Summary
Background
BTK inhibitors have been concurrently administered with anti-CD20 monoclonal antibodies (mAbs) in chronic lymphocytic leukemia (CLL). However, the optimal regimen for combining these two drugs remains pending.
Methods
This multi-center phase 2 study aimed to analyze whether consolidation with ofatumumab improved the response in patients with CLL receiving front-line treatment with ibrutinib. Patients received 12 cycles of ibrutinib monotherapy. Those who achieved CR after this induction were maintained on ibrutinib. Conversely, those who did not attain CR continued with ibrutinib in addition to a consolidation, which involved 7 doses of ofatumumab. The primary objective was the complete response (CR) rate at cycle 20. This study is registered within the EU Clinical Trials Register (EudraCT 2016-004937-26).
Findings
Between September 8, 2017, and May 21, 2018, 84 patients (median age, 69 years) were included. After completion of 12 cycles of ibrutinib (n = 80), 4 patients (5%) were in CR, 67 (84%) in partial response (PR), and 6 patients (7%) had a PR with lymphocytosis (PRL). After consolidation with ofatumumab, 20 patients improved the response from PR to CR and 6 patients with PRL obtained a PR. Seventy-one patients (85%) completed 20 cycles of treatment, with a CR rate of 24/71 (34%). According to the intention-to-treat analysis at cycle 20, the ORR was 69/84 (82.2%), with a CRR of 24/84 (28.6%). Progression-free survival and overall survival at 48-months were 89.9% (CI: 82.4–95.5) and 92.2% (CI: 85.3–97.1), respectively.
Interpretation
These findings underscore the potential for a consolidation strategy in CLL, wherein the addition of a mAb in patients with low tumor burden might enhance the quality of the response.
Funding
The study was funded by Janssen that also supplied ibrutinib, whereas ofatumumab was supplied by Novartis.
Keywords: Chronic lymphocytic leukemia, Treatment, Consolidation
Research in context.
Evidence before this study
Several studies have analysed the combination of inhibitors of BTK (BTKi) with anti-CD20 monoclonal antibodies (mAbs) in patients with chronic lymphocytic leukemia (CLL), both in the frontline and relapsed or refractory settings. We searched PubMed for articles published up to March 2024 containing clinical efficacy and safety data for combination therapies with BTKi and anti-CD20 mAbs including the following keyword for the search: ibrutinib, acalabrutinib, zanubrutinib, rituximab, obinutuzumab, ofatumumab, CLL.
We identified six studies evaluating ibrutinib in combination with rituximab, three with obinutuzumab, one with ofatumumab, two studies examining acalabrutinib with obinutuzumab, and one study analyzing the combination of zanubrutinib and obinutuzumab.
These prior studies have demonstrated tolerability and encouraging results of BTKi in combination with anti-CD20 mAbs, particularly with second-generation anti-CD20 mAbs obinutuzumab, but few of these studies evaluated different combination schedules of these agents.
Added value of this study
To our knowledge, this is the first clinical study to evaluate the efficacy and safety of a consolidation strategy with an anti-CD20 mAb, ofatumumab, after 12 months of ibrutinib monotherapy in treatment naïve CLL. Our study demonstrated that consolidation with anti-CD20 monoclonal antibody therapy following 12 cycles of treatment with ibrutinib was well-tolerated and achieved a deeper response.
Implications of all the available evidence
Different combination therapies are being evaluated for the treatment of patients with CLL with the aim of achieving deeper responses, and potentially developing limited-duration combination strategies. Optimal combinations and the most appropriate schedules for these strategies are still to be determined.
Introduction
Treatment with inhibitors of the Bruton's tyrosine kinase (BTKi) yields high response rates and sustains long-term control of the disease in patients with chronic lymphocytic leukemia (CLL), even in the presence of poor risk genetic features. Consequently, BTKi have emerged as a cornerstone of the treatment of CLL.1, 2, 3, 4, 5, 6, 7 However, complete responses, especially those with undetectable minimal residual disease (uMRD), are rarely observed with BTKi treatment, and these treatments require continuous administration. To enhance response rates, especially the quality of responses, BTKi have been combined with other effective treatments in CLL, such as the BCL2 inhibitor venetoclax8, 9, 10, 11 and anti-CD20 monoclonal antibodies (mAbs).1, 2, 3, 4,7,12, 13, 14, 15, 16, 17, 18 Regarding the latter combination strategy, initial studies combining the BTKi ibrutinib with rituximab showed limited effect on response rates compared to ibrutinib in monotherapy, with no clear impact of this combination on patient outcomes.2,12,13 Beyond these studies, BTKi have been combined with second-generation anti-CD20 mAbs, such as ofatumumab and obinutuzumab. Ofatumumab, a human anti-CD20 mAb with more potent complement-dependent cytotoxicity (CDC) than rituximab, showed tolerability and clinical activity when combined with ibrutinib in patients with CLL.14,19 Furthermore, the combination of BTKi with obinutuzumab obtained higher response rates, including increased rates of uMRD, potentially offering a benefit in terms of progression-free survival (PFS) compared to BTKi monotherapy.3,4 Nonetheless, despite these encouraging results, the optimal treatment regimen for combining BTKi and anti-CD20 mAbs to achieve maximum benefits from this combination has yet to be determined. In this regard, preliminary results suggested a greater impact on MRD response rate and the depth of response when the anti-CD20 mAb was administered after more than one year of prior ibrutinib exposure, particularly when the tumor bulk was low.20
In this study, we aimed to investigate whether consolidation with an anti-CD20 mAb added after 12 months of exposure to BTKi improves the quality of the response in patients with CLL. Thus, we hereby present a multi-center, non-randomized phase 2 study designed to assess the effectiveness and safety of combining ofatumumab with ibrutinib in patients who have not attained a complete response following 12 months of ibrutinib monotherapy as a frontline treatment.
Methods
Study design and patients
This is a multi-center, non-randomized, open-label, double agent, phase 2 study of the Spanish Group of CLL (GELLC) designed to evaluate the efficacy and safety of the combination of ibrutinib and ofatumumab in patients not attaining a complete response following 12 months of treatment with ibrutinib monotherapy as front-line therapy. The study included an initial induction phase consisting of 12 cycles (28-day) of ibrutinib in monotherapy, and a consolidation phase adding six cycles of ofatumumab in patients not obtaining a complete response (CR) after the induction. Patients considered in CR after the induction phase were maintained on ibrutinib until progression.
Patients aged ≥18 years with previously untreated CLL or SLL requiring treatment per iwCLL criteria21 were eligible for the study. Additional inclusion criteria were cumulative illness rating scale (CIRS) score <6, Eastern Cooperative Oncology Group performance status (ECOG) status 0–1, and adequate hepatic, renal (estimated glomerular filtration rate [Cockroft-Gault] > 40 mL/min), and hematologic function. Patients with active autoimmune hemolytic anemia, autoimmune thrombocytopenia, or those requiring or receiving anticoagulation with warfarin or equivalent vitamin K antagonists were excluded.
The study was conducted in accordance with International Conference on Harmonization guidelines for Good Clinical Practice and principles of the Declaration of Helsinki. The Protocol was approved by institutional review boards or independent ethics committees of all participating institutions. All patients provided written informed consent. This study is registered within the EU Clinical Trials Register (EudraCT 2016-004937-26).
Treatment and procedures
Patients received an induction phase with single-agent oral ibrutinib (420 mg once daily) continuously for 12 cycles (28-days). Following 12 cycles of ibrutinib, patients who did not attain a complete response (CR) were treated with the combination of ibrutinib and ofatumumab. Ofatumumab was administered by IV infusion, 300 mg on Day 1 and 1000 mg on Day 8 of cycle 13, followed by 5 monthly infusions of 1000 mg (Day 1 of subsequent 28-day cycles for cycles C14, C15, C16, C17, and C18). Conversely, patients who achieved CR after 12 cycles of ibrutinib continued with ibrutinib in monotherapy.
Outcomes and assessments
The primary endpoint of the study was the CR rate evaluated after 20 cycles of treatment (2 months after completing ofatumumab consolidation) in the intention to treat population (ITT). A per-protocol analysis for response was also performed. The response was assessed after 12 cycles of ibrutinib, determining whether patients continued with ibrutinib in monotherapy (for patients in CR) or received consolidation with ofatumumab (for patients not in CR).
Secondary endpoints included overall response rate (ORR), evaluation of minimal residual disease (MRD), safety, duration of response, progression-free survival (PFS), and overall survival (OS).
The ORR was defined as the proportion of patients who achieved a CR, CR with incomplete hematologic recovery (CRi), nodular partial response (nPR), or PR during the study. Patients who attained a PR with lymphocytosis were included in the ORR calculation. Additionally, the best overall response was also determined, which was defined as the best response documented from the start of treatment until progressive disease or recurrence. The iwCLL guidelines (Hallek, 2008)22 were used to assess response in CLL patients, with the modification that isolated treatment-related lymphocytosis was not considered as disease progression.23
The assessments encompassed clinical response using physical examination, laboratory evaluations, computed tomography (CT) scans, and bone marrow biopsy to confirm CR, as well as MRD status by flow cytometry in peripheral blood and bone marrow. Safety evaluations were conducted at each visit.
Statistical analysis
The sample size was calculated based on the primary end point (CR rate, CRR). We assumed a CRR of 13% (CR rate reported for ibrutinib as first line therapy in CLL) as P0 (null hypothesis), and a CRR of 25% (representing a 12% improvement) as P1 (alternative hypothesis). Applying Simon 2-stage design approach,24 to obtain 80% power with type I error probability of α = 0.05, a number of 76 patients was required. Considering an anticipated 10% dropout rate, enrollment of 84 patients was deemed necessary. The study was planned to terminate prematurely if fewer than 4 complete responses were observed among the first 28 patients evaluated.
Data from all subjects who received at least one dose of the study drugs were included in the efficacy and safety analyses.
Distribution of PFS and OS were summarized using the Kaplan Meier estimate of median and its corresponding 95% confidence interval (CI). PFS and OS curves were compared by the log-rank test stratified for IGHV mutational status (mutated vs. unmutated IGHV). Patients who withdrew from the study or were considered lost to follow-up without prior documentation of disease progression were censored on the date of the last adequate disease assessment.
Safety evaluations were summarized descriptively. Adverse events (AEs) were classified using the NCI CTCAE (v 4.0). Subsets of AEs were summarized including serious AEs (SAEs), events of all CTCAE grade severities, events classified as NCI CTCAE grade 3 or higher, and events that resulted in withdrawal of study medication.
All statistical analyses were performed using the SPSS 25.0 software program for Windows (SPSS Inc., Chicago, IL).
Role of funding
The study was funded by Janssen that also supplied ibrutinib, whereas ofatumumab was supplied by Novartis.
Results
Patient characteristics
Between September 8, 2017, and May 21, 2018, 91 patients were enrolled in the study. Seven patients did not meet the selection criteria and were considered screening-failures. These patients did not receive any dose of the study treatment. Eighty-four patients were finally included in the analysis of effectiveness and safety of the study. Median age of the patients was 69 years (range, 38–84 years), and 58 (69%) patients were aged 65 years or older. Median of the CIRS score was 2 (range, 0 to 5), whereas 35 (42%) patients had a creatinine clearance rate <70 mL/min. Most patients (82%) had Binet stage B or C, and 61% had an unmutated IGHV status. In addition, 13/84 (15%) of the patients had high-risk biologic features including 17p deletion (6%), mutation of TP53 (7%), or complex karyotype (8%). The main clinical characteristics of the patients are shown in Table 1.
Table 1.
Characteristics of the patients, n = 84.
| Median age (range), years | 69 (38–84) |
|---|---|
| Gender, male, | 60/84 (71%) |
| ECOG, n (%) | |
| 0 | 54 (64.3)28 (33.3) |
| 1 | 2 (2.4) |
| 2 | |
| Binet stage, n (%) | |
| A | 15 (18.3) |
| B | 40 (48.8) |
| C | 27 (32.9) |
| Rai stage, n (%) | |
| 0 | 3 (3.7) |
| I | 14 (17.1) |
| II | 29 (35.4) |
| III | 14 (17.1) |
| IV | 22 (26.8) |
| CIRS score, median, range | 2 (0–5) |
| IGHV mutational status, n (%) | |
| Mutated | 28/72 (39) |
| Unmutated | 44/72 (61) |
| Genetic features of CLL,a n (%) | |
| 17 deletion | 5/84 (6) |
| 11q deletion | 9/84 (10.7) |
| 13q deletion | 37/84 (44.1) |
| Trisomy 12 | 20/84 (23.8) |
| Complex karyotype,b n (%) | 7/84 (8.3) |
| TP53 mut, n (%) | 6/84 (7.1) |
CIRS, Cumulative illness rating scale; ECOG, Eastern Cooperative Oncology Group performance status.
Data available in 78 cases.
Data available in 60 cases.
Treatment disposition and efficacy analysis
Induction phase
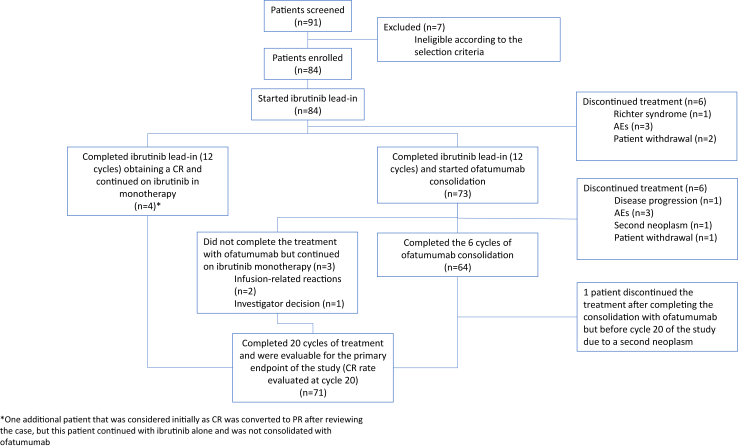
The 84 patients included in the study received initial treatment with ibrutinib monotherapy. Overall, 80 of the 84 patients (95%) completed the induction phase treatment. Following completion of 12 cycles of ibrutinib (n = 80), 4 patients (5%) were in CR, 67 patients (84%) in PR, 6 patients (7%) in PRL, and 3 patients (4%) in SD. Four patients who discontinued the treatment during the induction phase, and two additional patients who discontinued the treatment at cycle 12, were not included in the consolidation phase of the study (See Fig. 1). Causes leading to discontinuation in these patients included progression of the disease with transformation to Richter syndrome (n = 1), infections (n = 2), gastric toxicity (n = 1), and patient withdrawal (n = 2).
Fig. 1.
Patient flow and treatment disposition.
Consolidation phase
Seventy-three patients initiated the consolidation treatment with ofatumumab, whereas the 4 patients in CR continued ibrutinib monotherapy. Additionally, one patient initially classified as CR was subsequently reevaluated as having a PR. However, this patient continued with ibrutinib alone and was not consolidated with ofatumumab. Sixty-four patients (88%) completed the planned six cycles of ofatumumab, while 9 patients (12%) received fewer than 6 cycles of ofatumumab. Two patients who experienced grade 3 infusion-related reactions, and one patient due to investigator decision, did not complete the treatment with ofatumumab but continued with ibrutinib. Additionally, six patients discontinued treatment during the consolidation phase due to disease progression (n = 1), hematological toxicity (n = 1), infections (n = 1), second neoplasms (n = 1), seizures (n = 1), and patient withdrawal (n = 1). Furthermore, one additional patient discontinued treatment after completing the consolidation phase with ofatumumab but before cycle 20 of the study due to a second neoplasm (Fig. 1).
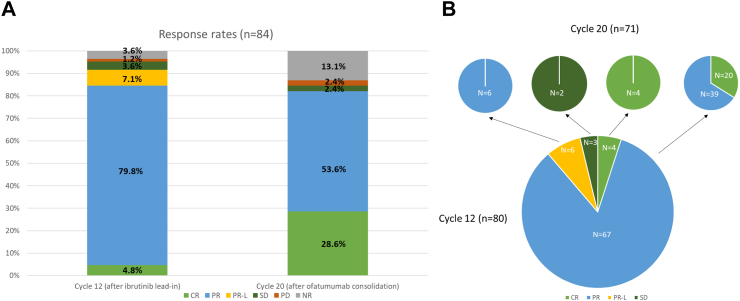
According to the ITT analysis at cycle 20, the ORR was 69/84 (82.2%), with a CRR of 24/84 (28.6%) and a PR rate of 45/84 (53.6%). Thirteen patients (15.5%) had discontinued the study, six patients (7.1%) during the induction phase with ibrutinib (≤ cycle 12), and seven patients (8.3%) during the consolidation phase with ibrutinib plus ofatumumab. At cycle 20, 71 patients (85%) out of the 84 patients remained in the study. Among these 71 patients, 24 (34%) were in CR (including two patients with CRi), 45 (63%) in PR, and 2 patients (3%) were considered in SD. Following consolidation with ofatumumab, 20 patients improved their response from PR to CR, 6 patients with PRL obtained PR, 39 patients maintained a PR, while 2 patients remained in SD. The four patients who were in CR after cycle 12 and continued with ibrutinib alone remained in CR (Fig. 2). No differences in the CR rate were observed according to IGHV mutational status (mutated IGHV vs. unmutated IGHV), or to 17p deletion or TP53 mutation.
Fig. 2.
Response rates (A) evaluated at cycle 12 (ibrutinib lead–in treatment) and at cycle 20 (2 cycles after consolidation treatment with ofatumumab); (B) Evolution of response from cycle 12 to cycle 20. CR, complete response; PR, partial response; PR-L, partial response with lymphocytosis; SD, stable disease; PD, progressive disease; NR, not reached.
When considering all patients included in the study, the best ORR achieved at any time during the study was 80/84 (95%), including a best CR rate of 36/84 (43%). MRD analysis was performed using flow cytometry (sensitivity ≥10−4) in patients considered to be in CR (n = 24), and it was undetectable in 1 patient.
Progression-free survival and overall survival
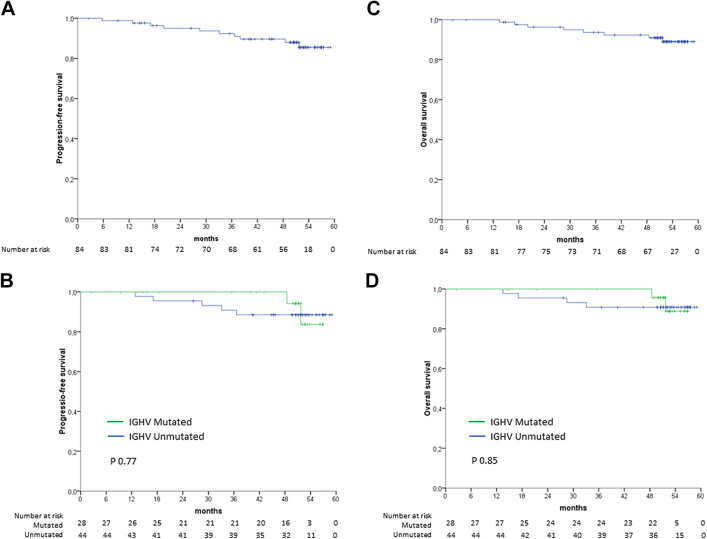
With a median follow-up of 52.4 months (2–59.2 months), the ITT analysis estimated the 48-months PFS and OS to be 89.9% (95% CI: 82.4–95.5) and 92.2% (CI: 85.3–97.1), respectively. According to the IGHV mutational status, patients with mutated IGHV had a 48-months PFS and OS of 94.1% (95% CI: 82.9–99.9) and 95.7% (95% CI: 87.3–99.9), respectively. In contrast, patients with unmutated IGHV had a 48-months PFS and OS of 88.5% (95% CI: 78.9–98.1) and 90.8% (95% CI: 82.2–99.4), respectively (Fig. 3).
Fig. 3.
Progression-free survival (PFS) (A), PFS by IGHV mutation status (B), overall survival (OS) (C), and OS by IGHV mutation status (D). P value was based on stratified log-rank test.
Safety
As of the data cut-off date on September 27, 2022, 24 out of the 84 (29%) patients had discontinued the study. Among them, six patients discontinued during the induction phase with ibrutinib monotherapy, another six patients discontinued during consolidation with ofatumumab, and the remaining 12 patients discontinued at a later point of the study. Ten patients, in addition to 1 death with unknown cause, (13%) discontinued the study due to AEs. The primary reasons for patient discontinuation included infections (5/24 [21%] patients), second neoplasm (5/24 [21%] patients), patient decision (3/24 [13%] patients), and disease progression (2/24 [8%] patients).
The most common AEs of any grade are summarized in Table 2, and included bleeding (50% [49% G1-2, 1% G ≥ 3]), diarrhea (43% [39% G1-2, 4% G ≥ 3]), upper respiratory tract infection (39% [38% G1-2, 1% G ≥ 3]), and infusion-related reactions (31% [26% G1-2, 5% G ≥ 3]). All patients reported at least one AE of any grade, with fifty-three (63%) patients experiencing a grade 3 or higher AE. Serious AEs were observed in 42 patients (50%). The most common grade ≥3 AEs were neutropenia (17%), anemia (8%), pneumonia (7%), infusion-related reactions (5%), diarrhea (4%), and hypertension (4%).
Table 2.
Summary of adverse events, n = 84.
| Most common adverse events | Number of patients, n (%) |
||
|---|---|---|---|
| Any grade | Grade 1–2 | Grade ≥3 | |
| Bleeding | 42 (50%) | 41 (49%) | 1 (1%) |
| Diarrhea | 36 (43%) | 33 (39%) | 3 (4%) |
| Upper respiratory tract infection | 33 (39%) | 32 (38%) | 1 (1%) |
| Infusion related reaction | 26 (31%) | 22 (26%) | 4 (5%) |
| Anemia | 25 (30%) | 18 (21%) | 7 (8%) |
| Rash | 24 (29%) | 23 (27%) | 1 (1%) |
| Hypertension | 21 (25%) | 18 (21%) | 3 (4%) |
| Pyrexia | 21 (25%) | 21 (25%) | – |
| Neutropenia | 19 (23%) | 5 (6%) | 14 (17%) |
| Arthralgia | 18 (21%) | 17 (20%) | 1 (1%) |
| Asthenia | 15 (18%) | 15 (18%) | – |
| Thrombocytopenia | 14 (17%) | 13 (16%) | 1 (1%) |
| Peripheral edema | 12 (14%) | 12 (14%) | – |
| Pruritus | 12 (14%) | 12 (14%) | – |
| Atrial fibrillation | 11 (13%) | 10 (12%) | 1 (1%) |
| Pneumonia | 10 (12%) | 4 (5%) | 6 (7%) |
| Dizziness | 10 (12%) | 9 (11%) | 1 (%) |
| Muscle spasms | 9 (11%) | 9 (11%) | – |
| Vomiting | 9 (11%) | 9 (11%) | – |
| Covid-19 | 8 (10%) | 4 (5%) | 4 (5%) |
| Cellulitis | 8 (10%) | 6 (7%) | 2 (2%) |
| Fatigue | 8 (10%) | 8 (10%) | – |
| Nausea | 8 (10%) | 8 (10%) | – |
Atrial fibrillation was reported in 13% (12% G1-2, 1% G ≥ 3) of the patients, and no events of ventricular arrhythmias were reported. Furthermore, no cases of tumor lysis syndrome were observed. Eight patients suffered from COVID-19 infections, 4 of them being grade ≥3 events, and 1 patient died from a COVID-19 pneumonia. Lastly, second neoplasms were observed in 9 patients (11%) including cutaneous basal cell carcinoma (n = 2), cutaneous squamous cell carcinoma (n = 3), sigmoid adenocarcinoma (n = 1), urothelial carcinoma (n = 1), prostate adenocarcinoma (n = 1), and a diffuse astrocytoma (n = 1).
Discussion
Although anti-CD20 monoclonal antibodies have been previously investigated in combination with BTKi, there is a scarcity of studies examining the most appropriate combination schedule. In this multi-center phase 2 study, consolidation with ofatumumab after 12 months of ibrutinib monotherapy significantly improved the response in treatment-naïve CLL patients over ibrutinib alone. Specifically, 20/71 (28%) patients transitioned from PR to CR after consolidation. Furthermore, the highest ORR achieved during the study was 95%, with a CR rate of 43%. These results exhibit favorable CR rates compared to those reported for ibrutinib monotherapy or its combination with rituximab. They are also similar to or even higher than the response rates observed with ibrutinib or with the second generation BTKi acalabrutinib when combined with the type II anti-CD20 monoclonal antibody obinutuzumab.1, 2, 3, 4, 5,13,25,26
Ibrutinib in monotherapy achieved a CRR of 4% as initial therapy for CLL in the first report of the RESONATE-2 study. However, with extended follow-up, the CRR increased, reaching 13% at 2-year and 34% at the last update of the study (8-year follow-up).5,25 Prior studies, including two randomized clinical trials, showed no clear benefit of adding rituximab to ibrutinib. For instance, in the Alliance A041202 study2,13 evaluating the combination of ibrutinib and rituximab in older patients with CLL (≥65 years), a CRR of 12% was observed. This was only slightly higher than the CRR in the same study for the ibrutinib monotherapy arm (CRR of 7%).2 In a younger population, the combination of rituximab and ibrutinib obtained a CRR of 17.2% in the E1912 study.1 Additionally, when obinutuzumab was combined with ibrutinib, it resulted in a CRR of 19% determined by an independent review committee (IRC) and 41% according to investigator assessment in the iLLUMINATE trial.3 Finally, the combination of obinutuzumab and acalabrutinib achieved a best IRC-assessed CRR of 13% in the primary analysis of the ELEVATE-TN study, which increased to 30.7% in a 4-year follow-up of the study.4,26 Nevertheless, despite the high CRR obtained in our study, MRD response was low (1%). This might be explained by the lower efficacy of ofatumumab in eradicating MRD compared to other mAbs, particularly obinutuzumab.
It is noteworthy that in most studies that combine BTKi and mAbs, the antibody has been incorporated from the beginning of the treatment regimen. Conversely, only a limited number of studies have explored alternative treatment schedules within the context of combination therapies. For instance, Jaglowski et al.14 evaluated the activity of the combination of ofatumumab and ibrutinib in a previous phase 1b/2 study in 3 different administration sequences in patients with relapsed CLL: ibrutinib lead–in (4 weeks prior to ofatumumab), concurrent start, or ofatumumab lead–in. All three administration sequences demonstrated activity, with ORR of 100%, 79%, and 71%, and estimated 12-month PFS of 89%, 85%, and 75%, respectively. However, the group that received a lead–in with ofatumumab showed the lowest response to therapy, reflecting the inferior efficacy of ofatumumab compared with ibrutinib. It is worth noting that even in the ibrutinib lead–in cohort, patients received ofatumumab only 4 weeks after commencing treatment with ibrutinib. More recently, preliminary reports by Rawstron et al. suggested a significant impact on the MRD response rate when the anti-CD20 monoclonal antibody obinutuzumab was introduced after a treatment period of 1 year with ibrutinib, when the tumour burden of the patients was low.20 Despite the relatively modest number of patients, it is notable that 10 patients receiving obinutuzumab more than 1 year after starting ibrutinib monotherapy (at median of 16.2 months [range 13–19]) obtained a higher response rate (CR/CRi 50% vs. 30%) and MRD response (<0.01% BM MRD in 50% vs. 6%) compared to 30 patients who initiated both ibrutinib and obinutuzumab concurrently. The high CRR observed in our study suggests, in accordance with the notion pointed out by Rawstron et al., that adding the monoclonal antibody in patients with low tumor burden might improve the quality of the response and optimize its combination with BTKi.
The responses obtained in our study were durable, with an estimated 48-month PFS and OS of 89.9% and 92.2%, respectively. However, the absence of a control arm in our study precludes us from determining the specific impact of achieving a deeper response by adding an anti-CD20 antibody to BTKi on the long-term outcomes of the patients. In this regard, phase 3 studies have obtained mixed results. For instance, the phase 3 Alliance A041202 study did not demonstrate advantage in terms of PFS of the combination of rituximab with ibrutinib compared to ibrutinib alone.2 Conversely, the ELEVATE-TN study showed a favorable 48-month PFS rate of the acalabrutinib combined with obinutuzumab vs. acalabrutinib monotherapy arm.4,26 Finally, the question of whether it is feasible to safely discontinue BTKi treatment in patients attaining profound responses through the use of BTKi and anti-CD20 monoclonal antibodies, particularly in instances with undetectable MRD, remains unresolved.
Limitations of our study include its single-arm design, without the presence of a control arm, and the low rate of MRD achieved associated with the CD20 mAb administered in our study.
Most patients included in the study were older (median age 69 years, with 69% of the patients being ≥65 years) but without significant comorbidities (median CIRS score 2), although 42% had a creatinine clearance <70 mL/min. The safety findings in our study were consistent with the previous reports with each agent, with no new safety signals identified. The most common grade ≥3 AEs were hematological toxicity and infections, while grade ≥3 infusion-related reactions were observed in 5% of patients, and there were no reported cases of tumor lysis syndrome. Discontinuations due to AEs were similar between the first 12 months of treatment with ibrutinib monotherapy and during the consolidation phase with ofatumumab. Overall, the discontinuation rate of the study, 29%, compares favorably to that reported for first-line single-agent ibrutinib (42% in the RESONATE-2 study after 5 years of follow-up)27 or ibrutinib combined with anti-CD20 monoclonal antibodies (39.2% in the E1912 study with 5.8 years of follow-up, and 42% in the ILLUMINATE study with 45 months of median follow-up).28,29 However, it should be noted that the absence of significant comorbidities within the patient population included in this study may have contributed, in part, to these comparatively lower discontinuation rates. Notably, eight patients contracted COVID-19 infections, four of them grade ≥3, and one patient died from COVID-19 pneumonia. Anti-CD20 monoclonal antibodies may impair humoral immunity against SARS-CoV-2,.30,31 Therefore, emphasizing a complete vaccination regimen before the initiation of B-cell-directed agents in CLL patients is important to ensure their protection.
In conclusion, this study has provided evidence that the implementation of consolidation with anti-CD20 monoclonal antibody therapy following 12 cycles of treatment with ibrutinib was well-tolerated and elicited a deeper response. These findings support the potential role of a consolidation therapeutic strategy in the management CLL, where adding a monoclonal antibody in patients with low tumor burden appears to improve the quality of the treatment response.
Contributors
P.A. and F.B. designed the clinical study, accessed and verified the data, and wrote the manuscript with input and approval of the final version from all co-authors. PA, EG-B, CF, ER-H, MFM, JD, RA, JAH-R, MJT, MG, PB, JDS, AR, CB, CM, JAG-M, RC, LY, and LFC enrolled patients in the study. MBV contribute to the flow cytometry data analysis. All authors supervised the work, reviewed the manuscript and approved the final version.
Data sharing statement
Clinical trial data can be requested by qualified researchers for use in scientific research. Sharing of data is subject to protection of patient privacy and respect for the patient's informed consent. The data will be provided following review and approval of a research proposal and execution of a Data Sharing Agreement.
Declaration of interests
P.A. Consulting/advisory: Roche, Genmab, Janssen, BMS, AbbVie, AstraZeneca, BeiGene; Honoraria: Roche, Genmab, Janssen, BMS, AbbVie, AstraZeneca, Gilead, Incyte. E.G-B. Consultancy: Janssen, Abbvie, Gilead, Kiowa, EUSAPharma, Incyte, Lilly, Beigene, Novartis; Speaker: Janssen, Abbvie, Takeda, Kiowa, Roche, EUSAPharma, Incyte, Astra-Zeneca; Travel: Janssen, Abbvie, EUSAPharma. C. F: Consulting/advisory: Janssen, AbbVie, AstraZeneca, Gilead; Honoraria: Roche, Janssen, AbbVie, AstraZeneca, Gilead, BeiGene. R.A. Consulting/advisory: Roche, Janssen, AbbVie, AstraZeneca, Takeda. JAH-R. Consultant: Janssen, Roche, Abbvie, Gilead, BMS/Celgene, Amgen, Takeda, Rovi, AstraZeneca, Beigene, Sandoz Novartis, Celltrion, EusaPharm, Sanofi, Lilly, Menarini Stemline; Speaker's Bureau: Janssen, Roche, Abbvie, Gilead, BMS/Celgene, Amgen, Takeda, AstraZeneca, Beigene, Lilly; Grant/Research Support: BMS/Celgene, Janssen, Sanofi. A.R. Honoraria: Janssen, BMS, Novartis, Roche, EUSA Pharma, Abbvie, Astra Zeneca, Sanofi, Takeda, Incyte, GSK Consulting/Advisory: Janssen, BMS, Pfizer, Roche, Abbvie, Gilead, Novartis, Takeda; Astra Zeneca, Beigene. L.F.C. Advisory, consultancy and research founding: Janssen, Roche, Abvvie, Beigene, Astra-Zeneca and Loxo. F.B. has received research funding and honoraria from Roche, Celgene, Takeda, AstraZeneca, Novartis, Abbvie and Janssen. The other authors have no conflicts of interest to declare.
Acknowledgements
The authors thank the patients for their participation.
The study was funded by Janssen that also supplied ibrutinib, whereas ofatumumab was supplied by Novartis. This work was also supported in part by the Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias (PI17/00943, PI18/01392, PI22/01204), Gilead Sciences (GLD15/00348, M.G) and the Health Council of the Junta de Castilla y León (GRS 2036/A/19, M.G.).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102642.
Appendix A. Supplementary data
References
- 1.Shanafelt T.D., Wang X.V., Kay N.E., et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381(5):432–443. doi: 10.1056/NEJMoa1817073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woyach J.A., Ruppert A.S., Heerema N.A., et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–2528. doi: 10.1056/NEJMoa1812836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreno C., Greil R., Demirkan F., et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56. doi: 10.1016/S1470-2045(18)30788-5. [DOI] [PubMed] [Google Scholar]
- 4.Sharman J.P., Egyed M., Jurczak W., et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291. doi: 10.1016/S0140-6736(20)30262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger J.A., Tedeschi A., Barr P.M., et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd J.C., Furman R.R., Coutre S.E., et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillmen P., Pitchford A., Bloor A., et al. Ibrutinib and rituximab versus fludarabine, cyclophosphamide, and rituximab for patients with previously untreated chronic lymphocytic leukaemia (FLAIR): interim analysis of a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(5):535–552. doi: 10.1016/S1470-2045(23)00144-4. [DOI] [PubMed] [Google Scholar]
- 8.Wierda W.G., Allan J.N., Siddiqi T., et al. Ibrutinib plus venetoclax for first-line treatment of chronic lymphocytic leukemia: primary analysis results from the minimal residual disease cohort of the randomized phase II CAPTIVATE study. J Clin Oncol. 2021;39(34):3853–3865. doi: 10.1200/JCO.21.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam C.S., Allan J.N., Siddiqi T., et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: primary analysis of the CAPTIVATE FD cohort. Blood. 2022;139(22):3278–3289. doi: 10.1182/blood.2021014488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munir T., Moreno C., Owen C., et al. Impact of minimal residual disease on progression-free survival outcomes after fixed-duration ibrutinib-venetoclax versus chlorambucil-obinutuzumab in the GLOW study. J Clin Oncol. 2023;41(21):3689–3699. doi: 10.1200/JCO.22.02283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillmen P., Rawstron A.C., Brock K., et al. Ibrutinib plus venetoclax in relapsed/refractory chronic lymphocytic leukemia: the CLARITY study. J Clin Oncol. 2019;37(30):2722–2729. doi: 10.1200/JCO.19.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger J.A., Keating M.J., Wierda W.G., et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger J.A., Sivina M., Jain N., et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood. 2019;133(10):1011–1019. doi: 10.1182/blood-2018-10-879429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaglowski S.M., Jones J.A., Nagar V., et al. Safety and activity of BTK inhibitor ibrutinib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood. 2015;126(7):842–850. doi: 10.1182/blood-2014-12-617522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan C.E., Brander D.M., Barr P.M., et al. A phase 1b study of ibrutinib in combination with obinutuzumab in patients with relapsed or refractory chronic lymphocytic leukemia. Leukemia. 2023;37(4):835–842. doi: 10.1038/s41375-023-01830-2. [DOI] [PubMed] [Google Scholar]
- 16.Castro J.E., Lengerke-Diaz P.A., Velez Lujan J., et al. Ibrutinib plus obinutuzumab as frontline therapy for chronic lymphocytic leukemia is associated with a lower rate of infusion-related reactions and with sustained remissions after ibrutinib discontinuation: a single-arm, open-label, phase 1b/2 clinical trial NCT0231576. Adv Hematol. 2022;2022 doi: 10.1155/2022/4450824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woyach J.A., Blachly J.S., Rogers K.A., et al. Acalabrutinib plus obinutuzumab in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia. Cancer Discov. 2020;10(3):394–405. doi: 10.1158/2159-8290.CD-19-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam C.S., Quach H., Nicol A., et al. Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv. 2020;4(19):4802–4811. doi: 10.1182/bloodadvances.2020002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wierda W.G., Kipps T.J., Mayer J., et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawstron A.C., Hillmen P., Maycock S. Ibrutinib and obinutuzumab in CLL: MRD responses sutained for several years with deepest MRD depletion in patients with > 1 year prior ibrutinib exposure. Blood. 2020;136(Supplement 1):27–28. [Google Scholar]
- 21.Hallek M., Cheson B.D., Catovsky D., et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 22.Hallek M., Cheson B.D., Catovsky D., et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the national cancer institute-working group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson B.D., Byrd J.C., Rai K.R., et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30(23):2820–2822. doi: 10.1200/JCO.2012.43.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 25.Barr P.M., Owen C., Robak T., et al. Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022;6(11):3440–3450. doi: 10.1182/bloodadvances.2021006434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharman J.P., Egyed M., Jurczak W., et al. Efficacy and safety in a 4-year follow-up of the ELEVATE-TN study comparing acalabrutinib with or without obinutuzumab versus obinutuzumab plus chlorambucil in treatment-naïve chronic lymphocytic leukemia. Leukemia. 2022;36(4):1171–1175. doi: 10.1038/s41375-021-01485-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger J.A., Barr P.M., Robak T., et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787–798. doi: 10.1038/s41375-019-0602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanafelt T.D., Wang X.V., Hanson C.A., et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022;140(2):112–120. doi: 10.1182/blood.2021014960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno C., Greil R., Demirkan F., et al. First-line treatment of chronic lymphocytic leukemia with ibrutinib plus obinutuzumab. Haematologica. 2022;107(9):2108–2120. doi: 10.3324/haematol.2021.279012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vijenthira A., Gong I.Y., Fox T.A., et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiménez M., Roldán E., Fernández-Naval C., et al. Cellular and humoral immunogenicity of the mRNA-1273 SARS-CoV-2 vaccine in patients with hematologic malignancies. Blood Adv. 2022;6(3):774–784. doi: 10.1182/bloodadvances.2021006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.