Abstract
Backgrounds/aims:
To evaluate the outcomes of hepatocellular carcinoma (HCC) patients who underwent right anatomical hepatectomy using the combination of the extrahepatic Glissonean pedicle approach (Takasaki’s technique) and liver hanging maneuver (LHM) (Belghiti’s technique).
Patients and methods:
A retrospective analysis of 30 cases of HCC treated with right hepatectomy using extrahepatic Glissonean pedicle approach and LHM by only one surgeon at our department from March 2020 to August 2023. Clinical characteristics, pathological results, postoperative outcomes, and survival rate were analyzed.
Results:
Among the 30 HCC patients analyzed, males accounted for 96.7% of patients. The mean age was 54.9±11 years. 96.7% had normal preoperative liver function (Child-Pugh A). LHM with an extrahepatic Glissonean approach was feasible in 100% of cases with minor blood loss, no blood transfusion, intraoperative complications, or perioperative mortality. The mean operative time was 123.8±29.0 min. The mean hospital stay was 9.37±4.02 days. Postoperative liver failure accounted for 6.7%. Pathological results: 63.3% moderately differentiated HCC; 36.7% poorly differentiated HCC. 1-year, 2-year, and 3-year survival rates were 86.1, 73.8, and 59.0%, respectively. Recurrence was witnessed in 13 (43.3%) cases, with 6 (20%) cases in remnant liver. 1-year, 2-year, and 3-year disease-free survival were 69.3, 42.0, and 28.0%, respectively.
Conclusion:
Right anatomical hepatectomy using extrahepatic Glissonean pedicle approach combined LHM for HCC was feasible and safe at our high-volume oncology center in a developing country.
Keywords: extrahepatic Glissonean pedicle approach, hepatocellular carcinoma (HCC), liver hanging maneuver, right anatomical hepatectomy
Introduction
Highlights
The Global Cancer Observatory (GLOBOCAN) 2020 data reveal a somber reality in Vietnam: hepatocellular carcinoma reigns supreme as the most prevalent and fatal cancer.
Surgical liver resection has to be considered as the only potential curative treatment for primary and secondary liver malignancies and major liver resections are frequently required to achieve complete tumor removal, with disease-free surgical margins.
For several decades, the majority of major hepatectomies performed at our institution were nonanatomical in nature, leading to increase incidence of postoperative complications and compromised oncological outcomes.
Right hepatectomy is a major hepatectomy. An extrahepatic Glissonean pedicle approach combined with a liver hanging maneuver in the anatomical right hepatectomy is safe and feasible at specialized centers.
Vietnam, a developing country, has applied a wide range of treatments for hepatocellular carcinoma (HCC), which ranks second in incidence and first in deaths1. Surgical liver resection has to be considered as the only potential curative treatment for primary and secondary liver malignancies and major liver resections are frequently required to achieve complete tumor removal, with disease-free surgical margins. For several decades, the majority of major hepatectomies performed at our institution were nonanatomical in nature. This practice stemmed from limitations in the experience of our surgical and anesthesia teams, as well as constraints in available surgical instrumentation. As a consequence, this approach was associated with an increased incidence of postoperative complications and compromised oncological outcomes.
In 1986, Takasaki published an article on the extrahepatic Glissonean pedicle approach without the need to dissect each component of the portal pedicle2. This technique minimizes the potential risk of vascular and biliary injury. In 2001, Belghiti described a technique of hanging the liver with a tape passed in an avascular tunnel on the anterior surface of the inferior vena cava (IVC), between the right and middle hepatic vein3. This marked a safe border to guide a linear resection between the two lobes and limit blood loss during parenchymal resection.
Right hepatectomy is a major hepatectomy. An extrahepatic Glissonean pedicle approach combined with a liver hanging maneuver (LHM) in the anatomical right hepatectomy is safe and feasible at specialized centers.
Methods
Registration
This article has been reported in line with the preferred reporting of case series in surgery (PROCESS) criteria4 (Supplemental Digital Content 1, http://links.lww.com/MS9/A436).
Our procedures adhered to the Declaration of Helsinki. This article was register in ‘ResearchRegistry.com’ with identifying number ‘researchregistry10043’.
Study design
Retrospective, case series, single-center, and consecutive.
Settings and time-frames
Thirty HCC patients underwent right anatomical hepatectomy using the extrahepatic Glissonean pedicle approach combined with LHM in our department from March 2020 to November 2023.
Quantitative variables were presented as mean±SD, while qualitative variables were expressed as percentages. Kaplan–Meier analysis was applied for overall survival and disease-free survival outcomes. All analyses were performed using SPSS software (version 20.0; SPSS, Inc.). A P-value of less than 0.05 was considered statistically significant.
Participants
The patients were classified the A and B staging based on Barcelona Clinic Liver Cancer (BCLC) guidelines. We have routinely applied this combined technique to right hepatectomy, with the exclusion criteria of large tumors adjacent to the liver hilum or IVC, which risk tumor rupture and uncontrollable bleeding during LHM and pedicle dissection.
Preintervention patient optimization
Epidemiological characteristics, clinical examination findings, laboratory test results, and diagnostic imaging interpretations of the patients were performed.
Inclusion criteria:
Patients with HCC classified as stages I to IIIA according to the TNM classification of AJCC (2018) using preoperative multisequence computed tomography or MRI.
Liver function: classified as Child-Pugh A.
Future remnant liver volume to body weight ratio ≥0.8%.
Patients undergoing right anatomical hepatectomy using extrahepatic glissonean pedicle approach combined liver hanging manoeuvre.
Postoperative pathology: HCC.
Patient agree to participate in the study.
Exclusion criteria:
Tumor in the hilar area or IVC involvement.
The patient has had previous surgery on the liver hilum area.
Interventions
Right anatomical hepatectomy using extrahepatic glissonean pedicle approach combined LHM.
Intervention details
The patient was positioned supine under general anesthesia. An incision was made in J-shape to clearly expose the surgical cite. Cholecystectomy was first performed (Supplementary Video 1, Supplemental Digital Content 2, http://links.lww.com/MS9/A437).
First, performed a cholecystectomy and then removed the connective tissue of the triangle of Calot from the liver bed. The right Glissonean pedicle and the liver parenchyma of the hepatic hilum came into view. All tissue was totally cleared from around the right Glissonean pedicle right up to the point where the pedicle enters the opening of the hepatic hilum. Then, detached the right Glissonean pedicle from the surrounding liver tissue as much as possible. In some cases, it was difficult to introduce forceps behind the right branch, because of some resistance. We did not try to insert the forceps by force, this must be done by making a very fine line – cut just between the connective tissue of the right Glissonean pedicle and the liver parenchyma in order to prevent bleeding and to keep the operative field clean. There usually appeared a few tiny branches that were all ligated. Then chose one special forcep with varied curvature, to introduce tape around the pedicles. After clamping the right Glissonean pedicle, the area of the middle segment could be recognized by its change of color. Marked the demcarcation line with electrocautery (Supplementary Video 2, Supplemental Digital Content 3, http://links.lww.com/MS9/A438).
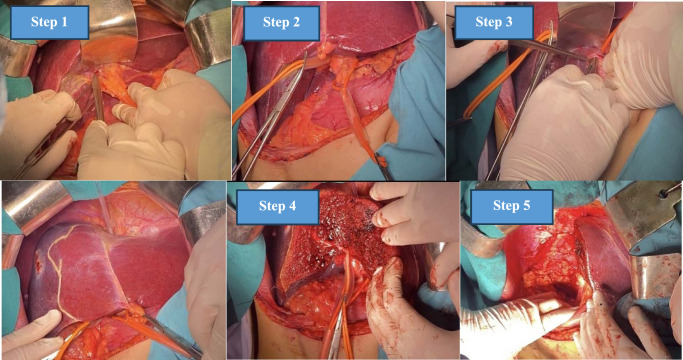
Then, Belghiti’s LHM was performed using a plastic tube (Supplementary Video 3, Supplemental Digital Content 4, http://links.lww.com/MS9/A439). Following the demarcation line, parenchyma was dissected, then right Glissonean pedicle and right hepatic vein were consecutively transected by an Ethicon ECHELON ENDOPATH™ Reload (60 mm) white (Supplementary Video 4, Supplemental Digital Content 5, http://links.lww.com/MS9/A440, Supplementary Video 5, Supplemental Digital Content 6, http://links.lww.com/MS9/A441). Finally, hemostasis at the liver resection site and abdominal closure (Fig. 1). Postoperative specimen, including resected liver tumor and gallbladder were forwarded for a comprehensive histopathological evaluation (Fig. 2).
Figure 1.
Surgical protocol of right hepatectomy applying Takasaki Glissonean approach combined. with Belghiti’s hanging maneuver. (1) Cholecystectomy and right liver mobilization. (2) Glissonean pedicle dissection and control. (3) Hanging maneuver. (4) Parenchymal resection. (5) Hemostasis
Figure 2.
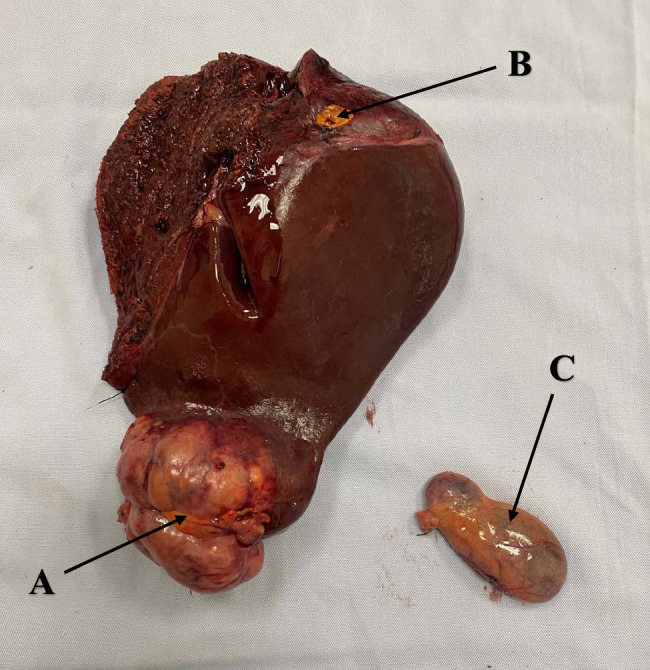
Postoperative specimen: A. Primary tumor. B. Satellite node. C. Gallbladder.
Macroscopic examination revealed the tumor’s location, size, presence of satellite nodules, vascular invasion, and portal vein thrombosis morphology. Microscopic evaluation by a team of experienced oncological pathologists further defined the histological differentiation, microvascular invasion, lymph node metastasis, and concomitant liver cirrhosis.
Operator details
All of the operations were performed by only Dr ATP, the head of department, experienced surgeon at our high-volume oncology center. Dr ATP, a resident physician in Surgery since 2004, successfully defended his doctoral thesis in Hepatobiliary and Pancreatic Surgery in 2014. Holding the distinction of the most experienced researcher at our department, Dr ATP is also a highly accomplished surgeon with nearly 2000 successful hepatectomy procedures performed to date.
Quality control
This study was conducted with the informed consent of patient and received the requisite ethical approval from the Scientific Council of our hospital. The council comprises expert representatives from relevant specialties, including hepatobiliary surgeons, radiologists, oncologists, gastroenterologists, and pathologists.
Follow-up
A follow-up regimen in our center was implemented for the first month, comprising scheduled examinations after 2 months, 3 months, and 6 months for the first year, then every 6 months. Each follow-up visit entailed comprehensive data collection, physical examinations, complete blood count analysis, liver function and tumor marker tests, and imaging assessments through computed tomography and MRI. This systematic follow-up protocol closely monitored all surviving patients until November 2023.
Results
We included 30 cases of HCC undergoing a combined approach of extrahepatic Glissonaean approach and LHM. Most of the patients were male, 29 cases (96.7%). The mean age was 52.2±13.6, and the oldest patient was 77 years old. One experienced surgeon performed all operations to ensure homogeneity in surgical technique. The diagnosis of HCC was confirmed with postoperative pathological results.
The underlying liver disease was analyzed, with 21 (70%) cases of cirrhosis, 22 (73.3%) cases of hepatitis B, and only 2 (6.7% with hepatitis C). Mild abdominal pain was noticed in 14 (46.7%) cases, and the others were asymptomatic and were accidentally diagnosed with a liver tumor on the general check-ups. Most of our patients had preserved liver function with Child-Pugh A, accounting for 96.7%, and there was only 1 case with Child-Pugh B (3.3%). 27 (90%) of the patients had a single tumor, and 3 (10%) had 2 to 3 tumors. The mean tumor size was 9.1 cm. This made most of our cases BCLC A (93.3%), and only two cases (6.7%) with multinodal lesions over 3 cm were BCLC B (Table 1).
Table 1.
Clinical characteristics of patients in study (n=30)
| Results | |
|---|---|
| Male, n (%) | 29 (96.7) |
| Age, years, mean (range) | 52.2±13.6 (24–77) |
| Liver disease | |
| Cirrhosis | 21 (70.0%) |
| HBV | 22 (73.3%) |
| HCV | 2 (6.7%) |
| Clinical manifestations | |
| Asymptomatic | 16 (53.3%) |
| Upper abdominal pain | 14 (46.7%) |
| Jaundice | 0 |
| Preoperative imaging | |
| Number of tumors | |
| 1 | 27 (90.0%) |
| 2–3 | 3 (10.0%) |
| >3 | 0 |
| Tumor size (cm) | 9.1±3.7 (3–20) |
| Lab test | |
| Liver function | |
| Child-Pugh A | 29 (96.7%) |
| Child-Pugh B | 1 (3.3%) |
| Alpha Fetoprotein, n (%) | |
| <20 ng/ml | 5 (16.7) |
| 20–400 ng/ml | 8 (26.7) |
| >400 ng/ml | 17 (56.6) |
| Operative techniques | |
| Pedicle control | |
| Intermittent Pringle Maneuver | 22 (73.3%) |
| Nonclamping | 8 (26.7%) |
| Lymph node resection | 8 (26.7%) |
| Pedicle clamping time (minutes) | 13.5±5.8 |
| Operative time (minutes) | 123.8±29.0 (80–240) |
| Intraoperative incidence | 0 |
| Intraoperative blood loss (ml) | 20.3±1.2 |
The extrafascial approach was feasible in 100% of our cases. The intermittent clamping of hepatic pedicle time is 15 min, unclamping is 5 min for 22 (73.3%), and ‘no clamping’ is used for eight (26.7%) cases. Suspected lymph nodes located at the hepatoduodenal ligament along the common hepatic artery and retro-pancreatic space were resected in eight cases (26.7%). The mean operative time was 123.8±29.0 min, ranging from 80 to 240 min. Blood loss was minimal, and no intraoperative incidence was recorded. None of our patients required perioperative blood transfusion (Table 1).
The postoperative period witnessed two cases of liver failure, which were successfully managed with watchful waiting, and was finally discharged on postoperative days 20 and 22. No other complications were recorded. The mean postoperative stay was 9.4 days. No perioperative mortality was recorded.
Pathology results revealed 19 (63.3%) cases with grade 2 (moderately differentiated) carcinoma, 10 (33.3%) cases with grade 3, and 1 (3.3%) cases with grade 4. All eight cases with suspected lymph nodes resected were negative, and all specimens reached a clear margin (Table 2).
Table 2.
Postoperative outcomes (n=30)
| Perioperative period | |
| Postoperative complications, n (%) | |
| Liver failure | 2 (6.7) |
| Infection | 0 |
| Bleeding | 0 |
| Pathology | |
| Grade 2 | 19 (63.3) |
| Grade 3 | 10 (33.3) |
| Grade 4 | 1 (3.3) |
| R0 surgical margin | 30 (100) |
| Postoperative hospital stay (days) | 9.4±4.0 (6–22) |
| POD of drain removal | 8.6±2.5 (6–15) |
| Mortality within 30 days | 0 |
| Lab test | |
| Maximum AST (UI/l) | 204.8±35.8 |
| Maximum ALT (UI/l) | 164.3±46.2 |
| Maximum bilirubin (mmol/l) | 26.3±6.4 |
| Minimum prothrombin acitivity (%) | 71.3±10.3 |
| Follow-up | |
| Recurrence | 13 (43.3%) |
| Contralateral liver | 6 (20.0%) |
| Extrahepatic metastasis | 7 (23.3%) |
| Mortality | 6 (20%) |
AST, alanine aminotransferase; ALT, alanine aminotransferase.
After a mean follow-up of 16 months (ranging from 2 to 45 months), mortality was seen in four cases, primarily due to other comorbidities. Recurrence was witnessed in 13 cases, with six (20%) cases in the remnant liver and seven cases (23.3%) with extrahepatic metastasis, including lungs, adrenal gland, and peritoneal lymph nodes (Table 2). Among six cases of intrahepatic recurrence, four cases were treated successfully with transarterial chemoembolization; the others refused further treatment.
One year, 2-year, and 3-year survival rates were 86.1, 73.8, and 59.0%, respectively. One year, 2-year, and 3-year disease-free survival were 69.3, 42.0, and 28.0%, respecetively (Fig. 3).
Figure 3.
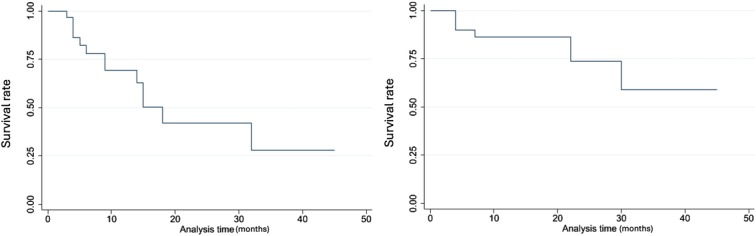
Kaplan–Meier analysis (1) Disease-free survival (2) Overall survival.
Discussion
Our study reported 30 cases of HCC undergoing right anatomical hepatectomy using an extrahepatic Glissonean pedicle approach combined with LHM.
The extrahepatic Glissonean approach was to dissect pedicles at the liver hilum without resection into the parenchyma. The technique was introduced in reports by Couinaud and Takasaki and later developed by Sugioka, using Laenec’s capsule and proposing ‘gates’ to isolate the pedicles2,5. The Glissonean approach allowed the exclusion of specific pedicles, accurate delineation of the ischemic anatomical region, and sufficient parenchymal resection without compromising liver function, especially in cases with tumors limited within segments6,7. A recent systemic review by Moris comparing with traditional technique reported a significantly lower recurrence rate (P=0.0013), a higher 5-year survival rate (67%, P<0.01), and lower recurrence-related death (11.4%, P<0.01) among patients undergoing hepatectomy with Glissonean approach7. Extrafascial dissection also saves time dissecting pedicle components and avoids injury to the hilar vessels and biliary tree. At our center, liver-sparing surgery with an extrafascial Glissonean approach has become our standard technique. The Extrafascial Glissoneal approach was feasible in 100% of our cases. However, the approach may be a challenge in cases with past surgery at the hilum; tumors invaded near the hilum, anatomical variations of portal components, or cirrhosis, which risked uncontrollable bleeding during hilar dissection7,8. Mouly reported a failure rate of 25% due to incomplete clamping of the right pedicle or incidental clamping of the left pedicle8. Makdissi reported 1/30 cases of posterior pedicle exclusion due to anatomical variation of the anterior sectoral pedicle from the left pedicle identified during parenchymal resection9. The authors underlined the importance of careful anatomical variation assessment via imaging study for the success of this technique2,8,9.
LHM was introduced by Belghetti in 2001, followed by numerous articles proving its feasibility and safety for anatomical right hepatectomy as a strategy to minimize blood loss and guide parenchymal resection posteriorly in an avascular plane to the IVC3,10. In 2020, Tzedakis proposed modification to this technique for hepatectomy of different segments other than right hepatectomy10. Contra-indication of this technique was the invasion of tumors to bifurcations of pedicles and hepatocaval junctions, which may induce bleeding and tumor seeding during the maneuver10. Blood loss in our study was insignificant (20.3 ml). Li et al.11, reviewing 16 studies including 1109 patients, showed that LHM was associated with enhanced perioperative results, including significantly minor blood loss, lower transfusion rate, less transaction time, lower length of stay, lower complication rate, and more prolonged overall survival. Nanashima also noted that intraoperative blood loss was less in the LHM population (1566 ml vs. 2017 ml) and stated that LHM was feasible for tumors over 5 cm12. Meanwhile, a study by Makdissi implied the feasibility of a combined technique of LHM and intrahepatic Glissonean approach9.
‘Nonclamping’ was applied in eight (26.7%) cases. This technique was hypothesized to reduce ischemic injury to the remnant liver and, therefore, maintain postoperative function2,5,9. A systemic review by Mobarak pointed out a significant decrease in overall complications rate, blood loss, transfusion requirements, air embolism, liver failure, multiorgan failure, and mortality in the selective hepatic pedicle exclusion group undergoing major hepatectomy6.
The mean operative time of our combined strategy was 123.8 min, ranging from 80 to 240 min, depending on the tumor size and location. Our result was significantly less than in many studies: Makdissi et al.9 reported 326 min; Nanashima et al.12 conveyed a mean operative time of 488 min, insignificantly less than non-LHM group (544 min, P=0.385); Li’s et al.11 systemic review of hepatectomy with LHM reported 277 min.
The postoperative maximum aminotransferase level in our study was 160–200 UI/l, lower than that reported by Nanashima et al.12 (425 UI/l), indicating minimal hepatic cell injury in our case series. Our mean minimum postoperative prothrombin activity was 71.3%, higher than that of Nanashima et al.12 (54%), while bilirubin level was higher (26.4 mmol versus 11.6 mmol). We recorded two (6.7%) cases of liver insufficiency, which were successfully managed medically, and the mean hospital stay was 9.4 days. Literature reported the overall rate of liver failure was 0–10%, of postoperative complications 0–30%, a mean hospital stay of 15–23 days11.
Oncological results were expressed in our study as postoperative pathologic results, recurrence rate, and disease-free survival. Pathologically, the R0 margin was ensured in all of our cases. Our study recorded tumor size ranging from 1.5 to 15 cm, with a mean size of 9.1 cm, slightly larger than Mouly’s report (mean 6 cm, range 2–12 cm8. Sixty percent of our cases had tumors >5 cm, considerable to that of Nanashima (62%). Compared with smaller tumors, liver resection in tumors >5 cm did not significantly differ in disease-free and survival outcome13. Makdissi et al.9, researching patients with tumors >5 cm, showed no perioperative morbidity or mortality, free oncological margin, and overall and disease-free of 59 and 37%, respectively. This proved the feasibility and treatment efficacy of this LHM, mainly for large tumors. Since LHM helped avoid liver mobilization, less manipulation of peripheral tumors allowed less dissemination and enhanced oncological outcomes.
The recurrence rate in our study was 36.7% within an average of 16 months (2–45 months) of follow-up. All patients received preoperative multisequence CT-scan or MRI that could detect HCC lesions in left liver if suspected. For localized recurrence cases, we prioritize radical intervention while multifocal recurrence cases will be treated with transarterial chemoembolization. Our result was comparable to that of Nanashima et al.13 in the HCC group, reporting a recurrence rate of 57% within 46 months, with more than half of the cases in the remnant liver. Based on Kaplan–Meier analysis shown in Figure 3, 1-year, 2-year, and 3-year survival rates in our study were 86.1, 73.8, and 59.0%, respectively. Nanashima et al.13 reported a survival rate at 1, 3, and 5 years of the LHM group for HCC was ~90, 70, and 60%, significantly higher than that of the non-LHM group (90, 55, and 50%, respectively). Similarly, our disease-free survival rate was analyzed as 69.3, 42.0, and 28.0%, respectively. Although recorded in a shorter follow-up, our result was comparable to that of Nanashima et al.13, with 1-year, 3-year, 5-year disease-free survival of 58, 30, and 30%, respectively.
Many studies have confirmed these favorable results for LHM in both overall and disease-free survival included in Li’s et al.11 meta-analysis, even in patient populations other than HCC.
Conclusion
In conclusion, right anatomical hepatectomy using an extrahepatic Glissonean pedicle approach combined with LHM for HCC was feasible and safe at our high-volume oncology center in a developing country. Among the 30 HCC patients analyzed, LHM with the extrahepatic Glissonean approach was feasible in 100% of cases with minor blood loss. The mean operative time was 123.8±29.0 min. The mean hospital stay was 9.37±4.02 days. Postoperative liver failure accounted for 6.7%. One year, 2-year, and 3-year survival rates were 86.1, 73.8, and 59.0%, respectively. Recurrence was witnessed in 13 (43.3%) cases, with 6 (20%) cases in remnant liver within 16 months of follow-up.
Ethical approval
This study was conducted with the informed consent of patient and received the requisite ethical approval from the Scientific Council of Vietnam National Cancer Hospital. The council comprises expert representatives from relevant specialties, including hepatobiliary surgeons, radiologists, oncologists, gastroenterologists, and pathologists. Their comprehensive review and endorsement ensured adherence to the highest ethical standards throughout the research process. Our procedures adhered to the Declaration of Helsinki. The authors reported no conflicts of interest.
Consent
Written informed consent was obtained from the patients for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
None.
Author contribution
A.T.P. and C.M.T.: conceptualization, writing – original draft, and writing – review and editing. All authors contributed in data curation, methodology, and visualization.
Conflicts of interest disclosure
The author declares no conflict of interest.
Research registration unique identifying number (UIN)
This article has been reported in line with the PROCESS criteria.
Guarantor
Anh The Pham.
Data availability statement
Our procedures adhered to the Declaration of Helsinki. This article was register in ‘ResearchRegistry.com’ with identifying number being ‘researchregistry10043’.
Provenance and peer review
None.
Supplementary Material
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/annals-of-medicine-and-surgery.
Published online 24 April 2024
Contributor Information
Anh T. Pham, Email: theanhbenhvienk@gmail.com.
Cuong M. Truong, Email: cuongsur@gmail.com.
Phuong H. Trinh, Email: trinhhuyphuong1996@gmail.com.
Chinh Thi Nguyen, Email: chinhbvnn2387@gmail.com.
My H. Pham, Email: hoanmy.yds@gmail.com.
Quoc H. Dang, Email: quocqh97@gmail.com.
References
- 1. Ferlay JEM, Lam F, Laversanne M, et al. Global cancer observatory: Cancer Today. International Agency for Research on Cancer. 2024. https://gcoiarcwhoint/today [Google Scholar]
- 2. Takasaki K. Glissonean pedicle transection method for hepatic resection: a new concept of liver segmentation. J Hepatobiliary Pancreat Surg 1998;5:286–291. [DOI] [PubMed] [Google Scholar]
- 3. Belghiti J, Guevara OA, Noun R, et al. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg 2001;193:109–111. [DOI] [PubMed] [Google Scholar]
- 4. Mathew G, Sohrabi C, Franchi T, et al. Preferred reporting of case series in surgery (PROCESS) 2023 guidelines. Int J Surg 2023;109:3760–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sugioka A, Kato Y, Tanahashi Y. Systematic extrahepatic Glissonean pedicle isolation for anatomical liver resection based on Laennec’s capsule: proposal of a novel comprehensive surgical anatomy of the liver. J Hepatobiliary Pancreat Sci 2017;24:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mobarak S, Stott MC, Tarazi M, et al. Selective hepatic vascular exclusion versus pringle maneuver in major hepatectomy: a systematic review and meta-analysis. Front Surg 2022;9:860721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moris D, Rahnemai-Azar AA, Tsilimigras DI, et al. Updates and critical insights on glissonian approach in liver surgery. J Gastrointest Surg 2018;22:154–163. [DOI] [PubMed] [Google Scholar]
- 8. Mouly C, Fuks D, Browet F, et al. Feasibility of the Glissonian approach during right hepatectomy. HPB (Oxford) 2013;15:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makdissi FF, de Mattos BVH, Kruger JAP, et al. A combined “Hanging Liver Maneuver” and “Intrahepatic Extra-Glissonian Approach” for anatomical right hepatectomy: technique standardization, results, and correlation with portal pedicle anatomy. Front Surg 2021;8:690408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tzedakis S, Jeddou H, Boudjema K, et al. Hanging and modified liver hanging maneuver. J Visc Surg Dec 2020;157:511–518. [DOI] [PubMed] [Google Scholar]
- 11. Li H, Zhu B, Huang J, et al. Liver hanging maneuver versus conventional approach for open hepatectomy: a meta-analysis. HPB (Oxford) 2019;21:802–809. [DOI] [PubMed] [Google Scholar]
- 12. Nanashima A, Hiyoshi M, Imamura N, et al. Liver hanging maneuver is suitable in major hepatectomy for liver malignancies over 5 cm. Turk J Surg 2022;38:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nanashima A, Abo T, Takagi K, et al. Prognostic influence of the liver hanging maneuver for patients with hepatobiliary malignancies who underwent hepatic resections. Eur J Surg Oncol (EJSO) 2014;40:1540–1549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our procedures adhered to the Declaration of Helsinki. This article was register in ‘ResearchRegistry.com’ with identifying number being ‘researchregistry10043’.