically significant. The lack of observed association between quaternary CRS and PFS benefit could be attributed to several reasons. The first possibility is that the use of hormonal therapy may have a greater contribution to disease control compared to CRS at later lines of treatment (p = 0.01). Second, quaternary CRS may require stricter cytoreductive criteria for PFS benefit and may only be beneficial in those with no gross residual disease (rather than optimal residual disease ≤1 cm). Additionally, there were 20 of 47 patients who had quaternary CRS with unknown residual disease status and, therefore, there may have been higher proportions of patients with suboptimal CRS (residual disease >1 cm) that may have contributed to a non-statistically significant result for median PFS4. Given PFS and OS was observed to be inversely proportional to residual disease at the completion of secondary and quaternary CRS, this trend highlights the importance of achieving maximal cytoreduction; this follows a similar trend seen in epithelial ovarian cancer.18 Thus, further evaluation of the role of quaternary CRS is indicated.
Research Implications
Future collaborative studies are indicated to confirm the survival benefit of surgery and evaluate the role of quaternary CRS for recurrent aGCT. In our data, the majority of patients underwent an open approach for cytoreduction and therefore, future studies should examine the role of minimally invasive tumor cytoreductive approaches on survival benefit. This study was not designed to determine predictive factors for achieving no gross residual during CRS in the recurrent setting; future studies are needed to establish selection criteria for ideal candidates for CRS.
Strengths and limitations
Given the rarity of aGCT, the establishment of a prospective, randomized controlled trial to evaluate the role of successive CRS in the recurrent setting will be extremely challenging, if not impossible. Thus, well-conducted retrospective studies are crucial to guide clinical management. One of the strengths of this study is that it represents the largest study that evaluates CRS in patients with aGCT in the recurrent setting at the time of manuscript publication. Additionally, there is extensive follow-up with detailed information collected at each episode of disease progression to evaluate the contribution of CRS and other therapies to survival outcomes. However, this study has several limitations. Retrospective studies are subject to selection bias, which may consequently confound the effect of the studied intervention. Thus, at each progression, the study analyses were focused to only evaluate patients where tumor cytoreduction was feasible and therefore, excluded patients with unresectable disease or who were poor surgical candidates due to comorbidities or performance status (upon clinical chart review). Establishing criteria for eligible surgical patients was critical to minimizing bias and, relative to other published retrospective studies, may enable greater generalizability of the results to clinical practice. Another limitation includes temporal changes in management strategies or clinician preference of systemic therapies. Unfortunately, there have been few advances in the breadth of systemic agents for the management of recurrent aGCT over the decades. Furthermore, the CRS continued to demonstrate a favorable association with survival (PFS and OS) after adjustment for chemotherapy and prior adjuvant therapy on multivariable analysis.
Conclusions
In this large cohort of patients with recurrent aGCT, when surgically feasible, CRS was associated with improvements in OS and in PFS at multiple, successive disease progressions for resectable disease.
Supplementary Material
AJOG at a Glance:
A. Why was this study conducted? Few studies have evaluated the role of cytoreductive surgery compared to systemic therapy alone in recurrent adult-type granulosa cell tumors of the ovary.
B. What are the key findings? In a retrospective cohort of 149 patients with recurrent adult-type granulosa cell tumors of the ovary (with resectable disease and without surgical contraindications), secondary cytoreductive surgery was associated with a significant improvement in progression-free survival (HR 0.37) and overall survival (HR 0.28). Tertiary cytoreductive surgery was associated with progression-free survival (HR 0.43).
C. What does this study add to what is already known? When tumor resection is feasible in patients without contraindications to surgery, cytoreductive surgery may be associated with improved survival in recurrent adult-type granulosa cell tumors of the ovary.
Sources of Funding:
This research was supported in part by Cancer Prevention & Research Institute of Texas grants RR2000045 (R.T.H.) and RP170593 (K.F.H.). This work was also supported by the NIH/NCI under award numbers T32 CA101642 (D.G and A.L.B), P30CA016672 (supports the Biostatistics Resource Group, Tissue Biospecimen and Pathology Resource, and Clinical Trials Office). Additional funding sources include the Dr. Henry R. Shibata Fellowship Award/Cedars Cancer Foundation (J.A.H), Jennifer “Jenny” Song Fund for Granulosa Cell Tumor Research, The University of Texas MD Anderson Cancer Center Ovarian Cancer SPORE (NIH grant CA217685), the American Cancer Society (A.K.S.), the Ovarian Cancer Research Alliance (A.K.S.), and the Frank McGraw Memorial Chair in Cancer Research (A.K.S.). These aforementioned funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Disclosure of potential conflicts of interest: M.F is a consultant for Stryker and Astellas. M.F. reports research funding from AkesoBio. S.N.W is a consultant for ZielBio, AstraZeneca, Clovis Oncology, Eisai, Merck, Mereo, Mersana, Seagen, Nuvectis, Verastem, EQRx, GSK, Immunogen, Lilly, Zentalis, Roche/Genentech, Caris, Vincerx, and NGM Bio. A.K.S is a consultant for Kiyatec, Merck, GlaxoSmithKline, Onexo, ImmunoGen, Lylon, AstraZeneca. A.K.S. is a shareholder in BioPath. D.M.G is a consultant for Genentech and is a shareholder in Johnson & Johnson, Bristol Myers Squibb, and Procter and Gamble. J.A.H, A.L.F, K.F.H, B.F, K.I.F, D.G, V.K.V, A.L.B, B.L, L.M.R, and R.T.H report no conflicts of interests.
Condensation
Tweetable statement: In recurrent adult ovarian granulosa cell tumors, secondary and tertiary cytoreduction is associated with better progression-free survival. Also, secondary cytoreduction is associated with better overall survival.
References
- 1.Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. Mar 15 2003;21(6):1180–9. doi: 10.1200/JCO.2003.10.019 [DOI] [PubMed] [Google Scholar]
- 2.Malmstrom H, Hogberg T, Risberg B, Simonsen E. Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol Jan 1994;52(1):50–5. doi: 10.1006/gyno.1994.1010 [DOI] [PubMed] [Google Scholar]
- 3.Farkkila A, Haltia UM, Tapper J, McConechy MK, Huntsman DG, Heikinheimo M. Pathogenesis and treatment of adult-type granulosa cell tumor of the ovary. Ann Med. Aug 2017;49(5):435–447. doi: 10.1080/07853890.2017.1294760 [DOI] [PubMed] [Google Scholar]
- 4.Mangili G, Ottolina J, Gadducci A, et al. Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br J Cancer. Jul 9 2013;109(1):29–34. doi: 10.1038/bjc.2013.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans AT 3rd, Gaffey TA, Malkasian GD Jr., Annegers JF. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. Feb 1980;55(2):231–8. [PubMed] [Google Scholar]
- 6.Lee YK, Park NH, Kim JW, Song YS, Kang SB, Lee HP. Characteristics of recurrence in adult-type granulosa cell tumor. Int J Gynecol Cancer. Jul-Aug 2008;18(4):642–7. doi: 10.1111/j.1525-1438.2007.01065.x [DOI] [PubMed] [Google Scholar]
- 7.Gurumurthy M, Bryant A, Shanbhag S. Effectiveness of different treatment modalities for the management of adult-onset granulosa cell tumours of the ovary (primary and recurrent). Cochrane Database Syst Rev. Apr 21 2014;2014(4):CD006912. doi: 10.1002/14651858.CD006912.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fotopoulou C, Savvatis K, Braicu EI, et al. Adult granulosa cell tumors of the ovary: tumor dissemination pattern at primary and recurrent situation, surgical outcome. Gynecol Oncol. Nov 2010;119(2):285–90. doi: 10.1016/j.ygyno.2010.06.031 [DOI] [PubMed] [Google Scholar]
- 9.Abu-Rustum NR, Restivo A, Ivy J, et al. Retroperitoneal nodal metastasis in primary and recurrent granulosa cell tumors of the ovary. Gynecol Oncol. Oct 2006;103(1):31–4. doi: 10.1016/j.ygyno.2006.01.050 [DOI] [PubMed] [Google Scholar]
- 10.Mangili G, Sigismondi C, Frigerio L, et al. Recurrent granulosa cell tumors (GCTs) of the ovary: a MITO-9 retrospective study. Gynecol Oncol. Jul 2013;130(1):38–42. doi: 10.1016/j.ygyno.2013.04.047 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. Feb 2 2021;19(2):191–226. doi: 10.6004/jnccn.2021.0007 [DOI] [PubMed] [Google Scholar]
- 12.Karalok A, Ureyen I, Tasci T, et al. Maximum surgical effort is warranted for recurrent adult granulosa cell tumors of ovary. Tumori. Aug 3 2016;102(4):404–8. doi: 10.5301/tj.5000510 [DOI] [PubMed] [Google Scholar]
- 13.Hanvic B, Lecuru F, Vanacker H, et al. Impact of surgery and chemotherapy in ovarian sex cord-stromal tumors from the multicentric Salome study including 469 patients. A TMRG and GINECO group study. Gynecol Oncol. May 19 2023;174:190–199. doi: 10.1016/j.ygyno.2023.05.014 [DOI] [PubMed] [Google Scholar]
- 14.Chua TC, Iyer NG, Soo KC. Prolonged survival following maximal cytoreductive effort for peritoneal metastases from recurrent granulosa cell tumor of the ovary. J Gynecol Oncol. Sep 2011;22(3):214–7. doi: 10.3802/jgo.2011.22.3.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhuri TK, Butler-Manuel S, Karanjia N, Tailor A. Liver resection for metastases arising from recurrent granulosa cell tumour of the ovary--a case series. Eur J Gynaecol Oncol. 2010;31(3):342–4. [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. Apr 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. Jul 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. Mar 1 2002;20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


