Abstract
Endophytic bacteria, living inside plants, are competent plant colonizers, capable of enhancing immune responses in plants and establishing a symbiotic relationship with them. Endophytic bacteria are able to control phytopathogenic fungi while exhibiting plant growth-promoting activity. Here, we discussed the mechanisms of phytopathogenic fungi control and plant growth-promoting actions discovered in some major groups of beneficial endophytic bacteria such as Bacillus, Paenibacillus, and Pseudomonas. Most of the studied strains in these genera were isolated from the rhizosphere and soils, and a more extensive study of these endophytic bacteria is needed. It is essential to understand the underlying biocontrol and plant growth-promoting mechanisms and to develop an effective screening approach for selecting potential endophytic bacteria for various applications. We have suggested a screening strategy to identify potentially useful endophytic bacteria based on mechanistic phenomena. The discovery of endophytic bacteria with useful biocontrol and plant growth-promoting characteristics is essential for developing sustainable agriculture.
Keywords: Bacillus, Paenibacillus, Pseudomonas, Secondary metabolites, Screening approach, Agricultural sustainability
Graphical abstract
Highlights
-
•
Plant diseases are generally caused by phytopathogenic fungi.
-
•
Endophytic bacteria have specific and effective biological properties against phytopathogenic fungi.
-
•
Both biocontrol and growth-promoting features have been discovered in endophytic bacteria.
-
•
Various screening strategies for effective biocontrol and growth-promoting features in endophytic bacteria are discussed.
-
•
Useful endophytic bacteria are important for maintaining soil health and fertility, and contributing towards agricultural sustainability.
1. Introduction
It has been estimated that 60 % more food will need to be produced by 2050 to feed the population of more than 10 billion people worldwide [1,2]. The production increase must be maintained despite the loss caused by crop pests and diseases to reach the food demand [2]. The mean global yield losses from crop diseases and pests were approximately 30.3 % in rice, 21.5 % in wheat, 22.6 % in maize, 21.4 % in soybean, and 17.2 % in potato [2]. Plant diseases cause not only significant crop yield losses but also reductions in the quality of crops, which impacts human health [3].
Crop plants suffer from various diseases due to infection by phytopathogens. Phytopathogenic fungal infections cause the vast majority of diseases reported in plants. Cereal crops, such as rice, wheat, and maize, are vital sources of human foods, while crops such as tomato, banana, and kiwifruit are key sources of human nutrition. Various fungal pathogens very often colonize these crops. Magnaporthe oryzae, the rice blast pathogen, may cause a 10–35 % loss of rice [4]. Fusarium graminearum is a devastating fungus that may give rise to head blight, foot rot, and root rot [5] in wheat. This fungus does not only affect yield loss. Still, it may also adulterate wheat grain by producing deoxynivalenol and zearalenone as mycotoxins, which are hazardous to the health of humans and animals [6]. In maize cultivation, fungi are considered critical pathogens, with Fusarium spp. being causal agents for infecting roots, stalks, ears, and kernels [7]. In addition to yield loss, Fusarium can reduce grain quality by releasing fumonisin or deoxynivalenol [8,9]. Rhizoctonia solani may cause diseases in more than 200 species of various plants, including rice, tobacco, and horticultural crops such as tomato, brinjal, potato, pepper, etc. [10]. Botrytis cinerea can cause grey mold in various fruits. It can infect multiple fruits, such as grapes, strawberry, raspberry, blackberry, kiwifruit, apple, and pear [11]. It also causes diseases in cabbage, lettuce, broccoli, beans, and carrots [11]. Sclerotinia sclerotiorum is a serious cosmopolitan pathogen that causes soft rot or stem rot by infecting a broad range of plants, including sunflower, rapeseed, soybean, lentil, chickpea, peanut, onion, tulip, and various vegetables [12].
Plant disease control primarily depends on chemical pesticide application to manage plant pathogens or vectors of plant pathogens. However, the applications of pesticides may result in hazardous effects on the ecosystem and human lives. So, researchers and producers are searching for eco-friendly disease control techniques. As an alternative tool, plant growth-promoting bacteria (PGPB) can be used to biocontrol plant pathogens [13]. PGPB may inhabit the rhizosphere, episphere, and inside plants [14]. Endophytes inhabit plant tissues without causing disease [15], and some endophytes are also PGPB and thus are considered plant growth-promoting endophytic bacteria (PGPEB) [14].
Endophytes are getting more attention for applications as biocontrol agents and plant growth promoters [[16], [17]]. Endophytic bacteria can be used to control pre- and post-harvest plant pathogens [18]. These bacteria can restrict pathogens by occupying plant tissue space, producing lytic enzymes and secondary metabolites, and developing plant defenses [18,19].
Strains from the bacterial genera of Bacillus, Paenibacillus, Pseudomonas, Burkholderia, Enterobacter, Klebsiella, and Arthrobacter strains have been reported for their endophytic nature and tested for biocontrol and plant growth-promotion [[20], [21], [22], [23], [24], [25]]. Bacillus can produce endospores, thick-walled survival structures allowing microorganisms to bypass stress and adverse environmental situations. These bacteria have a wide spectrum of biocontrol potential, can enhance plant growth, and trigger plant defenses [26,27]. Paenibacillus can also produce endospores, live in adverse environments, and assist in controlling plant pathogens through antimicrobial production or triggering induced systemic resistance [28]. Pseudomonas species are versatile metabolically and can adapt to various environmental conditions. Members of Pseudomonas can also restrict phytopathogens and enhance plant growth [22,29].
Recently, many governments around the world have placed various strategies to reduce the usage of agri-chemicals for pest control. In addition, the selection of biocontrol agents and the study of their metabolic and genetic profiles can be extremely important for the scientific community and stakeholders involved in food supply chains. In this review, we discussed the various mechanisms of phytopathogenic fungi control and growth-promoting activity by endophytic bacteria, emphasizing the species belonging to Bacillus, Paenibacillus, and Pseudomonas genera. In addition, we proposed a rapid potential screening approach to identify effective biocontrol and plant-growth-promoting agents.
2. Biocontrol mechanisms of endophytic bacteria
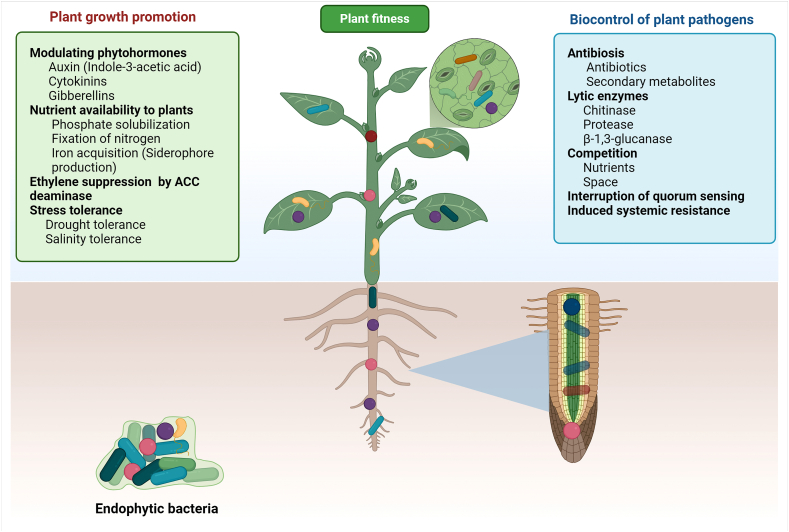
Endophytic bacteria may inhibit plant pathogens as these bacteria can produce various antimicrobial compounds and enzymes to control fungal growth. Endophytic bacteria may also stimulate defense systems through the induction of plant systemic resistance [30, [31]. The colonization of useful bacteria and their subsequent competition for nutrients and space can decrease the incidence of plant diseases [18,32]. A list of generalized biocontrol mechanisms is included in Fig. 1. Endophytic bacteria with antifungal and plant growth promotion reported in various studies are presented in Table 1. The biocontrol mechanisms of these bacteria can be described as direct and indirect mechanisms.
Fig. 1.
The schematic representation of endophytic bacterial effects on plant fitness by promoting plant growth and biocontrol of plant pathogens.
Table 1.
List of endophytic bacteria with biocontrol activity.
| Strain | Origin | Test fungal pathogens | Inhibition mechanisms | References |
|---|---|---|---|---|
| Bacillus | ||||
| B. subtilis CB2 | Wheat seeds | F. graminearum | Iturin | [33] |
| B. subtilis SG_JW.03 | Maize seeds | F. moniliforme | Fengycin Iturin |
[34] |
|
B. amyloliquefaciens YN201732 |
Tobacco seeds | Erysiphe cichoracearum | ISR | [35] |
| B. velezensis QSE-21 | Tomato stem | B. cinerea | ISR | [36] |
| B. velezensis DMW1 | Potato tubers |
R. solani S. sclerotiorum |
Fengycin Iturin |
[37] |
| B. siamensis WB1 | Walnut roots | C. acutatum | Fengycin, Iturin | [38] |
| B. safensis B21 | Sweet olive fruits | M. oryzae | Iturin | [39] |
| B. aryabhattai B003 | Sweet-grass root | B. cinerea | ISR | [40] |
| Paenibacillus | ||||
| P. polymyxa SF05 | Maize sheath | R. solani | ISR | [41] |
| P. polymyxa WLY78 | Bamboo roots | F. oxysporum f. sp. cucumerium | Fusaricidins ISR |
[42] |
| P. peoriae RP51 | Black locust nodule |
F. graminearum R. solani M. oryzae |
Fusaricidins | [43] |
| Paenibacillus sp. UY79 | Wild peanut nodule |
B. cinerea F. oxysporum R. solani |
Fusaricidins | [44] |
| Pseudomonas | ||||
| P. bijieensis XL17 | Rape crown gall | B. cinerea | DAPG | [45] |
| P. fluorescens HP72 | Bentgrass root | R. solani | DAPG | [46] |
2.1. Direct biocontrol mechanisms of endophytic bacteria
2.1.1. Antibiosis
Antibiosis is a process by which bacteria can restrict other microbes by producing antimicrobial compounds. Endophytic bacteria inhibit the growth of phytopathogenic microorganisms by synthesizing secondary metabolites with antifungal and antibacterial activities [47]. Secondary metabolites, including surfactin, iturin, fengycin, bacillaene, subtilosin A, fusaricidin, polymyxin, 2,4-diacetylephloroglucinol (DAPG), phenazine-1-carboxylic acid, 2-hydroxyphenazine, pyrrolnitrine, viscosinamide, and Orfamide are well known for their antimicrobial activity [18,[48], [49], [50], [51], [52]]. For example, B. subtilis produces fengycin and inhibits B. cinerea in apple fruits [18], P. fluorescence produces DAPG and inhibits Thielaviopsis basicola in tobacco [18], and P. polymyxa produces fusaricidins and inhibits Fusarium, Rhizoctonia, Sclerotinia, etc. [52].
2.1.2. Hydrolytic enzymes
Hydrolytic enzymes of endophytic bacteria may break down different polymeric components, including cellulose, chitin, proteins, and lipids [53]. These enzymes can degrade fungal cell walls [18]. The widely reported enzymes for biocontrol include protease, cellulase, β-1,3-glucanase, and chitinase. These enzymes can damage the cell walls of pathogens [54]. For instance, the extra-cellular chitinase of Pseudomonas aeruginosa was reported to be able to control Xanthomonas campestris, the causal agent of black rot disease in cruciferous crops [55]. The chitinase from B. subtilis, when incorporated into a PDA plate, showed a 42.3 % reduction in R. solani mycelial growth [56].
2.1.3. Volatile compounds
Endophytic bacteria also release volatile organic compounds (VOCs), which are reported to be able to inhibit phytopathogenic fungi, bacteria, and nematodes [57]. Pseudomonas putida, isolated from black pepper root, inhibited Phytopthora capsici, Athelia rolfsii, Giberella moniliformis, R. solani, Pythium myriotylum, and Colletotrichum gloeosporioides by its VOCs [58]. The B. subtilis strain DZSY21, isolated from Eucommia ulmoides leaves, inhibited Curvularia lunata by producing VOCs, isopentyl acetate, and 2- heptanone [59]. VOCs of B. velezensis ZSY-1 strongly suppressed F. oxysporum, Alternaria solani, B. cinerea, Colletotrichum lindemuthianum [60].
2.1.4. Siderophores
Some endophytes, including Bacillus, Paenibacillus, and Pseudomonas, can produce active low molecular weight compounds that can chelate iron (Fe), supply it in plant-available form, and deprive pathogens of iron [47]. Siderophores produced by endophytes such as hydroxymate, phenolate, catecholate, and pyoverdine have exhibited biocontrol activity [[61], [62], [63]]. For example, Yu et al. [64] reported that B. subtilis strain CAS15 inhibited fungal isolates of Colletotrichum, Fusarium, Magnaporthe, Pythium, and Phytopthora through bacillibactin (catecholate type siderophores) production.
2.1.5. Interruption of quorum sensing
Quorum sensing is a process that regulates activities like crosstalk among the cells, biofilm formation, reproduction, mutualism, adaption, and pathogenesis [65]. Some endophytes have been identified as having interrupted phytopathogenic signaling pathways by quenching quorum sensing [66]. Rhodococcus corynebacterioides, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia, which were isolated from various plants, destroyed quorum-sensing compounds, namely 3-hydroxy palmitic acid methyl ester of R. solanacearum, and thus suppress eggplant wilt [67].
2.1.6. Competition for nutrients and space
Endophytic bacteria may also inhibit pathogens by competition for nutrients and space. Lastochkina et al. [68] reported that B. subtilis suppressed Phytopthora infestans and F. oxysporum by competing effectively for nutrients and space inside the potato tubers.
2.2. Indirect biocontrol mechanisms of endophytic bacteria
The indirect biocontrol mechanism involves the induction of plant defenses associated with microbes. Two kinds of induced defenses have been proposed, namely induced systemic resistance (ISR) and systemic acquired resistance (SAR), based on the hormonal implications and the elicitor type [69]. ISR is triggered by rhizobacteria or other non-pathogenic microbes, while pathogenic microbes or chemical compounds trigger SAR [70]. ISR is operated by the jasmonic acid (JA) or ethylene (ET) pathways, and SAR is regulated by the salicylic acid (SA)-dependent signaling pathways following pathogenesis-related (PRs) proteins gene expression [[71], [72], [73]]. However, ISR may also depend on both SA and JA/ET signaling processes. The ISR driven by B. cereus strain AR156 depended on SA and JA/ET signaling process and NPR1 [74]. Another study showed that endophytic B. subtilis, producing antifungal lipopeptides (fengycin and iturin), protected maize from F. moniliforme and induced PR genes (PR-1 and PR-4) in maize [34].
Endophytic bacteria-mediated ISR can protect plants from phytopathogens [19,75]. For example, ISR was developed in saffron against F. oxysporum by Burkholderia gladioli [25], in grapevine and tomato against B. cinerea [76], and Verticillium dahliae [77] by Pseudomonas sp., in oak against Ceratocystis fagacearum by P. putida and P. denitrificans [78], in tomato against F. oxysporum f. sp. radicis-lycopersici by P. fluorescens [79], and pea against F. oxysporum f. sp. pisi by B. pumilus [80].
3. Biocontrol mechanisms of Bacillus
The Bacillus genus is ubiquitous and can live in soil, water, and air, on the surface, inside plant and rhizosphere, gastrointestinal tracts, and other extreme environments [21,81]. Some Bacillus are used in agriculture for easy industrial production, satisfactory biocontrol efficacy, and environmental safety [[82], [83], [84]]. The species of Bacillus species have divergent secondary metabolisms and various antagonistic compounds. B. subtilis strains may contain up to 5 % of their whole genome for secondary metabolite synthesis [85]. B. amyloliquefaciens FZB42 comprises 8 % of the genome for secondary metabolites synthesis such as polyketides, lipopeptides, antimicrobial peptides antimicrobial peptides, siderophores, and bacteriocins [86,87].
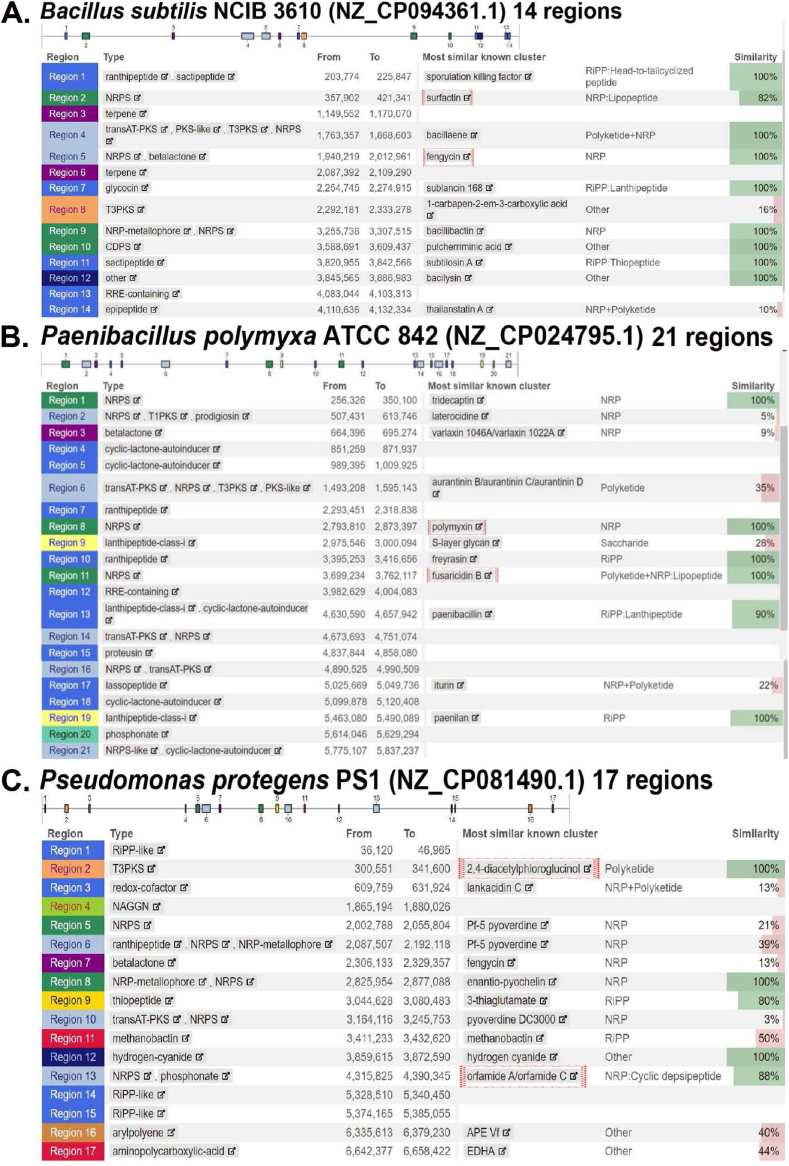
Previous studies revealed that biosynthetic gene clusters (BGCs) are phylogenetically conserved in the Bacillus genus, and multiple species or clade-specific molecules have been discovered [88]. Xia et al. [89] reported that BGCs distribution is related to their phylogenetic position based on large-scale Bacillus genome analysis. The BGCs in the cereus clade include non-ribosomally synthesized peptides (NRPS), fengycin, bacteriocin, bacillibactin, and petrobactin; thurincin, polyoxypeptin, and zwittermicin were found in some genomes of B. thuringiensis and B. cereus [89]. In the subtilis clade, fengycin, surfactin, bacillibactin, bacilysin, and T3PKS are primarily present (Fig. 2. A). Each group have specific BGCs like betalactone for B. pumilus, subtilosin and subtilin for B. subtilis, macrolactin and difficidin for B. velezensis and B. amyloliquefaciens too. Some genomes of B. velezensis and B. amyloliquefaciens contain plipastatin, mersacidin, and plantazolicin, and lichenysin may be produced by B. licheniformis [89]. In the megaterium clade, siderophore, surfactin, and T3PKS were found, and some can produce lanthipeptide, paeninodin, or bacteriocins. The major BGCs in the circulans clade were identified as T3PKS, and some produce siderophore, lanthipeptide, and bacteriocin [89]. The secondary metabolites of the Bacillus genus include NRPS, polyketide and lipopeptides, bacteriocins, and siderophores. NRPS and lipopeptides are a highly heterogeneous group consisting of amino acids, amino- or hydroxyl- fatty acids with various hydrocarbon chains, and sometimes these go under acylation, glycosylation, and methylation [81].
Fig. 2.
Biosynthetic gene clusters (BGCs) were identified by antiSMASH version 7.1.0 from whole genome sequences. (A)B. subtilis NCIB 3610 (NZ_CP094361.1). Fourteen BGCs were found, including surfactin, fengycin, bacilysin, bacillibactin, and T3PKS. B. subtilis NCIB 3610 is a representative strain in subtilis clade. (B) P. polymyxa ATCC 842 (NZ_CP024795.1). Twenty-one BGCs, including fusaricidin and polymyxin, were found. Paenibacillus polymyxa ATCC 842 is a representative strain in P. polymyxa complex. (C) P. protegens PS1 (NZ_CP081490.1). Seventeen BGCs were found, including 2,4-diacetylphloroglucinol (DAPG) and orfamide. Pseudomonas protegens PS1 is a representative strain of the P. corrugata subgroup under the P. fluorescens complex. Accession numbers were obtained from NCBI and submitted to antiSMASH on November 26, 2023 (https://antismash.secondarymetabolites) to search the biosynthetic gene clusters [90].
B. subtilis combines many useful features like plant colonization competence, growth-promoting activities, suppression of pathogens, and ISR activation [[83], [91]]. The motile nature and biofilm formation are very important for B. subtilis to colonize roots and for biocontrol of phytopathogens [92]. Furthermore, phytohormones, lipopeptides, siderophores, and volatile compounds enable B. subtilis to promote plant growth and induce the immune system of plants [93]. According to Cawoy et al. [94], some B. subtilis/B. amyloliquefaciens strains can inhibit fungal pathogens. Iturins and fengycins are key factors for fungal inhibition [95,96]. The fengycin (fen) gene cluster constitutes fenA, fenB, fenC, fenD, and fenE. All five genes are conserved in B. siamensis and B. velezensis, while B. amyloliquefaciens has fenA and fenE [97].
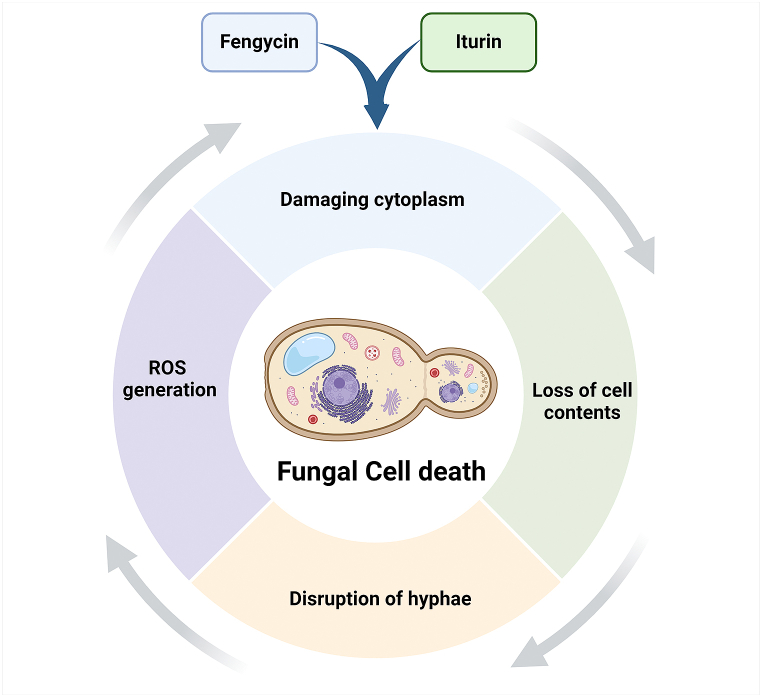
Fengycin mechanisms involving cell death of the pathogens may be related to interactions with the cell membrane and cell permeability modification [98]. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) revealed that the application of B. subtilis BS155 synthesized fengycin caused damage to M. oryzae hyphae, cytoplasm, plasma membrane, and loss of cell membrane integrity, resulting in cell death [98]. Fengycin can be applied to manage rice blast caused by M. grisea [98], barley head blight caused by F. graminearum [99], cucurbit powdery mildew caused by Podosphaera fusca [100], grey mold caused by B. cinerea [101] and maize disease caused by Rhizomucor variabilis [102], etc. Iturin LPs family includes iturin A, C, D, and E, bacillomycin D and F, bacillopeptin, and mycosubtilin [103]. Bacillomycin-D produced by B. velezensis showed antagonism against various pathogens, such as Xanthomonas campestris pv. cucurbitae [104], F. graminearum [105], Aspergillus flavus [106], and F. oxysporum f. sp. cucumerinum [107]. SEM and TEM examination determined that bacillomycin-D altered the morphology of the cytoplasmic membrane, cell wall, conidia, and hyphae of F. graminearum. Bacillomycin-D of B. velezensis induced the expression of thioredoxin and glutathione reductase genes of F. graminearum, which are involved in reactive oxygen species (ROS) synthesis. The genes encoding catalase and peroxidase enzymes were downregulated when F. graminearum was treated with bacillomycin-D. The bacillomycin-D-induced ROS was associated with F. graminearum cell death [105]. Bacillus strains may produce huge amounts of surfactins but have less fungal inhibitory action. It has antibiotic actions [107,108], with rare antifungal activity [94]. Gu et al. [92] reported that B. subtilis produced subtilosin A and bacilysin and controlled Acidovorax citrulli, causing fruit blotch. Agarwal et al. [109] found that B. pumilus can inhibit R. solani and F. oxysporum by chitinase and surfactin production. An illustration of Bacillus lipopeptides like fengycin and iturin-driven antifungal mechanisms of Bacillus is shown in Fig. 3.
Fig. 3.
A generalized schematic representation of antifungal mechanisms of lipopeptides synthesized by Bacillus.
4. Biocontrol mechanisms of Paenibacillus
Paenibacillus is well recognized for its secondary metabolites, including nonribosomal lipopeptides, polyketides, lassopeptides, bacteriocins, and lantibiotics, which are valuable in medicine and agrobiotechnology [52,110]. The most found bioactive compounds in Paenibacillus are lipopeptides with diverse linear and cyclic structures with peptide chains containing 6 to 13 amino acids and variable fatty acid chains [52]. These lipopeptides include fusaricidins and LiF-antibiotics [111,112], paenilipoheptins [113], octapeptins [114,115], polypeptins [114], pelgipeptins (cyclic lipononapeptides, β-hydroxy fatty acid) [116], polymyxins, tridecapeptins [114] and paenibacterins [117]. P. polymyxa bacteria are potent plant growth-promoter colonizing rhizoplane [118] and can also live as endophytes inside the plant [20]. These strains can synthesize four lipopeptides: polymyxins, fusaricidins, paenilipoheptins, and tridecaptins. All of these are comprised of various structural homologs. In specifics, fusaricidins are an uncommon complexity of isoforms, resulting in a broad range of parallel substances with strong antifungal activities. Fusaricidins and polymyxins biosynthesis is accomplished non-ribosomally at multifunctional protein templates [52,119]. Most P. polymyxa complex members possess the biosynthetic gene clusters of fusaricidins and polymyxin (Fig. 2. B).
Fusaricidin synthesis is controlled by the fusaricidin biosynthetic (fus) gene cluster. The cluster contains eight genes in the order of fusG, fusF, fusE, fusD, fusC, fusB, fusA, and fusTE; mutation analysis revealed that genes fusG, fusF, fusE, fusD, fusC, fusB, and fusA except fusTE were all responsible for the antifungal actions [42]. Among the eight genes, fusA is needed for the synthesis of cyclic polypeptide moiety, and the fusG, fusF, fusE, fusD, fusC, and fusB are essential for the synthesis of lipid moiety of fusaricidins. fusG, fusF, fusE, fusD, fusC, fusB, and fusA are arranged independently in a single operon, and its promoter transcribed fusTE [42]. The fusA gene contains modules of six amino acids activation and condensation to form a complete fusaricidin peptide chain [120].
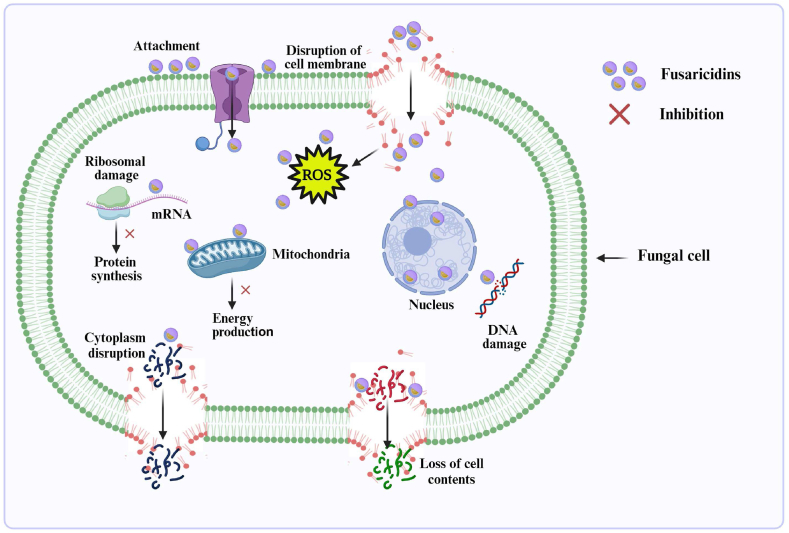
After discovering fusaricidins [121], other researchers conducted further detailed studies [122,123]. Fusaricidins are great broad-spectrum antifungal compounds against a range of phytopathogenic fungi. For example, Li and Chen [42] reported that fusaricidin produced by P. polymyxa WLY78 inhibited the fungal development of F. oxysporum f. sp. cucumerinum causal agent of cucumber wilt. Its fusaricidin inhibited spore development and damaged F. oxysporum f. sp. cucumerinum hyphae. It also elicited the plant's systemic resistance against fungal pathogens. Beatty and Jensen [124] observed that P. polymyxa PKB1 produced fusaricidin, which inhibited the Leptosphaeria maculans, a fungus of canola that causes blackleg disease. Mousa et al. [125] showed that fusaricidin produced by endophytic Paenibacillus inhibited F. graminearum, a causal agent of gibberella ear rot in maize. In a recent study, RNA-seq results revealed that fusaricidins producing P. polymyxa AF01 arrested some of the transcription as well as translation, hampered the structural dynamics of RNA and DNA, interrupted energy production and or conversion and transduction of signals, caused ROS accumulation, ultimately inhibited the biosynthesis of cell wall, altered membrane permeability and restricted protein biosynthesis [126]. A proposed mode of actions of fusaricidins has been illustrated based upon previous studies (Fig. 4).
Fig. 4.
Schematic representation of possible mechanisms of fusaricidins, most commonly produced by the members of the P. polymyxa complex.
5. Biocontrol mechanisms of Pseudomonas
The Pseudomonas genus consists of about 428 species (https://www.ncbi.nlm.nih.gov/genome/?term=pseudomonas, accessed on March 23, 2024) occurring in diverse habitat or niches including soils, water, animal guts, and plant tissues [127]. Many Pseudomonas species were isolated and characterized as PGPB for their useful functions to plants [128]. Many Pseudomonas species are associated with plants belonging to the P. fluorescens lineage, and they phylogenetically fall into five groups such as P. fluorescens, P. putida, P. syringae, P. lutea, and P. asplenii [129]. Among these, some are useful for biocontrol or growth promotion, especially the species under the P. fluorescens group, and some others show plant pathogenicity, mostly under the P. syringae or P. asplenii groups [130]. The plant-beneficial Pseudomonas exhibits plant benefits in various ways: direct pathogens inhibition, plant resistance development, effects on plant growth, utilization of minerals, and environmental stress tolerance [131].
Non-ribosomally synthesized polyketides and cyclic lipopeptides are major secondary metabolites with antimicrobial activity synthesized by PGPB Pseudomonas [132,133]. P. protegens strain Pf‐5, P. kilonensis strain F113, and P. fluorescens strains SBW25 and 2P24 produced 2,4‐diacetylphloroglucinol (DAPG), Orfamide, viscosin, phenazines, pyrrolnitrin, pyoluteorin, and amphisin showing direct phytopathogenic inhibition [134,135]. The cyclic lipopeptides may contribute to biofilm formation, swarming motility, pathogen virulence, and antifungal, antibacterial, anti-oomycete, antiviral, insecticidal, and anti-carcinogenic attributes [132,136]. The DAPG is a widely studied secondary metabolite with antibacterial, antifungal, antiviral, and antihelminthic properties [137]. Some strains of P. protegens and P. corrugata subgroups under the P. fluorescens complex comprise the DAPG biosynthetic gene cluster [135]. The biosynthetic gene clusters of a P. protegens strain are given here (Fig. 2. C). The phl gene cluster controls the DAPG production and consists of phlACBDE operon associated with phlF, phlG, phlH, and phlI genes [138,139]. The phlD is essential for monoacetylphloroglucinol (MAPG) synthesis, whereas phlA, phlB, and phlC are associated with transforming MAPG to DAPG [138].
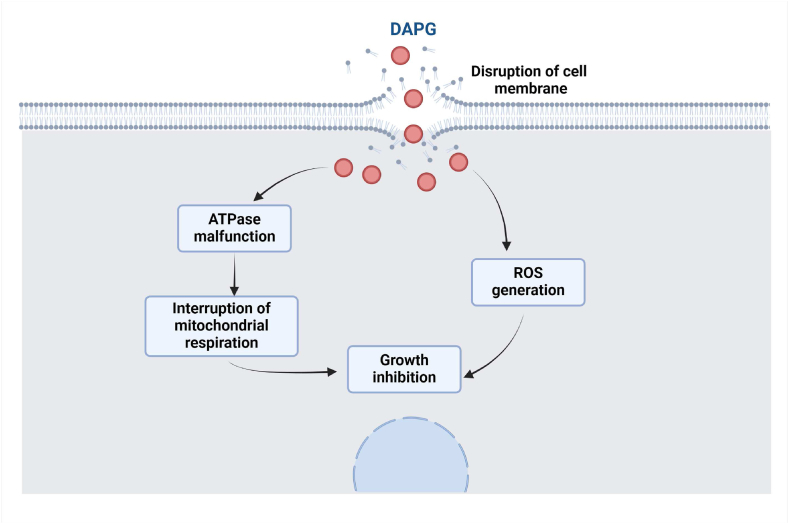
Kwak et al. [140] discovered the action mechanisms of DAPG by using a mutant library of Saccharomyces cerevisiae, and they identified 231 mutants that were DAPG sensitive. The selected mutants were subjected to chemical, biochemical, and genetic analyses, and they reported three prime physiological activities relevant to DAPG sensitivity: membrane permeability, ROS regulation, and homeostasis of cells. According to Stepanov et al. [141], the antifungal actions of DAPG include damaging cellular permeability, malfunctioning of H + ATPase, and disturbance of mitochondrial respiration. The primary adverse effects of DAPG are respiration intervention and ATP synthesis, leading to growth inhibition [142]. Ali et al. [45] observed a DAPG-producing strain, P. bijieensis XL17, belongs to the P. corrugata subgroup, has damaged cell membrane and cytoplasm, causing cell wall leakage, and tends to lose cell organelles. An illustration of the probable fungal inhibition mechanism of DAPG is given below (Fig. 5).
Fig. 5.
An illustration of possible antifungal mechanisms of DAPG.
In addition to secondary metabolite-driven antagonism against pathogens, biocontrol control of Pseudomonas is also connected to ISR and siderophore-mediated iron competition [143]. P. simiae strain WCS417, including other strains, showed colonization competence in plant roots and triggered ISR, resulting in higher protection against plant pathogens [143]. The Type VI secretion system (T6SS) in Pseudomonas spp. is important for its biocontrol activity. The T6SS is a syringe-like structure resembling a phage tail capable of secreting effector proteins into targeted prokaryotic and eukaryotic cells [144,145]. This system has been recognized as a powerful antifungal weapon, and fungal-specific effector proteins, Tfe1 and Tfe2, have been identified. These effector proteins act through specific mechanisms in various fungal species by causing cell death. Tef1 causes the depolarization of the plasma membrane, and Tef2 interrupts nutrient uptake and induces autophagy [146,147].
6. Endophytic bacteria in plant growth promotion
The PGPB includes free-living bacteria that may establish symbiosis with plants (e.g., Rhizobia spp.), endophytic bacteria in plant tissues, and cyanobacteria [[17], [31], [148]]. Despite the differences within different groups, all these bacteria utilize similar mechanisms. These bacteria contain some plant-growth promoting (PGP) traits. PGPB enhances plant growth by acquiring nutrients or phytohormone modulation or inhibiting various plant pathogens [[83], [149]] (Fig. 1). Some endophytic bacteria bearing plant growth-promoting attributes are listed in Table 2.
Table 2.
List of plant growth-promoting endophytic bacteria.
| Strain | Origin | Test plants | PGP traits | References |
|---|---|---|---|---|
| Bacillus | ||||
| B. subtilis 2S | Maize stem | Maize | IAA Siderophore |
[21] |
| B. subtilis 135 | Honeysuckle root | Wheat | IAA Siderophore |
[150] |
| B. cereus EPP5 | Pearl millet stem | Pearl millet | IAA Siderophore Phosphate solubilization Potassium solubilization |
[151] |
| B. amyloliquefaciens EPP62 | Pearl millet root | Pearl millet | IAA Siderophore Phosphate solubilization Potassium solubilization |
[151] |
| B. thuringiensis CR71 | Tomatillo root | Cucumber | IAA Siderophore |
[152] |
| B. altitudinis SB001 | Sweet-grass root | Tobacco Maize Soybean |
IAA | [153] |
| Paenibacillus | ||||
| P. polymyxa SK1 | Tiger lily bulb | Tiger lily | IAA Siderophore Nitrogen fixation Phosphate solubilization |
[154] |
| P. polymyxa 122 | Honeysuckle root | Wheat | IAA Siderophore Phosphate solubilization |
[155] |
| P. peoriae RP51 | Black locust nodule | Wheat | IAA Siderophore Nitrogen fixation Phosphate solubilization |
[156] |
| Pseudomonas | ||||
| P. bijieensis XL17 | Rape crown gall | Rice | IAA Phosphate solubilization |
[45] |
| Pseudomonas sp. CI-3 | Chickpea root | Chickpea | IAA Siderophore Phosphate solubilization |
[155] |
| Pseudomonas sp. n00132 | Rice leaf | Rice | IAA Siderophore Phosphate solubilization |
[156] |
| P. aeruginosa KAS6 | Pearl millet seed | Pearl millet | IAA Phosphate solubilization |
[157] |
| P. flourescens L228 | Elephant grass leaf | Pea | Phosphate solubilization | [158] |
| P. flourescens L321 | Elephant grass leaf | Rapeseed | IAA Siderophore Phosphate solubilization |
[159] |
| P. aeruginosa Ld-08 | Lily bulb | Lily | IAA Siderophore Phosphate solubilization |
[160] |
6.1. Nutrient acquisition
Nutrient or resource acquisition is the best-studied phenomenon of PGPB by which bacteria can provide nutrient resources (nitrogen, iron, phosphorus, etc.) in available form to plants [[32], [161], [162]]. Some endophytic bacteria can biologically fixed atmospheric nitrogen to the available state for utilization by plants using nitrogenase [163]. Nitrogen-fixing bacteria, such as Azospirillum brasilense and Azoarcus sp. BH72, Burkholderia spp., Herbaspirillum seropedicae, and Gluconacetobacter diazotrophicus increased plant growth by N2 fixing in controlled environments [164].
Endophytic bacteria may solubilize fixed phosphates and become available to plants [[161], [162], [165]]. These endophytic bacteria may also assimilate soluble phosphorus by preventing phosphate fixation and its adsorption in phosphate-limiting conditions [166]. In this way, they can serve as reservoirs of phosphorus for plants.
Endophytic bacteria produce siderophores, chelate insoluble ferric ions, and supply iron to plants [167]. Pseudomonas strain GRP3, a siderophore-producing bacterium, was inoculated in mung beans under iron deficit conditions, which increased chlorophyll levels compared to untreated plants [168]. P. flourescens synthesized Fe-pyoverdine complex was uptaken by Arabidopsis thaliana, increasing iron levels in plants with increasing plant growth [169].
6.2. Modulating phytohormones
Endophytes produce phytohormones that regulate plant physiology, growth and development [[31], [170], [171]]. Indole-3-acetic acid (IAA), cytokinins, gibberellins and ethylene are important hormones in the interactions between plants and bacteria [[84], [161], [167], [171]]. IAA of endophytic bacteria increases root volume, surface area, and lateral roots in plants [172]. For example, Tsavkelova et al. [173] observed that the IAA of orchids inhabiting endophytic bacteria had effects on increasing root length and number of roots of kidney beans used during bioassay.
Biotic and abiotic stresses can trigger ethylene synthesis in plants [31][84]. A higher level of ethylene may deter plant root development. Endophytic bacteria synthesize 1-aminocyclopropane-1- carboxylate (ACC) deaminase and can hydrolyze ACC, the prior product of plant ethylene. The ACC deaminase-producing bacteria break ACC into α-ketobutyrate and ammonia [174]. Hence, these bacteria can improve plant growth by ameliorating stress conditions [14].
Endophytic bacteria can also produce cytokinins and gibberellins [[84], [171], [175]]. Cytokinins regulate cell division, seed germination, elongation of the root, differentiation of chloroplast and xylem, axillary and flowering bud growth, fruit set, leaf senescence, and increase biotic and abiotic tolerance [[176], [177], [178], [179], [180], [181]]. The root inoculation of P. flourescens strain G20–18, which can produce cytokinins, promoted tomato growth and increased drought tolerance [182]. Endophytic P. resinovorans strain Gp e1 and P. polymyxa strain Gp e2 were shown to produce cytokinin-like substances [183]. Gibberellins enhance seed germination, promote stem and leaf growth, develop flowers and fruits, and restrict plant ageing [184,185]. B. amyloliquefaciens RWL-1 and P. pseudomonas strains improved plant growth by gibberellins production [186,187].
6.3. Stress tolerance
Biotic and abiotic stresses reduce crop growth and in some scenarios, give negative effects to soil fertility and health [[17], [188]]. Endophytic bacteria can inhibit pathogens through biocontrol potentials and improve systemic plant resistance. They can reduce abiotic stresses by osmotic adjustments, antioxidant enzyme production, phytohormone alteration, and modification of plant morphology and signal system [[17], [188], [189]]. Heat-tolerant endophytes and rhizobacteria like B. amyloliquefaciens and P. putida compensate for heat stress in rice and wheat by modulating antioxidant defense enzymes and plant hormones [190,191]. Endophytic B. cereus enhanced rice salinity tolerance by modulating IAA, abscisic acids, and gibberellins [20]. P. polymyxa CR1 primed drought stress tolerance and root growth of soybean and arabidopsis [192]. It was found that endophytes can transfer and solubilize nutrient elements like phosphorus, nitrogen, potassium, and other micronutrients and make them available to plants [193,194].
7. Future perspectives and the suggested screening approaches to identify biocontrol and plant growth-promoting agents
Endophytic bacteria are capable of living inside the plant system. Therefore, it is a great opportunity to select the most promising ones from these bacterial diversity for applications in sustainable agriculture. Research on effectiveness, perspectives, and implications for the usage of endophytic bacteria in biological control are crucial, in addition to the antifungal activity. Many endophytic bacteria have been selected for biocontrol and plant growth promotion. However, most of them were evaluated under in vitro conditions, and further operational evaluation should be confirmed under field conditions. When proven successful, these bacteria can be used to replace the agrochemicals in delivering natural biocontrol functionality in agriculture.
In this review, we suggested a rapid approach to screen and identify the broad-spectrum biocontrol bacteria belonging to various known groups, for example, Bacillus subtilis clade, Paenibacillus polymyxa complex, and Pseudomonas fluorescens complex, etc. The screening strategies may include the following steps:
First step: Confrontation cultures of bacterial isolates and pathogens screen out antimicrobial bacteria.
Second step: 16S rRNA gene sequences analysis phylogenetically to identify antimicrobial strains of known taxonomic groups, which are most probably able to produce plant growth-promoting IAA, fix N2, and produce broad-spectrum antimicrobials.
Third step: Matrix-assisted laser desorption ionization-time of flight-mass spectrometry (MALDI-TOF-MS) analyses to identify lipopeptide producers.
Fourth step: PCR amplification and sequencing of key biosynthesis genes, such as phlD and nifH, to identify a key biocontrol or plant growth-promoting trait.
Fifth step: Gnotobiotic assay of plant seedlings, a biocontrol strain, and a target pathogen to screen biocontrol strains with plant colonizing capacity and in planta biocontrol ability.
Sixth step: Field trials using promising biocontrol and plant growth-promoting strains to determine practical application.
8. Conclusion
Endophytic bacteria are effective and useful alternative biological tools to agrochemicals. They can control phytopathogenic fungi and promote plant growth without negatively impacting the agroecosystem. The underlying antifungal mechanisms mostly involve lipopeptides and polyketides. Fengycin, fusaricidins, and DAPG are key metabolites for antifungal activity in the case of endophytic Bacillus, Paenibacillus, and Pseudomonas, exhibiting dual biocontrol and plant-growth promotion functionality. Proper screening of these endophytic bacteria is crucial. Most studies on endophytic bacteria-driven biocontrol and plant growth promotion are conducted under laboratory conditions. Moving forward, more endophytic bacteria should be screened initially in the laboratory, and tests should be extended to realistic and operational conditions (e.g. farm level) for their utilization in developing sustainable agriculture.
Data availability
No data was used for the research described in the article.
Ethics declarations
Review and approval by an ethics committee was not required for this study because it is a literature review and does not address the ethical considerations of animal, cell, and human experimentation.
Funding
There was no funding for this study.
CRediT authorship contribution statement
Md Arshad Ali: Writing – original draft, Data curation, Conceptualization. Temoor Ahmed: Writing – review & editing, Conceptualization. Ezzeldin Ibrahim: Writing – original draft, Data curation. Muhammad Rizwan: Writing – original draft, Data curation. Khim Phin Chong: Writing – review & editing, Supervision, Conceptualization. Jean Wan Hong Yong: Writing – review & editing, Supervision.
Declaration of generative AI and AI-assisted technologies in the writing process
While preparing this work, the author(s) used Grammarly Software to improve the language. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the publication's content.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Khim Phin Chong, Email: chongkp@ums.edu.my.
Jean Wan Hong Yong, Email: jean.yong@slu.se.
References
- 1.Fedoroff N.V. Food in a future of 10 billion. Agric. Food Secur. 2015;4:11. doi: 10.1186/s40066-015-0031-7. [DOI] [Google Scholar]
- 2.Ristaino J.B., Anderson P.K., Bebber D.P., Brauman K.A., Cunniffe N.J., Fedoroff N.V., Finegold C., Garrett K.A., Gilligan C.A., Jones C.M., Martin M.D., MacDonald G.K., Neenan P., Records A., Schmale D.G., Tateosian L., Wei Q. The persistent threat of emerging plant disease pandemics to global food security. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2022239118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savary S., Willocquet L., Pethybridge S.J., Esker P., McRoberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 4.Talbot N.J. On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003;57:177–202. doi: 10.1146/annurev.micro.57.030502.090957. [DOI] [PubMed] [Google Scholar]
- 5.Colombo E.M., Kunova A., Pizzatti C., Saracchi M., Cortesi P., Pasquali M. Selection of an endophytic Streptomyces sp. strain DEF09 from wheat roots as a biocontrol agent against Fusarium graminearum. Front. Microbiol. 2019;10:2356. doi: 10.3389/fmicb.2019.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/cmr.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Román S.G., Quiroz-Chávez J., Villalobos M., Urías-Gutiérrez V., Nava-Pérez E., Ruíz-May E., Singh R.K., Sharma L., Quiroz-Figueroa F.R. A global screening assay to select for maize phenotypes with a high tolerance or resistance to Fusarium verticillioides (Sacc.) nirenberg rots. Agronomy. 2020;10:1990. doi: 10.3390/agronomy10121990. [DOI] [Google Scholar]
- 8.Nagy E., Voichiţa H., Kadar R. The influence of fusarium ear infection on the maize yield and quality (Transylvania-Romania) Commun. Agric. Appl. Biol. Sci. 2006;71:1147–1150. PMID: 17390871. [PubMed] [Google Scholar]
- 9.Lizárraga-Sánchez G.J., Leyva-Madrigal K.Y., Sánchez-Peña P., Quiroz-Figueroa F.R., Maldonado-Mendoza I.E. Bacillus cereus sensu lato strain B25 controls maize stalk and ear rot in Sinaloa, Mexico. Field Crops Res. 2015;176:11–21. doi: 10.1016/j.fcr.2015.02.015. [DOI] [Google Scholar]
- 10.Gondal A.S., Rauf A., Naz F. Anastomosis groups of Rhizoctonia solani associated with tomato foot rot in Pothohar region of Pakistan. Sci. Rep. 2019;9:3910. doi: 10.1038/s41598-019-40043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson B., Tudzynski B., Tudzynski P., van Kan J.A. Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 2007;8:561–580. doi: 10.1111/j.1364-3703.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 12.Bolton M.D., Thomma B.P., Nelson B.D. Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006;7:1–16. doi: 10.1111/j.1364-3703.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 13.Kannojia P., Choudhary K.K., Srivastava A.K., Singh A.K. In: PGPR Amelioration in Sustainable Agriculture. Singh A.K., Kumar A., Singh P.K., editors. Woodhead Publishing; 2019. Chapter Four - PGPR Bioelicitors: induced systemic resistance (ISR) and proteomic perspective on biocontrol; pp. 67–84. [DOI] [Google Scholar]
- 14.Santoyo G., Moreno-Hagelsieb G., del Carmen Orozco-Mosqueda M., Glick B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Hallmann J., Quadt-Hallmann A., Mahaffee W.F., Kloepper J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997;43:895–914. doi: 10.1139/m97-131. [DOI] [Google Scholar]
- 16.Falade A.O., Adewole K.E., Ekundayo T.C. Aptitude of endophytic microbes for production of novel biocontrol agents and industrial enzymes towards agro-industrial sustainability. Beni-Suef Univ. J. Basic Appl. Sci. 2021;10:61. doi: 10.1186/s43088-021-00146-3. [DOI] [Google Scholar]
- 17.de Vries FT, Griffiths RI, Knight CG, Nicolitch O, Williams A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science. 2020;368(6488):270–274. doi: 10.1126/science.aaz5192. [DOI] [PubMed] [Google Scholar]
- 18.Morales-Cedeño L.R., Orozco-Mosqueda M.d.C., Loeza-Lara P.D., Parra-Cota F.I., de los Santos-Villalobos S., Santoyo G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: fundamentals, methods of application and future perspectives. Microbiol. Res. 2021;242 doi: 10.1016/j.micres.2020.126612. [DOI] [PubMed] [Google Scholar]
- 19.Oukala N., Aissat K., Pastor V. Bacterial endophytes: the hidden actor in plant immune responses against biotic stress. Plants. 2021;10:1012. doi: 10.3390/plants10051012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan M.A., Asaf S., Khan A.L., Adhikari A., Jan R., Ali S., Imran M., Kim K.-M., Lee I.-J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020;22:850–862. doi: 10.1111/plb.13124. [DOI] [PubMed] [Google Scholar]
- 21.Bolivar-Anillo H.J., González-Rodríguez V.E., Cantoral J.M., García-Sánchez D., Collado I.G., Garrido C. Endophytic bacteria Bacillus subtilis, isolated from zea mays, as potential biocontrol agent against Botrytis cinerea. Biology. 2021;10:492. doi: 10.3390/biology10060492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christakis C.A., Daskalogiannis G., Chatzaki A., Markakis E.A., Mermigka G., Sagia A., Rizzo G.F., Catara V., Lagkouvardos I., Studholme D.J., Sarris P.F. Endophytic bacterial isolates from halophytes demonstrate phytopathogen biocontrol and plant growth promotion under high salinity. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.681567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mowafy A.M., Fawzy M.M., Gebreil A., Elsayed A. Endophytic Bacillus, Enterobacter, and Klebsiella enhance the growth and yield of maize. Acta Agric. Scand. B Soil Plant Sci. 2021;71:237–246. doi: 10.1080/09064710.2021.1880621. [DOI] [Google Scholar]
- 24.Mutungi P.M., Wekesa V.W., Onguso J., Kanga E., Baleba S.B.S., Boga H.I. Culturable bacterial endophytes associated with shrubs growing along the draw-down zone of lake bogoria, Kenya: assessment of antifungal potential against Fusarium solani and induction of bean root rot protection. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.796847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad T., Bashir A., Farooq S., Riyaz-Ul-Hassan S. Burkholderia gladioli E39CS3, an endophyte of Crocus sativus Linn., induces host resistance against corm-rot caused by Fusarium oxysporum. J. Appl. Microbiol. 2022;132:495–508. doi: 10.1111/jam.15190. [DOI] [PubMed] [Google Scholar]
- 26.Compant S., Duffy B., Nowak J., Clément C., Barka E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005;71:4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafi J., Tian H., Ji M. Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol. Biotechnol. Equip. 2017;31:446–459. doi: 10.1080/13102818.2017.1286950. [DOI] [Google Scholar]
- 28.Grady E.N., MacDonald J., Liu L., Richman A., Yuan Z.-C. Current knowledge and perspectives of Paenibacillus: a review. Microb. Cell Factories. 2016;15:203. doi: 10.1186/s12934-016-0603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig K., Johnson B.R., Grunden A. Leveraging Pseudomonas stress response mechanisms for industrial applications. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.660134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pérez-Montaño F., Alías-Villegas C., Bellogín R.A., del Cerro P., Espuny M.R., Jiménez-Guerrero I., López-Baena F.J., Ollero F.J., Cubo T. Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol. Res. 2014;169:325–336. doi: 10.1016/j.micres.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Glick B.R. Beneficial plant-bacterial interactions. Springer Cham; 2020. [DOI] [Google Scholar]
- 32.Glick B.R. Plant growth-promoting bacteria: mechanisms and applications. Sci. Tech. Rep. 2012;2012 doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taheri E., Tarighi S., Taheri P. An endophytic bacterium with biocontrol activity against important wheat pathogens. Biol. Control. 2023;183 doi: 10.1016/j.biocontrol.2023.105243. [DOI] [Google Scholar]
- 34.Gond S.K., Bergen M.S., Torres M.S., White J.F., Jr. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015;172:79–87. doi: 10.1016/j.micres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Jiao R., Munir S., He P., Yang H., Wu Y., Wang J., He P., Cai Y., Wang G., He Y. Biocontrol potential of the endophytic Bacillus amyloliquefaciens YN201732 against tobacco powdery mildew and its growth promotion. Biol. Control. 2020;143 doi: 10.1016/j.biocontrol.2019.104160. [DOI] [Google Scholar]
- 36.Xu Y., Wang L., Liang W., Liu M. Biocontrol potential of endophytic Bacillus velezensis strain QSE-21 against post-harvest grey mould of fruit. Biol. Control. 2021;161 doi: 10.1016/j.biocontrol.2021.104711. [DOI] [Google Scholar]
- 37.Yu C., Chen H., zhu L., Song Y., Jiang Q., Zhang Y., Ali Q., Gu Q., Gao X., Borriss R., Dong S., Wu H. Profiling of antimicrobial metabolites synthesized by the endophytic and genetically amenable biocontrol strain Bacillus velezensis DMW1. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.00038-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng X., Xu R., Zhao N., Wang D., Cun M., Yang B. Isolation, identification, and characterization of endophytic Bacillus from walnut (Juglans sigillata) root and its biocontrol effects on walnut anthracnose. Agriculture. 2022;12:2102. doi: 10.3390/agriculture12122102. [DOI] [Google Scholar]
- 39.Rong S., Xu H., Li L., Chen R., Gao X., Xu Z. Antifungal activity of endophytic Bacillus safensis B21 and its potential application as a biopesticide to control rice blast. Pestic. Biochem. Physiol. 2020;162:69–77. doi: 10.1016/j.pestbp.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Portieles R., Xu H., Yue Q., Zhao L., Zhang D., Du L., Gao X., Gao J., Portal Gonzalez N., Santos Bermudez R., Borrás-Hidalgo O. Heat-killed endophytic bacterium induces robust plant defense responses against important pathogens. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-91837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen B., Han H., Hou J., Bao F., Tan H., Lou X., Wang G., Zhao F. Control of maize sheath blight and elicit induced systemic resistance using Paenibacillus polymyxa strain SF05. Microorganisms. 2022;10:1318. doi: 10.3390/microorganisms10071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y., Chen S. Fusaricidin produced by Paenibacillus polymyxa WLY78 induces systemic resistance against Fusarium wilt of cucumber. Int. J. Mol. Sci. 2019;20:5240. doi: 10.3390/ijms20205240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali M.A., Lou Y., Hafeez R., Li X., Hossain A., Xie T., Lin L., Li B., Yin Y., Yan J., An Q. Functional analysis and genome mining reveal high potential of biocontrol and plant growth promotion in nodule-inhabiting bacteria within Paenibacillus polymyxa complex. Front. Microbiol. 2021;11 doi: 10.3389/fmicb.2020.618601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa A., Corallo B., Amarelle V., Stewart S., Pan D., Tiscornia S., Fabiano E. Paenibacillus sp. Strain UY79, Isolated from a root nodule of Arachis villosa, displays a broad spectrum of antifungal activity. Appl. Environ. Microbiol. 2022;88 doi: 10.1128/aem.01645-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ali M.A., Luo J., Ahmed T., Zhang J., Xie T., Dai D., Jiang J., Zhu J., Hassan S., Alorabi J.A., Li B., An Q. Pseudomonas bijieensis strain XL17 within the P. corrugata subgroup producing 2,4-diacetylphloroglucinol and lipopeptides controls bacterial canker and gray mold pathogens of kiwifruit. Microorganisms. 2022;10:425. doi: 10.3390/microorganisms10020425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuxi H., Shino S., Toshihiro A., Hiroshi O. Importance of 2,4-DAPG in the biological control of brown patch by Pseudomonas fluorescens HP72 and newly identified genes involved in 2,4-DAPG biosynthesis. Soil Sci. Plant Nutr. 2004;50(8):1287–1293. doi: 10.1080/00380768.2004.10408606. [DOI] [Google Scholar]
- 47.Fadiji A.E., Babalola O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020;8:467. doi: 10.3389/fbioe.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel P.S., Huang S., Fisher S., Pirnik D., Aklonis C., Dean L., Meyers E., Fernandes P., Mayerl F. Bacillaene, a novel inhibitor of procaryotic protein synthesis produced by Bacillus subtilis: production, taxonomy, isolation, physico-chemical characterization and biological activity. J. Antibiot. 1995;48:997–1003. doi: 10.7164/antibiotics.48.997. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen T.H., Christophersen C., Anthoni U., Sørensen J. Viscosinamide, a new cyclic depsipeptide with surfactant and antifungal properties produced by Pseudomonas fluorescens DR54. J. Appl. Microbiol. 1999;87:80–90. doi: 10.1046/j.1365-2672.1999.00798.x. [DOI] [PubMed] [Google Scholar]
- 50.Ma Z., Geudens N., Kieu N.P., Sinnaeve D., Ongena M., Martins J.C., Höfte M. Biosynthesis, chemical structure, and structure-activity relationship of orfamide lipopeptides produced by Pseudomonas protegens and related species. Front. Microbiol. 2016;7:382. doi: 10.3389/fmicb.2016.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masum M.M.I., Liu L., Yang M., Hossain M.M., Siddiqa M.M., Supty M.E., Ogunyemi S.O., Hossain A., An Q., Li B. Halotolerant bacteria belonging to operational group Bacillus amyloliquefaciens in biocontrol of the rice brown stripe pathogen Acidovorax oryzae. J. Appl. Microbiol. 2018;125:1852–1867. doi: 10.1111/jam.14088. [DOI] [PubMed] [Google Scholar]
- 52.Mülner P., Schwarz E., Dietel K., Herfort S., Jähne J., Lasch P., Cernava T., Berg G., Vater J. Fusaricidins, polymyxins and volatiles produced by Paenibacillus polymyxa strains DSM 32871 and M1. Pathogens. 2021;10 doi: 10.3390/pathogens10111485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H., Carvalhais L.C., Crawford M., Singh E., Dennis P.G., Pieterse C.M.J., Schenk P.M. Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017;8:2552. doi: 10.3389/fmicb.2017.02552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mota M.S., Gomes C.B., Souza Júnior I.T., Moura A.B. Bacterial selection for biological control of plant disease: criterion determination and validation. Braz. J. Microbiol. 2017;48:62–70. doi: 10.1016/j.bjm.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mishra S., Arora N.K. Evaluation of rhizospheric Pseudomonas and Bacillus as biocontrol tool for Xanthomonas campestris pv campestris. World J. Microbiol. Biotechnol. 2012;28:693–702. doi: 10.1007/s11274-011-0865-5. [DOI] [PubMed] [Google Scholar]
- 56.Saber W.I., Ghoneem K.M., Al-Askar A.A., Rashad Y.M., Ali A.A., Rashad E.M. Chitinase production by Bacillus subtilis ATCC 11774 and its effect on biocontrol of rhizoctonia diseases of potato. Acta Biol. Hung. 2015;66:436–448. doi: 10.1556/018.66.2015.4.8. [DOI] [PubMed] [Google Scholar]
- 57.Khare E., Mishra J., Arora N.K. Multifaceted interactions between endophytes and plant: developments and prospects. Front. Microbiol. 2018;9:2732. doi: 10.3389/fmicb.2018.02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheoran N., Valiya Nadakkakath A., Munjal V., Kundu A., Subaharan K., Venugopal V., Rajamma S., Eapen S.J., Kumar A. Genetic analysis of plant endophytic Pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiol. Res. 2015;173:66–78. doi: 10.1016/j.micres.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Xie S., Liu J., Gu S., Chen X., Jiang H., Ding T. Antifungal activity of volatile compounds produced by endophytic Bacillus subtilis DZSY21 against Curvularia lunata. Ann. Microbiol. 2020;70:2. doi: 10.1186/s13213-020-01553-0. [DOI] [Google Scholar]
- 60.Gao Z., Zhang B., Liu H., Han J., Zhang Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control. 2017;105:27–39. doi: 10.1016/j.biocontrol.2016.11.007. [DOI] [Google Scholar]
- 61.Rajkumar M., Ae N., Prasad M.N.V., Freitas H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010;28:142–149. doi: 10.1016/j.tibtech.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Dimopoulou A., Theologidis I., Benaki D., Koukounia M., Zervakou A., Tzima A., Diallinas G., Hatzinikolaou D.G., Skandalis N. Direct antibiotic activity of bacillibactin broadens the biocontrol range of Bacillus amyloliquefaciens MBI600. mSphere. 2021;6 doi: 10.1128/msphere.00376-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu H., Zeng Q., Yalimaimaiti N., Wang W., Zhang R., Yao J. Comprehensive genomic analysis of Bacillus velezensis AL7 reveals its biocontrol potential against Verticillium wilt of cotton. Mol. Genet. Genom. 2021;296:1287–1298. doi: 10.1007/s00438-021-01816-8. [DOI] [PubMed] [Google Scholar]
- 64.Yu X., Ai C., Xin L., Zhou G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011;47:138–145. doi: 10.1016/j.ejsobi.2010.11.001. [DOI] [Google Scholar]
- 65.Hosni T., Moretti C., Devescovi G., Suarez-Moreno Z.R., Fatmi M.B., Guarnaccia C., Pongor S., Onofri A., Buonaurio R., Venturi V. Sharing of quorum-sensing signals and role of interspecies communities in a bacterial plant disease. ISME J. 2011;5:1857–1870. doi: 10.1038/ismej.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kusari P., Kusari S., Lamshöft M., Sezgin S., Spiteller M., Kayser O. Quorum quenching is an antivirulence strategy employed by endophytic bacteria. Appl. Microbiol. Biotechnol. 2014;98:7173–7183. doi: 10.1007/s00253-014-5807-3. [DOI] [PubMed] [Google Scholar]
- 67.Achari G.A., Ramesh R. Characterization of bacteria degrading 3-hydroxy palmitic acid methyl ester (3OH-PAME), a quorum sensing molecule of Ralstonia solanacearum. Lett. Appl. Microbiol. 2015;60:447–455. doi: 10.1111/lam.12389. [DOI] [PubMed] [Google Scholar]
- 68.Lastochkina O., Baymiev A., Shayahmetova A., Garshina D., Koryakov I., Shpirnaya I., Pusenkova L., Mardanshin I.d., Kasnak C., Palamutoglu R. Effects of endophytic Bacillus subtilis and salicylic acid on post-harvest diseases (Phytophthora infestans, Fusarium oxysporum) development in stored potato tubers. Plants. 2020;9:76. doi: 10.3390/plants9010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pieterse C.M., van Wees S.C., van Pelt J.A., Knoester M., Laan R., Gerrits H., Weisbeek P.J., van Loon L.C. A novel signaling pathway controlling induced systemic resistance in arabidopsis. Plant Cell. 1998;10:1571–1580. doi: 10.1105/tpc.10.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mauch-Mani B., Baccelli I., Luna E., Flors V. Defense priming: an adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017;68:485–512. doi: 10.1146/annurev-arplant-042916-041132. [DOI] [PubMed] [Google Scholar]
- 71.Ward E.R., Uknes S.J., Williams S.C., Dincher S.S., Wiederhold D.L., Alexander D.C., Ahl-Goy P., Metraux J.P., Ryals J.A. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell. 1991;3:1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Loon L.C., Van Strien E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999;55:85–97. doi: 10.1006/pmpp.1999.0213. [DOI] [Google Scholar]
- 73.Van Oosten V.R., Bodenhausen N., Reymond P., Van Pelt J.A., Van Loon L.C., Dicke M., Pieterse C.M.J. Differential effectiveness of microbially induced resistance against herbivorous insects in arabidopsis. Mol. Plant Microbe Interact. 2008;21:919–930. doi: 10.1094/mpmi-21-7-0919. [DOI] [PubMed] [Google Scholar]
- 74.Niu D., Wang X., Wang Y., Song X., Wang J., Guo J., Zhao H. Bacillus cereus AR156 activates PAMP-triggered immunity and induces a systemic acquired resistance through a NPR1-and SA-dependent signaling pathway. Biochem. Biophys. Res. Commun. 2016;469:120–125. doi: 10.1016/j.bbrc.2015.11.081. [DOI] [PubMed] [Google Scholar]
- 75.Pérez-García A., Romero D., de Vicente A. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr. Opin. Biotechnol. 2011;22:187–193. doi: 10.1016/j.copbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Barka E.A., Belarbi A., Hachet C., Nowak J., Audran J.-C. Enhancement of in vitro growth and resistance to gray mould of Vitis vinifera co-cultured with plant growth-promoting rhizobacteria. FEMS Microbiol. Lett. 2000;186:91–95. doi: 10.1111/j.1574-6968.2000.tb09087.x. [DOI] [PubMed] [Google Scholar]
- 77.Sharma V.K., Nowak J. Enhancement of verticillium wilt resistance in tomato transplants by in vitro co-culture of seedlings with a plant growth promoting rhizobacterium (Pseudomonas sp. strain PsJN) Can. J. Microbiol. 1998;44:528–536. doi: 10.1139/w98-017. [DOI] [Google Scholar]
- 78.Brooks D.S., Gonzalez C., Appel D.N., Filer T.H. Evaluation of endophytic bacteria as potential biological control agents for oak wilt. Biol. Control. 1994;4:373–381. doi: 10.1006/bcon.1994.1047. [DOI] [Google Scholar]
- 79.M'Piga P., Bélanger R.R., Paulitz T.C., Benhamou N. Increased resistance to Fusarium oxysporum f. sp. radicis-lycopersici in tomato plants treated with the endophytic bacterium Pseudomonas fluorescens strain 63-28. Physiol. Mol. Plant Pathol. 1997;50:301–320. doi: 10.1006/pmpp.1997.0088. [DOI] [Google Scholar]
- 80.Benhamou N., Kloepper J.W., Quadt-Hallman A., Tuzun S. Induction of defense-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol. 1996;112:919–929. doi: 10.1104/pp.112.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fira D., Dimkić I., Berić T., Lozo J., Stanković S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018;285:44–55. doi: 10.1016/j.jbiotec.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 82.Wang Y., Zhang C., Liang J., Wang L., Gao W., Jiang J., Chang R. Surfactin and fengycin B extracted from Bacillus pumilus W-7 provide protection against potato late blight via distinct and synergistic mechanisms. Appl. Microbiol. Biotechnol. 2020;104:7467–7481. doi: 10.1007/s00253-020-10773-y. [DOI] [PubMed] [Google Scholar]
- 83.Poveda J., González-Andrés F. Bacillus as a source of phytohormones for use in agriculture. Appl. Microbiol. Biot. 2021;105(23):8629–8645. doi: 10.1007/s00253-021-11492-8. [DOI] [PubMed] [Google Scholar]
- 84.Wong W.S., Tan S.N., Ge L., Chen X., Yong J.W.H. In: Bacterial Metabolites in Sustainable Agroecosystem. Maheshwari D.K., editor. Springer International; Switzerland: 2015. The importance of phytohormones and microbes in biofertilizers: a critical review; pp. 105–158. [Google Scholar]
- 85.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol. Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen X.H., Koumoutsi A., Scholz R., Schneider K., Vater J., Süssmuth R., Piel J., Borriss R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2009;140:27–37. doi: 10.1016/j.jbiotec.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 87.Rückert C., Blom J., Chen X., Reva O., Borriss R. Genome sequence of B. amyloliquefaciens type strain DSM7(T) reveals differences to plant-associated B. amyloliquefaciens FZB42. J. Biotechnol. 2011;155:78–85. doi: 10.1016/j.jbiotec.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Steinke K., Mohite O.S., Weber T., Kovács Á T. Phylogenetic distribution of secondary metabolites in the Bacillus subtilis species complex. mSystems. 2021;6(21) doi: 10.1128/mSystems.00057-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xia L., Miao Y., Cao A., Liu Y., Liu Z., Sun X., Xue Y., Xu Z., Xun W., Shen Q., Zhang N., Zhang R. Biosynthetic gene cluster profiling predicts the positive association between antagonism and phylogeny in Bacillus. Nat. Commun. 2022;13:1023. doi: 10.1038/s41467-022-28668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blin K., Shaw S., Augustijn H.E., Reitz Z.L., Biermann F., Alanjary M., Fetter A., Terlouw B.R., Metcalf W.W., Helfrich E.J.N., van Wezel G.P., Medema M.H., Weber T. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023;51:W46–W50. doi: 10.1093/nar/gkad344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hashem A., Tabassum B., Fathi Abd_Allah E. Bacillus subtilis: a plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019;26:1291–1297. doi: 10.1016/j.sjbs.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gu X., Zeng Q., Wang Y., Li J., Zhao Y., Li Y., Wang Q. Comprehensive genomic analysis of Bacillus subtilis 9407 reveals its biocontrol potential against bacterial fruit blotch. Phytopathol. Res. 2021;3:4. doi: 10.1186/s42483-021-00081-2. [DOI] [Google Scholar]
- 93.Franco-Sierra N.D., Posada L.F., Santa-María G., Romero-Tabarez M., Villegas-Escobar V., Álvarez J.C. Bacillus subtilis EA-CB0575 genome reveals clues for plant growth promotion and potential for sustainable agriculture. Funct. Integr. Genom. 2020;20:575–589. doi: 10.1007/s10142-020-00736-x. [DOI] [PubMed] [Google Scholar]
- 94.Cawoy H., Debois D., Franzil L., De Pauw E., Thonart P., Ongena M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2015;8:281–295. doi: 10.1111/1751-7915.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ongena M., Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2008;16:115–125. doi: 10.1016/j.tim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Falardeau J., Wise C., Novitsky L., Avis T.J. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 2013;39:869–878. doi: 10.1007/s10886-013-0319-7. [DOI] [PubMed] [Google Scholar]
- 97.Zeng Q., Xie J., Li Y., Chen X., Gu X., Yang P., Hu G., Wang Q. Organization, evolution and function of fengycin biosynthesis gene clusters in the Bacillus amyloliquefaciens group. Phytopathol. Res. 2021;3:26. doi: 10.1186/s42483-021-00103-z. [DOI] [Google Scholar]
- 98.Zhang L., Sun C. Fengycins, cyclic lipopeptides from marine Bacillus subtilis strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 2018;84(18) doi: 10.1128/aem.00445-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim K., Lee Y., Ha A., Kim J.-I., Park A.R., Yu N.H., Son H., Choi G.J., Park H.W., Lee C.W., Lee T., Lee Y.-W., Kim J.-C. Chemosensitization of Fusarium graminearum to chemical fungicides using cyclic lipopeptides produced by Bacillus amyloliquefaciens strain JCK-12. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.02010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Romero D., de Vicente A., Rakotoaly R.H., Dufour S.E., Veening J.W., Arrebola E., Cazorla F.M., Kuipers O.P., Paquot M., Pérez-García A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant Microbe Interact. 2007;20:430–440. doi: 10.1094/mpmi-20-4-0430. [DOI] [PubMed] [Google Scholar]
- 101.Toral L., Rodríguez M., Béjar V., Sampedro I. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 2018;9:1315. doi: 10.3389/fmicb.2018.01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zihalirwa Kulimushi P., Argüelles Arias A., Franzil L., Steels S., Ongena M. Stimulation of fengycin-type antifungal lipopeptides in Bacillus amyloliquefaciens in the presence of the maize fungal pathogen Rhizomucor variabilis. Front. Microbiol. 2017;8:850. doi: 10.3389/fmicb.2017.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen X.H., Koumoutsi A., Scholz R., Borriss R. More than anticipated - production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J. Mol. Microbiol. Biotechnol. 2009;16:14–24. doi: 10.1159/000142891. [DOI] [PubMed] [Google Scholar]
- 104.Zeriouh H., Romero D., Garcia-Gutierrez L., Cazorla F.M., de Vicente A., Perez-Garcia A. The iturin-like lipopeptides are essential components in the biological control arsenal of Bacillus subtilis against bacterial diseases of cucurbits. Mol. Plant Microbe Interact. 2011;24:1540–1552. doi: 10.1094/mpmi-06-11-0162. [DOI] [PubMed] [Google Scholar]
- 105.Gu Q., Yang Y., Yuan Q., Shi G., Wu L., Lou Z., Huo R., Wu H., Borriss R., Gao X. Bacillomycin D produced by Bacillus amyloliquefaciens is involved in the antagonistic interaction with the plant-pathogenic fungus Fusarium graminearum. Appl. Environ. Microbiol. 2017;83(17) doi: 10.1128/AEM.01075-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moyne A.L., Shelby R., Cleveland T.E., Tuzun S. Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. J. Appl. Microbiol. 2001;90:622–629. doi: 10.1046/j.1365-2672.2001.01290.x. [DOI] [PubMed] [Google Scholar]
- 107.Xu H.-M., Rong Y.-J., Zhao M.-X., Song B., Chi Z.-M. Antibacterial activity of the lipopetides produced by Bacillus amyloliquefaciens M1 against multidrug-resistant Vibrio spp. isolated from diseased marine animals. Appl. Microbiol. Biotechnol. 2014:127–136. doi: 10.1007/s00253-013-5291-1. [DOI] [PubMed] [Google Scholar]
- 108.Bais H.P., Weir T.L., Perry L.G., Gilroy S., Vivanco J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- 109.Agarwal M., Dheeman S., Dubey R.C., Kumar P., Maheshwari D.K., Bajpai V.K. Differential antagonistic responses of Bacillus pumilus MSUA3 against Rhizoctonia solani and Fusarium oxysporum causing fungal diseases in Fagopyrum esculentum Moench. Microbiol. Res. 2017;205:40–47. doi: 10.1016/j.micres.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 110.Xie J., Shi H., Du Z., Wang T., Liu X., Chen S. Comparative genomic and functional analysis reveal conservation of plant growth promoting traits in Paenibacillus polymyxa and its closely related species. Sci. Rep. 2016;6 doi: 10.1038/srep21329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vater J., Herfort S., Doellinger J., Weydmann M., Dietel K., Faetke S., Lasch P. Fusaricidins from Paenibacillus polymyxa M-1, a family of lipohexapeptides of unusual complexity-a mass spectrometric study. J. Mass Spectrom. 2017;52:7–15. doi: 10.1002/jms.3891. [DOI] [PubMed] [Google Scholar]
- 112.Qiu S., Avula B., Guan S., Rao Ravu R., Wang M., Zhao J., Khan I.A., Hinchee M., Li X.C. Identification of fusaricidins from the antifungal microbial strain Paenibacillus sp. MS2379 using ultra-high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2019;1586:91–100. doi: 10.1016/j.chroma.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 113.Vater J., Herfort S., Doellinger J., Weydmann M., Borriss R., Lasch P. Genome mining of the lipopeptide biosynthesis of Paenibacillus polymyxa E681 in combination with mass spectrometry: discovery of the lipoheptapeptide paenilipoheptin. Chembiochem. 2018;19:744–753. doi: 10.1002/cbic.201700615. [DOI] [PubMed] [Google Scholar]
- 114.Cochrane S.A., Vederas J.C. Lipopeptides from Bacillus and Paenibacillus spp.: a gold mine of antibiotic candidates. Med. Res. Rev. 2016;36:4–31. doi: 10.1002/med.21321. [DOI] [PubMed] [Google Scholar]
- 115.Velkov T., Roberts K.D., Li J. Rediscovering the octapeptins. Nat. Prod. Rep. 2017;34:295–309. doi: 10.1039/c6np00113k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qian C.D., Liu T.Z., Zhou S.L., Ding R., Zhao W.P., Li O., Wu X.C. Identification and functional analysis of gene cluster involvement in biosynthesis of the cyclic lipopeptide antibiotic pelgipeptin produced by Paenibacillus elgii. BMC Microbiol. 2012;12:197. doi: 10.1186/1471-2180-12-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo Y., Huang E., Yuan C., Zhang L., Yousef A.E. Isolation of a Paenibacillus sp. strain and structural elucidation of its broad-spectrum lipopeptide antibiotic. Appl. Environ. Microbiol. 2012;78:3156–3165. doi: 10.1128/AEM.07782-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eastman A.W., Heinrichs D.E., Yuan Z.C. Comparative and genetic analysis of the four sequenced Paenibacillus polymyxa genomes reveals a diverse metabolism and conservation of genes relevant to plant-growth promotion and competitiveness. BMC Genom. 2014;15:851. doi: 10.1186/1471-2164-15-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li J., Jensen S.E. Nonribosomal biosynthesis of fusaricidins by Paenibacillus polymyxa PKB1 involves direct activation of a D-amino acid. Chem. Biol. 2008;15:118–127. doi: 10.1016/j.chembiol.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 120.Vater J., Niu B., Dietel K., Borriss R. Characterization of novel fusaricidins produced by Paenibacillus polymyxa-M1 using MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 2015;26:1548–1558. doi: 10.1007/s13361-015-1130-1. [DOI] [PubMed] [Google Scholar]
- 121.Kajimura Y., Kaneda M. Fusaricidins B, C and D, new depsipeptide antibiotics produced by Bacillus polymyxa KT-8: isolation, structure elucidation and biological activity. J. Antibiot. 1997;50:220–228. doi: 10.7164/antibiotics.50.220. [DOI] [PubMed] [Google Scholar]
- 122.Kurusu K., Ohba K., Arai T., Fukushima K. New peptide antibiotics LI-F03, F04, F05, F07, and F08, produced by Bacillus polymyxa. I. Isolation and characterization. J. Antibiot. 1987;40:1506–1514. doi: 10.7164/antibiotics.40.1506. [DOI] [PubMed] [Google Scholar]
- 123.Kuroda J., Fukai T., Nomura T. Collision-induced dissociation of ring-opened cyclic depsipeptides with a guanidino group by electrospray ionization/ion trap mass spectrometry. J. Mass Spectrom. 2001;36:30–37. doi: 10.1002/jms.101. [DOI] [PubMed] [Google Scholar]
- 124.Beatty P.H., Jensen S.E. Paenibacillus polymyxa produces fusaricidin-type antifungal antibiotics active against Leptosphaeria maculans, the causative agent of blackleg disease of canola. Can. J. Microbiol. 2002;48:159–169. doi: 10.1139/w02-002. [DOI] [PubMed] [Google Scholar]
- 125.Mousa W.K., Shearer C.R., Limay-Rios V., Zhou T., Raizada M.N. Bacterial endophytes from wild maize suppress Fusarium graminearum in modern maize and inhibit mycotoxin accumulation. Front. Plant Sci. 2015;6:805. doi: 10.3389/fpls.2015.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin S., Chen X., Xie L., Zhang Y., Zeng F., Long Y., Ren L., Qi X., Wei J. Biocontrol potential of lipopeptides produced by Paenibacillus polymyxa AF01 against Neoscytalidium dimidiatum in pitaya. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1188722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nikel P.I., Martínez-García E., de Lorenzo V. Biotechnological domestication of pseudomonads using synthetic biology. Nat. Rev. Microbiol. 2014;12:368–379. doi: 10.1038/nrmicro3253. [DOI] [PubMed] [Google Scholar]
- 128.Gu Y., Wang J., Xia Z., Wei H.-L. Characterization of a versatile plant growth-promoting rhizobacterium Pseudomonas mediterranea strain S58. Microorganisms. 2020;8:334. doi: 10.3390/microorganisms8030334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garrido-Sanz D., Meier-Kolthoff J.P., Göker M., Martín M., Rivilla R., Redondo-Nieto M. Genomic and genetic diversity within the Pseudomonas fluorescens complex. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weller D.M. Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology. 2007;97:250–256. doi: 10.1094/phyto-97-2-0250. [DOI] [PubMed] [Google Scholar]
- 131.Rosier A., Bishnoi U., Lakshmanan V., Sherrier D.J., Bais H.P. A perspective on inter-kingdom signaling in plant-beneficial microbe interactions. Plant Mol. Biol. 2016;90:537–548. doi: 10.1007/s11103-016-0433-3. [DOI] [PubMed] [Google Scholar]
- 132.Geudens N., Martins J.C. Cyclic lipodepsipeptides from Pseudomonas spp. - biological swiss-army knives. Front. Microbiol. 2018;9:1867. doi: 10.3389/fmicb.2018.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stringlis I.A., Zhang H., Pieterse C.M.J., Bolton M.D., de Jonge R. Microbial small molecules - weapons of plant subversion. Nat. Prod. Rep. 2018;35:410–433. doi: 10.1039/c7np00062f. [DOI] [PubMed] [Google Scholar]
- 134.Ramette A., Frapolli M., Saux M.F.-L., Gruffaz C., Meyer J.-M., Défago G., Sutra L., Moënne-Loccoz Y. Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin. Syst. Appl. Microbiol. 2011;34:180–188. doi: 10.1016/j.syapm.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 135.Almario J., Bruto M., Vacheron J., Prigent-Combaret C., Moënne-Loccoz Y., Muller D. Distribution of 2,4-diacetylphloroglucinol biosynthetic genes among the Pseudomonas spp. reveals unexpected polyphyletism. Front. Microbiol. 2017;8:1218. doi: 10.3389/fmicb.2017.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oni F.E., Geudens N., Adiobo A., Omoboye O.O., Enow E.A., Onyeka J.T., Salami A.E., De Mot R., Martins J.C., Höfte M. Biosynthesis and antimicrobial activity of pseudodesmin and viscosinamide cyclic lipopeptides produced by pseudomonads associated with the cocoyam rhizosphere. Microorganisms. 2020;8:1079. doi: 10.3390/microorganisms8071079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang W., Zhao Z., Zhang B., Wu X.G., Ren Z.G., Zhang L.Q. Posttranscriptional regulation of 2,4-diacetylphloroglucinol production by GidA and TrmE in Pseudomonas fluorescens 2P24. Appl. Environ. Microbiol. 2014;80:3972–3981. doi: 10.1128/aem.00455-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bangera M.G., Thomashow L.S. Identification and characterization of a gene cluster for synthesis of the polyketide antibiotic 2,4-diacetylphloroglucinol from Pseudomonas fluorescens Q2-87. J. Bacteriol. 1999;181:3155–3163. doi: 10.1128/jb.181.10.3155-3163.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moynihan J.A., Morrissey J.P., Coppoolse E.R., Stiekema W.J., O'Gara F., Boyd E.F. Evolutionary history of the phl gene cluster in the plant-associated bacterium Pseudomonas fluorescens. Appl. Environ. Microbiol. 2009;75:2122–2131. doi: 10.1128/AEM.02052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kwak Y.S., Han S., Thomashow L.S., Rice J.T., Paulitz T.C., Kim D., Weller D.M. Saccharomyces cerevisiae genome-wide mutant screen for sensitivity to 2,4-diacetylphloroglucinol, an antibiotic produced by Pseudomonas fluorescens. Appl. Environ. Microbiol. 2011;77:1770–1776. doi: 10.1128/AEM.02151-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stepanov A.A., Poshvina D.V., Vasilchenko A.S. 2,4-diacetylphloroglucinol modulates Candida albicans virulence. J. Fungi. 2022;8:1018. doi: 10.3390/jof8101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Troppens D.M., Dmitriev R.I., Papkovsky D.B., O'Gara F., Morrissey J.P. Genome-wide investigation of cellular targets and mode of action of the antifungal bacterial metabolite 2,4-diacetylphloroglucinol in Saccharomyces cerevisiae. FEMS Yeast Res. 2013;13:322–334. doi: 10.1111/1567-1364.12037. [DOI] [PubMed] [Google Scholar]
- 143.Berendsen R.L., van Verk M.C., Stringlis I.A., Zamioudis C., Tommassen J., Pieterse C.M.J., Bakker P.A.H.M. Unearthing the genomes of plant-beneficial Pseudomonas model strains WCS358, WCS374 and WCS417. BMC Genom. 2015;16:539. doi: 10.1186/s12864-015-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Navarro-Monserrat E.D., Taylor C.G. T6SS: a key to Pseudomonas's success in biocontrol? Microorganisms. 2023;11:2718. doi: 10.3390/microorganisms11112718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yin R., Cheng J., Lin J. The role of the type VI secretion system in the stress resistance of plant-associated bacteria. Stress Biol. 2024;4:16. doi: 10.1007/s44154-024-00151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Trunk K., Coulthurst S.J., Quinn J. A new front in microbial warfare—delivery of antifungal effectors by the type VI secretion system. Journal of Fungi. 2019;5:50. doi: 10.3390/jof5020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Trunk K., Peltier J., Liu Y.-C., Dill B.D., Walker L., Gow N.A.R., Stark M.J.R., Quinn J., Strahl H., Trost M., Coulthurst S.J. The type VI secretion system deploys antifungal effectors against microbial competitors. Nature Microbiology. 2018;3:920–931. doi: 10.1038/s41564-018-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moreira-Grez B., Tam K., Cross A.T., Yong J.W.H., Kumaresen D., Nevill P., Farrell M., Whiteley A.S. The bacterial microbiome associated with arid biocrusts and the biogeochemical influence of biocrusts upon the underlying soil. Front. Microbiol. 2019;10:e2143. doi: 10.3389/fmicb.2019.02143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Glick B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995;41:109–117. doi: 10.1139/m95-015. [DOI] [Google Scholar]
- 150.Zhao L., Xu Y., Lai X.H., Shan C., Deng Z., Ji Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz. J. Microbiol. 2015;46:977–989. doi: 10.1590/s1517-838246420140024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kushwaha P., Kashyap P.L., Srivastava A.K., Tiwari R.K. Plant growth promoting and antifungal activity in endophytic Bacillus strains from pearl millet (Pennisetum glaucum) Braz. J. Microbiol. 2020;51:229–241. doi: 10.1007/s42770-019-00172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Flores A., Diaz-Zamora J.T., Orozco-Mosqueda M.d.C., Chávez A., de los Santos-Villalobos S., Valencia-Cantero E., Santoyo G. Bridging genomics and field research: draft genome sequence of Bacillus thuringiensis CR71, an endophytic bacterium that promotes plant growth and fruit yield in Cucumis sativus L. 3 Biotech. 2020;10:220. doi: 10.1007/s13205-020-02209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang D., Xu H., Gao J., Portieles R., Du L., Gao X., Borroto Nordelo C., Borrás-Hidalgo O. Endophytic Bacillus altitudinis strain uses different novelty molecular pathways to enhance plant growth. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.692313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Khan M.S., Gao J., Chen X., Zhang M., Yang F., Du Y., Moe T.S., Munir I., Xue J., Zhang X. Isolation and characterization of plant growth-promoting endophytic bacteria Paenibacillus polymyxa SK1 from Lilium lancifolium. BioMed Res. Int. 2020;2020 doi: 10.1155/2020/8650957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Brígido C., Singh S., Menéndez E., Tavares M.J., Glick B.R., Félix M.d.R., Oliveira S., Carvalho M. Diversity and functionality of culturable endophytic bacterial communities in chickpea plants. Plants. 2019;8:42. doi: 10.3390/plants8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Maghboli Balasjin N., Maki J.S., Schläppi M.R., Marshall C.W. Plant growth-promoting activity of bacteria isolated from asian rice (Oryza sativa L.) depends on rice genotype. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.02787-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kumar K., Verma A., Pal G.Anubha, White J.F., Verma S.K. Seed endophytic bacteria of pearl millet (Pennisetum glaucum L.) promote seedling development and defend against a fungal phytopathogen. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.774293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Otieno N., Lally R., Kiwanuka S., Lloyd A., Ryan D., Germaine K., Dowling D. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lally R.D., Galbally P., Moreira A.S., Spink J., Ryan D., Germaine K.J., Dowling D.N. Application of endophytic Pseudomonas fluorescens and a bacterial consortium to Brassica napus can increase plant height and biomass under greenhouse and field conditions. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.02193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khan M.S., Gao J., Zhang M., Xue J., Zhang X. Pseudomonas aeruginosa Ld-08 isolated from Lilium davidii exhibits antifungal and growth-promoting properties. PLoS One. 2022;17 doi: 10.1371/journal.pone.0269640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pang J., Ryan M.H., Wen Z., Lambers H., Liu Y., Zhang Y., Tueux G., Jenkins S., Mickan B., Wong W.S., Yong J.W.H., Siddique K.H.M. Enhanced nodulation and phosphorus acquisition from sparingly-soluble iron phosphate upon treatment with arbuscular mycorrhizal fungi in chickpea. Physiol. Plantatum. 2023;13873 doi: 10.1111/ppl.13873. [DOI] [PubMed] [Google Scholar]
- 162.Xu J., Liu S., Song S., Guo H., Tang J., Yong J.W.H., Ma Y., Chen X. Arbuscular mycorrhizal fungi and the associated soil bacterial community influence decomposition under different soil phosphorus. Soil Biol. Biochem. 2018;120:181–190. [Google Scholar]
- 163.Montañez A., Blanco A.R., Barlocco C., Beracochea M., Sicardi M. Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in vitro. Appl. Soil Ecol. 2012;58:21–28. doi: 10.1016/j.apsoil.2012.02.009. [DOI] [Google Scholar]
- 164.Bhattacharjee R.B., Singh A., Mukhopadhyay S.N. Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: prospects and challenges. Appl. Microbiol. Biotechnol. 2008;80:199–209. doi: 10.1007/s00253-008-1567-2. [DOI] [PubMed] [Google Scholar]
- 165.Van Der Heijden M.G.A., Bardgett R.D., Van Straalen N.M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008;11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 166.Khan K.S., Joergensen R.G. Changes in microbial biomass and P fractions in biogenic household waste compost amended with inorganic P fertilizers. Bioresour. Technol. 2009;100:303–309. doi: 10.1016/j.biortech.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 167.Afzal I., Shinwari Z.K., Sikandar S., Shahzad S. Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019;221:36–49. doi: 10.1016/j.micres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 168.Sharma A., Johri B.N., Sharma A.K., Glick B.R. Plant growth-promoting bacterium Pseudomonas sp. strain GRP3 influences iron acquisition in mung bean (Vigna radiata L. Wilzeck) Soil Biol. Biochem. 2003;35:887–894. doi: 10.1016/S0038-0717(03)00119-6. [DOI] [Google Scholar]
- 169.Vansuyt G., Robin A., Briat J.F., Curie C., Lemanceau P. Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Mol. Plant Microbe Interact. 2007;20:441–447. doi: 10.1094/mpmi-20-4-0441. [DOI] [PubMed] [Google Scholar]
- 170.Sturz A.V., Christie B.R., Nowak J. Bacterial endophytes: potential role in developing sustainable systems of crop production. Crit. Rev. Plant Sci. 2000;19:1–30. doi: 10.1080/07352680091139169. [DOI] [Google Scholar]
- 171.Yong J.W.H., Letham D.S., Wong S.C., Farquhar G.D. Rhizobium-induced elevation in xylem cytokinin delivery in pigeonpea induces changes in shoot development & leaf physiology. Funct. Plant Biol. 2014;41:1323–1335. doi: 10.1071/FP14066. [DOI] [PubMed] [Google Scholar]
- 172.Taghavi S., Garafola C., Monchy S., Newman L., Hoffman A., Weyens N., Barac T., Vangronsveld J., van der Lelie D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl. Environ. Microbiol. 2009;75:748–757. doi: 10.1128/AEM.02239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tsavkelova E.A., Cherdyntseva T.A., Botina S.G., Netrusov A.I. Bacteria associated with orchid roots and microbial production of auxin. Microbiol. Res. 2007;162:69–76. doi: 10.1016/j.micres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 174.Sun Y., Cheng Z., Glick B.R. The presence of a 1-aminocyclopropane-1-carboxylate (ACC) deaminase deletion mutation alters the physiology of the endophytic plant growth-promoting bacterium Burkholderia phytofirmans PsJN. FEMS Microbiol. Lett. 2009;296:131–136. doi: 10.1111/j.1574-6968.2009.01625.x. [DOI] [PubMed] [Google Scholar]
- 175.Kaur M., Karnwal A. Screening of plant growth-promoting attributes bearing endogenous bacteria from abiotic stress resisting high altitude plants. J. Agric. Food Res. 2023;11 doi: 10.1016/j.jafr.2022.100489. [DOI] [Google Scholar]
- 176.Kieber J.J. Tribute to folke skoog: recent advances in our understanding of cytokinin biology. J. Plant Growth Regul. 2002;21:1–2. doi: 10.1007/s003440010059. [DOI] [PubMed] [Google Scholar]
- 177.Arkhipova T.N., Prinsen E., Veselov S.U., Martinenko E.V., Melentiev A.I., Kudoyarova G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil. 2007;292:305–315. doi: 10.1007/s11104-007-9233-5. [DOI] [Google Scholar]
- 178.Akhtar S.S., Mekureyaw M.F., Pandey C., Roitsch T. Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front. Plant Sci. 2020;10 doi: 10.3389/fpls.2019.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Letham DS, Palni LMS. The biosynthesis and metabolism of cytokinins. Ann. Rev. Plant Physiol. 1983;34:163–197. [Google Scholar]
- 180.Jameson P.E. Zeatin: The 60th anniversary of its identification. Plant Physiol. 2023;192:34–55. doi: 10.1093/plphys/kiad094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tarkowski P., Ge L., Yong J.W.H., Tan S.N. Analytical methods for cytokinins. Trends Anal. Chem. 2009;28:323–335. [Google Scholar]
- 182.Mekureyaw M.F., Pandey C., Hennessy R.C., Nicolaisen M.H., Liu F., Nybroe O., Roitsch T. The cytokinin-producing plant beneficial bacterium Pseudomonas fluorescens G20-18 primes tomato (Solanum lycopersicum) for enhanced drought stress responses. J. Plant Physiol. 2022;270 doi: 10.1016/j.jplph.2022.153629. [DOI] [PubMed] [Google Scholar]
- 183.Bhore S.J., Ravichantar N., Loh C.Y. Screening of endophytic bacteria isolated from leaves of sambung nyawa [Gynura procumbens (Lour.) Merr.]aleti for cytokinin-like compounds. Bioinformation. 2010;5:191–197. doi: 10.6026/97320630005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Albermann S., Linnemannstöns P., Tudzynski B. Strategies for strain improvement in Fusarium fujikuroi: overexpression and localization of key enzymes of the isoprenoid pathway and their impact on gibberellin biosynthesis. Appl. Microbiol. Biotechnol. 2013;97:2979–2995. doi: 10.1007/s00253-012-4377-5. [DOI] [PubMed] [Google Scholar]
- 185.Rana K.L., Kour D., Kaur T., Devi R., Yadav A.N., Yadav N., Dhaliwal H.S., Saxena A.K. Endophytic microbes: biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. Antonie Leeuwenhoek. 2020;113:1075–1107. doi: 10.1007/s10482-020-01429-y. [DOI] [PubMed] [Google Scholar]
- 186.Sandhya V., Shrivastava M., Ali S.Z., Sai Shiva Krishna Prasad V. Endophytes from maize with plant growth promotion and biocontrol activity under drought stress. Russ. Agric. Sci. 2017;43:22–34. doi: 10.3103/S1068367417010165. [DOI] [Google Scholar]
- 187.Shahzad R., Waqas M., Khan A.L., Asaf S., Khan M.A., Kang S.-M., Yun B.-W., Lee I.-J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016;106:236–243. doi: 10.1016/j.plaphy.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 188.Kamran M., Imran Q.M., Ahmed M.B., Falak N., Khatoon A., Yun B.-W. Endophyte-mediated stress tolerance in plants: a sustainable strategy to enhance resilience and assist crop improvement. Cells. 2022;11:3292. doi: 10.3390/cells11203292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gupta A, Rico-Medina A, Caño-Delgado AI. The physiology of plant responses to drought. Science. 2020;368(6488):266–269. doi: 10.1126/science.aaz7614. [DOI] [PubMed] [Google Scholar]
- 190.Ali S.Z., Sandhya V., Grover M., Linga V.R., Bandi V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant Interact. 2011;6:239–246. doi: 10.1080/17429145.2010.545147. [DOI] [Google Scholar]
- 191.Tiwari S., Prasad V., Chauhan P.S., Lata C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu W., Sikora E., Park S.-W. Plant growth-promoting rhizobacterium, Paenibacillus polymyxa CR1, upregulates dehydration-responsive genes, RD29A and RD29B, during priming drought tolerance in arabidopsis. Plant Physiol. Biochem. 2020;156:146–154. doi: 10.1016/j.plaphy.2020.08.049. [DOI] [PubMed] [Google Scholar]
- 193.White J.F., Kingsley K.L., Zhang Q., Verma R., Obi N., Dvinskikh S., Elmore M.T., Verma S.K., Gond S.K., Kowalski K.P. Review: endophytic microbes and their potential applications in crop management. Pest Manag. Sci. 2019;75:2558–2565. doi: 10.1002/ps.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ali M.A., Hafiz M.H.R., Salehin A., Hayashi S., Itoh K. NifH gene analysis of endophytic bacteria of sweet potato under various climatic locations. Research Journal of Biotechnology. 2022;17:90–93. doi: 10.25303/1702rjbt9093. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.