Abstract
Background:
Measles, a highly contagious and vaccine-preventable disease, continues to present global public health challenges. This retrospective study focused on measles outbreaks in Hormozgan province, southern Iran, spanning from 2014 to 2019.
Methods:
Between 2014 and 2019, patients suspected of having measles, as reported by medical centers in Hormozgan, were subject to a comprehensive evaluation. The diagnosis of measles was conclusively established through the use of real-time polymerase chain reaction (RT-PCR) testing. A detailed collection of pertinent data was undertaken. SPSS software, version 21, was employed for statistical analysis.
Results:
In the current study, out of 1291 clinically suspected measles cases, 151 were PCR-confirmed, with an average age of 16.77 years (±10.46), comprising 50.9% males and 49.1% females. The annual distribution showed varied incidence: 8.4% in 2014, peaking at 18.8% in 2015, then fluctuating to 11.4% in 2016, 0.8% in 2017, and 17.9% in 2018, with no cases in 2019. Among confirmed cases, 16.5% were vaccinated, while 68.2% were not, and 15.23% had unknown vaccination status.
Conclusion:
This retrospective study highlights the ongoing challenge of measles in Hormozgan province, Iran, from 2014 to 2019. Despite measles being preventable by vaccination, a significant number of cases were confirmed among both vaccinated and unvaccinated individuals, indicating gaps in immunization coverage and effectiveness. The fluctuating annual incidence, with a peak in 2015 and no cases in 2019, suggests variable success in disease control efforts. This underscores the need for enhanced surveillance, improved vaccination strategies, and public health interventions to effectively combat measles outbreaks in this region.
Keywords: epidemiology, Iran, measles, outbreak, public health, vaccination
Introduction
Highlights
The study emphasizes the fluctuation in measles cases in southern Iran between 2018 and 2019.
Vulnerability in infants underscores the need for targeted vaccination efforts.
Unvaccinated cases, particularly in those under one year old, highlight the pivotal role of vaccination in measles prevention.
Measles stands out as one of the most highly contagious illnesses known to mankind1,2. This vaccine-preventable disease triggers a range of distressing symptoms, including the development of a maculopapular rash, respiratory complications, and elevated body temperature. Its incubation period typically spans 7–21 days3–5.
Regrettably, measles remains a substantial contributor to both child mortality and morbidity on a global scale, accounting for a significant 4% of the annual six million deaths among children below the age of 56.
Due to the remarkably high level of contagiousness exhibited by the measles virus and its capacity for airborne transmission, even a solitary case of measles can lead to a substantial number of secondary exposures, potentially culminating in a pandemic outbreak7,8.
Efforts aimed at measles elimination draw upon strategic guidelines derived from the Global Measles and Rubella Strategic Plan 2012–20209. A cornerstone of these strategies involves maintaining robust population protection through vaccination, striving to achieve a minimum vaccine coverage rate of at least 95%10.
Between the years 2000 and 2019, an impressive vaccination effort unfolded, encompassing over 670 million individuals ranging from 6 months to 60 years of age. This massive undertaking involved the implementation of 257 national or subnational Supplementary Immunization Activities (SIAs)9.
Despite the availability of a safe and effective measles vaccine, measles outbreaks continue to loom as a significant global public health concern11. In the period spanning from 2016 to 2019, a sharp upswing in measles cases was observed, with an alarming tally of over 500 000 confirmed cases reported to the WHO12.
In the Eastern Mediterranean Region (EMRO), the prevention of vaccine-preventable infections, including measles, stands as a paramount concern13. EMRO nations have adopted regional policies aimed at measles elimination, albeit with varying levels of efficacy and success10. However, it is crucial to acknowledge that this region has encountered substantial challenges due to increased measles outbreaks. Several countries, including Afghanistan, Pakistan, and Syria, have grappled with healthcare sector issues exacerbated by these outbreaks. Alarmingly, in certain nations such as Afghanistan, Sudan, Somalia, Djibouti, and Pakistan, measles vaccination coverage remained below 60%13.
In Iran, measles has consistently represented a well-diagnosed infectious disease and a substantial public health challenge14. Prior to the implementation of mass immunization programs, the country experienced a considerable burden of measles cases. In non-epidemic years, measles cases in Iran reached levels as high as 150 000, while during epidemic years, this figure soared to a staggering 500 00011. Iran has made substantial progress in measles vaccination coverage over the years. From 1980 to 2005, the country witnessed a remarkable increase in vaccination coverage, with rates soaring from 38 to 95%15. These achievements align with Iran’s National Immunization Program, which mandates that all children receive a minimum of two doses of the measles vaccine during their early childhood. These doses are administered at the ages of 12 and 18 months16.
The primary objective of this study was to comprehensively characterize the recent measles outbreak in southern Iran, focusing on epidemiological patterns and vaccination status among affected individuals. Additionally, the study aims to offer a set of recommendations aimed at advancing efforts toward achieving measles elimination in the region. This research endeavours to shed light on the current status of measles-elimination initiatives in southern Iran and provide valuable insights to inform future public health strategies.
Methods
Study design and setting
The present study conducted a retrospective descriptive analysis of all measles cases reported to the health centers in Hormozgan province’s database between 2014 and 2019. The study protocol was registered with the Ethical Committee of Hormozgan University of Medical Sciences under registration ID: IR.HUMS.REC.1399.471. All participants were informed about the study, and written consent was obtained from each of them. The study adhered to the Helsinki principles and ethical guidelines17.
Measles diagnosis
We adhered to the WHO-recommended measles case definition, which incorporates both clinical and laboratory criteria for diagnosis; both criteria were utilized to define confirmed cases18.
The measles cases reported by the health centers in Hormozgan province were classified into two categories: clinically suspected cases and laboratory-confirmed cases, determined by positive real-time polymerase chain reaction (RT-PCR) results in this study19.
To accurately confirm measles in clinically suspected patients, a thorough evaluation process was implemented. Every individual suspected of having measles underwent a comprehensive assessment. This included the collection of urine and pharyngeal samples within 5 days following the onset of the rash.
The collected samples were then dispatched to the measles reference laboratory at the Faculty of Health, Tehran University of Medical Sciences. Upon arrival at the laboratory, these specimens were immediately processed and subsequently stored at a temperature of –75°C. This storage protocol was maintained until the time of laboratory diagnosis. The diagnostic procedure employed was the RT-PCR, a method known for its high specificity and sensitivity in detecting the measles virus.
Inclusion and exclusion criteria
In our study, we focused on individuals diagnosed with measles over a six-year period, spanning from 1 January 2014 to 31 December 2019. The inclusion criteria comprised cases reported from all health centers within Hormozgan province, ensuring extensive geographical coverage.
To be included, cases had to be confirmed through RT-PCR among clinically suspected individuals. There were no restrictions based on age or sex; thus, individuals from all demographic groups who met these criteria were encompassed in the analysis. Individuals who exhibited measles-like symptoms but were not officially diagnosed with measles were not included. This exclusion was pivotal in ensuring that the data accurately reflected confirmed measles cases.
Data collection
The present study was conducted in accordance with the guidelines of STROCSS20. Relevant information, including age, sex, nationality, number of cases, measles disease summary, symptoms, and vaccination status (including the number of doses administered), was collected.
To study measles complications, the medical records of measles patients were reviewed. The surveillance system for measles elimination identified suspected cases (i.e. fever, rash, cough, conjunctivitis, and/or coryza), and cases were reported to the health centers in Hormozgan province by physicians and health institutions using an enhanced surveillance system.
The vaccination status of measles cases was validated by collecting individual vaccination certificates and searching the electronic health records database. It is noteworthy that ‘without’ vaccination status included children older than one year, and ‘unknown’ included individuals with an unknown vaccination history.
Statistical analysis
Statistical analyses were performed using SPSS software, version 21. Descriptive statistics were utilized to summarize the demographic characteristics of the study population, including means and standard deviations for continuous variables and frequencies and percentages for categorical variables. According to the Kolmogorov–Smirnov test it was indicated that the distribution of all variables was normal.
Results
Baseline characteristics
From 2014 to 2019, a total of 1291 clinically suspected cases of measles were identified, of which 151 were confirmed through PCR. The mean age of the study population was 16.77 ± 10.46 years, with 77 cases being men (50.9%) and 74 (49.1%) being women. Eighty-one were under 1 year old, 19 were between 1 and 4 years old, 10 were between 5 and 9 years old, 7 were between 10 and -14 years old, 7 were between 15 and 24 years old, and 27 people were above 25 years old. The majority of individuals were from Iran (n=140), followed by Afghani (n=8). The detailed information of study individuals is presented in Table 1.
Table 1.
Nationality of patients included in the study.
| Under 1 year | 1–4 years | 5–9 years | 10–14 years | 15–24 years | More than 25 years | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nationality | Iranian | Afghan | Pakistani | Iranian | Iranian | Afghan | Iranian | Afghan | Iranian | Afghan | Iranian | Other |
| 2014 | 2 | 1 | 1 | 1 | 4 | |||||||
| 2015 | 21 | 6 | 3 | 1 | 2 | 3 | 1 | 6 | 1 | |||
| 2016 | 9 | 1 | 1 | |||||||||
| 2017 | 1 | |||||||||||
| 2018 | 46 | 2 | 1 | 12 | 3 | 3 | 1 | 3 | 15 | |||
Pattern of measles outbreaks by year, 2014–2019
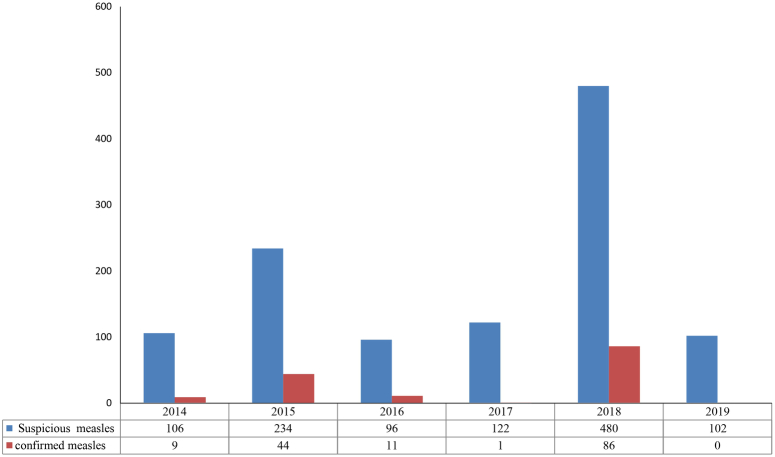
Figure 1 shows the trend in measles outbreaks from 2014 to 2019, highlighting variations in outbreak size and year. The data reveals a fluctuating pattern: 9 confirmed cases (8.4%) in 2014 out of 106 suspected, an increase to 44 cases (18.8%) in 2015 out of 234 suspected, 11 cases (11.4%) in 2016 out of 96 suspected, a drop to one case (0.8%) in 2017 out of 122 suspected, and a peak in 2018, with 86 cases (17.9%) out of 480 suspected. Notably, 2019 marked a significant decrease, with no reported cases, as depicted in Fig. 1.
Figure 1.
Measles outbreaks patterns from 2014 to 2019.
Distribution of clinical symptoms in patients
According to the study findings, the most prevalent complications during these outbreaks were skin rash, affecting 100% of cases (n=151), followed by fever at 95.36% (n=144). Conversely, the least common complications included cough, reported in 64.90% of cases (n=98), rhinorrhea in 56.95% (n=86), and conjunctivitis in 54.96% (n=83) (Table 2). In this study, Measles caused no deaths, and we discharged all patients after 2–4 days.
Table 2.
Symptoms of patients included in the study.
| Symptoms | Total number | Rashes | Percentage of rashes% | Fever | The percentage of fever% | Cough | The percentage of cough% | Runny nose | The percentage of runny nose% | The redness of the conjunctiva | The percentage of redness of the conjunctiva% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Under 1 year | 81 | 81 | 100 | 76 | 93.8 | 53 | 65.4 | 48 | 59.2 | 41 | 50.6 |
| 1–4 years | 19 | 19 | 100 | 19 | 100 | 9 | 47.7 | 9 | 47.7 | 8 | 42.1 |
| 5–9 years | 10 | 10 | 100 | 9 | 90 | 6 | 60 | 5 | 50 | 5 | 50 |
| 10–14 years | 7 | 7 | 100 | 7 | 100 | 4 | 57.1 | 6 | 85.7 | 5 | 71.4 |
| 15–24 years | 7 | 7 | 100 | 7 | 100 | 5 | 71.4 | 4 | 57.1 | 5 | 71.4 |
| More than 25 years | 27 | 27 | 100 | 26 | 96.3 | 21 | 77. 8 | 14 | 51.8 | 19 | 70.8 |
Incidence of measles outbreaks by nationality and age
In 2014, there were nine confirmed cases, comprising six Iranians and three Afghans. The following year, 2015, 44 confirmed cases were witnessed, including 41 Iranians, two Afghans, and one from another nationality. In 2016, all 11 confirmed cases were Iranian. Moving to 2017, there was only one confirmed case involving an Iranian individual. The year 2018 saw a surge with 86 confirmed cases, primarily among Iranians (82 cases), followed by three Afghans and one Pakistani. The majority of measles cases in 2018 occurred among Iranians.
Analyzing the nationality breakdown for Iranian case-patients from 2014 to 2019 revealed that children aged younger than 1 year had the highest incidence of measles (n=76), followed by those aged older than or equal to 25 years (n=26). Other age groups included 19 cases in the 1–4-year range, 8 cases in the 5–9-year range, and 6 cases in each of the 10–14 and 15–24-year ranges.
For individuals of Afghan nationality from 2014 to 2018, children aged younger than 1 year also had the highest incidence (n=4), mirroring the trend in Iranians. The second most affected age group was 10–14 years old (n=2), with one case in each of the 5–9 and 15–24-year age groups. Only one case from Pakistan was reported, and that individual was under 1 year old.
Further details on the trend of the annual number of measles cases in the south of Iran are presented in Table 1.
Vaccination status
Figure 2 provides an overview of the age distribution among the 151 confirmed cases based on their vaccination status. Of the 151 subjects investigated, 81 patients under 1 year old (53.6%) did not receive any measles, mumps, and rubella (MMR) vaccine doses, while 4 cases (2.6%) had received 1 MMR vaccine dose. Moreover, 21 cases (13.9%) had received 2 MMR vaccine doses, and 22 cases (14.5%) had no vaccination throughout their entire life.
Figure 2.
Vaccination status of individuals.
Analysis of the results from 2014 to 2018 revealed that, on average, 16.5% (n=25) of individuals who contracted measles were vaccinated (either one or two MMR vaccine doses) against the virus. In contrast, a substantial majority, averaging 68.2% (n=103) of the cases, were confirmed to be non-vaccinated. Additionally, 15.23% (n=23) of cases had an unknown vaccination status.
Discussion
Eliminating measles, a key vaccine-preventable disease, is a top priority in the Eastern Mediterranean Regional Office (EMRO), highlighting the need for superior immunization efforts and impactful vaccination campaigns13.
The results of our study revealed that children under one year old were highly susceptible to measles. In addition, most of the infected children were unvaccinated against the virus. Early in life, children often acquire passive immunity through maternal antibodies, offering protection against the virus. However, these antibodies typically deplete from a newborn’s serum within six months, rendering the children more susceptible to infection. It is important to note that immunized children may still be at risk due to factors such as prematurity, which can impact maternal antibody levels and result in declining maternal immunity21,22.
To curb measles transmission, it is advised to maintain a population immunity level of 89–94%. Administering only the first dose of the measles-containing vaccine (MCV1) to nine-month-old children falls short of attaining this immunity threshold. Consequently, to ensure and sustain measles elimination, the WHO recommends achieving greater than or equal to 95% coverage with the two-dose measles-containing vaccine (MCV2)23,24. Typically, the second dose of the measles-containing vaccine is administered between 4 and 6 years of age. However, depending on the prevailing measles status in a region, it may be recommended to administer the second dose just a month after the first25. To achieve sufficient immunity levels and effectively halt transmission, two doses of the MMR vaccine are essential7. The increasing likelihood of measles outbreaks can be attributed to the rising trend of vaccine refusal, which the WHO recognized as one of the top ten global health threats in 201926.
Our study revealed that while the majority of measles cases occurred in children, 27.8% involved adults. WHO reports show that 77% of measles cases globally occur in children under 1525. Although measles predominantly affects children, especially in low-vaccination regions, the importance of adult cases should not be underestimated27. Vaccine efficacy in adults is affected by age, lifestyle, occupation, and health status. The aging process and immunosenescence diminish the immune response to vaccines, making the protection of adults increasingly difficult28.
In our study of adults over 25, 10 out of 27 patients were unvaccinated, while the vaccination history of the remainder was unknown. The occurrence of measles in adults who were previously vaccinated points to a possible decline in immunity over time post-vaccination29. Vaccination failure in measles is classified into primary and secondary forms. Primary failure denotes the absence of immunity development post-initial vaccination, whereas secondary failure represents a reduction in immunity over time following initially successful vaccination30,31. Notably, 13.9% (n=21) of our cases had received two vaccine doses yet developed measles again. It is important to note that primary failure of the measles vaccine is more prevalent than secondary failure. Most epidemiological studies indicate that immune suppression following vaccination is uncommon29.
Our findings revealed that the majority of cases were unvaccinated and below the vaccination age, underscoring the critical need for vaccination in measles prevention. It is crucial to acknowledge potential factors that might influence the accuracy of these figures. One such factor is the number of vaccine doses administered; individuals reporting measles contraction despite vaccination might have received an insufficient number of doses. Additionally, only 16.5% of cases (n=25) had a documented number of vaccine doses received (either one or two doses). This falls considerably short of the WHO’s target of 80%, indicating a shortfall in the immunization system32. Our findings show that 68.22% of measles cases (n=103) occurred in unvaccinated individuals. It is noteworthy that outbreaks can still occur in populations where only 10% are susceptible to measles24. Given the potential clustering of non-vaccinated individuals, the estimated vaccination coverage rates may not accurately reflect the overall population but rather a high-risk subpopulation33. Chains of measles transmission are frequently observed in enclosed settings or close-contact environments, such as healthcare facilities, schools, and households24.
Vaccine-preventable diseases such as rubella and measles may pose higher susceptibility risks among international migrants, yet there is limited data on the immune status among various migrant groups34. In our study, specific immigrant cases were identified in the measles outbreaks between 2014 and 2018. Notably, nine confirmed cases were traced to individuals originating from Pakistan and Afghanistan. To improve measles vaccination coverage, many countries have implemented supplemental immunization campaigns since 1994. However, some nations, including Afghanistan, Sudan, Somalia, Djibouti, and Pakistan, have reported measles vaccination coverage levels below 60%13. Contrastingly, measles vaccinations in Iran have increased dramatically, from 38% in 1980 to 99% in 2005, with nearly 99% of the population being immunized during this 25-year period35. Research emphasizing the importance of maintaining consistently high vaccination coverage has highlighted instances of measles outbreaks with imported cases from neighbouring countries, as observed in Australia. A similar situation exists in Iran, particularly concerning travellers arriving from neighbouring countries with widespread measles15. Therefore, immunization against measles and rubella is essential for immigrant groups who may not have been adequately immunized34.
In our study, the most common complication observed was a skin rash, followed by fever. An acute measles infection progresses through four stages: the incubation process, the prodrome, the exanthem, and the recovery and immunity phases. After the incubation phase, the prodrome is clinically characterized by symptoms such as cough, coryza, and conjunctivitis. The characteristic exanthem typically appears behind the ears and on the face 3–4 days after the onset of fever. In uncomplicated cases, clinical recovery is marked by a persistent cough and skin desquamation, which can be more pronounced in malnourished children. The persistence of fever during this stage may indicate potential complications related to measles36,37. Patients who are unvaccinated face a higher risk of complications, especially those who are very young or very old, are malnourished, live in crowded conditions, have vitamin A deficiency, are immunodeficient, or have been intensively exposed to measles38,39.
In our current study, no deaths attributable to measles were reported. This contrasts with findings from other studies: Mishra and colleagues reported a single death in their study, Murhekar and colleagues reported six deaths, which constituted a 1.5% Case Fatality Rate (CFR), Pomerai and colleagues reported five deaths (4% CFR), and Mishra and colleagues recorded 14 deaths (6.2% CFR)40–43. The absence of fatalities in our study might be attributed to the immediate medical attention provided to the cases and the prophylactic administration of Vitamin A, which may have significantly influenced the outcome.
In 2018 and 2019, several countries in the European and American regions, as well as in the Eastern Mediterranean Region (EMRO), lost their measles-elimination status. Given this context, it is not surprising that Iran also experienced a similar situation in 2018 with a dramatic outbreak. However, in 2019, when the EMRO was experiencing its greatest surge in measles cases, Iran was awarded a certificate for measles elimination in October 2019, which aligns with our findings of zero cases in that year. Following the detection of an outbreak, a catch-up vaccination campaign was promptly implemented. Additionally, during the follow-up phase, the number of measles cases reduced substantially44.
The implementation of the measles care system in Iran began primarily with the vaccination of children, predominantly in the 1990s45. Iran has implemented a measles-elimination surveillance plan utilizing a case-based system for suspected cases identified through symptoms like fever and maculopapular rashes. This approach involves reporting, laboratory confirmation, clinical and epidemiological studies, and the registration of cases using individual forms. The cohesive surveillance system, coupled with high vaccination rates for children under two years old, has contributed to a significant reduction in indigenous measles cases in Iran. As Iran approaches the final stages of measles elimination, there is a heightened need for sensitive reporting of suspected cases and the maintenance of vaccine coverage to ensure the preservation of elimination status46. On 28 May 2019, Iran received the certificate of elimination of measles44; however, attention must be paid to the disease care system, tracking the transmission chain and the source of the disease, following up on all cases of suspected disease, better implementation of case care, and maintaining vaccination coverage, including vaccinating immigrants. These measures are essential for the elimination of measles. Additionally, keeping population immunity high can reduce the occurrence of measles outbreaks47–49.
Strengths and limitation
The strengths of this study include its potential to enhance the direct uptake of generated evidence and its translation by the health system. Additionally, this simple analysis has demonstrated that health managers could use surveillance data in a timely manner to investigate program challenges and design appropriate interventions. Secondly, since the confirmation of measles cases is an important indicator of the performance of the measles-elimination program, our study utilized RT-PCR for confirmation rather than relying on IgG or IgM testing.
However, it is important to acknowledge certain limitations of the study. The retrospective nature of the analysis may be subject to constraints related to data availability and potential underreporting. Additionally, the study’s focus on a specific province in southern Iran may limit the generalizability of the findings to broader geographical contexts. The accuracy of vaccination status relies on available records, and discrepancies in reporting may exist. Furthermore, the absence of mortality in our study should be interpreted with caution, as it may be influenced by factors such as healthcare access and timely intervention.
Another limitation was the lack of data on the vaccination status in some cases. This weakness limits the ability to assess vaccination history comprehensively. In this regard, some modelling patterns could be useful tools to combine information from different data sources, helping to address some of the biases in the surveillance data and providing more robust evidence50.
Ethics statement
The study received approval from the Research Ethics Committee of Hormozgan University of Medical Sciences (with the ethics committee protocol number: IR.HUMS.REC.1399.471).
Consent to participate
Informed consent was obtained from participants involved in the study. All methods were conducted in accordance with relevant guidelines and regulations.
Source of funding
None.
Authors contributions
Study concept or design: M.S., S.A.Y. Data collection: L.D.S., G.G.H. Data analysis or interpretation: S.G., A.G. Writing the paper: all authors.
Conflicts of interest disclosure
The authors have no conflict of interest to declare.
Research registration unique identifying number (UIN)
Regrettably, we acknowledge that we did not register our study prospectively in a publicly accessible database before the recruitment of the first subject, as per your journal's submission guidelines. We sincerely apologize for this deviation from the prescribed procedure. In light of our oversight, we kindly request an exception to the registration policy and the opportunity to rectify this situation by registering our study retrospectively.
Guarantor
Seyed Hossein Asadi Yousefabad.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available within the article.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 3 April 2024
Contributor Information
Mehdi Hassani Azad, Email: mehdi.hassaniazad@gmail.com.
Laya Dehghani Sargazi, Email: Layadehghani20@gmail.com.
Mojtaba Salari, Email: mojtaba.salari.1994@gmail.com.
Samaneh Jahangiri, Email: smnaryn69@gmail.com.
Seyyed Mohammad Hashemi, Email: mohammadhashemi281@gmail.com.
Seyedeh Sahar Asadi, Email: Asadi.sahar6545@yahoo.com.
Ghasem Ghaedi Hengami, Email: ghaedi96@yahoo.com.
Arezoo Ghazalgoo, Email: arezooghazalgoo@gmail.com.
Mohammad-Hossein Keivanlou, Email: mh.keivanlou@gmail.com.
Ehsan Amini-Salehi, Email: ehsanaminisalehi1998@gmail.com.
Seyed Hossein Asadi Yousefabad, Email: hossein.asadi@hums.ac.ir.
References
- 1. Estofolete CF, de Aguiar Milhim BHG, de França CCG, et al. Prevalence of measles antibodies in São José do Rio Preto, São Paulo, Brazil: a serological survey model. Sci Rep 2020;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kondamudi NP, Waymack JR, Measles, StatPearls, StatPearls Publishing., StatPearls Publishing LLC, 2024. [PubMed] [Google Scholar]
- 3. Leung AK, Hon K, Leong K, et al. Measles: a disease often forgotten but not gone. Hong Kong Med J 2018;24:512–520. [DOI] [PubMed] [Google Scholar]
- 4. Thompson KM, Odahowski CL. The costs and valuation of health impacts of measles and rubella risk management policies. Risk Anal 2016;36:1357–1382. [DOI] [PubMed] [Google Scholar]
- 5. Stein-Zamir C, Shoob H, Abramson D. Measles clinical presentation, hospitalization and vaccination status among children in a community-wide outbreak. Vaccine 2023;41:2764–2768. [DOI] [PubMed] [Google Scholar]
- 6. Farooqi SS, The World Health Report 2005 - Make Every Mother and Child Count. Ann Saudi Med. 2005;25:516–7. [Google Scholar]
- 7. McLean HQ, Fiebelkorn AP, Temte JL, et al. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep Recommend Rep 2013;62:1–34. [PubMed] [Google Scholar]
- 8. Kobayashi T, Nishiura H. Transmission Network of Measles During the Yamagata Outbreak in Japan, 2017. J Epidemiol 2022;32:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orenstein WA, Cairns L, Hinman A, et al. Measles and Rubella Global Strategic Plan 2012-2020 midterm review report: Background and summary. Vaccine. 2018;36:A35–A42. [DOI] [PubMed] [Google Scholar]
- 10. Teleb N, Atta H, Hajjeh R. Measles and rubella elimination in the Eastern Mediterranean Region: successes and challenges. East Mediterr Health J 2019;25:667–668. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Lu P, Hu Y, et al. Cross-sectional surveys of measles antibodies in the Jiangsu Province of China from 2008 to 2010: the effect of high coverage with two doses of measles vaccine among children. PLoS One 2013;8:e66771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Probert WS, Glenn-Finer R, Espinosa A, et al. Molecular Epidemiology of Measles in California, United States—2019. J Infect Dis 2021;224:1015–1023. [DOI] [PubMed] [Google Scholar]
- 13. Gaafar T, Moshni E, Lievano F. The challenge of achieving measles elimination in the Eastern Mediterranean Region by 2010. J Infect Dis 2003;187(suppl 1):S164–S171. [DOI] [PubMed] [Google Scholar]
- 14. Salimi V, Abbasi S, Zahraei SM, et al. Implementation of a national measles elimination program in Iran: phylogenetic analysis of measles virus strains isolated during 2010–2012 outbreaks. PLoS One 2014;9:e94846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macintyre CR, Karki S, Sheikh M, et al. The role of travel in measles outbreaks in Australia—an enhanced surveillance study. Vaccine 2016;34:4386–4391. [DOI] [PubMed] [Google Scholar]
- 16. Izadi S, Zahraie S-M, Sartipi M. An investigation into a measles outbreak in southeast Iran. Jpn J Infect Dis 2012;65:45–51. [PubMed] [Google Scholar]
- 17. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization , Measles Outbreak Toolkit, 2020. https://www.who.int/emergencies/outbreak-toolkit/disease-outbreak-toolboxes/measles-outbreak-toolbox#:~:text=Case%20definitions%20for%20case%20finding&text=Any%20person%20with%20fever%20and,(i.e.%2C%20red%20eyes
- 19. Michel Y, Saloum K, Tournier C, et al. Rapid molecular diagnosis of measles virus infection in an epidemic setting. J Med Virol 2013;85:723–730. [DOI] [PubMed] [Google Scholar]
- 20. Mathew G, Agha R, Albrecht J, et al. 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 21. Manikkavasagan G, Ramsay M. Protecting infants against measles in England and Wales: a review. Arch Dis Child 2009;94:681–685. [DOI] [PubMed] [Google Scholar]
- 22. Ozbek S, Vural M, Tastan Y, et al. Passive immunity of premature infants against measles during early infancy. Acta Paediatr (Oslo, Norway: 1992) 1999;88:1254–1257. [DOI] [PubMed] [Google Scholar]
- 23. Thompson KM. Evolution and use of dynamic transmission models for measles and rubella risk and policy analysis, risk analysis: an official publication of the. Soc Risk Anal 2016;36:1383–1403. [DOI] [PubMed] [Google Scholar]
- 24. Rota PA, Moss WJ, Takeda M, et al. Measles. Nat Rev Dis Primers 2016;2:16049. [DOI] [PubMed] [Google Scholar]
- 25. What is the optimal age to give MCV2? Recommendations on timing. Accessed 23 February 2019. https://www.who.int/immunization/sage/8_Optimal_age_MCV2_GOODMAN.pdf?ua=1
- 26. Strebel PM, Orenstein WA. Measles. N Eng J Med 2019;381:349–357. [DOI] [PubMed] [Google Scholar]
- 27. El Zarif T, Kassir MF, Bizri N, et al. Measles and mumps outbreaks in Lebanon: trends and links. BMC Infect Dis 2020;20:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roses M, Bonvehí PE. Vaccines in adults. Medicina (Buenos Aires) 2019;79(6/1):552–558. [PubMed] [Google Scholar]
- 29. Mokhtari AT, Alavi MM, Yadegari D, et al. Epidemiologic survey of documented measles outbreak in Tehran, 2004.
- 30. Cherry JD, Feigin RD, Shackelford PG, et al. A clinical and serologic study of 103 children with measles vaccine failure. J Pediatr 1973;82:802–808. [DOI] [PubMed] [Google Scholar]
- 31. Nkowane BM, Bart SW, Orenstein WA, et al. Measles outbreak in a vaccinated school population: epidemiology, chains of transmission and the role of vaccine failures. Am J Public Health 1987;77:434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel MK, Orenstein WA. Classification of global measles cases in 2013–17 as due to policy or vaccination failure: a retrospective review of global surveillance data. Lancet Glob Health 2019;7:e313–e320. [DOI] [PubMed] [Google Scholar]
- 33. Althaus CL, Salathé M. Measles vaccination coverage and cases among vaccinated persons. Emerg Infect Dis 2015;21:1480–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hagstam P, Böttiger B, Winqvist N. Measles and rubella seroimmunity in newly arrived adult immigrants in Sweden. Infect Dis (London, England) 2019;51:122–130. [DOI] [PubMed] [Google Scholar]
- 35. Esteghamati A, Gouya MM, Zahraei SM, et al. Progress in measles and rubella elimination in Iran. Pediatr Infect Dis J 2007;26:1137–1141. [DOI] [PubMed] [Google Scholar]
- 36. Moss WJ. Measles. Lancet (London, England) 2017;390:2490–2502. [DOI] [PubMed] [Google Scholar]
- 37. Alves Graber EM, Andrade FJ, Jr, Bost W, et al. An Update and review of measles for emergency physicians. J Emerg Med 2020;58:610–615. [DOI] [PubMed] [Google Scholar]
- 38. Perry RT, Halsey NA. The clinical significance of measles: a review. J Infect Dis 2004;189(suppl 1):S4–S16. [DOI] [PubMed] [Google Scholar]
- 39. Beckford AP, Kaschula RO, Stephen C. Factors associated with fatal cases of measles. A retrospective autopsy study. South African Med J Suid-Afrikaanse tydskrif vir geneeskunde 1985;68:858–863. [PubMed] [Google Scholar]
- 40. Mishra PP, Chauhan NT. Double outbreak of measles in the talaja block of bhavnagar district, gujarat, India 2011: a need for improving the vaccine coverage and the community participation. J Clin Diagn Res 2012;6:1713–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murhekar MV, Hutin YJ, Ramakrishnan R, et al. The heterogeneity of measles epidemiology in India: implications for improving control measures. J Infect Dis 2011;204(suppl 1):S421–S426. [DOI] [PubMed] [Google Scholar]
- 42. Pomerai KW, Mudyiradima RF, Gombe NT. Measles outbreak investigation in Zaka, Masvingo Province, Zimbabwe, 2010. BMC Res Notes 2012;5:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mishra A, Mishra S, Lahariya C, et al. Practical observations from an epidemiological investigation of a measles outbreak in a district of India. Indian J Commun Med 2009;34:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Namaki S, Gouya MM, Zahraei SM, et al. The elimination of measles in Iran. Lancet Glob Health 2020;8:e173–e174. [DOI] [PubMed] [Google Scholar]
- 45. Zahraei SM, Gouya MM, Azad TM, et al. Successful control and impending elimination of measles in the Islamic Republic of Iran. J Infect Dis 2011;204(suppl 1):S305–S311. [DOI] [PubMed] [Google Scholar]
- 46. Karami M, Rahmani K, Moradi G, et al. Measles surveillance system in the Islamic Republic of Iran: History, structures and achievements. Iranian J Epidemiol 2020;16:81–89. [Google Scholar]
- 47. Perry RT, Gacic-Dobo M, Dabbagh A, et al. Progress toward regional measles elimination--worldwide, 2000-2013. Morb Mortal Wkly Rep 2014;63:1034–1038. [PMC free article] [PubMed] [Google Scholar]
- 48. Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev 1993;15:265–302. [DOI] [PubMed] [Google Scholar]
- 49. Celiloğlu C, Tolunay O, Çelik Ü. Evaluation of pediatric measles cases hospitalized in 2019. Turk Arch Pediatr 2021;56:328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ntirampeba D, Neema I, Kazembe L. Modelling spatio-temporal patterns of disease for spatially misaligned data: An application on measles incidence data in Namibia from 2005-2014. PLoS One 2018;13:e0201700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.