Abstract
Diabetes mellitus is a major, rapidly growing endocrine disorder in most countries. The high cost and side effects of conventional drugs for the management of this disease have shifted attention to medicinal plants. Solanum anguivi (S. anguivi) fruits has been reported to be a very good and rich source of polyphenols such as flavonoids, that can be exploited. Flavonoids are plant secondary metabolites widely found in vegetables, fruits and seeds and are known to be of medicinal significance in different range of diseases like diabetes. This study involved in vitro and ex vivo assays on the antioxidant, anti-inflammatory, and antidiabetic properties of flavonoid-rich fractions of S. anguivi fruits. Healthy male Wistar rats (n = 5) weighing 150–180 g were used for ex vivo antioxidant and antidiabetic studies, their liver was exercised for the experiment. The percentage yields of the three flavonoid-rich fractions (Fr. A, B, and C) of S. anguivi fruits obtained from the column chromatographic technique were 15.53 ± 0.75, 11.53 ± 0.80, and 10.17 ± 0.49 mg/g quercetin equivalents. The three fractions (A, B, and C) of S. anguivi fruits significantly scavenged both 2,2-diphenyl-1-picrylhydrazyl (DPPH) with fraction A having the lowest IC50 value (26.14 ± 1.06 μg/ml) compared with fraction B (37.78 ± 5.12 μg/ml) and fraction C (38.24 ± 2.40 μg/ml) when compared with ascorbic acid with the least IC50 value (15.27 ± 0.34 μg/ml). While fraction A (19.61 ± 1.19 μg/ml) scavenged nitric oxide (NO) radicals better than fraction B (22.97 ± 0.55 μg/ml) and fraction C (49.95 ± 6.18 μg/ml). Although ascorbic acid had better scavenging ability than the three fractions (17.23 ± 0.16 μg/ml). The flavonoid-rich fraction A shows better result in inhibiting α-glucosidase with IC50 value of 16.24 μg/ml compared to fraction B (128.04 μg/ml) and fraction C (143.16 μg/ml). For α-amylase, flavonoid-rich fraction A had an IC50 of 31.50 μg/ml compared to B (84.32 μg/ml) and C (145.40 μg/ml). The various controls also showed promising results with acarbose having IC50 of 3.93 μg/mL and 15.66 μg/mL respectively for α-glucosidase and α-amylase. Our findings also showed that FeSO4-induced tissue damage decreased the levels of GSH, SOD, and CAT activities while increasing the levels of MDA. In contrast, following treatment with the three flavonoid fractions of S. anguivi fruits helped to restore these parameters to near-normal levels, by significantly increasing the potential of GSH, SOD, CAT and reducing the levels of MDA which signifies that flavonoid-rich fractions of S. anguivi have great potential to address complications arising from oxidative stress. In addition, the three flavonoid-rich fractions A, B, and C of S. anguivi fruits exhibited ex vivo anti-inflammatory properties via reduced nitric oxide levels in iron-induced oxidative damage. Data obtained from this study shows that the flavonoid-rich fraction of S. anguivi possess anti-diabetic property via inhibition of α-glucosidase and α-amylase and antioxidant property via free radical scavenging. Also, comparing all the fractions, flavonoid-rich fraction A appears to be more potent compared to the fractions B and C. Further research will be needed in isolating and as well applying the fractions in real life situations in the management of diabetes.
Keywords: S. anguivi fruits, Flavonoids, Diabetes mellitus, Antioxidant, Anti-inflammatory
1. Introduction
Medicinal plants are groups of plants with vital roles in alleviating human illness [1]. According to a survey done by the World Health Organization, medicinal plants have long been regarded for their medicinal potentials and nutritional importance, both of which aid in the treatment of various ailments including oxidative stress-related diseases and drug development [2]. Several experimental studies on medicinal plants have found significant differences in chemical composition (total flavonoid and total phenolic contents), mineral count, and biological activity between geographical regions and places around the world [3,4]. Solanum anguivi Lam. (SAG) is a rare ethnomedicinal herb belonging to the family Solanaceae. It is called African eggplant in English. The chronic administration and antioxidant activity of saponin extract of S. anguivi fruits in Wistar rats was reported by Ref. [5]. Their structure consists of 15 carbon skeletons and two aromatic rings (A and B) connected by three carbon chains. Flavonoids are further classified into 6 subclasses: flavonols, flavones, flavanones, isoflavones, flavanols, and anthocyanidins [6].
Diabetes mellitus (DM) is a chronic metabolic disease that is characterized by a deficiency in insulin production, insulin action or both [7]. Diabetes usually presents with polydipsia, polyuria, and microvascular problems involving the eyes, kidney and peripheral nerves along with cardiovascular challenges, including hypertension [7]. Despite efforts to curb the prevalence of diabetes mellitus, the disease is still on the rise. Hence, an effective therapeutic approach targeting diabetes precursor enzymes such as α-amylase and α‐glucosidase for treating diabetes will be of significant benefit [8,9]. The α-amylase enzyme converts oligosaccharides to polysaccharides [10], while α-glucosidase catalyzes the final phase of carbohydrate hydrolysis, resulting in absorbable monosaccharides [11]. However, inhibition of the α-amylase enzyme reduces the rate of glucose absorption, especially after meals [12]. Oxidative stress is a main metabolic abnormality involved in the development of diabetic complications [13]. This results when there is an increased production of reactive oxygen species beyond the capacity of the antioxidant system. ROS production could increase as a result of abnormal metabolism of glucose, free fatty acids and other reactive metabolites in the diabetic mellitus state [14]. Chronic inflammation and oxidative stress are involved in the pathophysiology of diabetes mellitus. Proinflammatory cytokines can indirectly incite oxidative stress by activating macrophages, which play an essential role in removing the pathogen via the production of reactive oxygen species. Hence, diabetes mellitus, oxidative stress and inflammation act in a vicious cycle. Oxidative damage has been linked to the onset and progression of numerous liver diseases, including fibrosis, liver cancer, and cirrhosis [15]. Solanum anguivi has been reported as a natural source of antioxidant. Several studies have justified the testing of S. anguivi in an animal model. The administration of saponin from S. anguivi fruit showed marked hypoglycemic, antiperoxidative and antihyperlipidemic effects in alloxan-induced diabetic rats [16]. In addition, Elekofehinti et al. [16] reported that the methanolic extract of S. anguivi fruit exhibited antioxidant activity. Furthermore, the saponins extract from S. anguivi fruit was also reported to exhibit cholesterol lowering effect [17]. Despite the few pharmacological activities reported on the S. anguivi fruits, there is little information of its effects on metabolites and pathways involved in oxidative-mediated injury in liver tissues. Therefore, the aim of this study was to evaluate the antioxidant, antidiabetic, and anti-inflammatory activities of flavonoid-rich fractions of Solanum anguivi LAM. Fruit via in vitro and ex vivo studies.
2. Materials and methods
2.1. Collection of plant material
The fruits of Solanum anguivi were obtained from Oja Oba's Market in Ado Ekiti, Ekiti State, Nigeria. It was authenticated at the Herbarium Unit of the Department of Plant Science, Ekiti State University, Ado-Ekiti, with herbarium number UHAE 202/377. The fruits of Solanum anguivi were air-dried for four weeks, ground to a fine powder using a mechanical blender (Kenwood, Model BL490, China), and then stored in an airtight container for further investigation.
2.2. Preparation of methanolic and flavonoid-rich fractions of S. anguivi fruits
The extraction of flavonoids was carried out from the defatted S. anguivi fruits using petroleum ether [18]. The powdered plant was macerated in 80 % methanol to obtain the hydroalcoholic crude extract using an Erlenmeyer flask for 2 days at 25 °C. After 48 h, the filtrate was filtered using filter paper (Whatman No. 1). The flavonoid-rich lipids were further extracted using the method of [19]. The filtrate collected was concentrated to dryness using a rotary evaporator. Column chromatographic separation was carried out by preparing a homogenous slurry of the flavonoid-rich extract in a solvent system of chloroform and methanol (60:40). Next, the adsorption of the sample onto silica gel (20 g) was determined. The sample was then allowed to dry at room temperature to form powder. The powder was added from the top of the column with the aid of a funnel. The solvent system of chloroform and methanol (60:40) was carefully added from the top of the column by means of a funnel. Isocratic elution was further carried out to obtain the eluates. Eluates (20 mL each) were collected in test tubes marked fractions 1–19. The eluates with similar retention factor (Rf) values were pooled together using a thin layer chromatographic procedure, which yielded three [3] subfractions, A (5.8 g), B (3.9 g) and C (3.4 g).
2.3. Determination of the total flavonoid content of flavonoid-rich fractions of S. anguivi fruits
Total flavonoids were analyzed using the aluminum chloride colorimetric method with slight modifications according to Ref. [20]. Quercetin was used to make the calibration curve. Ten [10] mg of quercetin was dissolved in 96 % ethanol and diluted to 2, 4, 6, 8 and 10 μg/mL. One [1] ml of each concentration of standard solutions as well as 1 ml of each sample solution were mixed with 3 mL 96 % ethanol, 0.2 mL 10 % aluminum chloride, 0.2 mL 1 M potassium acetate and 5.6 mL distilled water. The mixture was incubated at room temperature for 10 min with intermittent shaking. The absorbance of the resulting mixture was measured at 376 nm against a blank without aluminum chloride using a UV–Vis spectrophotometer. Total flavonoids were calculated as the mean ± SD (n = 3) and expressed as the weight of quercetin equivalent (QE) in 100 mg extract.
2.4. Evaluation of in vitro antioxidant capacity
2.4.1. Assay of 2,2-diphenyl-1-picryl-hydrazyl radical scavenging ability
The method described by Ref. [21] was used to determine the 2,2-diphenyl-1-picryhydrazyl scavenging effect of the flavonoid-rich fractions of S. anguivi fruit. One (1 ml) milliliter of flavonoid-rich fractions of S. anguivi fruit (15–240 μg/ml) or reference compound was added to a methanol solution of DPPH (0.4 mM). The mixture was left in the dark for 30 min before reading the absorbance at 517 nm. These tests were performed in triplicate using ascorbic acid as a reference. The radical scavenging ability was calculated as the percentage of DPPH discoloration as follows.
Calculation
where Ablank is the absorbance with extract without DPPH.
Asample is the absorbance of the reacting mixture with fractions and DPPH.
2.5. Assay of nitric oxide radical scavenging ability
The nitric oxide-scavenging activity of flavonoid-rich fractions of S. anguivi fruit was determined according to the method described by Ref. [22]. Various concentrations (15–240 μg/ml) of flavonoid-rich fractions of S. anguivi fruit and standards were prepared. Sodium nitroprusside (2.5 ml, 10 mM) in phosphate buffered saline (PBS) pH 7.4 was added to 0.5 ml of different concentrations of flavonoid-rich fractions of S. anguivi fruit and standard. The reaction mixture was incubated at 25 °C for 150 min. After incubation, 0.5 ml of Griess reagent (1 % (w/v) sulphanilamide, 2 % (v/v) H3PO4 and 0.1 % (w/v) naphthylethylenediamine hydrochloride) was added. Sodium nitroprusside in PBS (2 ml) was used as a control. The nitric oxide radical scavenging activity of the flavonoid-rich fractions of S. anguivi fruit and ascorbic acid was calculated according to the following equation.
Calculation
The nitric oxide radical scavenging activity was calculated as follows:
2.6. Ferric reducing antioxidant ability
The reducing power of flavonoid-rich fractions of S. anguivi fruit was estimated according to the method of [23]. Various concentrations (15–240 μg/ml) of flavonoid-rich fractions of S. anguivi fruit were dissolved in 2.5 ml (200 mM) sodium phosphate buffer (pH 6.6) and 2.5 ml of 1 % potassium ferricyanide. The mixtures were incubated at 50 °C for 20 min, and then 2.5 ml of 10 % TCA was added to terminate the reaction, followed by centrifugation at 3000×g for 10 min. Then, 5 ml of the supernatant was mixed with an equal volume of water and 1 ml of 0.1 % ferric chloride. The same treatments were performed with standard ascorbic acid solution, and the absorbance was read at 700 nm. The reducing power was calculated and expressed as ascorbic acid equivalent.
2.7. Total antioxidant capacity
The total antioxidant capacity of flavonoid-rich fractions of S. anguivi fruit was estimated according to the method of [24]. One (1 ml) milliliter of the reagent (28 mM sodium phosphate, 4 mM ammonium molybdate and 0.6 M sulfuric acid) and 100 μl (0.1 ml) of the various concentrations (15–240 μg/ml) of flavonoid-rich fractions of S. anguivi fruit were placed in test tubes. The test tubes were then capped using foil paper and incubated in a water bath at 95 °C for approximately 90 min. The samples were then cooled, and the absorbance was read at 695 nm.
2.8. Determination of enzyme inhibitory activities of Solanum anguivi flavonoid-rich fraction
The flavonoid-rich fractions of S. anguivi fruit were evaluated for their antidiabetic activity using in vitro assays, such as α-amylase and α-glucosidase inhibitory activity [25,26].
2.9. Determination of alpha-amylase inhibitory activity
A volume of 250 μl of the samples at different concentrations (15–240 μg/ml) was incubated in a water bath with 500 μl of porcine pancreatic amylase (2 U m/L) in 100 mmol/L phosphate buffer (pH 6.8) at 37 °C for 20 min. A known volume (250 μl) of 1 % starch dissolved in 100 mmol/L phosphate buffer (pH 6.8) was added to the reaction mixture and incubated at 37 °C for 1 h. Then, one ml of dinitrosalicylic acid (1 % 3,5-dinitrosalicylic acid, 0.2 % phenol, 0.05 % Na2SO3 and 1 % sodium hydroxide) color reagent was added and heated at 100 °C for 10 min. After cooling to room temperature in a cold water bath, the absorbance of the resulting mixture was read at 540 nm. Acarbose was used as a reference. The experiment was carried out in triplicate.
Calculation
The percentage inhibition was calculated as follows:
where Asample is the absorbance of the sample and Acontrol is the absorbance of the control.
2.10. Determination of alpha-glucosidase inhibitory activity
One (1 mg) milligram of α-glucosidase (Saccharomyces cerevisiae, Sigma‒Aldrich, USA) was dissolved in 100 ml of phosphate buffer (pH 6.8) containing 200 mg of bovine serum albumin. The reaction mixture consisting of 10 μl of sample at varying concentrations (15–240 μg/ml) of flavonoid-rich fractions of S. anguivi fruit was premixed with 490 μl of phosphate buffer pH 6.8 and 250 μl of 5 mM p-nitrophenyl α-d-glucopyranoside. After preincubating at 37 °C for 15 min. The reaction was terminated by the addition of 2000 μl of 200 mM Na2CO3. α-Glucosidase activity was determined spectrophotometrically at 400 nm by measuring the quantity of p-nitrophenol released from p-NPG. Acarbose was used as a positive control for the α-glucosidase inhibitor.
Calculation
The percentage inhibition was calculated as follows:
where Asample is the absorbance of the sample and Acontrol is the absorbance of the control.
2.11. Ex vivo antioxidant study
2.11.1. Experimental animals
Healthy male Wistar rats (n = 5) weighing 150–180 g were used for ex vivo studies. They were obtained from the Animal House of the College of Medicine, Ekiti State University, Ado-Ekiti, Ekiti State. The animals were housed in aluminum cages under standard laboratory conditions (12-h light/dark cycle, 25 ± 2 °C). They were fed rat feed (Top Feeds, a product of Premier Feed Mills of Nigeria PLC, Ogorode, Sapele, Delta State, Nigeria) and tap water ad libitum. The animals were acclimatized for two weeks before the start of the experiment. This study was approved, and an ethical clearance certificate (ORDI/AD/EAC/2023/118) was issued by the University Ethical Committee through the Office of Research, Development, and Innovation (ORDI), Ekiti State University.
2.11.2. Induction of tissue oxidative stress and treatment
After being fasted overnight, the rats were anesthetized with halothane, and the livers were harvested. The harvested livers were homogenized and centrifuged at 15,000 rpm and 40 °C. The supernatant was separated into tubes for ex vivo investigations. Oxidative stress was induced in isolated hepatic tissue as described by Ref. [27]. A total of 30 μl of 15 mM ferrous phosphate (FeSO4) was incubated with 100 μl of different concentrations of the column chromatographic fractions (CCF) and 100 μl of hepatic tissue homogenate for 30 min at 37 °C. Thereafter, catalase, superoxide dismutase activities, reduced glutathione levels, and MDA levels were determined.
2.11.3. Measurement of lipid peroxidation
The level of lipid peroxidation was measured according to a previously reported method by Ref. [28]. Then, 200 μl of 8.1 % SDS solution, 750 μl of 20 % acetic acid, 2 ml of 0.25 % TBA solution and 850 μl of water were added to 200 μL of column chromatographic fractions (CCF) with different concentrations (15–240 μg/ml) and boiled for 1 h. The absorbance was measured at 532 nm after cooling. The MDA concentrations of the samples were calculated from the MDA standard curve.
2.11.4. Determination of catalase activity
Catalase activity was determined using a spectrophotometric method as described by Ref. [29]. The sample (100 μl) was mixed and incubated with 1000 μl of 65 μM H2O2 in 6.0 mM sodium phosphate buffer, pH 7.4 at 37 °C for 2 min. The reaction was stopped by adding 4000 μl of 32.4 mM ammonium molybdate, and the absorbance was measured at 347 nm against the reagent blank. A standard tube contained all the reagents except the tissue sample, and the control tube contained all the reagents except H2O2. Catalase activity was calculated using the following formula:
t: time; S°: absorbance of standard tube; S: absorbance of test tube; M: absorbance of control test (correction factor); Vt: total volume of reagents in test tube; Vs: volume of test sample.
2.11.5. Determination of superoxide dismutase (SOD) enzyme activity
This assay was carried out according to the method of [30]. Samples were treated as above. Then, 170 μl of 0.1 mM diethylenetriaminepentaacetic acid (DETAPAC) and 15 μl of the infusion were placed in a 96-well plate. Then, 15 μl of 1.6 mM 6-hydroxydopamine (6-HD) was added, and the mixture was quickly mixed by gently tapping all four sides of the plates. The absorbance was recorded at 492 nm for 3 min at 1 min intervals. SOD enzyme activity was calculated using the following formula:
| Activity = (A1-Ab)/ε490* RV * Df/Sv |
ε490 = Molar absorptivity at 490 nm = 1.742/mM/cm; A1 and Ab = Reaction rate for sample and blank, respectively; RV=Reaction volume; Df = Dilution factor; Sv = Sample volume.
2.11.6. Determination of nitric oxide level
The level of nitric oxide (NO) in the tissues was determined using Griess’ method as described previously [31]. Then, 100 μl of the samples or distilled water (blank) was incubated with 100 μl of Griess reagent at 25 °C in the dark for 30 min. Absorbance was read at 548 nm.
2.12. Data analysis
Data were expressed as the means ± SD of three determinations. GraphPad Prism 9 (GraphPad Software Inc., San Diego, California, USA) was used to conduct statistical analysis on the data using one-way Analysis of variance (ANOVA), as well as the calculation of IC50. The data were considered statistically significant at p < 0.05.
3. Results
3.1. Total flavonoid content
The total flavonoid contents of the flavonoid-rich fractions (A, B, and C) of S. anguivi were 15.53 ± 0.75, 11.53 ± 0.80, and 10.17 ± 0.49 mg/g quercetin equivalent, respectively.
3.2. In vitro antioxidant activity
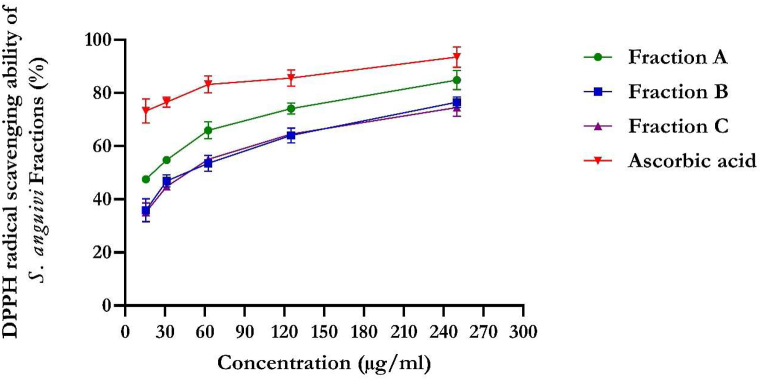
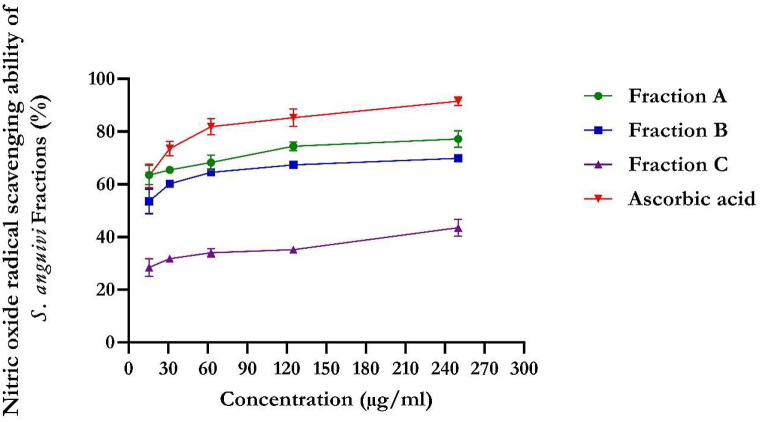
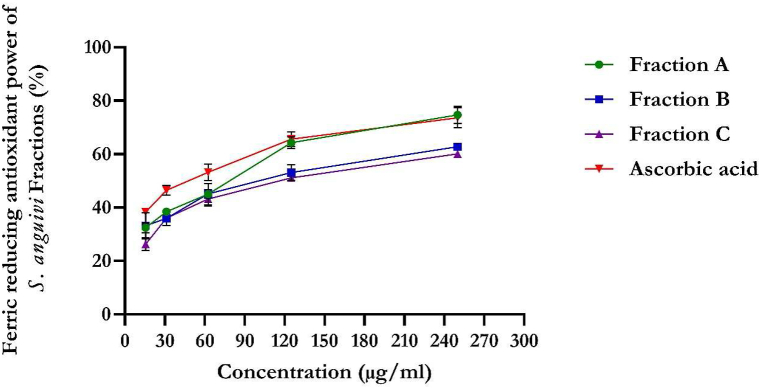
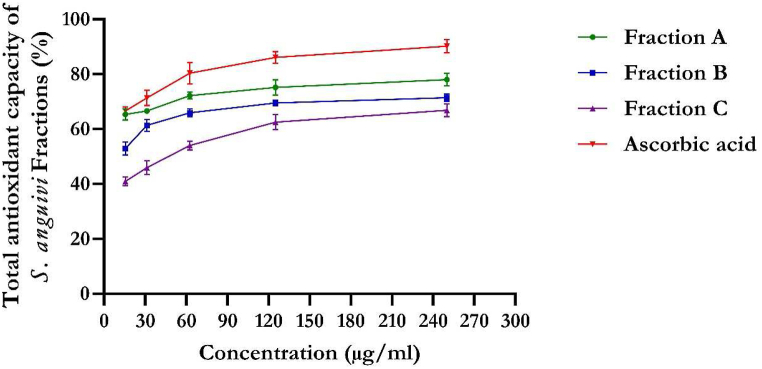
The flavonoid-rich fractions of S. anguivi showed significant (p < 0.05) antioxidant activities compared to ascorbic acid measured by DPPH, NO, ferric reducing antioxidant power (FRAP), and total antioxidant capacity methods, as depicted in Fig. 1, Fig. 2, Fig. 3, Fig. 4. The flavonoid-rich fractions (A, B, and C) of S. anguivi significantly (p < 0.05) scavenged free radicals with increasing concentrations. The three fractions (A, B, and C) of S. anguivi fruits significantly scavenged both 2,2-diphenyl-1-picrylhydrazyl (DPPH) with fraction A having the lowest IC50 value (26.14 ± 1.06 μg/ml) compared with fraction B (37.78 ± 5.12 μg/ml) and fraction C (38.24 ± 2.40 μg/ml) when compared with ascorbic acid with the least IC50 value (15.27 ± 0.34 μg/ml). While fraction A (19.61 ± 1.19 μg/ml) scavenged nitric oxide (NO) radicals better than fraction B (22.97 ± 0.55 μg/ml) and fraction C (49.95 ± 6.18 μg/ml). Although ascorbic acid had better scavenging ability than the three fractions (17.23 ± 0.16 μg/ml). However, flavonoid-rich fraction A of S. anguivi showed the most potent antioxidant activity of all three fractions.
Fig. 1.
DPPH scavenging activity of flavonoid-rich fractions of S. anguivi fruits. Values represent the mean ± standard deviation (n = 3).
Fig. 2.
NO scavenging activity of flavonoid-rich fractions of S. anguivi fruits. Values represent the mean ± standard deviation (n = 3).
Fig. 3.
Ferric reducing antioxidant power of flavonoid-rich fractions of S. anguivi fruits. Values represent the mean ± standard deviation (n = 3).
Fig. 4.
Total antioxidant capacity of flavonoid-rich fractions of S. anguivi fruits. Values represent the mean ± standard deviation (n = 3).
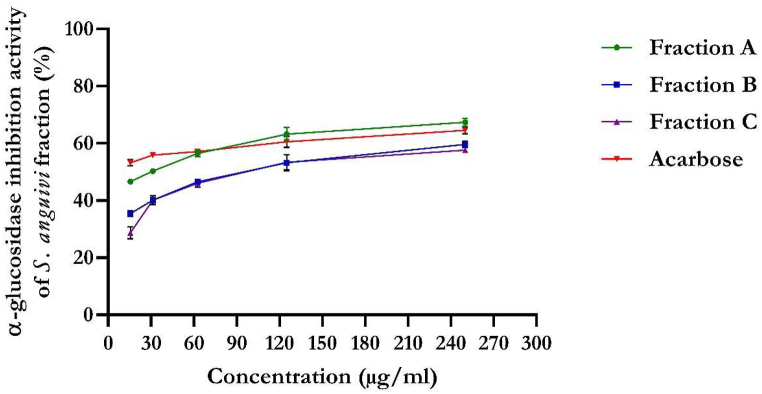
3.2.1. In vitro enzyme inhibitory activities
The flavonoid-rich fractions of S. anguivi fruits showed a remarkable inhibitory effect on α-glucosidase activity in a dose-dependent manner, as shown in Fig. 5. Flavonoid-rich fraction A of S. anguivi fruits showed better inhibitory activity (IC50 = 16.24 μg/ml) against α-glucosidase than fractions B (IC50 = 128.04 μg/ml) and C (IC50 = 143.16 μg/ml). The standard, acarbose, showed 64.57 % inhibition of α-glucosidase (IC50 = 3.93 μg/ml).
Fig. 5.
α-Glucosidase activity of flavonoid-rich fractions of S. anguivi fruits. Values represent the mean ± standard deviation (n = 3).
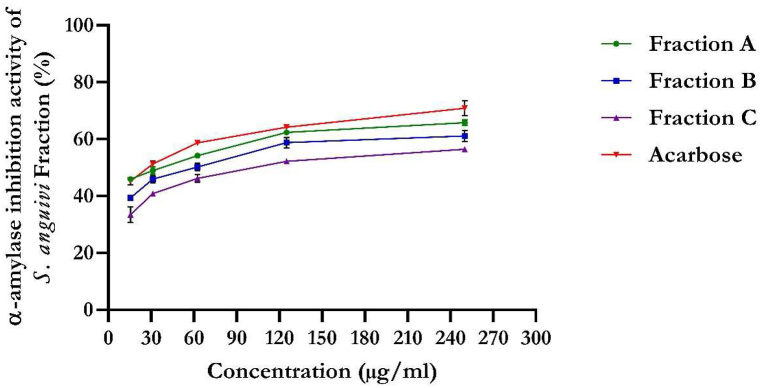
The flavonoid-rich fractions of S. anguivi fruits also led to a significant inhibitory effect (IC50 = 31.50 μg/ml) against α-amylase activity in a dose-dependent manner (Fig. 6) compared to flavonoid-rich fractions B (IC50 = 84.32 μg/ml) and C (IC50 = 145.40 μg/ml). The standard, acarbose, showed 70.88 % inhibition of α-amylase (IC50 = 15.66 μg/ml).
Fig. 6.
α-amylase activity of flavonoid-rich fractions of S. anguivi fruits. Values represent the mean ± standard deviation (n = 3).
3.3. Ex vivo antioxidative studies
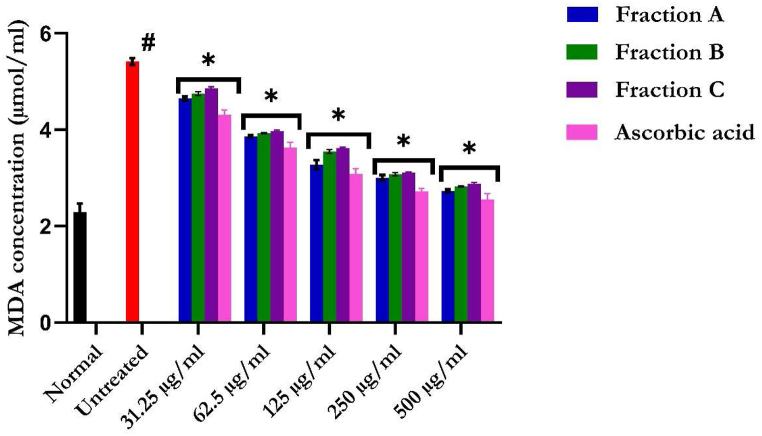
3.3.1. Lipid peroxidation
The inhibitory potentials of the flavonoid-rich fractions of S. anguivi fruits on lipid peroxidation are shown in Fig. 7. Upon induction of oxidative injury in hepatic tissue, there was an increase in MDA levels. However, treatment with the flavonoid-rich fractions of S. anguivi fruits led to a significant and dose-dependent reduction in MDA levels. Those treated with flavonoid-rich fractions A possesses a greater reduction in the inhibitory activity of the plant compared to the B and C fractions, which also show a reduction but not up to the control.
Fig. 7.
Lipid peroxidation inhibitory activity of flavonoid-rich fractions of S. anguivi fruits. Values represent the mean ± standard deviation (n = 3). *Statistically significant (p < 0.05) compared with untreated tissues; #statistically significant (p < 0.05) compared with normal tissues. Normal: liver tissues not treated with FeSO4 and/or flavonoid-rich fractions of S. anguivi fruits. Untreated: liver tissues treated with FeSO4 only.
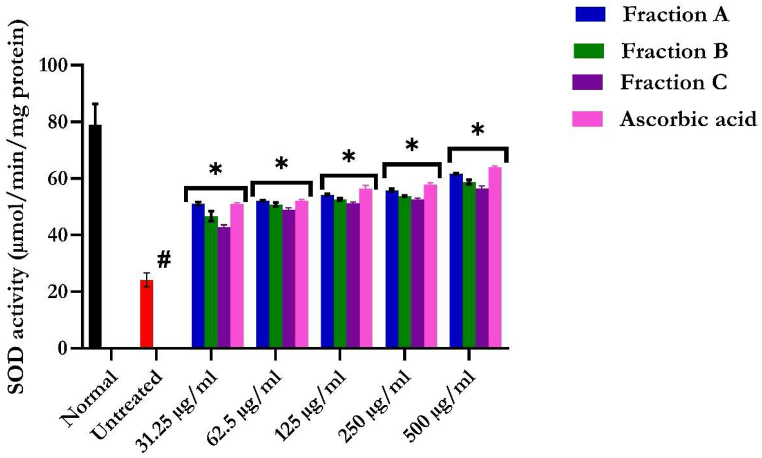
3.3.2. Superoxide dismutase enzyme activity
The reduced SOD activities in hepatic tissues further indicate an occurrence of oxidative injury upon incubation with FeSO4, as shown in Fig. 8. SOD enzyme activity was significantly increased in the flavonoid-rich fractions of S. anguivi fruit-treated tissues compared with the untreated tissue. The group treated with flavonoid-rich fraction A possesses a significant (p < 0.05) increase in the SOD activity of the liver compared to the fractions B and C.
Fig. 8.
Superoxide dismutase activity of flavonoid-rich fractions of S. anguivi fruits. Values represent the mean ± standard deviation (n = 3). *Statistically significant (p < 0.05) compared with untreated tissues; #statistically significant (p < 0.05) compared with normal tissues. Normal: liver tissues not treated with FeSO4 and/or flavonoid-rich fractions of S. anguivi fruits. Untreated: liver tissues treated with FeSO4 only.
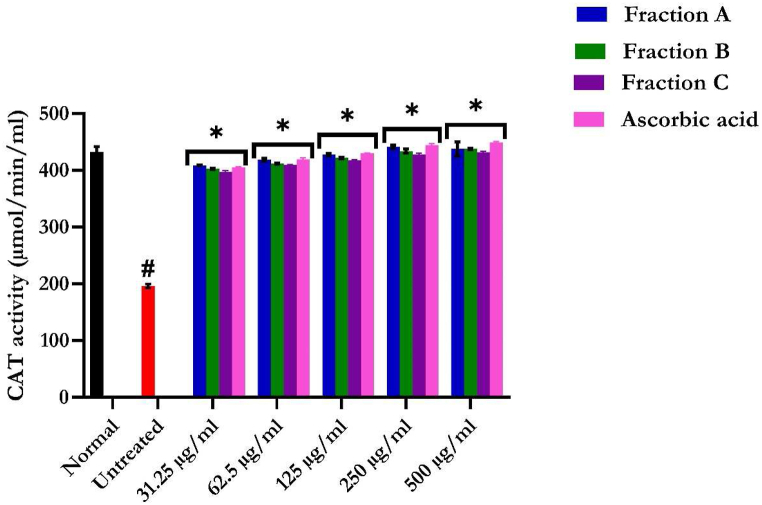
3.3.3. Catalase activity
The reduced CAT activities in hepatic tissues further indicate an occurrence of oxidative injury upon incubation with FeSO4, as shown in Fig. 9. Flavonoid-rich fractions of S. anguivi fruits significantly reduced the amount of H2O2 remaining in Fe2+-induced oxidative stress, as shown in Fig. 9, indicating enhanced catalase activity as it converted H2O2 to H2O. The group treated with flavonoid-rich fraction A possesses a significant (p < 0.05) increase in the CAT activity of the liver compared to the fractions B and C.
Fig. 9.
Catalase activity of flavonoid-rich fractions of S. anguivi fruits in oxidative hepatic injury. Data are presented as the mean ± SD (n = 3). *Statistically significant (p < 0.05) compared with untreated tissues; #statistically significant (p < 0.05) compared with normal tissues. Normal: liver tissues not treated with FeSO4 and/or flavonoid-rich fractions of S. anguivi fruits. Untreated: liver tissues treated with FeSO4 only.
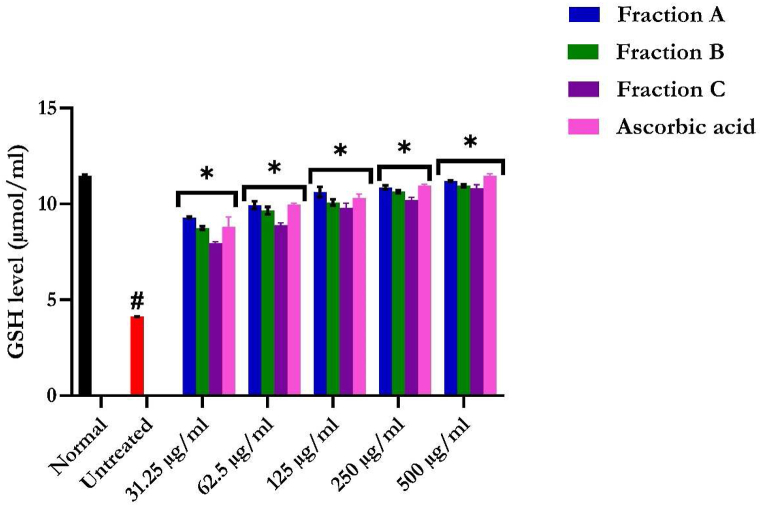
3.3.4. Glutathione reduced (GSH) level
Incubation of hepatic tissues with FeSO4 caused a significant (p < 0.05) reduction in GSH levels, indicating an incidence of oxidative injury, as shown in Fig. 10. Treatment with flavonoid-rich fractions of S. anguivi fruits significantly (p < 0.05) elevated the levels compared to ascorbic acid, with the highest concentration of flavonoid-rich fractions of S. anguivi fruits showing an increase that portrays potent activity. The flavonoid-rich fractions A extract treated group, possess a significant (p < 0.05) increase in the GSH activity of the liver compared to the fractions B and C.
Fig. 10.
Reduced glutathione (GSH) level of flavonoid-rich fractions of S. anguivi fruits in oxidative hepatic injury. Data are presented as the mean ± SD (n = 3). *Statistically significant (p < 0.05) compared with untreated tissues; #statistically significant (p < 0.05) compared with normal tissues. Normal: liver tissues not treated with FeSO4 and/or flavonoid-rich fractions of S. anguivi fruits. Untreated: liver tissues treated with FeSO4 only.
3.4. Ex vivo inflammatory studies
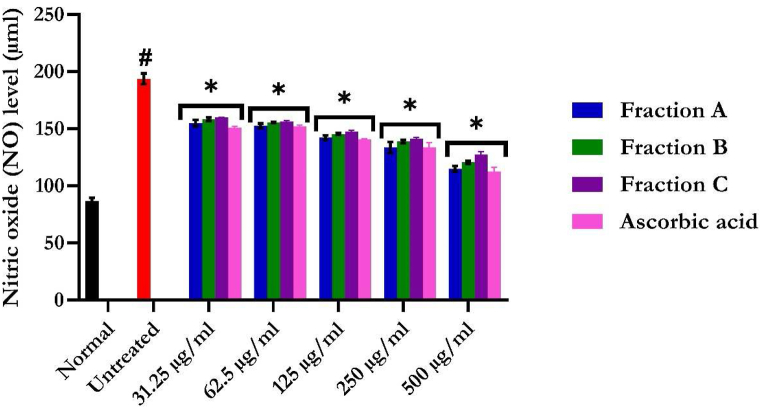
3.4.1. Nitric oxide (NO) level
Induction of hepatotoxicity significantly (p < 0.05) elevated hepatic NO levels, as shown in Fig. 11. The dose-dependent NO depletion upon treatment with flavonoid-rich fractions of S. anguivi fruits may therefore indicate a pro-inflammatory-protective effect on iron-induced hepatotoxicity.
Fig. 11.
Nitric oxide (NO) level of flavonoid-rich fractions of S. anguivi fruits in oxidative hepatic injury. Data are presented as the mean ± SD; n = 3. *Statistically significant (p < 0.05) compared with untreated tissues; #statistically significant (p < 0.05) compared with normal tissues. Normal: liver tissues not treated with FeSO4 and/or flavonoid-rich fractions of S. anguivi fruits. Untreated: liver tissues treated with FeSO4 only.
4. Discussion
Flavonoids help regulate cellular activity and fight off radicals that causes oxidative damage on the body. Methanolic extract is a frequent choice for isolating flavonoids from S. anguivi fruits because compared to other solvents like water or ethanol, methanol can penetrate plant cell walls more efficiently, leading to better extraction of intracellular flavonoids [32]. In a study published by Ref. [33] found that a methanolic extract of S. anguivi fruits had the highest total phenolic and flavonoid content compared to other solvents like water and ethanol. In addition, another study by Ref. [34] demonstrated the antioxidant and anti-inflammatory activity of a methanolic extract from S. anguivi fruits, attributed to its flavonoid content. Hence the reason why we selected the solvent methanol in extraction our flavonoid-rich extract of S. anguivi fruits for this study.
Several techniques have been used to determine the antioxidant activity in vitro to allow rapid screening of substances since substances that have low antioxidant activity in vitro will probably show little activity in vivo [35]. Free radicals are known to play a definite role in a wide variety of pathological manifestations. Antioxidants fight against free radicals and protect us from various diseases. They exert their action either by scavenging reactive oxygen species or protecting antioxidant defense mechanisms [36]. The electron donation ability of natural products can be measured by 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) purple-colored solution bleaching [35]. A large decrease in the absorbance of the reaction mixture indicates significant free radical scavenging activity of the compound under test [37]. In the present study, among all the fractions tested, the flavonoid-rich fraction A of S. anguivi fruits showed a markedly higher inhibition percentage and was positively correlated with the total flavonoid content. This result also correlates with several other studies that carried out DPPH in-vitro analysis on flavonoid rich extracts, and they all show remarkable promising results [[38], [39], [40], [41]]. The results of this study suggest that the flavonoid-rich fractions of S. anguivi have a hydrogen donating ability that stabilizes free radicals and scavenges potential damage.
Excessive nitric oxide concentration has been associated with diabetes mellitus. Flavonoid-rich fractions of S. anguivi fruits exhibited effectiveness in scavenging NO, which could be attributed to their antioxidant properties. The bioactive agents of S. anguivi compete with oxygen to react with nitric oxide, thereby suppressing nitrite generation. The results align with previously reported studies [42].
In the reducing power assay, the yellow color of the test solution changes to green depending on the reducing power of the test specimen. The presence of the reductants in the solution causes the reduction of the Fe3+/ferricyanide complex to the ferrous form. Therefore, Fe2+ can be monitored by absorbance measurements at 700 nm. Previous reports have suggested that reducing power exerts antioxidant action by donating a hydrogen atom to break the free radical chain [40]. Increasing absorbance at 700 nm indicates an increase in reducing ability. The antioxidants present in fractions A, B, and C of S. anguivi caused their reduction of the Fe3+/ferricyanide complex to the ferrous form and thus proved the reducing power. The present study demonstrated that the flavonoid-rich fraction A of S. anguivi exhibited the highest total antioxidant capacity. Recent studies have shown that many flavonoids and related polyphenols contribute significantly to the antioxidant capacity of medicinal plants [43,44]. The scavenging abilities of the flavonoid-rich fractions of S. anguivi could serve in managing diabetes mellitus, as free radicals are involved in the pathogenesis of diabetes mellitus. This results correlates with previously reported studies on antioxidant activities of flavonoid-rich extract of Syzygium cumini and Dalbergiella welwitschii [44,45].
Inhibitors of the α-glucosidase and α-amylase enzymes involved in carbohydrate metabolism are a recognized technique in the management of diabetes mellitus for decreasing postprandial hyperglycemia [8]. Efficient control of the glycemic index in diabetes requires moderate levels of α-amylase inhibitors and potent levels of α-glucosidase inhibitors, as they aid in effectively regulating the available dietary sugar needed for absorption in the small intestine [46]. The inhibitory effect of the flavonoid-rich fractions of S. anguivi on the activity of α-glucosidase and α-amylase was shown to be concentration dependent and could serve as an indicator of the potential antidiabetic effect of the fractions.
The liver, as the main detoxifying organ, helps to maintain metabolic homeostasis. The organ metabolizes various compounds that produce ROS [47], causing liver parenchymal cells to be more vulnerable to oxidative stress. Disequilibrium in the levels of pro-oxidant and antioxidant causes oxidative stress, which has been linked to the pathogenesis and development of hepatic toxicity [48,49]. In this study, the effect of FeSO4 on the liver caused oxidative injury and significantly reduced CAT and SOD activities, as well as GSH levels, with an accompanying increase in MDA levels, showing the development of oxidative stress and inflammation. When administered with flavonoid-rich fractions of S. anguivi, significant reversion of these alterations caused by FeSO4 was observed, confirming the extract's resolving effect on hepatic oxidative stress. The most prominent hepatoprotective effect was evident in fraction A. This finding correlates with earlier studies on the flavonoid-rich fraction of Dalbergiella welwitschii leaf [45].
Proinflammation has also been related to the advancement of oxidative hepatic toxicity, with ROS generation identified as a major mechanistic link. The increased production of O2•-interacts with NO, resulting in the formation of peroxynitrite (ONOO−), a powerful free radical, as documented by Erukainure et al. [50]. The increased levels of NO observed in FeSO4-exposed liver tissues show a pro-inflammatory effect. Hence, these results support the anti-inflammatory effect of flavonoid-rich fractions of S. anguivi in oxidative hepatic injury. Our findings are in agreement with earlier study published by Refs. [51,52].
5. Conclusion
The results emphasize the importance of investigating natural sources, such as flavonoid-rich fractions, for their potential therapeutic advantages in diabetes treatment. The study investigated different fractions of the flavonoid-rich fraction (A, B, and C) and also compared them with one another and standard drug. They all show promising effects, but the most potent one according to this study, is fraction A when it was compared to both B and C. The fractions were able to provide anti-inflammatory, anti-diabetic and anti-oxidant potentials to the studied organ. In addition, S. anguivi flavonoid-rich fractions could be used to treat oxidative hepatic injury in a variety of ways, all of which point to the therapeutic and protective potential of S. anguivi flavonoid-rich fractions for oxidative hepatic injury. More research into the therapeutic properties of S. anguivi and their flavonoid capacity should be studied further especially in the management of and treatments of diabetes.
Funding
This study was self-funded.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Adebola Busola Ojo: Writing – review & editing, Writing – original draft, Validation, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Issac Gbadura Adanlawo: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Baquar S.R. ist ed. Ferozsons (Pvt) Limited; Lahore, Pakistan: 2001. Textbook of Economic Botany; pp. 121–156. [Google Scholar]
- 2.Zahnit W., Smara O., Bechki L., Bensouici C., Messaoudi M., Benchikha N., Larkem I., Awuchi C.G., Sawicka B., Simal-Gandara J. Phytochemical profiling, mineral elements, and biological activities of Artemisia campestris L. Grown in Algeria. Horticulturae. 2022;8(10):914. doi: 10.3390/horticulturae8100914. [DOI] [Google Scholar]
- 3.Messaoudi M., Rebiai A., Sawicka B., Atanassova M., Ouakouak H., Larkem I., Egbuna C., Awuchi C.G., Boubekeur S., Ferhat M.A., et al. Effect of extraction methods on polyphenols, flavonoids, mineral elements, and biological activities of essential oil and extracts of Mentha pulegium L. Molecules. 2022;27(1):11. doi: 10.3390/molecules27010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benchikha N., Chelalba I., Debbeche H., Messaoudi M., Begaa S., Larkem I., Amara D.G., Rebiai A., Simal-Gandara J., Sawicka B., et al. Lobularia libyca: phytochemical profiling, antioxidant and antimicrobial activity using in vitro and in silico studies. Molecules. 2022;27(12):3744. doi: 10.3390/molecules27123744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adanlawo I.G., Akanji M.A. Effect of chronic administration of saponin extract from the fruits of Solanum anguivi Lam. on alkaline phosphatase activities of some rat tissues. Nigerian Journal of Biochemistry and Molecular Biology. 2003;18:59–62. [Google Scholar]
- 6.Elekofehinti O.O., Adanlawo I.G., Fakoya A., Saliu J.A., Sodehinde S.A. Effect of saponins from Solanum anguivi Lam. fruit on heart and kidney superoxide dismutase, catalase and malondialdehyde in rat. Curr. Res. J. Biol. Sci. 2012;4:530–533. [Google Scholar]
- 7.Abd-Alla H.I., Albalawy M.A., Aly H.F., Shalaby N.M., Shaker A.H. Flavone composition and anti-hypercholesterolemic and anti-hyperglycemic activities of Chrysanthemum coronarium leaves. Z. Naturforsch. 2014;69:199–208. doi: 10.5560/znc.2013-0115. [DOI] [PubMed] [Google Scholar]
- 8.Adefegha S.A., Oboh G. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside-induced lipid peroxidation in rat pancreas by water extractable phytochemicals from some tropical spices. Pharmaceut. Biol. 2012;50(7):857–865. doi: 10.3109/13880209.2011.641022. [DOI] [PubMed] [Google Scholar]
- 9.Jadalla B.M.I.S., Moser J.J., Sharma R., Etsassala N.G.E.R., Egieyeh S.A., Badmus J.A., Marnewick J.L., Beukes D., et al. In Vitro Alpha-Glucosidase and AlphaAmylase Inhibitory Activities and Antioxidant Capacity of Helichrysum Cymosum and Helichrysum Pandurifolium Schrank Constituents. 2022. [Google Scholar]
- 10.Peyrot des Gachons C., Breslin P.A.S. Salivary amylase: digestion and metabolic syndrome. Curr. Diabetes Rep. 2016;16(10) doi: 10.1007/s11892-016-0794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alqahtani A.S., Hidayathulla S., Rehman T., Elgamal A.A., Dib R.A. El, Alajmi M.F. Biomolecules-10-00061.Pdf. Biomolecules. 2020;10(61):2–19. Available: https://pubmed.ncbi.nlm.nih.gov/31905962/ [Google Scholar]
- 12.Salahuddin M.A.H., Ismail A., Kassim N.K., Hamid M., Ali M.S.M. Phenolic profiling and evaluation of in vitro antioxidant, α-glucosidase and α-amylase inhibitory activities of Lepisanthes fruticosa (Roxb) Leenh fruit extracts. Food Chem. 2020;331(1473) doi: 10.1016/j.foodchem.2020.127240. [DOI] [PubMed] [Google Scholar]
- 13.Xu J., Zou M.H. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266–1286. doi: 10.1161/CIRCULATIONAHA.108.835223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott J.A., King G.L. Oxidative stress and antioxidant treatment in diabetes. Ann. N. Y. Acad. Sci. 2004;1031:204–213. doi: 10.1196/annals.1331.020. [DOI] [PubMed] [Google Scholar]
- 15.Anderson E.R., Shah Y.M. Iron homeostasis in the liver. Compr. Physiol. 2013;3(1):315–330. doi: 10.1002/cphy.c120016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elekofehinti O.O., Kamdem J.O., Kade I.J., Rocha J.B.T., Adanlawo I.G. Hypoglycemic, antiperoxidative and antihyperlipidemic effects of saponins from Solanum anguivi Lam. fruits in alloxan-induced diabetic rats. South Afr. J. Bot. 2013;88:56–61. [Google Scholar]
- 17.Adanlawo I.G., Akanji M.A. Effect of saponin extract from Solanum anguivi Lam. fruits on serum cholesterol concentration of albino rats. Rece. Prog. Med. Pla. 2008;9(19):1–7. [Google Scholar]
- 18.Kumar S., Mishra A., Pandey A.K. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Compl. Alternative Med. 2013;13:120–129. doi: 10.1186/1472-6882-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu Y.F., Sun J., Wu X., Liu R.H. Antioxidant and antiproliferative activity of common vegetables. J. Agric. Food Chem. 2002;50:6910–6916. doi: 10.1021/jf020665f. [DOI] [PubMed] [Google Scholar]
- 20.Sulastri E., Zubair M.S., Anas N.I., Abidin S., Hardani R., Yulianti R., Aliyah A. Total phenolic, total flavonoid, quercetin content and antioxidant activity of standardized extract of Moringa oleifera leaf from regions with different elevation. Phcog. J. 2018;10(6):104–108. [Google Scholar]
- 21.More G.K., Makola R.T. vols. 1–9. 2020. In vitro analysis of free radical scavenging activities and suppression of LPS-induced. (ROS Production in Macrophages by Solanum Sisymbriifolium Extracts). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagetia G.C., Baliga M.S. Evaluation of the radioprotective action of geriforte in mice exposed to different doses of gamma-radiation. Am. J. Chin. Med. 2004;32(4):551–567. doi: 10.1142/S0192415X04002193. [DOI] [PubMed] [Google Scholar]
- 23.Pulido R., Bravo L., Saura-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000;48:396–402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- 24.Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 25.Shai L., Masoko P., Mokgotho M. Yeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South. South Afr. J. Bot. 2010;76:465–470. [Google Scholar]
- 26.Kazeem M.I., Akanji M.A., Yakubu M.T., Ashafa A.O.T. Protective effect of free and bound polyphenol extracts from ginger (Zingiber officinale Roscoe) on the hepatic antioxidant and some carbohydrate metabolizing enzymes of streptozotocin-induced diabetic rats. Evid. base Compl. Alternative Med. 2013:7. doi: 10.1155/2013/935486. Article ID 935486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erukainure O.L., Chukwuma C.I., Matsabisa M.G., Salau V.F., Koorbanally N.A., Islam M.S. Buddleja saligna Willd (Loganiaceae) inhibits angiotensinconverting enzyme activity in oxidative cardiopathy with concomitant modulation of nucleotide hydrolyzing enzymatic activities and dysregμlated lipid metabolic pathways. J. Ethnopharmacol. 2020;248 doi: 10.1016/j.jep.2019.112358. [DOI] [PubMed] [Google Scholar]
- 28.Oboh G., Olasehinde T.A., Ademosun A.O. Inhibition of enzymes linked to type-2 diabetes and hypertension by essential oils from peels of orange and lemon. Int. J. Food Prop. 2017:S586–S594. [Google Scholar]
- 29.Winterbourn C.C. Superoxide as an intracellular radical sink. Free Radic. Biol. Med. 1993;14(1):85–90. doi: 10.1016/0891-5849(93)90512-s. [DOI] [PubMed] [Google Scholar]
- 30.Zelko I.N., Manriani Y.J., Folz R.J. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 31.Erukainure O.L., Reddy R., Islam M.S. Raffia Palm (Raphia hookeri) wine extenuates redox imbalance and modulates activities of glycolytic and cholinergic enzymes in hyperglycemia induced testicular injury in type 2 diabetes Rats. J. Food Biochem. 2019;43 doi: 10.1111/jfbc.12764. [DOI] [PubMed] [Google Scholar]
- 32.Jaimez-Ordaz J., Contreras-López E., Hernández-Sánchez T., González-Olivares L.G., Añorve-Morga J., Ramírez-Godínez J. Comparative evaluation of four extraction methods of antioxidant compounds from decatropis bicolor in aqueous medium applying response surface design. Molecules. 2021;26(4):1042. doi: 10.3390/molecules26041042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daramola B. Effects of extraction solvent, morphological parts and ripening stage on antioxidative activity of Solanum anguivi fruit. Int. Food Res. J. 2015;22(2):644–650. [Google Scholar]
- 34.Ghildiyal S., Gautam M.K., Joshi V.K., Goel R.K. Anti-inflammatory activity of two classical formulations of Laghupanchamula in rats. J. Ayurveda Integr. Med. 2013 Jan;4(1):23–27. doi: 10.4103/0975-9476.109546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira X., Souza F., da S., Almeida J.R.G., de Lima J.T., Arajo Ribeiro L.A. de, Quintans Jnior L.J., et al. Biological oxidations and antioxidant activity of natural products [Internet]. Phytochemicals as nutraceuticals - global approaches to their role in nutrition and health. InTech; 2012. Available from: [DOI] [Google Scholar]
- 36.Umamaheswari M., Chatterjee T.K. In vitro antioxidant activities of the fractions of Coccinnia grandis L. leaf extract. Afr. J. Tradit., Complementary Altern. Med. 2008;5:61–73. [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnaiah D., Sarbatly R., Nithyanandam R.R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;2011(89):217–233. [Google Scholar]
- 38.Dobrecky C., Marchini T., Ricco R., Garcés M., Gadano A., Carballo M.…Evelson P. Antioxidant activity of flavonoid rich fraction of Ligaria cuneifolia (Loranthaceae) Chem. Biodivers. 2020;17(10) doi: 10.1002/cbdv.202000302. [DOI] [PubMed] [Google Scholar]
- 39.Usmani S., Hussain A., Wahab S., Kushwaha P., Khatoon S., Arif M.…Kamal M. Antioxidant potential of crude extract, flavonoid-rich fractions, and a new compound from the seeds of Cordia dichotoma. Indian Journal of Natural Products and Resources (IJNPR)[Formerly Natural Product Radiance (NPR)] 2021;12(3):437–444. [Google Scholar]
- 40.Bencheikh N., Bouhrim M., Merrouni I.A., Boutahiri S., Kharchoufa L., Addi M.…Elachouri M. Antihyperlipidemic and antioxidant activities of flavonoid-rich extract of Ziziphus lotus (L.) lam. fruits. Appl. Sci. 2021;11(17):7788. [Google Scholar]
- 41.Meziant L., Bachir-bey M., Bensouici C., Saci F., Boutiche M., Louaileche H. Assessment of inhibitory properties of flavonoid-rich fig (Ficus carica L.) peel extracts against tyrosinase, α-glucosidase, urease and cholinesterases enzymes, and relationship with antioxidant activity. European Journal of Integrative Medicine. 2021;43 [Google Scholar]
- 42.Saeed N., Khan M.R., Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Compl. Alternative Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan R.A., Khan M.R., Sahreen S. Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens. Chem. Cent. J. 2012;6:43. doi: 10.1186/1752-153X-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ajiboye B.O., Ojo O.A., Akuboh O.S., Abiola O.M., Idowu O., Amuzat A.O. Anti-hyperglycemic and anti-inflammatory activities of polyphenolic-rich extract of Syzygium cumini linn leaves in alloxan-induced diabetic rats. Journal of evidence-based integrative medicine. 2018;23 doi: 10.1177/2515690X18770630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ajiboye B.O., Oyinloye B.E., Ojo O.A., Lawal O.E., Jokomba Y.A., Balogun B.A., Adeoye A.O., Ajuwon O.R. Effect of flavonoid-rich extract from Dalbergiella welwitschii leaf on redox, cholinergic, monoaminergic, and purinergic dysfunction in oxidative testicular injury: ex vivo and in silico studies. Bioinf. Biol. Insights. 2022;16:1–17. doi: 10.1177/11779322221115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojo O.A., Amanze J., Oni A.P., Grant S., Iyobhebhe M., Elebiyo T.C., Rotimi D., Asogwa N.T., Oyinloye B.E., Ajiboye B.E., Ojo A.B. Antidiabetic activity of avocado seeds (Persea americana Mill.) in diabetic rats via activation of PI3K/AKT signaling pathway. Sci. Rep. 2022;12:2919. doi: 10.1038/s41598-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasanuzzaman M., Bhuyan M.H.M., Anee T.I., Parvin K., Nahar K., Mahmud J.A., Fujita M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants. 2019;8(9):384. doi: 10.3390/antiox8090384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aseervatham G.S.B., Ananth D.A., Sivasudha T. The Liver. Academic Press; 2018. The liver: oxidative stress and dietary antioxidants; pp. 239–246. [Google Scholar]
- 49.Grohmann M., Wiede F., Dodd G.T., Gurzov E.N., Ooi G.J., Butt T., Rasmiena A.A., Kaur S., GμLati T., Goh P.K., Treloar A.E. Obesity drives STAT-1-dependent NASH and STAT-3-dependent HCC. Cell. 2018;175(5):1289–1306. doi: 10.1016/j.cell.2018.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erukainure O.I., Hafizur R.M., Kabir N., Choudhary M.I., Atolani O., Banerjee P., Preissner R., Chukwuma C.I., Muhammad A., Amonsou E.O., Islam M.S. Suppressive effects of Clerodendrum volubile P Beauv. [Labiatae] methanolic extract and its fractions on type 2 diabetes and its complications. Front. Pharmacol. 2018;9(2018):8. doi: 10.3389/fphar.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ojo O.A., Agboola O., Ogunro O.B., Rotimi D.E., Iyobhebhe M., Elebiyo T.C., Ayeni J.F., Ojo A.B., Odugbemi A.I., Egieyeh S.A., Oluba O.M. Beet leaf (Beta vulgaris L.) extract attenuates iron-induced testicular toxicity via modulation of redox imbalance, cholinergic, and purinergic dysfunctions, and glucose metabolizing enzymes activities. Heliyon. 2023;9(7) doi: 10.1016/j.heliyon.2023.e17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solcan M.B., Fizeșan I., Vlase L., Vlase A.M., Rusu M.E., Mateș L.…Popa D.S. Phytochemical profile and biological activities of extracts obtained from young shoots of blackcurrant (Ribes nigrum L.), European blueberry (Vaccinium myrtillus L.), and mountain cranberry (Vaccinium vitis-idaea L.) Horticulturae. 2023;9(11):1163. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.