Abstract
Background:
Firefighters are faced with a broad range of toxic exposures during their work, including known and suspected carcinogens. The current study is an update to the previously published meta-analysis of cancer risk among firefighters by Soteriades and colleagues, and focuses on studies published from 2008 to 2020.
Methods:
A comprehensive search of the literature was conducted, including electronic databases and bibliographies of recently published papers. Analyses include stratification of studies conducted in the United States (US) versus other countries. Cancer incidence and mortality rates were compared to the relevant general population. Random effects models were used to calculate summary risk estimates and their 95% confidence intervals.
Results:
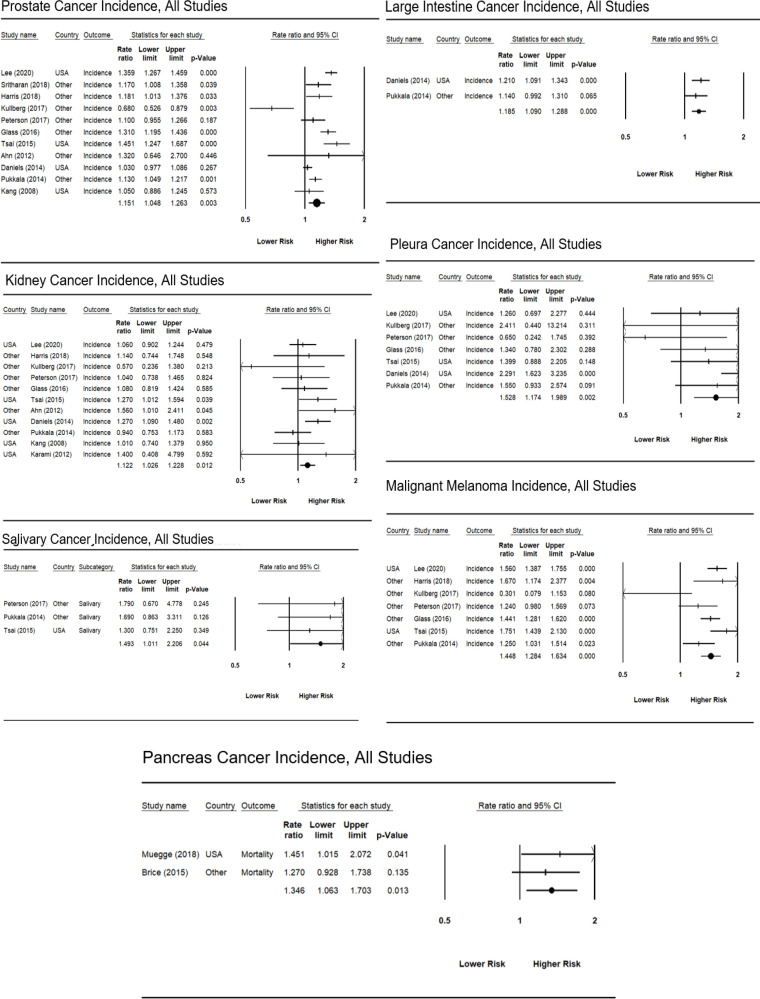
A total of 24 studies were included in the meta-analysis. Among the 42 cancer types covered, incidence was associated with firefighting in US samples for colon, kidney, large intestine, pleura, and prostate cancer, as well as malignant melanoma. There was an increased incidence of Hodgkin’s Disease and malignant melanoma and a significantly lower risk of kidney cancer for non-US samples. Significant cancer mortality estimates for US samples included oral/buccal/mouth, other parts of the buccal cavity, pharynx, colon, esophagus, large intestine, lung, Non-Hodgkin’s Lymphoma, pancreas, pleura, rectum, and soft tissue sarcoma. No cancer had a significantly higher rate of mortality among non-US samples.
Conclusions:
The findings underscore the global cancer burden among firefighters, and indicate that geographically stratifying studies afford a more nuanced risk perspective. Further research should investigate why US firefighters exhibit higher cancer mortality rates compared to international counterparts.
Key Words: Firefighter, cancer, occupational health, meta-analysis, public safety
Introduction
Firefighting is an inherently dangerous job that requires a broad range of exposures to known and suspected carcinogens [1]. In addition, firefighting involves shift work, which has been identified as a possibly carcinogenic risk [2]. The confluence of risks prompted a significant level of research on the relationship between firefighting and cancer risks starting as early as the 1960s with a study entitled “Lung Cancer in New York City Firemen” [3]. While growth in the field was slow for decades, it accelerated – particularly in the US – after the World Trade Center attacks on September 11, 2001, and in conjunction with the government funding firefighter health research, specifically through the Federal Emergency Management Agency (FEMA).
With mounting scientific evidence, the International Agency for Research on Cancer (IARC) reviewed the literature on firefighting and cancer in 2022 [2]. As an update to their Group 2A Classification in 2010 [4], the expert panel concluded that firefighting as an exposure should be classified as “carcinogenic to humans” (Group 1) due to new literature. The assessment supported “sufficient” evidence for firefighting leading to mesothelioma and bladder cancer. “Limited” evidence was identified for colon, prostate, and testicular cancers, melanoma and non-Hodgkin’s lymphoma. ‘Limited evidence of carcinogenicity’ is used by IARC to describe situations where a positive association has been observed between exposure to an agent and cancer, but where other explanations, such as chance, bias, or confounding factors, could not be confidently ruled out.
While there is a growing body of literature on the relationship between cancer and firefighting, inconsistent results across studies make conclusions challenging. Several recent meta-analyses focused on meaningfully combining studies to provide more insights by weighting and pooling study results [5-8]. Yet, meta-analyses also have their limitations. As noted by Guidotti [9], occupational health research often is underpowered and, even when bringing studies together, can miss significant relationships particularly for very rare outcomes such as cancer. He also notes that differences in study samples may mask significant results that exist within a particular group.
Soteriades and colleagues [10] published a 2019 meta-analysis of findings on firefighters and cancer from 1966-2007. The authors found statistically significant associations between firefighting and bladder, testicular, CNS, brain, colorectal, non-Hodgkin’s lymphoma, skin melanoma, and prostate cancers. They also found statistically significant but inconsistent relationships for cancers of the pancreas, kidney, non-Hodgkin’s lymphoma, leukemia, lymphosarcoma and reticulosarcoma, and multiple myeloma. Study limitations included an end date of 2007, but results were similar to those reported by LeMasters et al. [7]. Further, as with other reviews in this literature, some cancers were grouped into large categories (e.g., Buccal Cavity) rather than broken down into more specific anatomical regions (e.g., tongue or lip) as reported in the primary studies.
The current study extends the previous work by updating the literature review from 2008 to 2020. In addition to replicating the assessment of incidence and mortality with publications post 2008, we adopted a “detailed” rather than a “broad” approach to reporting data on cancer sites. That is, we present data on cancer sites as they were described in the primary studies rather than grouping into broader anatomical regions. Both approaches have benefits, however, the detailed approach can provide more precise information about cancer rates in these specific areas and may be better able to identify certain risk factors or patterns.
Given most studies identified used US-based firefighters, analyses were stratified by US versus non-US samples to determine if geographic differences and different practices in strategies/tactics, exposure types, and use of personal protective equipment (PPE) may lead to differences in incidence and mortality. The current analysis was designed to examine whether some differences in reported results may be due to combining all studies rather than dividing them into similar sub categories of exposures and practices.
Materials and Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Details for this checklist are presented in Appendix A Table S1.
Search Strategy
The electronic literature search was conducted in the PubMed, NIOSHTIC2, and Google Scholar databases with adapted search terms for each database. The search terms included any combination of firefighter, firefighting, fire, or first responder and cancer, lymphoma, mesothelioma, myeloma, melanoma, leukemia, malignancy, malignant, tumor, or carcinoma (Appendix A Table S2). References of published literature, as well as references identified in literature reviews, were included and evaluated.
Eligibility Criteria
Study inclusion criteria included: 1) English version of the abstract available; 2) reports included standardized mortality ratios (SMR), proportionate mortality ratio (PMR), relative risk (RR), standardized incidence ratio (SIR), or case/control mortality odds ratio (OR), or any results sufficient to calculate risk estimates and focused on firefighters and cancer risk; and 3) had necessary information for derivation of meta-analytic risks. Consideration was given to studies published between 1 January 2009 and 30 April 2020 since the current study aimed to extend the results from previous meta-analysis work. In addition, studies on volunteers, trainees, or wildland firefighters and those reported effect size on cancers related to the World Trade Center or 9/11 event were excluded. If more than one article was published with overlapping populations, preference was given to the article with the most comprehensive information. Review articles identified in the search were included in the secondary search of the bibliography.
Study Selection
Three independent researchers (NJ, CK, and BH) performed the study identification and eligibility assessment by first screening the titles and abstracts to determine whether the inclusion criteria were met. Then the full texts of the studies included were reviewed independently by NJ and CK. A third researcher (WCP) was consulted if no agreement could be reached between NJ and CK. The reasons for exclusion were recorded for each excluded study.
Data Extraction
Data extraction was performed independently by two researchers (CC and BN) and double-checked by another researcher (NJ). In case there were discrepancies in the results, the data extraction was discussed together until a consensus was reached. The following information was collected from each included study: authors’ names, year of publication, country, time period of case ascertainment, sample size, demographics (age, years serving in the fire service, sex), occupational information (source, occupation coding system), source of control population, information assessed on exposures, study design, cancer (source, classification information, type), cancer incidence and/or mortality, and risk estimate and its 95% CI (ORs, SIRs, RRs, PMRs, or SMRs). Classification of the cancer types was adapted to the tenth revision of the international classification of disease (ICD-10) (Appendix A Table S3).
Quality Assessments
Two researchers (NJ and CK) independently assessed the quality of case-control and cohort studies using the Newcastle-Ottawa Quality Assessment Scale (NOS) [11]. The NOS assesses the quality of both case-control and cohort studies based on three main domains: selection of study groups (four items, one point each), comparability of study groups (one item, up to two points), and ascertainment of exposure or outcome (three items, one point each). The sum of points ranges from 0 to 9, with higher scores indicating better quality. Any disagreement of ratings was discussed, and a consensus was reached mutually or by consulting a third author (WCP) if an earlier consensus could not be reached.
Statistical Analyses
Risk estimates, pooled estimates, and confidence intervals were derived using Comprehensive Meta Analysis (CMA) software version 3.3.070 [12]. Weights were given to each primary study based on the inverse of the variance of the effect size, and random effects models were used to pool the results. As with previous firefighter cancer meta-analyses, summary incidence risk estimates (SIREs) and mortality risk estimates (SMREs) were calculated by pooling the risk estimates provided in the primary studies (ORs, SIRs, RRs, PMRs, or SMRs). The logarithm of the risk estimate and its standard error were entered and back-transformed for data presentation. In addition to summary estimates, forest plots were used to examine the summary estimates overall and stratified by country and cancer type [13].
Given the aims of the review, data were analyzed separately for studies based on firefighters from the US versus other countries. When risk ratios were presented based on more than one reference group, the group that most approximated the general public was selected. Given their greater occupational exposures, we focused on risk estimates for career firefighters. When multiple risk estimates were provided based on different sets of covariates, the estimate with the most comprehensive set of covariates was used. One primary study [14] presented risk estimates separately for female firefighters. Pinkerton et al. [15] provided updated mortality risk estimates for Daniels and colleagues [16]; thus, only the estimates from Pinkerton were included in the analyses except for cancers only reported by Daniels. Bigert et al. [17] presented an additional four years of follow-up for the Swedish subsample of the Pukkala et al. NOCCA study [18]. Given that Pukkala and colleagues [18] provided a more comprehensive sample from multiple countries and the non-independence of the two cohorts, the additional outcomes presented by Bigert et al. [17] are provided in Appendix B Table 30.
Statisticians have cautioned against using heterogeneity statistics such as I2 for meta-analyses based on a small number of primary studies [19]. Even with a sample size of seven primary studies, bias in heterogeneity statistics can range from 12-28 percentage points, which is substantial given that in the Cochrane Library, the median I2 estimate is 21 percent. In cases like the present meta-analysis, von Hippel suggests a focus on confidence intervals rather than point estimates [19]. In addition, point estimates and confidence intervals of summary effect sizes were obtained from a random effects model. In the case of significant heterogeneity, a random-effects model will usually yield wider confidence intervals than a fixed-effects model, reflecting the uncertainty introduced by the variability between studies [20-22]. Finally, given the relatively small sample size of women in this study (n = 168), the risk analysis for this subgroup was reported separately from the pooled data.
Results
Characteristics of Included Studies
The literature search resulted in 4,285 articles, with 2,681 duplicates. We excluded 1,485 records (agreement = 88%) based on screening titles and abstracts and another 97 records (agreement = 90%) by reviewing full texts (Figure 1). As a result, 22 unique studies [23-27, 16, 28-32, 14, 33-36, 15, 37-40] were included in the analysis, with most coming from the US (33%), Canada (19%), and Norway/Denmark/Sweden (19%). Twelve studies focused specifically on firefighters, with the remainder including multiple occupations (Table 1). A total of 17 studies investigated the association between cancer incidence and firefighting occupation and seven articles investigated the association between firefighting and cancer mortality. An overview of the extracted risk estimates from a total of 22 studies is displayed in Appendix A Table S4 and S5.
Figure 1.
Forrest Plot of Significantly Increased Cancers, Incidence for All Studies
Table 1.
Characteristics of Studies Reporting on Firefighting and Cancer Incidence and Mortality
| Study’s First | Publication Date | Location | Case Ascertainment |
Occupational Focus | Sample Size of Firefighters | Average Age (SD) | Control Population(s) | Quality |
|---|---|---|---|---|---|---|---|---|
| Author† | Rating | |||||||
| Pinkerton | 2020 | US | 1950-2016 | Firefighters | 29,992 | NP | National | 8 |
| Langevin | 2020 | US | 1999-2011 | Firefighters | 13 | 60.2(10.6) | Local/Regional | 7 |
| Lee | 2019 | US | 1982-2014 | Firefighters | 3,928 | 57.2(12.6) | Local/Regional | 7 |
| Muegge | 2018 | US | 1982-2013 | Firefighters | 2,818 | 71.3(NP) | Local/Regional | 7 |
| Sritharan | 2018 | Canada | 1991-2010 | Multiple Occupations | NP | NP | Local/Regional | 8 |
| Petersen | 2018 | Denmark | 1970-2014 | Firefighters | 11,775 | 57.0 (13.8) | National | 8 |
| Other | ||||||||
| Harris | 2018 | Canada | 1992-2010 | Multiple Occupations | 4,535 | 41.0 (9.7) | National | 9 |
| Police | ||||||||
| Other | ||||||||
| Kullberg | 2017 | Sweden | 1931-1958 | Firefighters | 1,080 | 38.0 (NP) | Local/Regional | 7 |
| Petersen | 2017 | Denmark | 1968-2014 | Firefighters | 9,061 | 59.0 (12.9) | National | 8 |
| Other | ||||||||
| Bigert | 2016 | Europe, Canada, New Zealand, China | 1985-2010 | Firefighters | 190 | 62.9 (8.4) | National | 2 |
| Other | ||||||||
| Glass | 2016 | Australia | 1980-2011 | Firefighters | 30,057 | 49.9 (12.8) | National | 8 |
| Tsai | 2015 | US | 1988-2007 | Firefighters | 3,996 | 63.3 (NP) | Local/Regional | 7 |
| Brice | 2015 | France | 1979-2008 | Firefighters | 10,829 | 30 (NP) | National | 6 |
| Ahn | 2015 | Korea | 1980-2007 | Multiple Occupations | 29,453 | 41.3 (9.2) | National | 8 |
| Daniels | 2014 | US | 1950-2009 | Firefighters | 29,993 | 60.0 (16.0) | National | 7 |
| Pukkala | 2014 | Nordic | 1961-2005 | Firefighters | 16,422 | NP | National | 8 |
| Page-Bailly | 2013 | France | 2001-2007 | Multiple Occupations | 25 | NP | National | 7 |
| Ahn | 2012 | Korea | 1980-2007 | Multiple Occupations | 29,458 | 41.8 (9.3) | National | 9 |
| Karami | 2012 | US | 2002-2007 | Multiple | 8 | NP | Local/Regional | 5 |
| Corbin | 2011 | New Zealand | 2007-2008 | Multiple | 3 | NP | National | 7 |
| Occupations | ||||||||
| Villeneuve | 2011 | Canada | 1994-1997 | Multiple | 22 | NP | National | 7 |
| Occupations | ||||||||
| Kang | 2008 | US | 1986-2003 | Multiple Occupations | 2,125 | NP | Local/Regional | 7 |
| Police |
†See References for full citation. Note: NP, data not provided
More details on the characteristics of the included studies are presented in Appendix A Table S6 and S7. Briefly, 17 studies have investigated the association between cancer incidence and firefighting occupation (Appendix A Table S6). Of these, 9 were case-control (53%), and 8 were cohort (47%) studies. The time period of case ascertainment was between 1931 and 2014, with a maximum of 30,057 firefighters included. The studies on cancer incidence used more than one source to collect information on cancer incidence, and the most dominant source was cancer registries (16 studies). Seven articles investigated the association between firefighting and cancer mortality (Appendix A Table S7). Of these, six were based on cohort (86%), and one was case-control (14%). The time-period of case ascertainment was between 1950 and 2016, with a maximum of 30,057 firefighters. All studies extracted the outcome data from death certificates.
Methodological Quality of the Studies
The quality of studies ranged from 2 to 9 (9 = maximum possible rating, median = 7). However, all but two studies (two case-control studies) were rated as being in the highest category of methodological quality (scores from 7 to 9; see Appendix A Table S8). Consequently, the meta-analysis was founded on primary studies that predominantly exhibited good methodological rigor.
Cancer Incidence and Mortality among Firefighters
For most cancer types, firefighters did not have a significantly higher incident risk compare to the general public (Table 2). Estimates based on all studies regardless of location suggested firefighters were not at increased risk for all cancer types and were at lower risk for multiple cancer sites within the Buccal Cavity (SIRE = 0.907, 95% CI = 0.845-0.974). For both US and non-US studies combined, firefighters had an increased risk of Salivary (SIRE = 1.493, 95% CI = 1.011-2.206) and Large Intestine cancer (SIRE = 1.185, 95% CI = 1.090-1.288), as well as Pleura (SIRE = 1.538, 95% CI = 1.174-1.989) and Malignant Melanoma (SIRE = 1.448, 95% CI = 1.284-1.34).
Table 2.
Pooled Risk Estimates for Firefighter Cancer Incidence by Type: US vs. Other Locations
| All Studies | US Studies | Non-US Studies | |
|---|---|---|---|
| CancerType | Risk Estimate (95% CI) | Risk Estimate (95% CI) | Risk Estimate (95% CI) |
| [N of Studies] | [N of Studies] | [N of Studies] | |
| All Cancers | 1.025 (0.977 – 1.075) [7] | 1.090 (1.060 – 1.120) [1] | 1.008 (0.951 – 1.068) [6] |
| Bladder | 1.050 (0.961 – 1.147) [10] | 1.055 (0.942 – 1.181) [4] | 1.030 (0.875 – 1.212) [6] |
| Bone | 1.068 (0.656 – 1.740) [4] | 0.720 (0.360 – 1.440) [1] | 1.571 (0.792 – 3.120) [3] |
| Brain | 1.052 (0.900 – 1.231) [10] | 1.202 (0.966 – 1.496) [4] | 0.891 (0.748 – 1.060) [6] |
| OtherCNS | --- | --- | 1.073 (0.786 – 1.465) [2] |
| Meninges | --- | --- | 1.22 (0.637 – 2.339) [1] |
| Breast | 1.134 (0.718 – 1.791) [4] | 0.984 (0.640 – 1.515) [3] | 2.171 (0.893 – 5.274) [1] |
| Buccal Cavityand Pharynx | |||
| Multiple Sites1 | 0.907 (0.845 – 0.974) [3] | 0.904 (0.840 – 0.974) [1] | 0.950 (0.722 – 1.250) [1] |
| Oral/Buccal Cavity/Mouth | 1.099 (0.575 – 2.098) [5] | 0.824 (0.513 - 1.322) [2] | 1.548 (0.390 – 6.142) [3] |
| Lip | 1.094 (0.801 – 1.495) [5] | 1.374 (0.881 – 2.144) [2] | 0.877 (0.566 – 1.360) [3] |
| Tongue | 1.255 (0.928 – 1.616) [3] | 1.181 (0.820 – 1.700) [1] | 1.288 (0.841 – 1.972) [2] |
| Salivary | 1.493 (1.011 – 2.206) [3] | 1.30 (0.751 – 2.250) [1] | 1.721 (0.989 – 2.998) [2] |
| Pharynx | 0.999 (0.789 – 1.265) [3] | 1.06 (0.749 – 1.499) [1] | 0.950 (0.688 – 1.311) [2] |
| Oropharynx | --- | --- | 1.90 (0.382 – 9.461) [1] |
| Nasopharynx | --- | 1.310 (0.321 – 5.340) [1] | ---- |
| Hypopharynx | --- | --- | 3.099 (0.580 – 16.556) [1] |
| Colon | 1.055 (0.953 – 1.167) [9] | 1.12 (1.024 – 1.227) [4] | 0.968 (0.792 – 1.183) [5] |
| Esophagus | 1.018 (0.778 – 1.331) [9] | 1.115 (0.726 – 1.712) [4] | 0.929 (0.732 – 1.178) [5] |
| Eye | 0.880 (0.473 – 1.638) [2] | 0.880 (0.419 – 1.846) [1] | 0.880 (0.281 – 2.753) [1] |
| Hodgkin’s Disease | 1.271 (0.934 – 1.729) [7] | 1.022 (0.757 – 1.382) [3] | 1.587 (1.012 – 2.489) [4] |
| Kidney | 1.122 (1.035 – 1.231) [11] | 1.169 (1.063 – 1.285) [4] | 1.056 (0.901 – 1.237) [6] |
| Renal Pelvis | --- | 1.459 (0.787 – 2.708) [1]3 | --- |
| Large Intestine | 1.185 (1.090 – 1.288) [2] | 1.210 (1.091 – 1.343) [1] | 1.140 (0.992 – 1.310) [1] |
| Larynx | 0.724 (0.565 – 0.926) [7] | 0.611 (0.446 – 0.837) [3] | 0.924 (0.712 – 1.198) [4] |
| Leukemia | 1.018 (0.906 – 1.145) [9] | 1.064 (0.855 – 1.325) [4] | 0.952 (0.797 – 1.139) [5] |
| Lymphatic | --- | --- | 0.910 (0.549 – 1.509) [1] |
| Myeloid | --- | --- | 0.760 (0.398 – 1.452) [1] |
| Liver | 0.837 (0.0731 – 0.959) [9] | 0.918 (0.651 – 1.296) [3] | 0.821 (0.690 – 0.977) [6]2 |
| Gallbladder | --- | --- | 1.196 (0.818 – 1.747) [3] |
| Lung | 0.922 (0.83 – 1.024) [13] | 0.965 (0.793 – 1.176) [4] | 0.888 (0.799 – 0.987) [9] |
| Multiple Myeloma | 0.982 (0.813 – 1.187) [9] | 0.925 (0.685 – 1.249) [4] | 1.077 (0.860 – 1.349) [5] |
| Non-Hodgkin’s Lymphoma | 1.017 (0.925 – 1.117) [10] | 1.013 (0.869 – 1.181) [4] | 1.029 (0.900 – 1.177) [6] |
| Other Male Genital Organs | 0.884 (0.551 – 1.417) [4] | 0.659 (0.425 – 1.021) [2] | 1.303 (0.743 – 2.285) [2] |
| Pancreas | 1.041 (0.933 – 1.161) [9] | 0.923 (0.778 – 1.096) [3] | 1.154 (0.994 – 1.340) [6] |
| Pleura (mesothelioma) | 1.528 (1.174 – 1.989) [7] | 1.670 (1.130 – 2.468) [3] | 1.344 (0.957 – 1.888) [4] |
| Prostate | 1.151 (1.048 – 1.263) [11] | 1.208 (1.004 – 1.453) [4] | 1.117 (0.999 – 1.248) [7] |
| Rectum | 1.065 (0.983 – 1.154) [8] | 1.048 (0.937 – 1.172) [3] | 1.083 (0.966 – 1.216) [5] |
| Skin | 1.083 (0.905 – 1.297) [4] | 1.040 (0.768 – 1.408) [1] | 1.089 (0.858 – 1.384) [3] |
| Malignant Melanoma | 1.448 (1.284 – 1.634) [7] | 1.609 (1.454 – 1.779) [2] | 1.340 (1.142 – 1.571) [5] |
| Small Intestine | 1.270 (0.888 – 1.816) [3] | 1.150 (0.692 – 1.911) [1] | 1.507 (0.739 – 3.076) [2] |
| Soft Tissue Sarcoma | 1.034 (0.836 – 1.279) [5] | 1.028 (0.801 – 1.319) [3] | 1.051 (0.699 – 1.582) [2] |
| Stomach | 1.012 (0.883 – 1.160) [10] | 0.934 (0.765 – 1.141) [4] | 1.079 (0.885 – 1.316) [6] |
| Testis | 1.219 (0.976 – 1.523) [8] | 1.247 (0.883 – 1.759) [4] | 1.167 (0.832 – 1.638) [4] |
| Thyroid | 1.298 (0.975 – 1.727) [8] | 1.403 (0.820 – 2.401) [3] | 1.175 (0.916 – 1.506) [5] |
Note: Bold texts indicated statistically significant effect sizes (i.e., 95% confidence interval does not include 1.0); 1. Risk estimates for multiple sites within Buccal Cavity and Pharynx (e.g., Lip, Oral Cavity, and Pharynx); 2. Three non-US studies reported liver and gallbladder incidence separately. The first effect sizes represent either liver and gallbladder or liver only. The second effect sizes represent gallbladder only.
US firefighters had a statistically significant increase in incident risk of all cancers (SIRE = 1.090, 95% CI = 1.060-1.120) (Table 2). The only cancer with increased incident risk for both US and non-US firefighters was Malignant Melanoma (SIRE US = 1.609, 95% CI = 1.454-1.779; SIRE non-US = 1.340,95% CI = 1.142-1.571). US firefighters had a significantly lower incident risk of cancer within multiple sites of the Buccal Cavity (0.904, 95% CI = 0.840-0.974) and higher incident risk of Colon (SIRE = 1.12, 95% CI = 1.024-1.227), Kidney (1.169, 95% CI = 1.064-1.285), Large Intestine (SIRE = 1.210, 95% CI = 1.091-1.343), Pleura (SIRE = 1.670, 95% CI = 1.130-2.468), and the Prostate (SIRE = 1.208, 95% CI = 1.004-1.453), along with Malignant Melanoma (SIRE = 1.609, 95% CI = 1.454-1.779). In contrast, non-US firefighters were at increased incident risk of Hodgkin’s Disease (SIRE = 1.587, 95% CI = 1.012-2.489) and significantly lower incident risk of Liver cancer (SIRE = 0.821, 95% CI = 0.,690-0.977).
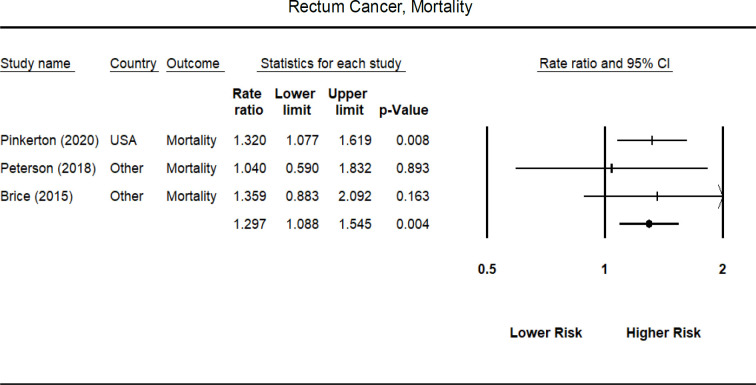
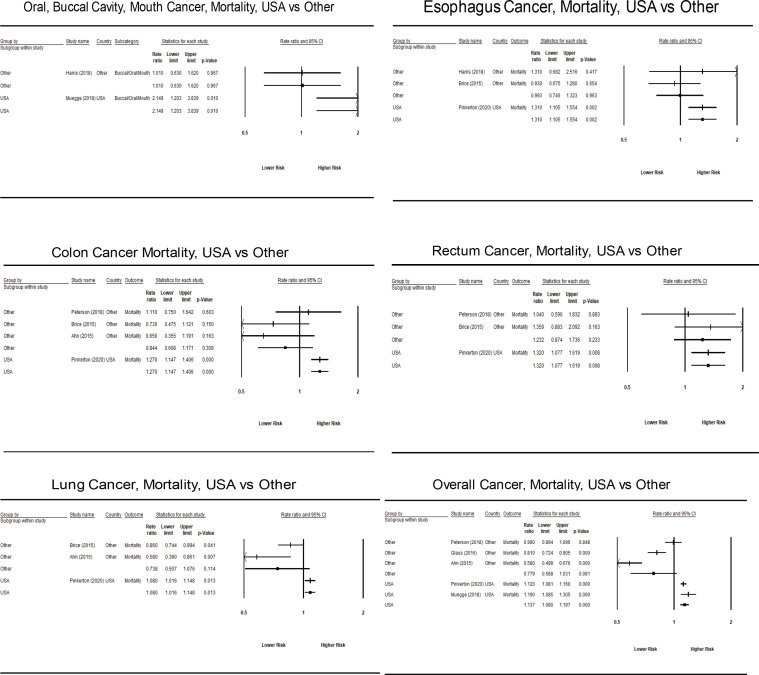
Not surprisingly, few studies provided estimates for cancer mortality; thus, estimates were typically based on a small number of primary studies when stratified by US vs. non-US location. None of the cancer types demonstrated higher mortality risk for non-US firefighters (Table 3). For US firefighters, a statistically significant higher mortality risk was found for all cancers (SIMR = 1.137, 95% CI = 1.108-1.197). Significantly higher mortality risk was found for a large number of cancer types (Oral/Buccal cavity mouth, other parts of Buccal cavity, Pharynx, Colon, Esophagus, Large Intestine, Lung, Non-Hodgkin Lymphoma, Pancreas, Pleura, Rectum, and soft tissue Sarcoma; see Table 3 for individual SIMRs). However, most of the mortality estimates for US firefighters were based on a single primary study. Forest plots for each specific cancer site and for incident and mortality risk are summarized in Appendix B (Figures 1-4).
Table 3.
Pooled Risk Estimates for Firefighter Cancer Mortality by Type and US vs. Other Locations
| All Studies | US Studies | Non-US Studies | |
|---|---|---|---|
| Cancer Type | Risk Estimate (95% CI) | Risk Estimate (95% CI) | Risk Estimate (95% CI) |
| [N of Studies] | [N of Studies] | [N of Studies] | |
| All Cancers | 0.917 (0.756 – 1.111) [5] | 1.137 (1.108 – 1.197) [2] | 0.779 (0.588 – 1.031) [3] |
| Bladder | 0.946 (0.785 – 1.141) [2] | 0.980 (0.807 – 1.190) [1] | 0.730 (0.425 1.254) [1] |
| Brain | --- | 1.357 (0.690 – 2.669) [2] | --- |
| Breast | 1.204 (0.626 – 2.316) [2] | 1.240 (0.632 – 2.433) [1] | 0.760 (0.052 – 11.060) [1] |
| Buccal Cavity and Pharynx | --- | --- | 1.150 (0.899 – 1.472) [1] |
| Oral/Buccal Cavity/Mouth | 1.445 (0.690 – 3.025) [2] | 2.149 (1.203 – 3.839) [1] | 1.010 (0.630 – 1.620) [1] |
| Other Parts Buccal Cavity | --- | 3.999 (1.069 – 14.955) [1] | --- |
| Oral Cavity and Esophagus | --- | --- | 1.270 (0.851 – 1.894) [1] |
| Lip | ---- | --- | 2.090 (0.867 – 5.038) [1] |
| Salivary | --- | --- | 2.340 (0.613 – 8.923) [1] |
| Pharynx | --- | 2.259 (1.073 – 4.758) [1] | --- |
| Colon | 0.971 (0.702 – 1.343) [4] | 1.270 (1.147 – 1.406) [1] | 0.844 (0.608 – 1.171) [3] |
| Esophagus | 1.170 (0.916 – 1.494) [3] | 1.310 (1.105 – 1.554) [1] | 0.993 (0.746 – 1.323) [2] |
| Kidney | 1.201 (0.770 – 1.872) [3] | 1.426 (0.965 – 2.107) [2] | 0.630 (0.320 – 1.239) [1] |
| Large Intestine | --- | 1.310 (1.160 – 1.479) [1] | --- |
| Larynx | --- | --- | 1.100 (0.745 – 1.624) [1] |
| Leukemia | 1.039 (0.740 – 1.459) [2] | 1.110 (0.939 – 1.311) [1] | 0.660 (0.269 – 1.616) [1] |
| Liver/Gallbladder | --- | --- | 0.777 (0.394 – 1.532) [2] |
| Lung | 0.869 (0.672 – 1.125) [3] | 1.080 (1.016 – 1.148) [1] | 0.738 (0.507 – 1.076) [2] |
| Multiple Myeloma | --- | 0.930 (0.707 – 1.223) [1] | --- |
| Non-Hodgkin’s Lymphoma | --- | 1.210 (1.031 – 1.433) [1] | --- |
| Other Male Genitalia | --- | 0.390 (0.129 – 1.175) [1] | --- |
| Pancreas | 1.346 (1.063 – 1.703) [2] | 1.451 (1.077 – 1.619) [1] | 1.270 (0.928 – 1.738) [1] |
| Pleura (mesothelioma) | --- | 1.861 (1.138 – 3.043) [1] | --- |
| Prostate | 0.765 (0.474 – 1.236) [3] | 1.080 (0.972 – 1.201) [1] | 0.599 (0.420 – 0.853) [2] |
| Rectum | 1.297 (1.088 – 1.545) [3] | 1.320 (1.192 – 1.600) [1] | 1.232 (0.874 – 1.736) [2] |
| Skin | 1.025 (0.821 – 1.279) [2] | 1.050 (0.837 – 1.318) [1] | 0.650 (0.242 – 1.742) [1] |
| Small Intestine | --- | 1.660 (0.779 – 3.538) [1] | --- |
| Soft Tissue Sarcoma | --- | 2.499 (1.037 – 6.026) [1] | --- |
| Stomach | 1.081 (0.749 – 1.560) [4] | 1.060 (0.881 – 1.274) [1] | 1.107 (0.592 – 2.070) [3] |
| Testis | --- | 0.730 (0.193 – 2.756) [1] | --- |
Note: Bold texts indicated statistically significant effect sizes (i.e., 95% confidence interval does not include 1.0).
Figure 4.
Forrest Plot of Significantly Increased Cancers, Mortality, All Studies
Figure 2.
Forrest Plot of Significantly Increased Cancers, Incidence, USA vs Other
Figure 3.
Forrest Plot of Significantly Increased Cancers, Mortality, USA vs Other
Discussion
This meta-analysis was conducted to investigate the relationship between firefighting and various cancers. Although there is no universally accepted cut-off for the size of risk estimates, it has been suggested that a risk ratio of 1.2 or less (or 0.83 or more if < 1) is small, between 1.2 and 2.0 (or 0.83 and 0.50 if < 1) is medium, and 2.0 or greater (or 0.5 or less if < 0.5) is large [41]. The results indicated a small increase in incident risk for cancer of the large intestine, and a moderate increase for salivary cancer, mesothelioma, and malignant melanoma across all studies. There was a small decrease in incident risk for cancer found in multiple sites of the buccal cavity for all studies combined. When the studies were stratified based on the US versus non-US locations, the incidence of all cancers, colon, and kidney cancer demonstrate a small increase in incident risk while cancer of the large intestine, mesothelioma, prostate, and malignant melanoma were found to be moderately elevated in the US studies. Cancer in multiple sites in the buccal cavity demonstrated a small decrease in risk. In contrast, only Hodgkin’s disease and malignant melanoma had an increased incidence risk in the non-US studies, and both were in the moderate range.
Non-US firefighters were not at increased mortality risk for any cancer type. For firefighters from the US, all cancers and lung cancer demonstrated a small increase in mortality risk, while cancer of the colon, esophagus, large intestine non-Hodgkin’s lymphoma, pancreas, pleura, and rectum demonstrated a moderately increased risk for cancer mortality. Large increases in mortality risk were found for cancers of the oral/buccal cavity/mouth, other parts of the buccal cavity, pharynx, and soft tissue sarcoma.
Differences in results between the current analysis and Soteriades [10] may be due to a number of reasons. As suggested by Casjen and colleagues [5], improvements in PPE available and more consistent use, as well as firefighting tactics, may be leading to a reduction in cancers over time. However, as studies from Underwriters Laboratory have found, current building materials and furnishings are leading to fires that burn more quickly and “dirtier” than in the past. Based on their work, it was found that, while legacy fires typically took up to 15 minutes to engulf a home, current synthetic materials do the same in approximately four minutes.
The guiding mission of our study was to provide a quantitative summary of the published peer-reviewed literature. These are the findings policymakers are interested in considering and which most influence organizational practice. Unlike other areas of research, conducting high-quality studies of cancer risk in firefighters is a considerable undertaking, and the likelihood of a large file drawer effect (many unpublished studies) is low. The following factors suggest that the impact of unpublished, high-quality epidemiological studies on our meta-analysis is likely to be minimal. We rigorously examined the quality of the included studies to ensure that they could provide accurate effect size estimates for the populations studied. The research in this domain is primarily conducted by research institutions like large universities and the National Institute for Occupational Safety and Health (NIOSH). These organizations have no vested interest in not publishing a study of firefighter cancer risk given the large undertaking and lack of vested interests in the outcomes. Our estimates have relatively narrow 95% confidence intervals. For example, for all cancers across all studies, the effect size was 1.025, with a 95% CI of 0.977–1.075. Narrow intervals like these indicate less variability in effect sizes across studies of different sample sizes, which argues against publication bias. Effects sizes with large confidence intervals were not significant. Since publication bias tends to overestimate effects, this provides evidence against the notion that the studies in our sample were biased positively. Publication bias typically manifests as a preponderance of statistically significant findings. In our meta-analysis, most effect sizes were not statistically significant, making publication bias less likely. We employed random-effects models rather than fixed-effects models. This approach is more robust to the presence of heterogeneity and provides a more conservative estimate of the overall effect size, thereby controlling for potential bias.
As with any study, our study had some limitations. With the rapidly growing field of firefighter health research and new studies emerging regularly, recent studies published after the current one may alter the results. Continuing to consider emerging literature and examining different ways to stratify data analysis holds the promise of unique insights to shape worldwide intervention and prevention efforts for the fire service. Also, the current meta-analysis was focused on updating the work of Soteriades [10] with emerging literature, so it was only assessed from 2008 forward. The foundational work of IARC [2] classifying firefighting as a Group 1 carcinogen provides a comprehensive review of existing literature. Still, those findings can only be sensitive to some of the strata within the fire service.
The current analysis results provide unique insights and potential future directions for research. The strength of the study is that it explores differences between the U.S. and non-U.S. based studies and points to the importance of exploring regional/national differences. As the research continues to grow, additional research is needed to examine sub-categories within broad cancer classifications. For instance, while the general category of skin cancer was not found to be elevated among US firefighters, there was a significant increase in malignant melanomas. Future meta-analyses also should account for where study populations are being drawn from, as different countries and regions may see dissimilarities in cancer incidence and mortality. Differences may be related to the products that are burning, fire suppression strategies and tactics, or use of PPE. As the field expands, findings may impact prevention efforts and can point to new best practice approaches for cancer prevention. Location specific cancers may also be used to inform screening efforts.
Future research also should include additional studies with detailed descriptions of the personnel being assessed. While large cohorts from registries have the advantage of large sample sizes, classifications typically include a broad variety of personnel (e.g. high and low call volume, structural and wildland firefighters, etc.) and may also mask the results of more nuanced analyses [39]. NIOSH launched the National Firefighter Registry as a prospective cohort of firefighters within the US in early 2023 [42]. A noted benefit of the registry is that it asks personnel to include details of their years of service, roles, exposures and health behaviors. As other countries focus on increasing research on cancer among their firefighters, mirroring data collection internationally will provide additional benefits to understanding how and why firefighters are contracting cancer and how to reduce the risk.
Author Contribution Statement
All authors contributed equally in this study.
Acknowledgements
The authors would like to acknowledge Kathy Crosby Bell and the Last Call Foundation for their financial support. The dedication of their founder and the organization to making fire and emergency services healthier and safer is a testament to how tragedy can lead to a better world. The authors also would like to thank Carrie Sutherland for her contributions to this manuscript, including data extraction and preparation, as well as assistance with editing and formatting the final document. The NDRI USA, Inc. Institutional Review Board (IRB) deemed the project not human subjects’ research. Author contributions: SJ, CP, and CH conceptualized the project, designed data collection, and collaborated on data collection and analysis. BH, CC and BN assisted in data extraction and coding. SJ, CP, and CH provide expert testimony on cancer coverage for firefighters.
Supplementary materials
Supplementary materials
References
- 1.Jahnke SA, Jitnarin N, Kaipust CK, Hollerbach BH, Naylor BM, Crisp C. Fireground exposure of firefighters: A literature review. Quincy, MA: Fire Protection Research Foundation; 2021. [Google Scholar]
- 2.Demers PA, DeMarini DM, Fent KW, Glass DC, Hansen J, Adetona O, et al. Carcinogenicity of occupational exposure as a firefighter. Lancet Oncol. 2022;23(8):985–6. doi: 10.1016/S1470-2045(22)00390-4. [DOI] [PubMed] [Google Scholar]
- 3.Saland G, Seley GP. Lung cancer in new york city firemen. N Y State J Med. 1963:63:92–4. [PubMed] [Google Scholar]
- 4.International Agency for Research on C. Painting, firefighting, & shiftwork . 2010. [Google Scholar]
- 5.Casjens S, Bruning T, Taeger D. Cancer risks of firefighters: A systematic review and meta-analysis of secular trends and region-specific differences. Int Arch Occup Environ Health. 2020;93(7):839–52. doi: 10.1007/s00420-020-01539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jalilian H, Ziaei M, Weiderpass E, Rueegg CS, Khosravi Y, Kjaerheim K. Cancer incidence and mortality among firefighters. Int J Cancer. 2019;145(10):2639–46. doi: 10.1002/ijc.32199. [DOI] [PubMed] [Google Scholar]
- 7.LeMasters GK, Genaidy AM, Succop P, Deddens J, Sobeih T, Barriera-Viruet H, et al. Cancer risk among firefighters: A review and meta-analysis of 32 studies. J Occup Environ Med. 2006;48(11):1189–202. doi: 10.1097/01.jom.0000246229.68697.90. [DOI] [PubMed] [Google Scholar]
- 8.Mehlum IS, Johannessen HA, Kjaerheim K, Grimsrud TK, Nordby KC. Risk of prostate cancer in firefighters: A review and meta-analysis of studies published after 2007. Eur J Public Health. 2018;28:r. [Google Scholar]
- 9.Guidotti TL. Interpreting the literature. In: Guidotti TL, editor. Health risks and fair compensation in the fire service. Risk, systems and decisions. Cham: Springer International Publishing; 2016. pp. 41–62. [Google Scholar]
- 10.Soteriades ES, Kim J, Christophi CA, Kales SN. Cancer incidence and mortality in firefighters: A state-of-the-art review and meta-analysis. Asian Pac J Cancer Prev. 2019;20(11):3221–31. doi: 10.31557/APJCP.2019.20.11.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses. 2012. [Google Scholar]
- 12.Borentstein M, Hedges L, Higgins J. Comprehensive meta-analysis software (cma) Englewood: NJ: Biostat; 2021. [Google Scholar]
- 13.Alavi M, Hunt GE, Visentin DC, Watson R, Thapa DK, Cleary M. Seeing the forest for the trees: How to interpret a meta-analysis forest plot. J Adv Nurs. 2021;77(3):1097–101. doi: 10.1111/jan.14721. [DOI] [PubMed] [Google Scholar]
- 14.Lee DJ, Koru-Sengul T, Hernandez MN, Caban-Martinez AJ, McClure LA, Mackinnon JA, et al. Cancer risk among career male and female florida firefighters: Evidence from the florida firefighter cancer registry (1981-2014) Am J Ind Med. 2020;63(4):285–99. doi: 10.1002/ajim.23086. [DOI] [PubMed] [Google Scholar]
- 15.Pinkerton L, Bertke SJ, Yiin J, Dahm M, Kubale T, Hales T, et al. Mortality in a cohort of us firefighters from san francisco, chicago and philadelphia: An update. Occup Environ Med. 2020;77(2):84–93. doi: 10.1136/oemed-2019-105962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniels RD, Kubale TL, Yiin JH, Dahm MM, Hales TR, Baris D, et al. Mortality and cancer incidence in a pooled cohort of us firefighters from san francisco, chicago and philadelphia (1950–2009) Occup Environ Med. 2013:oemed-2013. doi: 10.1136/oemed-2013-101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bigert C, Martinsen JI, Gustavsson P, Sparen P. Cancer incidence among swedish firefighters: An extended follow-up of the nocca study. Int Arch Occup Environ Health. 2020;93(2):197–204. doi: 10.1007/s00420-019-01472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pukkala E, Martinsen JI, Weiderpass E, Kjaerheim K, Lynge E, Tryggvadottir L, et al. Cancer incidence among firefighters: 45 years of follow-up in five nordic countries. Occup Environ Med. 2014;71(6):398–404. doi: 10.1136/oemed-2013-101803. [DOI] [PubMed] [Google Scholar]
- 19.von Hippel PT. The heterogeneity statistic i(2) can be biased in small meta-analyses. BMC Med Res Methodol. 2015;15:35. doi: 10.1186/s12874-015-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to meta-analysis. John Wiley & Sons; 2009. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn Y-S, Jeong KS. Mortality due to malignant and non-malignant diseases in korean professional emergency responders. PLoS ONE. 2015;10:3. doi: 10.1371/journal.pone.0120305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn YS, Jeong KS, Kim KS. Cancer morbidity of professional emergency responders in korea. Am J Ind Med. 2012;55(9):768–78. doi: 10.1002/ajim.22068. [DOI] [PubMed] [Google Scholar]
- 25.Amadeo B, Marchand J-L, Moisan F, Donnadieu S, Gaëlle C, Simone M-P, et al. French firefighter mortality: Analysis over a 30-year period. Am J Ind Med. 2015;58(4):437–43. doi: 10.1002/ajim.22434. [DOI] [PubMed] [Google Scholar]
- 26.Bigert C, Gustavsson P, Straif K, Taeger D, Pesch B, Kendzia B, et al. Lung cancer among firefighters: Smoking-adjusted risk estimates in a pooled analysis of case-control studies. J Occup Environ Med. 2016;58(11):1137–43. doi: 10.1097/JOM.0000000000000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corbin M, McLean D, Mannetje A, Dryson E, Walls C, McKenzie F, et al. Lung cancer and occupation: A new zealand cancer registry-based case-control study. Am J Ind Med. 2011;54(2):89–101. doi: 10.1002/ajim.20906. [DOI] [PubMed] [Google Scholar]
- 28.Glass DC, Pircher S, Monaco AD, Hoorn SV, Sim MR. Mortality and cancer incidence in a cohort of male paid australian firefighters. Occup Environ Med. 2016:oemed-2015. doi: 10.1136/oemed-2015-103467. [DOI] [PubMed] [Google Scholar]
- 29.Harris MA, Kirkham TL, MacLeod JS, Tjepkema M, Peters PA, Demers PA. Surveillance of cancer risks for firefighters, police, and armed forces among men in a canadian census cohort. Am J Ind Med. 2018;61(10):815–23. doi: 10.1002/ajim.22891. [DOI] [PubMed] [Google Scholar]
- 30.Kang D, Davis LK, Hunt P, Kriebel D. Cancer incidence among male massachusetts firefighters, 1987-2003. Am J Ind Med. 2008;51(5):329–35. doi: 10.1002/ajim.20549. [DOI] [PubMed] [Google Scholar]
- 31.Karami S, Colt JS, Schwartz K, Davis FG, Ruterbusch JJ, Munuo SS, et al. A case–control study of occupation/industry and renal cell carcinoma risk. BMC Cancer. 2012;12:344 . doi: 10.1186/1471-2407-12-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kullberg C, Andersson T, Gustavsson P, Selander J, Tornling G, Gustavsson A, et al. Cancer incidence in stockholm firefighters 1958-2012: An updated cohort study. Int Arch Occup Environ Health. 2018;91(3):285–91. doi: 10.1007/s00420-017-1276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muegge CM, Zollinger TW, Song Y, Wessel J, Monahan PO, Moffatt SM. Excess mortality among indiana firefighters, 1985-2013. Am J Ind Med. 2018;61(12):961–7. doi: 10.1002/ajim.22918. [DOI] [PubMed] [Google Scholar]
- 34.Paget-Bailly S, Guida F, Carton M, Menvielle G, Radoï L, Cyr D, et al. Occupation and head and neck cancer risk in men: Results from the icare study, a french population-based case-control study. J Occup Environ Med. 2013;55(9):1065–73. doi: 10.1097/JOM.0b013e318298fae4. [DOI] [PubMed] [Google Scholar]
- 35.Kirstine Ugelvig Petersen K, Pedersen JE, Bonde JP, Ebbehoej NE, Hansen J. Long-term follow-up for cancer incidence in a cohort of danish firefighters. Occup Environ Med. 2018;75(4):263–9. doi: 10.1136/oemed-2017-104660. [DOI] [PubMed] [Google Scholar]
- 36.Petersen KU, Pedersen JE, Bonde JP, Ebbehøj NE, Hansen J. Mortality in a cohort of danish firefighters; 1970-2014. Int Arch Occup Environ Health. 2018;91(6):759–66. doi: 10.1007/s00420-018-1323-6. [DOI] [PubMed] [Google Scholar]
- 37.Pukkala E, Martinsen JI, Weiderpass E, Kjaerheim K, Lynge E, Tryggvadottir L, et al. Cancer incidence among firefighters: 45 years of follow-up in five nordic countries. Occup Environ Med. 2014;71(6):398–404. doi: 10.1136/oemed-2013-101803. [DOI] [PubMed] [Google Scholar]
- 38.Sritharan J, Pahwa M, Demers PA, Harris SA, Cole DC, Parent M-E. Prostate cancer in firefighting and police work: A systematic review and meta-analysis of epidemiologic studies. Environ Health: Glob Access Sci. 2017;16(1):124 . doi: 10.1186/s12940-017-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai RJ, Luckhaupt SE, Schumacher P, Cress RD, Deapen DM, Calvert GM. Risk of cancer among firefighters in california, 1988-2007. Am J Ind Med. 2015;58(7):715–29. doi: 10.1002/ajim.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villeneuve S, Cyr D, Lynge E, Orsi L, Sabroe S, Merletti F, et al. Occupation and occupational exposure to endocrine disrupting chemicals in male breast cancer: A case-control study in europe. Occup Environ Med. 2010;67(12):837–44. doi: 10.1136/oem.2009.052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2022. [Google Scholar]
- 42.CDC. National firefighter registry (NFR) for Cancer. niosh: cdc: 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.