Abstract
We characterized the populations of primary sensory neurons that become latently infected with herpes simplex virus (HSV) following peripheral inoculation. Twenty-one days after ocular inoculation with HSV strain KOS, 81% of latency-associated transcript (LAT)-positive trigeminal ganglion (TG) neurons coexpressed SSEA3, 71% coexpressed TrkA (the high-affinity nerve growth factor receptor), and 68% coexpressed antigen recognized by monoclonal antibody (MAb) A5; less than 5% coexpressed antigen recognized by MAb KH10. The distribution of LAT-positive, latently infected TG neurons contrasted sharply with (i) the overall distribution of neuronal phenotypes in latently infected TG and (ii) the neuronal distribution of viral antigen in productively infected TG. Similar results were obtained following ocular and footpad inoculation with KOS/62, a LAT deletion mutant in which the LAT promoter is used to drive expression of the Escherichia coli lacZ gene. Thus, although all neuronal populations within primary sensory ganglia appear to be capable of supporting a productive infection with HSV, some neuronal phenotypes are more permissive for establishment of a latent infection with LAT expression than others. Furthermore, expression of HSV LAT does not appear to play a role in this process. These findings indicate that there are marked differences in the outcome of HSV infection among the different neuronal populations in the TG and highlight the key role that the host neuron may play in regulating the repertoire of viral gene expression during the establishment of HSV latent infection.
During primary infection of sensory ganglia with herpes simplex virus type 1 (HSV-1), there is an early divergence of the latent and productive pathways of viral gene transcription (17, 28, 41, 43). Since neither viral gene expression nor viral DNA replication appears to be necessary for HSV to establish a latent infection (2, 3, 14, 28, 37, 38, 43), it is likely that host cell factors play important roles in regulating establishment of the latent state. Experiments carried out in vitro suggest that host expression of Oct-1, Oct-2, gamma interferon, and nerve growth factor (NGF) may affect the outcome of infection with HSV by either direct or indirect interaction with the transcriptional regulatory complex responsible for transcription of the HSV immediate-early (IE) genes (7, 23, 31, 44, 45). However, many of these experiments were carried out with nonneuronal cell lines, transformed cell lines, and hybrid cell lines. Thus, the relevance of these studies to our understanding of the regulation of HSV infection in sensory neurons in vivo remains in question.
Primary sensory neurons are a diverse population of cells that can be classified according to cellular morphology, physiological response properties, neuropeptide content, synthesis of cytoplasmic enzymes, and expression of cell surface receptors and glycoconjugates (36). It has long been our working hypothesis that different populations of host sensory neurons may be capable of differentially regulating the outcome of an infection with HSV; in a previous study, we presented preliminary data indicating that a latent pattern of viral gene expression was more likely to occur in some murine dorsal root ganglion (DRG) neuronal phenotypes (SSEA3 immunoreactive) than in others (LD2 immunoreactive) (28). In the present study, we extend these findings by examining patterns of viral gene expression in eight different populations of primary sensory neurons. Distinguishing features of the neuronal populations that we have chosen to study include expression of (i) TrkA, the high-affinity NGF receptor, which is primarily expressed in neurons that convey nociceptive information (40); (ii) the neuropeptides, calcitonin gene-related peptide (CGRP) and substance P (SP); (iii) b-NOS (brain nitric oxide synthase); (iv) the developmentally regulated globoseries glycoconjugate SSEA3 (9); (v) the neurofilament protein recognized by monoclonal antibody (MAb) RT97, which specifically labels the “large light” neuron population (20); and (vi) the lactoseries glycoconjugates recognized by MAbs KH10 and A5 (10). MAbs KH10 and A5 identify the same populations of primary sensory neurons as MAbs LD2 and 1B2 (10), antisera used in prior studies of HSV-infected sensory ganglia (19, 27, 28).
The results of our study indicate that although all neuronal populations of the trigeminal ganglion (TG) are capable of supporting a productive infection with HSV, some neuronal populations (e.g., KH10 immunoreactive) are much more permissive for this course of infection than others. Of the TG neuronal populations studied, HSV infection of the A5-immunoreactive population was the most likely to result in a latent pattern of viral infection. Our results were reproducible independent of inoculation site (footpad or eye), survival time (7 or 21 days), HSV latency-associated transcript (LAT) expression, and method of detecting latently infected neurons (in situ hybridization [ISH] for LAT versus immunohistochemistry [IH] for β-galactosidase [β-Gal]). These findings highlight the key role that the host neuron may play in regulating the repertoire of viral gene expression during establishment of HSV latent infection. They also highlight the importance of considering the complex neuronal composition of primary sensory ganglia in interpreting the results of in vivo studies of HSV infection.
MATERIALS AND METHODS
Cells and virus stocks.
Viral stocks were propagated in rabbit skin cells maintained in minimal essential medium supplemented with 5% fetal calf serum, 250 mg of penicillin per ml, and 250 mg of streptomycin per ml. KOS, KOS/62, and KOS/1 were used in the course of these studies. KOS/62 and KOS/1 have been described in detail elsewhere (11, 26, 28, 35). KOS/62 has a deletion of the 5′ ends of both copies of the LAT coding region with an insertion of the Escherichia coli lacZ gene immediately downstream of both copies of the LAT TATA box. As assayed by Northern blot analysis, KOS/62 does not express LATs (42). KOS/1 has a single LAT promoter-lacZ cassette inserted in the glycoprotein C coding region. Viral titers in the range of 108 to 109 PFU/ml were typically obtained for the three viruses.
Animals and inoculations.
Four-week-old female Swiss Webster mice were anesthetized by intraperitoneal injection with 1.5% pentobarbital. Following corneal scarification, eyes and the ocular adnexa were inoculated with 15 μl of viral stock (108 PFU/ml). Footpad inoculation was carried out with 30 μl of viral stock (15). Following inoculation, mice were allowed to recover from anesthesia and monitored daily.
Tissue preparation.
Three to 21 days postinoculation (p.i.) mice were euthanized by carbon dioxide inhalation and thoracotomy. Cardiac perfusion with 0.1 M phosphate-buffered saline (PBS; pH 7.2) was performed immediately, followed by perfusion with 4% paraformaldehyde in 0.1 M PBS. Dissected sensory ganglia from groups of five mice were combined and immersion fixed in 4% paraformaldehyde at 4°C for either 30 min (for immunofluorescence) or 4 to 6 h (for combined IH-ISH). All fixed tissue was subsequently equilibrated with 10% sucrose, embedded in OCT (Miles Inc., Ind.), and snap frozen in liquid nitrogen. Serial sections (6 μm) were collected as five alternate sets onto Superfrost* Plus slides (Fisher). Cut tissue was stored at −20°C for immunofluorescence and −70°C for combined IH-ISH.
Primary antisera.
The following rabbit polyclonal antisera were used in the course of this study: anti-CGRP (Amersham, Arlington Heights, Ill.), anti-SP (Incstar Corp., Stillwater, Minn.), anti-b-NOS (Signal Transduction Laboratory Lexington, Ky.), fluorescein isothiocyanate (FITC)-conjugated rabbit anti-HSV (DAKO, Carpinteria, Calif.), and anti-TrkA (a gift from Douglas Clary and Louis Reichardt). The TrkA-specific antiserum has been characterized previously (1). Mouse MAbs anti-SSEA3, KH10, A5, and RT97 were obtained from the Developmental Studies Hybridoma Bank (Iowa City, Iowa). Antisera KH10 and A5 identify the same populations of neurons as antisera LD2 and 1B2, respectively (10). Mouse monoclonal anti-β-Gal antiserum (Boehringer Mannheim) was labeled with biotin, using a biotinylation kit from Amersham.
IH.
For dual immunofluorescence studies, of β-Gal and neuronal cell markers tissue sections were incubated with primary antisera for 16 to 48 h at 4°C, washed with 1% normal goat serum in PBS and then incubated with secondary antisera (FITC-conjugated goat anti-rabbit immunoglobulin G [IgG] [Vector Laboratories, Burlingame, Calif.] or FITC-conjugated goat anti-mouse IgM [Vector]) for 40 min at room temperature (RT). Slides were then sequentially incubated with biotinylated mouse anti-β-Gal antiserum for 1 h, Rhodamine600 Avidin D (Vector) diluted 1:1,200 for 40 min, biotinylated goat anti-avidin D (Vector Laboratories) diluted 1:200 for 40 min, and again Rhodamine600 Avidin D for 40 min. The slides were then washed and mounted with glass coverslips by using Vectashield (Vector Laboratories). For dual immunofluorescence studies of HSV antigen and neuronal cell markers, a similar protocol was used except that the secondary antiserum for the neuronal markers was trimethyl rhodamine isocyanate (TRITC)-conjugated goat anti-rabbit IgG or TRITC-conjugated goat anti-mouse IgM (TAGO Inc., Burlingame, Calif.) and FITC-conjugated rabbit anti-HSV-1 (DAKO) was used to detect HSV antigen-positive cells. To minimize bias in the selection and evaluation of experimental tissue, sets of tissue representing every fifth tissue section (see “Tissue preparation” above) were stained and analyzed with a given combination of antisera. Results were then expressed as percentage of ganglionic neurons positive for a given combination of antisera; the total number of neurons analyzed for any combination of antisera is given in Tables 1 to 4. Data were expressed in this fashion for ease of comparison to results obtained by combined IH-ISH.
TABLE 1.
Infected neuronal populations of the TG
| Study population (day p.i.) | No. of neurons evaluated | Neuronal composition (%)a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SSEA3 | A5 | KH10 | CGRP | SP | TrkA | b-NOS | RT97 | ||
| Uninfected | 16,539 | 38 | 12 | 15 | 37 | 17 | 26 | 3 | 41 |
| KOS/62 infected | |||||||||
| All neuronsb (7) | 21,691 | 34 | 10 | 13 | 32 | 16 | 24 | 3 | 39 |
| HSV Ag+c (3) | 6,118 | 23 | 6 | 17 | 33 | 15 | 30 | 2 | 34 |
| β-Gal+d (7) | 2,516 | 80 | 61 | 2 | 43 | 5 | 59 | 2 | 54 |
| β-Gal+ (21) | 968 | 83 | 75 | 3 | 53 | 7 | 69 | 5 | 49 |
| KOS/1 infected | |||||||||
| HSV Ag+ (3) | 4,167 | 28 | 6 | 24 | 32 | 23 | 25 | 2 | 36 |
| β-Gal + (7) | 1,693 | 75 | 54 | 5 | 43 | 5 | 59 | 2 | 54 |
Since some neurons label with more than one of the antisera used in this study, the neuronal composition in each row adds up to greater than 100%.
Infected and noninfected.
Productively infected neurons; Ag+, antigen positive.
Latently infected neurons with detectable LAT promoter activity as assayed by immunofluorescence for β-Gal.
TABLE 4.
Infected neuronal populations of the TG: outcomes analysisa
| Antiserum | HSV Ag+
|
LAT+
|
HSVAg+/LAT+ | ||
|---|---|---|---|---|---|
| % | n | % | n1 | ||
| SSEA3 | 28 | 976 | 81 | 973 | 1:1 |
| TrkA | 21 | 732 | 71 | 853 | 1:1.2 |
| A5 | 7 | 244 | 68 | 817 | 1:3.3 |
| KH10 | 16 | 567 | 2 | 24 | 23:1 |
| RT97b | 49 | 1,707 | 34 | 408 | 4.2:1 |
| b-NOS | 2 | 70 | 3 | 36 | 1.9:1 |
| CGRP | 31 | 1,080 | 51 | 613 | 1.8:1 |
| SP | 12 | 418 | 20 | 240 | 1.7:1 |
| Total | 3,483 | 1,201 | 2.9:1 | ||
n, number of HSV antigen-positive (Ag+) neurons at 3 days p.i.; n1, number of LAT-positive neurons at 21 days p.i.
Since we have no data on the percentage of neurons latently infected with KOS that coexpress RT97, for the purposes of the analysis presented here we used data from mice infected with KOS/62.
NADPH diaphorase histochemistry.
Tissue sections were washed in 0.1 M PBS (pH 8.0), incubated in a solution containing 1 mM NADPH (Sigma), 2 mM nitroblue tetrazolium (Sigma), and 0.3% Triton X-100 in 0.1 M PBS (pH 8.0) for 25 min at 37°C, and washed in PBS again to stop the reaction. Stained sections were assessed by light microscopy.
Combined IH-ISH. (i) IH.
IH was performed by immunoperoxidase staining. To maintain the integrity of the RNA signal for further ISH, gloves were used for all procedures, glassware was rinsed with diethylpyrocarbonate (DEPC)-treated water, and all reagents were prepared with DEPC-treated water. Prior to incubation with primary antisera, all tissue sections were treated with 3% H2O2 in KPBS (0.02 M potassium phosphate [pH 7.2 to 7.4], 0.15 M NaCl) to quench endogenous peroxidase activity. Tissue sections were incubated with primary antisera (anti-TrkA, A5, KH10, anti-SSEA3, anti-SP, and anti-CGRP), diluted with KPBS containing bovine serum albumin (20 μg/ml), heparin (5 mg/ml), Triton X-100 (3 μl/ml), and human placental RNase inhibitor (180 U/ml) for 16 to 36 h at 4°C. Sections were subsequently rinsed with KPBS, incubated with the appropriate secondary antisera (biotin-conjugated goat anti-rabbit IgG or biotin-conjugated goat anti-mouse IgM) for 1 h at RT, rinsed again with KPBS, and incubated with ABC reagent (Vector) for 30 min at RT. The sections were again washed with KPBS and reacted with metal-enhanced diaminobenzidine substrate working solution (Pierce, Rockford, Ill.).
ISH was carried out as described by Cunningham and DeSouza (5). Labeled riboprobes, specific for the stable LAT intron, were prepared by using plasmid pATD-19 as a template (28). Radioactively labeled riboprobes were prepared as described by Cunningham and DeSouza (5), and digoxigenin-labeled riboprobes were prepared by using a commercial RNA labeling kit (Boehringer Mannheim). Digoxigenin-labeled riboprobes were ethanol precipitated, air dried, and resuspended in Tris-EDTA buffer. All labeled riboprobes were stored at −70°C.
FISH prehybridization.
Following IH, tissue sections were prepared for fluorescent ISH (FISH) by sequential incubation with 10% neutral buffered formalin (30 min), 0.001% proteinase K (30 min), 0.1 M triethanolamine (3 min), 0.25% acetic anhydride in 0.1 M triethanolamine (3 min), and prehybridization solution for 2 h at 45°C. Prehybridization solution consisted of 50% formamide, 0.3 M NaCl, 20 mM Tris (pH 8.0), 1 mM EDTA, 1× Denhardt's solution, 500 μg of yeast tRNA per ml, 100 μg of salmon sperm DNA per ml, 0.1% sodium dodecyl sulfate, and 100 mM dithiothreitol. Tissue sections that had not been previously reacted for IH served as controls.
FISH.
The digoxigenin-labeled riboprobe was dissolved in hybridization solution, which contained 25 ml of formamide, 3 ml of 5 M NaCl, 1 ml of 1 M Tris (pH 8.0), 100 μl of 0.5 M EDTA, 2.5 ml of 20× Denhardt's solution, 25 mg of yeast tRNA, 5 g of dextran sulfate, 0.5 ml of single-stranded DNA (10 mg/ml), 0.5 ml of 10% sodium dodecyl sulfate, and 0.75 g of dithiothreitol, brought to 37.5 ml with DEPC-treated water. The probe concentration in the hybridization solution was approximately 0.5 μg/ml. The hybridization cocktails were heated to 65°C for 10 min, and aliquots of this cocktail were applied to each slide of prehybridized tissue sections, followed by careful placement of a glass coverslip. Hybridization was then carried out in a humidified chamber at 65°C for 16 to 18 h. Following hybridization, the coverslips were removed by immersion in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and the uncovered sections were rinsed in 2× SSC for 10 min followed by RNase A solution at 37°C for 30 min. Sections were then taken through 2× SSC for 10 min, 1× SSC for 10 min, 0.5× SSC for 10 min, 0.1× SSC for 30 min at 60°C, 0.1× SSC for 3 min, 2× SSC for 2 min, rinse buffer (100 mM Tris, 150 mM NaCl) for 2 min, and 1% blocking reagent for nucleic acid hybridization and detection (Boehringer Mannheim) for 30 min. The sections were then incubated with FITC-conjugated antidigoxigenin antiserum (1:10; Boehringer Mannheim) for 30 min and washed in rinse buffer.
Coverslips were mounted with Vectashield. Tissue sections were evaluated and photographed by alternate fluorescence and bright-field microscopy. In accordance with the protocol for evaluating immunohistochemical-stained tissue, we assayed and evaluated sets of tissue representing every fifth tissue section. Results were then expressed as a percentage of ganglionic neurons positive for a given combination of antiserum and ISH probe. We chose to express data in this fashion since the technically challenging nature of the combined IH-FISH protocol frequently left us with a variable number of tissue sections (or portions thereof) that did not stain well enough for analysis.
RESULTS
Patterns of viral gene expression during acute infection of the mouse TG.
As assayed by immunofluorescent staining using polyclonal antisera to HSV, productive infection of TG neurons with KOS/62 was present as early as 2 days after ocular inoculation and peaked 3 days p.i. By 7 days p.i., productively infected neurons were no longer found in infected ganglia, but a few nonneuronal cells, mostly leukocytes and glia, continued to stain for HSV antigen. Immunofluorescent staining for β-Gal, a marker of LAT promoter activity, was also present in ganglionic neurons as early as 2 days after inoculation with KOS/62. Staining for β-Gal persisted in ganglionic neurons until at least 21 days p.i., the latest time point studied; however, the intensity of β-Gal staining decreased markedly between 7 and 21 days p.i. This progressive drop in lacZ reporter gene expression is similar to that previously described for DRG neurons latently infected with KOS/62 (26).
Three different patterns of KOS/62 gene expression were observed in immunofluorescently stained TG neurons. Most labeled neurons stained only with the polyclonal antisera to HSV. The remaining labeled neurons stained for β-Gal, either alone or in conjunction with HSV antigens. At the peak of the productive phase of TG infection (day 3), 65% of β-Gal-positive neurons coexpressed HSV antigen, a much greater percentage than observed at the peak of acute DRG infection (28). These results are similar to those reported by Sawtell and Thompson (35), who used 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining for β-Gal rather than the immunofluorescence approach used here. No labeled neurons were observed at any time point in mice inoculated with sterile medium.
Similar patterns of immunofluorescent labeling of TG neurons were observed following ocular inoculation with KOS/1. However, one striking difference was noted. For all time points studied, there were fewer β-Gal-expressing neurons in KOS/1-infected tissue than in KOS/62-infected tissue; by 21 days p.i., immunofluorescent staining for β-Gal in KOS/1-infected ganglionic neurons was so weak that it was difficult to identify β-Gal-positive neurons with certainty. This observation is in contrast to the report of Sawtell and Thompson (35), who found significantly more β-Gal-positive neurons in KOS/1-infected TG than in those infected with KOS/62. The most likely explanation for the difference between our results and those of Sawtell and Thompson is that we assayed for β-Gal-positive neurons by using a compound immunofluorescence assay, whereas they used histochemical staining with X-Gal. For dual-labeling studies, we have found that the immunofluorescence assay provides markedly superior morphology and antigen preservation than X-Gal staining. We have also found that X-Gal staining of KOS/1- and KOS/62-infected sensory ganglia is extremely sensitive to fixation conditions, with minor differences in fixative freshness and fixation time significantly affecting the outcome of quantitative assays (data not shown).
KOS/62-infected neuronal populations in the mouse TG.
Following ocular inoculation with KOS/62, the TG of infected mice were assayed by dual immunofluorescence to characterize differences in TG neuronal populations that hosted productive and latent patterns of viral gene expression. Based on the results of experiments described above, we chose to study productively infected, HSV antigen-positive neurons at 3 days p.i. and latently infected, β-Gal-positive neurons at 7 and 21 days p.i. The results of these experiments are summarized in Table 1, and representative examples of dual-stained tissue are presented in Fig. 1.
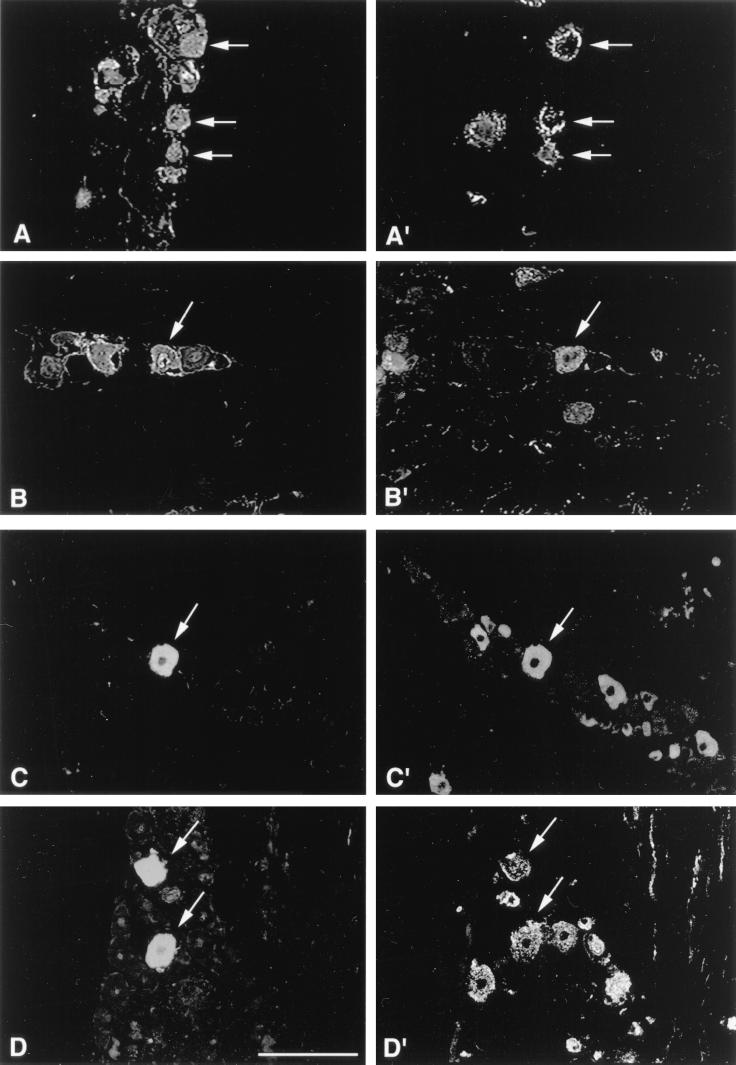
FIG. 1.
Representative examples of KOS/62-infected TG tissue sections stained by dual immunofluorescence to identify neuronal subpopulations expressing β-Gal or HSV antigen. At 3 days p.i., HSV antigen expression (A and B) can be seen colocalizing to KH10- and CGRP-immunoreactive neurons (A′ and B′, respectively). At 7 days p.i., β-Gal expression (C and D) can be seen colocalizing to TrkA- and A5-immunoreactive neurons (C′ and D′ respectively). Magnification bar represents 100 μm.
The neuronal distributions of β-Gal expression in the TG were similar at 7 and 21 days p.i. and differed markedly from both the neuronal distribution HSV antigen at 3 days p.i. and the overall neuronal composition of uninfected TG. The most marked differences at both 7 and 21 days p.i. were the relatively high proportion of β-Gal-expressing neurons that were SSEA3 (83%), TrkA (69%), and A5 (75%) immunoreactive and the relatively low proportion that colabeled with MAb KH10 (3%). It is unlikely that these difference were a consequence of changes in the expression of neuronal markers in response to HSV infection since the neuronal composition of the mouse TG at 7 days p.i. was very similar to that found in uninfected mice.
Based on these observations, we conclude that the outcome of infection of individual TG neurons with HSV (productive or latent) is mediated, at least in part, by the phenotype of the host neuron. Specifically, HSV infection of SSEA3-, A5-, and TrkA-positive neurons is more likely to result in a latent pattern of viral gene expression than infection of other neuronal populations, especially those which are KH10 positive.
KOS/1-infected neuronal populations in the mouse TG.
Since it has been suggested that LAT may play a role in the establishment of HSV latent infection in the mouse TG (35), we wondered whether the LAT deletion in KOS/62 was playing a role in the preferential establishment of latent infection in neurons with the SSEA3, TrkA, and A5 phenotypes. To examine this issue, TG of mice were assayed by dual immunofluorescence 7 days following ocular inoculation with KOS/1, and the results were compared to those observed with KOS/62. TG neurons from mice 21 days after inoculation with KOS/1 were not evaluated due to the consistently poor immunofluorescent staining for β-Gal at this time point.
Infection with KOS/1 resulted in a pattern of latent and productive viral gene expression in different populations of TG neurons that closely resembled that observed with KOS/62 (Table 1). A relatively high proportion of β-Gal-positive neurons in KOS/1-infected TG were SSEA3 (75%), TrkA (59%), and A5 (54%) immunoreactive, and a relatively low proportion colabeled with MAb KH10 (5%). As with KOS/62, the neuronal distribution of β-Gal expression in the TG 7 days after inoculation with KOS/1 differed markedly from both the neuronal distribution of HSV antigen in the TG 3 days p.i. and the overall neuronal composition of uninfected TG. Based on these observations, we conclude that LAT plays little, if any, role in the preferential establishment of a latent infection in certain populations of ganglionic neurons.
KOS/62-infected neuronal populations in the mouse DRG.
To determine whether the observed patterns of viral gene expression in different neuronal populations of the TG were unique to the sensory neurons innervating ocular tissues, we studied productive and latent patterns of infection in different neuronal populations of DRG following footpad inoculation with KOS/62. HSV antigen-positive neurons were assayed 4 days p.i.; the peak of productive neuronal infection in the DRG (28) and β-Gal-positive neurons were assayed 8 days p.i., at which time we could no longer detect HSV antigen-positive neurons.
Infection of DRG with KOS/62 by footpad inoculation resulted in a pattern of latent and productive viral gene expression in different populations of DRG neurons that closely resembled that observed in the TG (Table 2). As in the infected TG, we found that a high proportion of β-Gal-positive neurons were SSEA3 (86%), TrkA (65%), and A5 (48%) immunoreactive, whereas a very low proportion colabeled with MAb KH10 (1%). As expected, the neuronal distribution of β-Gal expression in the DRG at 8 days after infection with KOS/62 differed significantly from both the neuronal distribution of HSV antigen in the DRG at 4 days p.i. and the overall neuronal composition of uninfected DRG. Since our findings for infected DRG were similar to those for the TG, we conclude that differential viral gene expression in different populations of primary sensory neurons is not unique to the TG, as a consequence of either its somatotopic organization, unique embryological origins, or highly rich innervation of ocular surface structures.
TABLE 2.
KOS/62-infected neuronal populations of DRG
| Study population (day p.i.) | No. of neurons evaluated | Neuronal composition (%)a
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SSEA3 | A5 | KH10 | CGRP | SP | TrkA | b-NOS | RT97 | ||
| Uninfected | 6,104 | 39 | 12 | 23 | 39 | 15 | 26 | 3 | 39 |
| Infected | |||||||||
| All neuronsb (8) | 13,753 | 38 | 12 | 29 | 43 | 12 | 26 | 2 | 37 |
| HSV Ag+c (4) | 3,227 | 25 | 8 | 29 | 33 | 16 | 29 | 1 | 40 |
| β-Gal+d (8) | 1,104 | 86 | 48 | 1 | 38 | 6 | 65 | 1 | 45 |
Since some neurons label with more than one of the antisera used in this study, the neuronal composition in each row adds up to greater than 100%. HSV Ag = herpes simplex virus antigen, β-gal = beta-galactosidase, d4 = 4 days.
Infected and noninfected.
Productively infected neurons; Ag+, antigen positive.
Latently infected neurons with detectable LAT promoter activity as assayed by immunofluorescence for β-Gal.
KOS-infected neuronal populations in the mouse TG.
In the course of these studies, it became clear that the number of β-Gal-positive neurons in ganglia latently infected with KOS/62, whether detected by indirect immunofluorescence or by enzymatic reaction, was far less than the number of LAT-positive neurons in ganglia latently infected by KOS as assayed by ISH. This was not surprising since we have previously reported that β-Gal expression is not stable in ganglionic neurons latently infected with KOS/62 (26). We were thus concerned that the observed difference in viral gene expression in phenotypically distinct neuronal populations was a consequence of the use of β-Gal-expressing viral vectors instead of wild-type HSV.
To address this issue, we analyzed the neuronal distribution of LAT expression in the TG of mice infected with wild-type KOS. However, this meant first developing protocols for combined IH-ISH in which the sensitivity of each component of the assay approached that of either component used alone. We found that this could be satisfactorily accomplished for all but two (RT97 and anti-b-NOS) of the neuron-specific antisera by sequential immunoperoxidase staining followed by ISH for LAT, using a digoxigenin-labeled riboprobe visualized with FITC-conjugated antidigoxigenin antiserum. Double labeling for LAT and bNOS was accomplished by substituting a histochemical stain for NADPH diaphorase in lieu of the anti-b-NOS antiserum (6). Representative examples of KOS-infected tissue dual labeled by the IH-ISH protocol are presented in Fig. 2. Immunoperoxidase staining followed by ISH for LAT with 35S-labeled riboprobe produced satisfactory results for all but three of the neuronal markers (RT97, b-NOS, and A5). With these IH-ISH protocols, the overall distribution of neuronal phenotypes in infected TG (Table 3) was similar to that observed by immunofluorescence evaluation alone (Table 1). Furthermore, as determined by assaying consecutive alternate sections of latently infected TG (21 days p.i.), the number of LAT-positive neurons detected by IH-ISH was equal to or greater than the number detected by ISH alone, using either a 35S-labeled or digoxygenin-conjugated riboprobe (data not shown). The percentage of TG neurons expressing LAT as determined by the IH-FISH protocol (3.7% of 10,869 neurons) approached that reported for LAT detection by in situ PCR as reported by Mehta et al. (29).
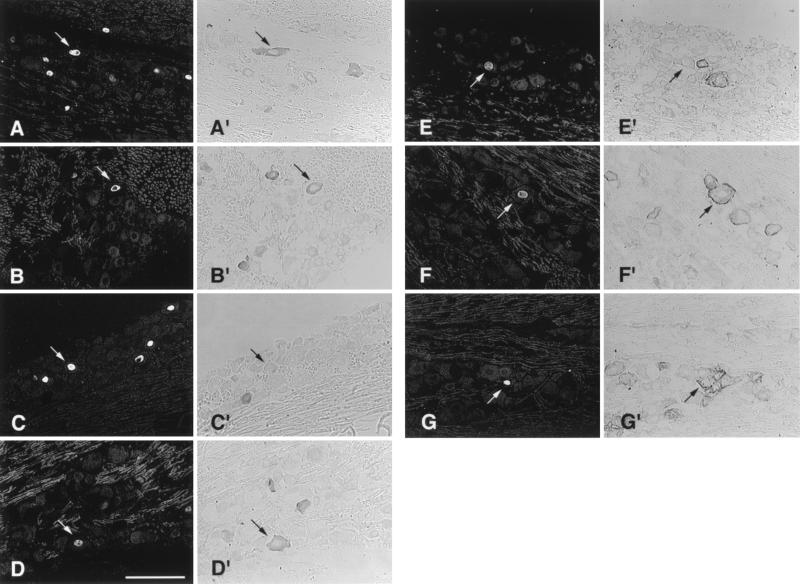
FIG. 2.
Representative examples of KOS-infected TG tissue sections, 21 days p.i., assayed by FISH for LAT (A to G) and immunoperoxidase for specific neuronal subpopulations (A′ to G′). Panels A′ to G′ are examples of neurons labeled with TrkA, CGRP, b-NOS, SP, KH10, A5, and SSEA3 antisera, respectively. Magnification bar represents 100 μm.
TABLE 3.
KOS-infected neuronal populations of the TG
| Study population (day p.i.) | No. of neurons evaluated | Neuronal composition (%)a
|
||||||
|---|---|---|---|---|---|---|---|---|
| SSEA3 | A5 | KH10 | CGRP | SP | TrkA | NADPH | ||
| All neuronsb (21) | 7,058 | 40 | 9 | 16 | 33 | 17 | 29 | 3 |
| HSV Ag+c (3) | 1,973 | 28 | 7 | 16 | 31 | 12 | 21 | 2 |
| LAT+ (21) | 2,563 | 81 | 68 | 2 | 51 | 20 | 71 | 3 |
| FISHd | ||||||||
| 35S | 1,614 | 78 | 5 | 50 | 19 | 62 | ||
Since some neurons label with more than one of the antisera used in this study, the neuronal composition in each row adds up to greater than 100%. No labeling with RT97 was detected.
Infected and noninfected.
Productively infected neurons; Ag+, antigen positive.
Latently infected neurons with LAT detectable by FISH.
As summarized in Table 3, ocular infection with KOS resulted in a pattern of HSV antigen and LAT expression in different populations of TG neurons that closely resembled that following ocular infection with KOS/62 or KOS/1. Thus, differential viral gene expression in different populations of primary sensory neurons is not unique to infection with the viral constructs KOS/62 and KOS/1; it is observed with the wild-type virus as well.
Risk of a latent infection in different neuronal populations of the mouse TG.
Although the data presented thus far provide valuable insights into which subpopulations of TG neurons are more, or less, likely to harbor latent infection with HSV, a more complete picture could be established if the relative number of TG neurons that host productive and latent patterns of HSV infection following ocular inoculation were known. To obtain an estimate of this ratio, KOS-infected TG were batched in groups of five, sectioned in a serial fashion, and assayed by either immunoperoxidase staining for HSV antigen or ISH for LAT. Light microscopic analysis of every fifth section revealed 3,484 neuronal profiles with HSV antigen expression at the peak of productive infection (3 days p.i.) and 1,201 neuronal profiles with autoradiographic signal for LAT at 21 days p.i.
Using these data, we calculated, for each population of TG neurons, the relative chance of acute HSV infection resulting in either a productive (HSV antigen-positive) or latent (LAT-positive) pattern of viral gene expression (Table 4). The results clearly demonstrate differences in the outcome of HSV infection in different neuronal populations. Most marked were the relatively high percentage of A5-immunoreactive neurons (77%) and low percentage of KH10-immunoreactive neurons (4%) that survive acute HSV infection to express LAT at 21 days p.i. Moreover, although SSEA3- and TrkA-immunoreactive neurons make up the largest percentage of LAT-positive neurons in latently infected ganglia, these data indicate that infection of A5-immunoreactive neurons was more likely to result in a latent pattern of viral gene expression.
DISCUSSION
In this study, we characterized the populations of TG neurons that become infected with HSV following peripheral inoculation. Although all neuronal subpopulations were capable of supporting a productive viral infection, some neuronal phenotypes were more permissive for establishment of a latent infection with LAT expression than others. We found this observation to be independent of (i) survival time, (ii) site of inoculation, (iii) viral LAT expression, and (iv) the use of β-Gal constructs in lieu of wild-type virus. These findings confirm and extend our earlier observations (28) on the differential regulation viral gene expression in different populations of primary sensory neurons and further support the hypothesis that the host neuron plays a key role in regulating the repertoire of HSV gene expression.
Evidence in support of host cell influence on HSV gene expression comes from a number of different laboratories and investigative approaches. Perhaps the earliest indication of the role that neurons might play a key role in the regulation of viral gene expression comes from the simple observation that HSV readily establishes a latent infection in neurons but not in other cell types. More recently it has become clear that a productive pattern of viral gene expression is critically dependent on transactivation of the viral IE genes by a complex formed between the HSV virion protein Vmw65, the cellular transcription factor Oct-1, and one or more additional host cell factors (18, 31, 32). Host cell factors may similarly act to inhibit productive infection with HSV. For example, Lillycrop et al. (22, 23) have provided evidence that two neuronal isoforms of Oct-2 (Oct 2.4 and Oct 2.5) may act as repressors of HSV IE transcription by binding to octamer-related TAATGARAT motifs in the viral IE promoters. In this way viral IE gene expression, and thus the outcome of neuronal infection with HSV, might be dictated by the balance of specific host transcription factors in a given cell. Strong evidence in support of this possibility comes from studies of transgenic mice containing the promoter regulatory region of the HSV-1 ICP4 gene coupled to the E. coli lacZ reporter gene (30). These mice exhibited neuronal β-Gal expression, indicative of ICP4 promoter activity, in the absence of HSV infection and markedly different levels of β-Gal expression in different neuronal populations.
One way in which HSV gene expression in neurons might be regulated is through the action of NGF. In this study, we found that a disproportionately large percentage of latently infected primary sensory neurons expressed TrkA and were thus capable of responding to NGF. In contrast, latent infection was rarely found in KH10-immunoreactive neurons, a population that express little or no TrkA (L. Yang et al., unpublished data). Thus, our data support the hypothesis that in vivo, the ability to respond to NGF may play some role in determining which HSV-infected neurons progress to a latent infection during primary infection. Since many of the A5- and SSEA3-immunoreactive neurons of the mouse TG coexpress TrkA (Yang et al., unpublished data), this may explain the tendency of these neurons to progress to a latent infection. The exact mechanism by which NGF exerts its influence on HSV gene expression is not clear, but the same second messengers and signal transduction pathways that are responsible for mediating the effects of NGF on neuronal gene expression appear to play some role in mediating the effects of NGF on HSV gene expression in infected neurons (12, 21, 39). However, neuronal responsiveness to NGF does not completely explain our results since (i) almost half of the HSV-infected TrkA-immunoreactive neurons exhibited a productive pattern of viral gene expression and (ii) about 30% of latently infected neurons did not express TrkA.
Another way in which neurons might be capable of regulating HSV gene expression is through the production of NO. The antimicrobial properties of NO, including its ability to inhibit replication of HSV-1 (4, 16), have been well documented (34). A subset of neurons in the TG and DRG produce NO, identifiable by immunological and histochemical staining for the enzyme NOS-1 (also known as b-NOS or n-NOS), but we found no evidence that acute HSV infection of these neurons preferentially resulted in a latent pattern of infection. This might be explained by the relatively small amount of NO produced by neurons compared to that produced by macrophages. Alternatively, since neuronal NO is largely packaged for secretion as a neurotransmitter, the antiviral action of this molecule is likely to be greatest in tissues immediately adjacent to, or innervated by, NOS-positive neurons. In this regard it was interesting that in a number of tissue sections we observed multiple NOS-positive neurons immediately adjacent to latently infected LAT-positive neurons (data not shown). Determination of any role that neuronal NO might play in the outcome of infection with HSV requires further study.
Reports from two different groups suggest that LAT deletion viruses, including KOS/62, establish a latent infection in the mouse TG less efficiently than viruses with a wild-type LAT coding region but are as efficient as the wild-type virus at establishing latent infection in the murine DRG (8, 35). This has led to the conclusion that the effect of LAT on the establishment of latency is anatomical site specific. In our studies, we found no evidence for a site-specific effect of LAT on the distribution of latently infected neuronal phenotypes in primary sensory ganglia. We obtained similar results following ocular inoculation with either KOS/62, a LAT deletion virus, or viruses with the wild-type LAT coding region, KOS and KOS/1. We also found that results for the DRG following footpad inoculation with KOS/62 were similar to those obtained following ocular inoculation. Based on these observations, we conclude that LAT plays little or no role in differential regulation of viral gene expression in different populations of primary sensory neurons during establishment of latency. Our findings are of particular interest in light of recent data indicating that a LAT-associated function reduces expression of IE viral gene expression during acute infection of neurons in vitro (24) and in vivo (13).
In the course of this study, we have relied on markers of LAT promoter activity to identify neurons latently infected with HSV. This approach seemed reasonable since for years it has been the standard used in studies of HSV latency. However, it could be argued that the higher rate of colocalization of LAT with some neuronal phenotypes reflects differential accumulation of LAT by these cells rather than their relative permissiveness to harbor a latent infection. In latently infected TG, Maggioncalda et al. (25) found two to three times as many neurons positive for HSV DNA by in situ PCR than were positive for LAT by ISH. Thompson and Sawtell (42) have similarly claimed to have found many more HSV DNA-positive neurons in latently infected TG by contextual analysis than by ISH but failed to demonstrate control ISH data for assays using the same viruses and inoculation conditions as used in this study. Although it has been assumed that differential accumulation of LAT among TG neurons is a consequence of differences in latent viral load (33), differential expression and/or degradation of LAT among different neuronal populations of the TG may also contribute to this process. There are two reasons why we do not believe that differential LAT accumulation among latently infected TG neuronal populations accounts for the higher rate of localization of LAT in TrkA-, SSEA3-, and A5-positive neurons than with other TG neuronal populations. First, despite marked cell-to-cell differences in LAT ISH signal strength in tissue sections of TG latently infected with KOS, we did not observe differences in LAT signal strength among the different neuronal phenotypes studied. Second, we obtained virtually identical colocalization results whether we identified latently infected neurons by ISH for LAT or by immunofluorescent labeling for LAT promoter-driven β-Gal expression, despite the markedly lower sensitivity of the immunofluorescence assay for identifying latently infected cells. Assuming that differential LAT accumulation among latently infected TG neuronal populations accounts for the higher rate of LAT colocalization with the TrkA-, SSEA3-, and A5-positive neurons, then we would have expected a higher rate of LAT colocalization with these neuronal phenotypes in the less sensitive assay for identifying latently infected cells.
In summary, in this study we have demonstrated that some neuronal populations of the TG are more permissive for establishment of a latent infection with LAT expression than others. Of the neuronal populations that were studied, infection of A5-immunoreactive neurons was mostly likely to result in a latent pattern of viral gene expression, whereas infection of KH10-immunoreactive neurons was most likely to result in a productive pattern of infection. The differences in the susceptibility of these two neuronal populations to productive infection with HSV were dramatic. Whereas 23 of 24 KH10-immunoreactive neurons that became infected proceeded to a productive infection, only 1 of 4 HSV-infected A5-immunoreactive neurons followed this course of viral gene expression. These findings lend further support for the hypothesis that the host neuron may play a key role in regulating the repertoire of HSV gene expression and that closer attention to differences in the transcriptional regulation among primary sensory neurons may help us understand how transcription of the HSV genome is differentially regulated in productive and latent infection. Our findings also highlight the importance of attention to the complex neuronal makeup of sensory ganglia in order to rationally plan and interpret in vivo experimental studies with HSV.
ACKNOWLEDGMENTS
We thank Aaron Ellison for helpful discussions and Anita Edgecombe for help in preparation of the manuscript. MAbs A5, KH10, SSEA3, and RT97, developed by E. Fenderson, T. M. Jessell, D. Solter, and J. N. Wood, respectively, were obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Biological Sciences, University of Iowa, Iowa City, under contract N01-HD-7-3263 from the NICHD.
This work was supported by grants NIH 10008 and NIH 02162 and by a Research to Prevent Blindness Lew Wasserman Merit Award (T.P.M.).
REFERENCES
- 1.Clary D O, Weskamp G, Austin L R, Reichardt L F. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements G B, Stow N D. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J Gen Virol. 1989;70:2501–2506. doi: 10.1099/0022-1317-70-9-2501. [DOI] [PubMed] [Google Scholar]
- 3.Coen D M, Kosz-Vnenchak M, Jacobson J G, Leib D A, Bogard C L, Schaffer P A, Tyler K L, Knipe D M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Croen K D. Evidence for an antiviral effect of nitric oxide. J Clin Investig. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham E T, Jr, DeSouza E B. Localization of type I IL-1 receptor mRNA in brain and endocrine tissues using in situ hybridization histochemistry. In: DeSouza E B, editor. Methods in neuroscience, part A. Neurobiology of cytokines. New York, N.Y: CRC Press; 1994. pp. 112–127. [Google Scholar]
- 6.Dawson T M, Bredt D S, Fotuhi M, Hwang P M, Snyder S H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci USA. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Stasio P R, Taylor M W. Specific effect of interferon on the herpes simplex virus type 1 transactivation event. J Virol. 1990;64:2588–2593. doi: 10.1128/jvi.64.6.2588-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devi-Rao G B, Bloom D C, Stevens J G, Wagner E K. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J Virol. 1994;68:1271–1282. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodd J, Jessell T M. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J Neurosci. 1985;5:3278–3294. doi: 10.1523/JNEUROSCI.05-12-03278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd J, Solter D, Jessell T M. Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurones. Nature. 1984;311:469–472. doi: 10.1038/311469a0. [DOI] [PubMed] [Google Scholar]
- 11.Farrell M J, Margolis T P, Gomes W A, Feldman L T. Effect of the transcription start region of the herpes simplex virus type 1 latency-associated transcript promoter on expression of productively infected neurons in vivo. J Virol. 1994;68:5337–5343. doi: 10.1128/jvi.68.9.5337-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frazier D A, Cox D, Godshalk E M, Schaffer P A. The herpes simplex virus type 1 latency-associated transcript promoter is activated through Ras and Raf by nerve growth factor and sodium butyrate in PC12 cells. J Virol. 1996;70:7424–7432. doi: 10.1128/jvi.70.11.7424-7432.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garber D A, Schaffer P A, Knipe D M. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J Virol. 1997;71:5885–5893. doi: 10.1128/jvi.71.8.5885-5893.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho D Y, Mocarski E S. Herpes simplex virus latent RNA (LAT) is not required for latent infection in the mouse. Proc Natl Acad Sci USA. 1989;86:7596–7600. doi: 10.1073/pnas.86.19.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Javier R T, Stevens J G, Dissette V B, Wagner E K. A herpes simplex virus transcript abundant in latently infected neurons is dispensable for establishment of the latent state. Virology. 1988;166:254–257. doi: 10.1016/0042-6822(88)90169-9. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu T, Bi Z, Reiss C S. Interferon-γ induced type 1 nitric oxide synthase activity inhibits viral replication in neurons. J Neuroimmunol. 1996;68:101–108. doi: 10.1016/0165-5728(96)00083-5. [DOI] [PubMed] [Google Scholar]
- 17.Kosz-Vnenchak M, Coen D M, Knipe D M. Restricted expression of herpes simplex virus lytic genes during establishment of latent infection by thymidine kinase-negative mutant viruses. J Virol. 1990;64:5396–5402. doi: 10.1128/jvi.64.11.5396-5402.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristie T M, LeBowitz J H, Sharp P A. The octamer-binding proteins form multi-protein-DNA complexes with the HSV αTIF regulatory protein. EMBO J. 1989;8:4229–4238. doi: 10.1002/j.1460-2075.1989.tb08608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaVail J H, Johnson W E, Spencer L C. Immunohistochemical identification of trigeminal ganglion neurons that innervate the mouse cornea: relevance to intercellular spread of herpes simplex virus. J Comp Neurol. 1993;327:133–140. doi: 10.1002/cne.903270111. [DOI] [PubMed] [Google Scholar]
- 20.Lawson S N, Harper A A, Harper E I, Garson J A, Anderton B H. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol. 1984;228:263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- 21.Leib D A, Nadeau K C, Rundle S A, Schaffer P A. The promoter of the latency-associated transcripts of herpes simplex virus type 1 contains a functional cAMP-response element: role of the latency-associated transcripts and cAMP in reactivation of viral latency. Proc Natl Acad Sci USA. 1991;88:48–52. doi: 10.1073/pnas.88.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lillycrop K A, Estridge J K, Latchman D S. The octamer binding protein Oct-2 inhibits transactivation of the herpes simplex virus immediately-early genes by the virion protein Vmw65. Virology. 1993;196:888–891. doi: 10.1006/viro.1993.1552. [DOI] [PubMed] [Google Scholar]
- 23.Lillycrop K A, Dent C L, Wheatley S C, Beech M N, Ninkina N N, Wood J N, Latchman D S. The octamer-binding protein Oct-2 represses HSV immediate-early genes in cell lines derived from latently infectable sensory neurons. Neuron. 1991;7:381–390. doi: 10.1016/0896-6273(91)90290-g. [DOI] [PubMed] [Google Scholar]
- 24.Mador N, Goldenberg D, Cohen O, Panet A, Steiner I. Herpes simplex virus type 1 latency-associated transcripts suppress viral replication and reduce immediate-early gene mRNA levels in a neuronal cell line. J Virol. 1998;72:5067–5075. doi: 10.1128/jvi.72.6.5067-5075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maggiocalda J, Mehta A, Su Y H, Fraser N W, Block T M. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology. 1996;225:72–81. doi: 10.1006/viro.1996.0576. [DOI] [PubMed] [Google Scholar]
- 26.Margolis T P, Bloom D C, Dobson A T, Feldman L T, Stevens J G. Decreased reporter gene expression during latent infection with HSV LAT promoter constructs. Virology. 1993;197:585–592. doi: 10.1006/viro.1993.1632. [DOI] [PubMed] [Google Scholar]
- 27.Margolis T P, Dawson C R, LaVail J H. Herpes simplex viral infection of the mouse trigeminal ganglion. Investig Ophthalmol Visual Sci. 1992;33:259–267. [PubMed] [Google Scholar]
- 28.Margolis T P, Sedarati F, Dobson A T, Feldman L T, Stevens J G. Pathways of viral gene expression during acute neuronal infection with HSV-1. Virology. 1992;189:150–160. doi: 10.1016/0042-6822(92)90690-q. [DOI] [PubMed] [Google Scholar]
- 29.Mehta A, Maggioncalda J, Bagasra O, Thikkavarapu S, Saikumari P, Valyi-Nagy T, Fraser N W, Block T M. In situ DNA PCR and RNA hybridization detection of herpes simplex virus sequences in trigeminal ganglia of latently infected mice. Virology. 1995;206:633–640. doi: 10.1016/s0042-6822(95)80080-8. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell W J. Neurons differentially control expression of a herpes simplex virus type 1 immediate-early promoter in transgenic mice. J Virol. 1995;69:7942–7950. doi: 10.1128/jvi.69.12.7942-7950.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Hare P, Goding C R, Haigh A. Direct combinatorial interaction between a herpes simplex virus regulatory protein and a cellular octamer-binding factor mediates specific induction of virus immediate-early gene expression. EMBO J. 1988;7:4231–4238. doi: 10.1002/j.1460-2075.1988.tb03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preston C M, Frame M C, Campbell M E M. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988;52:425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- 33.Ramakrishnan R, Poliani P L, Levine M, Glorioso J C, Fink D J. Detection of herpes simplex virus type 1 latency-associated transcript expression in trigeminal ganglia by in situ reverse transcriptase PCR. J Virol. 1996;70:6519–6523. doi: 10.1128/jvi.70.9.6519-6523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiss C S, Komatsu T. Does nitric oxide play a critical role in viral infections? J Virol. 1998;72:4547–4551. doi: 10.1128/jvi.72.6.4547-4551.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawtell N M, Thompson R L. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site-dependent establishment and reactivation from latency. J Virol. 1992;66:2157–2169. doi: 10.1128/jvi.66.4.2157-2169.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott S A. Sensory neurons: diversity, development and plasticity. New York, N.Y: Oxford University Press; 1992. [Google Scholar]
- 37.Sears A E, Halliburton I W, Meignier B, Silver S, Roizman B. Herpes simplex virus 1 mutant deleted in the α22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J Virol. 1985;55:338–346. doi: 10.1128/jvi.55.2.338-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sedarati F, Margolis T P, Stevens J G. Latent infection can be established with drastically restricted transcription and replication of the HSV-1 genome. Virology. 1993;192:687–691. doi: 10.1006/viro.1993.1089. [DOI] [PubMed] [Google Scholar]
- 39.Smith R L, Pizer L I, Johnson E M, Jr, Wilcox C L. Activation of second-messenger pathways reactivates latent herpes simplex virus in neuronal cultures. Virology. 1992;188:311–318. doi: 10.1016/0042-6822(92)90760-m. [DOI] [PubMed] [Google Scholar]
- 40.Snider W D, Wright D E. Neurotrophins cause a new sensation. Neuron. 1996;16:229–232. doi: 10.1016/s0896-6273(00)80039-2. [DOI] [PubMed] [Google Scholar]
- 41.Speck P G, Simmons A. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J Virol. 1991;65:4001–4005. doi: 10.1128/jvi.65.8.4001-4005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson R L, Sawtell N M. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997;71:5432–5440. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valyi-Nagy T, Deshmane S L, Spivack J G, Steiner I, Ace C I, Preston C M, Fraser N W. Investigation of herpes simplex virus type 1 (HSV-1) gene expression and DNA synthesis during the establishment of latent infection by an HSV-1 mutant, in 1814, that does not replicate in mouse trigeminal ganglia. J Gen Virol. 1991;72:641–649. doi: 10.1099/0022-1317-72-3-641. [DOI] [PubMed] [Google Scholar]
- 44.Wilcox C L, Smith R L, Freed C R, Johnson E M., Jr Nerve growth factor-dependence of herpes simplex virus latency in peripheral sympathetic and sensory neurons in vitro. J Neurosci. 1990;10:1268–1275. doi: 10.1523/JNEUROSCI.10-04-01268.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood J N, Lillycrop K A, Dent C L, Ninkina N N, Beech M M, Willoughby J J, Winter J, Latchman D S. Regulation of expression of the neuronal POU protein Oct-2 by nerve growth factor. J Biol Chem. 1992;267:17787–17791. [PubMed] [Google Scholar]