Abstract
Background
Increased homogentisic acid (HGA) in alkaptonuria (AKU) causes severe arthritis. Nitisinone reduces the production of HGA, but whether it also decreases arthroplasty was examined in 237 AKU patients.
Patients and methods
Patients attending the United Kingdom National Alkaptonuria Centre (NAC) and the Suitability of Nitisinone in Alkaptonuria 2 (SONIA 2) study were studied. Assessments included questionnaires eliciting details of arthroplasty. Nitisinone was administered from baseline, 2 mg in the NAC and 10 mg in SONIA 2. In SONIA 2, subgroups consisted of those with baseline arthroplasty on and not on nitisinone (BR + N+, BR + N-), as well as those without baseline arthroplasty on and not on nitisinone (BR-N+, BR-N-).
Results
In the SONIA2 subgroups, new joint replacement (JR) probabilities after baseline were significantly different (BR + N+, BR + N-, BR-N+, BR-N-) (χ2 = 23.3, p < 0.001); mean (SD) was 3.8 (0.1) years in BR-N-, 3.7 (0.1) years in BR-N+, 3.4 (0.3) years in BR + N-, and 3.0 (0.3) years in BR + N+. Further, the BR + N- showed more JR than the BR-N- subgroup (p < 0.01), while BR + N+ similarly showed more JR than the BR-N+ subgroup (p < 0.001).
In the NAC, the BR- group had a mean age of 51.6 (7.0) years at baseline but 57.7 (8.7) years at final follow up during nitisinone therapy and showed only 7 incident JR. The BR+ group had an age at baseline of 57.4 (8.5) years and had undergone 94 JRs at baseline.
Conclusion
The incidence of arthroplasty was earlier and more frequent after the first JR and was not affected by nitisinone.
Keywords: Ochronosis, Alkaptonuria, Joint replacement, Nitisinone, Prevalence, Incidence
1. Introduction
Arthroplasty is a transformative but palliative therapy eliminating pain and allowing functional recovery for severe joint disease in conditions such as osteoarthritis and alkaptonuria (AKU). AKU has been described as a severe genetic osteoarthritis phenotype in standard textbooks of rheumatology and osteoarthritis [1,2]. AKU is a very rare Mendelian disease with a prevalence of 1 in 250,000 in most populations around the world [3,4]. Few published studies have fully characterised arthroplasty in substantial numbers of patients with AKU [5]. Only one previous publication explored the effect of homogentisic acid (HGA)-lowering nitisinone therapy on preventing arthroplasty in AKU and failed to find an effect [6].
The Mendelian defect in AKU (OMIM#203500) is present from birth. Biallelic defective homogentisate dioxygenase gene loci result in inability to break down HGA due to lack of homogentisate 1,2 dioxygenase activity (EC:1.13.11.5) [7,8]. Accumulating pigmentation derived from HGA in connective tissues including joint and spine cartilage, tendons, and ligaments, is a process termed ochronosis [9,10]. The breakdown of these pigmented stiffened and brittle joint tissues leads to joint failures and replacements.
An earlier report in patients attending the National Alkaptonuria Centre (NAC), established by the United Kingdom National Health Service Highly Specialised Services in the Royal Liverpool University Hospital (RLUH) since 2012, estimated the baseline prevalence of arthroplasty at 36.8%, with similar frequency in males and females, and these were carried out mainly on the knees, hips, and shoulders. Multiple arthroplasties were found in 33.3% in the report [6]. The incidence of post-baseline arthroplasty was 6.5% in the nitisinone group and 7.1% in the no-nitisinone group; the dose of nitisinone used was 2 mg daily. The conclusion reached was that nitisinone had no significant effect on the incidence of post-baseline arthroplasty over the period of follow up. Other than this report, there is little other objective data focussing on arthroplasty in AKU.
A randomised clinical trial of nitisinone in patients with AKU (Suitability of Nitisinone in Alkaptonuria 2; SONIA 2) examined the effect of a higher dose of nitisinone, where 69 patients received nitisinone and 69 were untreated during a 4-year period; the dose of nitisinone was 10 mg daily [11]. At Year 4, no notable difference in post-baseline incidence of arthroplasty between the treatment groups was observed. The conclusion reached agreed with that of the NAC report, i.e., that nitisinone did not reduce arthroplasty over the study time frame.
Nitisinone is a remarkably potent inhibitor of the p-hydroxyphenyl-pyruvate dioxygenase (HPPD) (EC:1.13.11.27) enzyme [12,13], with the resulting decrease in accumulation of HGA. Because of the direct connection between HGA and ochronotic pigment, a decrease in HGA could decrease ochronotic arthropathy and therefore the need for arthroplasty. A previous study examining low-dose nitisinone therapy in 40 AKU patients over 3 years reported no effect of nitisinone on clinical parameters such as hip-joint range of motion and did not describe any effect of nitisinone on joint replacements (JR), having found just two new JRs during the study [12].
The earlier reports on arthroplasty in AKU, both in the NAC and SONIA 2, did not assess whether nitisinone was able to prevent JRs in those who had not yet had an arthroplasty [6]. This is crucial in a condition considered to be largely irreversible. No detailed analysis of JRs in SONIA 2 was published [11].
The previously published data from the NAC reflects real-world evidence from a clinical service where all patients are entitled to receive nitisinone. The lack of a matched untreated control group made it difficult to characterise the effect of nitisinone on arthroplasty in AKU. Therefore, the current data analysis included data from the SONIA 2 research study, while at the same time the NAC data was also reviewed after more patients were followed up for a longer time. It offers an opportunity to further characterise the frequency and pattern of arthroplasty in AKU and to examine the effect of low-dose nitisinone on arthroplasty in a large cohort of AKU patients in terms of primary prevention, i.e., those who have not had a JR compared to secondary prevention i.e., those who have already had a JR at the time of starting nitisinone.
2. Patients and methods
2.1. Patients
2.1.1. The NAC cohort
Patients with biochemically confirmed AKU namely a documented increase in urine homogentisic acid, age 16 years and over, attended the RLUH between 2007 and 2023 at least once. The NAC provides oral nitisinone, mainly 2 mg daily but some on 5 mg and 10 mg dose since 2022. No patient received nitisinone prior to 2012. Patients who started attending the NAC annually after June 2012, are included in the nitisinone group, there being a lack of a group not on nitisinone. The numbers of patients in the different groups are shown in Fig. 1. The duration of nitisinone 2 mg therapy varied among the patients.
Fig. 1.
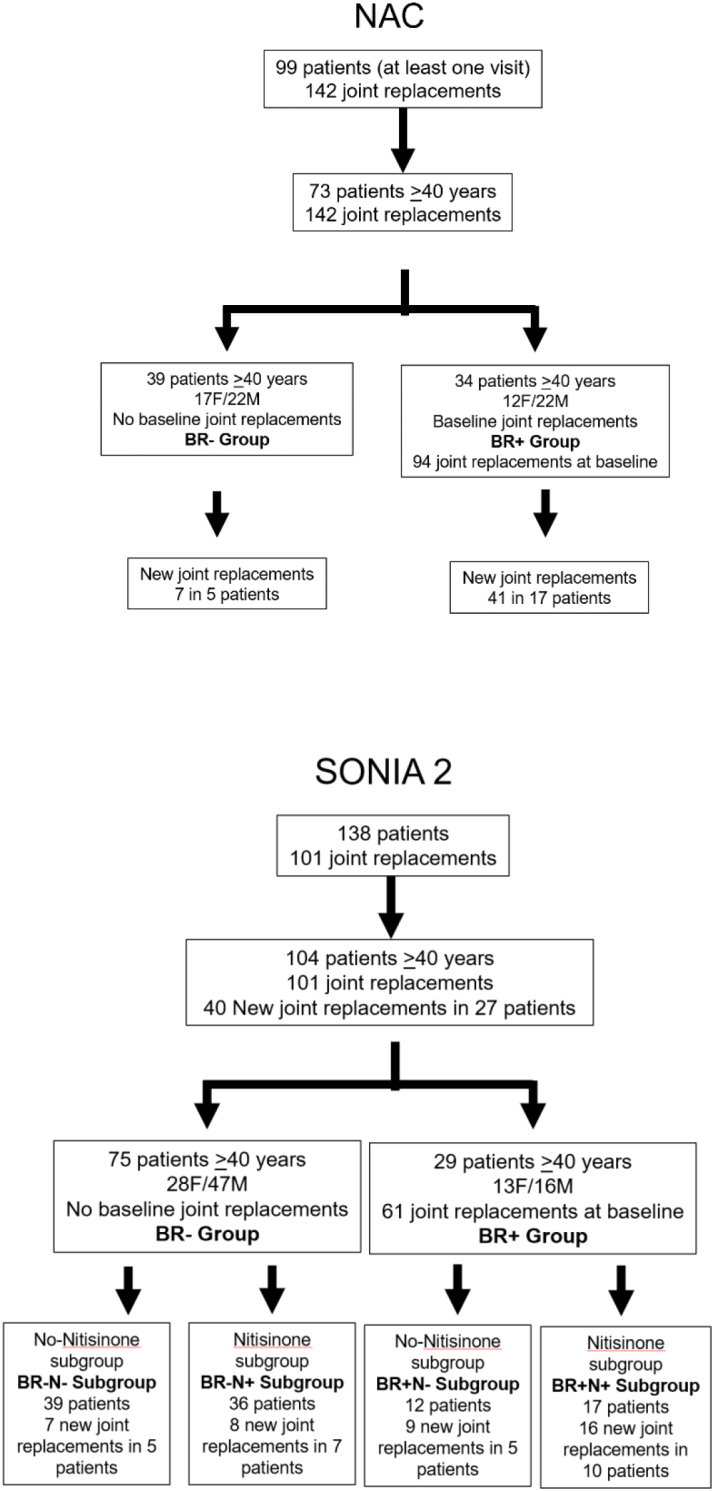
Patient disposition in the NAC and SONIA 2.
2.1.2. The SONIA 2 study cohort
SONIA 2 was a four-year, open-label, evaluator-blinded, multicentre, randomised, no-treatment controlled, parallel-group study [11]. The numbers of patients are described in Fig. 1. The study sites were Liverpool (UK), Paris (France) and Piešťany (Slovakia). The study included 138 patients, 69 in control and 69 randomised to oral nitisinone 10 mg daily, aged 25 years or older, with diagnosed AKU and any clinical manifestation in addition to increased HGA. The visits were once a year after an initial 3-month visit (baseline, months 3, 12, 24, 36 and 48). Independent ethics committees at each centre approved the study. All patients provided written informed consent prior to inclusion.
2.1.3. Assessment at every visit
Historical data such as number and type of JRs were collected via a structured questionnaire both in the NAC and the SONIA 2. Information collected included details of all treatment patients were receiving at each visit, and elicited details of complications such as JRs. Only total numbers of JRs were used in the data analysis. The exact age of the patients at the time of JRs was only available for the NAC cohort. Demographic, body weight, and BMI data were also available for both the NAC and SONIA 2 cohorts. In both studies, measurements of 24-h urine HGA (uHGA24), and fasting serum HGA (sHGA) were carried out by tandem mass spectrometry. HGA was measured on acidified urine and serum samples from each visit as previously described [14,15].
Photographs of the eyes and ears were taken at each visit, under standardised conditions and examined for ochronotic pigmentation. Nasal and temporal aspects of each eye were scored for pigment as previously described both in the NAC and SONIA 2. Photographs of both ears were scored for ochronosis, as previously described in the NAC and SONIA 2 patients [11,16,17]. Scores of both eyes and ears were then summed to generate a combined score.
2.2. Calculations
The prevalence of JRs (%), confined to those aged 40 years and over, was calculated using the following formula: [Number of patients with JR at baseline age /number of patients at baseline] × 100.
The incidence of new JRs (%), confined to those aged 40 years and over, was calculated using the following formula: [(Number of patients with new JRs/number of patients at baseline) ÷ years of follow up] × 100.
2.2.1. Nitisinone therapy
Nitisinone was commenced in eligible patients after tests including serum and 24-h urine collections were completed both in the NAC and SONIA 2 cohorts at the first visit. The daily dose of nitisinone was 2 mg in the NAC cohort and 10 mg in the SONIA 2 cohort.
2.2.2. Statistical analysis
Continuous variables for metabolites uHGA24, sHGA, sTYR, and sNIT were compared using ANOVA with Tukey-Kramer test for multiple comparison using Instat™ GraphPad 3. Two-sided 95% confidence intervals corresponding to a two-sided 5% level of significance were used throughout the analyses. SONIA 2 analyses described here were post-hoc. Kaplan-Meier analysis of time to JR were performed using IBM SPSS Statistics version 19 on the SONIA 2 dataset (SPSS Inc., Chicago, IL, USA). Patients who were prematurely withdrawn from the SONIA2 study were considered censored cases in the Kaplan-Meier analysis. Chi-square tests with Yates correction were also employed to assess the levels of statistical significance between groups of data for JRs in the SONIA 2 dataset (Instat™ GraphPad 3).
2.2.3. Role of the funding source
The funding sources were not involved in the study design, collection, analysis and interpretation of data, the writing of the manuscript, or in the decision to submit the manuscript for publication.
3. Results
3.1. The NAC (cohort, & groups)
In the total 99 patients, there were 142 JRs with the first replacement at age 42 years. Therefore, only data from 73 patients who were 40 years and over were analysed, which still included all 142 JRs (Table 1).
Table 1.
Demographic and metabolic data in the over 40 years groups in the NAC and SONIA 2 cohorts.
|
SONIA 2 |
NAC |
|||
|---|---|---|---|---|
| Baseline Replacement (BR+) group (n = 29) | No Baseline (BR-) Replacement group (n = 75) |
Baseline Replacement (BR+) group (n = 34) | No Baseline (BR-) Replacement group (n = 39) |
|
| Weight Kg | 73.6 (16.6) | 73.9 (15.2) | 74.3 (17.1) | 72.8 (16.3) |
| BMI Kg/M2 | 28.1 (4.7) | 26.4 (4.3) | 27.6 (5.0) | 26.6 (4.4) |
| Sex | 13F/16M | 27F/48M | 12F/22M | 17F/22M |
| sHGA μmol/L (baseline, pre-nitisinone) | 34.6 (11.9) | 28.9 (9.1) ** (16.5% lower) | 32.9 (14.4) | 35.6 (16.2) (0.08% higher) |
| uHGA24 μmol/day (baseline, pre-nitisinone) | 26,281 (11764) | 23,276 (10620) (0.04% lower) | 22,125 (10663) | 23,540 (11195) (0.06% higher) |
| Ochronosis scores (baseline) | 20.9 (8.9) | 28.2 (10.1) *** | 12.1 (8.8) | 21.5 (8.7) *** |
| SONIA 2 (effect of nitisinone) | |||||
|---|---|---|---|---|---|
| Baseline Replacement (BR+) Group (n = 29) |
No Baseline Replacement (BR-) Group (n = 75) |
||||
| No NIT (N-) subgroup (n = 12) |
NIT subgroup (N+) (n = 17) | No NIT (N-) subgroup (n = 39) |
NIT (N+) subgroup (n = 36) | ||
| Age (baseline) years | Baseline | 58.4 (5.5) | 57.5 (7.7) | 50.0 (6.3) | 52.0 (6.9) |
| Final | 61.6 (5.1) | 61.0 (7.2) | 53.8 (6.3) | 55.8 (6.9) | |
| sHGA μmol/L | Baseline | 33.4 (10.9) | 35.5 (12.9) ¥ | 27.3 (7.5) | 30.7 (10.3) ¥ |
| Final | 29.1 (7.8) (12.9% lower) | 1.2 (2.2) (96.6% lower) | 28.2 (10.3) (0.03% higher) | 0.5 (0.8) (98.4% lower) | |
| uHGA24 μmol/day | Baseline | 23,787 (12722) | 28,041 (11088) ¥ | 22,632 (10641) | 23,993 (10701) ¥ |
| Final | 19,601 (12890) (17.6% lower) | 127 (254) (99.6% lower) | 22,923 (12111) (0.01% lower) | 100 (70) (99.6% lower) | |
| NAC (effect of nitisinone) | |||
|---|---|---|---|
| Baseline Replacement (BR+) Group (n = 34) | No Baseline Replacement (BR-) Group (n = 39) | ||
| Age (baseline) years | Baseline | 57.4 (8.5) | 52.0 (9.3) |
| Final | 61.4 (9.5) | 57.7 (8.7) | |
| sHGA μmol/L | Baseline | 32.9 (14.4) ¥ | 35.6 (16.2) ¥ |
| Final | 3.6 (1.3) (89.1% lower) | 4.5 (3.4) (87.4% lower) | |
| uHGA24 μmol/day | Baseline | 22,125 (10663) ¥ | 23,540 (11195) ¥ |
| Final | 852 (663) (96.1% lower) | 1105 (1933) (95.3% lower) | |
¥ denotes comparison between baseline and final values showing p < 0.0001.
*, **, *** denote p values <0.05, 0.01, and 0.001 respectively for other comparisons between Baseline JR and No Baseline JR groups.
Values expressed as mean (SD).
The 73-patient cohort was further subdivided into two groups, 39 patients who did not have a JR at baseline (No Baseline Replacement: BR-), and 34 patients who had JRs at baseline (Baseline Replacement: BR+) (Fig. 1, Table 1).
There were no differences in age, body weight, BMI, sHGA or uHGA24 between the BR- and BR+ groups in the NAC cohort (Table 1). Ochronosis scores, combined for eyes and ears, were significantly higher in the BR+ compared with the BR- groups both in the NAC and SONIA 2 (Table S1, Fig. 2).
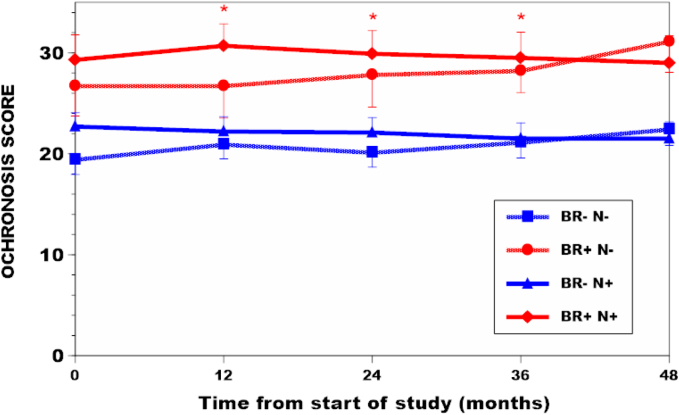
Fig. 2.
Ochronosis scores in the groups and subgroups of SONIA 2 over the four years of study. Comparison of BR-N+ with BR + N+ subgroups showed significantly higher ochronosis scores for BR + N+ subgroup at 12, 24 and 36 months (indicated by red asterisks) (p < 0.05).
39 patients in the BR- group with a mean (SD) age of 52.8 (9.5) years, 22 males and 17 females, were followed up for a mean of 5.0 (3.6) years during 2 mg daily nitisinone treatment (Table 1). Five patients had seven new JRs during the follow-up.
34 patients in the BR+ group with 94 JRs at baseline, had a mean age of 58.9 (8.4) years, 22 males and 12 females, who were followed up for a mean of 4.0 (4.1) years during 2 mg daily nitisinone treatment (Table 1). 17 of the 34 patients had 41 new JRs during the follow-up.
3.2. The SONIA 2 (cohort, groups & subgroups)
In the SONIA 2 cohort, there were 101 JRs at baseline in the 138 patients, and like in the NAC, there were no JRs before the age of 40 years. Therefore, only patients 40 years and over were included in the final analyses; this group comprised of 104 patients and included all 101 JRs. Of these, there were 75 patients in the BR- group and 29 in the BR+ (Fig. 1, Table 1).
There were 28 female and 47 male patients in the BR-, who then had 15 new JRs over the 4 years of follow-up. In this BR- group, there were 39 patients with mean age 50.0 (6.3) years at baseline who did not receive nitisinone (BR-N- subgroup); among these, there were seven JRs in five patients over 3.8 (6.3) years of follow-up. In the BR- group that did receive nitisinone (BR-N+ subgroup) there were 36 patients with mean age 52.0 (6.9) years at baseline; there were eight JRs in seven patients in this subgroup over a mean follow-up duration of 3.8 (6.8) years (Table 1).
In the BR+ group of 29 patients, there were 13 females and 16 males, in whom 25 JRs occurred during follow-up. Of these, there were 12 patients with a mean age 58.4 (5.5) years who had 26 baseline JRs and who did not receive nitisinone (BR + N- subgroup); among these, there were nine new JRs in five patients over 3.2 (5.1) years of follow-up. The remaining 17 patients who received nitisinone (BR + N+ subgroup) had a mean age 57.5 (7.7) years had 35 baseline JRs; among these, there were 16 JRs in ten patients over a mean follow-up duration of 3.5 (7.2) years (Table 1).
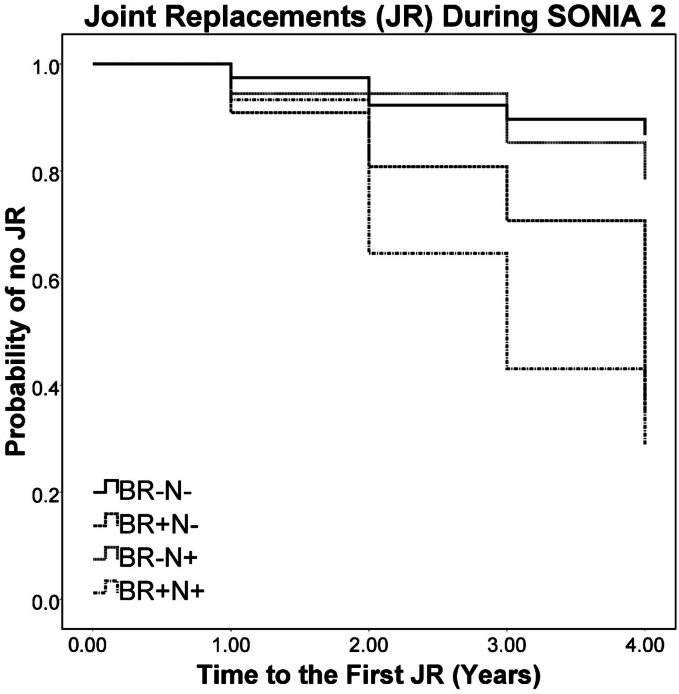
The log rank (Mantel-Cox) test showed that Kaplan-Meier survival curves representing time to JR during the SONIA2 study were significantly (χ2 = 23.3, p < 0.001) different among the BR-N-, BR + N-, BR-N+ and BR + N+ subgroups (Fig. 3). In patients not on nitisinone those who had no previous joint replacement had significantly lower probability of subsequent replacements (BR-N- vs BR + N-, χ2 = 7.77, p = 0.005). In the nitisinone group patients with no previous joint replacement had significantly lower probability of subsequent replacements (BR-N+ vs BR + N+, χ2 = 12.29, p < 0.001). The longest mean time to JR was observed in BR-N- patients [3.8 (0.1) years], followed by NBR-N+ [3.7 (0.1) years], BR + N- [3.4 (0.3) years] and BR + N+ subgroups [3.0 (0.3) years]. The number of censored cases namely those patients withdrawn from the SONIA 2 were as follows: BR-N- (7 patients), BR + N- (5 patients), BR-N+ (7 cases) and BR + N+ (4 cases).
Fig. 3.
Kaplan-Meier curves for joint replacements during SONIA 2 comparing BR-N-, BR + N-, BR-N+ and BR + N+ subgroups during the four years of study. There is a significant difference among the four subgroups (χ2 = 23.3, p < 0.001). The BR- subgroups with and without nitisinone showed fewer JRs during the study. The BR+ subgroups however showed greater number of JRs during the study period. Comparison between BR-N- and BR + N- showed significantly greater JRs in the BR + N- subgroup (p < 0.01), while BR-N+ and BR + N+ subgroups similarly showed significantly greater JRs in the BR + N+ subgroup (p < 0.001).
The BR+ group tended to have a larger BMI (28.1 ± 4.7) kg/m2 compared to the BR- group [26.4 (4.3)] (p < 0.08). sHGA was significantly higher in the BR+ group [34.6 (11.9)], compared to the BR- group [28.9 (9.1)] (p < 0.01); the uHGA24 was similar between the BR- and BR+ groups (Table 1). Ochronosis scores were significantly higher at all visits in the BR+ compared with the BR- group (Fig. 2, Table S1).
3.2.1. Age analysis of the NAC cohort to assess nitisinone effect on joint replacements
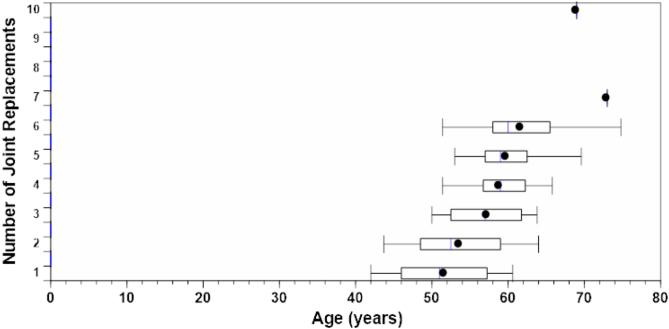
Data were collected on the age at each joint replacement (Fig. 4). The mean age at first JR in the BR+ group was 51.6 (7.0) years. Within 10 years of the first JR most of the JRs had occurred during the follow-up.
Fig. 4.
Serial JRs in the NAC (box plots) showing the age [mean (SD)] at serial JRs in the BR+ group. JRs eight to ten had too few patients to be depicted by a boxplot. There were no JRs before age 42 years. Within 10 years of first JR, most patients had six JRs.
The mean age of the BR+ group at baseline in the NAC was 57.4 (8.5) years. In comparison, the mean baseline age of the BR- group was 52.0 (9.3) years; the mean age of this BR- group at the end of current follow-up was 57.7 (8.7) years, an age comparable to that at baseline of the BR+ group; this means that they were older than the age at first JR of the BR+ group but having experienced fewer new JRs (Table 2).
Table 2.
NAC cohort – age at first JR compared to age in the BR+ (at baseline), BR- (at baseline), and BR- (at final visit) groups.
| Groups (all were 40 years and older) | n | Age (years) [Mean (SD)] | Number of JRs |
|---|---|---|---|
| Age at First JR in BR+ group⁎ | 34 | 51.6 (7.0) | – |
| Age at baseline visit in BR+ group | 34 | 57.4 (8.5) | 94 |
| Age at current final follow-up visit in BR+ group | 34 | 63.6 (8.9) | 135 |
| Age at baseline visit in BR- group | 39 | 52.0 (9.3) | – |
| Age at current final follow-up visit in BR- group | 39 | 57.7 (8.7) | 7 |
BR+: group with baseline JR; BR-: group with no JR at baseline.
BR- group at final follow up show far fewer new JRs despite being older than the BR+ group at first baseline visit, both groups being on nitisinone.
Youngest patient was 42 years of age and oldest was 67 years of age.
3.2.2. Serial joint replacements in the NAC and SONIA 2 cohorts
Age at JRs data were collected from the BR+ group and similar data was also collected for JRs from BR+ and BR- during follow up. Thirty-nine out of a total of 73 patients in the NAC ((aged 40 years and over with and without baseline replacements) had JRs, a prevalence of approximately 46.6%. The incidence of new JRs in those aged 40 years and over was 6.7% during 4.5 years of follow-up.
In the NAC cohort, there were no replacements before age 42 years and the oldest patient was 67 years at first JR in the BR+ group and 69 years in the BR- group. Within 10 years from first JR, most of the JRs, which was on average six per patient, had already occurred. The number of patients with 1, 2, 3, 4, 5, 6, 7 and 10 JRs were 2, 8, 6, 6, 2, 8, 1 and 1 respectively in the BR+ group (Fig. 4).
In the SONIA 2 cohort, 29 patients had baseline JRs and 27 patients had new JRs during 3.6 years of follow-up. The prevalence of JRs was 27.9% in the 40-year age analysis. The incidence of JRs was 7.2% in the 40-year age analysis.
3.2.3. Effect of nitisinone on HGA
In the NAC cohort, baseline mean (SD) sHGA decreased significantly by 87.4% during nitisinone therapy both in the BR- [pre-nitisinone: 35.6 (16.2), post-nitisinone 4.5 (3.4) μmol/L; p < 0.0001] and by 89.1% in the BR+ groups [pre-nitisinone: 32.9 (14.4), post-nitisinone 3.6 (1.3) μmol/L; p < 0.0001] (Table 1).
Similarly, in the NAC cohort, baseline mean (SD) uHGA24 decreased significantly by 95.3% during nitisinone therapy both in the BR- [pre-nitisinone: 23540 (11195), post-nitisinone 1105 (1933) μmol/day; p < 0.0001] and by 96.1% in the BR+ groups [pre-nitisinone: 22125 (10663), post-nitisinone 852 (663) μmol/day; p < 0.0001] (Table 1).
In the SONIA 2 cohort, baseline mean (SD) sHGA decreased significantly by 98.2% during nitisinone therapy both in the BR- [pre-nitisinone: 28.9 (9.1), post-nitisinone 0.49 (0.82) μmol/L; p < 0.0001] and by 97.5% the BR+ groups [pre-nitisinone: 34.6 (11.9), post-nitisinone 1.2 (2.2) μmol/L; p < 0.0001] (Table 1, Fig. S1).
In the SONIA 2 cohort, baseline mean (SD) uHGA24 decreased significantly by 99.6% during nitisinone therapy both in the BR- [pre-nitisinone: 23276 (10,620, post-nitisinone 100 (70) μmol/day; p < 0.0001] and by 99.5% in the BR+ groups [pre-nitisinone: 26281 (11764), post-nitisinone 127 (254) μmol/day; p < 0.0001] (Table 1, Fig. S2).
3.2.4. Chi-Square analysis for comparison of joint replacement incidence in SONIA 2
The Chi Square analysis in the SONIA 2 cohort for the difference between the BR- and the BR+ groups showed significantly higher incident JRs in the BR+ group (Chi Square statistic of 12.1, p < 0.001). The similarly higher incident JRs during nitisinone therapy in the BR+ group compared with the BR- group during nitisinone was also noted (Chi Square statistic of 7.2, p < 0.01) (Table 3).
Table 3.
Chi-square table showing comparison of number of patients with new JR in the various groups and subgroups in the SONIA 2 cohort.
|
Table 3. Chi Square analysis using numbers of patients with new joint replacements (columns 3 & 4) and numbers of new joint replacements (columns 5 &6) | |||
|---|---|---|---|
| Groups & Subgroups | Number of patients | Number of new joint replacements | Chi-square statistic (statistical significance) |
| BR- | 75 | 15 | 13.3 *** |
| BR+ | 29 | 25 | |
| BR-N- | 39 | 7 | 0.01 |
| BR-N+ | 36 | 8 | |
| BR + N- | 12 | 9 | 0.02 |
| BR + N+ | 17 | 16 | |
| BR-N- | 39 | 7 | 4.6 * |
| BR + N- | 12 | 9 | |
| BR-N+ | 36 | 8 | 6.7 ** |
| BR + N+ | 17 | 16 | |
*p < 0.05; **p < 0.01; *** p < 0.001.
4. Discussion
Severe arthropathy is a major cause of morbidity in AKU requiring arthroplasty. The current analysis aimed to characterise the SONIA 2 cohort for arthroplasty to complement the one previously carried out in the NAC [6]. The NAC being a service provided real-world data compared to the SONIA 2 which was a research study. Both the NAC and SONIA 2 data showed that the first JR surgery occurred after the age of 40 years [6]. The JR per patient ratio in the analysis confined to those 40 years and older the ratio was 1.95 in the NAC compared with 0.97 in the SONIA 2.
The current analyses led to some main findings. Patients with arthroplasty are more predisposed to further JRs. Incident arthroplasty is more rapid in those with existing JRs. Those with prior arthroplasty have more numerous new JRs. Serial arthroplasty occurs rapidly following the first JR. Nitisinone-induced HGA-lowering apparently does not prevent further arthroplasty in those with an existing JR at the time of initiating nitisinone therapy over the medium term, i.e. secondary prevention. Each of these findings is examined in more detail in the following sections.
Patients with arthroplasty in AKU are predisposed to further JRs more quickly and more frequently than those without. Time to event log-rank analysis showed that time to incident arthroplasty during SONIA 2 was shortest in the BR + N+ with BR + N- not much further behind. Time to incident arthroplasty was longest for BR-N- and BR-N+ (Fig. 3). Further, chi-square analysis also showed that BR+ group had greater frequency of incident joint replacement than the BR- group (p < 0.001). The reason for this finding could be ochronotic pigmentation of cartilage reaching a ‘threshold’ following which tissue breaks down. Ochronotic pigmentation is not reversible and thus once a threshold is reached, joint failures follow more often, especially since all joint tissues are exposed to the increased circulating HGA leading to diffuse ochronosis. In support of this hypothesis, external ochronosis scores in the eyes and ears were higher in the BR+ groups and subgroups compared to the BR- group and subgroups. Unlike serum HGA which provides only a snapshot in time, ochronosis is consequent to lifetime tissue exposure to circulating HGA and provides a better indication of the metabolic burden of HGA in AKU [6,18].
In the NAC cohort, serial arthroplasty occurred in 0.18% of BR- patients compared with 1.12% in the BR+ group, a 6.7-fold increase in the BR- group (Fig. 4). Within ten years of the first JR, most JRs had occurred and is consistent as being due to with the above-mentioned hypothesis of circulating HGA producing a comparable degree of ochronosis in all joints. Multiple JRs of this magnitude are uncommon. A previous study examining JR surgery in a single hospital reported multiple JRs in rheumatoid arthritis rather than osteoarthritis [19]. Another study of 85,616 osteoarthritis patients reported a high risk of subsequent replacement of the contralateral joint and a relatively low risk of subsequent replacement of a different joint within 5 to 8 years after a hip, knee, or shoulder replacement, very different from the data discussed here [20].
Age is an important factor in the evolution of the need for arthroplasty with previous studies showing that JRs occur later in life in this inborn error of metabolism [11,16,17]. The reason for the later development of morbidity may be due to length of exposure to circulating HGA needed to cause threshold ochronosis leading to joint damage, failure and replacement i.e., the older the patient the more the exposure to circulating HGA. However, circulating HGA also increases with age possibly due to decreased renal elimination [21]. In other words, both factors namely duration of exposure and higher HGA circulating level with age are important and may account for the increase in ochronosis with age [22]. The increasing age-related ochronosis may in turn account for the pattern of joint failure requiring arthroplasty seen in AKU. The mean age at first JR in the NAC cohort was 51.6 years. The BR+ group was older with a mean age of 57.4 years compared with 52.0 years in the BR- at baseline. This is consistent with the view that greater ochronosis in the older BR+ groups of the NAC compared to the BR- group may cause more severe joint disease, joint failures and arthroplasty.
In the SONIA 2 cohort, the number of incident JRs examined by the chi-square analysis of the BR-N-, BR + N-, BR-N+ and BR + N+ subgroups showed no beneficial effect of nitisinone in decreasing JRs (Table 3). To understand the lack of efficacy of nitisinone despite extremely effective HGA-lowering, we turned to the NAC data analysis which followed patients for a longer time than in the SONIA 2. We considered that age may have been a factor in the differences in arthroplasty in the various subgroups. The mean age of the over 40 years group had increased from 51.6 to 57.4 years in the BR+ group while the BR- group had increased from 52.0 to 57.7 years by the end of the study duration. Nitisinone has been used to lower HGA in all patients in the BR+ and the BR- groups in the NAC. Incident JRs were 6.7-fold lower in the BR- group even though their mean age at final follow-up was greater than the mean age of the BR+ group at baseline (Table 2). If age was the major determinant of arthroplasty, it would be reasonable to expect more JRs in the BR- group at final follow-up when they were older than the mean age of the BR+ group; that this was not the case may be an indication that lowering HGA through nitisinone could prevent progression of cartilage ochronosis to the ‘threshold’ state. This could also support a beneficial effect of HGA-lowering on JR in the BR- group. Longer follow-up of the BR- group in the NAC may show whether this conclusion is justified.
The place of nitisinone use in joint failure prevention in AKU needs to be considered. To minimise arthroplasty in AKU, more efforts to prevent the first JR i.e., primary prevention of arthroplasty in AKU, is needed; this could be achieved by earlier HGA-lowering through nitisinone therapy. Further, since the BR+ group continued to have JRs despite nitisinone it appears that HGA-lowering is less effective in secondary prevention of arthroplasty in AKU. Patients currently present with advanced disease whereby they receive nitisinone too late as by this time their load bearing cartilage is irreversibly altered by pigment and therefore irreversibly primed for serial joint failures. Ochronosis is the key pathophysiological factor responsible for the arthropathy and arthroplasty in AKU as discussed elsewhere and the mechanisms of pigment formation from HGA is still incompletely understood [9,10]. This is not to preclude the use of nitisinone in advanced AKU especially those who have undergone JRs, since nitisinone-induced HGA lowering may benefit other aspects of AKU disease such as stones, bone, tendons, ligaments, and the heart as previously shown [11].
There were limitations in the datasets presented here. Due to the rarity of AKU, the numbers of patients in the SONIA 2 subgroups were still relatively small despite SONIA 2 being the largest studies ever carried out in the inherited metabolic disease speciality. The NAC is a service provided to AKU patients and since patients attending the NAC are eligible to receive nitisinone, there was no opportunity to have a designated no-treatment arm. However, the NAC group provides the opportunity to continue longer-term follow-up patients not limited by regulatory consideration such as trial duration.
To summarise, an increased degree of ochronosis may cause more severe disease, joint failures, and replacements. Serial joint failures leading to arthroplasty are common following the first JR. There is therefore a suggestion that starting HGA-lowering therapy before the first joint failure and replacement may prevent or delay arthroplasty and more research is needed to confirm this important finding. Further, HGA-lowering therapy may benefit other manifestations of AKU even if it is less effective in those with existing arthroplasty.
Ethical issues in the NAC cohort
Standard patient information sheets and signed consent forms were used in the research study between 2007 and 2011. Data from the NHS HSS approved service was collected and analysed as part of annual institutional audit (Audit no. ACO3836). A booklet describing all processes carried out in the NAC was given to each participant before they agreed to come to the NAC; specific information was provided in the booklet that data collected would be published but subjects would not be identifiable from the dissemination process. Further, specific consent was obtained for the ear and eye photographs using the institutional consent mechanisms. Data from the NAC has been published in peer-reviewed journals previously along similar justifications. The highest standards of ethical and clinical practices were followed in delivering the service at the NAC.
Ethics issues in SONIA 2 cohort
All patients were recruited following written informed consent.
The reference numbers for the ethics approvals are as follows:
EC Liverpool (NRES Committee North-West – Liverpool Central) Reference number: 13/NW/0567.
EC Piešťany (NURCH Ethical Committee, National Institute of Rheumatic Diseases, Ivana Krasku 4,92101 Piešťany, Slovak Republic) Reference number: 04196/0029/001/001.
EC Paris (EC Ile De France II, hospital Necker 149 Rue de Sevres 75 743 Paris Cedex 15, Porte N2, 1er etage, France). Reference number: 2013-08-08.
AI was not employed in the writing of this manuscript.
Details of the contributions of individual authors
LRR is the corresponding author and conceived and wrote the manuscript.
MK was involved in the conduct of the study as well as editing the manuscript.
BPN and JHH carried out chemical analyses on samples and edited the manuscript.
RI and JBA conducted the study in Slovakia and France in SONIA 2 and edited the manuscript.
BO and MR carried out SONIA 2 data analyses and edited the manuscript.
RI assisted in the statistical analysis.
GBG & JAG helped conceive the study and edited the manuscript.
Patient and public involvement
The patient charities (UK National Alkaptonuria Society and the French patient society ALCAP) were partners and part of the DevelopAKUre consortium which included the clinical trial SONIA 2 (Suitability of Nitisinone in Alkaptonuria 2). Patients were involved in the design, securing funding, the conduct of the study in terms of recruitment and retention, as well as dissemination.
The patient charity (UK National Alkaptonuria Society) is an embedded partner in the service and responsible for patient affairs and advocacy.
Data-sharing statement for SONIA 2 and NAC
SONIA 2 data access will be granted in response to qualified research requests. All de-identified individual participant data, for patients with separate consent signed for this purpose, can be made available to researchers. Data will be shared based on: the scientific merit of the proposal – i.e. the proposal should be scientifically sound, ethical, and have the potential to contribute to the advancement of public health as well as the feasibility of the research proposal – i.e. the requesting research team must be scientifically qualified and have the resources to conduct the proposed project. The data files would exclude data dictionaries that require user licenses. Data could be made available following finalized regulatory authority review and end of any data exclusivity periods and ending after 36 months or until corresponding author is able to fulfil this obligation whichever is earlier. Further, the study protocol and statistical analysis plan can be made available. Proposals should be directed to j.a.gallagher@liverpool.ac.uk to gain access. Data requestors will need to sign a data access agreement.
The data from the NAC can also similarly made available. Requests for data can be made to lrang@liv.ac.uk or milad.khedr@liverpoolft.nhs.uk.
CRediT authorship contribution statement
L.R. Ranganath: Writing – original draft, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. M. Khedr: Writing – review & editing, Investigation. B.P. Norman: Writing – review & editing, Methodology. J.H. Hughes: Methodology, Conceptualization. R. Imrich: Writing – original draft, Software, Formal analysis, Conceptualization. J.B. Arnoux: Investigation, and writing. B. Olsson: Writing – original draft, Project administration. M. Rudebeck: Writing – review & editing, Validation, Methodology. J.A. Gallagher: Writing – review & editing, Supervision, Conceptualization. G. Bou-Gharios: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
Lakshminarayan Ranganath received fees for lectures and consultations from Swedish Orphan Biovitrum (Sobi).
Birgitta Olsson and Mattias Rudebeck are ex-Sobi employees.
Milad Khedr, Brendan Norman, Juliette Hughes, Richard Imrich, Jean-Baptiste Arnoux, James Gallagher and George Bou-Gharios do not have anything to declare and have no conflicts to publication of this manuscript.
Acknowledgements
We acknowledge the funding support from NHS England Highly Specialised Services, UK. We would like to thank the European Commission for the Framework 7 grant award (DevelopAKUre, project number: 304985) that was crucial to allow SONIA 2 to be carried out.
We would like to thank all patients in SONIA 2, a very demanding four-year participation, as well as the patient societies supporting the patients in the study and for their immense efforts in successfully recruiting these many patients.
We would like to thank all patients and the large multidisciplinary team in the NAC, a continuing service, as well as the UK AKU society supporting the patients and the service.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2024.101097.
Contributor Information
L.R. Ranganath, Email: lrang@liv.ac.uk.
G. Bou-Gharios, Email: ggharios@liverpool.ac.uk.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- 1.Hochberg M.C., Silman A.J., Smoleń J., Weinblatt M.E., Kraus V.B. 2015. Rare Osteoarthritis: Ochronosis and Kashin-Beck Disease - Kraus VB. [Google Scholar]
- 2.Schumacher R., Jr., Chen L.X., Buckwalter J. In: Osteoarthritis – Diagnosis and Medical/Surgical Management. 4th edition. Moskowitz R.W., Altman R.D., Hchberg M.C., Buckwalter J.A., Goldberg V.M., editors. Lippincott Williams & Watkins, a Walter Kluwer Business; Philadelphia, USA: 2007. Secondary osteoarthritis: ochronosis, pages 235–237. [Google Scholar]
- 3.Ascher D.B., Spiga O., Sekelska M., et al. Homogentisate 1,2-dioxygenase (HGD) gene variants, their analysis and genotype–phenotype correlations in the largest cohort of patients with AKU. Eur. J. Hum. Genet. 2019;27:888–902. doi: 10.1038/s41431-019-0354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Usher J.L., Ascher D.B., Pires D.E., et al. Analysis of HGD gene mutations in patients with alkaptonuria from the United Kingdom: identification of novel mutations. JIMD Rep. 2015;24:3–11. doi: 10.1007/8904_2014_380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phornphutkul C., Introne W.J., Perry M.B., et al. Natural history of alkaptonuria. N. Engl. J. Med. 2002;347:2111–2121. doi: 10.1056/NEJMoa021736. [DOI] [PubMed] [Google Scholar]
- 6.Ranganath L.R., Davidson J., Vinjamuri S., Gallagher J.A. Characterising the arthroplasty in spondyloarthropathy in a large cohort of eighty-seven patients with alkaptonuria. J. Inherit. Metab. Dis. 2020;44:656–665. doi: 10.1002/jimd.12340. [DOI] [PubMed] [Google Scholar]
- 7.Davison A.S., Hughes A.T., Milan A.M., et al. Alkaptonuria - many questions answered, further challenges beckon. Ann. Clin. Biochem. 2020;57:106–120. doi: 10.1177/0004563219879957. [DOI] [PubMed] [Google Scholar]
- 8.La Du B.N., Zannoni V.G., Laster L., Seegmiller J.E. The nature of the defect in tyrosine metabolism in alcaptonuria. J. Biol. Chem. 1958;230:251–260. [PubMed] [Google Scholar]
- 9.Zannoni V.G., Lomtevas N., Goldfinger S. Oxidation of homogentisic acid to ochronotic pigment in connective tissue. Biochim. Biophys. Acta. 1969;177:94–105. doi: 10.1016/0304-4165(69)90068-3. [DOI] [PubMed] [Google Scholar]
- 10.Chow W.Y., Norman B.P., Roberts N.B., et al. Pigmentation chemistry and radical-based collagen degradation in Alkaptonuria and osteoarthritic cartilage. Angew. Chem. Int. Ed. Eng. 2020;59:11937–11942. doi: 10.1002/anie.202000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranganath L.R., Psarelli E.E., Arnoux J.B., et al. Suitability of Nitisinone in Alkaptonuria 2 (SONIA 2) - a randomised study on the efficacy and safety of nitisinone in alkaptonuria. Lancet Diabet. Endocrinol. 2020;8:762–772. doi: 10.1016/S2213-8587(20)30228-X. [DOI] [PubMed] [Google Scholar]
- 12.Introne WJ, Perry MB, Troendle J, et al. A 3-year randomized therapeutic trial of nitisinone in Alkaptonuria. Mol. Genet. Metab. 20122;103:307–14. [DOI] [PMC free article] [PubMed]
- 13.Ranganath L.R., Milan A.M., Hughes A.T., et al. Suitability of Nitisinone in Alkaptonuria 1 (SONIA 1): an international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose-response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann. Rheum. Dis. 2016;75:362–367. doi: 10.1136/annrheumdis-2014-206033. [DOI] [PubMed] [Google Scholar]
- 14.Hughes A.T., Milan A.M., Christensen P., et al. Urine homogentisic acid and tyrosine: simultaneous analysis by liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;963:106–112. doi: 10.1016/j.jchromb.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Hughes A.T., Milan A.M., Davison A.S., et al. Serum markers in alkaptonuria: simultaneous analysis of homogentisic acid, tyrosine and nitisinone by liquid chromatography tandem mass spectrometry. Ann. Clin. Biochem. 2015;52:597–605. doi: 10.1177/0004563215571969. [DOI] [PubMed] [Google Scholar]
- 16.Ranganath L.R., Khedr M., Milan A.M., et al. Nitisinone arrests ochronosis and decreases rate of progression of Alkaptonuria: evaluation of the effect of nitisinone in the United Kingdom national Alkaptonuria Centre. Mol. Genet. Metab. 2018;125:127–134. doi: 10.1016/j.ymgme.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Griffin R., Psarelli E.E., Cox T.F., et al. Data on items of AKUSSI in Alkaptonuria collected over three years from the United Kingdom National Alkaptonuria Centre and the impact of nitisinone. Mol. Genet. Metab. 2018;20:1620–1628. doi: 10.1016/j.dib.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranganath L.R., Norman B.P., Gallagher J.A. Ochronotic pigmentation is caused by homogentisic acid and is the key event in Alkaptonuria leading to the destructive consequences of the disease – a review. J. Inherit. Metab. Dis. 2019;42:776–792. doi: 10.1002/jimd.12152. [DOI] [PubMed] [Google Scholar]
- 19.Walker D.J., Usher K., O’Morchoe M., et al. Outcome from multiple joint replacement surgery to the lower limbs. Br. J. Rheumatol. 1989;28:139–142. doi: 10.1093/rheumatology/28.2.139. [DOI] [PubMed] [Google Scholar]
- 20.Lamplot J.D., Bansal A., Nguyen J.T., Brophy R.H. Risk of subsequent joint arthroplasty in contralateral or different joint after index shoulder, hip, or knee arthroplasty: association with index joint, demographics, and patient-specific factors. J. Bone Joint Surg. Am. 2018;100:1750–1756. doi: 10.2106/JBJS.17.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranganath L.R., Milan A.M., Hughes A.T., et al. Homogentisic acid is not only eliminated by glomerular filtration and tubular secretion but also produced in the kidney in alkaptonuria. J. Inherit. Metab. Dis. 2020;43:737–747. doi: 10.1002/jimd.12181. [DOI] [PubMed] [Google Scholar]
- 22.Ranganath L.R., Milan A.M., Hughes A.T., et al. Reversal of ochronotic pigmentation in alkaptonuria following nitisinone therapy: analysis of data from the United Kingdom National Alkaptonuria Centre. JMDR. 2020;55:75–87. doi: 10.1002/jmd2.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.