Abstract
α,β-Dehydrogenation of aliphatic acids has been realized through both enolate and β-C─H metalation pathways. However, synthesis of isolated β,γ-unsaturated aliphatic acids via dehydrogenation has not been achieved to date. Herein, we report the ligand-enabled β,γ-dehydrogenation of abundant and inexpensive free aliphatic acids, which provides a new synthetic disconnection as well as a versatile platform for downstream functionalization of complex molecules at remote γ-sites. A variety of free aliphatic acids, including acyclic and cyclic systems with ring sizes from 5-membered to macrocyclic, undergo efficient dehydrogenation. Notably, this protocol features good chemoselectivity in the presence of more accessible α-C─H bonds and excellent regioselectivity in fused bicyclic scaffolds. The utility of this protocol has been demonstrated by the late-stage functionalization of a series of bioactive terpene natural products at the γ-sites. Further functionalization of the β,γ-double bond allows for the installation of covalent warheads including epoxides, aziridines, and β-lactones into complex natural product scaffolds, which are valuable for targeted covalent drug discovery.
Graphical Abstract
1. Introduction
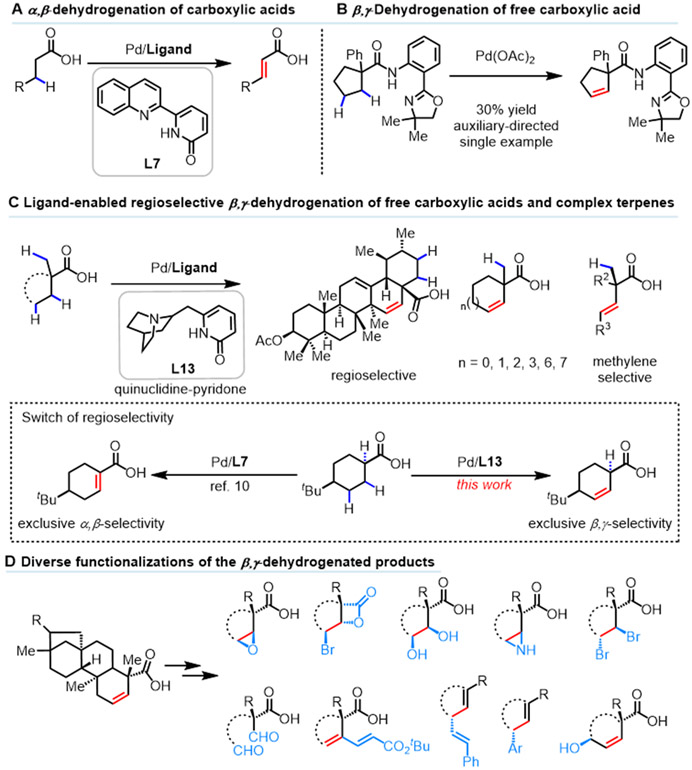
Desaturation of aliphatic substrates is a broadly useful transformation in modern organic chemistry.1, 2 Despite significant advances in the α,β-desaturation of carbonyls, such as aldehydes,3 ketones,4-6 esters,7 amides8, 9 and recently, free carboxylic acids10, 11 (Scheme 1A), efficient β,γ-dehydrogenation of carbonyls remains an unsolved problem. While α,β-desaturation generates conjugated olefins as Michael acceptors, β,γ-dehydrogenation would generate a versatile double bond remote from the existing carbonyl functional group that can be used to functionalize γ-positions. Although a diverse range of oxidative pathways can potentially lead to desaturation, we have focused on dehydrogenation via β and γ-methylene C─H activation since 2008.12 The feasibility of this approach was first demonstrated in a single example of cyclopentane carboxylic acid using a bidentate oxazoline-amide directing group with low efficiency (30% yield) (Scheme 1B).12 Through ligand development, we have recently achieved Pd(II)-catalyzed α,β-dehydrogenation of aliphatic acids.10 However, catalytic β,γ-dehydrogenation has not been achieved to date due to the product inhibition: the double bond in β,γ-unsaturated aliphatic acid preferentially bind to Pd(II) catalysts and vinylic C─H activation can occur thereby derailing the desired catalytic cycle. In our previous report, we designed a dehydrogenation-vinyl C─H olefination cascade to rescue the hijacked catalytic cycle.13 However, this approach prevents us from obtaining the desired β,γ-unsaturated aliphatic acids which are highly versatile and enabling synthetic intermediates.
Scheme 1.
Remote Dehydrogenation of Carboxylic Acids via β-C─H Activation
Herein, we report an efficient Pd-catalyzed β,γ-dehydrogenation of free carboxylic acids enabled by two types of recently developed bidentate ligands, namely, quinuclidine-pyridone and pyridine-pyridone ligands10, 14 (Scheme 1C). Both acyclic and cyclic aliphatic carboxylic acids with ring sizes ranging from five-membered rings to macrocycles are compatible with this catalyst. A series of biologically significant natural products also underwent β,γ-dehydrogenation to generate remote olefins, allowing for the incorporation of covalent warheads, such as epoxides and β-lactones, into complex natural product scaffolds (Scheme 1D). Strikingly, previously observed α,β-dehydrogenation10 was switched to β,γ-dehydrogenation by using our protocol with quinuclidine-pyridone ligand (Scheme 1C).
2. Results and Discussion
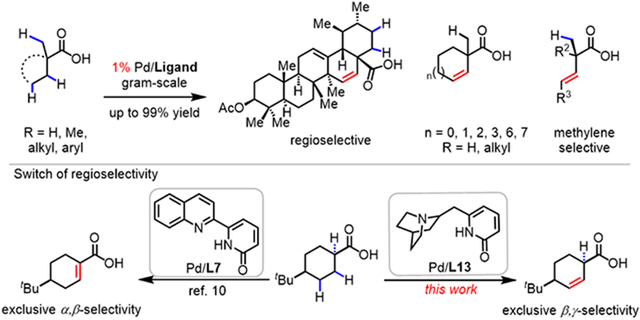
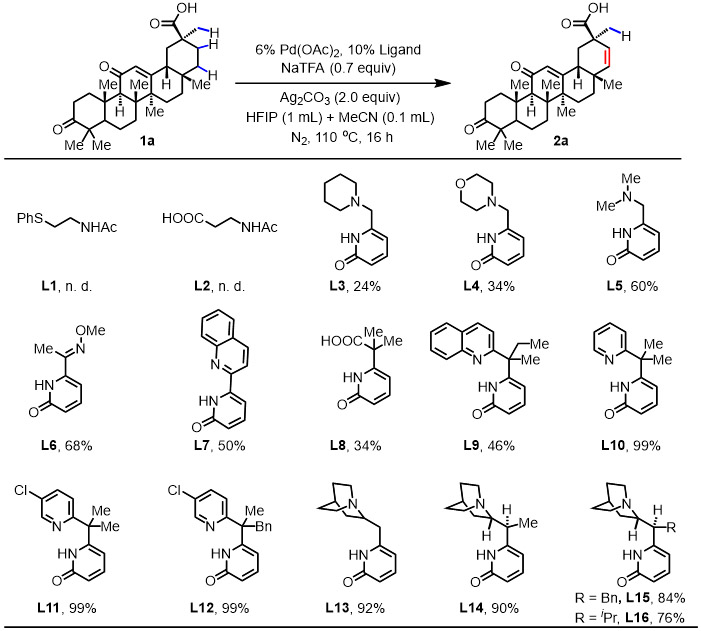
Terpene natural products containing carboxylic acid have been found to be useful in the prevention and therapy of several diseases and have exhibited diverse bioactivity such as antiviral, anticancer, and anti-inflammatory properties.15 For instance, ursolic acid and oleanolic acid have been shown to reduce leukemia cell growth16 and inhibit the proliferation of several transplantable tumors in animals.17 The introduction of β,γ-double bond into these natural products will afford a wealth of opportunities for subsequent elaboration and functionalization at remote γ-sites. Therefore, the β, γ-dehydrogenation was initiated with triterpenoid 3-oxoglycyrrhetinic acid 1a as the model substrate for condition optimization. Our previous study revealed that the formation of the desired free β,γ-unsaturated products could derail the catalytic cycle and trapping the intermediates with olefin was necessary for the catalytic cycle to proceed.5 With this insight in mind, we launched further efforts to test and develop more ligands to achieve selective β,γ-dehydrogenation reaction (see SI for details). A variety of five-membered chelating ligands previously developed18-25 in our laboratory to enable the C─H functionalization of free carboxylic acids were investigated (Table 1). The bidentate thioether ligand (L1) and mono-N-protected amino acid (MPAA) ligand (L2), previously demonstrated to promote a wide range of primary C(sp3)─H functionalization of free acids, did not provide any of the desaturated product.18, 19 Among the tertiary amine-pyridone ligands (L3-L5) and oxime ether-pyridone ligand (L6),13 previously enabling dehydrogenation-vinyl C─H olefination, L6 gave the highest yield (68%).
Table 1.
|
Conditions: 1a (0.1 mmol), Pd(OAc)2 (6 mol%), ligand (L) (10 mol%), NaTFA (0.7 equiv), Ag2CO3 (2.0 equiv), HFIP/MeCN (1.0 mL/0.1 mL), 110 °C, 16 h.
The yields were determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard.
Our recent work has shown that inhibition by α,β-unsaturated acids can be overcome by changing the bite angle of bidentate ligands (five vs. six-membered chelation).10 Accordingly, a series of six-membered chelating ligands (L8-L12) were tested, with new pyridine-pyridone ligands (L10-L12) leading to nearly quantitative yield (99%). Replacing the pyridine with quinuclidine as the L donor, quinuclidine-pyridone ligands (L13 and L14) were prepared to test β,γ-dehydrogenation, with L13 giving 92% yield of the desaturated product. Further modification with the quinuclidine-pyridone scaffold by installing bulky substituents, such as benzyl (L15) and isopropyl (L16) did not improve yields further (84% and 76%, respectively). It is noteworthy that only the desired product and unreacted starting material were observed by 1H NMR in the crude reaction mixture, without any visible formation of byproducts from vinyl or methyl C─H functionalization. Enone and carbonyl moieties are tolerated under the optimal conditions. Notably, the use of a mixture of HFIP: CH3CN (10:1) is crucial for the β,γ-dehydrogenation. Specifically, in the absence of CH3CN, no desired product was observed (Table S3).
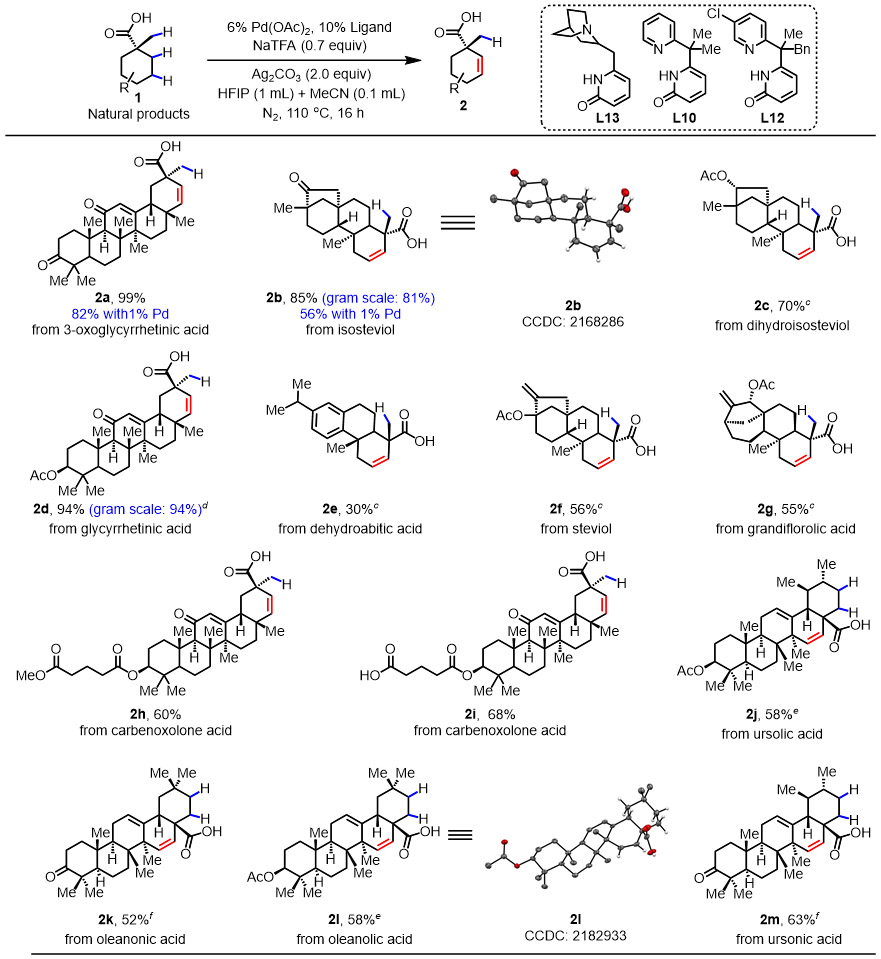
Then, the substrate scope of the late-stage desaturation of terpene natural products was investigated. As is showcased in Table 2, a series of biologically and synthetically significant terpenes participated in the β, γ-dehydrogenation and provided the corresponding products in up to 99% yield with good compatibility for other functional groups. Pharmacologically important isosteviol26 reacted to form the desaturation product 2b in 85% yield, the structure of which was confirmed with x-ray crystallography. Glycyrrhetic acid was also compatible with our protocol and afforded the corresponding product 2d with excellent yield (94%). Terminal alkene-containing steviol and grandiflorolic acid underwent smooth β,γ-desaturation to afford the target molecules with 56% and 55% yield, respectively (2f and 2g). Carbenoxolone acid, which contains two carboxylic acids, regioselectively dehydrogenated on the cyclohexyl ring, leading to similar yield to the one with carboxylic acid protected (2h-2i). Excellent regioselectivity was observed with ursolic acid and oleanolic acid, both of which could potentially undergo β,γ-dehydrogenation on two cyclohexane rings. Surprisingly, only one ring underwent desaturation, which might be due to steric shielding of the other ring (2j-2m). Notably, gram-scale reactions were carried out with isosteviol and glycyrrhenic acid, delivering the products (2b, 2d) with almost no diminishment of the yield (81% and 94%). With the 3-oxoglycyrrhetic acid, an 82% yield was obtained even when the Pd loading was decreased to 1% (2a). The ability of the reaction to tolerate increased scale and decreased catalyst loading indicates that it may be viable for large scale applications.
Table 2.
|
Conditions: 3 (0.1 mmol), Pd(OAc)2 (6 mol%), L10 (10 mol%), NaTFA (0.7 equiv), Ag2CO3 (2.0 equiv), HFIP/MeCN (1 mL/ 0.1 mL), N2, 110 °C, 16 h.
Isolated yields.
L13 was used instead of L10, 100 °C.
Pd(OAc)2 (10 mol%), L12 (15 mol%), NaTFA (1 equiv), Ag2O (2.0 equiv), HFIP/MeCN (1 mL/ 0.1 mL), 100 °C, 24 h.
100 °C. fL12 was used instead of L10, 100 °C.
Next, a wide range of cyclic and acyclic free carboxylic acids were examined under the optimized conditions (Table 3). Cyclopentane carboxylic acids with a variety of α-substituents (4a-4j), such as methyl, benzyl, chlorobutyl, and aryl, all afforded β,γ-dehydrogenation products in 42% to 66% yields with excellent mass balance. Notably, dehydrogenation was exclusively observed within the cyclopentane ring despite the presence of additional β,γ-activation sites in 4b-4g. The regioselectivity between the acyclic chain or cyclic ring sensitively depends on the ligand structure. With these bidentate pyridone ligands, the entire reactive organometallic intermediate and transition state has a spiro structure with palladium in the center, hence, the conformation of the cyclopentyl is drastically different from the acyclic chain. In addition to cyclopentane carboxylic acids, substrates containing six (4k-4n, 4r-4s), seven (4t), and eight (4w) membered rings also afforded the desired cis-β,γ-dehydrogenation products as single stereoisomers with moderate to good yields. Interestingly, cyclic aliphatic acids containing eleven (4x) and twelve (4y) membered rings produced a mixture of isomers favoring trans-olefins. β,γ-dehydrogenation of acyclic carboxylic acids was also realized, as demonstrated by the examples of 4o-4q. Strikingly, in contrast to our previous α,β-dehydrogenation reaction of α-hydrogen containing aliphatic acids (4u-4w),3 this catalytic protocol switched regioselectivity to β,γ-dehydrogenation (Scheme S2). It is noteworthy that remaining unreacted carboxylic acid substrates were observed from crude 1H NMR. No α,β-dehydrogenation product or any other byproduct was observed during the reaction. Notably, deuteration studies with cyclohexanoic acid 3v using ligand L13 showed that palladation is reversible and highly preferred at the β-position, occurring primarily syn relative to the directing group (Scheme S1). The subsequent syn-stereospecific β-hydride elimination occurred at the γ-position only, affording the desired product 4v in exclusive site selectivity. The observed influence of ligand on site selectivity site selectivity suggests potential ligand involvement in facilitating subsequent dehydrogenation step. Further computational studies on the origins of the observed site selectivity are underway in our group.
Table 3.
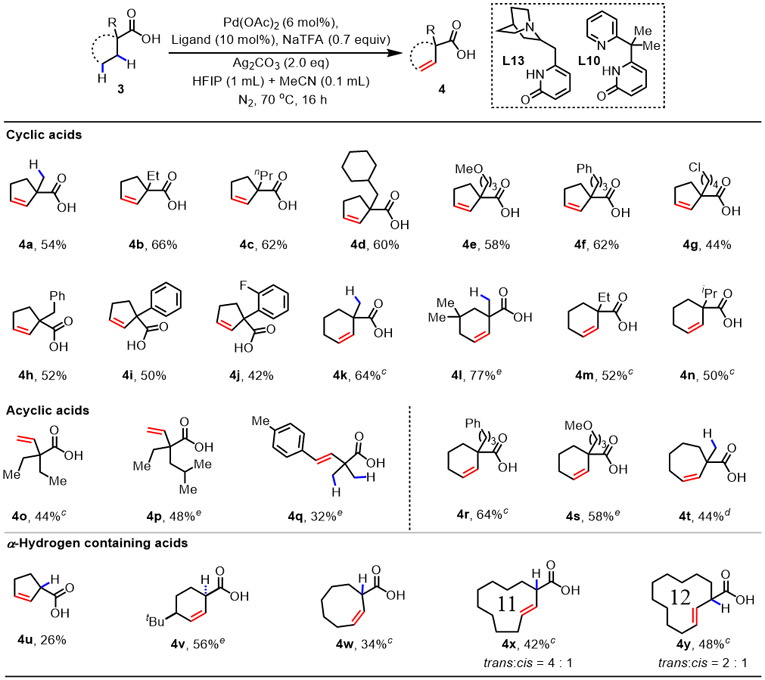
|
Conditions: 1 (0.1 mmol), Pd(OAc)2 (6 mol%), L10 (10 mol%), NaTFA (0.7 equiv), Ag2CO3 (2.0 equiv), HFIP/MeCN (1.0 mL/ 0.1 mL), N2, 70 °C, 16 h.
Isolated yields.
L13 (10 mol%) was used instead of L10, 100 °C.
L11 (10 mol%) was used instead of L10, 100 °C.
L13 (10 mol%) was used instead of L10, 80 °C.
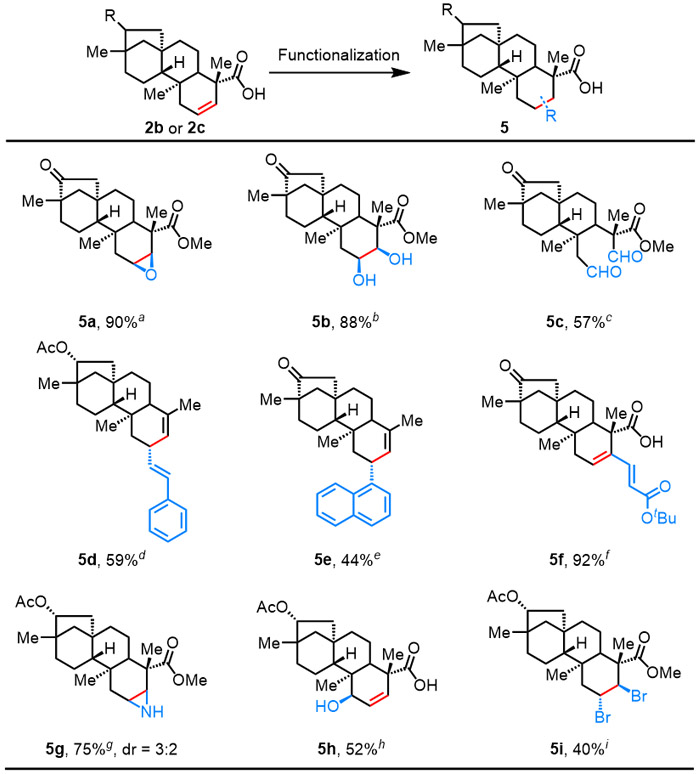
To illustrate the synthetic applications of this protocol, the β,γ-dehydrogenation products of isosteviol 2b and dihydroisosteviol 2c were used for subsequent derivatizations. Isosteviol and its derivatives represent pharmacologically significant drug candidates with potential anti-inflammatory, antibacterial, antiviral, and anticancer properties.26 However, previous chemical modifications of the isosteviol molecule are limited to the pre-existing reactive functional groups such as the ketone and carboxylic acid. Therefore, the introduction of a new versatile functionality by inert C─H bond functionalization would enable the synthesis of novel classes of isosteviol derivatives. As is demonstrated in Table 4, several structurally diverse products were generated from the β,γ-desaturated isosteviol, constructing new C─C and C─heteroatom bonds at remote positions. After treatment with m-chloroperoxybenzoic acid (m-CPBA) the versatile epoxide 5a was obtained in 90% yield. Traditional transformations such as dihydroxylation and ozonolysis were also carried out to provide the target molecules 5b and 5c in 88% and 57% yield, respectively. More recently developed transformations were also applied to this β,γ-desaturated substrate, such as the highly stereospecific Pd-catalyzed decarboxylative olefination reaction,27 which installed an additional olefin group at the γ-position of the original carboxylic acid (5d). Decarboxylative arylation was also carried out with 1-iodonaphthalene as the coupling partner (5e). A derivatization via the palladium-catalyzed C(alkenyl) ─H olefination directed by free carboxylic acid was also performed to deliver 5f in 92% yield.28 Prior studies have found that the installation of N-containing functionality into isosteviol derivatives increases their antitubercular activity.26 Therefore, we performed the aziridination of 2c, which provided the corresponding aziridine 5g in 75% yield with a diastereomeric ratio of 3:2. Allylic hydroxylation of the newly formed olefin provided the synthetically versatile allylic alcohol 5h in 52% yield, achieving β, γ, and δ functionalization over the two step sequence. Dibromination of the alkene was also demonstrated, leading to the trans-dibromo product in a synthetically useful yield (5i). The diversified derivatization of the newly introduced functionality might provide more opportunities for further exploration of the biological activities of carboxylic acid containing terpene natural products in medicinal chemistry.
Table 4.
|
Conditions: am-CPBA, CHCl3, 0 °C 1h, rt 24h. bOsO4, K3[Fe(CN)6], K2CO3, DABCO, tBuOH/H2O (1:1), 40 °C, overnight. c(1) O3, MeOH, 4h, −78 °C, (2) MeS, rt, 8h. dVinyl bromide, Pd(OAc)2, Cs2CO3, toluene, argon, 110 °C, 3h. e1-Iodonaphthalene, Cs2CO3, Pd(dba)2, toluene, argon, 110 °C, 26h. fAgOAc, Cu(OAc)2·H2O, Pd(OAc)2, dry DMSO/dioxane (1:20), olefin, 80 °C, 24h. gHOSA, pyridine, Rh2(esp)2, HFIP, rt, 3h. hSeO2, dioxane, 70 °C, 24 h. iNBS, DMSO, DCM, rt, 10 min. jIsolated yields. kThe dr value was determined by 1H NMR analysis of the crude product.
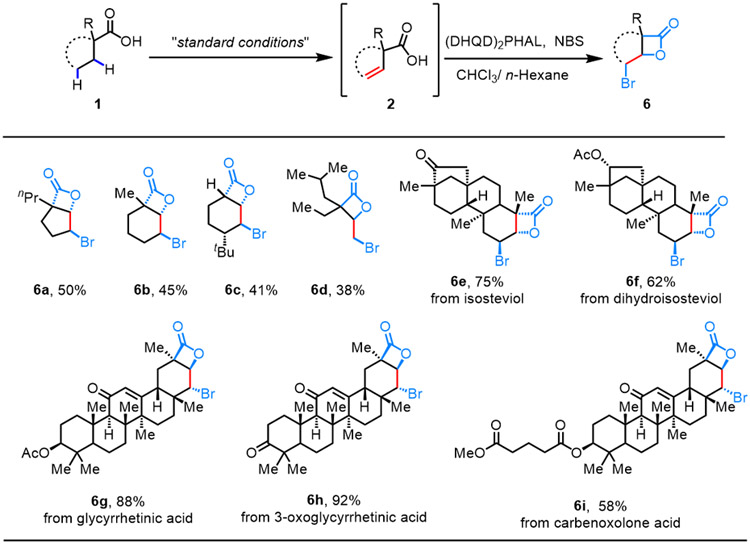
β-Lactone-containing natural products and derivatives have attracted significant attention in cell biology due to their ability to covalently modify proteins.29 Installation of β-lactones onto natural products presents new opportunities for activity-based protein profiling, which can facilitate the identification of previously unknown cellular targets of these natural products.30 Therefore, we developed a telescoped synthesis of a series of β-lactones through β,γ-dehydrogenation of free carboxylic acids, followed by bromo-β-lactonization31 (Table 5). As is demonstrated, the telescoped process went well with the simple five- and six-membered carboxylic acids to afford the corresponding β-lactones with 41%-50% overall yield (6a-6c). A branched chain carboxylic acid was also compatible with this telescoped process (6d). Notably, the sequential desaturation/bromolactonization also proved effective with a series of structurally and biologically diversified terpenes, enabling the late-stage incorporation of a β-lactone motif into these natural products with good to excellent overall yields (58%-92%) (6e-6i).
Table 5.
|
Isolated yields.
Conditions: (DHQD)2PHAL, CHCl3/Hexane, NBS, 0 °C- rt, overnight.
3. Conclusion
In summary, we have developed an unprecedented example of ligand-enabled β,γ-dehydrogenation of abundant free aliphatic acids, which provides a versatile platform for downstream functionalization at remote γ-sites. A variety of free aliphatic acids, including acyclic and cyclic systems with ring sizes from five-membered to macrocyclic are compatible. The utility of this protocol has been demonstrated through the late-stage installation of important structural motifs such as epoxides and β-lactone onto complex natural product scaffolds.
4. Experimental Section
The general procedure for the β,γ-dehydrogenation and vinyl C─H olefination reaction is as follows: In a sealed tube equipped with a magnetic stir bar was charged with Pd(OAc)2 (1.3 mg, 6 mol%), Ligand (10 mol%), the appropriate carboxylic acid substrate (0.1 mmol), Ag2CO3 (55.0 mg, 0.2 mmol), NaTFA (10 mg, 0.075 mmol). HFIP (1.0 mL) and MeCN (0.1 mL) were then added before the tube was briefly flushed with nitrogen. Subsequently, the vial was capped and closed tightly. The reaction mixture was then stirred at the rate of 300 rpm at 80 °C-110 °C for 16-24 h. After being allowed to cool to room temperature, the mixture was diluted with ethyl acetate and acidified with 0.2 mL of formic acid. The mixture was passed through a pad of celite with acetone as the eluent to remove any insoluble precipitate. The resulting solution was concentrated, and the residual mixture was purified using pTLC (hexane/ethyl acetate = 10/1 to 5/1).
Supplementary Material
Acknowledgments.
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, R01GM084019) for financial support. We thank D. A. Strassfeld for his help with the editing of the manuscript.
Footnotes
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website. Experimental details, full characterization of new compounds including 1H and 13C NMR spectra, HRMS data (PDF)
The authors declare no competing financial interest.
References
- 1.Gnaim S; Vantourout JC; Serpier F; Echeverria P-G; Baran PS Carbonyl desaturation: where does catalysis stand? ACS Catalysis 2021, 11, 883–892. [Google Scholar]
- 2.Parasram M; Chuentragool P; Wang Y; Shi Y; Gevorgyan V General, auxiliary-enabled photoinduced Pd-catalyzed remote desaturation of aliphatic alcohols. J. Am. Chem. Soc 2017, 139, 14857–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M-M; Ning X-S; Qu J-P; Kang Y-B Dehydrogenative synthesis of linear α, β-unsaturated aldehydes with oxygen at room temperature enabled by tBuONO. ACS Catalysis 2017, 7, 4000–4003. [Google Scholar]
- 4.Reich HJ; Reich IL; Renga JM Organoselenium chemistry alpha-Phenylseleno carbonyl compounds as precursors for alpha, beta-unsaturated ketones and esters. J. Am. Chem. Soc 1973, 95, 5813–5815. [Google Scholar]
- 5.Nicolaou K; Zhong Y-L; Baran P A new method for the one-step synthesis of α, β-unsaturated carbonyl systems from saturated alcohols and carbonyl compounds. J. Am. Chem. Soc 2000, 122, 7596–7597. [Google Scholar]
- 6.Gnaim S; Takahira Y; Wilke HR; Yao Z; Li J; Delbrayelle D; Echeverria P-G; Vantourout JC; Baran PS Electrochemically driven desaturation of carbonyl compounds. Nat. Chem 2021, 13, 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharpless K; Lauer R; Teranishi A Electrophilic and nucleophilic organoselenium reagents. New routes to alpha, beta-unsaturated carbonyl compounds. J. Am. Chem. Soc 1973, 95, 6137–6139. [Google Scholar]
- 8.Wang Z; He Z; Zhang L; Huang Y Iridium-catalyzed aerobic α, β-dehydrogenation of γ, δ-unsaturated amides and acids: activation of both α-and β-C─H bonds through an allyl–iridium intermediate. J. Am. Chem. Soc 2018, 140, 735–740. [DOI] [PubMed] [Google Scholar]
- 9.Chen M; Dong G Direct catalytic desaturation of lactams enabled by soft enolization. J. Am. Chem. Soc 2017, 139, 7757–7760. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z; Hu L; Chekshin N; Zhuang Z; Qian S; Qiao JX; Yu J-Q Ligand-controlled divergent dehydrogenative reactions of carboxylic acids via C─H activation. Science 2021, 374, 1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y; Chen Y; Newhouse TR Allyl-palladium-catalyzed α, β-dehydrogenation of carboxylic acids via enediolates. Angew. Chem., Int. Ed 2017, 129, 13302–13305. [DOI] [PubMed] [Google Scholar]
- 12.Giri R; Maugel N; Foxman BM; Yu J-Q Dehydrogenation of inert alkyl groups via remote C─H activation: converting a propyl group into a π-allylic complex. Organometallics 2008, 27, 1667–1670. [Google Scholar]
- 13.Sheng T; Zhuang Z; Wang Z; Hu L; Herron AN; Qiao JX; Yu J-Q One-step synthesis of β-alkylidene-γ-lactones via ligand-enabled β, γ-dehydrogenation of aliphatic acids. J. Am. Chem. Soc 2022, 144, 12924–12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang G; Strassfeld DA; Sheng T; Chen C-Y; Yu J-Q Transannular C─H functionalization of cycloalkane carboxylic acids. Nature 2023, 618, 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paduch R; Kandefer-Szerszeń M; Trytek M; Fiedurek J Terpenes: substances useful in human healthcare. Arch. Immunol. Ther. Exp 2007, 55, 315–327. [DOI] [PubMed] [Google Scholar]
- 16.Cipak L; Grausova L; Miadokova E; Novotny L; Rauko P Dual activity of triterpenoids: apoptotic versus antidifferentiation effects. Arch. Toxicol 2006, 80, 429–435. [DOI] [PubMed] [Google Scholar]
- 17.Liu J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol 1995, 49, 57–68. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y; Chen X; Yuan C; Li G; Zhang J; Zhao Y Pd-catalysed ligand-enabled carboxylate-directed highly regioselective arylation of aliphatic acids. Nat. Commun 2017, 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh KK; van Gemmeren M Pd-catalyzed β-C(sp3)─H arylation of propionic acid and related aliphatic acids. Chem. Eur. J 2017, 23, 17697–17700. [DOI] [PubMed] [Google Scholar]
- 20.Shen P-X; Hu L; Shao Q; Hong K; Yu J-Q Pd (II)-catalyzed enantioselective C(sp3)─H arylation of free carboxylic acids. J. Am. Chem. Soc 2018, 140, 6545–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhuang Z; Yu C-B; Chen G; Wu Q-F; Hsiao Y; Joe CL; Qiao JX; Poss MA; Yu J-Q Ligand-enabled β-C(sp3)─H olefination of free carboxylic acids. J. Am. Chem. Soc 2018, 140, 10363–10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu L; Shen PX; Shao Q; Hong K; Qiao JX; Yu J-Q PdII-catalyzed enantioselective C(sp3)─H activation/cross-coupling reactions of free carboxylic acids. Angew. Chem., Int. Ed 2019, 58, 2134–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang Z; Yu J-Q Lactonization as a general route to β-C(sp3)─H functionalization. Nature 2020, 577, 656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang Z; Herron AN; Yu J-Q Synthesis of cyclic anhydrides via ligand-enabled C─H carbonylation of simple aliphatic acids. Angew. Chem., Int. Ed 2021, 133, 16518–16523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan HSS; Yang J-M; Yu J-Q Catalyst-controlled site-selective methylene C─H lactonization of dicarboxylic acids. Science 2022, eabq3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullah A; Munir S; Mabkhot Y; Badshah SL Bioactivity profile of the diterpene isosteviol and its derivatives. Molecules 2019, 24, 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou CM; Chatterjee I; Studer A Stereospecific palladium-catalyzed decarboxylative C(sp3)─C(sp2) coupling of 2, 5-cyclohexadiene-1-carboxylic acid derivatives with aryl iodides. Angew. Chem., Int. Ed 2011, 50, 8614–8617. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y-C; Huang Y-H; Tsai H-C; Basha RS; Chou C-M Palladium-catalyzed proaromatic C(Alkenyl)─H olefination: synthesis of densely functionalized 1, 3-dienes. Org. Lett 2020, 22, 6765–6770. [DOI] [PubMed] [Google Scholar]
- 29.Böttcher T; Sieber SA β-Lactams and β-lactones as activity-based probes in chemical biology. MedChemComm 2012, 3, 408–417. [Google Scholar]
- 30.Jouanneau M; Vellalath S; Kang G; Romo D Natural product derivatization with β-lactones, β-lactams and epoxides toward ‘infinite’ binders. Tetrahedron 2019, 75, 3348–3354. [Google Scholar]
- 31.Ikeuchi K; Ido S; Yoshimura S; Asakawa T; Inai M; Hamashima Y; Kan T Catalytic desymmetrization of cyclohexadienes by asymmetric bromolactonization. Org. Lett 2012, 14, 6016–6019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.