Abstract
Introduction:
There has been increased interest in the use of Statins in total hip and knee arthroplasty (THA and TKA) patients to improve outcomes and reduce postoperative complications. This study was performed to systematically review the evidence on Statin use in total joint arthroplasty, specifically its benefits and complications.
Methods:
Adhering to the PRISMA guidelines, a systematic review of PubMed, Embase, Scopus, Web of Science, and the Cochrane database was performed to find studies reporting on the effects of Statin use on outcomes of THA and TKA. Two authors independently selected relevant papers to include.
Results:
A total of 18 papers were included in the final analysis. Most were retrospective studies, with heterogeneous patient selection and outcome measures. The evidence on the risks and benefits of Statin use on outcomes of total joint arthroplasty was very limited and heterogeneous. Studies were focusing on perioperative cardiac outcomes, clinical outcomes and complications, renal, pulmonary, and gastrointestinal outcomes. Due to the heterogeneity of reported data, a formal meta-analysis was not possible.
Conclusions:
There is some evidence in the literature suggesting that perioperative use of Statins, especially in Statin-naïve patients, may reduce cardiac (e.g. atrial fibrillation) and noncardiac (e.g. delirium) complications, while not increasing the risk of muscle or liver toxicity. The authors also found low levels of evidence that Statin use may reduce the long-term risk for revision surgery and osteolysis.
Keywords: joint arthroplasty, joint replacement, outcome, Statin, total hip arthroplasty, total knee arthroplasty
Introduction
Highlights
There has been increased interest in the use of Statins in the total joint arthroplasty literature to improve outcomes and reduce postoperative complications.
The potential benefits of Statins on outcomes of orthopedic surgeries are controversial.
Antiarrhythmia effects, protecting against postoperative atrial fibrillation, as well as reducing mortality following surgery are amongst the benefits of using statins.
Over 1 million total hip and knee arthroplasties (THA and TKA) a year are performed in the United States, a number that is projected to almost double by 20301. With the increasing demand for total joint arthroplasty (TJA), patient optimization to decrease perioperative and long-term complications is becoming increasingly important.
Statins are 5-hydroxy-3-methylglutaryl-co-enzyme A (HMG-CoA) reductase inhibitors and are commonly used lipid lowering agents. Since their introduction, Statins have dramatically reshaped the prevention and treatment of cardiovascular diseases, and have been found to substantially decrease the risk of cardiovascular events, sudden death, myocardial infarction (MI), and overall mortality2. While Statins are generally very safe, the most common complications include muscle and liver toxicity. On the other hand, in addition to being cholesterol lowering agents, Statins have also been linked to decreased inflammation, antioxidative effects, and improved endothelial function in preclinical and clinical studies3. Furthermore, there is some evidence that Statins may have a beneficial effect on bone homeostasis by modulating cytokine responses, promoting osteoblast-directed bone formation, and reducing osteoclastic-related bone resorption4. The potential benefits of Statins on outcomes of nonorthopaedic surgeries have been extensively studied, with several papers reporting improved outcomes in cardiac5, noncardiac vascular6, and elective procedures7.
There has been increased interest in the use of Statins in the TJA literature to improve outcomes and reduce postoperative complications. The available data, however, is not conclusive and has not been systematically reviewed. Therefore, the purpose of this study was to systematically review the literature to determine the evidence on Statin use in TJA, specifically its benefits and complications.
Methods
The PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Supplemental Digital Content 1, http://links.lww.com/MS9/A453) guidelines were followed to perform this systematic review8. Also, the AMSTAR 2 checklist (Supplemental Digital Content 2, http://links.lww.com/MS9/A454) was completed to evaluate the quality of study9. The study protocol was registered in PROSPERO (Code = CRD42023493244).
Search strategy
We performed a systematic review on 20 February 2022. We searched PubMed, MEDLINE, Embase, Scopus, Web of Science, and the Cochrane library. Search queries were personalized to follow each database’s rules and regulations, with the general terms of (‘Total Hip Arthroplasty’ AND ‘Statin’) and (‘Total Knee Arthroplasty’ AND ‘Statin’). An update search was performed on 01 October 2023.
PICO and inclusion/exclusion criteria
Original studies that reported any outcomes after THA or TKA, comparing Statin user and nonusers were included. Clinical trials, retrospective, and prospective observational studies with a study population of adults were selected. The intervention of interest was THA and TKA. The study’s primary outcome measures were risks and benefits of Statin in TJA. Non-English studies, as well as experimental, nonhuman studies were excluded. There was no limit to the publication year of selected studies.
Quality assessment
The MINORS (Methodological Index for Non-Randomized Studies) criteria was utilized to assess study quality. MINORS is a framework for scoring nonrandomized studies such as observational and descriptive studies. MINORS includes 12 items graded from 0 to 2, with maximum scores of 16 for noncomparative studies and 24 for comparative studies. Higher scores indicate a higher quality of evidence. Scores of 0–8 or 0–12 were considered low quality, 9–12 or 13–18 were deemed to be moderate quality, and 13–16 or 19–24 were regarded as high quality, respectively, for noncomparative and comparative studies (Appendix A).
Data extraction
EndNote version 20 (Clarivate) was used to screen the articles. Two reviewers (M.S. and M.B.) independently reviewed the titles and abstracts of each paper to select relevant papers. Discrepancies were addressed by a third author (S.H.Sh). Data from the selected studies were collected by two reviewers (M.S. and M.B.).
Results
The initial search yielded 96 records pertaining to TKA and 49 to THA. After multiple rounds of screening, 18 studies were selected for the final analysis (Fig. 1)10–27. The 18 included studies were published between 2006 and 2021. Cohort studies constituted the majority of papers (11 out of 18), followed by case–control and randomized controlled trials (3 studies each), and cross-sectional design (1 paper). Sample size ranged from 20 (a controlled trial) to 189 227 (cohort study). Table 1 summarizes the basic characteristics of the included studies.
Figure 1.
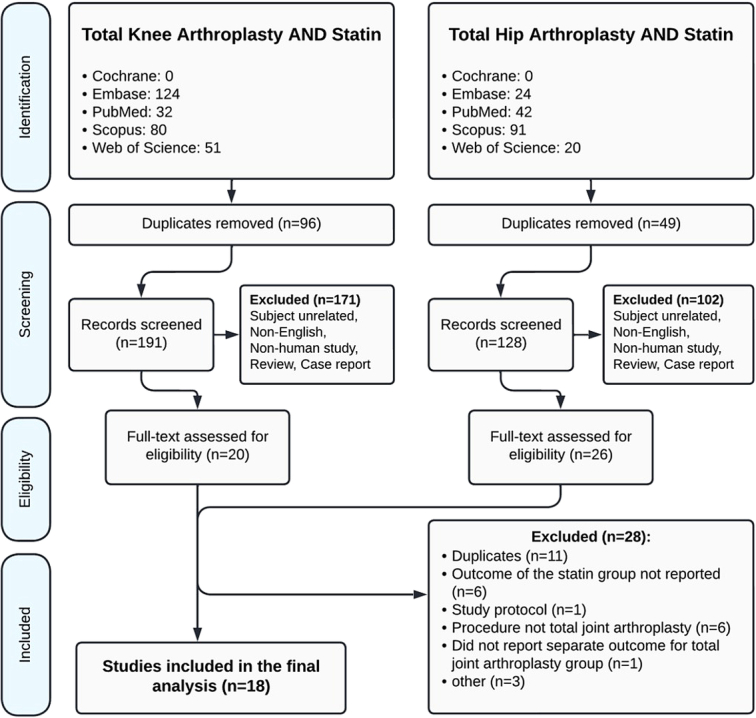
PRISMA flowchart showing identification, screening, and inclusion of studies for review.
Table 1.
Characteristics of the studies included in the systematic review.
| First author, year | Study design | Inclusion/exclusion criteria | Sample size | Male, female | Mean age (yrs) | Medication type/dosage | Treatment protocol | Follow-up | Procedure | Country |
|---|---|---|---|---|---|---|---|---|---|---|
| Bass 201510 | Cohort | Inclusion Criteria: individuals ≥ 65 years of age | 394 | 136, 258 | 71.8 | Statin | Being on statin regime | 2 days | THA and TKA | United States |
| Bass 2017 | Randomized controlled trial | Inclusion Criteria: age ≥ 65 years, hip Fx surgery, or elective unilateral primary THR or TKR for osteoarthritis, life expectancy of >3 months, ability to speak English and participate in the informed consent process. Exclusion Criteria: taking a statin within the previous 30 days or intolerance of statins, pathologic hip fracture, coronary artery disease, muscle disorder, liver disease, creatinine clearance < 30 cm^3 /min, treatment with an HIV protease inhibitor, hepatitis C protease inhibitor, erythromycin, clarithromycin, niacin, or an azole antifungal agent, hip Fx patients with history of peripheral arterial disease, transient ischemic attack, or stroke | 20 | 6, 14 | 73 | Atorvastatin 40 mg daily | Hip Fx patients started study drug (atorvastatin or matched placebo) at least 4 h prior to surgery. THR and TKR patients started study drug 4 days prior to surgery. Study drug was continued until POD 45 | 90 days | THA and TKA and hip fracture surgery | United States |
| Bonano 202112 | Cohort | Exclusion criteria: revision TJA, uni compartmental TKA, and oncologic procedures | 1769 | 688, 1079 (2 missing) | 65.66 | Simvastatin 80 mg or statin | 231 received simvastatin 80 mg daily first day of procedure and continued every day of hospitalization (perioperative statin) + 572 patients were on statin regime prior to surgery (prior statin) | 90 days | THA and TKA | United States |
| Chen 2018 | Case–control | Exclusion criteria: Patients with prior arrhythmia | 46521 | 19732, 26789 | – | Statin | Statin consumption within 1 year prior to their procedure | 90 days | THA | United States |
| Cook 202014 | Cohort | Inclusion Criteria: primary THA or TKA procedure. Exclusion Criteria: age <40 years, history of hip fracture, inflammatory arthritis at the time of primary THA/TKA | 151305 | 62066, 89239 | 69.7 | Statin | Post operation and preoperation consumption of statin | 3.9 years | THA and TKA | United Kingdom |
| Dastrup 201715 | Cohort | Inclusion Criteria: primary THA patients without a history of statin use | 8370 | – | – | statin | consumption of statins during the 365 days before their primary THA | – | THA | Denmark |
| Jo 201016 | Cohort | – | 143 | 14, 129 | 69.1 | Statin | Being on regular statin therapy | 7 days | TKA | South Korea |
| Kim 201617 | Cohort | Inclusion Criteria: patients with primary unilateral TKA, a tertiary teaching hospital in Seoul, Exclusion Criteria: patients with bilateral TKA, 1 or 2 weeks apart from each other, combined operation, preoperative serum creatinine (sCr) level >1.5 mg/dl or chronic kidney disease, and with incomplete data | 1309 | 1,541,155 | 68.8 | Statin | being on regular statin therapy | 4.2 | TKA | South Korea |
| Laisalmi-kokki 201018 | Case–control | Inclusion Criteria: patients aged 50 to 84 years. Exclusion criteria: muscular, hepatic, or renal disease or insufficiency, surgery within one month of the present surgery, and, in patients with statin therapy, discontinuation of statin therapy within 48 h of surgery | 48 | 23, 25 | 70.75 | Statin | Being on regular statin therapy (mean 4 years) (statin group) | 72 h | THA and TKA | Finland |
| Lalmohamed 201619 | Cohort | Inclusion Criteria: patients with an elective primary TJR during the study period. Age >40 years with no record of hip or knee fracture in the previous 3 months, and had no history of rheumatoid arthritis | 189277 | 77421, 111856 | 69.23 | Statin | Post operation consumption of statin | 4.9 years | TJR | United Kingdom and Denmark |
| Lübbeke 201320 | Cohort | Inclusion Criteria: all primary THAs via a lateral approach and patients who had received the same uncemented cup (Morscher press fit cup) and a 28 mm head | 735 | 337, 398 | 67.8 | Statin | Any use of statins during the period between surgery and the 5-year follow-up | 5 years | THA | Switzerland |
| Oh 201822 | Cross-sectional | Inclusion Criteria: unilateral TKA under spinal anesthesia. Exclusion Criteria: incomplete medical records pertaining to statin use or general anesthesia with endotracheal intubation | 6020 | 509, 5511 | 71 | Statin | Consumption of statins from 1 month before surgery | – | TKA | South Korea |
| Oh 201921 | Cohort | Inclusion Criteria: elective TKA under spinal anesthesia. Exclusion Criteria: incomplete medical records and patients who had undergone a previous TKA, which has been reported to induce more severe pain | 1088 | 108, 980 | 71.6 | Statin | Patients who took statins (atorvastatin, rosuvastatin, simvastatin, pravastatin, pitavastatin, or fluvastatin) daily for at least 1 month before surgery and who continued to take statins from POD 0 until discharge | 3 days | TKA | South Korea |
| Pritchett 2006 | Clinical trial | Inclusion Criteria: hip and knee replacements for osteoarthritis, osteonecrosis, or osteoporosis. Exclusion Criteria: patients with any known condition associated with hyperlipidemia, other than idiopathic, use of any medication known to increase or decrease lipid concentrations, current alcoholism, and metabolic disease other than osteoporosis or osteonecrosis | 84 | 34, 50 | 71 | Statin, fish oil | Post operation consumption of statin or fish oil | Approximately less than 120 days | THA and TKA | – |
| Sutton 202124 | Cohort | Inclusion Criteria: Patients with either TKA or THA with osteoarthrosis or osteoporosis. Exclusion Criteria: trauma, fracture, or infection | 45934 | 44292, 1642 | 66.9 | Statin | Consumption of statin before surgery | 3 years | THA and TKA | United States |
| Takeshita 202025 | Cohort | Inclusion Criteria: THA due to severe hip pain. Exclusion Criteria: bilateral THA within 3 months, revision THA, and hemodialysis, and incomplete data | 203 | 34, 169 | 65.3 | Statin | Statins consumption before surgery | 7 days | THA | Japan |
| Thillemann 201026 | Case–control | Exclusion Criteria: patients could not be properly followed up. | 4698 | 2144, 2554 | – | Statin | Post operation consumption of statin | 10 years | THA | Denmark |
| Zhang 2017 | Clinical trial | Inclusion Criteria: hypercholesterolemia patients with femoral neck fracture treated by primary THA The exclusion criteria: (1) any disorder that might affect bone or mineral metabolism, (2) consumption of corticosteroids, diuretics and any agent for osteoporosis, (3) prior hip preserving surgery or revision arthroplasty. Exclusion Criteria: consumption of any lipid-lowering drugs for the past 6 months | 42 | 27, 15 | 69 | simvastatin 40 mg/day | 12 months treatment with oral administrations of simvastatin at the dosage of 40 mg/day started on the day after surgery | 12 months | THA | – |
Study quality
As shown in Appendix A (Supplemental Digital Content 3, http://links.lww.com/MS9/A455), the included studies had an average MINORS score of 19.75 (range, 7–23), indicating a moderate to high quality of evidence. There were 11 studies with high quality, 6 with moderate quality, and 1 with low quality.
Patient population, inclusion, and exclusion criteria
Studies were heterogeneous in terms of their patient characteristics. Two studies included patients >65 years old10,11, two included patients >40 years old14,19, one study included patients 50–85 years old18, and the rest did not have a limited age range. Eight studies included both THA and TKA patients10–12,14,18,19,23,24, six papers reported on THA patients only13,15,20,25–27, and the remaining four studies reported on TKA only16,17,21,22. Exclusion criteria also varied from revision procedures to a list of underlying medical conditions (Table 1). All except two papers had a larger female population (Sutton et al. study on veterans24, and Zhang et al. study including patients with hypercholesterolemia27).
One study used Atorvastatin 40 mg daily11, and two used Simvastatin 40–80 mg daily12,27. The remaining studies did not limit the medication type or dosage used. The treatment protocol included preoperative use of Statins in 12 studies10,12–18,21,22,24,25. However, the duration of Statin treatment required for inclusion ranged from ‘any consumption of Statins in the year prior to surgery’15, to regular use of Statins16–18. Two studies started Statins perioperatively, either the day of surgery or postoperative day one11,12. Six papers reported the use of Statins postoperatively, up to 12 months after arthroplasty14,19,20,23,26,27.
Outcome measures
There was a total of 51 unique outcome reported in the studies in a wide range of categories. While a formal meta-analysis was not possible because of the heterogeneity, outcomes were extracted and analyzed in six groups: cardiovascular, serum markers, clinical outcomes and complications, pulmonary, renal, and gastrointestinal outcomes (Table 2). All reported outcomes are listed in Appendix B (Supplemental Digital Content 4, http://links.lww.com/MS9/A456), along with their effect size for each study.
Table 2.
Reported outcomes among the studies included in this systematic review.
| Reported outcomes | References |
|---|---|
| Cardiovascular | |
| Acute MI | Bass 201510/Laisalmi-kokki 201018 |
| 90-day total arrhythmia (bradycardia, atrial fibrillation, atrial tachycardia, atrial flutter, paroxysmal supraventricular, tachycardia, and ventricular tachycardia) | Bonano 202112/Chen 2018 |
| 90-day atrial fibrillation | Bonano 202112 |
| 90-day venous thromboembolic event | Bonano 202112 |
| 90-day deep venous thrombosis | Chen 2018 |
| 90-day pulmonary embolism | Chen 2018 |
| 90-day blood transfusion | Chen 2018 |
| 90-day anemia | Chen 2018 |
| 90-day congestive heart failure | Chen 2018 |
| 90-day MI | Chen 2018 |
| 6 weeks MI | Lalmohamed 201619 |
| Serum markers | |
| Changes in hs-cTnI level | Bass 201811 |
| Changes in IL-6 levels | Bass 201811 |
| Changes in postoperative serum-myoglobin (μg/l) | Laisalmi-kokki 201018 |
| Changes in serum lactate dehydrogenase (U/l) | Laisalmi-kokki 201018 |
| Changes in alkaline phosphatase (ALP) | Zhang 2017 |
| Changes in serum total cholesterol (TC) | Zhang 2017 |
| Changes in serum triglycerides (TG) | Zhang 2017 |
| Changes in serum low-density lipoprotein cholesterol (LDL-C) | Zhang 2017 |
| Changes in serum high-density lipoprotein cholesterol (HDL-C) | Zhang 2017 |
| Clinical outcomes and complications | |
| 90-day death | Chen 2018 |
| 90-day readmission | Bonano 2021 |
| 90-day manipulation under anesthesia | Bonano 202112 |
| 90-day infection (included both superficial and deep wound infections, urinary tract infections, respiratory and gastrointestinal infections.) | Bonano 202112 |
| 90-day dislocation | Bonano 202112 |
| 90-day bleeding | Chen 2018 |
| 90-day sepsis/shock | Chen 2018 |
| Revision arthroplasty | Lalmohamed 201619/Cook 202014/Thillemann 201026 |
| Long-term death | Kim 201617 |
| Acute muscular pain | Laisalmi-kokki 201018 |
| Acute muscular weakness | Laisalmi-kokki 201018 |
| Postoperative nausea and vomiting | Laisalmi-kokki 201018 |
| Postoperative paresthesia | Laisalmi-kokki 201018 |
| Postoperative allergic reaction | Laisalmi-kokki 201018 |
| Postoperative muscle fasciculation | Laisalmi-kokki 201018 |
| Long-term femoral osteolysis | Lübbeke 201320 |
| Postoperative delirium | Oh 201822 |
| Postoperative pain score | Oh 201921 |
| Postoperative analgesics requirement | Oh 201922 |
| Long-term changes in amount of bone marrow lipids and quality of lipids in joint fluid | Pritchett 2006 |
| Long-term prosthetic joint infection | Sutton 202124 |
| Long-term changes in BMD | Zhang 2017 |
| Pulmonary | |
| 90-day pneumonia | Chen 2018 |
| 90-day respiratory failure | Chen 2018 |
| Renal | |
| 90-day acute renal failure | Chen 2018 |
| Long-term AKI | kim 201617 |
| Dark urine | Laisalmi-kokki 201018 |
| Changes in plasma creatinine (μmol/l) | Laisalmi-kokki 201018 |
| Urinary tract infection | Laisalmi-kokki 201018 |
| reduction of eGFR by ≥10 ml/min/1.73 m² 7 days after THA | Takeshita 202025 |
| Gastrointestinal | |
| GI bleeding | Chen 2018 |
Cardiovascular outcome
Five studies reported the effects of Statin use on postoperative MI, arrythmias, thromboembolic events, and blood transfusion10,12,13,18,19. None of the studies found a significant association between Statin use and acute, 6-week, or 90-day risk of MI in patients undergoing joint arthroplasty10,13,18,19.
Two studies found significantly lower cardiac arrythmias among Statin users. Chen et al. queried a national database for Statin users who underwent joint arthroplasty and their sex-matched and age-matched controls of non-Statin users. Despite supposedly being at a higher risk for other cardiac complications, Statin users showed a 20% reduction in the relative risk of cardiac arrythmias in the first 90 days after surgery (OR=0.86, 95% CI: 0.78–0.94, P=0.001)13.
The same group published another study in patients undergoing primary PJA, comparing non-Statin users with a group of patients who received postoperative Simvastatin, beginning the day of surgery and continued for the duration of admission. The authors reported that Statin-naïve patients had a 10-fold decrease in the relative risk of developing postoperative arrhythmias (P=0.003). The risk was still lower when controlled for a history of beta-blocker use or atrial fibrillation, although not significantly (P=0.22)12.
Serum markers
Bass et al. performed a double-blind RCT in patients undergoing TJA and hip fracture surgery, and randomized them to placebo or Atorvastatin starting preoperatively and continued up to postoperative day 45. None of their patients had a cardiovascular event in either group. They also measured serum levels of high-sensitivity cardiac troponin I (hs-cTnI), Interleukin-6 (IL-6), and high-sensitivity C-reactive protein (hs-CRP) on postoperative day 2, as measures of subclinical myocardial injury and inflammation. All of their patients had increased levels of IL-6, while 20% showed a postoperative increase in hs-cTnI levels. Atorvastatin did not seem to blunt any of these serum markers, although they were only able to recruit 22 patients11.
Laisalmi-kokki reviewed 48 patients undergoing TKA and THA, half of which were receiving long-term Statin therapy. They measured myoglobin levels, serum lactate dehydrogenase (LDH), and creatine kinase to assess muscular adverse events associated with Statin use. All patients had increased levels of serum markers, with no difference noted between treated with or without Statins18.
Clinical outcomes and complications
Lalmohamed et al. reported on a large database from the United Kingdom and Denmark to determine whether Statin use influences the risk of revision surgery. They used several statistical methods to eliminate the confounding effect of time from surgery, Statin use duration, and follow-up duration. They found an adjusted incidence rate ratio of (IRR)=0.90, (95% CI: 0.85–0.96), showing a slight decrease in the risk of revision with Statin use. In contrast, their time-fixed cohort design yielded substantially lower risk estimates of IRR=0.36 (95% CI: 0.34–0.38), possibly overestimating the effects of Statin use19.
Thillemann et al. queried the national Danish registry of TJA to perform a nested, case–control study. They included all revision procedures performed during a 10-year period, and selected propensity-matched controls who did not have a revision to evaluate the impact of Statin use on the risk of revision. They found an 8.9% rate of revision surgery among all-comers. They found a significant decrease in the risk of revision associated with Statin use, and reported an adjusted relative risk of revision of 0.34 (95% CI: 0.28–0.41) in Statin users. Furthermore, the risk of revision from all causes, including deep infection, dislocation, aseptic loosening, and periprosthetic fracture, was lower among Statin users26.
In a similar study, Cook et al. analyzed the risk of revision surgery from a national database, and found that Statin use significantly reduced the risk of revision (HR=0.82, 95% CI: 0.75–0.90). They also compared the risk of revision with different durations of Statin use. Interestingly, they found that the duration of treatment has a direct effect on the risk of revision, with patients being treated >5 years had a significantly lower rate of revision compared to those treated for <1 year (HR=0.74, 95% CI: 0.62–0.88)14.
Chen et al., in their study querying a national database of TJA patients, found that Statin users were medically sicker at baseline compared to controls, and had a higher blood transfusion rate (OR=1.11, 95% CI: 1.05–1.17, P<0.001), acute renal failure (OR=1.23, 95% CI: 1.10–1.36, P<0.001), and death from all causes (OR=1.66, 95% CI: 1.41–1.95, P<0.001) up to 90 days postoperatively13. Bonano et al. found similar rates of 90 days admission, the need for manipulation under anesthesia, infection, or dislocation among their patients randomized to receive Simvastatin or placebo12.
Oh et al. found that patients treated with Statins were significantly less likely to develop postoperative delirium after TKA (OR=0.66, 95% CI: 0.45–0.97, P=0.036). Furthermore, they found that Simvastatin was associated with more risk reduction compared to Atorvastatin22.
Lubbeke et al. performed a case–control study in patient with femoral osteolysis within 5 years after THA and compared those with and without Statin use. They found that Statin users had a significantly lower risk of femoral osteolysis, with a risk ratio of 0.38 (95% CI: 0.15–0.99) after adjusting for age, sex, diagnosis, BMI, and stem type20. Along the same lines, Zhang et al. followed patients treated with and without Statin for 1 year postoperatively to determine the changes in bone marrow density (BMD), as determined by dual-energy X-ray absorptiometry (DEXA). They found that Simvastatin users had a significantly lower BMD loss compared to non-Statin users (P<0.005 for all comparisons), and they actually had increased BMD in some locations, which was not seen in the control group27.
Laisalmi-kokki et al.18 did not find an increased incidence of muscle weakness or other muscular or liver complications among Statin users who undergo TJA.
Sutton et al. evaluated the impact of Statin exposure on the risk of periprosthetic joint infection (PJI) following TJA in veterans. They found that Statin significantly decreased the risk of PJI (hazard ratio=0.869, 95% CI: 0.79–0.956)24.
Pulmonary
In the Chen et al.13 study, Statin users had a significantly higher incidence of 90-day respiratory failure and pneumonia compared to nonusers. However, Statin users were medically sicker at baseline.
Renal
Laisalmi-kokki et al.18 reported similar rates of dark urine, postoperative myoglobin levels, and changes in serum creatinine in patients treated with and without Statins.
Takeshita et al.25 reviewed 203 patients who underwent unilateral THA and did not find Statin use as a significant risk factor for acute kidney injury.
In contrast, Statin users had a significantly higher incidence if acute kidney injury in the study by Chen et al.13, although they were medically sicker at baseline.
Gastrointestinal
Chen et al.13 did not find Statin use to be a significant risk factor for postoperative bleeding in their national database study (OR=1.11, 95% CI: 0.96–1.28, P=0.147).
Discussion
In this systematic review, we sought to appraise the current evidence for the use of Statins in the joint arthroplasty literature. Our most important finding was that the level of evidence on the subject is very low, and further research is needed to settle the questions about Statin use in TJA. However, the limited evidence suggests that Statins are very low-risk in THA and TKA, and may have potential benefits in short-term and long-term outcomes of patients undergoing TJA.
While the primary use of Statins is as cholesterol-lowering agents, they are considered a ‘pleiotropic’ class of drugs, and have been associated with anti-inflammatory function as well. The effects of Statins on the outcomes of cardiac surgery have been extensively studied, and they have shown antiarrhythmia effects, protecting against postoperative atrial fibrillation, as well as reducing mortality following surgery28. This has been replicated in noncardiac surgeries as well29. With these in mind, and the experimental evidence that Statins are beneficial in bone metabolism, it is only reasonable that joint surgeons, in an effort to improve outcomes, would study the effects of Statins on TJA outcomes30,31.
In this systematic review, we were only able to find 18 studies reporting the use of Statins in TJA. The majority were retrospective, with no definition of what ‘Statin use’ is, with some studies including patients with any exposure to Statins in the year prior to TJA13,15. The heterogeneity of patient selection also extended to the treatment regimen. Twelve studies reported on preoperative use of Statins, six on postoperative treatment, and two on perioperative use. Outcome measures were also heterogeneous, and therefore, a meta-analysis to quantify the findings was not possible.
We grouped the outcomes based on the system involved. Five studies were reported the short-term (up to 90 days) effects of Statins on cardiac outcomes following TJA10,12,13,18,19. As mentioned earlier, there is compelling evidence in the nonorthopaedic literature on the benefits of Statins in decreasing postoperative cardiac arrythmias. This was echoed in two studies reviewed here, one a national database registry, and the other a single-center retrospective study12,13. Both studies showed a strong effect on early cardiac arrythmias, with a larger effect size in Statin-naïve patients. Despite these findings, none of the studies found Statins to significantly change the risk of postoperative MI in patients undergoing TJA. However, given that TJAs are elective surgeries with a low-risk of mortality, a very large sample size would be needed to find a definitive answer.
We found two studies that measured serum markers of muscle damage (LDH, CK) and inflammation (CRP, IL-6) to determine whether Statins may increase muscular complications after TJA. Neither of the studies found a significant difference between Statin users and nonusers, further consolidating the observation that Statins do not increase the complications post-TJA11,18.
Several studies reported the clinical outcomes and complications associated with Statin use in TJA. One study found a significant reduction in the rate of femoral osteolysis with postoperative Statin use20, which was further reported by Zhang et al.27, who found that Simvastatin users had a significantly lower BMD loss around the femoral stem. The risk of revision was also found to be significantly lower among Statin users in two studies. Interestingly, the length of Statin use correlated with the revision risk, with patients who were treated for more than 5 years having significantly lower revision rates14,26. However, a third study focused on the statistical basis of these findings, and suggested that we may have been overestimating the benefits of Statins on revision rate, although they eventually found a significant reduction in revision rate with their more stringent analysis as well19. Another study found that Statins may also decrease the risk of PJI24. The PJI incidence might be correlated to many factors and a causal relationship will be hard to be discussed between Statin use and PJI due to its multifactorial nature32. However, further studies must be performed to thoroughly evaluate it.
Due to the possibility of drug-drug interactions that could impact bleeding risks, particularly in the setting of surgery, research has been conducted on the interaction between Statins and anticoagulants, including warfarin. While some evidence suggests that Statins may raise the risk of bleeding when combined with warfarin, other studies suggest that Statins may lower the risk of bleeding in people using DOACs33,34. The effects may differ based on the particular statin and anticoagulant used, as well as patient-specific factors, and the evidence is not totally consistent. Drug interactions are a serious consideration for clinicians. Statins and some antibiotics, especially macrolides like erythromycin and clarithromycin, can interact dangerously and raise the risk of adverse consequences like renal damage and rhabdomyolysis, which is the breakdown of muscle35. The hepatic uptake transporters and cytochrome P450 (CYP) 3A4 enzyme, which are involved in the metabolism of several Statins, are inhibited by these antibiotics, raising the blood levels of the drug.
It should be noted that while the evidence we found in this systematic review was not strong, the prospects of future trials with level I evidence is not very bright. As mentioned by the authors of an RCT reviewed here, there are certain difficulties in designing and running RCTs on Statin use11. First, a large number of patients are already taking Statins, who would be ineligible to enter the study. Also, there are ethical considerations for randomizing patients at a high risk for postoperative complications to not receive Statins. Finally, with the low complication rate after TJA, especially in the early postoperative period, achieving the sample size needed to detect significant results is simply not feasible. Therefore, while further studies are encouraged on the topic, researchers should be aware of the potential limitations and expectations.
We acknowledge several limitations to this study. As with any systematic review, the quality of the literature determines the quality of the appraised data. Most of the studies reviewed here were retrospective, limiting the generalizability of the findings. As mentioned earlier, over 50 outcomes were assessed in these studies, which made a formal meta-analysis of the data impossible. Furthermore, the majority of retrospective studies did not have a strict inclusion criteria, and patients were included regardless of whether they had adhered to the Statin regimen or not. Despite these limitations, this is, to our knowledge, the first systematic review on the effects, benefits, and potential complications of Statins in TJA patients. Future research may adopt a randomized design, choose tangible, yet reproducible outcome measures, to possibly shed light on the true effects of Statins on outcomes of TJA.
Conclusion
In this systematic review, we found that the evidence on the risks and benefits of Statin use on outcomes of TJA is very limited and heterogeneous. However, some evidence suggests that perioperative use of Statins, especially in Statin-naïve patients, may reduce cardiac (e.g. atrial fibrillation) and noncardiac (e.g. delirium) complications, while not increasing the risk of muscle or liver toxicity. We also found low levels of evidence that Statin use may reduce the long-term risk for revision surgery and osteolysis.
Ethical approval
Ethics approval was not required for this systematic review.
Consent
Informed consent was not required for this systematic review.
Consent for publication: There is complete consent from the authors of the article for the publication of the article.
Sources of funding
There was no financial support for this study.
Author contributions
All the study authors have participated in various parts of the project.
Conflicts of interest disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Research registration unique identifying number (UIN)
The study protocol was registered in PROSPERO (CRD42022310240).
Guarantor
Corresponding author: Seyyed Hossein Shafiei, MD Assistant professor of orthopedic surgery, hip and pelvis Fellowship, Orthopedic Surgery Research Centre, Sina University Hospital, Tehran University of Medical Sciences, Tehran, Iran. Tel.: 0098 21 63121294.
Data availability statement
The data of this study is at the disposal of the authors and there is no restriction on its publication.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
The authors thank the staff of Sina Hospital.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/annals-of-medicine-and-surgery.
Published online 29 April 2024
Contributor Information
Soroush Baghdadi, Email: Baghdadi.soroush@gmail.com.
Mazyar Babagoli, Email: m-babagoli@student.tums.ac.ir.
Mohammad Soleimani, Email: mohammad.sly72@yahoo.com.
Akam Ramezani, Email: akamramezani@gmail.com.
Amirhossein Ghaseminejad-Raeini, Email: ahgnr1999@gmail.com.
Babak Siavashi, Email: siavashi@tums.ac.ir.
Mehrdad Sheikhvatan, Email: med.msv@gmail.com.
Yousef Fallah, Email: fallah2us@yahoo.com.
Seyyed H. Shafiei, Email: dr_hshafiei@yahoo.com.
References
- 1. Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the US, 2014 to 2030. JBJS 2018;100:1455–1460. [DOI] [PubMed] [Google Scholar]
- 2. Kapoor AS, Kanji H, Buckingham J, et al. Strength of evidence for perioperative use of statins to reduce cardiovascular risk: systematic review of controlled studies. BMJ 2006;333:1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellosta S, Bernini F, Paoletti R, et al. Non-lipid-related effects of statins. Ann Med 2000;32:164–176. [DOI] [PubMed] [Google Scholar]
- 4. von Knoch F, Heckelei A, Wedemeyer C, et al. The effect of simvastatin on polyethylene particle-induced osteolysis. Biomaterials 2005;26:3549–3555. [DOI] [PubMed] [Google Scholar]
- 5. Kuhn EW, Slottosch I, Wahlers T, et al. Preoperative statin therapy for patients undergoing cardiac surgery. Cochrane Database Syst Rev 2015;8:CD008493. [DOI] [PubMed] [Google Scholar]
- 6. Le Manach Y, Godet G, Coriat P. The impact of postoperative discontinuation or continuation of chronic statin therapy on cardiac outcome after major vascular surgery. J Vasc Surg 2007;46:1081. [DOI] [PubMed] [Google Scholar]
- 7. Molnar AO, Coca SG, Devereaux PJ, et al. Statin use associates with a lower incidence of acute kidney injury after major elective surgery. J Am Soc Nephrol 2011;22:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 9. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed) 358 2017:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bass AR, Rodriguez T, Hyun G, et al. Myocardial ischaemia after hip and knee arthroplasty: incidence and risk factors. Int Orthop 2015;39:2011–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bass AR, Szymonifka JD, Rondina MT, et al. Postoperative myocardial injury and inflammation is not blunted by a trial of atorvastatin in orthopedic surgery patients. HSS J 2018;14:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonano JC, Aratani AK, Sambare TD, et al. Perioperative statin use may reduce postoperative arrhythmia rates after total joint arthroplasty. J Arthroplasty 2021;36:3401–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen MJ, Bala A, Huddleston JI, III, et al. Statin use is associated with less postoperative cardiac arrhythmia after total hip arthroplasty. HIP Int 2019;29:618–623. [DOI] [PubMed] [Google Scholar]
- 14. Cook MJ, Sorial AK, Lunt M, et al. Effect of timing and duration of statin exposure on risk of hip or knee revision arthroplasty: a population-based cohort study. J Rheumatol 2020;47:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dastrup AS, Pottegard A, Overgaard S, et al. Statin treatment is not associated with the postoperative risk of cardiovascular events or death after total hip arthroplasty surgery. A Population-Based Study from the Danish Hip Arthroplasty Register 2017. PHARMACOEPIDEMIOLOGY AND DRUG SAFETY. WILEY; 111 RIVER ST, HOBOKEN 07030-5774. 373–374. [Google Scholar]
- 16. Jo S-H, Kim H-S, Choi Y-J, et al. AS-55: Statin is associated with lower incidence of deep vein thrombosis confirmed by computed tomographic angiography in patients undergoing total knee replacement arthroplasty. Am J Cardiol 2010;105:24A. [Google Scholar]
- 17. Kim H-J, Koh W-U, Kim S-G, et al. Early postoperative albumin level following total knee arthroplasty is associated with acute kidney injury: a retrospective analysis of 1309 consecutive patients based on kidney disease improving global outcomes criteria. Medicine 2016;95:e4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laisalmi-Kokki M, Tolonen K, Miettinen H, et al. Perioperative chronic use of statins and the risk of muscle complaints in patients undergoing knee and hip endoprosthesis surgery. J Clin Anesth 2010;22:81–87. [DOI] [PubMed] [Google Scholar]
- 19. Lalmohamed A, van Staa TP, Vestergaard P, et al. Statins and risk of lower limb revision surgery: the influence of differences in study design using electronic health records from the United Kingdom and Denmark. Am J Epidemiol 2016;184:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lübbeke A, Garavaglia G, Rothman KJ, et al. Statins may reduce femoral osteolysis in patients with total hip arthroplasty. J Orthop Res 2013;31:814–820. [DOI] [PubMed] [Google Scholar]
- 21. Oh TK, Chang CB, Shin H-J, et al. Association between perioperative statin use and postoperative pain after total knee arthroplasty. Reg AnesthPain Med 2019;44:221–226. [DOI] [PubMed] [Google Scholar]
- 22. Oh TK, Park HY, Shin H-J, et al. The role of perioperative statin use in the prevention of delirium after total knee replacement under spinal anesthesia. J Arthroplasty 2018;33:3666–3671. e1. [DOI] [PubMed] [Google Scholar]
- 23. Pritchett JW. Statins and dietary fish oils improve lipid composition in bone marrow and joints. Clin Orthop Relat Res 2007;456:233–237. [DOI] [PubMed] [Google Scholar]
- 24. Sutton SS, Magagnoli JC, Cummings TH, et al. Statin exposure and risk of prosthetic joint infection after total knee or hip arthroplasty among US veterans. J Arthroplasty 2021;36:3584–3588. e1. [DOI] [PubMed] [Google Scholar]
- 25. Takeshita S, Sonohata M, Kitajima M, et al. Acute deterioration of kidney function after total hip arthroplasty. Malays Orthop J 2020;14:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thillemann TM, Pedersen AB, Mehnert F, et al. The risk of revision after primary total hip arthroplasty among statin users: a nationwide population-based nested case-control study. JBJS 2010;92:1063–1072. [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, Sun Y, Xie H, et al. The effect of simvastatin on periprosthetic bone mineral density in the hypercholesterolaemic patients after total hip arthroplasty. Int Orthop 2018;42:59–64. [DOI] [PubMed] [Google Scholar]
- 28. Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation 2006;114:1455–1461. [DOI] [PubMed] [Google Scholar]
- 29. Bhave PD, Goldman LE, Vittinghoff E, et al. Statin use and postoperative atrial fibrillation after major noncardiac surgery. Heart Rhythm 2012;9:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim JY, Lee EY, Lee EB, et al. Atorvastatin inhibits osteoclastogenesis by decreasing the expression of RANKL in the synoviocytes of rheumatoid arthritis. Arthritis Res Ther 2012;14:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oxlund H, Dalstra M, Andreassen T. Statin given perorally to adult rats increases cancellous bone mass and compressive strength. Calcif Tissue Int 2001;69. [DOI] [PubMed] [Google Scholar]
- 32. Basile G, Gallina M, Passeri A, et al. Prosthetic joint infections and legal disputes: a threat to the future of prosthetic orthopedics. J Orthop Traumatol 2021;22:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu HH, Chang SH, Lee TH, et al. Concurrent use of statins decreases major bleeding and intracerebral hemorrhage in non-valvular atrial fibrillation patients taking direct oral anticoagulants-A nationwide cohort study. Front Cardiovasc Med 2022;9:969259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Engell AE, Svendsen ALO, Lind BS, et al. Drug-drug interaction between warfarin and statins: A Danish cohort study,. Br J Clin Pharmacol 2021;87:694–699. [DOI] [PubMed] [Google Scholar]
- 35. Abu Mellal A, Hussain N, Said AS. The clinical significance of statins-macrolides interaction: comprehensive review of in vivo studies, case reports, and population studies. Ther Clin Risk Manag 2019;15:921–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study is at the disposal of the authors and there is no restriction on its publication.


