Abstract
The chemokine receptors CCR5 and CXCR4 function as the principal coreceptors for human immunodeficiency virus type 1 (HIV-1). Coreceptor function has also been demonstrated for a variety of related receptors in vitro. The relative contributions of CCR5, CXCR4, and other putative coreceptors to HIV-1 disease in vivo have yet to be defined. In this study, we used sequential primary isolates and recombinant strains of HIV-1 to demonstrate that CXCR4-using (X4) viruses emerging in association with disease progression are highly pathogenic in ex vivo lymphoid tissues compared to CXCR4-independent viruses. Furthermore, synthetic receptor antagonists that specifically block CXCR4-mediated entry dramatically suppressed the depletion of CD4+ T cells by recombinant and clinically derived X4 HIV-1 isolates. Moreover, in vitro specificity for the additional coreceptors CCR3, CCR8, BOB, and Bonzo did not augment cytopathicity or diminish sensitivity toward CXCR4 antagonists in lymphoid tissues. These data provide strong evidence to support the concept that adaptation to CXCR4 specificity in vivo accelerates HIV-1 disease progression. Thus, therapeutic intervention targeting the interaction of HIV-1 gp120 with CXCR4 may be highly valuable for suppressing the pathogenic effects of late-stage viruses.
Human immunodeficiency virus type 1 (HIV-1) typically uses CD4 and a coreceptor to gain entry into target cells (reviewed in reference 3). The chemokine receptors CCR5 and CXCR4 are considered the major coreceptors in HIV-1 infection, since most or all isolates exhibit specificity for one or both of these receptors. The discovery of these entry cofactors unraveled the molecular basis underlying the differences in cellular tropism in vitro among HIV-1 strains. Whereas non-syncytium-inducing (NSI) macrophagetropic viruses use CCR5 to enter primary CD4+ T cells and macrophages, syncytium-inducing (SI) viruses infect primary CD4+ T cells and T-cell lines via CXCR4. NSI CCR5-using (R5) viruses represent the predominant virus population early after infection and persist throughout all stages of disease, whereas SI CXCR4-using (X4) viruses and/or viruses with dual tropism (R5/X4) typically emerge later in disease in temporal association with rapid CD4+ T-cell decline and progression to AIDS (11, 23, 29, 47, 51, 54, 56). The importance of CCR5 as an HIV-1 coreceptor in vivo became apparent with the discovery that individuals homozygous for a mutant CCR5 allele (CCR5 Δ32) are highly resistant to HIV-1 infection (12, 27, 34, 46). These findings collectively suggest an important contribution of X4 variants to HIV-1 pathogenesis in later stages of disease, whereas R5 variants appear to be crucial for transmission and establishment of infection.
Numerous in vitro studies have revealed that HIV-1 coreceptor specificity is not limited to CXCR4 and CCR5. Several other human chemokine receptors and related orphan receptors exhibit coreceptor activity for select HIV-1 strains in cellular infection assays (reviewed in reference 3). In addition to CCR5 and CXCR4, the potential coreceptor repertoire of HIV-1 includes the human chemokine receptors CCR2b, CCR3, CCR8, CCR9, and CX3CR1 (V28) and the orphan receptors GPR1, BOB (GPR15), Bonzo (STRL33), APJ, and ChemR23. In addition, the leukotriene B4 receptor and the human cytomegalovirus-encoded, chemokine-like receptor US28 permit cellular entry by some HIV-1 isolates in vitro. To date, all HIV-1 isolates found to use any of these receptors also use CCR5 and/or CXCR4. Although there is evidence that many of these receptors are expressed in relevant CD4+ HIV-1 target cells (9, 13, 19, 26, 38, 39, 45, 52), the importance of these additional coreceptors for HIV-1 pathogenesis in vivo remains undefined.
The discovery of HIV-1 coreceptors provided fundamental insights into the mechanism of viral entry and viral evolution, rendering the virus-coreceptor interaction a novel and highly attractive target for therapeutic intervention in HIV-1 disease. In view of the large number of potential alternate coreceptors and the ability of the virus to change its coreceptor specificity readily through evolution within the env gene (16, 36, 53), defining the in vivo significance of the various coreceptors in HIV-1 transmission and disease progression is crucial for the design of such antiviral strategies.
The aim of this study was to elucidate the contributions of distinct coreceptor specificities to HIV-1 disease by evaluating these properties in human lymphoid histocultures. This ex vivo system recapitulates key aspects of viral pathogenesis within the tissue context of dominant HIV-1 replication sites in vivo (20, 21). We reported recently that the specificity of recombinant HIV-1 strains for CXCR4 promotes aggressive depletion of CD4+ T cells in this model system (40), which illustrates the potential in vivo impact of coreceptor specificity. The present study demonstrates that the X4 phenotype of recombinant HIV-1 strains and primary HIV-1 isolates is linked to a highly pathogenic phenotype in ex vivo lymphoid tissues, which is substantially inhibited by interfering with CXCR4-mediated entry. Furthermore, we show that promiscuous use of the additional coreceptor CCR3, CCR8, BOB, or Bonzo in vitro does not promote accelerated CD4+ T-cell depletion in ex vivo lymphoid tissues. Finally we provide evidence that in vitro coreceptor specificity does not necessarily reflect relevant coreceptor affinities in biological systems. Together, our findings further highlight the critical role of gp120-CXCR4 interactions in the depletion of CD4+ T cells by HIV-1 and provide direct evidence that antagonism of this interaction may have therapeutic value for suppressing the pathogenic effects of HIV-1 contributing to disease progression.
MATERIALS AND METHODS
Cell lines and receptor plasmids.
293T cells and COS-7 cells were cultured as described previously (5). Plasmids expressing CD4 (pCD4neo), CXCR4 (pCMVFCXCR4), CCR5 (pCMVFCCR5), CCR3 (pCDNA3-CCR3p), and CCR8 (pAW-CCR8F) were previously described (1, 5, 22, 40). Plasmids expressing BOB/GP15 (pcDNA-GP15) and Bonzo/STRL33 (pcDNA-STRL33) were a gift of Kuldeep Neote.
Construction of recombinant viruses.
NL4-3 provirus expression plasmid p4-14 (7), containing unique restriction sites flanking the C2 and V3 regions of the viral env gene, was used as the isogenic viral backbone for constructing C2- and V3 replacements. C2-V3 regions were obtained by PCR with oligonucleotide primers containing the StuI and NheI cloning sites from plasmids holding the envelope sequences of the primary isolates HIV-1 92UG037-8 and 92HT593-1 (AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health) and HIV-1 ADA (55). Cloned fragments were ligated into the StuI/NheI-digested backbone of plasmid p4-14, a gift of Bruce Chesebro. Construction of V3 recombinants 4-9 and 6-4 has been described elsewhere (5).
Preparation of virus stocks.
Molecular clones pNL4-3 and pNL-Luc-R−E− were obtained from Malcolm Martin and Ned Landau, respectively, via the AIDS Research and Reference Reagent Program. The molecular clones 49-5 (7), 242 (8), and 241 (8) were generous gifts from Bruce Chesebro. Infectious virus stocks were prepared as previously described (5, 22). The p24 Gag concentration was assessed by enzyme-linked immunosorbent assay (NEN Life Sciences, Boston, Mass.). Pseudotyped virus stocks carrying the luciferase gene with the various envelopes were prepared similarly by cotransfecting pNL-Luc-R−E− along with full-length proviral plasmids, and supernatant was harvested as described earlier (5). The primary isolates 1/85, 7/86, 12/86, and 11/89 (11) were expanded by infection of heterologous peripheral blood mononuclear cells (PBMC). PBMC from four different donors were isolated from whole blood buffy coats (Stanford Blood Bank, Palo Alto, Calif.) by Ficoll-Hypaque density gradient centrifugation (Histopaque 1077; Sigma, St. Louis, Mo.) and cultured for 48 h in medium (RPMI 1640; Mediatech, Washington, D.C.) supplemented with 10% fetal calf serum (Gemini Biological Products) and phytohemagglutinin (1 μg/ml; Sigma). The PBMC were then washed and cultured for at least 7 days in RPMI 1640 medium with 10% fetal calf serum and recombinant human interleukin-2 (5 U/ml; courtesy of Chiron Corp.).
Coreceptor utilization assay for single-round infection.
293T cells were seeded in six-well plates and transfected by the CaPO4 method (Promega, Madison, Wis.) with pCD4neo alone or in combination with pCMVFCXCR4, pCMVFCCR5, pcDNA-CCR3p, pAW-CCR8F, pcDNA-GP15, or pcDNA-STRL33 according to the manufacturer's instructions. COS-7 cells were transfected by the Lipofectamine method as described earlier (1, 5). After 24 h, cells were infected with pseudotype viruses, and luciferase expression was assessed 48 h postinfection by measuring enzymatic activity (relative light units) as instructed by the manufacturer (Analytic Luminescence Laboratory, San Diego, Calif.).
Selectivity of CXCR4 antagonists assessed in single-round infections.
COS-7 cells were transiently transfected with pCD4neo in combination with pCMVFCXCR4 or pCMVFCCR5 as described above. Transfected cells were inoculated with pseudotype reporter viruses in the presence and absence of the CXCR4 antagonist AMD3100 (250 nM) or T22 (1 μM) (custom preparation by American Peptide Company, Inc., Sunnyvale, Calif.) and subsequently infected with luciferase-expressing reporter viruses. Luciferase expression was assessed 48 h postinfection.
Infection of human lymphoid tissues ex vivo.
Human noninflammatory spleen tissue derived postsurgery or postautopsy (provided by National Diabetes Research Interchange, Philadelphia, Pa.) and tonsil tissue removed during routine tonsillectomy (provided by San Francisco General Hospital and Kaiser-San Francisco, San Francisco, Calif., and Kaiser-San Rafael, San Rafael, Calif.) were prepared and inoculated with HIV-1 strains (approximately 0.1 ng for primary isolates and up to 5 ng/block for HIV-1 recombinants) as described previously (21, 40). Productive HIV-1 infection was assessed by measuring the amount of p24 antigen that accumulated in the culture medium between the successive changes of medium; 10 to 14 days postinfection, cells were mechanically isolated from infected and uninfected control tissue and analyzed by flow cytometry (fluorescence-activated cell sorting [FACS]) (see below).
Inhibition of CXCR4-mediated entry in ex vivo lymphoid histocultures by CXCR4-specific antagonists.
At 12 to 24 h prior to infection, the culture medium was supplemented with the CXCR4 antagonist AMD3100 (250 nM) or T22 (1 μM). Virus stocks were diluted 1:1 with medium containing the relevant antagonist in the indicated concentrations and applied on top of the tissue blocks. Untreated tissue was infected with equal amount of virus diluted 1:1 with plain culture medium. Plain medium or medium containing the relevant antagonist was successively changed in the untreated or treated cultures. Viral replication was assessed by measuring the p24 concentration in the culture medium that accumulated between medium changes.
Assessment of CD4+ T-cell depletion by flow cytometry.
Dispersed cells from infected and uninfected lymphoid histocultures were stained for cell surface markers CD3, CD4, CD8, and CCR5 as described previously (40) by using anti-CD3 (clone SK7, allophycocyanin conjugated), anti-CD4 (clone SK3, fluorescein isothiocyanate conjugated), anti-CD8 (clone SK1, peridinin chlorophyll protein conjugated) (Becton Dickinson, San Jose, Calif.), and anti-CCR5 (clone 2D7, phycoerythrin conjugated; Pharmingen, San Diego, Calif.) monoclonal antibodies; 5,000 to 10,000 lymphocytes positive for CD3 surface marker were counted, and the data were analyzed with CellQuest software (Becton Dickinson). To facilitate comparison among experiments, CD4+ T-cell depletion was assessed by measuring the ratio of CD4+ to CD8+ T cells. This value was normalized to the CD4/CD8 ratio of control (uninfected) samples to yield the mean relative CD4/CD8 ratio. Results are reported as mean relative CD4/CD8 ratio with standard error of the mean (SEM).
RESULTS
Minimal changes in the HIV-1 envelope are associated with increased depletion of CD4+ T cells.
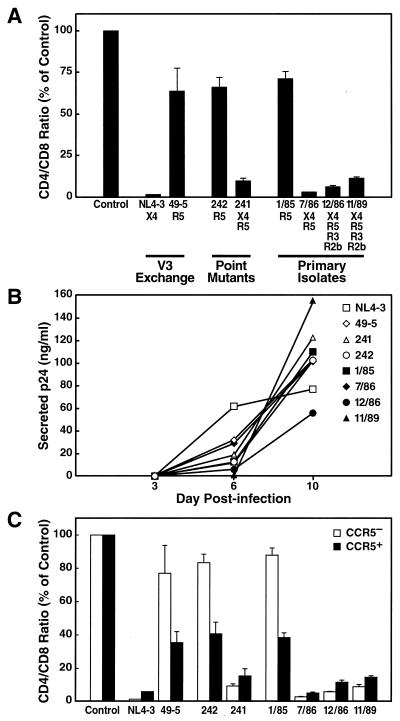
In a prior study, we found that CXCR4 coreceptor specificity alone is sufficient for HIV-1 to cause severe CD4+ T-cell depletion in human lymphoid histocultures (40). A cloned HIV-1 strain (NL4-3) that exhibits selectivity for CXCR4 (53) induced severe CD4+ T-cell depletion in ex vivo tonsil cultures, as represented by a profound decrease of the CD4/CD8 ratio among CD3+ lymphocytes, whereas an isogenic R5 strain (49-5) (7) induced only mild depletion (Fig. 1A). This finding was extended by assessing T-cell depletion in tonsil histocultures upon infection with a second isogenic virus pair, 242 and 241, which differ by a single amino acid substitution within the V3 loop (R5 [242] or R5/X4 [241]) (8, 53). The CCR5-restricted recombinant 242 caused only mild depletion in tonsil histocultures, whereas the dualtropic virus 241 severely depleted CD4+ T cells (Fig. 1A). Comparable virus-specific effects were observed in spleen tissue (data not shown). Viruses 241 and 242 replicated with similar kinetics in the histoculture system, as demonstrated by progressive p24 secretion into the medium (Fig. 1B). These results indicate that minimal changes in the env gene that enable HIV-1 to engage CXCR4 in addition to CCR5 result in dramatic enhancement of CD4+ T-cell depletion compared to the variant that is restricted to CCR5. In this setting of a dualtropic envelope, the aggressive T-cell depletion phenotype linked to CXCR4 usage dominates over the mild depletion phenotype linked to CCR5 usage.
FIG. 1.
Coreceptor specificity for CXCR4 by recombinant and primary isolates is linked with heightened virulence in ex vivo lymphoid tissues. (A) Previously determined coreceptor specificities (11, 40, 53) for each recombinant or primary virus are indicated. CD4+ T-cell depletion in ex vivo tonsil cultures was assessed by FACS analysis on day 10 postinfection; shown are mean relative CD4/CD8 ratios (n = 3) with SEM. (B) Viral replication was monitored by assessing the accumulation of p24 in the culture medium between successive medium changes on days 3, 6, and 10 postinfection. (C) In the same infection, T-cell depletion within the CCR5+ and CCR5− subsets of CD4+ T cells was analyzed by multiparameter FACS on day 10 postinfection. Shown are mean relative CD4/CD8 ratios (n = 3) with SEM for CCR5+ and CCR5− CD4+ T cells.
The emergence of X4 variants in vivo is associated with heightened virulence.
The results obtained with recombinants 242 and 241 may reflect the phenotypic evolution of HIV-1 in vivo. To test directly the hypothesis that the X4 variants emerging in patients accelerate CD4+ T-cell decline, we assessed the depletion potential of longitudinal primary HIV-1 isolates derived from an infected individual. These previously described biological isolates reflect the evolution from CCR5-restricted viruses early in infection to viruses with expanded coreceptor specificities, including CXCR4, with disease progression (11). CD4+ T-cell depletion and viral replication were assessed upon infection of tonsil histocultures with the sequential isolates. The earliest isolate tested, 1/85 (R5), induced mild depletion of CD4+ T cells, whereas all subsequent isolates (7/86 [R5/X4], 12/86 [R5/X4/R3/R2b], and 11/89 [R5/X4/R3/R2b]) that had adapted to use CXCR4 aggressively depleted CD4+ T cells in these cultures (Fig. 1A). Despite differences in coreceptor specificity (11), all of these primary isolates replicated to comparable levels, as measured by the amount of viral p24 protein released into the culture medium (Fig. 1B). No significant difference in the CD4+ T-cell depletion potential was found among isolate 7/86 (R5/X4) and the later isolates (12/86 and 11/89 [R5/X4/R2b/R3]), indicating that specificity for CXCR4 is sufficient to cause profound CD4+ T-cell depletion. These results strongly suggest that based on increased T-cell depletion in ex vivo lymphoid tissue, the adaptation of viral strains in vivo to use CXCR4 is likely to promote disease progression and rapid CD4+ T-cell loss.
Target cell specificity is linked to coreceptor expression.
Assessment of CXCR4 and CCR5 expression via flow cytometry in human spleen and tonsil specimens revealed differential expression of the viral coreceptors (24; J. C. Grivel, M. L. Penn, D. Eckstein, B. Schramm, R. F. Speck, N. W. Abbey, B. Herndier, L. Margolis, and M. A. Goldsmith, submitted for publication). Whereas CXCR4 typically is present on the vast majority of the CD4+ T lymphocytes, CCR5 is found on the surface of a minority of CD4+ T cells and varies with different donors. Therefore, most CD4+ T cells are potential targets for X4 HIV-1 strains, whereas fewer cells are predicted to be targets for R5 strains. To determine whether coreceptor specificity is linked to depletion of select subsets of CD4+ T cells by individual strains, multiparameter flow cytometry was used to detect the CD4, CD3, and CCR5 surface markers. This analysis revealed that R5 recombinant viruses (49-5 and 242) preferentially depleted the CCR5+ T-cell subset relative to the CCR5− subset, while X4 recombinants (NL4-3 and 241) potently depleted both subsets (Fig. 1C). Comparable results were obtained for the longitudinal primary isolates. The early CCR5-dependent isolate (1/85) preferentially depleted CCR5+ cells, whereas isolates with expanded coreceptor tropism, including CXCR4, depleted aggressively in both CCR5+ and CCR5− subsets (Fig. 1C). Therefore, the depletion patterns generated by the recombinant and primary strains correspond to the expression patterns of CCR5 and CXCR4 among CD4+ T cells.
Interference with CXCR4-mediated entry blocks CD4+ T-cell depletion.
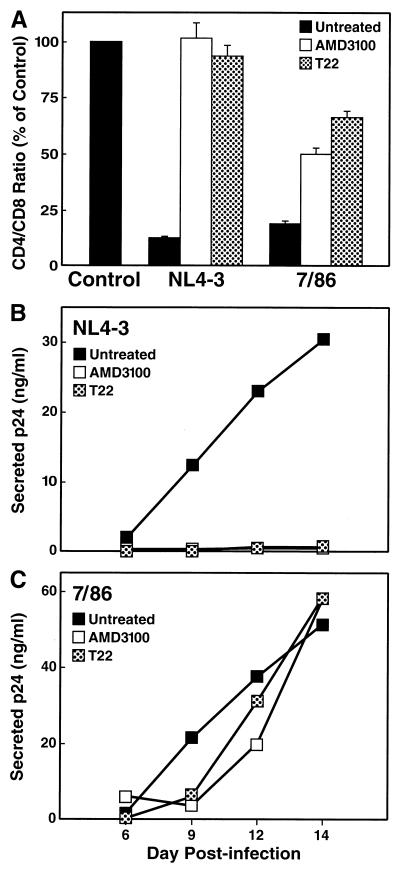
Based on these findings, we predicted that interfering with CXCR4-mediated entry by recombinant or primary HIV-1 isolates would attenuate the severe depletion of CD4+ T cells. To test this hypothesis, we infected spleen histocultures with the cloned X4 strain NL4-3 and the primary isolate 7/86 in the presence and absence of two independent CXCR4 antagonists, T22 (1 μM), a synthetic peptide that specifically and potently inhibits infection by X4 HIV-1 strains (37), and the bicyclam AMD3100, which is highly potent in blocking X4 HIV-1 replication in suspension cultures (14, 50). CD4+ T-cell depletion was assessed on day 14 postinfection. As expected, in the absence of CXCR4 antagonists, NL4-3 markedly depleted CD4+ T cells, as represented by a decrease of the CD4/CD8 ratio to 11% of the uninfected control sample (Fig. 2A). Both depletion (Fig. 2A) and viral replication (Fig. 2B) by NL4-3 were completely abrogated in the presence of either T22 or AMD3100. Furthermore, cellular depletion by the primary isolate 7/86 was substantially, although not completely, suppressed in the presence of T22 or AMD3100 (Fig. 2A). In the absence of the CXCR4 antagonists, isolate 7/86 decreased the CD4/CD8 ratio to 18% of uninfected control tissue, whereas CD4+ T-cell depletion in the presence of T22 or AMD3100 was markedly attenuated, as indicated by CD4/CD8 ratio of 66 or 47%, respectively, of uninfected control cultures (Fig. 2A), demonstrating the impact of CXCR4 usage on the cytopathic effect of this isolate.
FIG. 2.
The CXCR4 antagonists T22 and AMD3100 abrogate replication and depletion of the cloned HIV-1 strain NL4-3 and substantially diminish virulence of the primary isolate 7/86. (A) CD4+ T-cell depletion in ex vivo cultures of human spleen tissue was assessed on day 14 postinfection in the absence or presence of the CXCR4 antagonist AMD3100 (250 nM) or T22 (1 μM) in the culture medium. Shown are mean relative CD4/CD8 ratios (n = 3) with SEM. Viral replication was monitored for NL4-3 (B) and 7/86 (C) by assessing the accumulation of p24 in the culture medium between medium changes on days 6, 9, 12, and 14 postinfection.
Compared with the influence on cellular depletion, the effect of the CXCR4 antagonists on viral replication of the primary isolate was more modest, causing only a slight delay of p24 output compared to untreated infections (Fig. 2C). The molecular basis of this discordance is uncertain, although it may represent selective outgrowth of CXCR4-independent and/or inhibitor-insensitive strains within the uncloned isolate. In any event, this finding implies that virus replication per se does not necessarily equate to pathogenesis and emphasizes the importance of monitoring both virologic and cellular responses to a potential therapy.
Collectively these depletion studies with the CXCR4 antagonists confirm that the specificity for CXCR4 is a major determinant for the aggressive virulence of recombinant and primary isolates, and they provide additional evidence that the emergence of viruses with CXCR4 specificity contributes directly to acceleration of CD4+ T-cell depletion in vivo.
Contribution of additional coreceptors to CD4+ T-cell depletion.
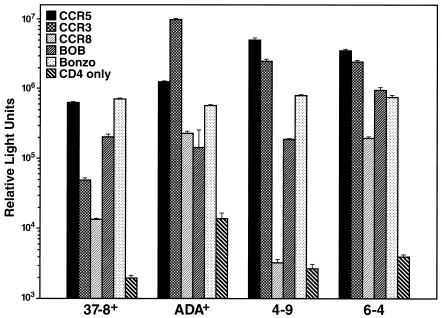
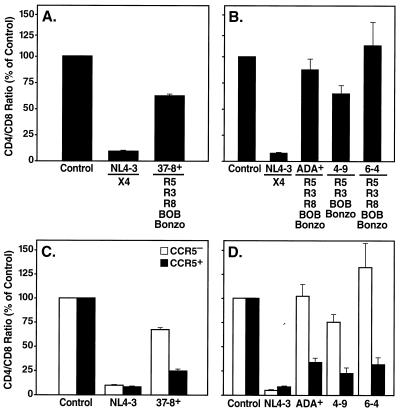
HIV-1 coreceptor usage is not strictly limited to CXCR4 and CCR5, since a number of other human chemokine receptors and related orphan receptors have been shown to serve as coreceptors for HIV-1 in vitro (reviewed in reference 3). To investigate the contribution of these additional coreceptors to pathogenicity of HIV-1, we assessed CD4+ T-cell depletion in lymphoid histocultures upon infection with multitropic viruses. For these experiments we used recombinant HIV-1 strains differing only in coreceptor determinants in the env gene in order to assign a singular causative role to coreceptor preferences if enhanced T-cell depletion was observed. Isogenic viruses were constructed by subcloning select envelope determinants of coreceptor specificity into a constant viral background (NL4-3). NL4-3 recombinants encoding the C2-V3 regions of the NSI R5 primary isolate 92UG037.8 and NSI R5 HIV-1 ADA (ADA+ and 37-8+) were characterized for their coreceptor utilization patterns in a standard single-round infection assay. 293T cells transiently expressing CD4 alone or in combination with various coreceptors were infected with luciferase reporter viruses pseudotyped with the corresponding envelopes of viruses ADA+ and 37-8+. Both recombinants efficiently infected cells expressing CCR5 along with CD4, whereas only background level signals were obtained with CD4 alone (Fig. 3). In addition, 37-8+ infected cells via Bonzo and to a somewhat lesser extent via BOB, CCR3, and CCR8 (Fig. 3). The ADA+ recombinant exhibited specificity for CCR5, CCR3, CCR8, BOB, and Bonzo (Fig. 3). The relative signals obtained for infections with different coreceptors varied somewhat among multiple assays, but the utilization patterns were consistent. We also used two R5 NL4-3 recombinants that we recently described, 4-9 and 6-4, encoding the V3 regions of colon-derived HIV-1 envelope sequences (5). Both 4-9 and 6-4 used CCR5 and CCR3 in the entry assay, whereas 6-4, but not 4-9, exhibited entry via CCR8 (Fig. 3). In addition, both viruses exhibited specificity for BOB and Bonzo (Fig. 3). To determine whether the ability to enter cells via additional coreceptors in vitro affects the depletion potential of such viruses in biological systems, we infected tonsil and spleen histocultures with these V3 exchange mutants and assessed CD4+ T-cell depletion. The recombinant 37-8+ only mildly depleted CD4+ T-cells in ex vivo spleen cultures, whereas the CXCR4-specific parent NL4-3 profoundly depleted CD4+ cells in the same tissues (Fig. 4A). In analogous experiments performed with tonsil tissues, the multitropic recombinants ADA+, 4-9, and 6-4 likewise displayed a modest T-cell depletion potential in contrast to the severe depletion of CD4+ T cells by NL4-3 (Fig. 4B). All NL4-3 recombinants replicated to levels comparable to those for NL4-3, as assessed by p24 release in the culture supernatant (see the legend to Fig. 4). These results demonstrate that the ability of R5 strains of HIV-1 to enter cells via CCR3, CCR8, BOB, or Bonzo in vitro does not correspond to enhanced T-cell depletion potential as assessed in ex vivo spleen and tonsil histocultures.
FIG. 3.
Coreceptor utilization pattern of gp120 exchange mutants. 293T cells transiently expressing CD4 alone or in combination with the coreceptor CCR5, CCR3, CCR8, BOB, or Bonzo were infected with a pseudotype reporter virus carrying the envelope of the recombinant ADA+, 37-8+, 4-9, or 6-4; 48 h postinfection, luciferase expression was assessed by measuring enzymatic activity (relative light units). The relative signals obtained for different coreceptors varied somewhat between multiple experiments, but the coreceptor pattern for a given virus was consistent.
FIG. 4.
CD4+ T-lymphocyte depletion by multitropic HIV-1 recombinants. (A) CD4+ T-cell depletion in human spleen tissue infected with HIV-1 recombinant NL4-3 or 37-8+. Shown are mean relative CD4/CD8 ratios with SEM determined on day 14 postinfection. (B) CD4+ T-cell depletion in human tonsil tissue infected by the recombinants NL4-3, ADA+, 6-4, and 4-9 as measured on day 12 postinfection. (C and D) Subset analysis of data from panels A and B, respectively, with CD4+ T-cell depletion stratified into CCR5+ and CCR5− subsets. The results shown are representative of two to three experiments performed with spleen and tonsil tissues from different donors for the viruses. All viruses replicated to similar levels in the corresponding spleen (total of accumulated p24 in panel A, 134 [NL4-3] and 127 [37-8+] ng/ml) and tonsil (total of accumulated p24 in panel B, 126 [NL4-3], 212 [ADA+], 196 [4-9], and 160 [6-4] ng/ml) tissue.
As another strategy for determining the pathogenic significance of alternate coreceptor usage, we measured specific cellular subset depletion patterns as described earlier. To this end, CD4+ T cells from experiments shown in Fig. 4A and B were analyzed for expression of CCR5. Viruses 37-8+, ADA+, 4-9, and 6-4 exhibited preferential depletion of CCR5+ CD4+ T cells in spleen (Fig. 4C) and tonsil (Fig. 4D) tissues irrespective of their ability to use additional coreceptors in vitro, in contrast to the depletion of both CCR5+ and CCR5− CD4+ T-cell subsets by NL4-3 (Fig. 1C, 4C, and 4D).
Overall, these results demonstrate that the ability to use CCR3, CCR8, BOB, and Bonzo in in vitro entry assays does not alter the preference of R5 viruses to deplete the CCR5+ subset of CD4+ T cells, suggesting that the use of alternate coreceptors does not confer additional virulence in ex vivo lymphoid tissues.
Blockade of CXCR4 abrogates replication and depletion by a multitropic HIV-1.
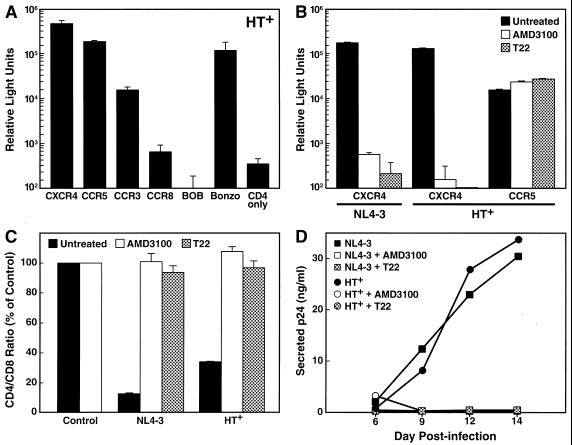
Our results demonstrated that aggressive depletion of CD4+ T cells by HIV-1 infection in lymphoid histocultures requires the use of CXCR4 as a coreceptor, whereas specificity for CCR5 in combination with additional coreceptors results in mild depletion of CD4+ T cells. We sought to confirm the linkage between CXCR4 specificity and high pathogenicity in the context of a multitropic HIV-1 recombinant. We therefore created the recombinant HT+, encoding the C2-V3 envelope regions of the primary HIV-1 isolate 92HT593.1 in the NL4-3 backbone. The in vitro coreceptor utilization pattern of HT+ was determined in the single-round luciferase reporter assay using COS-7 cells transiently expressing CD4 and the respective coreceptors. HT+ exhibited specificity for CXCR4, CCR5, and Bonzo, with less pronounced usage of CCR3 (Fig. 5A); usage of CCR8 and BOB was in the range of background levels obtained with CD4 alone. When spleen cultures were infected with HT+, strong depletion of CD4+ T cells was observed, which was similar to the effect of the NL4-3 control virus (Fig. 5C). To determine the relative contribution of individual coreceptors to this effect, we performed infections in the presence and absence of two independent CXCR4 antagonists, T22 (37) (1 μM) and AMD3100 (14, 50) (250 nM). Somewhat surprisingly, cellular depletion and viral replication by the multitropic HT+ was completely suppressed in the presence of T22 and AMD3100, similar to results for the CXCR4-restricted NL4-3 (Fig. 5C and D). In single-round infection assays using luciferase reporter viruses, we verified the selective effects of both antagonists in the concentrations used in the histoculture infections. Infection of COS-7 cells transiently expressing CD4 and CXCR4 by NL4-3 and HT+ was completely blocked in the presence of either T22 (1 μM) or AMD3100 (250 nM), while infection of CD4+ CCR5+ cells by HT+ was not affected (Fig. 5B). Together, these findings imply that the CD4+ T-cell depletion and replication by HT+ in ex vivo lymphoid tissue is solely a result of its specificity for CXCR4. Therefore, the qualitative ability to enter cells via certain receptors in vitro does not necessarily enable the virus to infect productively or to deplete lymphocytes via these receptors in biological systems in which quantitative or relative usage may be an important variable.
FIG. 5.
Inhibitory effect of CXCR4 antagonists on CD4+ T-cell depletion and replication by the multitropic HIV-1 recombinant HT+. (A) In vitro coreceptor specificity of HT+ assessed by infection of COS-7 cells transiently expressing CD4 alone or in combination with the corresponding coreceptor. Pseudotype reporter viruses expressing luciferase and carrying the envelope of HT+ were used, and luciferase activity was measured. (B) Selective inhibition of CXCR4-mediated entry by CXCR4 antagonists AMD3100 and T22. Transfected COS-7 cells were infected with the HT+ reporter viruses in the absence or presence of the antagonist AMD3100 (250 nM) or T22 (1 μM). (C) CD4+ T-cell depletion in spleen histocultures upon infection with replication-competent NL4-3 or HT+ in the absence or presence of the antagonist AMD3100 (250 nM) or T22 (1 μM). Shown are mean relative CD4/CD8 ratios with SEM determined on day 14 postinfection. (D) Viral replication of NL4-3 and HT+ in the experiment shown in panel C was monitored by assessing the accumulation of p24 in the culture medium between successive changes of medium.
DISCUSSION
A compelling relationship between HIV-1 coreceptor specificity and disease pathogenesis has been suggested by prior epidemiologic evidence (11, 12, 27, 28, 34, 46, 47). A valuable system in which to elucidate this relationship directly is ex vivo human lymphoid histocultures (20, 21) since lymphoid tissues are crucial sites of viral replication and immune system destruction in vivo. We have shown that HIV-1 envelope specificity for CXCR4 is linked to aggressive depletion of the CD4+ T-cell population in such cultures (40). The profound influence of coreceptor specificity on virulence appears to be largely attributable to differences in the availability of target cells expressing individual coreceptors (24; Grivel et al., submitted).
In this study, we used lymphoid histocultures to elucidate further the linkage between coreceptor specificity and the pathogenic potential of HIV-1. Our earlier work demonstrated that subtle changes in the HIV-1 envelope glycoprotein, gp120, are sufficient to drive drastic changes in coreceptor specificity (53). We found here that a single amino acid substitution causing a shift from R5 to R5/X4 phenotype dramatically enhanced HIV-1-induced pathogenicity as defined by CD4+ T-cell depletion, which parallels the typical viral evolution pattern in vivo. Thus, the heightened virulence linked to the X4 phenotype is dominantly expressed, as has been observed in another model system (36). To verify the biological relevance of these experimental viruses, we also assessed the pathogenic potential of primary viral isolates that had been obtained longitudinally from an HIV-1-infected individual who eventually progressed to AIDS (11). This panel of sequential clinical isolates reflects the typical broadening of coreceptor specificity in association with disease progression. The results of this analysis demonstrate that the expansion to R5/X4 phenotype in vivo is similarly linked to a highly pathogenic phenotype for lymphoid tissues ex vivo, confirming that the emergence of viral specificity for CXCR4 likely accelerates CD4+ T-cell depletion in vivo.
The interaction between HIV-1 and its coreceptor is an attractive target for antiviral therapy, since it may be possible to disable HIV-1 infection at the level of initial cellular entry. Both the remarkable resistance to HIV-1 infection found for individuals homozygous for a CCR5-null allele (12, 27, 32, 34, 46) and the inhibitory effects of the natural receptor ligands or modified chemokines in vitro (4, 6, 10, 33) emphasize the possible therapeutic value of targeting virus-coreceptor interactions. However, the potential of HIV-1 to evade antiviral strategies was suggested by the recent observation that anti-CCR5 agents can select for variants with CXCR4 specificity in a xenotransplant model of HIV disease (36). Our observation that CXCR4 utilization is a key factor in HIV-1 virulence further emphasizes the need to consider this as a possible target for antiviral therapy. Certain synthetic CXCR4 antagonists selectively and potently block CXCR4-mediated cell entry of HIV-1 in vitro (14, 37, 50). Here we investigated the effects of antagonists on recombinant and primary viral isolates in ex vivo cultures of lymphoid tissue. In the presence of the CXCR4 antagonists T22 (37) and AMD3100 (14, 50), replication and CD4+ T-cell depletion by the X4 clone NL4-3 were completely abrogated and the pathogenicity of the R5/X4 primary isolate 7/86 was markedly suppressed, demonstrating that interference with CXCR4-mediated entry dramatically reduces pathogenicity of X4 HIV-1 isolates in lymphoid tissues. Together these data provide additional evidence that specificity for CXCR4 is a major determinant of the greater virulence of viruses from late-stage patients, and they underscore the causative relationship between viral specificity for CXCR4 and rapid CD4+ T-cell decline in vivo. Furthermore, these findings emphasize the potential therapeutic value of blocking CXCR4-mediated entry in combating HIV-1 disease progression in vivo.
Interestingly, replication of the primary isolate 7/86 was only slightly delayed in the presence of the CXCR4 antagonist T22 or AMD3100 despite marked suppression of CD4+ T-cell depletion. Based on the reported dualtropic character of isolate 7/86 (11), which likely reflects a heterogeneous virus population, robust viral replication and rather mild depletion of CD4+ T cells in the presence of the inhibitor might be due to the outgrowth of variants with specificity for CCR5 that are less aggressive in depleting CD4+ T cells. This observation is in agreement with a recent study performed in vitro, demonstrating that the X4 phenotype of clinical isolates was completely suppressed in the presence of the CXCR4 antagonist AMD3100, resulting in the outgrowth of R5 variants (16). Incomplete inhibition of X4 strains as a result of differences in receptor affinity and/or receptor engagement also cannot be excluded, as it might contribute to some extent to the residual T-cell depletion and the overall viral replication potential of isolate 7/86 observed here. Precedence for differential engagement of CXCR4 as a cause of CXCR4 ligand or antagonist insensitivity was shown in an earlier study by Schols et al., demonstrating that a T-cell line-adapted X4 HIV-1 strain was able to overcome the inhibitory effects of SDF-1 and AMD3100 through the accumulation of mutations in gp120 that preserved its dependence on CXCR4 (49). These observations are potentially of great interest with respect to the development of potent antiviral agents targeting virus-coreceptor interactions but require further investigation including a broader test range of primary isolates.
In addition to CCR5 and CXCR4, more than 10 chemokine receptors and related orphan receptors have been shown to confer HIV-1 entry in vitro (reviewed in reference 3). Although the in vivo expression pattern of these additional coreceptors is not well defined, preliminary reports based mainly on detection of mRNA expression levels have suggested that most of these candidate receptors are expressed on CD4+ HIV-1 target cells (9, 13, 19, 26, 38, 39, 45, 52). In general, alternative coreceptors are irregularly used by HIV-1 and appear to be less efficient than CCR5 and CXCR4 (5, 15, 42, 45, 57). Contradictory results for HIV-1 coreceptor specificities of certain HIV-1 strains have been obtained depending on the assay system used (2, 15, 42, 44). Furthermore, differences in coreceptors expression levels can influence coreceptor activity (30, 41, 44). Therefore, most in vitro studies on coreceptor usage should be interpreted qualitatively rather than quantitatively. In view of the high mutation rate of HIV-1, high viral turnover in vivo, and the resulting potential of HIV-1 to escape coreceptor antagonism by simply changing its coreceptor specificities, it is critical to elucidate the in vivo relevance of alternative coreceptors to the natural history of disease and/or possible therapeutic intervention. We addressed this issue by assessing the lymphocyte depletion potential of isogenic viruses differing only in their envelope-specified in vitro coreceptor utilization patterns, which allowed us to focus on cytopathic effects driven by coreceptor specificity. The recombinants used were specific for the principal coreceptor CCR5 and displayed additional usage of CCR3, CCR8, BOB, or Bonzo based on conventional single-round luciferase reporter assays in vitro. Infection of lymphoid tissues with these multitropic viruses resulted in rather mild CD4+ T-cell depletion in contrast to severe CD4+ T-cell depletion caused by X4 strains. Another strategy for detecting changes in target cell specificity is the stratification of CD4+ T cells based on coreceptor expression to seek differences in the subsets of cells depleted by such viruses. Subset analysis based on CCR5 expression following infection with these multitropic viruses revealed no evidence for an expansion of the target cell population as a result of additional specificity for alternate coreceptors. Therefore, CCR5 and CXCR4 appear to be the dominant coreceptors influencing killing of T cells in these lymphoid tissues.
We also extended the analysis to a multitropic gp120 recombinant HIV-1 (HT+) with specificity for CXCR4, CCR5, CCR3, and Bonzo in vitro and high pathogenic potential in ex vivo lymphoid tissues. To uncover the coreceptor specificities that are responsible in this multitropic context for high virulence in lymphoid cultures, we sought to inhibit viral entry via CXCR4. Somewhat surprisingly, this multitropic strain displayed great sensitivity toward two CXCR4 antagonists, T22 (37) and the bicyclam AMD3100 (14, 50). Both CD4+ T-cell depletion and viral replication were completely abrogated in the presence of the inhibitors, similar to the effects obtained with the CXCR4-restricted strain NL4-3. A dominant interaction between the CXCR4 antagonists and the multitropic envelope that would result in interference with viral entry via coreceptors other than CXCR4 can be excluded as explanation for this effect since CCR5−, CCR3−, or Bonzo-mediated cellular entry was not abrogated by either T22 or AMD3100 in vitro (Fig. 5B, data not shown, and reference 31). These results imply that replication and pathogenicity of the recombinant HT+ in lymphoid tissues derive largely from CXCR4-dependent processes, despite additional coreceptor specificity in vitro. Since CCR5 is clearly expressed in these tissues at levels that confer productive infection by CCR5-restricted viruses, the coreceptor utilization of this particular virus in vitro apparently does not reflect its coreceptor preferences in the relevant biological context. This discordance leads us to conclude that coreceptor activity as assessed in highly sensitive cell-cell fusion and/or reporter entry assays does not necessarily reflect relevant coreceptor affinities of HIV-1 in vivo, since these conventional assays do not allow assessment of the relative preference of one coreceptor versus another. Therefore, although virus phenotyping is of some clinical and scientific utility, the results must be viewed as qualitative and thus be interpreted with caution.
Overall, no evidence was found in our studies for a contribution by the additional coreceptors to viral pathogenicity and/or replication in lymphoid tissues cultured ex vivo. Similarly, a recent study found that in vitro usage of additional coreceptors other than CCR5 or CXCR4 was irrelevant for replication of HIV-1 in activated human peripheral blood cultures (58). In addition, viruses isolated longitudinally from an HIV-1-infected individual who is homozygous for the CCR5 Δ32 null allele exhibited exclusive specificity for CXCR4 (35). These findings collectively argue that additional coreceptors should not preclude the development of therapeutic strategies that aim to interfere with HIV-1 cellular entry. However, it remains possible that alternative coreceptors play important roles for viral replication and pathogenicity in select tissue compartments where HIV-1 infection is manifest, such as thymus, brain, and mucosal surfaces (17, 18, 25, 43, 48).
In conclusion, we have shown with recombinant and biological strains that the in vivo adaptation toward CXCR4 use is linked to heightened virulence in lymphoid tissues as detected in ex vivo histocultures. Furthermore, interference with CXCR4-mediated entry significantly reduces the aggressive CD4+ T-cell depletion potential of recombinant and primary HIV-1 in lymphoid tissues, demonstrating the impact of CXCR4 specificity on pathogenicity. Together, these findings provide strong evidence that the acquisition of CXCR4 specificity in vivo directly contributes to acceleration of HIV-1 disease progression. No evidence was found that coreceptors other than CXCR4 and CCR5 contribute to HIV-1 pathogenicity in ex vivo lymphoid tissue. Although potential side effects must be examined carefully, these findings indicate that specific inhibition of CXCR4-mediated cellular entry may be highly valuable for suppressing HIV-1 pathogenicity in key sites of viral replication in vivo. A combined therapeutic strategy targeting the principal HIV-1 coreceptors CCR5 and CXCR4 in conjunction with established antiviral agents affecting other steps in the viral life cycle should potently combat HIV-1 infection and disease.
ACKNOWLEDGMENTS
B.S. and M.L.P. contributed equally to this work.
We thank Bruce Chesebro, Malcolm Martin, Ned Landau, and Kuldeep Neote for kindly providing plasmids and Dee J. Holthe, Cecilia Stewart, Claudette Delphis, Ursula Perotti, Nancy W. Abbey, Mark D. Weinstein, and the surgical staffs at Kaiser hospitals (San Rafael and San Francisco, Calif.) and San Francisco General Hospital for generous assistance in obtaining posttonsillectomy samples.
We acknowledge the technical assistance of Eric Wieder and Lisa Gibson in the conduct of these experiments and the assistance of Heather Gravois, John Carroll, and Neile Shea in preparation of the manuscript.
M.L.P. and S.Y.C. were supported by the Biomedical Sciences Graduate Program and the National Institutes of Health Medical Scientist Training Program at UCSF. R.F.S. was supported by the UCSF-GIVI Center for AIDS Research, B.S. was supported by the Boehringer Ingelheim Fund, and R.I.C. was supported by the Irene Diamond Fund. This work was supported in part by NIH grant R01-AI43695 (M.A.G.) and the J. David Gladstone Institutes (M.A.G.).
REFERENCES
- 1.Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. doi: 10.1126/science.274.5294.1924. [DOI] [PubMed] [Google Scholar]
- 2.Bazan H, Alkhatib G, Broder C, Berger E. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 4.Bleul C, Farzan M, Hyeryun C, Parolin C, Clark-Lewis I, Sodroski J, Springer T. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 5.Chan S Y, Speck R F, Power C, Gaffen S L, Chesebro B, Goldsmith M A. V3 recombinants indicate a central role for CCR5 as a coreceptor in tissue infection by human immunodeficiency virus type 1. J Virol. 1999;73:2350–2358. doi: 10.1128/jvi.73.3.2350-2358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J D, Bai X, Yang A G, Cong Y, Chen S Y. Inactivation of HIV-1 chemokine co-receptor CXCR-4 by a novel intrakine strategy. Nat Med. 1997;3:1110–1116. doi: 10.1038/nm1097-1110. [DOI] [PubMed] [Google Scholar]
- 7.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesebro B, Wehrly K, Nishio J, Perryman S. Mapping of independent V3 envelope determinants of human immunodeficiency virus type 1 macrophage tropism and syncytium formation in lymphocytes. J Virol. 1996;70:9055–9059. doi: 10.1128/jvi.70.12.9055-9059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe H, Farzan M, Konkel M, Martin K, Sun Y, Marcon L, Cayabyab M, Berman M, Dorf M E, Gerard N, Gerard C, Sodroski J. The orphan seven-transmembrane receptor Apj supports the entry of primary T-cell-line-tropic and dualtropic human immunodeficiency virus type 1. J Virol. 1998;72:6113–6118. doi: 10.1128/jvi.72.7.6113-6118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 11.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O'Brien S J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 13.Deng H, Unutmaz D, KewalRamani V, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–299. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 14.Donzella G A, Schols D, Lin S W, Esté J A, Nagashima K A, Maddon P J, Allaway G P, Sakmar T P, Henson G, De Clercq E, Moore J P. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. doi: 10.1038/nm0198-072. [DOI] [PubMed] [Google Scholar]
- 15.Edinger A L, Hoffman T L, Sharron M, Lee B, O'Dowd B, Doms R W. Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology. 1998;249:367–378. doi: 10.1006/viro.1998.9306. [DOI] [PubMed] [Google Scholar]
- 16.Este J A, Cabrera C, Blanco J, Gutierrez A, Bridger G, Henson G, Clotet B, D. S, De Clercq E. Shift of clinical human immunodeficiency virus type 1 isolates from R5 to X4 and prevention of emergence of the syncytium-inducing phenotype by blockade of CXCR4. J Virol. 1999;73:5577–5585. doi: 10.1128/jvi.73.7.5577-5585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabuzda D, He J, Ohagen A, Vallat A V. Chemokine receptors in HIV-1 infection of the central nervous system. Semin Immunol. 1998;10:203–213. doi: 10.1006/smim.1998.0133. [DOI] [PubMed] [Google Scholar]
- 18.Gaulton G N. Viral pathogenesis and immunity within the thymus. Immunol Res. 1998;17:75–82. doi: 10.1007/BF02786432. [DOI] [PubMed] [Google Scholar]
- 19.Gerber B O, Zanni M P, Uguccioni M, Loetscher M, Mackay C R, Pichler W J, Yawalkar N, Baggiolini M, Moser B. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr Biol. 1997;7:836–843. doi: 10.1016/s0960-9822(06)00371-x. [DOI] [PubMed] [Google Scholar]
- 20.Glushakova S, Baibakov B, Margolis L B, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- 21.Glushakova S, Baibakov B, Zimmerberg J, Margolis L B. Experimental HIV infection of human lymphoid tissue: correlation of CD4+ T cell depletion and virus syncytium-inducing/non-syncytium-inducing phenotype in histocultures inoculated with laboratory strains and patient isolates of HIV type 1. AIDS Res Hum Retroviruses. 1997;13:461–471. doi: 10.1089/aid.1997.13.461. [DOI] [PubMed] [Google Scholar]
- 22.Goldsmith M A, Warmerdam M T, Atchison R E, Miller M D, Greene W C. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J Virol. 1995;69:4112–4121. doi: 10.1128/jvi.69.7.4112-4121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goudsmit J. The role of viral diversity in HIV pathogenesis. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;10(Suppl. 1):S15–S19. [PubMed] [Google Scholar]
- 24.Grivel J-C, Margolis L B. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat Med. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- 25.Grody W W, Fligiel S, Naeim F. Thymus involution in the acquired immunodeficiency syndrome. Am J Clin Pathol. 1985;84:85–95. doi: 10.1093/ajcp/84.1.85. [DOI] [PubMed] [Google Scholar]
- 26.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 28.Ida S, Gatanaga H, Shioda T, Nagai Y, Kobayashi N, Shimada K, Kimura S, Iwamoto A, Oka S. HIV type 1 V3 variation dynamics in vivo: long-term persistence of non-syncytium-inducing genotypes and transient presence of syncytium-inducing genotypes during the course of progressive AIDS. AIDS Res Hum Retroviruses. 1997;13:1597–1609. doi: 10.1089/aid.1997.13.1597. [DOI] [PubMed] [Google Scholar]
- 29.Koot M, Keet I P, Vos A H, de Goede R E, Roos M T, Coutinho R A, Miedema F, Schellekens P T, Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Kozak S L, Platt E J, Madani N, Ferro F E, Jr, Peden K, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labrosse B, Brelot A, Heveker N, Sol N, Schols D, De Clercq E, Alizon M. Determinants for sensitivity of human immunodeficiency virus coreceptor CXCR4 to the bicyclam AMD3100. J Virol. 1998;72:6381–6388. doi: 10.1128/jvi.72.8.6381-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 33.Mack M, Luckow B, Nelson P J, Cihak J, Simmons G, Clapham P R, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells T N, Schlöndorff D, Proudfoot A E. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michael N L, Chang G, Louie L G, Mascola J R, Dondero D, Birx D L, Sheppard H W. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat Med. 1997;3:338–340. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 35.Michael N L, Nelson J A, KewalRamani V N, Chang G, O'Brien S J, Mascola J R, Volsky B, Louder M, White II G C, Littman D R, Swanstrom R, O'Brien T R. Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 Δ32. J Virol. 1998;72:6040–6047. doi: 10.1128/jvi.72.7.6040-6047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosier D E, Picchio G R, Gulizia R J, Sabbe R, Poignard P, Picard L, Offord R E, Thompson D A, Wilken J. Highly potent RANTES analogues either prevent CCR5-using human immunodeficiency virus type 1 infection in vivo or rapidly select for CXCR4-using variants. J Virol. 1999;73:3544–3550. doi: 10.1128/jvi.73.5.3544-3550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami T, Nakajima T, Koyanagi Y, Tachibana K, Fujii N, Tamamura H, Yoshida N, Waki M, Matsumoto A, Yoshie O, Kishimoto T, Yamamoto N, Nagasawa T. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–1393. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owman C, A. S, Boketoft A, Olde B. Leukotriene B4 is the functional ligand binding to and activating the cloned chemoattractant receptor, CMKRL1. Biochem Biophys Res Commun. 1997;240:162–166. doi: 10.1006/bbrc.1997.7628. [DOI] [PubMed] [Google Scholar]
- 39.Patterson B K, Landay A, Andersson J, Brown C, Behbahani H, Jiyamapa D, Burki Z, Stanislawski D, Czerniewski M A, Garcia P. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. Am J Pathol. 1998;153:481–490. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penn M L, Grivel J C, Schramm B, Goldsmith M A, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci USA. 1999;96:663–668. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pöhlmann S, Krumbiegel M, Kirchhoff F. Coreceptor usage of BOB/GPR15 and Bonzo/STRL33 by primary isolates of human immunodeficiency virus type 1. J Gen Virol. 1999;80:1241–1251. doi: 10.1099/0022-1317-80-5-1241. [DOI] [PubMed] [Google Scholar]
- 43.Rosenzweig M, Clark D P, Gaulton G N. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS. 1993;7:1601–1605. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samson M, Edinger A L, Stordeur P, Rucker J, Verhasselt V, Sharron M, Govaerts C, Mollereau C, Vassart G, Doms R W, Parmentier M. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur J Immunol. 1998;28:1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 46.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 47.Scarlatti G, Tresoldi E, Björndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyö E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 48.Schneider T, Ullrich R, Zeitz M. Immunopathology of human immunodeficiency virus infection in the gastrointestinal tract. Springer Semin Immunopathol. 1997;18:515–533. doi: 10.1007/BF00824056. [DOI] [PubMed] [Google Scholar]
- 49.Schols D, Este J A, Cabrera C, De Clercq E. T-cell-line-tropic human immunodeficiency virus type 1 that is made resistant to stromal cell-derived factor 1α contains mutations in the envelope gp120 but does not show a switch in coreceptor use. J Virol. 1998;72:4032–4037. doi: 10.1128/jvi.72.5.4032-4037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schols D, Struyf S, Van Damme J, Esté J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimizu N, Soda Y, Kanbe K, Liu H Y, Jinno A, Kitamura T, Hoshino H. An orphan G protein-coupled receptor, GPR1, acts as a coreceptor to allow replication of human immunodeficiency virus types 1 and 2 in brain-derived cells. J Virol. 1999;73:5231–5239. doi: 10.1128/jvi.73.6.5231-5239.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speck R F, Wehrly K, Platt E J, Atchison R E, Charo I F, Kabat D, Chesebro B, Goldsmith M A. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spijkerman I, de Wolf F, Langendam M, Schuitemaker H, Coutinho R. Emergence of syncytium-inducing human immunodeficiency virus type 1 variants coincides with a transient increase in viral RNA level and is an independent predictor for progression to AIDS. J Infect Dis. 1998;178:397–403. doi: 10.1086/515627. [DOI] [PubMed] [Google Scholar]
- 55.Westervelt P, Gendelman H E, Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci USA. 1991;88:3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao L, Rudolph D L, Owen S M, Spira T J, Lal R B. Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. AIDS. 1998;12:F137–F143. doi: 10.1097/00002030-199813000-00001. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, He T, Talal A, Wang G, Frankel S S, Ho D D. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y J, Moore J P. Will multiple coreceptors need to be targeted by inhibitors of human immunodeficiency virus type 1 entry? J Virol. 1999;73:3443–3448. doi: 10.1128/jvi.73.4.3443-3448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]