Abstract
Background:
Albumin acts as a scavenger of reactive oxygen species and an inhibitor of inflammatory processes that underlie hepatic encephalopathy (HE). However, the role of albumin in hepatic encephalopathy is not well-established. The authors performed this meta-analysis to evaluate the efficacy and safety of albumin in the management of hepatic encephalopathy.
Methods:
The authors carried out an extensive search across multiple databases, including MEDLINE (via PubMed), Embase, CENTRAL, and various trial registries, to identify randomized controlled trials (RCTs) evaluating the impact of albumin administration in HE. The authors used a random-effects model for analyses and presented dichotomous outcomes and continuous outcomes as relative risk and mean difference, along with corresponding 95% CIs, respectively. Heterogeneity was assessed using both the I2 index and χ2 test.
Results:
Our meta-analysis included 4 RCTs involving 306 patients. Our primary outcomes, mortality, and persistence of HE were reported by all four studies. Albumin was found to significantly decrease mortality in patients with HE [risk ratio (RR) 0.52, 95% CI 0.32–0.83; I2 =0%]. Persistence of HE was found to be comparable between the two groups (RR 0.83, 95% CI 0.68–1.00; I2 =24%). There was no significant difference between the albumin and control groups regarding length of hospital stay (MD −1.55, 95% CI −3.5 to 0.14; I2 =41%), adverse events (RR 1.00, 95% CI 0.87–1.16; I2 =0%), and severe adverse events (RR 0.89, 95% CI 0.59–1.35).
Conclusion:
Albumin administration in patients with hepatic encephalopathy decreases mortality but does not significantly impact the persistence of HE. Further high-quality, large-scale randomized controlled trials are needed to provide conclusive evidence.
Keywords: albumin, cirrhosis, hepatic encephalopathy, meta-analysis
Introduction
Highlights
Hepatic encephalopathy (HE) is the most frequent complication of cirrhosis with a cumulative incidence of 42% within 5–10 years.
Our meta-analysis included 4 randomized controlled trials involving 306 patients.
Our findings suggest that albumin administration decreases mortality in patients with HE, but no significant benefit was established regarding resolution of HE and length of hospital stay. Further high-quality, large-scale randomized controlled trials are needed to provide conclusive evidence.
Hepatic encephalopathy (HE) is a syndrome most seen in patients with liver failure and/or portosystemic shunting, which manifests as a wide symptomatic range of neuropsychiatric abnormalities from mild cognitive impairment and behavioural changes to marked disorientation, confusion, and coma1. It has become clear that it is also the most frequent complication of cirrhosis with a cumulative incidence of 42% within 5–10 years2,3. Studies have reported that the 30-day mortality in HE patients with grades 3 and 4 (West Haven clinical severity scoring) is 38%, independent of other organ system failure4.
The multi-factorial pathophysiology of HE suggests that compounds like ammonia, inflammatory cytokines, aromatic amino acids, as well as gut flora in the setting of decompensated liver disease, are shunted into the systemic circulation, and cause neuronal dysfunction and cerebral oedema due to their deleterious effects on the blood-brain barrier3. HE is a clinical diagnosis made after excluding other etiologies of encephalopathy.
Hepatic encephalopathy, however, is a reversible neuro-cognitive defect; hence, focusing on its treatment modalities warrants attention. Currently, non-absorbable disaccharides and non-absorbable antibiotics like lactulose and rifaximin, respectively, are the standard treatment for HE5. Albumin is a unique molecule in that its properties not only include intravascular volume expansion due to its bulky-sized molecule, which corrects effective arterial hypovolemia6, but it has also been shown to act as a scavenger of reactive oxygen species and is also an inhibitor of inflammatory mediators that are known to cause HE5,6. Albumin therapy has been shown to reduce the severity of HE with an improved 90-day survival6,7. Albumin therapy has also been shown to reverse HE compared to using disaccharides alone in HE6. However, the efficacy and safety of albumin therapy in patients with hepatic encephalopathy is not yet established. Previous trials evaluating the efficacy and safety of albumin in HE have yielded inconsistent results7–10.
Since the previous meta-analysis6 included only two trials and two more trials have been conducted since then, we aimed to consolidate the data from all the randomized controlled trials to assess the efficacy and safety of albumin therapy in HE.
Material and methods
We conducted our systematic review and meta-analysis following the Cochrane Handbook for Systematic Reviews and Interventions guidelines11. This meta-analysis was reported according to the PRISMA statement12, Supplemental Digital Content 1, http://links.lww.com/MS9/A424. We registered the study in the International Prospective Register of Systematic Reviews (PROSPERO) with the identifier number CRD42023460615. Our study did not require ethical committee approval. We have also evaluated the quality of our systematic review through AMSTAR 2 criteria13, Supplemental Digital Content 2, http://links.lww.com/MS9/A425.
Eligibility criteria
The studies included in our systematic review were only randomized controlled trials (RCTs) evaluating the efficacy and safety of albumin in comparison to placebo or any standard treatment in patients with HE independent of the cause. We included studies that reported at least one of our review’s outcomes.
We excluded animal trials and all study designs other than RCTs, such as observational studies and reviews.
Information sources
We searched from inspection to August 2023 on the following databases: Cochrane Central Register of Controlled Trials (CENTRAL, using the Cochrane Library), Embase (using Ovid), MEDLINE (using PubMed), and ClinicalTrials.gov with no language restriction. We checked ProQuest Dissertations and Theses Global (PQDT) and OpenGrey sources for potential grey literature.
We looked up the reference lists of all the included studies and related systematic reviews to include relevant studies in our analysis. We also conducted forward citation searching to retrieve eligible studies.
The literature search used terms related to “albumin”, “hepatic encephalopathy”, and “cirrhosis”.
Selection process
The studies found in the online databases were organized using Zotero, a screening software tool, and removed the duplicate studies. Two authors sequentially conducted title, abstract, and full-text screening to include or exclude studies based on the study’s inclusion and exclusion criteria. A third author resolved any conflict between the two authors.
Data collection and data items
Two reviewers separately extracted data from the four finalized studies into an Excel spreadsheet with the following study characteristics: study ID, country of origin, trial design, follow-up duration, sample size, demographic variables like age and sex, details of experimental and comparator groups, disease-specific factors like aetiology of HE, Baseline model for end-stage liver disease (MELD) score, severity of HE, prior history of HE and outcome variables.
Our study’s primary outcome measures were mortality and persistence of HE. Secondary outcome measures analyzed were length of hospital stay, adverse events, and serious adverse events.
Risk of bias assessment
We used the revised Cochrane Risk of Bias tool for randomized trials (RoB 2.0) for assessing the quality of our included articles14. Two reviewers independently assessed studies for problems in the randomization process, any deviation from intended interventions, missed outcome data, outcome measurement, and reporting of results.
Data synthesis
We utilized Review Manager (RevMan version 5.4; The Cochrane Collaboration) for our analysis. Dichotomous outcomes were presented as relative risk with 95% CIs, while continuous outcomes were expressed as mean difference with corresponding 95% CIs. In order to ensure consistency in the analyses, we transformed medians and interquartile ranges into means and standard deviations, following the approach suggested by Wan and colleagues. To conduct meta-analyses, we employed the DerSimonian and Laird random-effects model. Heterogeneity was assessed using both the I2 index and χ2 test. To evaluate publication bias in analyses involving a minimum of 10 studies, we used a funnel plot and checked for any asymmetry through Egger’s test implemented in Jamovi software’s MAJOR module (version 1.8). A P value less than 10 indicated potential publication bias.
Results
Selection process
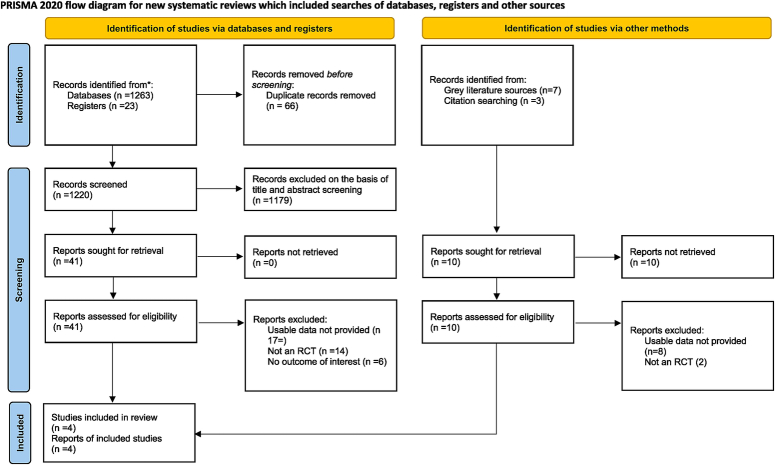
Our literature search yielded 1293 studies, which were then subjected to de-duplication and screening based on title and abstract. Fifty-one studies underwent full-text screening, but only four studies satisfied the inclusion criteria and were included in our review7–10. The detailed selection process is illustrated in a PRISMA flowchart (Fig. 1).
Figure 1.
PRISMA 2020 flowchart of included and excluded trials. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial..
Study characteristics
A total of four studies with a total of 306 patients were included in our meta-analysis7–10. While three of the included studies used normal saline as the control group7,9,10, one study used lactulose as the control intervention8. The method of administration of albumin was intravenous in all the studies, and the dose was set to be around 1.5 g/kg body weight/day. Fagan et al.10 only included patients with HE grade 0 (minimal hepatic encephalopathy), while all the other studies recruited patients with HE West Haven Grade II-IV. The detailed study characteristics of individual studies are shown in Table 1.
Table 1.
Characteristics of included studies.
| Characteristic | Ventura-Cots et al.9 | Fagan et al.10 | Sharma et al.8 | Simon-Talero et al.7 |
|---|---|---|---|---|
| Author and YOP | 2021 | 2023 | 2017 | 2013 |
| Country | Switzerland | Virginia | India | Spain |
| Sample size | 82 | 48 | 120 | 56 |
| Intervention | Albumin (n=40) | Albumin (n=24) | Lactulose + (n=60) | Albumin (n=26) |
| Control | Isotonic saline (n=42) | Isotonic saline (n=24) | Lactulose (n=60) | Isotonic saline (n=30) |
| Age (median/mean/frequency)a | 66.5 (59.9–73.6) vs. 69.1 (63.3–75.3) | 62.21±8.59 vs. 63.83±6.99 | 42.5±8.7 vs. 38.4±9.6 | 63.7±11.3 vs. 66.3±9.7 |
| Male sex, n (%) | 55 (67) | 43 (89.5) | 100 (83.3) | 42 (75) |
| Aetiology, n (%) | ||||
| Viral hepatitis | 11 (13.4) | 7 (14.5) | 36 (30) | 19 (33.9) |
| Alcohol | 41 (50) | 22 (45.8) | 67 (55.8) | 24 (42.9) |
| Other | 12 (14.6) | 2 (4.2) | 17 (14.2) | 13 (23.2) |
| MELD scorea | 17 (15–20) vs. 17 (16–20) | 11.75±3.78 vs. 10.46±3.36 | 26.4±5.8 vs. 25.8±5.1 | 16.8±3.8 vs. 16.1±5.1 |
| Duration of treatment | 3 days | 5 weeks | 10 days | 3 days |
| Severity of HE; n (%)a | ||||
| Grade II–III | 40 (100) vs. 41 (97.6) | -b | 41 (68.3) vs. 43 (71.6) | 22 (84.6) vs. 24 (80) |
| Grade IV | 0 vs. 1 (2.4) | -b | 19 (31.7) vs. 17 (28.4) | 4 (15.4) vs. 6 (20) |
HE, hepatic encephalopathy; MELD, model for end-stage liver disease; MHE, minimal hepatic encephalopathy; YOP, year of publication.
Intervention group compared to the control group, non-significant.
All patients had MHE (Grade 0).
Risk of bias in included studies
The detailed risk of bias assessment is illustrated in Figure 2. Two studies were found to have a low risk of bias, while the other two had some concerns of bias due to lack of information regarding a pre-specified analysis plan.
Figure 2.
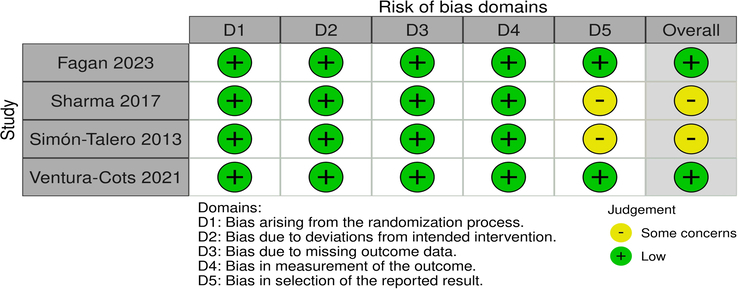
Quality assessment of randomized controlled trials.
Primary outcomes
Mortality
All the included studies evaluated mortality between albumin and control group [305 (150 vs. 155)]7–10. Our meta-analysis indicated a significant decrease in mortality in the albumin group compared to the control group [risk ratio (RR) 0.52, 95% CI 0.32–0.83; Fig. 3]. The estimated heterogeneity was low (I2=0%). After excluding Fagan and colleagues, the result did not change (RR 0.52, 95% CI 0.3–-0.83; Supplementary Figure 1, Supplemental Digital Content 3, http://links.lww.com/MS9/A426)
Figure 3.
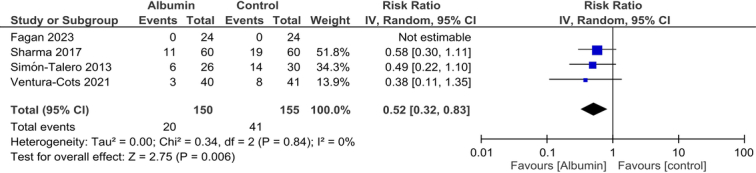
Comparison of mortality between patients receiving albumin or control in patients with hepatic encephalopathy.
Persistence of HE
There was no difference in the persistence of HE between the two groups (RR 0.83, 95% CI 0.68–1.00; Fig. 4), and the heterogeneity was found to be low (I2=24%). After excluding Fagan et al., the result did not change (RR 0.79, 95% CI 0.56–1.13; Supplementary figure 2, Supplemental Digital Content 3, http://links.lww.com/MS9/A426).
Figure 4.
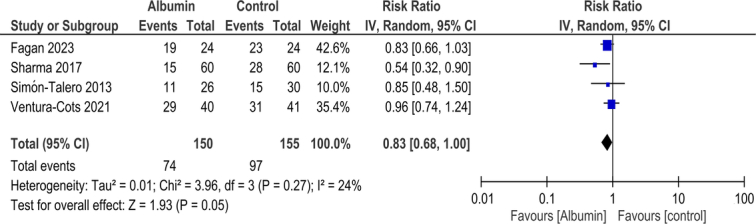
Comparison of persistence of hepatic encephalopathy (HE) between patients receiving albumin or control in patients with HE.
Secondary outcomes
Length of hospital stay
Length of hospital stay was evaluated by two studies and was found to be comparable between the two cohorts (MD −1.55, 95% CI −3.5 to 0.14; Supplementary figure 3, Supplemental Digital Content 3, http://links.lww.com/MS9/A426). The estimated heterogeneity for this outcome was moderate (I2=41%).
Adverse events
Only 2 studies evaluated adverse events between the two groups8,9. Use of albumin did not increase the risk of adverse events as compared to the control group (RR 1.00, 95% CI 0.87–1.16; Supplementary figure 4, Supplemental Digital Content 3, http://links.lww.com/MS9/A426). The interstudy heterogeneity was low (I2=0%).
Serious adverse events
The meta-analysis of two studies8,9 showed that there was no significant increase in serious adverse events with the use of albumin in HE as compared to control (RR 0.89, 95% CI 0.59–1.35; Supplementary figure 5, Supplemental Digital Content 3, http://links.lww.com/MS9/A426).
Publication bias
As studies are less than 10, we did not assess publication bias using funnel plot.
Discussion
The benefits of albumin infusion in the treatment of HE has been investigated in the past, but there is still a lack of data to advocate the use of albumin infusion as a standard of care in HE. The efficacy and safety of albumin administration in patients with hepatic encephalopathy have been examined in this meta-analysis of 4 RCTs involving 305 participants in 3 countries. It has been shown that albumin administration significantly reduced mortality in HE patients, but there was no significant improvement in terms of HE persistence or length of hospital stay. However, it is necessary to mention that there was a considerable trend towards the resolution of HE with albumin (RR 0.83, 95% CI 0.68–1.00). Albumin therapy did not increase the risk of adverse events and serious adverse events.
The main factor responsible for the development of HE is hyperammonemia15,16. The elevated ammonia levels in the brain causes an imbalance of various neurophysiological mechanisms causing abnormalities in neurotransmission, formation of pro-inflammatory cytokines, increases the oxidative stress and impairs the blood-brain barrier, thus leading to hepatic encephalopathy17–20. The role of albumin as a therapeutic agent for HE is based on the fact that it binds to reactive oxygen species, thus reducing oxidative stress, and it inhibits the pro-inflammatory cytokines production, thus reducing endothelial dysfunction and minimizing the vasodilation21. Albumin, when administered intravenously, can help restore colloid osmotic pressure, thereby promoting fluid retention within the vascular space and fluid accumulation in interstitial spaces. Albumin also has opsonizing properties, which help prevent the infectious complications that occur in patients with HE, such as spontaneous bacterial peritonitis22.
A similar meta-analysis by Bombassaro et al.6 done in 2021 showed that albumin was associated to significantly lower risks of hepatic encephalopathy and mortality but the fact that only 2 RCTs were included limits the scope of the results from their meta-analysis. The results of our meta-analysis are in accordance to the prior meta-analysis by Bombassaro et.al.6 in regard to the mortality benefit but deviated in regards to the resolution of HE. The inclusion of only two studies, lack of blinding by Sharma et al.8, heterogeneity between the two studies, and moderate risk of bias of those two included studies might have contributed to the difference observed.
Two of our included studies, Sharma et al.8 and Fagan et al.10 showed that albumin infusion can lead to the resolution of HE while the RCTs conducted by Simón-Talero et al.7 and Ventura-Cots9 found no significant difference between groups regarding the improvement in HE. The lack of blinding in the study by Sharma et al.8 might influence the assessment of HE in favour of the albumin by the investigators. The latest study by Fagan et al.10 included patients with prior overt HE with grade 0 on the West Haven scale that is minimal hepatic encephalopathy (MHE), who were already on rifaximin or lactulose for 2 months prior to enrolment, while the other three studies included patients with grade 2 or above on the West Haven scale. We performed a sensitivity analysis to address this potential source of heterogeneity as Fagan and colleagues only included patients with MHE. However, our results for mortality and persistence of HE did not change on the exclusion of Fagan and colleagues.
The study by Simón-Talero et al.7 was double-blinded and used an independent assessor for evaluation of HE but was limited by a small sample size. The study by Ventura-Cots9 was also a double-blind multi-centre trial but only an improvement in mortality with no significant difference in HE episodes between the groups during follow-up period. Another meta-analysis by Teh et al.23 showed that albumin infusion reduced the pooled risk of overt HE in cirrhosis patients and also lowered the pooled risk of developing overt HE in cirrhotic patients without OHE at baseline. There was no significant difference in the length of hospital stay or incidence of severe adverse events between the groups.
Our systematic review and meta-analysis include several notable strengths, which increase the reliability and comprehensiveness of our findings. To begin with, we expanded upon the 2021 published meta-analysis of Bombassaro and colleagues by incorporating two additional pertinent studies9,10, thereby enhancing the power and validity of our analysis. This augmentation allows for a more up-to-date and thorough evaluation of albumin’s efficacy in hepatic encephalopathy management. Secondly, the uniformity of the intervention method across all included studies is an important strength. The consistent administration of 20% albumin at a dose of 1.5 g/kg body weight/day IV enables an objective and matchable comparison between the different studies, minimizing potential confounding factors. Lastly, our study’s strength lies in the diversity and representativeness of the patient population across the selected studies. These studies incorporated patients from three countries, displaying an equal representation of genders and age groups. This diversity increases the external validity of our results, making them more applicable to a wider range of individuals affected by hepatic encephalopathy.
In contrary to the strengths mentioned above, it is of utmost importance to notice several limitations that could alter the interpretation of our findings. Only four studies with small sample sizes were included in our review, leading to decreased statistical power in evaluating our efficacy and safety outcomes. Our secondary outcomes were evaluated by only two studies, thus decreasing the power of analyses and may contribute to the absence of statistically significant differences in these outcomes. Moderate risk of bias in two of the four included studies and lack of blinding by Sharma and colleagues, which was the largest contributor to our meta-analysis, could also have affected the precision of the estimates. Moreover, the mortality benefit seen with albumin administration without significant resolution of HE cannot be attributed to albumin solely.
Additionally, the severity of hepatic encephalopathy of included patients varied a lot across the included studies. While some studies, like Fagan and colleagues included patients with only minimal symptoms according to Grade 0 of West Haven Criteria (MHE), others like Sharma and colleagues included over 70% patients with Grade III or IV of West Haven. Careful consideration of these differences is imperative when interpreting the clinical implications of our findings.
Differences in follow-up durations among the studies, beginning with as brief as 10 days to over 90 days, could introduce variability in our outcomes and potentially impact the generalizability. Another limitation of our review is that we could not assess the potential impact of precipitating factors of hepatic encephalopathy on the outcomes due to insufficient data. We also did not utilize Web of Science as a database during the study selection process. Finally, since patient-level data were not available to us, our meta-analysis was built using aggregate-level data.
To summarize, while our systematic review and meta-analysis offer valuable insights into the potential benefits of albumin in hepatic encephalopathy management, these findings should be interpreted cautiously, considering the limitations. Further research with larger sample sizes, and extended follow-up periods may yield additional clarity regarding the efficacy and safety of albumin in this clinical context. Consequently, there’s an increased need of further wide-range multi-centre randomized controlled trials assessing the role of albumin for the treatment of hepatic encephalopathy. In order to fully analyze the benefit of albumin in HE, we feel that other factors including oxidative stress, inflammation and sodium levels, should be controlled prior to the study so that the effect of these factors is minimized24.
Furthermore, it would be of great importance to compare the effect of albumin with currently already established and recommended treatment modalities, which are used in general clinical practice, like lactulose, ornithine and/or antibiotics (e.g. rifaximin). This would enable us to further investigate, whether there is a notifiable difference and benefit of albumin usage. Moreover, based on these results, secondary aspects like economic benefits, ecological advantages and/or benefits regarding the logistic availability of albumin in comparison to other established treatment options could be discussed in further stages.
Conclusion
Our meta-analysis suggests that albumin administration decreases mortality in patients with HE but did not significantly impact the resolution of HE. To achieve more definite conclusions regarding the efficacy and safety of albumin in HE, it is imperative to conduct further large-scale RCTs.
Ethical approval
Ethical approval was not required for this review.
Consent
No informed consents were required for the purpose of the current study.
Sources of funding
No financial support was received for this study.
Author contribution
F.M., M.M., M.E., and K.K. contributed to the conception and design of the study. O.F., S.S., S.N., V.N.N., V.P., A.T.S., Y.K. and L.C. contributed to the analysis, interpretation and writing of the original draft. F.M., M.M., M.E., K.K. and O.F. contributed to the interpretation and critical revision of the draft. All authors read and approved the final version and agree to be held accountable for it.
Conflicts of interest disclosure
The authors report no relationships that could be construed as a conflict of interest.
Research registration unique identifying number (UIN)
PROSPERO registration number: CRD42023460615.
Link: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=460615.
Guarantor
Kamal Kandel.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Human and animal participants
Research involving human participants and/or animals: No animals or human subjects were used in the current study.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/annals-of-medicine-and-surgery.
Published online 24 April 2024
Contributor Information
Farhan Murtaza, Email: farhanmurtaza3@gmail.com.
Midhun Mathew, Email: midhunmathew100@yahoo.com.
Oluwaseun Fagbamila, Email: Seun.f.fagbamila@gmail.com.
Sachin Subramani, Email: sachinsachu930@gmail.com.
Simran Nimal, Email: simranrnimal@gmail.com.
Veeramachaneni Naga Nyshita, Email: vnn9885@gmail.com.
Vishnu Priya, Email: priyaselvam06@gmail.com.
Abu Talha Sany, Email: dr.abutalha689@gmail.com.
Yamanth Kumar, Email: yamanthkumar67@gmail.com.
Laura Cicani, Email: Lcicani@gmail.com.
Muhammad Ehsan, Email: m.ehsanqadri@gmail.com.
Kamal Kandel, Email: Kamalkandel010@gmail.com.
References
- 1. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715–735. [DOI] [PubMed] [Google Scholar]
- 2. Hirode G, Vittinghoff E, Wong RJ. Increasing burden of hepatic encephalopathy among hospitalized adults: an analysis of the 2010–2014 national inpatient sample. Dig Dis Sci 2019;64:1448–1457. [DOI] [PubMed] [Google Scholar]
- 3. Elsaid MI, Rustgi VK. Epidemiology of hepatic encephalopathy. Clin Liver Dis 2020;24:157–174. [DOI] [PubMed] [Google Scholar]
- 4. Bajaj JS, O’Leary JG, Tandon P, et al. Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol 2017;15:565–574.e4. [DOI] [PubMed] [Google Scholar]
- 5. Fallahzadeh MA, Rahimi RS. Hepatic encephalopathy: current and emerging treatment modalities. Clin Gastroenterol Hepatol 2022;20:S9–S19. [DOI] [PubMed] [Google Scholar]
- 6. Bombassaro IZ, Tovo CV, de Mattos ÂZ, et al. Albumin in the management of hepatic encephalopathy: a systematic review and meta-analysis. Ann Hepatol 2021;26:100541. [DOI] [PubMed] [Google Scholar]
- 7. Simón-Talero M, García-Martínez R, Torrens M, et al. Effects of intravenous albumin in patients with cirrhosis and episodic hepatic encephalopathy: a randomized double-blind study. J Hepatol 2013;59:1184–1192. [DOI] [PubMed] [Google Scholar]
- 8. Sharma BC, Singh J, Srivastava S, et al. Randomized controlled trial comparing lactulose plus albumin versus lactulose alone for treatment of hepatic encephalopathy. J Gastroenterol Hepatol 2017;32:1234–1239. [DOI] [PubMed] [Google Scholar]
- 9. Ventura-Cots M, Simón-Talero M, Poca M, et al. Effects of albumin on survival after a hepatic encephalopathy episode: randomized double-blind trial and meta-analysis. J Clin Med 2021;10:4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fagan A, Gavis EA, Gallagher ML, et al. A double-blind randomized placebo-controlled trial of albumin in outpatients with hepatic encephalopathy: HEAL study. J Hepatol 2023;78:312–321. [DOI] [PubMed] [Google Scholar]
- 11. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). www.training.cochrane.org/handbook [Google Scholar]
- 12. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 13. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JPT Altman DG Gøtzsche PC et al.. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials BMJ 2011;343:d5928 [Google Scholar]
- 15. Felipo V, Urios A, Montesinos E, et al. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metab Brain Dis 2012;27:51–58. [DOI] [PubMed] [Google Scholar]
- 16. Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol 2002;67:259–279. [DOI] [PubMed] [Google Scholar]
- 17. Montgomery JY, Bajaj JS. Advances in the evaluation and management of minimal hepatic encephalopathy. Curr Gastroenterol Rep 2011;13:26–33. [DOI] [PubMed] [Google Scholar]
- 18. Butterworth RF, Giguère JF, Michaud J, et al. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol 1987;6:1–12. [DOI] [PubMed] [Google Scholar]
- 19. Skowrońska M, Albrecht J. Alterations of blood brain barrier function in hyperammonemia: an overview. Neurotox Res 2012;21:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li T, Li X, Zhou W, et al. Dynamic susceptibility contrast-enhanced first-pass perfusion MR imaging in patients with subclinical hepatic encephalopathy. J Neuroradiol J Neuroradiol 2012;39:290–294. [DOI] [PubMed] [Google Scholar]
- 21. Bortoluzzi A, Ceolotto G, Gola E, et al. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatol Baltim Md 2013;57:266–276. [DOI] [PubMed] [Google Scholar]
- 22. Bernardi M, Maggioli C, Zaccherini G. Human albumin in the management of complications of liver cirrhosis. Crit Care 2012;16:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teh KB, Loo JH, Tam YC, et al. Efficacy and safety of albumin infusion for overt hepatic encephalopathy: a systematic review and meta-analysis. Dig Liver Dis 2021;53:817–823. [DOI] [PubMed] [Google Scholar]
- 24. Bai Z, Bernardi M, Yoshida EM, et al. Albumin infusion may decrease the incidence and severity of overt hepatic encephalopathy in liver cirrhosis. Aging 2019;11:8502–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.