Abstract
When the electrocardiogram of acute pulmonary embolism is similar to that of acute myocardial infarction, it is difficult to distinguish between the two diseases quickly and effectively. We present the case of a 50-year-old man with acute pulmonary embolism. His electrocardiogram showed subtotal occlusion of the left main coronary artery with ST segment depression in I, II, aVF, V3 to V6, ST segment elevation in aVR, V1 and S1Q3T3. Invasive coronary angiography did not show coronary artery stenosis, then pulmonary angiography was performed quickly which showed massive bilateral acute pulmonary embolism. Electrocardiogram cannot effectively distinguish acute pulmonary embolism from subtotal occlusion of the left main coronary artery. For patients with hemodynamic instability, if ultrasound cannot be performed in time, the combination of invasive coronary angiography and pulmonary angiography can be an option to distinguish acute pulmonary embolism from subtotal occlusion of the left main coronary artery and to treat.
Keywords: Acute pulmonary embolism, Subtotal occlusion of the left main coronary artery, Invasive coronary angiography, Pulmonary angiography, Electrocardiogram
1. Introduction
Chest pain can occur in patients with both acute pulmonary embolism (APE) and acute myocardial infarction (AMI). When the electrocardiogram (ECG) of APE is similar to that of AMI, it is difficult to distinguish between the two diseases quickly and effectively. The diagnosis of APE is mainly based on computed tomography pulmonary angiography (CTPA), and the diagnosis of AMI is mainly based on invasive coronary angiography (ICA). The combination of pulmonary angiography (PAG) and ICA was rarely used.
2. Case presentation
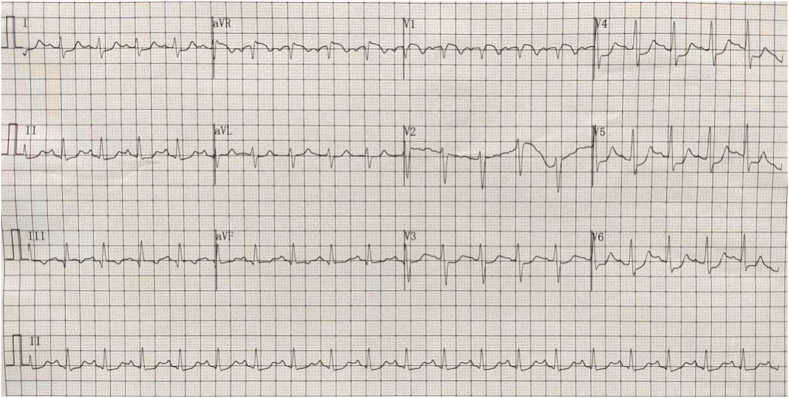
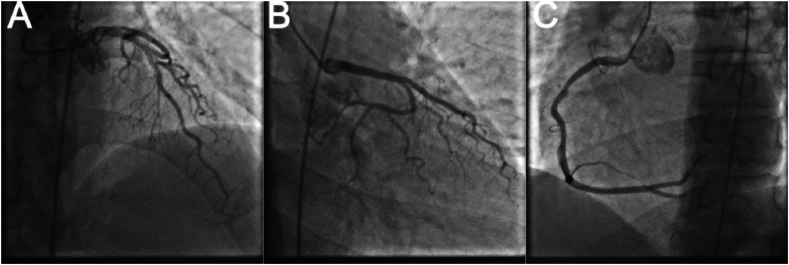
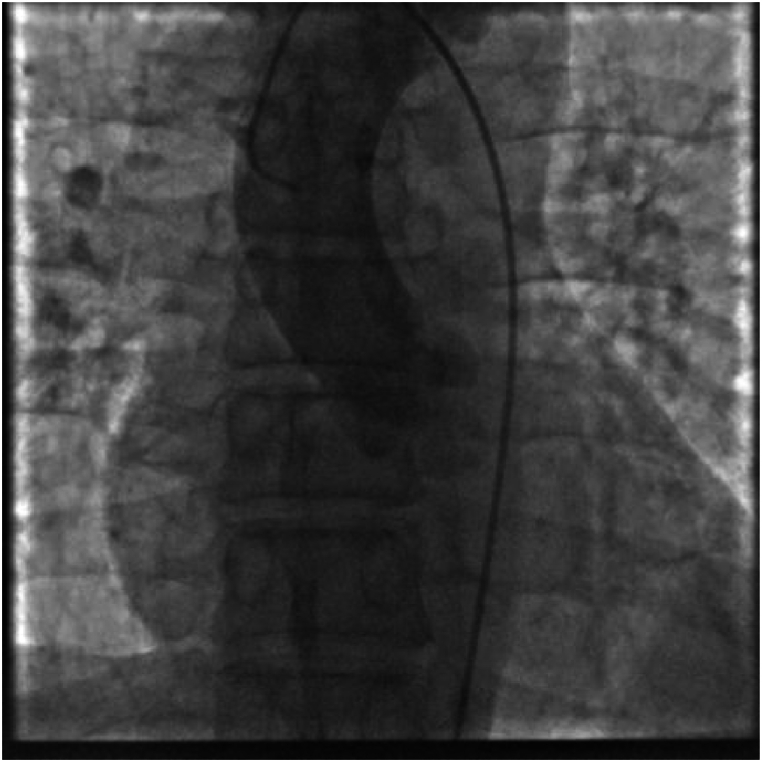
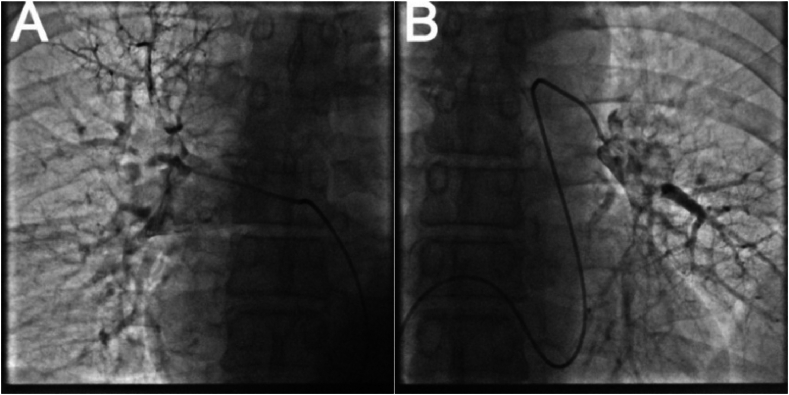
A 50-year-old men presented with chest pain and shortness of breath for 3 days. He had a history of smoking and drinking. In the past 1 month, he worked as a taxi driver. Initial observations in the emergency department showed blood pressure of 72/42 mmHg, heart rate of 122/min, respiratory rate of 25/min and 92 % room air oxygen saturation. His physical examination was unremarkable except for an appearance of acute stress. Fluid infusion and norepinephrine were given immediately to maintain mean arterial pressure about 70 mmHg. The ECG showed sinus tachycardia, ST segment depression in I, II, aVF, V3 to V6, ST segment elevation in aVR, V1 and S1Q3T3 (Fig. 1). According to the patient's symptoms and ECG, there was a high suspicion of subtotal occlusion of the left main coronary artery. Because ultrasound was not available in time, and it would take 1–2 hours to obtain laboratory test results, such as CK-MB, cTnI, Myoglobin, D-dimer, and the patient with hemodynamic instability was most likely to need intervention, we decided to transfer the patient to the catheterization laboratory immediately after chewed aspirin 300mg and clopidogrel 300mg. However, ICA did not show coronary artery stenosis (Fig. 2). Then an aortography was performed, which revealed no aortic dissection (Fig. 3). APE could not be ruled out, and CTPA was necessary. But the patient's hemodynamics was unstable. If CTPA was performed, the patient needed to be transported to CT room and it would take a longer time. This might lead to further deterioration of the patient's condition. Therefore, we finally decided to perform PAG directly in the catheterization laboratory. The PAG showed massive bilateral APE (Fig. 4). Mechanical fragmentation of the thrombus with in situ reduced-dose thrombolysis was immediately administered, followed by anticoagulation with low-molecular-weight heparin and the patient's condition gradually stabilized. After returning to the ward, laboratory test results were showed back: CK-MB<2.50ng/ml, cTnI 0.12ng/ml↑, Myoglobin 36.9ng/ml, D-dimer 6.41 μg/ml↑. Then we performed other relevant tests. Echocardiography showed enlarged right atrium and right ventricle, mild pulmonary hypertension, moderate tricuspid regurgitation, D-sign formation, and normal left ventricular systolic function (Fig. 5). Compression vascular ultrasound showed deep venous thrombosis (DVT) in the left femoral vein and popliteal vein. The patient was discharged 11 days later. The ECG returned to normal before discharge (Fig. 6). His symptoms were significantly relieved, and he felt better. Then he took oral rivaroxaban regularly. There are no adverse and unexpected events.
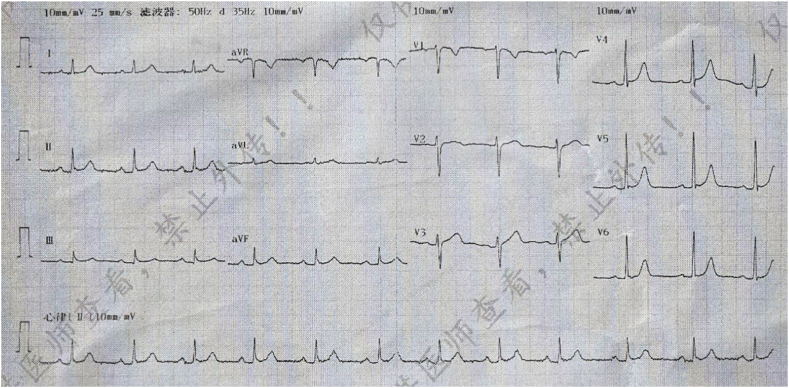
Fig. 1.
Electrocardiogram in the emergency department revealing sinus tachycardia, ST segment depression in I, II, aVF, V3 to V6, ST segment elevation in aVR, V1 and S1Q3T3.
Fig. 2.
Invasive coronary angiography revealing no coronary artery stenosis. A: left anterior descending coronary artery. B: left circumflex artery. C: right coronary artery.
Fig. 3.
Aortography revealing no aortic dissection.
Fig. 4.
Pulmonary angiography revealing massive bilateral acute pulmonary embolism. A: right pulmonary artery. B: left pulmonary artery.
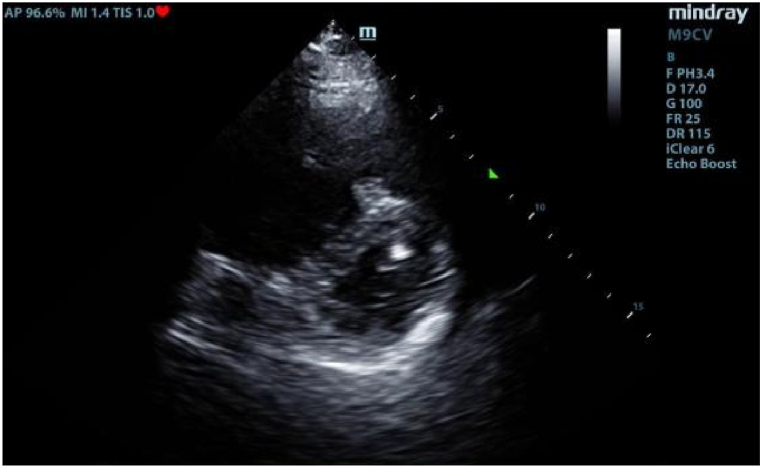
Fig. 5.
Echocardiography revealing D-sign formation.
Fig. 6.
Electrocardiogram returning to normal before discharge.
3. Discussion
APE can be distinguished from subtotal occlusion of the left main coronary artery in terms of medical history, clinical manifestations, physical examination, medical examinations. But patients are often in critical condition, we do not have enough time to collect comprehensive data. So, there are difficulties and challenges in the diagnosis.
The ECG plays a role in the differential diagnosis between APE and subtotal occlusion of the left main coronary artery because of its convenience and availability. The ECG of APE often shows sinus tachycardia, Right bundle branch block (RBBB), T wave inversion in V1 to V3/V4, S1Q3T3 or S1Q3, etc, due to increased right ventricular tension [1,2]. But these ECG patterns are most common in patients with massive APE, and the sensitivity and specificity are low, and even 15 %–27 % of patients with APE have normal ECG findings [3]. The ECG of subtotal occlusion of the left main coronary artery often shows ST-segment depression (mainly in leads I, II and V4-6), ST-segment elevation in leads aVR and V1 (aVR > V1), intraventricular conduction block (Left front-branch block, right bundle branch block), etc., due to ischemia of the left anterior descending artery and left circumflex artery [4,5]. Some patients with APE may also have anterior V3–V6 ST segment depression on ECG, and St segment depression is more common when shock is present [2]. This results in similar ECG patterns between the two diseases, which makes the diagnosis more difficult. As in our case of APE, the main cause of ECG appearance of subtotal occlusion of the left main coronary artery is the shock caused by massive APE, which leads to coronary insufficiency and myocardial injury [6]. It has been reported that another reason is that the dilated trunk of the pulmonary artery secondary to pulmonary hypertension oppresses the left main coronary artery, which leads to subtotal occlusion of the left main coronary artery [7].
APE and subtotal occlusion of the left main coronary artery are fatal diseases. It is important to make a diagnosis as soon as possible. In suspected high-risk APE, as indicated by the presence of hemodynamic instability, bedside echocardiography or emergency CTPA (depending on availability and clinical circumstances) is recommended for diagnosis [8]. For patients with acute chest pain and suspected AMI who are designated as high risk, ICA is recommended [9]. In the literature, many patients received a diagnosis of APE by CTPA after AMI was ruled out by ICA. Although this method can effectively distinguish the two diseases, it takes a long time and increases the risk of transportation. A combination of coronary computed tomography angiography (CCTA) and CTPA was reported in the literature to distinguish APE from AMI [10]. However, if the interventional therapy is necessary, the patient will have to be transported again to the interventional catheterization laboratory, which also takes a long time and increases the risk of transportation. Coronary revascularization is the main treatment for AMI. Percutaneous catheter-directed treatment is one of the treatment methods for PE [8]. In our case, echocardiography could not be performed in time to distinguish APE from subtotal occlusion of the left main coronary artery. We performed ICA firstly, considering that ACS was more likely. To avoid further aggravation of the patient's condition due to repeated transport, PAG was performed after the exclusion of ACS. Then APE was diagnosed, and percutaneous catheter-directed treatment was performed. Finally, the patient had a good prognosis. It is a pity for our case that the echocardiography was not performed in time. For patients with hemodynamic instability, if ultrasound cannot be performed in time, the combination of ICA and PAG can be an option to distinguish APE from subtotal occlusion of the left main coronary artery and to treat.
4. Conclusion
Electrocardiogram cannot effectively distinguish acute pulmonary embolism from subtotal occlusion of the left main coronary artery. For patients with hemodynamic instability, if ultrasound cannot be performed in time, the combination of Invasive coronary angiography and pulmonary angiography can be an option to distinguish acute pulmonary embolism from subtotal occlusion of the left main coronary artery and to treat.
Statement of ethics
Informed consent was acquired from the patient (or relative/guardian) and the patient (or relative/guardian) consented to the publishing of all images, clinical data, and other data included in the manuscript.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
YanZhang Shu: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. BaoLi Xu: Formal analysis, Data curation. XiaoJia Luo: Project administration, Funding acquisition, Data curation, Conceptualization. Yong Tang: Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
XiaoJia Luo, Email: 811722466@qq.com.
Yong Tang, Email: 1913671506@qq.com.
References
- 1.Shopp J.D., et al. Findings from 12-lead electrocardiography that predict circulatory shock from pulmonary embolism: systematic review and meta-analysis. Acad. Emerg. Med. 2015;22(10):1127–1137. doi: 10.1111/acem.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Digby G.C., et al. The value of electrocardiographic abnormalities in the prognosis of pulmonary embolism: a consensus paper. Ann. Noninvasive Electrocardiol. 2015;20(3):207–223. doi: 10.1111/anec.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ullman E., et al. Electrocardiographic manifestations of pulmonary embolism. Am. J. Emerg. Med. 2001;19(6):514–519. doi: 10.1053/ajem.2001.27172. [DOI] [PubMed] [Google Scholar]
- 4.Nikus K.C., Eskola M.J. Electrocardiogram patterns in acute left main coronary artery occlusion. J. Electrocardiol. 2008;41(6):626–629. doi: 10.1016/j.jelectrocard.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 5.Sen F., et al. Classical electrocardiographic clues for left main coronary artery disease. Indian Heart J. 2016;68(Suppl 2):S226–S227. doi: 10.1016/j.ihj.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan Z.Q., et al. Electrocardiogram patterns during hemodynamic instability in patients with acute pulmonary embolism. Ann. Noninvasive Electrocardiol. 2014;19(6):543–551. doi: 10.1111/anec.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safi M., et al. Extrinsic compression of left main coronary artery by the pulmonary trunk secondary to pulmonary hypertension documented using 64-slice multidetector computed tomography coronary angiography. Clin. Cardiol. 2009;32(8):426–428. doi: 10.1002/clc.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konstantinides S.V., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur. Heart J. 2020;41(4):543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 9.Gulati M., et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;144(22):e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 10.Maharaj V., et al. Double rule in: concomitant acute coronary occlusion and pulmonary embolism. JACC Case Rep. 2019;1(4):669–670. doi: 10.1016/j.jaccas.2019.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.