Abstract
Background
In many conditions characterised by septal hypertrophy, females have been shown to have worse outcomes compared to males. In clinical practice and research, similar cutoff points for septal hypertrophy are still used for both sexes. Here, we explore the association between different cutoff points for septal hypertrophy and survival in relation to sex.
Methods and results
We performed a retrospective analysis of consecutive patients undergoing echocardiography between March 2010 and February 2021 in a large tertiary referral centre. A total of 70,965 individuals were included. Over a mean follow-up period of 59.1 ± 37 months, 9631 (25 %) males and 8429 (26 %) females died. When the same cutoff point for septal hypertrophy was used for both sexes, females had worse prognosis than males. The impact of septal hypotrophy on survival became statistically significant at a lower threshold in females compared to males: 11.1 mm (HR 1.13, CI 95 %:1.03–1.23, p = 0.01) vs 13.1 mm (HR 1.21, CI 95 %: 1.12–1.32, p < 0.001). However, when indexed wall thickness was used, the cutoff points were 6 mm/body surface area (BSA) (HR 1.08, CI 95 %: 1–1.18, p = 0.04) and 6.2 mm/BSA (HR 1.07, CI 95 %: 1–1.15, p = 0.05) for females and males, respectively.
Conclusions
Septal hypertrophy is associated with increased mortality at a lower threshold in females than in males. This may account for the worse prognosis reported in females in many conditions characterised by septal hypertrophy. Applying a lower absolute value or using indexed measurements may facilitate early diagnosis and improve prognostication in females.
Keywords: Septal hypertrophy, Sex-related differences, Hypertrophic cardiomyopathy
1. Background
Septal hypertrophy is frequently encountered in the echocardiography laboratory. It is often the result of adaptive response to pressure overload (e.g. hypertension, aortic stenosis), volume overload (e.g. mitral regurgitation), sarcomeric mutations (e.g. hypertrophic cardiomyopathy) and infiltrative disease (e.g. amyloidosis). Septal and left ventricular hypertrophy (LVH) are associated with increased morbidity and mortality [1], [2], [3] and have an incremental prognostic value.[4], [5].
Females have lower normal wall thickness compared to males. According to current American Society of Echocardiography (ASE)/European Association of Echocardiography (EAE) guidelines, the normal range for septal thickness is 6–9 mm in women and 6–10 mm in men.[6] These differences may become more prominent in certain diseases as females have been shown to have a different response to cardiac hypertrophy when subjected to the same conditions as males. [7], [8], [9].
Nevertheless, data on the effect of sex on the association between septal wall thickness and survival is limited and uniform cutoff points for septal hypertrophy in men and women are often used in clinical practice and research. [10], [11] We aimed to investigate the differences between men and women in the association between septal wall thickness and survival in a large unselected cohort.
2. Methods
2.1. Study design and population
We retrospectively analysed consecutive echocardiographic examinations performed between March 2010 and February 2021 in the Tel-Aviv Medical Centre, a large tertiary referral centre in Israel. We excluded repeat tests and examinations with incomplete echocardiographic data. Baseline clinical characteristics and echocardiographic data were automatically retrieved from electronic records. Mortality was ascertained by linking the database to the population registry bureau mortality data.
The study was reviewed and approved by the Institutional Review Board in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, with a waiver of informed consent (TLV-0111-18).
2.2. Assessment of echocardiographic characteristics
Baseline echocardiography was performed per current American Society of Echocardiography guidelines in all patients and with the same equipment (iE33, Philips Medical Systems, Bothell, WA). Left ventricular (LV) diameters, inter-ventricular septal and posterior wall width, LV mass, relative wall thickness, LV ejection fraction)LVEF) were calculated as recommended by guidlines.[6] Valvular function was assessed by standard qualitative assessment using jet size according to the guidelines (normal/trivial = 1, mild = 2, moderate = 3, severe = 4). Left atrial volume was calculated using the biplane area length method at end systole. All readers were consultant cardiologists experienced in echocardiography.
2.3. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 27 software. Normally distributed continuous variables are presented as means ± SD. Categorical variables are reported as counts and percentages. Comparisons between groups were made using the independent t-test for continuous variables and Pearson's chi-square test for categorical variables. Kaplan-Meier survival curves were produced using the same software. Statistical significance was assumed at p value < 0.05. To build the different cutoff curves, cox hazard models were constructed for both sexes with septal thickness dichotomized at different cutoffs. The variables in the model were selected from Table 1 based on statistical and clinical significance. The hazard ratios with 95 % confidence intervals for each cutoff-sex pair were plotted on a continuous scale using spline functions. These graphs were plotted using the 'ggplot2′ package in R version 4.0.4 by R Foundation for Statistical Computing, Vienna, Austria. After we plotted the cutoff curve, we sought to determine the optimal statistically significant value. In order to do that we applied the same multivariate model across incremental cutoff values. For unindexed septal thickness, increments of 0.1 mm were used, while for indexed septal thickness, increments of 0.1 mm/BSA were employed. The lowest cutoff points that reached statistical significance were chosen.
Table 1.
Baseline characteristics by sex.
| Variable |
Male 38,546 (54 %) |
Female 32,419 (46 %) |
p value |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age (years) | 62 ± 18 | 65 ± 18 | < 0.001 |
| Hypertension | 11,800 (31 %) | 10,106 (31 %) | 0.1 |
| Dyslipidaemia | 9244 (24 %) | 7172 (22 %) | < 0.001 |
| Diabetes | 7375 (19 %) | 5203 (16 %) | < 0.001 |
| Ischemic heart disease | 9740 (25 %) | 3485 (11 %) | < 0.001 |
| Atrial fibrillation | 3937 (10 %) | 3671 (11 %) | < 0.001 |
| Congestive heart failure | 3012 (8 %) | 2221 (7 %) | < 0.001 |
| Stroke | 1810 (4.7 %) | 1467 (4.5 %) | 0.26 |
| Obesity | 2126 (5.5 %) | 1749 (5.4 %) | 0.46 |
| Malignancy | 1912 (5 %) | 1681 (5.2 %) | 0.17 |
| Echocardiographic characteristics | |||
| Septal thickness (mm) | 11.1 ± 2 | 10.3 ± 2 | < 0.001 |
| Septal thickness /BSA (mm/m2) | 6 ± 1.4 | 5.6 ± 1.4 | < 0.001 |
| LVM (gr) | 199 ± 67 | 155 ± 52 | < 0.001 |
| LVM /BSA (gr/m2) | 102 ± 33 | 89 ± 29 | < 0.001 |
| LVEDd /BSA (mm/m2) | 26.3 ± 4 | 25.6 ± 4 | < 0.001 |
| LVESd /BSA (mm/m2) | 17 ± 4 | 16 ± 4 | < 0.001 |
| Ejection fraction (%) | 55 ± 9 | 58 ± 6 | < 0.001 |
| LA volume /BSA (ml/m2) | 37 ± 15 | 36 ± 15 | 0.08 |
| AS ≥ moderate | 1441 (4 %) | 1446 (5 %) | < 0.001 |
| MR ≥ moderate | 1413 (4 %) | 1113 (3 %) | 0.1 |
| E/A | 1.2 ± 1 | 1.1 ± 1 | < 0.001 |
| E/e' average | 10.4 ± 5 | 11.7 ± 6 | < 0.001 |
LVM, left ventricular mass; BSA, body surface area; LVEDd, left ventricular end diastolic diameter; LVESd, left ventricular end systolic diameter; LA, left atrium; AS, aortic stenosis; MR, mitral regurgitation; Variables are n (%); mean ± SD.
3. Results
3.1. Study population and baseline characteristics
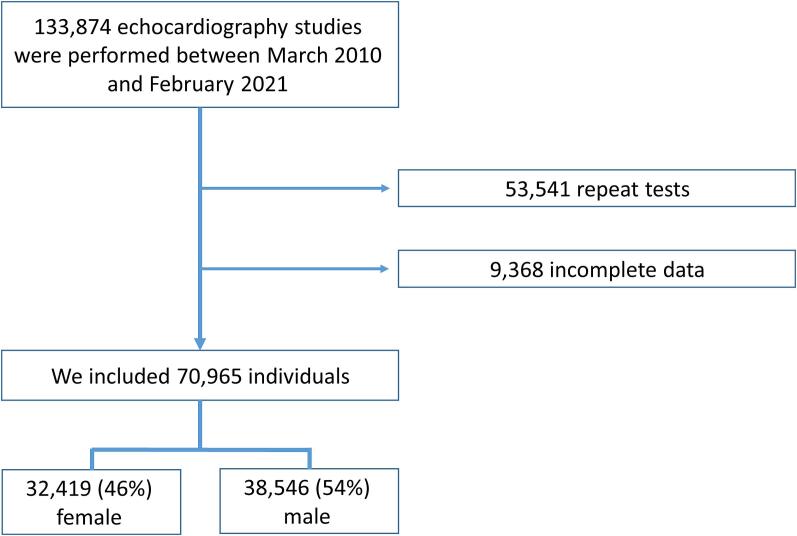
Between March 2010 and February 2021 we performed 133,874 transthoracic echocardiographic examinations. After excluding repeat tests (53,541 tests) and cases with incomplete echocardiographic data (9,368 tests), we remained with 70,965 patients (Fig. 1) which constituted our final cohort (40,239, 57 % inpatients and 30,726, 43 % outpatients). There were 32,419 (46 %) female and 38,546 (54 %) male examinees. Females were older than males (65 ± 18 years vs. 62 ± 18 years, p < 0.001), were less likely to have dyslipidaemia (22 % vs. 24 %, p < 0.001), diabetes (16 % vs. 19 %, p < 0.001) or ischemic heart disease (11 % vs. 25 %, p < 0.001) but more frequently had atrial fibrillation (11 % vs. 10 %, p < 0.001). Females had a thinner septal wall thickness (10.3 ± 2 mm vs. 11.1 ± 2 mm, p < 0.001), lower LV mass index (89 ± 29 gr/m2 vs. 102 ± 33 gr/m2, p < 0.001), lower LV end diastolic diameter index (25.6 ± 4 mm/m2 vs. 26.3 ± 4 mm/m2, p < 0.001) and lower LV end systolic diameter index (16 ± 4 mm/m2 vs. 17 ± 4 mm/m2, p < 0.001). Men had lower LVEF than women (55 % ± 9 vs. 58 % ± 6, p < 0.001) and were less likely to have at least moderate aortic stenosis (4 % vs. 5 %, p < 0.001). Females had also lower E to A ratio (1.1 ± 1 vs. 1.2 ± 1, p < 0.001) and higher E to e' ratio (11.7 ± 6 vs. 10.4 ± 5, p < 0.001).
Fig. 1.
Flow diagram that depicts individuals included in the study.
There was no significant difference between the sexes in indexed left atrial volume, arterial hypertension and the frequency of at least moderate mitral regurgitation. The complete baseline characteristics grouped by sex are shown in Table 1.
3.2. Septal thickness and survival by sex
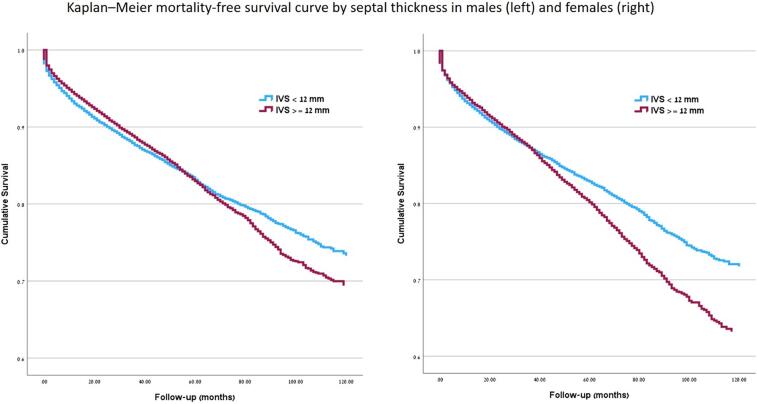
Over a mean follow-up of 59.1 ± 37 months, 9631 (25 %) males and 8429 (26 %) females died. The Kaplan-Meier mortality free survival curve by absolute septal thickness and sex is presented in Fig. 2.
Fig. 2.
Kaplan–Meier mortality-free survival curve by septal thickness and sex.
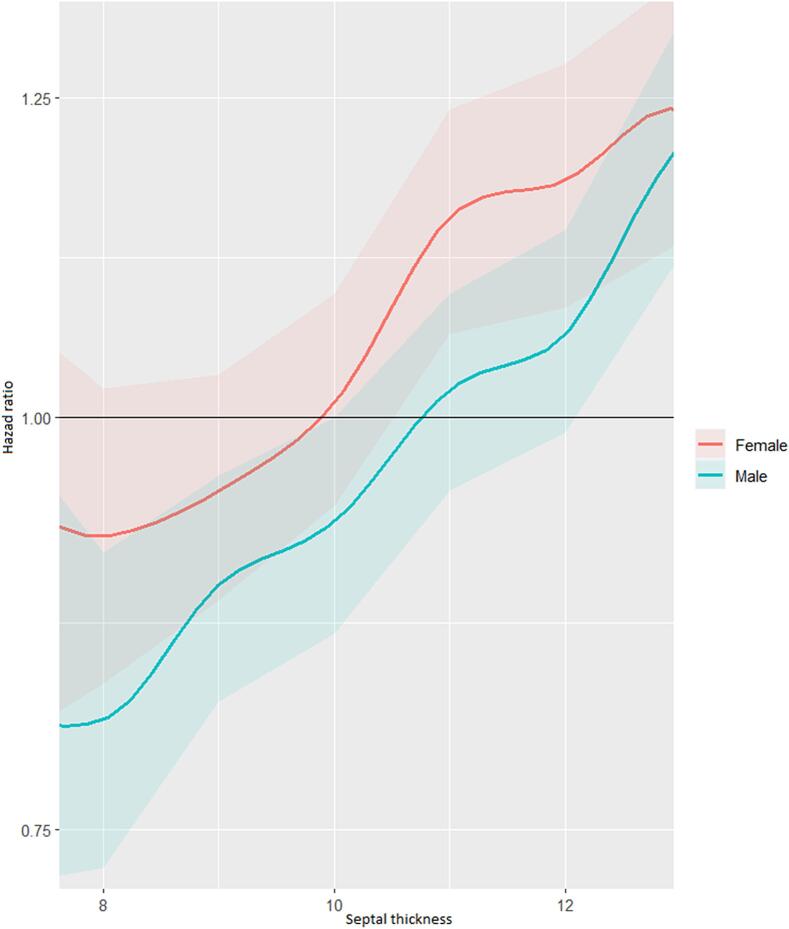
In multivariate cox regression analysis, septal thickness was an independent predictor for all-cause mortality for both men and women (Table 2). This was also evident when indexed septal thickness to BSA was used in this model instead of absolute values [HR 1.04 (CI 95 %: 1.01–1.07, p = 0.005) for men and HR 1.05 (CI 95 %: 1.02–1.08, p = 0.002) for women] (Table 3). When septal thickness was dichotomized at different cutoff points for this multivariate model, it became evident that septal thickness had an incremental prognostic significance in both sexes and that for each cutoff point, the hazard ratio for females was higher than in males (Fig. 3).
Table 2.
Cox regression model A for predicting all-cause mortality.
|
Male, p < 0.001 |
Female, p < 0.001 |
All, p < 0.001 |
||||
|---|---|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | HR (95 % CI) | p value | |
| Septal Thickness | 1.04 (1.01–1.06) | 0.002 | 1.05 (1.02–1.08) | < 0.001 | 1.04 (1.02–1.06) | < 0.001 |
| Age | 1.057 (1.054–1.059) | < 0.001 | 1.053 (1.05–1.055) | < 0.001 | 1.055 (1.053–1.057) | < 0.001 |
| Hypertension | 1.12 (1.06–1.19) | < 0.001 | 1.12 (1.05–1.19) | < 0.001 | 1.12 (1.07–1.16) | < 0.001 |
| Dyslipidaemia | 0.83 (0.19–0.89) | < 0.001 | 0.94 (0.87–0.98) | 0.007 | 0.87 (0.84–0.91) | < 0.001 |
| Diabetes | 1.57 (1.48–1.66) | < 0.001 | 1.49 (1.4–1.59) | < 0.001 | 1.54 (1.47–1.6) | < 0.001 |
| IHD | 0.92 (0.86–0.97) | 0.003 | 1.07 (0.99–1.15) | 0.08 | 0.97 (0.93–1.02) | 0.26 |
| Atrial fibrillation | 1.1 (1.03–1.18) | 0.006 | 1.05 (0.97–1.12) | 0.2 | 1.07 (1.02–1.13) | 0.05 |
| CHF | 1.5 (1.34–1.62) | < 0.001 | 1.58 (1.45–1.7) | < 0.001 | 1.54 (1.46–1.62) | < 0.001 |
| LVM /BSA | 0.996 (0.995–0.998) | < 0.001 | 0.998 (0.996–1) | 0.11 | 0.998 (0.996–0.999) | 0.003 |
| LVEDd /BSA | 1.02 (1––1.03) | 0.05 | 0.97 (0.96–0.99) | 0.005 | 0.99 (0.98–1) | 1.43 |
| LVESd /BSA | 1.02 (1–1.03) | 0.004 | 1.02 (1–1.03) | 0.006 | 1.02 (1.01–1.03) | < 0.001 |
| Ejection fraction | 0.99 (0.98–1) | < 0.001 | 0.98 (0.97–0.99) | < 0.001 | 0.99 (0.985–0.991) | < 0.001 |
| LA volume /BSA | 1.006 (1.004–1008) | < 0.001 | 1.008 (1.006–1) | < 0.001 | 1.007 (1.006–1.008) | < 0.001 |
| AS ≥ moderate | 0.98 (0.88–1.09) | 0.77 | 1.09 (0.99–1.2) | 0.07 | 1.04 (0.97–1.12) | 0.27 |
| MR ≥ moderate | 0.96 (0.85–1.07) | 0.45 | 1.13 (1.01–1.27) | 0.03 | 1.05 (0.97–1.13) | 0.27 |
HR, hazard ratio; CI, confidence interval; IHD, ischaemic heart disease; CHF, congestive heart failure;
LVM, left ventricular mass; BSA, body surface area; LVEDd, left ventricular end diastolic diameter; LVESd, left ventricular end systolic diameter; LA, left atrium; AS, aortic stenosis; MR, mitral regurgitation;
Table 3.
Cox regression model B for predicting all-cause mortality.
|
Male, p < 0.001 |
Female, p < 0.001 |
All, p < 0.001 |
||||
|---|---|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | HR (95 % CI) | p value | |
| Septal Thickness /BSA | 1.04 (1.01-1.07) | 0.005 | 1.05 (1.02-1.08) | 0.002 | 1.04 (1.02-1.06) | < 0.001 |
| Age | 1.06 (1.05–1.06) | < 0.001 | 1.05 (1.05–1.06) | < 0.001 | 1.05 (1.05–1.05) | < 0.001 |
| Hypertension | 1.14 (1.07–1.23) | < 0.001 | 1.13 (1.06–1.2) | < 0.001 | 1.13 (1.08–1.18) | < 0.001 |
| Dyslipidaemia | 0.82 (0.77–0.88) | < 0.001 | 0.9 (0.85–0.97) | 0.006 | 0.86 (0.82–0.91) | < 0.001 |
| Diabetes | 1.59 (1.49–1.69) | < 0.001 | 1.47 (1.37–1.57) | < 0.001 | 1.54 (1.46–0.61) | < 0.001 |
| IHD | 0.91 (0.85–0.97) | 0.006 | 1.08 (1–1.18) | 0.05 | 0.97 (0.96–1.02) | 0.23 |
| Atrial fibrillation | 1.09 (1–1.18) | 0.046 | 1.04 (0.96–1.13) | 0.3 | 1.06 (1–1.12) | 0.33 |
| CHF | 1.5 (1.38–1.63) | < 0.001 | 1.61 (1.48–1.75) | < 0.001 | 1.56 (1.47–1.65) | < 0.001 |
| LVM /BSA | 0.99 (0.99–0.99) | 0.001 | 1 (0.99–1) | 0.52 | 0.99 (0.99–1) | 0.19 |
| LVEDd /BSA | 1.01 (0.99–1.03) | 0.2 | 0.96 (0.95–0.98) | < 0.001 | 0.98 (0.97–0.99) | 0.006 |
| LVESd /BSA | 1.02 (1–1.04) | 0.006 | 1.02 (1–1.03) | 0.03 | 1.02 (1.01–1.03) | < 0.001 |
| Ejection fraction | 0.991 (0.987–0.995) | < 0.001 | 0.98 (0.98–0.99) | < 0.001 | 0.98 (0.98–0.99) | < 0.001 |
| LA volume /BSA | 1.007 (1.005–1.009) | < 0.001 | 1.008 (1.006–1.01) | < 0.001 | 1.007 (1.006–1.009) | < 0.001 |
| AS ≥ moderate | 1 (0.89–1.13) | 0.87 | 1.05 (0.94–1.18) | 0.4 | 1.03 (0.95–1.12) | 0.46 |
| MR ≥ moderate | 0.93 (0.82–1.06) | 0.93 | 1.05 (0.92–1.19) | 0.47 | 0.99 (0.9–1.09) | 0.89 |
HR, hazard ratio; CI, confidence interval; IHD, ischaemic heart disease; CHF, congestive heart failure;
LVM, left ventricular mass; BSA, body surface area; LVEDd, left ventricular end diastolic diameter; LVESd, left ventricular end systolic diameter; LA, left atrium; AS, aortic stenosis; MR, mitral regurgitation;
Fig. 3.
Multivariate adjusted risk for all-cause mortality by septal thickness and sex at different cutoff points.
When using a uniform absolute cut-point of 12 mm for septal thickness for both sexes (as often used in clinical practice), the unadjusted hazard ratio (HR) was 1.77 (CI 95 %: 1.70–1.84, p < 0.001) for men and 2.46 (CI 95 %: 2.35–2.57, p < 0.001) for women. The multivariate adjusted HR was 1 (CI 95 %: 0.94–1.08, p = 0.83) for males and 1.15 (CI 95 %: 1.07–1.25, p < 0.001) for females.
The optimal statistically significant unindexed cutoff in females was 11.1 mm (HR 1.13, CI 95 %:1.03–1.23, p = 0.01) vs 13.1 mm (HR 1.21, CI 95 %: 1.12–1.32, p < 0.001) in males. When using indexed septal thickness for BSA, the statistically significant cut-point was 6 mm/BSA for women, HR 1.08 (CI 95 %: 1–1.18, p = 0.04) and 6.2 mm/BSA for men, HR 1.07 (CI 95 %: 1–1.15, p = 0.05).
The differences in HR between using septal thickness as a continuous variable versus using it as a cutoff in the cox regression model indicates that septal thickness has a non-linear relationship with mortality, where the risk of death increases sharply above a certain threshold. This becomes especially valuable in clinical practice, where employing cutoffs for septal thickness makes it more interpretable and actionable.
In a subgroup analysis of 55,221 individuals who had a normal LV mass index (≤95 g/BSA for women and ≤ 115 g/BSA for men[6]), septal thickness remained an independent predictor for all-cause mortality [For absolute septal thickness, HR 1.11 (CI 95 %: 1.06–1.16, p < 0.001) for women and HR 1.07 (CI 95 %: 1.03–1.1, p < 0.001) for men, and for indexed septal thickness, HR 1.09 (CI 95 %: 1.03–1.15., p = 0.001) for females and HR 1.07 (CI 95 %: 1.03–1.11, p = 0.001) for males]. The appropriate statistically significant cut-point in this group was 10.1 mm for females [HR 1.36 (CI 95 %: 1.02–1.14, p = 0.023)] and 11.1 mm for males [HR 1.16 (CI 95 %: 1.06–1.27, p = 0.002)] and for indexed septal thickness was 6.1 mm/BSA for women [HR 1.14 (CI 95 %: 1.02–1.28, p = 0.02] and 6.2 mm/BSA for men [HR 1.13 (CI 95 %: 1.03–1.23, p = 0.01].
4. Discussion
Our study sought to assess the effect of sex on the relation between septal thickness and mortality in a large unselected cohort of individuals who underwent echocardiography examinations. We have demonstrated that septal thickness was predictive of all-cause mortality independently from sex, and that while it had an incremental prognostic significance in both sexes, its clinical importance became evident in females at a significantly lower threshold than males (11.1 mm vs 13.1 mm, respectively). This finding was also evident in a subgroup of individuals with normal LV mass index albeit at lower cut-points (10.1 mm in females and 11.1 in males).
Septal hypertrophy is not uncommon among individuals undergoing echocardiography studies. It can be found in isolation or in the context of an increased LV mass. [2], [12] Arterial hypertension, cardiac amyloidosis and sarcomeric mutations are important causes of septal and left ventricular hypertrophy.[13] Numerous studies have suggested that females with septal hypertrophy may have a more advanced disease and possibly worse prognosis than their male counterparts when uniform cut-points for septal thickness are used for both sexes.[13], [14], [15], [16], [17], [18].
Current guidelines on cardiac amyloidosis recommend a cut-points for septal thickness of 12 mm or greater to prompt evaluation for the disease[19]. However, females with transthyretin cardiac amyloidosis (ATTR) with a LV wall thickness greater than 12 mm are likely to have a more advanced disease compared to their male counterparts.[13], [14] In hypertensive patients, the presence of LVH has been shown to offset the sex-specific protection in cardiovascular risk suggesting that LVH is more harmful in females than in males. [20].
The 2023 European society of cardiology (ESC) guidelines on cardiomyopathies and the 2020 American heart association/American college of cardiology (AHA/ACC) guidelines on hypertrophic cardiomyopathy (HCM) recommend the use of left ventricular wall thickness of ≥ 15 mm (or ≥ 13 mm in case of a positive family history or genetic testing) for the clinical diagnosis of HCM for both men and women. [10], [11] Studies looking at the impact of sex on the clinical course and prognosis of HCM have shown that females experience more advanced New York Association class III/IV (NYHA) symptoms and were more likely to develop heart failure with preserved ejection fraction. [15], [16] Several studies have also demonstrated that females with HCM have excess mortality compared to men. [17], [18].
Our findings suggest that using the same cut-point for septal hypertrophy both in men and in women may at least partially explain the poorer outcomes reported in female patients in many diseases characterised by septal hypertrophy. Applying lower absolute values for females or using indexed measurements may facilitate early diagnosis and affect prognosis.
Relating adult wall thickness to BSA or height has been suggested as a way to avoid sex-bias and facilitate early diagnosis in HCM. [21], [22] Similarly, in ATTR amyloidosis, non-indexed wall thickness measurements may be responsible for the delayed diagnosis and potentially poorer prognosis observed in females. However, when indexed wall thickness measurements were used, there were no differences between the sexes.[13] Using Z-scores for septal thickness instead of unadjusted values may also prove beneficial as Z-scores are currently recommended by guidelines for the diagnosis of childhood HCM [23], and they are routinely used for aortic measurements in adults with aortic diseases. [24].
5. Limitations
This is a retrospective study performed in a single tertiary centre which could have resulted in referral bias. The design of our study was meant for the evaluation of sex-related differences in septal thickness in an unselected cohort of individuals undergoing echocardiography and hence no specific disease related conclusions can be derived. Data on outcomes other than all-cause mortality was not available for the entire cohort and therefore no insights into cardiovascular mortality or hospitalizations can be presented.
6. Conclusions
We have demonstrated in a large unselected cohort of patients that septal thickness was associated with poorer survival in females compared to males. We have also shown that the prognostic significance of septal thickness became evident in females at a lower threshold than males and persisted in a subgroup of patients without LVH. These findings may at least partially explain the delayed diagnosis and worse prognosis reported in females in many conditions characterised by septal hypertrophy. Using a lower cutoff point for septal hypertrophy in females or moving to indexed measurements may be required to facilitate early diagnosis and improve prognostication in female patients.
CRediT authorship contribution statement
Shafik Khoury: Writing – review & editing, Writing – original draft, Project administration, Conceptualization. Lior Zornitzki: Data curation. Michal Laufer-Perl: Writing – review & editing, Conceptualization. Raghav T. Bhatia: Writing – review & editing. Sarandeep Marwaha: Writing – review & editing. Maite Tome: Writing – review & editing, Conceptualization. Yoav Granot: Data curation. Moran Gvili Perelman: Formal analysis, Data curation. Ido Avivi: Data curation. Yacov Shacham: Writing – review & editing. Yishay Szekely: Writing – review & editing. Shmuel Banai: Writing – review & editing. Aviram Hochstadt: Formal analysis. Nir Flint: Writing – review & editing. Yan Topilsky: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.Ranasinghe I., Ayoub C., Cheruvu C., Freedman S.B., Yiannikas J. Isolated hypertrophy of the basal ventricular septum: characteristics of patients with and without outflow tract obstruction. Int. J. Cardiol. 2014;173(3):487–493. doi: 10.1016/j.ijcard.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 2.Loncaric F., Nunno L., Mimbrero M., et al. Basal ventricular septal hypertrophy in systemic hypertension. Am. J. Cardiol. 2020;125(9):1339–1346. doi: 10.1016/j.amjcard.2020.01.045. [DOI] [PubMed] [Google Scholar]
- 3.Liao Y., Cooper R.S., Mensah G.A., McGee D.L. Left ventricular hypertrophy has a greater impact on survival in women than in men. Circulation. 1995;92(4):805–810. doi: 10.1161/01.cir.92.4.805. [DOI] [PubMed] [Google Scholar]
- 4.O’Mahony C., Jichi F., Pavlou M., et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD) Eur. Heart J. 2014;35(30):2010–2020. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- 5.Elliott P.M., Gimeno Blanes J.R., Mahon N.G., Poloniecki J.D., McKenna W.J. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357(9254):420–424. doi: 10.1016/S0140-6736(00)04005-8. [DOI] [PubMed] [Google Scholar]
- 6.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Singh A., Chan D.C.S., Greenwood J.P., et al. Symptom onset in aortic stenosis: relation to sex differences in left ventricular remodeling. J. Am. Coll. Cardiol. Img. 2019;12(1):96–105. doi: 10.1016/j.jcmg.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Murphy E. Estrogen signaling and cardiovascular disease. Circ. Res. 2011;109(6):687–696. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas P.S., Katz S.E., Weinberg E.O., Chen M.H., Bishop S.P., Lorell B.H. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J. Am. Coll. Cardiol. 1998;32(4):1118–1125. doi: 10.1016/s0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 10.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: A report of the american college of cardiology/american heart association joint committee on clinical practice guidelines. Circulation. 2020;142(25):e558–e631. doi: 10.1161/CIR.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 11.Arbelo E., Protonotarios A., Gimeno J.R., et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023;44(37):3503–3626. doi: 10.1093/eurheartj/ehad194. [DOI] [PubMed] [Google Scholar]
- 12.Verdecchia P., Porcellati C., Zampi I., et al. Asymmetric left ventricular remodeling due to isolated septal thickening in patients with systemic hypertension and normal left ventricular masses. Am. J. Cardiol. 1994;73(4):247–252. doi: 10.1016/0002-9149(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 13.Patel R.K., Ioannou A., Razvi Y., et al. Sex differences among patients with transthyretin amyloid cardiomyopathy - from diagnosis to prognosis. Eur. J. Heart Fail. 2022;24(12):2355–2363. doi: 10.1002/ejhf.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zampieri M., Argirò A., Allinovi M., et al. Sex-related differences in clinical presentation and all-cause mortality in patients with cardiac transthyretin amyloidosis and light chain amyloidosis. Int. J. Cardiol. 2022;351:71–77. doi: 10.1016/j.ijcard.2021.12.048. [DOI] [PubMed] [Google Scholar]
- 15.Rowin E.J., Maron M.S., Wells S., Patel P.P., Koethe B.C., Maron B.J. Impact of sex on clinical course and survival in the contemporary treatment era for hypertrophic cardiomyopathy. J. Am. Heart Assoc. 2019;8(21):e012041. doi: 10.1161/JAHA.119.012041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivotto I., Maron M.S., Adabag A.S., et al. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2005;46(3):480–487. doi: 10.1016/j.jacc.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 17.Lorenzini M., Anastasiou Z., O’Mahony C., et al. Mortality among referral patients with hypertrophic cardiomyopathy vs the general european population. JAMA Cardiol. 2020;5(1):73–80. doi: 10.1001/jamacardio.2019.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geske J.B., Ong K.C., Siontis K.C., et al. Women with hypertrophic cardiomyopathy have worse survival. Eur. Heart J. 2017;38(46):3434–3440. doi: 10.1093/eurheartj/ehx527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Pavia P., Rapezzi C., Adler Y., et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021;42(16):1554–1568. doi: 10.1093/eurheartj/ehab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerdts E., Izzo R., Mancusi C., et al. Left ventricular hypertrophy offsets the sex difference in cardiovascular risk (the Campania Salute Network) Int. J. Cardiol. 2018;258:257–261. doi: 10.1016/j.ijcard.2017.12.086. [DOI] [PubMed] [Google Scholar]
- 21.Javidgonbadi D., Schaufelberger M., Östman-Smith I. Factors associated with excess female mortality in obstructive hypertrophic cardiomyopathy. Eur. J. Prev. Cardiol. 2022;29(11):1545–1556. doi: 10.1093/eurjpc/zwac078. [DOI] [PubMed] [Google Scholar]
- 22.Nijenkamp L.L.A.M., Bollen I.A.E., van Velzen H.G., et al. Sex differences at the time of myectomy in hypertrophic cardiomyopathy. Circ. Heart Fail. 2018;11(6):e004133. doi: 10.1161/CIRCHEARTFAILURE.117.004133. [DOI] [PubMed] [Google Scholar]
- 23.Authors/Task Force members, Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35(39):2733–2779. [DOI] [PubMed]
- 24.Campens L., Demulier L., De Groote K., et al. Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am. J. Cardiol. 2014;114(6):914–920. doi: 10.1016/j.amjcard.2014.06.024. [DOI] [PubMed] [Google Scholar]