Abstract
Studies on the CXCL12 rs1801157 polymorphism show that this polymorphism is involved in development of breast cancer, but its specific relationships or effects are not consistent. The purpose of this meta-analysis was to investigate the association between CXCL12 rs1801157 polymorphism and susceptibility to breast cancer. PubMed, Scopus, Embase, the Cochrane Library, Web of Science, and CNKI were searched for eligible studies through February 01, 2023. A total of ten studies with 2093 cases and 2302 controls were included in this meta-analysis. Overall, there is a significant association between CXCL12 rs1801157 polymorphism and risk of breast cancer under the homozygote genetic model (AA vs. GG, OR= 1.350, 95% CI: 1.050-1.734, p= 0.019). Stratified by ethnicity showed a significant association in Caucasian women, but not among Asian and mixed populations. This meta-analysis confirms that CXCL12 rs1801157 polymorphism is related to breast cancer risk, especially among Caucasian women. However, well-designed large-scale studies are required to further evaluate the results.
Key Words: Breast cancer, CXCL12, rs1801157, polymorphism, risk, meta-analysis
Introduction
Breast cancer is the most-commonly diagnosed malignant tumor in women in the world, as well as the first cause of death from malignant tumors [1-3]. Breast cancer patients account for as much as 36% of oncological patients. An estimated 287,850 new cases women were diagnosed with breast cancer in USA and 43,250 women will die from breast cancer in 2022 [4, 5]. The occurrence of breast cancer is associated with many risk factors, including genetic and hereditary predisposition [6, 7]. Breast cancers are highly heterogeneous [8, 9]. There is growing evidence that germline mutations in certain genes influence cancer susceptibility, tumor evolution, as well as clinical outcomes. For breast cancer, several genes such as BRCA1, BRCA2, PALB2, ATM, and CHEK2 act as high- to moderate-penetrance cancer susceptibility genes [10, 11]. Heritable predisposition genes are important risk factors for breast cancer susceptibility, accounting for 5.03% of all breast cancer cases [12].
A large number of genes associated with susceptibility to breast cancer contain single nucleotide polymorphisms (SNPs) [13-15]. The chemokine protein CXCL12 (also known as SDF1) and its receptor CXCR4 are involved in the proliferation, differentiation, and migration of specific cells in the body [16, 17]. SDF-1 belongs to the CXC subfamily of chemokines and is produced by stromal cells and mostly known for its pivotal role in the smooth muscle progenitor cells (SPCs) accumulation. The CXCL12 gene is located on chromosome 10q11.1. A single nucleotide polymorphism (SNP) in noncoding region 801 (G/A) a G-to-A base (G>A) at position 801 in the 3’untranslated region (UTR) of the CXCL12 gene up regulated the expression of SDF1 [18-21]. Growing evidence suggests that the SDF-1 rs1801157 polymorphism plays an important role in the pathogenesis of cancer. Razmkhah et al. reported that the SDF-1 rs1801157 polymorphism increased the risk of breast [22] and lung cancer [22, 23], but not colorectal and gastric cancers Iranian patients [24]. Kucukgergin and co-workers showed that the SDF-1 rs1801157 polymorphism was associated with bladder cancer susceptibility [25, 26]. Dommange et al. showed that CXCL12 801A carriers were associated with blast invasion in acute myelogenous leukemia (AML) [27, 28]. CXCL12 is closely related to invasion and metastasis of breast cancer through the CXCL12/CXCR4 axis, but it is unclear whether there is a risk associated with breast cancer [22]. Recently, studies have been conducted concerning the link between the CXCL12 rs1801157 polymorphism and the risk of breast cancer. Thus, we have performed this meta-analysis to evaluate the association between CXCL12 rs1801157 polymorphism and susceptibility to breast cancer.
Materials and Methods
Search Strategy
We conducted a comprehensive literature search on electronic databases including PubMed, EMBASE, Wed of Science, Elsevier, Google Scholar, Cochrane Library, SciELO, SID, WanFang, VIP, Chinese Biomedical Database (CBD) and Chinese National Knowledge Infrastructure (CNKI) to identify all relevant studies on the association of CXCL12 rs1801157 polymorphism with susceptibility to breast cancer up to February 01, 2023. The combination of following keywords and terms were used: (‘’breast cancer’’ OR “breast tumor” OR “breast neoplasm” OR “breast malignant tumor” OR “breast carcinoma’’) and (‘’stromal cell derived factor-1’’ OR ‘’C-X-C motif chemokine 12” “CXCL12” or “SDF1” OR ‘’CXCL12’’ OR ‘’SDF-1’’ OR ‘’rs1801157’’) AND (‘‘Polymorphism’’ OR ‘‘Mutation’’ OR ‘‘Genotype’’ OR ‘‘Allele’’ OR ‘‘Variation’’ OR ‘‘Variant’’). Languages were limited to English, Portuguese, Farsi and Chinese. In addition, hand searching of the references in retrieved reviews and eligible articles were performed as sources to find potential studies. Languages were limited to English and Chinese.
Inclusion and Exclusion Criteria
We have considered the studies to the meta-analysis that met the following predetermined inclusion criteria: (1) studies investigating the between CXCL12 rs1801157 polymorphism and breast cancer risk, (2) Studies with cohort and case-control design, (3) Studies provided sufficient data for estimating an odds ratio (OR) with a 95% confidence interval (95% CI) and (4) only conducted on the female breast cancer. The major exclusion criteria were as follow: (1) not conducted on human, (2) not breast cancer research, (3) investigated male breast cancer, (4) Only included cases, (5) duplicate of previous publications and (6) have not sufficient data for genotypes.
Data Extraction
We have extracted the following data about the eligible studies: first author name, year of publication, country of study, ethnicity of studied subjects, frequencies of genotypes in both case and control groups, and HWE. In this study the diverse ethnicity populations were categorized as Asian, Caucasian, African and Mixed. However, in the studies where the ethnicity of the case and controls was not clearly stated, we have inferred ethnicity on the basis of the largest ethnic group inhabiting the country of study. The data was extracted and confirmed by two authors; however, any disagreement was resolved by discussion among the three investigators.
Statistical Analysis
The strength of association between CXCL12 rs1801157 polymorphism with breast cancer risk was estimated by Odds ratios (ORs) with 95% confidence intervals (95% CIs). The significance of the pooled effect size was determined by Z-test, in which P<0.05 was considered statistically significant. The associations was evaluated under all five genetic models, i.e., allele (A vs. G), heterozygote (AG vs. GG), homozygote (AA vs. GG), dominant (AA+AG vs. GG) and recessive (AA vs. AG+GG). Between-study heterogeneity was evaluated by the Cochran Q-test, in which P ≤ 0.10 indicated significant heterogeneity was found. I2 statistic was also utilized to qualify between-study heterogeneity (range of 0 to 100%: I2=0-25%, no heterogeneity; I2=25-50%, moderate heterogeneity; I2= 50–75%, large heterogeneity; I2=75–100%, extreme heterogeneity) [29-31]. Therefore, a random-effects model (DerSimonian and Laird method) or fixed-effects model (Mantel-Haenszel method) was used to calculate pooled effect estimates in the presence or absence of heterogeneity, respectively [32-34]. Moreover, Hardy-Weinberg equilibrium (HWE) assessed by chi-square test was made in control group of each study [35-38], P>0.05 were considered to have reliable and representative controls. Subgroup analyses were conducted by stratification of ethnicity to identifying potential source of heterogeneity [39-41]. Begg’s funnel plot and Egger’s test were used to test any publication bias in the results [42-44]. On the other way, the underlying effects of each single study to overall results were evaluated by sensitivity analyses, with the method of deletion one independent study each time. All of the statistical calculations were performed using Comprehensive Meta-Analysis (CMA) software version 2.0 (Biostat, USA). Two-sided P-values < 0.05 were considered statistically significant.
Results
Characteristics of Eligible Studies
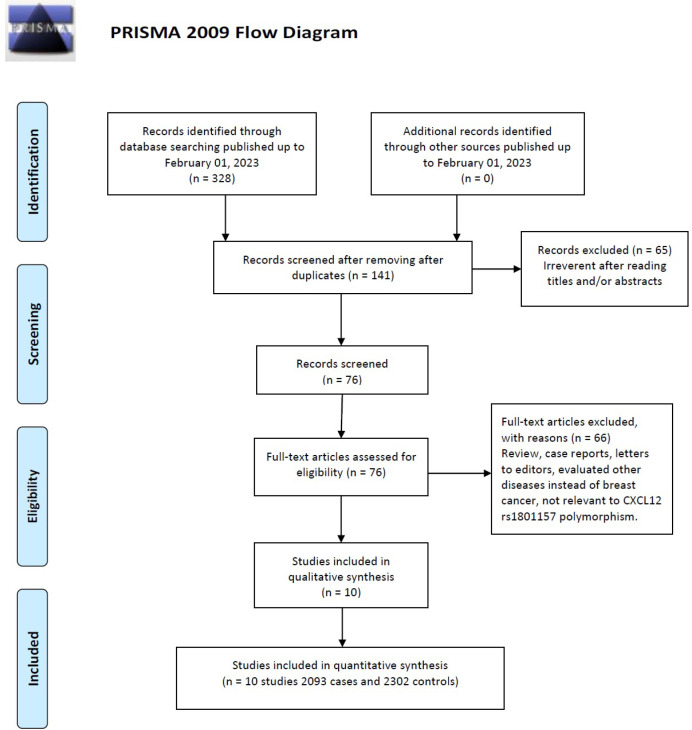
The flow chart of the literature selection process is shown in Figure 1. Initially, 328 potentially relevant published works were obtained with the initial search of databases. Of these studies, the first screening excluded 187 publications were excluded as duplicates, leaving 141 studies for further selection. Among these publications, 83 studies were excluded because they were review articles, case reports, meta-analysis, other polymorphisms of CXCL12 and related to cancer. Finally, a total of ten case-control studies [45-54] with 2093 cases and 2302 controls published from 2004 and 2022, were included were included in this meta-analysis. The basic information of each study is presented in Table 1. The countries of these studies included Greece, Iran, China, Brazil, Poland and Pakistan. Subjects in four of the studies with 1158 cases and 1207 controls belonged to Asian ethnicity [46, 49, 50, 53], three other studies with 718 cases and 30,649 controls were conducted on Caucasians [45, 51, 52] and three with 215 cases and 695 controls among mixed (Brazilian women) [47, 48, 54] populations. Moreover, six genotypic methods altogether were performed in all these studies using PCR-RFLP and MassARRAY. The genotype in the healthy control group for a study was not consistent with HWE (P < 0.05).
Figure 1.
Flow Diagram of the Study Selection Process
Table 1.
Characteristics of Studies Included in the Meta-Analysis of CXCL12 rs1801157 Polymorphism and Breast Cancer
| First author | Country (Ethnicity) |
SOC | Genotyping methods |
Case/ Control |
Cases | Controls | HWE | MAF | ||||||||
| Genotype | Allele | Genotype | Allele | |||||||||||||
| GG | AG | AA | G | A | GG | AG | AA | G | A | |||||||
| Zafiropoulos 2004 | Greece(Caucasian) | HB | PCR-RFLP | 264/212 | 98 | 136 | 30 | 332 | 196 | 101 | 92 | 19 | 294 | 130 | 0.764 | 0.307 |
| Razmkhah 2005 | Iran(Asian) | HB | PCR-RFLP | 278/181 | 105 | 139 | 34 | 349 | 207 | 101 | 67 | 13 | 269 | 93 | 0.681 | 0.257 |
| Lin 2009 | China(Asian) | HB | PCR-RFLP | 220/334 | 106 | 98 | 16 | 310 | 130 | 175 | 136 | 23 | 486 | 182 | 0.62 | 0.272 |
| de oliverira 2009 | Brazil(mixed) | HB | PCR-RFLP | 103/97 | 59 | 41 | 3 | 159 | 47 | 61 | 32 | 4 | 154 | 40 | 0.938 | 0.206 |
| Kruszyna 2010 | Poland(Caucasian) | PB | PCR-RFLP | 193/199 | 123 | 61 | 9 | 307 | 79 | 136 | 58 | 5 | 330 | 68 | 0.685 | 0.171 |
| de oliverira 2011 | Brazil (mixed) | HB | PCR-RFLP | 55/54 | 32 | 21 | 2 | 85 | 25 | 37 | 15 | 2 | 89 | 19 | 0.757 | 0.176 |
| Kontogianni 2013 | Greece (Caucasian) | HB | PCR-RFLP | 261/480 | 114 | 118 | 29 | 346 | 176 | 247 | 198 | 35 | 692 | 268 | 0.584 | 0.279 |
| Khalid 2017 | Pakistan (Asian) | HB | PCR-RFLP | 218/147 | 138 | 59 | 21 | 335 | 101 | 47 | 86 | 14 | 180 | 114 | 0.004 | 0.388 |
| Guembarovski 2018 | Brazil (mixed) | PB | PCR-RFLP | 59/150 | 37 | 19 | 3 | 93 | 25 | 109 | 38 | 3 | 256 | 44 | 0.882 | 0.147 |
| Lin 2022 | China(Asian) | NS | Mass ARRAY |
442/448 | 259 | 167 | 16 | 685 | 199 | 293 | 134 | 21 | 720 | 176 | 0.266 | 0.196 |
SOC, source of controls; HB, hospital based; PB, population based; NS, Not stated; PCR-RFLP, restriction fragment length polymorphism; HWE, Hardy-Weinberg equilibrium; MAF: minor allele frequency
Quantitative Syntheses
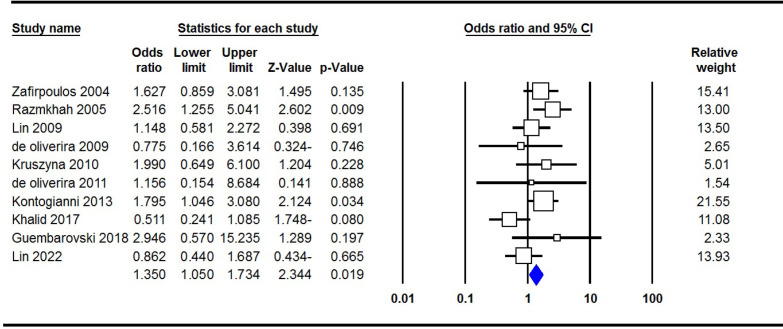
As is shown in Table 2, the main analyses performed on the CXCL12 rs1801157 polymorphism and breast cancer included association and heterogeneity tests. Pooled data showed that there was an increased CXCL12 rs1801157 polymorphism with breast cancer risk under the homozygote genetic model (A vs. GG, OR= 1.350, 95% CI: 1.050-1.734, p= 0.019, Figure 2). Moreover, after stratified by ethnicity, a significant association was revealed between this polymorphism and breast cancer among Caucasians under all five genetic models, i.e., allele (A vs. G, OR= 1.294, 95% CI: 1.117-1.531, p= 0.001), heterozygote (AG vs. GG, OR= 1.340, 95% CI: 1.071-1.640, p= 0.010), homozygote (AA vs. GG, OR= 1.646, 95% CI: 1.191-2.581, p= 0.004), dominant (AA+AG vs. GG, OR= 1.379, 95% CI: 1.128-1.696, p= 0.002) and recessive (AA vs. AG+GG, OR= 1.424, 95% CI: 1.038-2.179, p= 0.031), but not in Asian and mixed populations.
Table 2.
Meta-Analysis of the Association of CXCL12 rs1801157 Polymorphism and Breast Cancer
| Genetic Model | Type of Model | Heterogeneity | Odds ratio | Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|---|
| I2(%) | PH | OR | 95% CI | POR | PBeggs | PEggers | |||
| Overall | A vs. G | Random | 77.34 | ≤0.001 | 1.18 | 0.951-1.464 | 0.134 | 0.858 | 0.961 |
| AG vs. GG | Random | 84.21 | ≤0.001 | 1.183 | 0.843-1.655 | 0.331 | 0.858 | 0.709 | |
| AA vs. GG | Fixed | 38.6 | 0.101 | 1.35 | 1.050-1.734 | 0.019 | 0.858 | 0.909 | |
| AA+AG vs. GG | Random | 83.66 | ≤0.001 | 1.212 | 0.881-1.668 | 0.237 | 0.72 | 0.941 | |
| AA vs. AG+GG | Fixed | 0 | 0.676 | 1.266 | 0.994-1.613 | 0.056 | 0.72 | 0.941 | |
| Caucasians | A vs. G | Fixed | 0 | 0.957 | 1.308 | 1.117-1.531 | 0.001 | 1 | 0.45 |
| AG vs. GG | Fixed | 0 | 0.644 | 1.325 | 1.071-1.640 | 0.01 | 1 | 0.89 | |
| AA vs. GG | Fixed | 0 | 0.947 | 1.753 | 1.191-2.581 | 0.004 | 1 | 0.653 | |
| AA+AG vs. GG | Fixed | 0 | 0.724 | 1.383 | 1.128-1.696 | 0.002 | 1 | 0.819 | |
| AA vs. AG+GG | Fixed | 0 | 0.807 | 1.504 | 1.038-2.179 | 0.031 | 1 | 0.685 | |
| Asians | A vs. G | Random | 91.44 | ≤0.001 | 1.027 | 0.643-1.641 | 0.911 | 0.734 | 0.514 |
| AG vs. GG | Random | 94.41 | ≤0.001 | 0.952 | 0.438-2.070 | 0.902 | 0.734 | 0.378 | |
| AA vs. GG | Random | 69.83 | 0.019 | 1.071 | 0.566-2.024 | 0.833 | 1 | 0.52 | |
| AA+AG vs. GG | Random | 94.12 | ≤0.001 | 0.984 | 0.477-2.028 | 0.964 | 0.734 | 0.44 | |
| AA vs. AG+GG | Fixed | 9.38 | 0.346 | 1.104 | 0.787-1.547 | 0.568 | 1 | 0.947 | |
| Mixed | A vs. G | Fixed | 0 | 0.684 | 1.322 | 0.964-1.813 | 0.083 | 1 | 0.629 |
| AG vs. GG | Fixed | 0 | 0.922 | 1.436 | 0.976-2.114 | 0.066 | 0.296 | 0.137 | |
| AA vs. GG | Fixed | 0 | 0.5 | 1.372 | 0.514-3.661 | 0.528 | 1 | 0.948 | |
| AA+AG vs. GG | Fixed | 0 | 0.849 | 1.432 | 0.986-2.079 | 0.06 | 1 | 0.47 | |
| AA vs. AG+GG | Fixed | 0 | 0.494 | 1.212 | 0.458-3.204 | 0.698 | 1 | 0.967 | |
Figure 2.
Forest Plots for the Association of CXCL12 rs1801157 Polymorphism with Breast Cancer Risk under the Homozygote Genetic Model (AA vs. GG)
Sensitivity Analysis
Sensitivity analysis was performed to estimate the influence of some individual study on pooled results on CXCL12 rs1801157 and breast cancer by calculating the ORs before and after exclusion of a single article from meta-analysis in turn. No outlying study was observed to significantly change the pooled ORs after it was removed which confirmed our results were stable under the all five genetic models. Moreover, the test of HWE was conducted in this study, results of which indicate that results remain unchanged.
Publication Bias
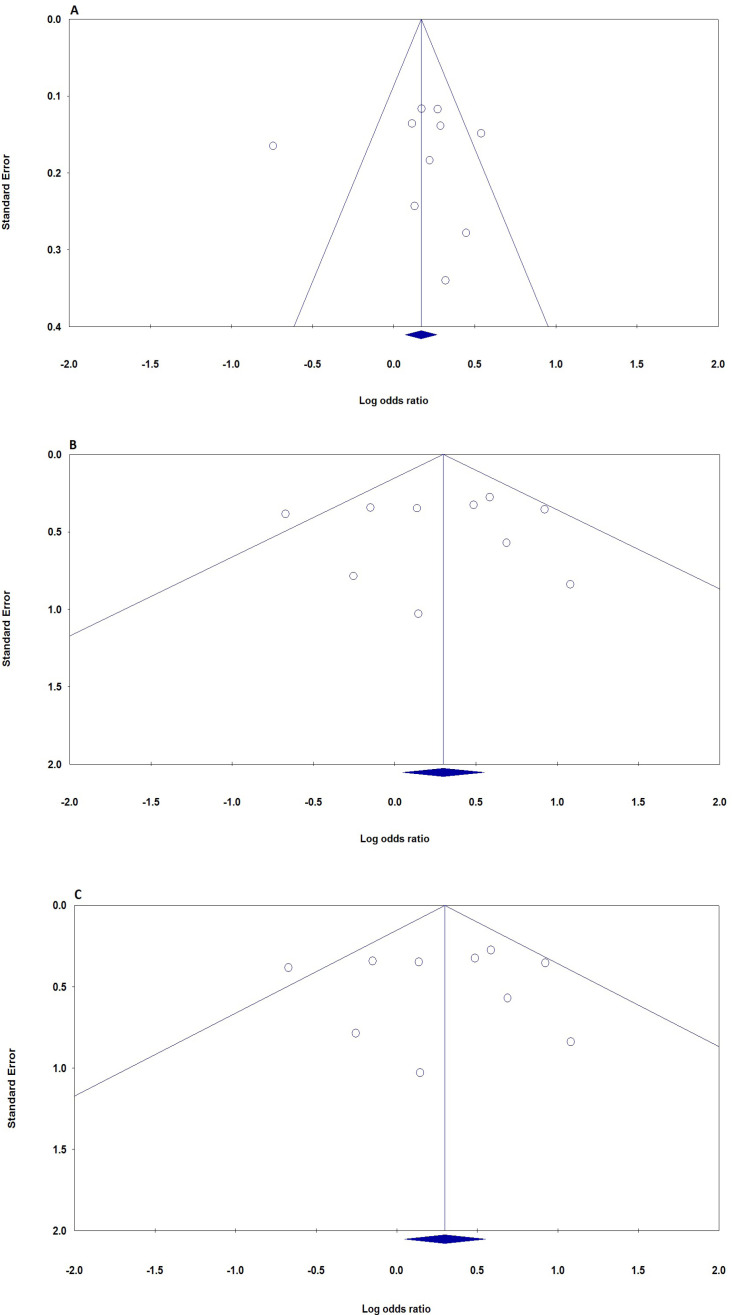
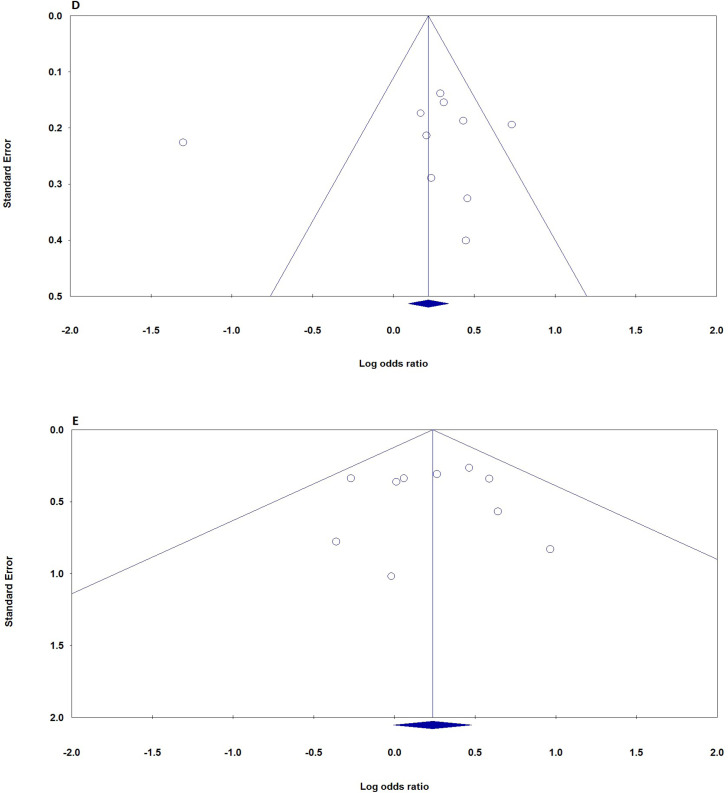
In this meta-analysis, the potential effect of publication bias in literatures was estimated by funnel plots (Figure 3) and the Egger’s test. No asymmetry was found in heterozygote and dominant plots for CXCL12 rs1801157 polymorphism association with breast cancer. Moreover, there was no statistically significant difference in the Egger’s test for CXCL12 rs1801157 polymorphism, which indicating no publication bias in the association. Thus, No significant publication bias was demonstrated in any genetic model of studied on CXCL12 rs1801157 polymorphism and breast cancer.
Figure 3.
Begg’s Funnel Plots (Publication Bias) for the association of CXCL12 rs1801157 Polymorphism with Breast Cancer Risk. A, allele (A vs. G); B, heterozygote (AG vs. GG); C, homozygote (AA vs. GG)
Figure 3.
Begg’s Funnel Plots (Publication Bias) for the association of CXCL12 rs1801157 Polymorphism with Breast Cancer Risk. D, dominant (AA+AG vs. GG); E, recessive (AA vs. AG+GG)
Discussion
In this study, our pooled data demonstrate significant association between CXCL12 rs1801157 polymorphism and breast cancer susceptibility under the homozygote genetic model (A vs. GG, OR= 1.350, 95% CI: 1.050-1.734, p= 0.019) from ten case-control studies. Several meta-analyses have explored the association between this polymorphism and breast cancer risk and it is difficult to judge if the analysis with small sample size would be more valid or not. An overall meta-analysis by, Xia et al., showed that the CXCL12 rs1801157 polymorphism was associated with breast cancer was in an allelic genetic model (OR: 1.214, 95%CI: 1.085- 1.358, p=0.001), a homozygote model (OR: 1.663, 95%CI: 1.240-2.232, p=0.001), a heterozygote model (OR: 1.392, 95%CI: 1.190-1.629, p≤0.001), a recessive genetic model (OR: 1.407, 95%CI: 1.060-1.868, p=0.018) and a dominant genetic model (OR: 1.427, 95%CI: 1.228-1.659, p=0.000). Moreover, their subgroup analysis based on ethnicity, significance was observed between the Caucasian women and the mixed group [55]. Zhu et al. [56] in a study based pooled data showed that CXCL12 rs1801157 was associated with risk of breast cancer, lung cancer, and other cancers. Moreover, their subgroup analysis revealed that this polymorphism was associated with cancer risk in the Asians under all genetic models. However, in the Caucasian subgroup, a significant association was only found under an additive genetic model and a dominant genetic model [56]. In 2012, Shen et al. [57] in a meta-analysis based on 5 case-control studies with 1,058 breast cancer cases and 1,023 controls evaluated the association of CXCL12 rs1801157 polymorphism with breast cancer. Their pooled data showed that the CXCL12 rs1801157 polymorphism was significantly associated with risk of breast cancer under three genetic models (AA vs. GG, OR = 1.64, 95% CI = 1.16-2.33; GA vs. GG, OR = 1.42, 95% CI = 1.18-1.71; and AA/GA vs. GG, OR = 1.44, 95% CI = 1.21-1.72) [57]. Ma et al. [58] in a meta-analysis of 16 publications with 2,888 cases and 3,611 controls examined the association of CXCL12 rs1801157 polymorphism with multiple kinds of malignant cancer. Their pooled data revealed that this polymorphism was associated with the increased risk of overall cancer under the homozygote model (AA vs. GG, OR=1.43, 95℅CI=1.07-1.91), the recessive model (AA vs. GG+GA, OR=1.26, 95℅CI=1.03-1.54), and the dominant model (GA+AA vs. GG, OR=1.35, 95℅CI=1.15-1.58). Their stratified analysis showed that CXCL12 rs1801157 polymorphism was associated in breast cancer, Asians and hospital-based controls groups [58]. In 2012, Gong et al., in a meta-analysis based on meta-analysis of 17 studies with 3048 cancer patients and 4522 controls assessed the association between the CXCL12 rs1801157 polymorphism and cancer risk. The meta-analysis showed that this variant polymorphism was associated with a significantly increased risk of all cancer types (OR=1.38, 95%CI=1.18-1.61 for GA vs. GG, and OR=1.36, 95%CI=1.17-1.59 for GA+AA vs. GG), especially in breast cancer (OR=1.64, 95% CI=1.16-2.33 for AA vs. GG, OR=1.42, 95%CI=1.18-1.71 for GA vs. GG, and OR=1.44, 95%CI=1.21-1.72 for GA+AA vs. GG) and lung cancer (OR=2.86, 95% CI=1.75-4.69 for AA vs. GG, OR=1.62, 95% CI=1.20-2.18 for GA vs. GG, OR=1.80, 95% CI=1.36-2.39 for GA+AA vs. GG, and OR=2.24, 95%CI=1.41-3.57 for AA vs. GA+GG) [59].
Heterogeneity in meta-analysis refers to the variation in study outcomes between studies. Thus, assessing heterogeneity in meta-analysis is critical for model selection and decision making [60-62]. High heterogeneity was found in this meta-analysis under three genetic models in overall population [63]. First, we used random models when significant heterogeneity. Second, we performed stratified analyses to explore sources of heterogeneity. In the subgroup analysis based on ethnicity, heterogeneity increased in Asians but decreased in Caucasian and mixed populations which suggest that ethnicity may be a factor in heterogeneity.
Although our study pooled a number of 2093 breast cancer cases and 2302 controls, limitations which might affect the objectivity of the results still exist. First, the moderate sample size in the meta-analysis of CXCL12 rs1801157 polymorphism might be still unable to draw a conclusion of the association between CXCL12 rs1801157 polymorphism and breast cancer. Second, our studies included data from only Asian, Caucasian, Brazilian population and none from the African women. Moreover, the amount of case-control studies in the stratified analysis was relatively small, which might cause the potential false associations. Third, there is significant heterogeneity for several studies in our meta-analysis which may distort the current meta-analysis. Fourth, limited data hampered our attempts to examine association of CXCL12 rs1801157 polymorphism and the clinical manifestation of breast cancer. As a multifactorial disease, breast cancer is influenced by genetic combined with environmental factors. Focusing on single gene region, this meta-analysis ignored the complex interaction between various factors such as age, gender, lifestyle, family history, and nutrient intake. Thus, gene-gene and gene-environment interactions should have been taken into consideration, if the relevant information was available.
In conclusion, this study showed that the CXCL12 rs1801157 polymorphism was significantly associated with breast cancer, with an increased breast cancer susceptibility among Asians, but not among Caucasian and mixed populations. Future work which takes into account gene-gene and gene-environment interactions is warranted for more precise evidence and to understand the mechanism of association between the CXCL12 rs1801157 polymorphism and breast cancer.
Author Contribution Statement
Conceptualization: Abolhasan Alijanpour, Ahmadreza Golshan, Nazanin Hajizadeh; Data curation: Mojgan Karimi-Zarchi, Nazanin Hajizadeh; Formal analysis: Abolhasan Alijanpour, Hossein Neamatzadeh; Investigation: Kazem Aghili, Maedeh Barahman; Sepideh Azizi Methodology: Maryam Aghasipour, Maryam Aghasipour; Supervision: Mohammad Vakili-Ojarood, Ahmad Shirinzadeh-Dastgiri; Validation: Mohammad Vakili-Ojarood, Ahmad Shirinzadeh-Dastgiri, Sepideh Azizi; Writing – original draft: Sahel Khajehnoori, Maedeh Barahman; Writing – review & editing: Amirhosein Naseri, Hossein Neamatzadeh.
Acknowledgements
Conflicts of interest/Competing interests
The authors declare that they have no conflict of interest.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors. An ethical approval was not necessary as this study was a meta-analysis based on previous studies.
References
- 1.de Medina P, Genovese S, Paillasse MR, Mazaheri M, Caze-Subra S, Bystricky K, et al. Auraptene is an inhibitor of cholesterol esterification and a modulator of estrogen receptors. Mol Pharmacol. 2010;78(5):827–36. doi: 10.1124/mol.110.065250. [DOI] [PubMed] [Google Scholar]
- 2.Kocanova S, Mazaheri M, Caze-Subra S, Bystricky K. Ligands specify estrogen receptor alpha nuclear localization and degradation. BMC Cell Biol. 2010;11:98. doi: 10.1186/1471-2121-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamali M, Hamadani S, Neamatzadeh H, Mazaheri M, Zare Shehneh M, Modaress Gilani M, et al. Association of xrcc2 rs3218536 polymorphism with susceptibility of breast and ovarian cancer: A systematic review and meta-analysis. Asian Pac J Cancer Prev. 2017;18(7):1743–9. doi: 10.22034/APJCP.2017.18.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistics, 2022. CA Cancer J Clin. 2022;72(6):524–41. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 5.Ghorbani S, Rezapour A, Eisavi M, Barahman M, Bagheri Faradonbeh S. Cost-benefit analysis of breast cancer screening with digital mammography: A systematic review. Med J Islam Repub Iran. 2023;37:89. doi: 10.47176/mjiri.37.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayad S, Abdi-Gamsae M, Jafari-Nedooshan J, Farbod M, Dastgheib SA, Karimi-Zarchi M, et al. Association of axin2 s2240308 c>t, rs1133683 c>t, rs7224837 a>g polymorphisms with susceptibility to breast cancer. Asian Pac J Cancer Prev. 2021;22(8):2717–22. doi: 10.31557/APJCP.2021.22.8.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayad S, Dastgheib SA, Farbod M, Asadian F, Karimi-Zarchi M, Salari S, et al. Association of pon1, lep and lepr polymorphisms with susceptibility to breast cancer: A meta-analysis. Asian Pac J Cancer Prev. 2021;22(8):2323–34. doi: 10.31557/APJCP.2021.22.8.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallian S, Rad MJ, Tavallaei M, Tavassoli M. Correlation of major histocompatibility complex class i related a (mica) polymorphism with the risk of developing breast cancer. Med Oncol. 2012;29(1):5–9. doi: 10.1007/s12032-010-9776-9. [DOI] [PubMed] [Google Scholar]
- 9.Forat-Yazdi M, Jafari M, Kargar S, Abolbaghaei SM, Nasiri R, Farahnak S, et al. Association between sult1a1 arg213his (rs9282861) polymorphism and risk of breast cancer: A systematic review and meta-analysis. J Res Health Sci. 2017;17(4):e00396. [PubMed] [Google Scholar]
- 10.Forat-Yazdi M, Neamatzadeh H, Sheikhha MH, Zare-Shehneh M, Fattahi M. Brca1 and brca2 common mutations in iranian breast cancer patients: A meta analysis. Asian Pac J Cancer Prev. 2015;16(3):1219–24. doi: 10.7314/apjcp.2015.16.3.1219. [DOI] [PubMed] [Google Scholar]
- 11.Neamatzadeh H, Shiryazdi SM, Kalantar SM. Brca1 and brca2 mutations in iranian breast cancer patients: A systematic review. J Res Med Sci. 2015;20(3):284–93. [PMC free article] [PubMed] [Google Scholar]
- 12.Azarpira MR, Ghilian MM, Sobhan MR, Mehdinezhad-Yazdi M, Aghili K, Miresmaeili SM, et al. Association of mthfr and tnf-α genes polymorphisms with susceptibility to legg-calve-perthes disease in iranian children: A case-control study. J Orthop. 2018;15(4):984–7. doi: 10.1016/j.jor.2018.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamali E, Tavassoli M, Hemati S. Association between the polymorphism of ca dinucleotide repeat in intron 1 of nfκb1 gene and risk of breast cancer. Journal of Shahrekord University of Medical Sciences. 2015;17:13–21. [Google Scholar]
- 14.Asadollahi S, Mazaheri MN, Karimi-Zarchi M, Fesahat B, Farzaneh A. The relationship of foxr2 gene expression profile with epithelial-mesenchymal transition related markers in epithelial ovarian cancer. Klin Onkol. 2020;33(3):201–7. doi: 10.14735/amko2020201. [DOI] [PubMed] [Google Scholar]
- 15.Ghorbani F, Javadirad SM, Amirmahani F, Fatehi Z, Tavassoli M. Associations of bcl2 ca-repeat polymorphism and breast cancer susceptibility in isfahan province of iran. Biochem Genet. 2021;59(2):506–15. doi: 10.1007/s10528-020-10013-y. [DOI] [PubMed] [Google Scholar]
- 16.Dar A, Kollet O, Lapidot T. Mutual, reciprocal sdf-1/cxcr4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in nod/scid chimeric mice. Exp Hematol. 2006;34(8):967–75. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Farajkhoda T, Khoshbin A, Enjezab B, Bokaei M, Karimi Zarchi M. Assessment of two emergency contraceptive regimens in iran: Levonorgestrel versus the yuzpe. Niger J Clin Pract. 2009;12(4):450–2. [PubMed] [Google Scholar]
- 18.Hirata H, Hinoda Y, Kikuno N, Kawamoto K, Dahiya AV, Suehiro Y, et al. Cxcl12 g801a polymorphism is a risk factor for sporadic prostate cancer susceptibility. Clin Cancer Res. 2007;13(17):5056–62. doi: 10.1158/1078-0432.CCR-07-0859. [DOI] [PubMed] [Google Scholar]
- 19.Jafari M, Jarahzadeh MH, Dastgheib SA, Seifi-Shalamzari N, Raee-Ezzabadi A, Sadeghizadeh-Yazdi J, et al. Association of pai-1 rs1799889 polymorphism with susceptibility to ischemic stroke: A huge meta-analysis based on 44 studies. Acta Medica (Hradec Kralove) 2020;63(1):31–42. doi: 10.14712/18059694.2020.13. [DOI] [PubMed] [Google Scholar]
- 20.Aladle D, Ghannam MA, El-Ashwah S, Ghobrial FEI, Mortada MI. Association of sdf-1 gene polymorphism with increased risk of acute myeloid leukemia patients. Asian Pac J Cancer Prev. 2021;22(4):1035–43. doi: 10.31557/APJCP.2021.22.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahadoram S, Davoodi M, Hassanzadeh S, Bahadoram M, Barahman M, Mafakher L. Renal cell carcinoma: An overview of the epidemiology, diagnosis, and treatment. G Ital Nefrol. 2022;39:3. [PubMed] [Google Scholar]
- 22.Razmkhah M, Doroudchi M, Ghayumi SM, Erfani N, Ghaderi A. Stromal cell-derived factor-1 (sdf-1) gene and susceptibility of iranian patients with lung cancer. Lung Cancer. 2005;49(3):311–5. doi: 10.1016/j.lungcan.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Razmjoo S, Jazayeri SN, Bahadoram M, Barahman M. A rare case of craniopharyngioma in the temporal lobe. Case Rep Neurol Med. 2017;2017:4973560. doi: 10.1155/2017/4973560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razmkhah M, Ghaderi A. Sdf-1alpha g801a polymorphism in southern iranian patients with colorectal and gastric cancers. Indian J Gastroenterol. 2013;32(1):28–31. doi: 10.1007/s12664-012-0283-0. [DOI] [PubMed] [Google Scholar]
- 25.Kucukgergin C, Isman FK, Dasdemir S, Cakmakoglu B, Sanli O, Gokkusu C, et al. The role of chemokine and chemokine receptor gene variants on the susceptibility and clinicopathological characteristics of bladder cancer. Gene. 2012;511(1):7–11. doi: 10.1016/j.gene.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Asgari M, Firouzi F, Abolhasani M, Bahadoram M, Barahman M, Madjd Z, et al. P53, ck20, and fgfr3 overexpression is associated with the characteristics of urothelial cell carcinoma of the bladder. Asian Pac J Cancer Prev. 2023;24(9):3125–31. doi: 10.31557/APJCP.2023.24.9.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dommange F, Cartron G, Espanel C, Gallay N, Domenech J, Benboubker L, et al. Cxcl12 polymorphism and malignant cell dissemination/tissue infiltration in acute myeloid leukemia. Faseb j. 2006;20(11):1913–5. doi: 10.1096/fj.05-5667fje. [DOI] [PubMed] [Google Scholar]
- 28.Abbaspour S, Abdollahi H, Arabalibeik H, Barahman M, Arefpour AM, Fadavi P, et al. Endorectal ultrasound radiomics in locally advanced rectal cancer patients: Despeckling and radiotherapy response prediction using machine learning. Abdom Radiol (NY) 2022;47(11):3645–59. doi: 10.1007/s00261-022-03625-y. [DOI] [PubMed] [Google Scholar]
- 29.Mashhadiabbas F, Neamatzadeh H, Nasiri R, Foroughi E, Farahnak S, Piroozmand P, et al. Association of vitamin d receptor bsmi, taqi, foki, and apai polymorphisms with susceptibility of chronic periodontitis: A systematic review and meta-analysis based on 38 case -control studies. Dent Res J (Isfahan) 2018;15(3):155–65. [PMC free article] [PubMed] [Google Scholar]
- 30.Davari HM, Rahim MM, Ershadi RM, Rafieian SM, Mardani PM, Vakili MM, et al. First iranian experience of the minimally invasive nuss procedure for pectus excavatum repair: A case series and literature review. Iran J Med Sci. 2018;43(5):554–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Shirinzadeh-Dastgiri A, Saberi A, Vakili M, Marashi SM. 21-year-old female with pneumothorax and massive air leak following blunt trauma; a photo quiz. Arch Acad Emerg Med. 2022;10(1):e24. doi: 10.22037/aaem.v10i1.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasanpour dargah m, samadi n, vakili j, et al. Comparative analysis of the effects of vasoperssin and norepinephrine on the renal function in patients undergoing cabg; a randomized clinical trial. Iranian red crescent medical journal, 2018;20:e67026. [Google Scholar]
- 33.Amini K, Vakili Ogharood M, Davari M, Ershadifard S, Asadi H. A case of a fractured fragment of tracheostomy tube entering the left bronchus: A case report. Journal of Babol University of Medical Sciences. 2021;23(1):393–7. [Google Scholar]
- 34.Farshid S, Alijanpour A, Barahman M, Dastgheib SA, Narimani N, Shirinzadeh-Dastgiri Z, et al. Associations of mthfr rs1801133 (677c>t) and rs180113 (1298a>c) polymorphisms with susceptibility to bladder cancer: A systematic review and meta-analysis. Asian Pac J Cancer Prev. 2022;23(5):1465–82. doi: 10.31557/APJCP.2022.23.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sohrevardi SM, Nosouhi F, Hossein Khalilzade S, Kafaie P, Karimi-Zarchi M, Halvaei I, et al. Evaluating the effect of insulin sensitizers metformin and pioglitazone alone and in combination on women with polycystic ovary syndrome: An rct. Int J Reprod Biomed. 2016;14(12):743–54. [PMC free article] [PubMed] [Google Scholar]
- 36.Moghimi M, Ahrar H, Karimi-Zarchi M, Aghili K, Salari M, Zare-Shehneh M, et al. Association of il-10 rs1800871 and rs1800872 polymorphisms with breast cancer risk: A systematic review and meta-analysis. Asian Pac J Cancer Prev. 2018;19(12):3353–9. doi: 10.31557/APJCP.2018.19.12.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moshtaghioun SM, Fazel-Yazdi N, Mandegari M, Shirinzadeh-Dastgiri A, Vakili M, Fazel-Yazdi H. Evaluation the presence of serpina5 (exon 3) and fto rs9939609 polymorphisms in papillary thyroid cancer patients. Asian Pac J Cancer Prev. 2021;22(11):3641–6. doi: 10.31557/APJCP.2021.22.11.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vakili Ojarood M, Khanghah AS, Belalzadeh M. Gangrenous ischemic colitis due to acute promyelocytic leukaemia, and myelofibrosis in a 62-year-old man suffering from esrd; case report. Int J Surg Case Rep. 2021;89:106663. doi: 10.1016/j.ijscr.2021.106663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gohari M, Neámatzadeh H, Jafari MA, Mazaheri M, Zare-Shehneh M, Abbasi-Shavazi E. Association between the p53 codon 72 polymorphism and primary open-angle glaucoma risk: Meta-analysis based on 11 case-control studies. Indian J Ophthalmol. 2016;64(10):756–61. doi: 10.4103/0301-4738.195002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirjalili SA, Moghimi M, Aghili K, Jafari M, Abolbaghaei SM, Neamatzadeh H, et al. Association of promoter region polymorphisms of interleukin-10 gene with susceptibility to colorectal cancer: A systematic review and meta-analysis. Arq Gastroenterol. 2018;55(3):306–13. doi: 10.1590/S0004-2803.201800000-66. [DOI] [PubMed] [Google Scholar]
- 41.Mirjalili H, Dastgheib SA, Shaker SH, Bahrami R, Mazaheri M, Sadr-Bafghi SMH, et al. Proportion and mortality of iranian diabetes mellitus, chronic kidney disease, hypertension and cardiovascular disease patients with covid-19: A meta-analysis. J Diabetes Metab Disord. 2021;20(1):905–17. doi: 10.1007/s40200-021-00768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Razmpoosh E, Safi S, Abdollahi N, Nadjarzadeh A, Nazari M, Fallahzadeh H, et al. The effect of nigella sativa on the measures of liver and kidney parameters: A systematic review and meta-analysis of randomized-controlled trials. Pharmacol Res. 2020;156:104767. doi: 10.1016/j.phrs.2020.104767. [DOI] [PubMed] [Google Scholar]
- 43.Cheraghi A, Barahman M, Hariri R, Nikoofar A, Fadavi P. Comparison of the pathological response and adverse effects of oxaliplatin and capecitabine versus paclitaxel and carboplatin in the neoadjuvant chemoradiotherapy treatment approach for esophageal and gastroesophageal junction cancer: A randomized control trial study. Med J Islam Repub Iran. 2021;35:140. doi: 10.47176/mjiri.35.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Razmpoosh E, Safi S, Nadjarzadeh A, Fallahzadeh H, Abdollahi N, Mazaheri M, et al. The effect of nigella sativa supplementation on cardiovascular risk factors in obese and overweight women: A crossover, double-blind, placebo-controlled randomized clinical trial. Eur J Nutr. 2021;60(4):1863–74. doi: 10.1007/s00394-020-02374-2. [DOI] [PubMed] [Google Scholar]
- 45.Zafiropoulos A, Crikas N, Passam AM, Spandidos DA. Significant involvement of ccr2-64i and cxcl12-3a in the development of sporadic breast cancer. J Med Genet. 2004;41(5):e59. doi: 10.1136/jmg.2003.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razmkhah M, Talei AR, Doroudchi M, Khalili-Azad T, Ghaderi A. Stromal cell-derived factor-1 (sdf-1) alleles and susceptibility to breast carcinoma. Cancer Lett. 2005;225(2):261–6. doi: 10.1016/j.canlet.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 47.de Oliveira KB, Oda JM, Voltarelli JC, Nasser TF, Ono MA, Fujita TC, et al. Cxcl12 rs1801157 polymorphism in patients with breast cancer, hodgkin’s lymphoma, and non-hodgkin’s lymphoma. J Clin Lab Anal. 2009;23(6):387–93. doi: 10.1002/jcla.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Oliveira KB, Guembarovski RL, Oda JM, Mantovani MS, Carrera CM, Reiche EM, et al. Cxcl12 rs1801157 polymorphism and expression in peripheral blood from breast cancer patients. Cytokine. 2011;55(2):260–5. doi: 10.1016/j.cyto.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Lin GT, Tseng HF, Yang CH, Hou MF, Chuang LY, Tai HT, et al. Combinational polymorphisms of seven cxcl12-related genes are protective against breast cancer in taiwan. Omics. 2009;13(2):165–72. doi: 10.1089/omi.2008.0050. [DOI] [PubMed] [Google Scholar]
- 50.Lin S, Zheng Y, Wang M, Zhou L, Zhu Y, Deng Y, et al. Associations of cxcl12 polymorphisms with clinicopathological features in breast cancer: A case-control study. Mol Biol Rep. 2022;49(3):2255–63. doi: 10.1007/s11033-021-07047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kruszyna Ł, Lianeri M, Rubis B, Knuła H, Rybczyńska M, Grodecka-Gazdecka S, et al. Cxcl12-3’ g801a polymorphism is not a risk factor for breast cancer. DNA Cell Biol. 2010;29(8):423–7. doi: 10.1089/dna.2010.1030. [DOI] [PubMed] [Google Scholar]
- 52.Kontogianni P, Zambirinis CP, Theodoropoulos G, Gazouli M, Michalopoulos NV, Flessas J, et al. The impact of the stromal cell-derived factor-1-3’a and e-selectin s128r polymorphisms on breast cancer. Mol Biol Rep. 2013;40(1):43–50. doi: 10.1007/s11033-012-1989-x. [DOI] [PubMed] [Google Scholar]
- 53.Khalid S, Hanif R. Association of rs1801157 single nucleotide polymorphism of cxcl12 gene in breast cancer in pakistan and in-silico expression analysis of cxcl12-cxcr4 associated biological regulatory network. PeerJ. 2017;5:e3822. doi: 10.7717/peerj.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guembarovski AL, Guembarovski RL, Hirata BKB, Vitiello GAF, Suzuki KM, Enokida MT, et al. Cxcl12 chemokine and cxcr4 receptor: Association with susceptibility and prognostic markers in triple negative breast cancer. Mol Biol Rep. 2018;45(5):741–50. doi: 10.1007/s11033-018-4215-7. [DOI] [PubMed] [Google Scholar]
- 55.Xia Y, Guo XG, Ji TX. The g801a polymorphism in the cxcl12 gene and risk of breast carcinoma: Evidence from a meta-analysis including 2,931 subjects. Asian Pac J Cancer Prev. 2014;15(6):2857–61. doi: 10.7314/apjcp.2014.15.6.2857. [DOI] [PubMed] [Google Scholar]
- 56.Zhu K, Jiang B, Hu R, Yang Y, Miao M, Li Y, et al. The cxcl12 g801a polymorphism is associated with cancer risk: A meta-analysis. PLoS One. 2014;9(9):e108953. doi: 10.1371/journal.pone.0108953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen W, Cao X, Xi L, Deng L. Cxcl12 g801a polymorphism and breast cancer risk: A meta-analysis. Mol Biol Rep. 2012;39(2):2039–44. doi: 10.1007/s11033-011-0951-7. [DOI] [PubMed] [Google Scholar]
- 58.Ma XY, Jin Y, Sun HM, Yu L, Bai J, Chen F, et al. Cxcl12 g801a polymorphism contributes to cancer susceptibility: A meta-analysis. Cell Mol Biol (Noisy-le-grand). 2012;58 Suppl:Ol1702–8. [PubMed] [Google Scholar]
- 59.Gong H, Tan M, Wang Y, Shen B, Liu Z, Zhang F, et al. The cxcl12 g801a polymorphism and cancer risk: Evidence from 17 case-control studies. Gene. 2012;509(2):228–31. doi: 10.1016/j.gene.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Antikchi MH, Neamatzadeh H, Ghelmani Y, Jafari-Nedooshan J, Dastgheib SA, Kargar S, et al. The risk and prevalence of covid-19 infection in colorectal cancer patients: A systematic review and meta-analysis. J Gastrointest Cancer. 2021;52(1):73–9. doi: 10.1007/s12029-020-00528-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Novin K, Fadavi P, Mortazavi N, Sanei M, Khoshbakht Ahmadi H, Barahman M, et al. Neutrophil-to-lymphocyte ratio (nlr) as a poor predictive biomarker for pathological response to neoadjuvant chemoradiation in locally advanced rectal cancer: A prospective study. Asian Pac J Cancer Prev. 2023;24(1):61–7. doi: 10.31557/APJCP.2023.24.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbaspour S, Barahman M, Abdollahi H, Arabalibeik H, Hajainfar G, Babaei M, et al. Multimodality radiomics prediction of radiotherapy-induced the early proctitis and cystitis in rectal cancer patients: A machine learning study. Biomed Phys Eng Express. 2023;10:1. doi: 10.1088/2057-1976/ad0f3e. [DOI] [PubMed] [Google Scholar]
- 63.Alemrajabi M, Khavanin Zadeh M, Hemmati N, Banivaheb B, Alemrajabi F, Jahanian S, et al. Inferior part of rectus abdominis muscle flap outcomes after abdominoperineal resection: A case series pilot study. World J Plast Surg. 2021;10(3):104–10. doi: 10.29252/wjps.10.3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]