Since the approval of the first immune checkpoint inhibitor (ICI) in 2011, ICIs have revolutionized the landscape of cancer therapy, with nearly 50% of all solid tumor patients now receiving ICI-based mono or combination therapy.1 ICIs are monoclonal antibodies administered as i.v. infusions every 2–4 weeks that target either cytotoxic T-lymphocyte associated protein-4 (CTLA-4) or programmed cell death protein 1 expression on host immune cells or the programmed death-ligand 1 expressed in tumor cells. The efficacy of ICIs arises largely from their restoration of host T-cell activity against tumor-specific Ag. However, these molecular effects also result in an array of immune-related adverse events (irAEs) due to loss of self-tolerance with resultant tissue damage. Dermatitis and colitis are most commonly encountered with ICI treatment (30%–40%) followed by hepatitis (10%–15%), pneumonitis (5%–10%), and other endocrine disorders (< 5%).1 Moderate to severe irAEs not only lead to delays and/or permanent discontinuation of therapy but also reduced anti-tumor efficacy. The incidence of immune-mediated liver injury from checkpoint inhibitors (ILICI) was lower (1%–5%) in carefully selected patients enrolled in clinical trials compared with the rates seen in clinical practice of 20%–30%.2,3 The aim of this review is to provide an update on the salient clinical features, recommended medical evaluation, and a stepwise approach to ILICI treatment.
CLINICAL PRESENTATION
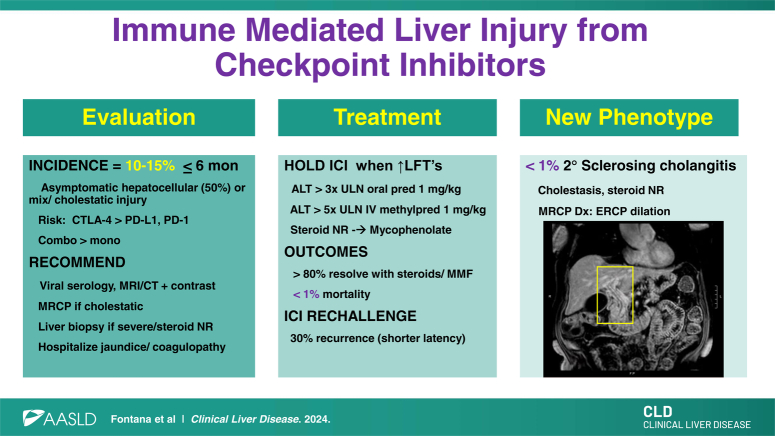
The National Comprehensive Cancer Network developed the Common Terminology Criteria for Adverse Events (version 5.0) to help standardize the diagnosis and staging of ILICI and other irAEs (Table 1).4 Most ICIs are administered on a protocolized basis with a pretreatment clinic visit and blood draw (complete blood count, comprehensive metabolic panel, and thyroid stimulating hormone) to assess for irAEs. ILICI most commonly arises within the first 6 months of therapy but may occur at any time during therapy.5,6,7 Most patients with ILICI are asymptomatic, but a minority of patients may present with jaundice, fever, or abdominal pain.5 The laboratory profile at ILICI onset is hepatocellular in 40%–50% of patients, while others present with a mixed or cholestatic lab profile.6 Most patients have a normal total bilirubin and international normalized ratio level at presentation. Of note, concomitant irAEs involving other organs may be present in up to 10% of patients at ILICI onset, and ILICI may develop after steroid tapering for other irAEs.
TABLE 1.
CTCAE (version 5.0) grading of immunotherapy hepatotoxicity
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|
| Serum AST/ ALT | 1–3 × ULN (abn baseline) | > 3–5 × ULN (abn baseline) | > 5–20 × ULN (abn baseline) | > 20 × ULN (abn baseline) | Death |
| Alkaline phosphatase | 2.5 × ULN (abn baseline) | > 2.5–5 × ULN (abn baseline) | > 5–20 × ULN (abn baseline) | > 20 × ULN (abn baseline) | Death |
| Total bilirubin | 1–1.5 × ULN (abn baseline) | > 1.5–3 × ULN (abn baseline) | 3–10 × ULN (abn baseline) | > 10 × ULN (abn baseline) | Death |
Abbreviations: abn, abnormal; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, common terminology criteria for adverse events; ULN, upper limit of normal.
A stepwise approach to the medical evaluation of patients receiving an ICI with liver injury is recommended (Table 2). Since ILICI is largely a clinical diagnosis of exclusion, all patients should be evaluated for competing causes of liver injury through a review of their alcohol intake, medical history, and concomitant medication and dietary supplement exposure. In addition, testing for viral hepatitis (hepatitis A/B/C serologies), screening for hepatotoxins, and autoantibody testing is recommended (Table 2). For patients with abdominal symptoms or ≥ grade 2 ILICI, liver imaging can assess the hepatic vasculature, potential for hepatic metastases, extrinsic biliary compression, and choledocholithiasis. Although liver ultrasound is widely available and easy to obtain, contrast-enhanced computerized tomography scan or MRI is preferred due to its superior sensitivity and specificity for hepatic metastases and vascular issues. Furthermore, magnetic resonance cholangiopancreatography can determine if secondary sclerosing cholangitis is present in those with mixed or cholestatic ILICI. In patients with atypical features such as fever, rash, or systemic symptoms, a more extensive laboratory evaluation for nonhepatotropic viruses may prove worthwhile (Table 2).
TABLE 2.
Recommended medical evaluation for immune checkpoint inhibitor-treated patients with liver injury
| Competing etiologies | Diagnostic testing |
|---|---|
| Other drugs or hepatotoxins | Medical history Drug and supplement use Alcohol intake Lab testing Urine toxicology Serum phosphatidyl ethanol |
| Viral hepatitis | Lab testing Hepatitis A IgM HBsAg, anti-HBc Anti-HCV and HCV-RNA Anti-HEV IgM and HEV-RNAa |
| Autoimmune hepatitis | Lab testing ANA, anti-SMA Quantitative immunoglobulins Liver biopsyb |
| Metastatic tumor to the liver (choledocholithiasis/biliary obstruction) | Imaging Liver ultrasound with Doppler CT or MRI with contrast MRCP |
| Liver vascular disease (hepatic ischemia/portal vein thromboses) | Medical history Prior hypotension/arrhythmia, heart disease Imaging Liver ultrasound with Doppler Liver CT or MRI with contrast |
| Secondary testing | |
| Opportunistic infections | Medical history Fever, skin rash Lab testing CMV-DNA, anti-CMV IgM HSV-DNA, anti-HSV IgM Heterophile ab, EBV-DNA, EBV serologies Liver biopsy |
| Other etiologies (Liver micrometastases, hepatic steatosis) | Liver biopsy |
Only in areas of high endemicity (not US).
Generally reserved when there is a high index of suspicion with elevated autoantibody titers and serum IgG levels.
Abbreviations: ANA, anti-nuclear antibody; CT, computerized tomography; ICI, immune checkpoint inhibitor; MRCP, magnetic resonance cholangiopancreatography; SMA, smooth muscle antibody.
A variety of histopathological findings have been reported in patients with ILICI, including noncaseating granulomas, lobular hepatitis, pericentral necrosis, and hepatocyte apoptosis.1,5 However, the histopathology of ILICI is distinct from that of autoimmune hepatitis, and very few patients have plasma cell infiltrates. One recent retrospective study of 100 patients with ILICI demonstrated that an alternative and unsuspected diagnosis was only seen in 10% and that performing a liver biopsy led to a delay in steroids in some patients (Table 3).8 Therefore, we recommend obtaining a liver biopsy only in selected patients, including those with atypical clinical features at presentation or evidence of synthetic dysfunction with a rising bilirubin or international normalized ratio. In addition, a liver biopsy is recommended if a Grade 2/3 patient does not respond to corticosteroids before initiating additional immunosuppression to better define and confirm the nature and severity of liver injury. Furthermore, a liver biopsy should be considered in the 20% of patients who do not respond to high-dose mycophenolate, if not previously done, to confirm the diagnosis.
TABLE 3.
Considerations regarding liver biopsy in liver injury associated with checkpoint inhibitors
| Benefits | Limitations |
|---|---|
| Diagnosis confirmation | Specificity of findings |
| Findings can strengthen diagnosis if uncertain or atypical presentation or labs (lobular inflammation, endotheliitis, granulomas, and apoptosis) | No pathognomonic histological findings for ILICI |
| Prognosis | Periprocedural risk |
| Eosinophils and granulomas have better outcomes | 1%–2% risk of severe bleeding/hospitalization |
| Severe necrosis and fibrosis have poorer outcomes | 30% require analgesics |
| Identify pre-existing liver disease | Logistics |
| Metabolic-associated liver disease in 10%–20% of general US population | Scheduling Delay in corticosteroids |
| Alternative etiology and management | Clinical impact |
| Malignant infiltration of the liver will worsen with immunosuppression | < 10% have an alternative etiology |
| Opportunistic viral infection (HSV, CMV) will worsen with immunosuppression | > 80% of Grade 2–4 patients rapidly respond to corticosteroids |
| Cholestatic patients may have small duct sclerosing cholangitis only seen on biopsy | — |
Abbreviations: CMV, cytomegalovirus; HSV, herpes simplex virus; ILICI, immune-mediated liver injury from checkpoint inhibitor.
CLINICAL RISK FACTORS
Patients who receive a CTLA-4 inhibitor (ipilimumab, tremelimumab) are at higher risk for various irAEs including ILICI in comparison to patients treated with a programmed cell death protein 1 (nivolumab, pembrolizumab, cemiplimab, dostarlimab, retifanlimab, and toripalimab) or programmed death-ligand 1 inhibitor (durvalumab, avelumab, and atezolizumab).2 Patients treated with a combination of a CTLA-4 and programmed cell death protein 1 inhibitor are at greatest risk of ILICI,2,9 and a CTLA-4 combined with other chemotherapeutic agents can also lead to synergistic hepatotoxicity.3,6,7,9 Some studies have found female gender and younger age as risk factors for ILICI, but these have not been replicated in other cohorts.3,9 The tumor type and stage also do not affect the risk of developing ILICI, with the exception that patients with HCC experience higher rates of liver injury.3,7 There is evolving data that the use of broad-spectrum antibiotics before ICI initiation may reduce the efficacy of treatment, but whether manipulation of the host microbiome may improve efficacy or reduce irAEs remains unclear.10
MANAGEMENT OF ILICI
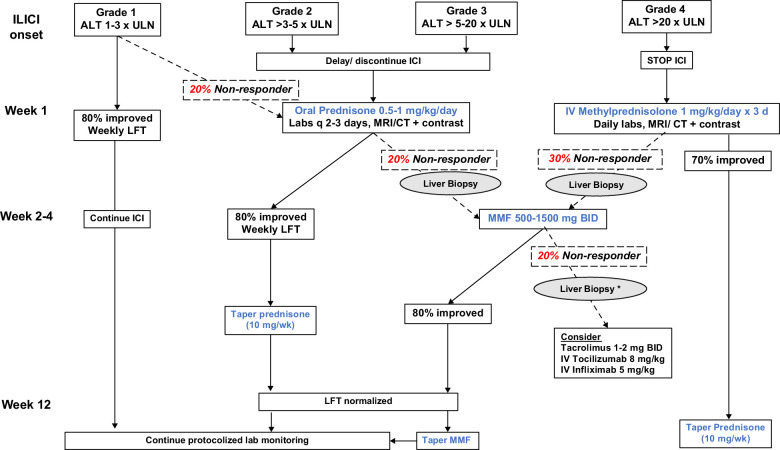
Once a diagnosis of ILICI is established, management largely depends on the severity (Figure 1). Grade 1 patients may continue immunotherapy with more frequent lab monitoring once the labs have normalized. Subjects with Grade 2 toxicity should have the ICI held and have labs repeated within 3 to 5 days. In those with worsening labs, oral corticosteroids should be initiated at a dose of prednisone 0.5–1 mg/kg/day. Although 80% of patients will respond to oral steroids, nonresponders should be considered for a liver biopsy before increasing immunosuppression. For patients with Grade 3 ILICI, the ICI should be held, and steroids initiated with either high-dose oral prednisone or i.v. methylprednisolone 1 mg/kg/day for up to 3 days with daily lab monitoring. Patients with progressive ILICI despite corticosteroids, evidence of synthetic dysfunction with jaundice or coagulopathy, or Grade 4 hepatitis should be hospitalized for a more rapid and thorough evaluation, including serological testing, contrast-enhanced liver imaging, and a liver biopsy if not previously done.4,11
FIGURE 1.
Treatment algorithm for immune-mediated liver injury from checkpoint inhibitors. The management of ILICI depends on its severity at presentation. In nonresolving or worsening Grade 1 or Grade 2/3 patients, evaluation for alternative causes, including a contrast-enhanced CT or MRI is recommended while withholding the ICI as well as oral prednisone with frequent lab checks. Approximately 20%–30% of Grade 2–4 patients will not improve with high-dose oral or i.v. corticosteroids, wherein a liver biopsy is recommended to confirm the diagnosis and exclude alternative causes. Oral mycophenolate mofetil leads to laboratory normalization in 80% of steroid nonresponders, allowing steroids to be safely tapered over 6–10 weeks. For mycophenolate nonresponders, a liver biopsy is recommended if not previously done before considering further immunosuppression. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, Computerized tomography; ICI, immune checkpoint inhibitor; ILICI, immune-mediated liver injury from checkpoint inhibitor; LFT, liver biochemistries; ULN, upper limit of normal.
Approximately 70%–80% of patients with Grade 1–4 ILCI will normalize their liver biochemistries or return to their pretreatment baseline after withholding the ICI and corticosteroid administration at a median of 5–7 weeks.12 For corticosteroid nonresponders, mycophenolate mofetil (MMF) can be used as a second-line agent at a maximum dose of 1500 mg twice daily with weekly monitoring of the complete blood count due to potential myelotoxicity.4 Some experts also consider azathioprine at doses of 1.5–2 mg/kg per day, but its onset of action is generally slower. Once the liver biochemistries improve, steroids can be tapered by 10 mg per week until discontinued, and MMF can be decreased by 250–500 mg BID/weekly over 6–8 weeks with ongoing lab monitoring to minimize the risk of rebound hepatitis. Patients that are expected to be on high-dose prednisone for > 4 weeks should be given trimethoprim-sulfamethoxazole or pentamidine for pneumocystis pneumoniae prophylaxis as well as calcium with vitamin D. If there is no laboratory response to corticosteroids or MMF, a liver biopsy and repeat liver imaging is advisable to ensure that an alternative diagnosis is not present. Additional agents to consider in MMF nonresponders include tocilizumab, infliximab, and tacrolimus, but the individual risk versus benefit of further immunosuppression should be carefully considered.4
In a recent meta-analysis, the incidence of fatal ILICI was very low at 0.07%.2 It is not known whether ILICI increases the risk of chronic liver disease, given the limited duration of follow-up of treated patients. However, some patients may develop prominent hepatic steatosis from corticosteroid-induced hyperglycemia, weight gain, and insulin resistance. There are limited data on the safety and efficacy of ICI rechallenge in patients with ILICI, but studies to date suggest that only 30% of highly selected patients experience another irAE following rechallenge (Table 4).13,14,15,16,17 In the reported series, most patients were retreated with a single agent, and anti-CTLA-4 was not used. Risk factors for rechallenge are not well established, although some series suggest that those with an underlying immune disorder or detectable anti-nuclear antibody may be at increased risk. However, many of the rechallenged patients were still on steroids at the time of rechallenge.
TABLE 4.
Outcomes with ICI rechallenge in patients with prior ILICI
| Series (n) | Li13 (31) | Partinely14 (66) | Riverio-Barciela15 (23) | Hountodji16 (51) |
|---|---|---|---|---|
| ≥ Prior Grade 3 | 31 (100) | 26 (39) | 23 (100) | 37 (72) |
| Tumor type | ||||
| Melanoma | 31 (100) | NR | 4 (17) | 21 (41) |
| Lung | 0 | — | 7 (30) | 13 (25) |
| Renal | 0 | — | 6 (26) | 6 (12) |
| Other | 0 | — | 6 (26) | 11 (21) |
| Time to initial ILICI (d) | 41 (30 to 80) | 61 (5 to 1189) | 56 (3 to 357) | 157 + 179 |
| Rechallenge drug | ||||
| Anti-CTLA-4 | 2 (6) | NR | 0 | 0 |
| Anti-PD-(L)1 | 29 (94) | — | 20 (85) | 44 (86) |
| Anti-CTLA-4 + PD-(L)1 | 0 | — | 3 (15) | 2 (4) |
| Recurrent ILICI | 6 (19) | 17 (26) | 8 (35) | 12 (23) |
| Time to recurrent ILICI (d) | 91 (21 to 448) | 32 | 115 (7 to 462) | NR |
| Risk factors for recurrence | None | NR | ANA + or known immune disorder (75% vs. 27%) | None |
Note: Data reported as n (%) or median (range).
Abbreviations: ANA, anti-nuclear antibody; CTLA-4, cytotoxic T-lymphocyte associated protein-4; ILICI, immune-mediated liver injury from checkpoint inhibitor; PD-L1, programmed death-ligand 1.
SECONDARY SCLEROSING CHOLANGITIS DUE TO ICI
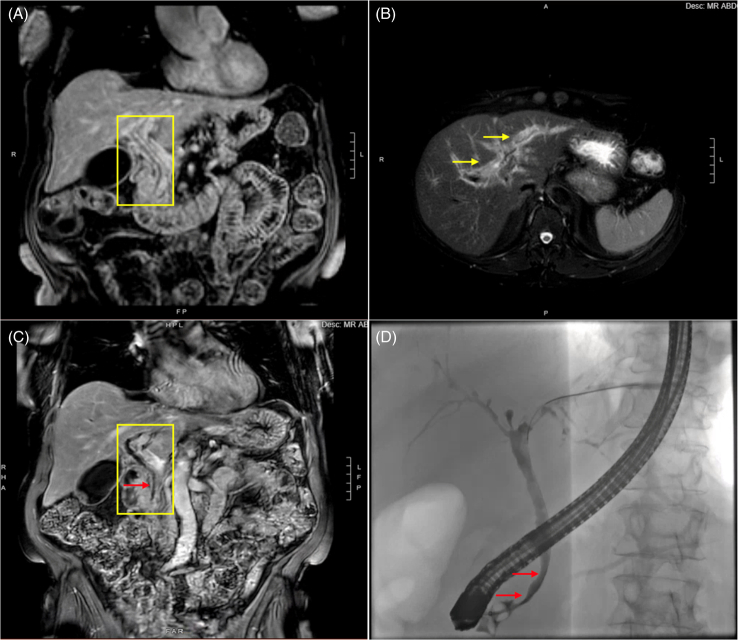
Recent data suggest that microscopic bile ducts may initially be affected in some patients with ILICI who present with a cholestatic lab profile.18 In addition, there are rare cases of macroscopic bile duct involvement termed “secondary sclerosing cholangitis” that is characterized by stenosis/stricturing and thickening or irregularity of the bile ducts, which can be segmental or diffuse (Figure 2). Although secondary sclerosing cholangitis is rare, with an estimated incidence of < 1%, it is important to diagnose since treatment and outcomes differ.18,19 Compared to the 70%–80% of patients with ILICI who respond to corticosteroids, only 8.5% of patients with secondary sclerosing cholangitis had complete resolution of their liver injury with steroids. Therefore, further immunosuppression in patients with secondary sclerosing cholangitis is not advisable, and endoscopic therapy for dominant strictures or a trial of ursodeoxycholic acid should be considered.17
FIGURE 2.
Representative images of secondary sclerosing cholangitis from ICI therapy. A 71-year-old female with metastatic melanoma presented with abdominal pain and AST 656 IU/l, ALT 1442 IU/l, and alkaline phosphatase of 503 IU/L after 3 doses of Nivolumab and relatlimab. Initial MRCP imaging showed (A) long-segment hyperenhancement of the common bile duct (yellow rectangle) and (B) significant peribiliary hyperenhancement (yellow arrows) on T2-weighted images. One month later, an MRCP showed (C) persistent long-segment hyperenhancement with new irregularity of the CBD and subtle distal CBD stricturing (red arrow). Endoscopic cholangiogram (D) confirmed a diagnosis of secondary sclerosing cholangitis with rarefication of intrahepatic ducts and beading and an irregular extrahepatic duct with 2 subtle short nonobstructing strictures (red arrows). Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBD, common bile duct; ICI, immune checkpoint inhibitor; MRCP, magnetic resonance cholangiopancreatography.
SUMMARY
Gastroenterologists will continue to encounter patients with cancer on ICIs who develop abrupt onset of liver injury. The diagnosis of ILICI is fundamentally based on clinical presentation and exclusion of competing causes of liver injury. Therefore, a stepwise approach to evaluation with serologic testing, contrast-enhanced computerized tomography or MRI imaging, and consideration of a liver biopsy in selected patients is recommended. Initial management of ILICI depends upon its severity and response to withholding ICI. Patients with progressive ILICI or severe ILICI may require corticosteroids and the addition of anti-metabolite such as MMF for steroid nonresponders to achieve laboratory normalization.
Acknowledgments
CONFLICTS OF INTEREST
Robert J. Fontana has received research support from Takeda Pharmaceuticals and Kezar Pharmaceuticals. Linnea A. Swanson and Fadi Hawa have no conflicts.
Footnotes
Abbreviations: CT, computerized tomography; CTLA-4, cytotoxic T-lymphocyte associated protein-4; ICI, immune checkpoint inhibitor; ILICI, immune-mediated liver injury from checkpoint inhibitors; irAE, immune-related adverse events; MMF, mycophenolate mofetil.
Contributor Information
Linnea A. Swanson, Email: linneasw@med.umich.edu.
Fadi Hawa, Email: hfadi@med.umich.edu.
Robert J. Fontana, Email: rfontana@med.umich.edu.
REFERENCES
- 1. Cunningham M, Gupta R, Butler M. Checkpoint inhibitor hepatotoxicity: Pathogenesis and management. Hepatology. 2023;79:198–2122. [DOI] [PubMed] [Google Scholar]
- 2. Zheng C, Huang S, Lin M, Hong B, Ni R, Dai H, et al. Hepatotoxicity of immune checkpoint inhibitors: What is currently known. Hepatol Commun. 2023;7:e0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atallah E, Welsh SJ, O’Carrigan B, Oshaughnessy A, Dolapo I, Kerr AS, et al. Incidence, risk factors and outcomes of checkpoint inhibitor-induced liver injury: A 10-year real-world retrospective cohort study. JHEP Reports. 2023;5:100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of Immunotherapy-related toxicity, version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:387–405. [DOI] [PubMed] [Google Scholar]
- 5. De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181–1190. [DOI] [PubMed] [Google Scholar]
- 6. Tsung I, Dolan R, Lao CD, Fecher L, Riggenbach K, Yeboah‐Korang A, et al. Liver injury is most commonly due to hepatic metastases rather than drug hepatotoxicity during pembrolizumab immunotherapy. Aliment Pharmacol Ther. 2019;50:800–808. [DOI] [PubMed] [Google Scholar]
- 7. Swanson LA, Kassab I, Tsung I, Schneider BJ, Fontana RJ. Liver injury during durvalumab-based immunotherapy is associated with poorer patient survival: A retrospective analysis. Front Oncol. 2022;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li M, Sack JS, Bell P, Rahma OE, Srivastava A, Grover S, et al. Utility of liver biopsy in diagnosis and management of high-grade immune checkpoint inhibitor hepatitis in patients with cancer. JAMA Oncol. 2021;7:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan J, Liu Y, Guo X, Bai Z, Levi Sandri GB, Méndez-Sánchez N, et al. Risk factors for immune-mediated hepatotoxicity in patients with cancer treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Expert Opin Drug Saf. 2022;21:1275–1287. [DOI] [PubMed] [Google Scholar]
- 10. Chang AE, Golob JL, Schmidt TM, Peltier DC, Lao CD, Tewari M. Targeting the gut microbiome to mitigate immunotherapy-induced colitis in cancer. Trends in Cancer. 2021;7:583–593. [DOI] [PubMed] [Google Scholar]
- 11. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO Guideline update. J Clin Oncol. 2021;39:4073–4126. [DOI] [PubMed] [Google Scholar]
- 12. Chen K, He J, Xu J, Chen J. Effectiveness of immunosuppressant use for the treatment of immune checkpoint inhibitor-induced liver injury: A systematic review and meta-analysis. Front Oncol. 2023;13:1088741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li M, Sack JS, Rahma OE, Hodi FS, Zucker SD, Grover S. Outcomes after resumption ofimmune checkpoint inhibitor therapy after high grade immune-mediated hepatitsi. Cancer. 2020;126:5088–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patrinely JR, McGuigan B, Chandra S, et al. A multicenter chacterization of hepatitis associated with immune chekcpoint inhibitors. Oncoimmunology. 2021;10:1875639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riverio-Barciela M, Barreira-Diaz A, Callejo-Perez A, Munoz-Couselo E, Diaz-Mejia N, Diaz-Gonzalez A, et al. Retreatment with immune checkpoint inhibitors after a severe immune-related hepatitis: Results from a prospective multicenter study. Clin Gastroenterol Hepatol. 2023;21:732–740. [DOI] [PubMed] [Google Scholar]
- 16. Hountondji L, Ferreira De Matos C, Lebossé F, Quantin Z, Lesage C, Palassin P, et al. Clinical pattern of checkpoint inhibitor-induced lliver injury in a multicentre cohort. J Hep Rep. 2023;5:100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Likhitsup A, Fontana RJ. Diagnosis and Management of Immune mediated liver injury from checkpoint inhibitors. Curr Opin gastroenterology. 2024;40:164–171. [DOI] [PubMed] [Google Scholar]
- 18. Pi B, Wang J, Tong Y, Yang Q, Lv F, Yu Y. Immune-related cholangitis induced by immune checkpoint inhibitors: A systematic review of clinical features and management. Eur J Gastroenterol Hepatol. 2021;33:E858–E867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meunier L, Hountondji L, Jantzem H, Faillie JL, Maria A, Palassin P, et al. Cholangitis induced by immune checkpoint inhibitors: Analysis of pharmacovigilance data. Clin Gastroenterol Hepatol. 2023;16:S1542–3565(23)01037-6. doi: 10.1016/j.cgh.2023.12.008 [DOI] [PubMed] [Google Scholar]