Abstract
Objective
To evaluate whether access to buprenorphine to treat opioid use disorder (OUD) was associated with the coronavirus disease pandemic, the relaxation of training requirements to obtain an X-Waiver to prescribe buprenorphine (April 2021), and the removal of the X-Waiver (December 2022).
Patients and Methods
The OptumLabs Data Warehouse, which includes claims from Commercial and Medicare Advantage enrollees, was used to evaluate trends in prescription fills from January 1, 2019, to June 30, 2023. We compared fill patterns of buprenorphine for OUD with acamprosate to treat alcohol use disorder and naltrexone to treat alcohol use disorder or OUD. We evaluated trends in the rate ratio (RR) of overall fills; RR by days supply; distribution of fills by daily dose; and distribution of fills by prescriber type.
Results
Coronavirus disease (RR, 1.06; 95% CI, 1.01-1.11) was associated with a slightly increased rate of fills for Commercial enrollees but not overall or for Medicare Advantage enrollees. There were also no significant increases (P>0.05) associated with the change in training requirements or removal of the X-Waiver. Over the study period, there was an increasing share of fills for 16+ mg for Commercial enrollees, and buprenorphine prescribers were more likely to be advanced practice nurses or physician assistants.
Conclusion
We did not find meaningful improvement in access in response to coronavirus disease or the changes in the X-Waiver. These findings suggest that interventions beyond removing the X-Waiver may be needed to improve buprenorphine access.
In 2020 and 2021, the number of opioid overdose deaths increased by 30% and 15% compared with the previous years, respectively.1 These trends highlight the need for effective treatment options to improve outcomes. Buprenorphine is a first-line medication for the management of opioid use disorder (OUD). Use of buprenorphine to treat OUD is associated with reductions in use of illicit opioids,2 opioid overdoses,3 and opioid-related deaths.4 Having access to treatment options like buprenorphine is increasingly important as the opioid crisis continues.
Despite buprenorphine’s established effectiveness5, 6, 7, 8 and the increasing need for treatment for people living with OUD, there remain critical barriers for access to buprenorphine. For example, buprenorphine is a controlled substance that has restrictions for prescribing.9,10 Historically, clinicians intending to prescribe buprenorphine for outpatient treatment of OUD have had to obtain a Drug Addiction Treatment Act of 2000 waiver, also known as the X-Waiver. To obtain a waiver, physicians had to complete an 8-hour training and advanced practice nurses or physician assistants (APN/PA) had to complete a 24-hour training. There were also limits on the number of patients treated at a time, reporting requirements, and the possibility of unannounced Drug Enforcement Agency (DEA) inpsections.11 In response to the increase in opioids overdose deaths,1 starting on April 27, 2021,12 the US Department of Health Human Services released new regulations to exempt from the training requirement prescribers treating up to 30 patients with buprenorphine at 1 time. Subsequently, the Consolidated Appropriations Act eliminated the X-Waiver entirely on December 29, 2022.13 It is important to understand how buprenorphine prescribing practices were affected by these changes to restrictions on prescribing. In addition, there may have been changes in access to buprenorphine during the pandemic including broader disruptions in access to care and new regulations allowing buprenorphine prescribing through telemedicine without an initial in-person visit.14, 15, 16, 17 Evaluating trends in buprenorphine fills would characterize the current state of access to buprenorphine and the potential impact of these changes.
In this analysis, we used administrative claims data to evaluate trends in fills of buprenorphine for OUD, along with 2 comparison medications that are also used to treat substance use disorders: acamprosate to treat alcohol use disorder (AUD) and naltrexone to treat AUD or OUD.
Patients and Methods
Data Sources
The data source was the OptumLabs Data Warehouse, which includes deidentified administrative claims for Commercial and Medicare Advantage (MA) (aged and disabled) enrollees.18 We included fills for acamprosate, buprenorphine, or naltrexone between January 2019 and June 2023. The analysis was deemed exempt from institutional board review. Reporting follows the Strengthening the Reporting of Observational Studies in Epidemiology checklist.19
Participants and Study Design
The population consisted of enrollees with at least 6 months of previous insurance enrollment, 1 or more prescription fill for a study medication, and a diagnosis of AUD or OUD. Included individuals had 1 or more fills for acamprosate, buprenorphine, or oral naltrexone. For buprenorphine, we used National Drug Codes to identify products that are specifically for the treatment of OUD rather than pain. Acamprosate is used to treat AUD,20 and naltrexone can be used to treat AUD or OUD.21 We included acamprosate and naltrexone as comparison medications for substance use disorders that would not be directly affected by the flexibility in telemedicine policies, the change in X-Waiver training requirements, or the elimination of the X-Waiver. In comparison, acamprosate and naltrexone face fewer restrictions on prescribing than buprenorphine and did not experience direct changes to restrictions on prescribing during the period.20,21 These 2 drugs would, however, be affected by coronavirus-related changes in substance use and disruptions in care delivery, making them important comparison groups to identify potential changes in buprenorphine fills owing to policy changes during coronavirus disease (COVID-19). Enrollees receiving acamprosate had to be diagnosed with AUD, enrollees receiving buprenorphine had to be diagnosed with OUD, and enrollees receiving naltrexone had to be diagnosed with either AUD or OUD but not both to facilitate the comparisons with the other medications that are only prescribed for AUD (acamprosate) or OUD (buprenorphine). Enrollees were determined to have AUD or OUD if they had 1 or more inpatient diagnosis or 2 or more outpatient diagnoses at least 1 day apart in the previous 6 months. We selected a lookback period of 6 months because patients who are receiving acamprosate, buprenorphine, or naltrexone should be continuously monitored. In addition, we wanted to minimize the risk of identifying individuals who are in remission because individuals in the denominator did not have to receive active treatment for AUD or OUD. We used the Elixhauser ICD-10-CM codes22,23 to identify AUD and the subset of Elixhauser ICD-10-CM codes specific to OUD22,23 to identify OUD (F11.x). Enrollees had to have at least 6 months of continuous coverage before the fill to measure medical comorbidities. We included all fills for enrollees meeting the inclusion criteria.
Variables
The main outcome was the trend in fills for each month. We first calculated the moving 3-month rate of fills by dividing the number of fills in the 3-month period for each medication by the number of enrollees in the 3-month period with AUD or OUD. The denominator included all enrollees with diagnoses of AUD for OUD regardless of whether they had fills for one of the study medications. We then calculated the rate ratio (RR) relative to the beginning of the study period by dividing each 3-month rate by the January-March 2019 3-month rate. The RRs were calculated separately by medication and insurance (Commercial or MA). Buprenorphine fills were stratified by the distribution of fills for days supply (1-7, 8-15, 16-27, and 28+ days) and daily dose (0-7.9, 8-11.9, 12-15.9, and 16+ mg). Longer initial days supply24 and doses above 24 mg25 have been associated with better outcomes. We separately calculated days supply and daily dose by Commercial and MA. We also evaluated the percentage of new fills by prescriber specialty. We limited the prescriber specialty analysis to the most common prescriber types: family practice, pain medicine, psychiatry, and APN/PA.
Statistical Analyses
We used descriptive statistics to analyze demographic characteristics and comorbidities including AUD, OUD, and serious mental illness.26 We used generalized estimating equations with a log link and a Poisson distribution to evaluate the statistical significance of shifts in the trends (Supplemental Material, available online at http://www.mcpiqojournal.org). We used this approach because we modeled the count of fills and needed to include an offset term for the log of total enrollment of individuals with AUD or OUD in each month. The key assumption of the Poisson distribution is equidispersion.27 The overdispersion parameter was smaller than 0.01 in all models, and we concluded that the assumption was met. In the regression models, we adjusted for calendar month and a continuous time trend to evaluate whether there was a shift beyond monthly variation and existing trends. We included indicators for each of the 3 study time points and analyzed the corresponding RR for changes to the level of fills. We then descriptively analyzed the trends in RRs, the distribution of fills by days supply and daily dose, and the percentage of fills by prescriber type. We focused on 3 time points: (1) the start of the pandemic: operationalized as a binary variable for months of March 2020 or after; (2) the change to training requirements: the change occurred on April 27, 2021, and we operationalized it as a binary variable for months of May 2021 or after; (3) the elimination of the X-Waiver: the change occurred on December 29, 2022 and we operationalized it as a binary variable for months of January 2023 or after. All analyses were performed using Stata 16 (StataCorp, 2019).
Results
Sample Characteristics
We identified 80,638 patients and 887,716 fills (Supplemental Figure 1: sample flow diagram, available online at http://www.mcpiqojournal.org). There were 21,002 acamprosate fills, 777,115 buprenorphine fills, 85,679 naltrexone AUD fills, and 3920 naltrexone OUD fills.
A large percentage (Supplemental Tables 1 and 2, available online at http://www.mcpiqojournal.org) of patients had serious mental illness (all fills: 76.3% (10,549) Commercial acamprosate, 36.4% (141,215) buprenorphine, 69.7% (2126) naltrexone for OUD, and 74.4% (46,915) naltrexone for AUD). Patients receiving AUD medications tended to be older than patients receiving OUD medications both for Commercial (age 40-64 years for all fills: acamprosate, 65.6% [9062]; naltrexone for AUD, 60.9% [38,417]; buprenorphine, 51.4% [199,789]; naltrexone for OUD 27.0% [825]) and MA (age 65+ years for all fills: acamprosate, 52.0% [3713]; naltrexone for AUD, 55.4% [12,471]; buprenorphine, 30.2% [116,048]; and naltrexone for OUD, 24.6% [208]) cohorts.
Overall Trends
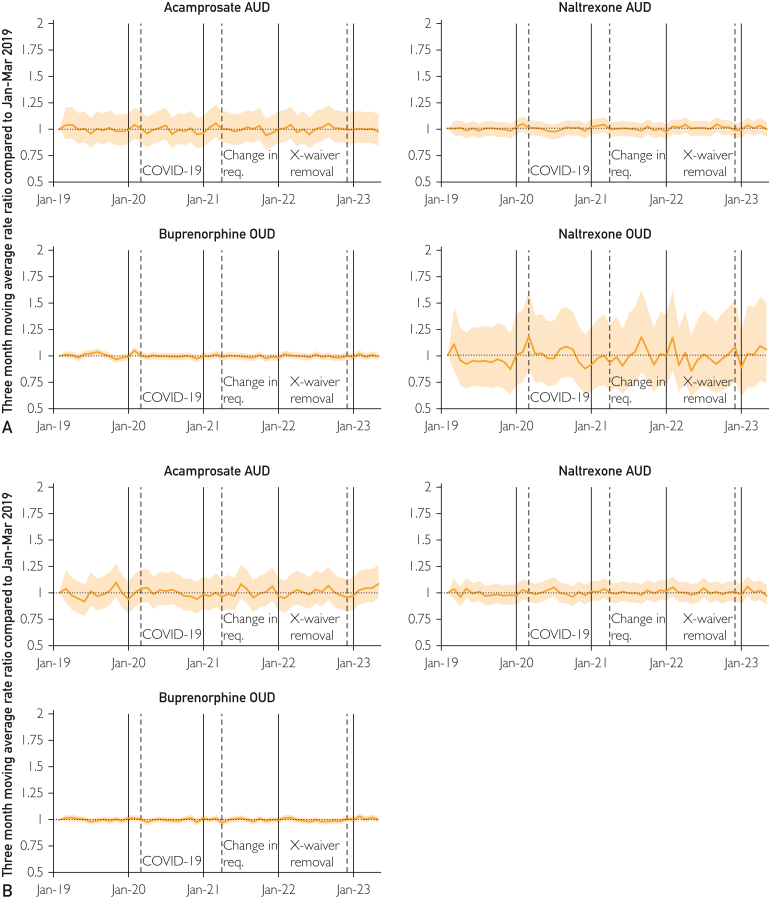
The RRs and regression results did not show meaningful differences in buprenorphine fills with respect to COVID-19, the change in training requirements, or the elimination of the X-Waiver (Table). The regression results for all buprenorphine fills were not statistically significant at any time point. However, the change with respect to COVID-19 was statistically significant for Commercial enrollees (RR, 1.06; 95% CI, 1.01-1.11) although not significant for MA enrollees. The 3-month moving rate reported patterns similar to the regression results with no clear shifts in the trend for buprenorphine fills (Figure 1; Supplemental Table 3, available online at http://www.mcpiqojournal.org).
Table.
Regression Results for Changes in the Rate of Fillsa
| Overall fills |
Days supply (d) |
Daily dose (mg) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acamp |
BUP |
NTX OUD |
NTX AUD |
1-7 |
8-15 |
16-27 |
28+ |
0-7.9 |
8-11.9 |
12-15.9 |
16+ |
|
| Rate ratio | ||||||||||||
| All | ||||||||||||
| COVID-19 | 1.03 (0.98-1.08) | 1.03 (0.99-1.06) | 1.13 (0.99-1.30) | 0.98 (0.95-1.01) | 0.91b (0.83-1.00) | 1.10c (1.05-1.15) | 0.94 (0.88-1.01) | 1.06c (1.03-1.08) | 0.99 (0.95-1.02) | 0.94c (0.92-0.97) | 1.11d (1.04-1.18) | 1.08c (1.03-1.12) |
| Change in training | 1.05b (1.00-1.10) | 0.97 (0.95-1.00) | 1.15 (0.98-1.35) | 1.06c (1.03-1.09) | 0.86c (0.79-0.93) | 1.00 (0.96-1.04) | 1.05 (0.98-1.11) | 1.00 (0.98-1.02) | 1.01 (0.98-1.05) | 1.06c (1.03-1.10) | 0.94b (0.89-0.99) | 0.94c (0.91-0.97) |
| Elimination of X-Waiver | 1.11c (1.06-1.16) | 1.03 (1.00-1.06) | 1.14 (0.95-1.37) | 1.05d (1.02-1.08) | 0.99 (0.90-1.08) | 1.02 (0.98-1.07) | 1.04 (0.98-1.11) | 1.05c (1.02-1.07) | 1.06d (1.02-1.10) | 1.06c (1.03-1.10) | 1.04 (0.98-1.10) | 1.00 (0.97-1.04) |
| Com. | ||||||||||||
| COVID-19 | 0.98 (0.93-1.03) | 1.06b (1.01-1.11) | 1.03 (0.83-1.26) | 0.99 (0.96-1.04) | 0.94 (0.82-1.07) | 1.15d (1.05-1.26) | 0.97 (0.89-1.05) | 1.07c (1.04-1.10) | 1.01 (0.96-1.06) | 0.98 (0.95-1.01) | 1.15d (1.06-1.26) | 1.12d (1.03-1.23) |
| Change in training | 1.01 (0.96-1.06) | 0.98 (0.93-1.03) | 1.05 (0.80-1.38) | 1.06d (1.02-1.10) | 0.84b (0.72-0.98) | 0.98 (0.88-1.08) | 1.02 (0.93-1.11) | 1.01 (0.98-1.04) | 1.03 (0.98-1.09) | 1.06d (1.02-1.11) | 0.93 (0.85-1.02) | 0.93 (0.85-1.01) |
| Elimination of X-Waiver | 1.07b (1.01-1.13) | 1.03 (0.97-1.08) | 1.05 (0.74-1.49) | 1.04b (1.00-1.08) | 0.99 (0.83-1.18) | 1.02 (0.90-1.15) | 1.07 (0.97-1.18) | 1.03 (1.00-1.06) | 1.06 (1.00-1.13) | 1.04 (0.99-1.09) | 1.02 (0.92-1.12) | 1.00 (0.92-1.09) |
| MA | ||||||||||||
| COVID-19 | 1.12 (0.99-1.28) | 0.98 (0.96-1.01) | 1.21 (0.95-1.55) | 0.92d (0.87-0.98) | 0.91 (0.81-1.01) | 1.01 (0.97-1.06) | 0.92b (0.86-0.98) | 1.03d (1.01-1.06) | 1.00 (0.95-1.05) | 0.89c (0.85-0.94) | 0.99 (0.94-1.04) | 1.00 (0.97-1.03) |
| Change in training | 1.10 (0.99-1.21) | 0.96c (0.94-0.98) | 1.49c (1.19-1.86) | 1.04 (1.00-1.08) | 0.86c (0.79-0.94) | 0.98 (0.94-1.01) | 1.05 (1.00-1.11) | 1.00 (0.98-1.02) | 0.93c (0.90-0.96) | 1.00 (0.96-1.04) | 0.93c (0.90-0.97) | 0.97d (0.95-0.99) |
| Elimination of X-Waiver | 1.19c (1.08-1.30) | 1.02 (1.00-1.04) | 1.37d (1.10-1.69) | 1.02 (0.99-1.06) | 0.99 (0.91-1.07) | 1.02 (0.98-1.05) | 1.01 (0.96-1.06) | 1.06c (1.04-1.08) | 1.00 (0.97-1.03) | 1.02 (0.98-1.06) | 1.06d (1.02-1.10) | 1.02 (1.00-1.04) |
Acamp, acamprosate; AUD, alcohol use disorder; BUP, buprenorphine; OUD, opioid use disorder; MA, Medicare Advantage; NTX, naltrexone.
P<.05.
P<.001.
P<.01.
Figure 1.
Rate ratio of fills, February 2019-May 2023 for commercial (A) and medicare advantage (B). The moving average rate ratio is calculated with respect to January-March 2019; shaded areas represent 95% CIs. AUD, alcohol use disorder; COVID-19, coronavirus disease; OUD, opioid use disorder.
Although the CIs were wider owing to lower counts, the patterns for the comparison medications did not show meaningful changes in access (Table). For all fills of naltrexone for OUD, none of the differences were statistically significant. For the AUD medications, there were increases for acamprosate and naltrexone for AUD with respect to the change in training requirement and the elimination of the X-Waiver. Figure 1 also presented a lack of meaningful shifts.
Days Supply
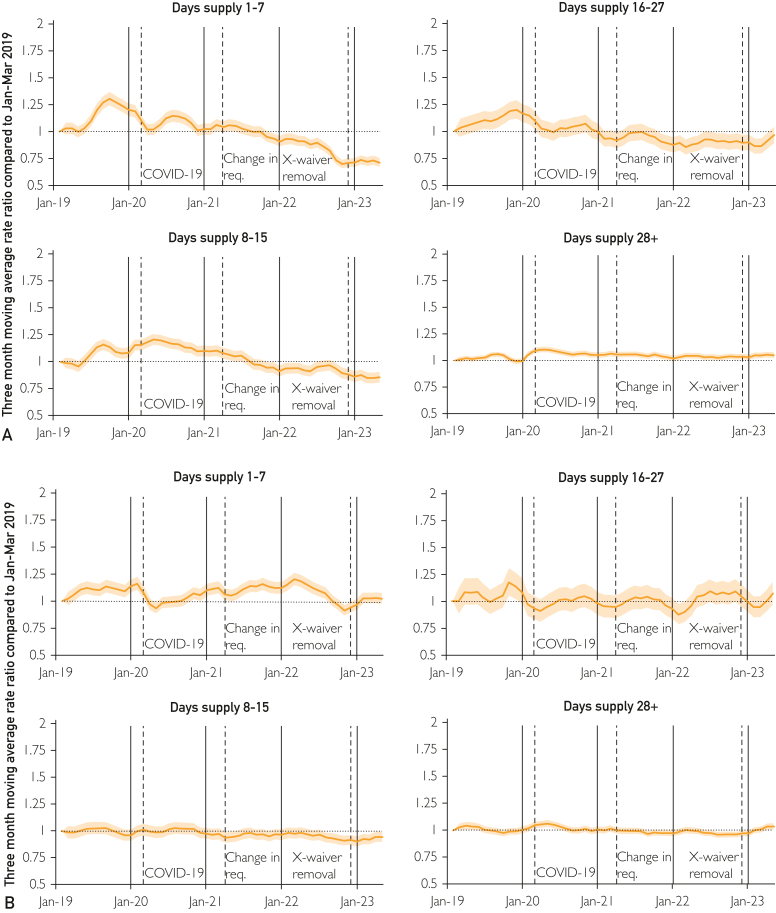
The rate of days supply showed modest shifts with respect to the time points of interest (Table). The regression results were statistically significant and reported a decrease for 1-7 days (RR for all enrollees, 0.91; 95% CI, 0.83-1.00) and increases for 8-15 days (1.10; 95% CI, 1.05-1.15) and 28+ days (1.06; 95% CI, 1.03-1.08) with respect to the COVID-19 pandemic. The elimination of the X-Waiver was also associated with an increase in fills for 28+ days (1.05; 95% CI, 1.02-1.07). Although the RR for the elimination of the X-Waiver with respect to 28+ days was statistically significant for MA, it was not for Commercial. The trends (Figure 2; Supplemental Table 4, available online at http://www.mcpiqojournal.org) found gradual decreases for 1-7 and 8-15 days and a gradual increase for 28+ days for Commercial.
Figure 2.
Distribution of fills for buprenorphine by days supply, February 2019-May 2023 for commercial (A) and medicare advantage (B). The moving average rate ratio is calculated with respect to January-March 2019; shaded areas represent 95% CIs. COVID-19, coronavirus disease.
Daily Dose
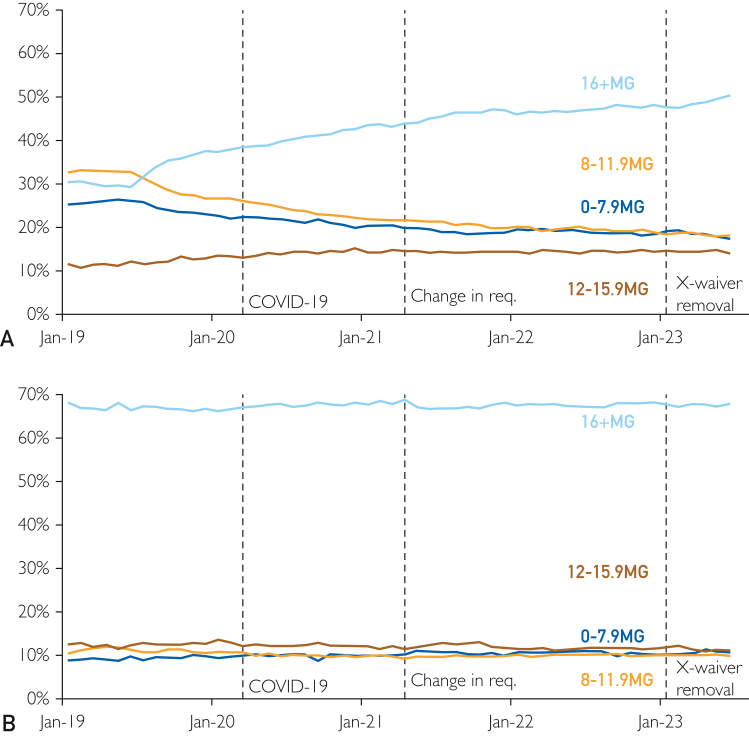
The rate of fills by daily dose reported some increases for higher doses with the onset of COVID-19 and decreases for higher doses around the change in the training requirements (Table). Specifically, there were increases for 12-15.9 and 16+ mg with respect COVID-19. In contrast, there were decreases for 12-15.9 and 16 mg associated with the changes in the training requirement. There were also increases for 0-7.9 and 8-11.9 mg associated with the elimination of the X-Waiver. Compared with MA enrollees, Commercial enrollees experienced greater increases for 12-15.9 and 16+ mg associated with COVID-19. There were also general trends for Commercial enrollees (Figure 3; Supplemental Table 5, available online at http://www.mcpiqojournal.org) with gradual increases for 12-15.9 and 16+ mg as well as gradual declines for 0-7.9 and 8-11.9 mg. In contrast, the percentages for MA enrollees were relatively stable over time.
Figure 3.
Distribution of fills by dose, January 2019-June 2023 for commercial (A) and medicare advantage (B). COVID-19, coronavirus disease.
Prescriber Specialty
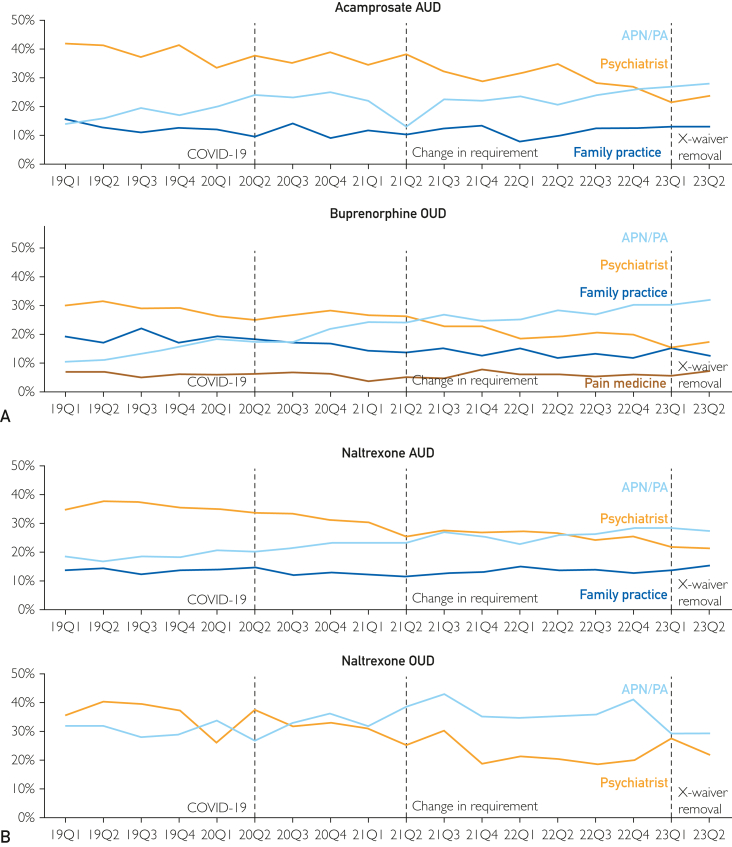
Although the percentage of fills by prescriber did not appear to change in response to the study events, there were gradual trends over the period (Figure 4; Supplemental Table 6, available online at http://www.mcpiqojournal.org). For acamprosate, buprenorphine, and naltrexone for AUD, there were substantial increases in the percentage of fills that were prescribed by APN/PA from 2019Q1 to 2023Q2 (14.2%-27.9% acamprosate; 10.7%-31.8% buprenorphine; and 19.5%-28.9% for naltrexone for AUD). In contrast, there were declines for psychiatry from 2019Q1 to 2023Q2 (42.0%-3.9% for acamprosate; 30.0%-17.2% for buprenorphine; and 36.7%-22.5% for naltrexone for AUD). For naltrexone for OUD, the percentages fluctuated and ended down for both APN/PA (31.8%-29.1%) and psychiatry (35.6%-21.8%).
Figure 4.
Percentage of fills by prescriber, 2019Q1 to 2023Q2. APN/PA, advanced practice nurse or physician assistant; AUD, alcohol use disorder, COVID-19, coronavirus disease; OUD, drug use disorder.
Discussion
In this national study of prescription fills trends, overall, there were no meaningful shifts in the trend for fills of buprenorphine to treat OUD compared with 2 other medications to treat substance use disorders around the start of the COVID-19 pandemic, the change in training requirements for obtaining X-Waivers, or the elimination of the X-Waiver. There were gradual trends for an increase in the rate of fills for 28+ days for Commercial enrollees. There was also an increase in the percentage fills with a daily dose of either 12-15.9 or 16+ mg among Commercial enrollees. We also found an increase in the percentage of fills prescribed by APN/PA.
Consistent with previous studies, we found that there were not improvements in access to buprenorphine in response to the care delivery changes and the flexibilities for prescribing buprenorphine during the COVID-19 pandemic or the change in the training requirements. Further, we extended the findings to the initial months after the elimination of the X-Waiver. Three previous studies using IQVIA™ data, which covers approximately 90% of fills at retail pharmacies, found that fills for buprenorphine remained mostly flat during COVID-19.28, 29, 30 One of these studies found a decrease in fills for new patients29 whereas another found an increase in the number of patients with buprenorphine fills.30 Our results were more consistent with the latter study because we found that COVID-19 was associated with a small increase in the rate of fills for Commercial enrollees. Another study using the Symphony Health database, which includes about 85% of fills, found that there was an initial decrease in buprenorphine fills for racial or ethnic minority patients during the early months of COVID-19 but not for White patients.31 The finding of disparities in access is consistent with another analysis using the OptumLabs Data Warehouse data that found improved access overall to buprenorphine in the emergency department (ED) from 2014 to 2020, but that disparities remained by race and ethnicity.32 With respect to the change in the training requirement, a report from the Assistant Secretary of Planning and Evaluation using IQVIA data and the Substance Abuse and Mental Health Services Administration Buprenorphine Waiver Notification System found an increase in treatment capacity, but no meaningful increase in the number of patients receiving buprenorphine.33 Our findings of decreased fills from psychiatrists and increased fills from APN/PA were also consistent with findings from analyses using IQVIA data34,35 and DEA Registrant Files.36 Taken together, these findings suggest that access to buprenorphine has not meaningfully improved.
Our findings have important implications for policy and practice. First, the lack of a decline in the rate of buprenorphine fills suggests that the policies to counteract COVID-19–related disruptions may have succeeded in maintaining access. In particular, the flexibility to prescribe buprenorphine through telemedicine without an initial in-person visit may have helped maintain access during the early period of COVID-19 when in-person care dropped precipitously.14 Recently, the DEA issued proposed rules that would restrict the ability to prescribe buprenorphine through telemedicine without an initial in-person visit. After receiving a record 38,000 comments, the DEA and the Substance Abuse and Mental Health Services Administration temporarily extended the telemedicine policies through November 11, 2023,37 and later extended them again through December 31, 2024.38 Given that the telemedicine flexibility may have helped maintain access amid the large increase in fatal opioid overdoses,1 preserving the telemedicine flexibilities may be important for ensuring access does not worsen. Second, the lack of meaningful shifts in buprenorphine fills after the change in training requirements for the X-Waiver and the elimination of the X-Waiver suggest that these policy changes on their own may not be enough to meaningfully improve access. A recent analysis that surveyed prescribers who received X-Waivers but had not actually prescribed buprenorphine found multiple barriers to prescribing including limited access to psychosocial services, behavioral health practitioners, addiction specialists, and psychiatric services for patients with mental health comorbidities.39 Another analysis found that from 2016 to 2021 buprenorphine fills increased at a slower rate than the number of prescribers, which meant that the average number of fills per provider decreased.35 These findings may suggest that the main challenge is not prescriber capacity but rather converting this capacity into better access to buprenorphine.
Interventions may be needed to improve prescriber self-efficacy and take advantage of the existing capacity to prescribe. One approach has focused on the ED setting because buprenorphine initiation in the ED has been shown to improve engagement with addiction treatment and reduce self-reported opioid use.5 Despite increasing buprenorphine prescribing rates after ED encounters in recent years, buprenorphine was still only prescribed for 7.6% of OUD ED encounters and 1.6% of opioid overdose encounters not resulting in an admission.40 The EMergency department initiated BuprenorphinE for opioid use Disorder (EMBED) pragmatic trial tested a clinical decision support intervention to promote the initiation of buprenorphine in the ED.41,42 Although there was no significant difference in the percentage of patients receiving buprenorphine, there was an increase in the percentage of physicians that initiated buprenorphine at least once.41 A separate intervention developed a triage screening and treatment protocol in the ED setting for patients with OUD.43 An observational analysis found that the intervention was associated with an increase in withdrawal assessment and buprenorphine prescribing.44 Another approach has focused on expanding access in rural areas, which have worse access to treatment for OUD.45 An example of an intervention to improve access is the University of North Carolina Extension for Community Healthcare Outcomes project. Extension for Community Healthcare Outcomes (ECHO) involves a hub and spoke model in which specialists at University of North Carolina provide a teleconferencing and coaching intervention to support primary care providers in rural and underserved areas to provide treatment for OUD.46 Qualitative findings suggest that participating providers have found value in this program.47 In addition to interventions such as these, there may also need to for accompanying policy changes such as more funding for training in addiction medicine and financial incentives for health systems to promote treatment for OUD.48
This analysis has several limitations. First, the population consisted of Commercial and MA enrollees from a large, national insurer. A large share of patients with OUD have either Medicaid coverage or lack health insurance,49 and the findings may not generalize to these populations. It is still important to characterize access for Commercial enrollees given that 58% of nonelderly adults with substance use disorder are enrolled in Commercial coverage compared with 21% in Medicaid.50 In addition, approximately 1.7 million Medicare enrollees have a substance use disorder51 and approximately half of all Medicare enrollees are covered by MA.52 It would be valuable for future research to explore the trends for individuals with Medicaid coverage or who are uninsured. Second, we only observed whether a medication was filled and were unable to assess how medications were used. Third, we did not directly observe the reason the medication was prescribed for the fills. Although we required patients to have diagnoses for AUD or OUD, it is possible that the prescriptions were not for these conditions. It is also possible that some individuals with diagnoses for AUD or OUD do not actually meet clinical criteria for the condition.53 Fourth, the analysis of prescriber specialty did not account for the possibility of prescriptions by APN/PA needing to be co-signed by physicians or state-level differences in policies that limit the ability of APN/PA to prescribe buprenorphine.54
Conclusion
We found that buprenorphine prescribing patterns were largely stable from January 2019 to June 2023 in the context of the emergence of COVID-19 policies to enable prescribing through telemedicine and escalating overdose deaths. Further, a change in the regulatory requirements for the X-Waiver and the elimination of the X-Waiver were not associated with improved access. Additional interventions to improve provider self-efficacy may be needed to improve access and narrow the treatment gap for individuals with OUD.
Potential Competing Interests
Dr Nath reports grants or contracts from American Medical Association – Practice Transformation Initiative Grant # 16118. Dr D’Onofrio reports support for attending meetings and/or travel from NIDA and participation on the National Institute on Drug Abuse (NIDA) Advisory Board. Dr Melnick reports grants or contracts from American Medical Association, Agency for Healthcare Research and Quality, Center for Medicare and Medicaid Services to the institution. The other authors declare no competing interest.
Footnotes
Grant Support: This work was supported by cooperative agreement (UH3DA047003) from the National Institute on Drug Abuse of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding source did not have involvement in study design; collection, analysis and interpretation of data; writing the report; or decision to submit the article for publication.
Data Previously Presented: This work was presented at the AcademyHealth Annual Research Meeting on June 27, 2023.
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.CDC National Center for Health Statistics U.S. overdose deaths in 2021 increased half as much as in 2020—but are still up 15% https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
- 2.Ling W., Amass L., Shoptaw S., et al. A multi-center randomized trial of buprenorphine–naloxone versus clonidine for opioid, detoxification: findings from the National Institute on Drug Abuse Clinical Trials Network. Addiction. 2005;100(8):1090–1100. doi: 10.1111/j.1360-0443.2005.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan J.R., Schackman B.R., Weinstein Z.M., Walley A.Y., Linas B.P. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend. 2019;200:34–39. doi: 10.1016/j.drugalcdep.2019.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larochelle M.R., Bernson D., Land T., et al. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Intern Med. 2018;169(3):137–145. doi: 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Onofrio G., O’Connor P.G., Pantalon M.V., et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–1644. doi: 10.1001/jama.2015.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronquest N.A., Willson T.M., Montejano L.B., Nadipelli V.R., Wollschlaeger B.A. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil. 2018;9:59–78. doi: 10.2147/SAR.S150253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schackman B.R., Leff J.A., Polsky D., Moore B.A., Fiellin D.A. Cost-effectiveness of long-term outpatient buprenorphine-naloxone treatment for opioid dependence in primary care. J Gen Intern Med. 2012;27(6):669–676. doi: 10.1007/s11606-011-1962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas C.P., Fullerton C.A., Kim M., et al. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatr Serv. 2014;65(2):158–170. doi: 10.1176/appi.ps.201300256. [DOI] [PubMed] [Google Scholar]
- 9.Fiscella K., Wakeman S.E., Beletsky L. Buprenorphine deregulation and mainstreaming treatment for opioid use disorder: X the X waiver. JAMA Psychiatry. 2019;76(3):229–230. doi: 10.1001/jamapsychiatry.2018.3685. [DOI] [PubMed] [Google Scholar]
- 10.Haffajee R.L., Bohnert A.S.B., Lagisetty P.A. Policy pathways to address provider workforce barriers to buprenorphine treatment. Am J Prev Med. 2018;54(6 suppl 3):S230–S242. doi: 10.1016/j.amepre.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Substance Abuse and Mental Health Services Administration (SAMHSA) Become a buprenorphine waivered practitioner. United States Department of Health and Human Services. https://www.samhsa.gov/medication-assisted-treatment/become-buprenorphine-waivered-practitioner
- 12.United State Department of Health and Human Services HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. https://www.hhs.gov/about/news/2021/04/27/hhs-releases-new-buprenorphine-practice-guidelines-expanding-access-to-treatment-for-opioid-use-disorder.html Updated April 27, 2022.
- 13.Consolidated Appropriations Act. H.R. Congress (2021 to 2022) sess (2022). Vol 2617. US Congress. 2023. https://www.congress.gov/bill/117th-congress/house-bill/2617 117th.
- 14.Patel S.Y., Mehrotra A., Huskamp H.A., Uscher-Pines L., Ganguli I., Barnett M.L. Variation in telemedicine use and outpatient care during the COVID-19 pandemic in the United States. Health Aff (Millwood) 2021;40(2):349–358. doi: 10.1377/hlthaff.2020.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Medicare and Medicaid Services Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet
- 16.America’s health insurance plans Health insurance providers respond to coronavirus (COVID-19) https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/
- 17.Drug Enforcement Administration (DEA) How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf
- 18.Wallace P.J., Shah N.D., Dennen T., Bleicher P.A., Crown W.H. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33(7):1187–1194. doi: 10.1377/hlthaff.2014.0038. [DOI] [PubMed] [Google Scholar]
- 19.Von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 20.Wilde M.I., Wagstaff A.J. Acamprosate. A review of its pharmacology and clinical potential in the management of alcohol dependence after detoxification. Drugs. 1997;53(6):1038–1053. doi: 10.2165/00003495-199753060-00008. [DOI] [PubMed] [Google Scholar]
- 21.Minozzi S., Amato L., Vecchi S., et al. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;2011(4) doi: 10.1002/14651858.CD001333.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Quan H., Sundararajan V., Halfon P., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 24.Meinhofer A., Williams A.R., Johnson P., Schackman B.R., Bao Y. Prescribing decisions at buprenorphine treatment initiation: do they matter for treatment discontinuation and adverse opioid-related events? J Subst Abuse Treat. 2019;105:37–43. doi: 10.1016/j.jsat.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers L.C., Hallowell B.D., Zullo A.R., et al. Buprenorphine dose and time to discontinuation among patients with opioid use disorder in the era of fentanyl. JAMA Netw Open. 2023;6(9) doi: 10.1001/jamanetworkopen.2023.34540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arizona health care cost containment system (AHCCCS). 320, Services with Special Circumstances. https://www.azahcccs.gov/shared/MedicalPolicyManual/
- 27.Frome E.L., Checkoway H. Epidemiologic programs for computers and calculators. Use of Poisson regression models in estimating incidence rates and ratios. Am J Epidemiol. 1985;121(2):309–323. doi: 10.1093/oxfordjournals.aje.a114001. [DOI] [PubMed] [Google Scholar]
- 28.Chua K.-P., Nguyen T.D., Zhang J., Conti R.M., Lagisetty P., Bohnert A.S. Trends in buprenorphine initiation and retention in the United States, 2016-2022. JAMA. 2023;329(16):1402–1404. doi: 10.1001/jama.2023.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Currie J.M., Schnell M.K., Schwandt H., Zhang J. Prescribing of opioid analgesics and buprenorphine for opioid use disorder during the COVID-19 pandemic. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali M.M., Creedon T.B., Jacobus-Kantor L., Sherry T.B. National trends in buprenorphine prescribing before and during the COVID-19 pandemic. J Subst Abuse Treat. 2023;144 doi: 10.1016/j.jsat.2022.108923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen T., Ziedan E., Simon K., et al. Racial and ethnic disparities in buprenorphine and extended-release naltrexone filled prescriptions during the COVID-19 pandemic. JAMA Netw Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens M.A., Tsai J., Savitz S.T., et al. Trends and disparities in access to buprenorphine treatment following an opioid-related emergency department visit among an insured cohort, 2014-2020. JAMA Netw Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali M.M., Creedon T., Jacobus-Kantor L., et al. Early changes in waivered clinicians and utilization of buprenorphine for opioid use disorder after implementation of the 2021 HHS Buprenorphine Practice Guidelines. US Department of Health and Human Services. December 2, 2022. https://aspe.hhs.gov/sites/default/files/documents/facbce1704035fded1034192d148304d/buprenorphine-practice-guideline-early-impacts.pdf
- 34.Creedon T.B., Ali M.M., Schuman-Olivier Z. Trends in buprenorphine prescribing for opioid use disorder by psychiatrists in the US from 2003 to 2021. JAMA Health Forum. 2023;4(4) doi: 10.1001/jamahealthforum.2023.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larochelle M.R., Jones C.M., Zhang K. Change in opioid and buprenorphine prescribers and prescriptions by specialty, 2016-2021. Drug Alcohol Depend. 2023;248 doi: 10.1016/j.drugalcdep.2023.109933. [DOI] [PubMed] [Google Scholar]
- 36.Spetz J., Hailer L., Gay C., et al. Buprenorphine treatment: advanced practice nurses add capacity. Health Aff (Millwood) 2022;41(9):1231–1237. doi: 10.1377/hlthaff.2022.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Substance Abuse and Mental Health Services Administration (SAMHSA) DEA, SAMHSA extend COVID-19 telemedicine flexibilities for prescribing controlled medications for six months while considering comments from the public. https://www.samhsa.gov/newsroom/press-announcements/20230509/dea-extend-covid19-telemedicine-flexibilities-prescribing-controlled-medications
- 38.Second temporary extension of COVID-19 telemedicine flexibilities for prescription of controlled medications. 2023. https://www.federalregister.gov/documents/2023/10/10/2023-22406/second-temporary-extension-of-covid-19-telemedicine-flexibilities-for-prescription-of-controlled#:∼:text=This%20Second%20Temporary%20Rule%2C%20like,since%20March%202020%20for%20prescribing [Google Scholar]
- 39.Jones C.M., Olsen Y., Ali M.M., et al. Characteristics and prescribing patterns of clinicians waivered to prescribe buprenorphine for opioid use disorder before and after release of new practice guidelines. JAMA Health Forum. 2023;4(7) doi: 10.1001/jamahealthforum.2023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Little D., Singletary A., Barkley E., Bartelt K., Stamp T. Buprenorphine prescriptions remain uncommon after opioid-related ED encounters. Epic Research. https://epicresearch.org/articles/buprenorphine-prescriptions-remain-uncommon-after-opioid-related-ed-encounters
- 41.Melnick E.R., Nath B., Dziura J.D., et al. User centered clinical decision support to implement initiation of buprenorphine for opioid use disorder in the emergency department: EMBED pragmatic cluster randomized controlled trial. BMJ. 2022;377 doi: 10.1136/bmj-2021-069271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray J.M., Ahmed O.M., Solad Y., et al. Computerized clinical decision support system for emergency department–initiated buprenorphine for opioid use disorder: user-centered design. JMIR Hum Factors. 2019;6(1) doi: 10.2196/13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowenstein M., McFadden R., Abdel-Rahman D., et al. Redesign of opioid use disorder screening and treatment in the ED. NEJM Catal Innov Care Deliv. 2022;3(1) doi: 10.1056/CAT.21.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowenstein M., Perrone J., McFadden R., et al. Impact of universal screening and automated clinical decision support for the treatment of opioid use disorder in emergency departments: a difference-in-differences analysis. Ann Emerg Med. 2023;82(2):131–144. doi: 10.1016/j.annemergmed.2023.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amiri S., McDonell M.G., Denney J.T., Buchwald D., Amram O. Disparities in access to opioid treatment programs and office-based buprenorphine treatment across the rural-urban and area deprivation continua: a US nationwide small area analysis. Value Health. 2021;24(2):188–195. doi: 10.1016/j.jval.2020.08.2098. [DOI] [PubMed] [Google Scholar]
- 46.University of North Carolina at Chapel Hill What is UNC ECHO? https://echo.unc.edu/unc-echo
- 47.Shea C.M., Gertner A.K., Green S.L. Barriers and perceived usefulness of an ECHO intervention for office-based buprenorphine treatment for opioid use disorder in North Carolina: a qualitative study. Subst Abus. 2021;42(1):54–64. doi: 10.1080/08897077.2019.1694617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakeman S.E., Beletsky L. Beyond the X—next steps in policy reforms to address the overdose crisis. N Engl J Med. 2023;388(18):1639–1641. doi: 10.1056/NEJMp2301479. [DOI] [PubMed] [Google Scholar]
- 49.Feder K.A., Mojtabai R., Krawczyk N., et al. Trends in insurance coverage and treatment among persons with opioid use disorders following the Affordable Care Act. Drug Alcohol Depend. 2017;179:271–274. doi: 10.1016/j.drugalcdep.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saunders H., Rudowitz R. Demographics and health insurance coverage of nonelderly adults with mental illness and substance use disorders in 2020. Kaiser Family Foundation. https://www.kff.org/medicaid/issue-brief/demographics-and-health-insurance-coverage-of-nonelderly-adults-with-mental-illness-and-substance-use-disorders-in-2020/
- 51.Parish W.J., Mark T.L., Weber E.M., Steinberg D.G. Substance use disorders among Medicare beneficiaries: prevalence, mental and physical comorbidities, and treatment barriers. Am J Prev Med. 2022;63(2):225–232. doi: 10.1016/j.amepre.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 52.Ochieng N., Biniek J.F., Freed M., Damico A., Neuman T. Medicare Advantage in 2023: enrollment update and key trends. Kaiser Family Foundation. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2023-enrollment-update-and-key-trends/
- 53.Lagisetty P., Garpestad C., Larkin A., et al. Identifying individuals with opioid use disorder: validity of International Classification of Diseases diagnostic codes for opioid use, dependence and abuse. Drug Alcohol Depend. 2021;221 doi: 10.1016/j.drugalcdep.2021.108583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andraka-Christou B., Gordon A.J., Spetz J., et al. Beyond state scope of practice laws for advanced practitioners: additional supervision requirements for buprenorphine prescribing. J Subst Abuse Treat. 2022;138 doi: 10.1016/j.jsat.2021.108715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.