Abstract
Purpose
Low-dose total skin electron beam therapy (TSEBT) is a proven treatment for managing cutaneous T-cell lymphoma (CTCL) and Sezary syndrome with skin burden. We performed a retrospective comparison of response rates and time to progression for patients receiving low-dose TSEBT based on dose per fractionation, total dose, and stage.
Methods and Materials
One hundred and ten patients with CTCL and Sezary syndrome were treated with 135 courses of low-dose (400-1500 cGy) TSEBT or subtotal skin electron therapy at multiple centers of a single institution between August 2003 and June 2023. Patients were stratified according to total dose, dose per fraction, and stage.
Results
The median follow-up was 301 days (IQR, 141, 767). The median age at treatment was 69.9 years (range, 29.7-96.5). T-stage distribution was as follows: 3 (2.7%) T1, 74 (67.3%) T2, 16 (14.5%) T3, and 17 (15.5%) T4. American Joint Committee on Cancer eighth edition stage distribution was as follows: 3 (2.7%) IA, 53 (48.2%) IB, 3 (2.7%) IIA, 16 (14.5%) IIB, 8 (7.3%) IIIA, 19 (17.3%) IVA, and 8 (7.3%) IVB. There was no significant difference in disease distribution between patients treated with different fractionation schemes. The overall response rate was 89.6%. Forty-four courses (32.6%), 34 courses (25.2%), and 43 (31.9%) resulted in a complete, near-complete, and partial response, respectively. Fourteen courses (10.4%) resulted in no clinical response. For all patients, the median time to response was 43.0 days (IQR, 23.0-70). The median time to skin progression for all patients was 107.5 days (IQR, 67.8-233.5).
Conclusions
This analysis demonstrated that CTCL patients treated with low-dose radiation therapy delivered over various fractionation schemes had similar overall response rates and median time to progression.
Introduction
Cutaneous T-cell lymphoma (CTCL) is a heterogeneous group of diseases varying from indolent patches on the skin to tumors and erythroderma.1 The most common form of CTCL, mycosis fungoides, typically follows an indolent course with intermittent, stable, or slow progression of lesions. It can be associated with burdensome skin-related symptoms. Rare types of CTCLs, eg, Sezary Syndrome, can significantly affect patients' quality of life. When other skin-directed therapies have failed to provide adequate relief and disease control, radiation therapy (RT) may be considered.2 Although focal RT may treat areas of limited disease, total skin electron beam therapy (TSEBT) allows treatment of the entire skin surface.3 With TSEBT, low-energy electrons deliver radiotherapeutic doses to the entire skin surface with very shallow penetration depths, sparing deeper internal organs and tissues.
Conventional doses of TSEBT of 30 to 36 Gy delivered over 8 to 10 weeks are associated with durable cutaneous responses.4 A shortened radiation treatment regimen of 30 Gy in 20 fractions delivered over 5 weeks was effectively implemented by the United Kingdom Cutaneous Lymphoma Group.5 Modern RT for CTCLs has transitioned to using lower doses of TSEBT. Clinical trials have demonstrated that low-dose RT, consisting of 12 Gy in 8 to 12 fractions, can lead to similar rates of clinical response and times to progression compared with prolonged courses.3,6
More recently, various approaches to reduce total treatment time, including further hypofractionation or decreased total dose, have been approached in the clinical setting.7,8 Due to the COVID-19 pandemic, it has become more routine to consider hypofractionation consisting of 12 Gy over 3 fractions (one fraction delivered weekly). In this retrospective review, we sought to evaluate the response rates, time to progression, and toxicity in patients receiving low-dose total skin or subtotal skin electron beam therapy through analysis of total dose, dose per fraction, and stage.
Methods and Materials
This retrospective study was approved by the Institutional Review Board (22-004274) and was conducted per the Declaration of Helsinki of 1975 (as revised in 1985). Because of the study's retrospective nature, the requirement for informed consent was waived. A retrospective chart review was conducted of 110 consecutive patients with CTCL, treated between August 2003 and April 2023, at 2 centers of a single institution.
Radiation treatment planning and delivery
Dose, fractionation, and decision for boosts for patients planned to undergo TSEBT or subtotal skin electron beam therapy (STSEBT) were selected according to clinical factors such as disease burden, age, and performance status and logistical factors such as distance traveled and interruptions in work. Often, 3 regimens were considered: 1 Gy per fraction, delivered over 10 to 15 fractions; 1.5 Gy per fractionation, delivered over 8 fractions; and 3 to 4 Gy, delivered over 2 to 4 fractions.9 Regimens delivering 1- 1.5 Gy per fraction were delivered daily. Hypofractionated regimens were delivered once weekly, typically for 2 to 4 treatments over 2 to 4 weeks. For this study, patients were divided into 3 groups according to the low-dose TSEBT/STSEBT regimen they received: 1 Gy per fraction, >1 to 2 Gy per fraction, or >2 Gy per fraction. Patients were divided into 2 groups based on the dose received: <12 Gy or ≥12 Gy.
TSEBT was delivered from an extended source using 6MeV electrons to surface distance to patients standing in a standard TSEBT cage. TSEBT was administered using the Stanford technique, which requires a patient to stand in 6 different positions, rotating in 60° increments, to treat most of the skin surface adequately. A shielding stand was used for STSEBT, with shielding adapted to ensure shielding of uninvolved skin regions.10, 11
All 12 Stanford fields (6 dual fields) were treated per treatment session. Separate en-face electron fields or focal orthovoltage treatments were delivered for areas requiring a boost, which, depending on the patient and clinical needs, include the scalp, perineum, skin regions shielded by pannus, inframammary folds, soles of feet, and axillae.
Endpoints
Time to failure endpoints were calculated from the last day of TSEBT or STSEBT. The primary endpoint was the clinical response rate. The response was determined by the percent clearance of skin lesions after RT. Clearance of the entire skin surface (100%) constituted a complete response (CR).3 Near complete response (NCR) was defined as >95% to 99% clearance of skin lesions.3 Subtotal response was the clearance of ≥50% to 95% of skin lesions.3 Secondary endpoints included toxicity and time to progression. Progression was defined as a >25% increase in skin disease from baseline after response.6 Toxicity after treatments was defined per Common Terminology Criteria for Adverse Events, version 5.0.
Follow-up
In follow-up, patients were evaluated by their dermatologist and radiation oncologist. The first follow-up was scheduled roughly 6 to 8 weeks after the completion of radiation. Follow-up may be scheduled earlier or later according to provider concerns regarding disease control, progression, toxicity, or patient preference. Additional radiation treatment data were collected, and treatment courses were defined as total skin, subtotal skin, or focal treatment. The time to subsequent treatment was defined as the end of TSEBT or STSEBT to the start of additional radiation treatment.
Statistical analysis
Categorical variables were summarized as counts and percentages, and continuous variables were summarized as median and IQR. Baseline group characteristics were compared using χ2 or Fisher's exact if categorical, as appropriate, and analysis of variance if continuous. The cumulative incidence function was used to determine the risk of progression as a function, accounting for the competing risk of mortality. Analyses were performed using R version 4.2.2 (R Foundation for Statistical Computing) and SAS version 9.3 (SAS Institute Inc).12,13 P values were derived from 2-tailed tests. P values less than .05 were considered statistically significant.
Results
One hundred and ten patients underwent a total of 135 courses of TSEBT or STSEBT. Ten patients received prior focal electron beam radiation for areas of CTCL. There were 122 TSEBT courses and 13 STSEBT courses. Table 1 lists patient and treatment characteristics. The median age at first treatment was 69.9 years (range, 29.7-96.5). Seventy-six (69.7%) patients were male, and 33 (30.3%) were female. Most patients had an Eastern Cooperative Oncology Group performance status of 0 to 1 (94.3%). Tumor stage distribution was as follows: 3 (2.7%) T1, 74 (67.3%) T2, 16 (14.5%) T3, and 17 (15.5%) T4. American Joint Committee on Cancer eighth edition stage distribution was as follows: 3 (2.7%) IA, 53 (48.2%) IB, 3 (2.7%) IIA, 16 (14.5%) IIB, 8 (7.3%) IIIA, 19 (17.3%) IVA, and 8 (7.3%) IVB. Of the 135 cases of TSEBT or STSEBT performed, 30 (22.2%) cases were treated with 1 Gy per fraction, 49 (36.3%) with >1 to 2 Gy per fraction, and 56 (41.5%) with >2 Gy.
Table 1.
Patient demographics and initial treatment, by dose per fraction
| Characteristics | 1 Gy/fx (N = 28) | Between 1 and 2 Gy/fx (N = 41) | >2 Gy/fx (N = 41) | Total (N = 110) | P value |
|---|---|---|---|---|---|
| Sex | .472* | ||||
| Male | 22 (78.6%) | 26 (65%) | 28 (68.3%) | 76 (69.7%) | |
| Female | 6 (21.4%) | 14 (35%) | 13 (31.7%) | 33 (30.3%) | |
| Missing | 0 | 1 | 0 | 1 | |
| Race | .591* | ||||
| Asian | 0 (0%) | 0 (0%) | 2 (4.9%) | 2 (1.9%) | |
| Black or African American | 1 (3.7%) | 2 (5%) | 2 (4.9%) | 5 (4.6%) | |
| White | 25 (92.6%) | 38 (95%) | 35 (85.4%) | 98 (90.7%) | |
| Native Hawaiian or Other Pacific Islander | 1 (3.7%) | 0 (0%) | 1 (2.4%) | 2 (1.9%) | |
| Some Other Race | 0 (0%) | 0 (0%) | 1 (2.4%) | 1 (0.9%) | |
| Missing | 1 | 1 | 0 | 2 | |
| Ethnicity | .981* | ||||
| Hispanic | 1 (4.2%) | 2 (4.9%) | 2 (5.3%) | 5 (4.9%) | |
| Not Hispanic | 23 (95.8%) | 39 (95.1%) | 36 (94.7%) | 98 (95.1%) | |
| Missing | 4 | 0 | 3 | 7 | |
| Age at first treatment | .195† | ||||
| Mean (SD) | 69.4 (11.3) | 65.6 (12.6) | 71.0 (15.7) | 68.6 (13.6) | |
| Median (Q1, Q3) | 69.3 (64.6, 79.6) | 66.3 (61.4, 73.1) | 74.9 (66.2, 81.7) | 69.9 (63.5, 79.2) | |
| Range | 35.5-87.9 | 29.7-88.4 | 33.7-96.5 | 29.7-96.5 | |
| Age at diagnosis | .448† | ||||
| Mean (SD) | 67.3 (12.8) | 61.7 (14.1) | 65.2 (17.8) | 64.5 (15.6) | |
| Median (Q1, Q3) | 69.7 (58.4, 77.4) | 63.1 (56.2, 70.7) | 68.7 (56.3, 77.7) | 67.7 (56.6, 76.4) | |
| Range | 33.8-85.0 | 29.6-87.9 | 0.0-93.7 | 0.0-93.7 | |
| Missing | 8 | 12 | 2 | 22 | |
| Treatment Type | .114* | ||||
| Total Skin | 28 (100%) | 35 (85.4%) | 37 (90.2%) | 100 (90.9%) | |
| Partial | 0 (0%) | 6 (14.6%) | 4 (9.8%) | 10 (9.1%) | |
| ECOG at treatment | .373* | ||||
| 0 | 8 (30.8%) | 18 (46.2%) | 11 (26.8%) | 37 (34.9%) | |
| 1 | 17 (65.4%) | 20 (51.3%) | 26 (63.4%) | 63 (59.4%) | |
| 2 | 1 (3.8%) | 1 (2.6%) | 2 (4.9%) | 4 (3.8%) | |
| 3 | 0 (0%) | 0 (0%) | 2 (4.9%) | 2 (1.9%) | |
| Missing | 2 | 2 | 0 | 4 | |
| T-stage | .494* | ||||
| T1 | 1 (3.6%) | 1 (2.4%) | 1 (2.4%) | 3 (2.7%) | |
| T2 | 16 (57.1%) | 29 (70.7%) | 29 (70.7%) | 74 (67.3%) | |
| T3 | 3 (10.7%) | 6 (14.6%) | 7 (17.1%) | 16 (14.5%) | |
| T4 | 8 (28.6%) | 5 (12.2%) | 4 (9.8%) | 17 (15.5%) | |
| N-stage | .151* | ||||
| N0 | 27 (96.4%) | 36 (87.8%) | 41 (100%) | 104 (94.5%) | |
| N1 | 1 (3.6%) | 3 (7.3%) | 0 (0%) | 4 (3.6%) | |
| N2 | 0 (0%) | 2 (4.9%) | 0 (0%) | 2 (1.8%) | |
| M-stage | .519* | ||||
| M0 | 24 (88.9%) | 39 (95.1%) | 39 (95.1%) | 102 (93.6%) | |
| M1 | 3 (11.1%) | 2 (4.9%) | 2 (4.9%) | 7 (6.4%) | |
| Missing | 1 | 0 | 0 | 1 | |
| B-stage | .363† | ||||
| B0 | 20 (71.4%) | 32 (78%) | 35 (87.5%) | 87 (79.8%) | |
| B1 | 1 (3.6%) | 1 (2.4%) | 2 (5%) | 4 (3.7%) | |
| B2 | 7 (25%) | 8 (19.5%) | 3 (7.5%) | 18 (16.5%) | |
| Missing | 0 | 0 | 1 | 1 | |
| Stage | .190† | ||||
| IA | 1 (3.6%) | 1 (2.4%) | 1 (2.4%) | 3 (2.7%) | |
| IB | 10 (35.7%) | 17 (41.5%) | 26 (63.4%) | 53 (48.2%) | |
| IIA | 0 (0%) | 3 (7.3%) | 0 (0%) | 3 (2.7%) | |
| IIB | 3 (10.7%) | 7 (17.1%) | 6 (14.6%) | 16 (14.5%) | |
| IIIA | 4 (14.3%) | 2 (4.9%) | 2 (4.9%) | 8 (7.3%) | |
| IVA | 7 (25%) | 9 (22.0%) | 3 (7.3%) | 19 (17.3%) | |
| IVB | 3 (10.7%) | 2 (4.9%) | 3 (7.3%) | 8 (7.3%) | |
| Type of cutaneous lymphoma | .450† | ||||
| Angioimmunoblastic T cell lymphoma | 0 (0%) | 1 (2.5%) | 0 (0%) | 1 (0.9%) | |
| CD4 positive T cell lymphoma | 1 (3.6%) | 0 (0%) | 0 (0%) | 1 (0.9%) | |
| Cutaneous B cell lymphoma | 0 (0%) | 0 (0%) | 1 (2.4%) | 1 (0.9%) | |
| Mycosis fungoides | 20 (71.4%) | 29 (72.5%) | 34 (82.9%) | 83 (76.1%) | |
| Natural killer T cell lymphoma | 0 (0%) | 0 (0%) | 1 (2.4%) | 1 (0.9%) | |
| Primary cutaneous aggressive | 1 (3.6%) | 0 (0%) | 0 (0%) | 1 (0.9%) | |
| Epidermotropic CD8+ cytotoxic T-cell lymphoma | |||||
| Primary cutaneous CD30-positive | 0 (0%) | 2 (5%) | 1 (2.4%) | 3 (2.8%) | |
| Lymphoproliferative disorders (pcCD30+LPD) | |||||
| Primary cutaneous gamma/delta T-cell | 0 (0%) | 1 (2.5%) | 1 (2.4%) | 2 (1.8%) | |
| Lymphoma Sezary syndrome | 6 (21.4%) | 7 (17.5%) | 3 (7.3%) | 16 (14.7%) | |
| Missing | 0 | 1 | 0 | 1 | |
| Total follow-up (d) | < .001* | ||||
| Mean (SD) | 722.1 (717.4) | 310.6 (422.0) | 913.4 (875.4) | 640.0 (738.1) | |
| Median (Q1, Q3) | 469.0 (148.5, 1033.5) | 217.0 (112.0, 310.0) | 753.0 (158.0, 1347.0) | 301.0 (141.0, 767.0) | |
| Range | 58.0-2442.0 | 13.0-2666.0 | 33.0-4136.0 | 13.0-4136.0 | |
| Alive at last follow-up | .210† | ||||
| Yes | 18 (64.3%) | 34 (82.9%) | 31 (75.6%) | 83 (75.5%) | |
| No | 10 (35.7%) | 7 (17.1%) | 10 (24.4%) | 27 (24.5%) | |
Pearson's χ2 test.
Linear model analysis of variance.
The median total dose for patients receiving 1 Gy per fraction was 12 Gy (range, 8-15 Gy). The median total dose for patients receiving >1 to 2 Gy per fraction was 12 Gy (range, 4-14.4). The median for patients receiving >2 Gy per fraction was 12 Gy (range, 4-15).
The median time between treatment start and end was 16 days (IQR, 15-21), 10 (IQR, 9-10), and 14 (IQR, 4-21) with 1 Gy per fraction, >1 to 2 Gy per fraction, and >2 Gy per fraction, respectively. In terms of the total dose, 34 patients received <12 Gy (range, 4-11.7 Gy), and 101 patients received ≥12 Gy (range, 12-15 Gy). There was no significant difference in dose/fractionation scheme between patients with early stages I to IIA and advanced stages IIB to IV (P = .038).
Treatments and responses by dose per fraction are listed in Table 2. The overall response rate for all patients was 89.6%. There were 44 (32.6%), 34 (25.2%), and 43 (31.9%) patients who had a CR, subtotal response, or PR. There were 14 (10.4%) patients who had no response. CR, NCR, and PR occurred in 8 (26.7%), 4 (13.3%), and 16 (53.3%) patients receiving 1 Gy per fraction, respectively (Table 2). CR, NCR, and PR occurred in 12 (24.5%), 12 (24.5%), and 19 (38.8%) patients for >1 to 2 Gy per fraction, respectively. CR, NCR, and PR occurred in 24 (41.4%), 18 (32.1%), and 8 (14.3%) patients for > 2 Gy per fraction, respectively. When analyzing by total dose (Table 3), rates of CR were 38.2% and 30.7% for <12 Gy or ≥12 Gy. Rates of NCR were 20.6% and 26.7% for <12 Gy or ≥12 Gy, respectively. PR rates were 14.7% and 37.6% for <12 Gy and ≥12 Gy, respectively. More patients had no clinical response to radiation with <12 Gy (26.5% vs 5%) compared with ≥12 Gy. Regarding stages (Table 4), CR, NCR, and PR rates were 35.2%, 28.2%, and 28.2% for stages I to IIA and 29.7%, 21.9%, and 35.9% for stages IIB to IV (P = .003). There were similar rates of no clinical response between staging groups.
Table 2.
Treatments and responses by dose per fraction
| ≤1 Gy/fx | Between 1 and 2 | >2 Gy/fx | |||
|---|---|---|---|---|---|
| Treatment Details | (N = 30) | Gy/fx (N = 49) | (N = 56) | Total (N = 135) | P value |
| Treatment type | .056* | ||||
| Total skin | 30 (100.0%) | 41 (83.7%) | 51 (91.1%) | 122 (90.4%) | |
| Partial | 0 (0.0%) | 8 (16.3%) | 5 (8.9%) | 13 (9.6%) | |
| Dose per fraction (Gy) | < .001† | ||||
| Mean (SD) | 1.0 (0.0) | 1.5 (0.1) | 3.8 (0.6) | 2.4 (1.3) | |
| Median (Q1, Q3) | 1.0 (1.0, 1.0) | 1.5 (1.5, 1.5) | 4.0 (4.0, 4.0) | 1.5 (1.5, 4.0) | |
| Range | 1.0-1.0 | 1.4-2.0 | 2.2-5.0 | 1.0-5.0 | |
| Radiation dose, total (cGy) | < .001† | ||||
| Mean (SD) | 1270.0 (184.1) | 1138.0 (197.5) | 991.6 (305.0) | 1106.6 (267.1) | |
| Median (Q1, Q3) | 1200.0 (1200.0, | 1200.0 (1200.0, | 1200.0 (800.0, | 1200.0 (1185.0, | |
| 1400.0) | 1200.0) | 1200.0) | 1200.0) | ||
| Range | 800.0-1500.0 | 400.0-1440.0 | 400.0-1500.0 | 400.0-1500.0 | |
| Fractions | < .001† | ||||
| Mean (SD) | 12.7 (1.8) | 7.5 (1.5) | 2.8 (1.1) | 6.7 (4.1) | |
| Median (Q1, Q3) | 12.0 (12.0, 14.0) | 8.0 (8.0, 8.0) | 3.0 (2.0, 3.0) | 8.0 (3.0, 8.0) | |
| Range | 8.0-15.0 | 2.0-9.0 | 1.0-5.0 | 1.0-15.0 | |
| Elapsed days between start and end | < .001† | ||||
| Mean (SD) | 18.0 (6.8) | 9.8 (3.4) | 13.4 (10.0) | 13.2 (8.1) | |
| Median (Q1, Q3) | 16.0 (15.0, 20.8) | 10.0 (9.0, 10.0) | 14.0 (4.0, 21.0) | 11.0 (9.0, 18.0) | |
| Range | 6.0-43.0 | 1.0-22.0 | 0.0-42.0 | 0.0-43.0 | |
| Missing | 0 | 1 | 0 | 1 | |
| Time to response (at first evaluation) | .839† | ||||
| Mean (SD) | 57.7 (37.9) | 56.4 (68.0) | 51.6 (34.8) | 54.7 (49.0) | |
| Median (Q1, Q3) | 48.5 (36.8, 62.2) | 36.5 (15.5, 84.5) | 43.0 (24.0, 69.0) | 43.0 (23.0, 70.0) | |
| Range | 4.0-158.0 | 1.0-385.0 | 1.0-157.0 | 1.0-385.0 | |
| Missing | 2 | 9 | 7 | 18 | |
| Type of response (at first evaluation) | .009* | ||||
| Complete response | 8 (26.7%) | 12 (24.5%) | 24 (42.9%) | 44 (32.6%) | |
| Near complete response | 4 (13.3%) | 12 (24.5%) | 18 (32.1%) | 34 (25.2%) | |
| Partial response | 16 (53.3%) | 19 (38.8%) | 8 (14.3%) | 43 (31.9%) | |
| No response | 2 (6.7%) | 6 (12.2%) | 6 (10.7%) | 14 (10.4%) | |
| Time to progression | .384† | ||||
| Mean (SD) | 209.6 (183.1) | 140.3 (174.0) | 177.8 (135.4) | 173.1 (159.4) | |
| Median (Q1, Q3) | 113.0 (88.0, 355.0) | 71.5 (39.2, 192.5) | 115.0 (72.5, 252.5) | 107.5 (67.8, 233.5) | |
| Range | 43.0-648.0 | 1.0-734.0 | 42.0-545.0 | 1.0-734.0 | |
Linear model analysis of variance.
Pearson's χ2 test.
Table 3.
Treatments and responses for patients receiving < 12 Gy and ≥12 Gy
| Response Evaluation | <12 Gy (N = 34) | ≥12 Gy (N = 101) | Total (N = 135) | P value |
|---|---|---|---|---|
| Time to response (at first evaluation) | .425* | |||
| Mean (SD) | 47.7 (34.1) | 56.6 (52.3) | 54.7 (49.0) | |
| Median (Q1, Q3) | 41.0 (22.0, 67.0) | 44.5 (24.8, 77.5) | 43.0 (23.0, 70.0) | |
| Range | 12.0-157.0 | 1.0-385.0 | 1.0-385.0 | |
| Type of response (at first evaluation) | < .001† | |||
| Complete response | 13 (38.2%) | 31 (30.7%) | 44 (32.6%) | |
| Near complete response | 7 (20.6%) | 27 (26.7%) | 34 (25.2%) | |
| Partial response | 5 (14.7%) | 38 (37.6%) | 43 (31.9%) | |
| No response | 9 (26.5%) | 5 (5.0%) | 14 (10.4%) | |
| Time to progression | .218* | |||
| Mean (SD) | 137.6 (134.9) | 187.5 (167.4) | 173.1 (159.4) | |
| Median (Q1, Q3) | 73.5 (64.8, 170.8) | 123.0 (74.0, 253.8) | 107.5 (67.8, 233.5) | |
| Range | 13.0-545.0 | 1.0-734.0 | 1.0-734.0 |
Linear model analysis of variance.
Pearson's χ2 test.
Table 4.
Treatments and responses by cancer stage groupings stages I-IIA and stages IIB-IV
| Response Evaluation | Stage I-IIA (N = 71) | Stage IIB-IV (N = 64) | Total (N = 135) | P value |
|---|---|---|---|---|
| Time to response (at first evaluation) | .467* | |||
| Mean (SD) | 57.6 (40.4) | 51.0 (58.2) | 54.7 (49.0) | |
| Median (Q1, Q3) | 46.0 (31.0, 79.0) | 39.5 (18.0, 56.2) | 43.0 (23.0, 70.0) | |
| Range | 1.0-173.0 | 6.0-385.0 | 1.0-385.0 | |
| Missing | 6 | 12 | 18 | |
| Type of response (at first evaluation) | .569† | |||
| Complete response | 25 (35.2%) | 19 (29.7%) | 44 (32.6%) | |
| Near complete response | 20 (28.2%) | 14 (21.9%) | 34 (25.2%) | |
| No response | 6 (8.5%) | 8 (12.5%) | 14 (10.4%) | |
| Partial response | 20 (28.2%) | 23 (35.9%) | 43 (31.9%) | |
| Time to progression | .171* | |||
| Mean (SD) | 195.1 (168.0) | 144.4 (145.0) | 173.1 (159.4) | |
| Median (Q1, Q3) | 130.0 (87.5, 259.5) | 76.0 (58.0, 189.0) | 107.5 (67.8, 233.5) | |
| Range | 1.0-734.0 | 13.0-524.0 | 1.0-734.0 |
Linear model analysis of variance.
Pearson's χ2 test.
For all patients, the median time to response was 43 days (IQR, 23-70). The median time to response was 48.5 days (IQR, 36.8-62.2), 36.5 days (IQR, 15.5-84.5), and 43 days (IQR, 24-69) for 1 Gy per fraction, >1 to 2 Gy per fraction, and >2 Gy per fraction, respectively. The median time to response was 41 days (IQR, 22-67) for patients receiving <12 Gy and 44.5 days (IQR, 24.8-77.5) for patients receiving ≥12 Gy.
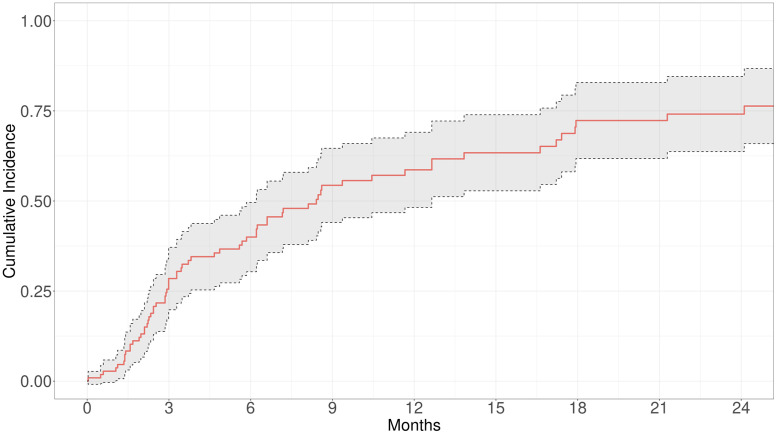
For all patients, the median time to skin progression was 107.5 days (IQR, 67.8-233.5). The median time to skin progression was 113 days (IQR, 88-355) for 1 Gy per fraction, 71.5 days (range, 39.2-192.5) for >1 to 2 Gy per fraction, and 115 days (IQR, 72.5-252.5) for >2 Gy per fraction (P = .384). The median time to progression was 73.5 days (IQR, 64.8-170.8) for patients receiving <12 Gy and 123 days (IQR, 74-253.8) for patients receiving ≥12 Gy (P = .218). For stages I to IIA and IIB to IV, the median time to progression was 130 days (IQR, 87.5-259.5) and 76 days (58-189), respectively. The cumulative incidence of progression is highlighted in Fig. 1. The cumulative incidence of skin progression requiring additional radiation treatment (TSEBT, STSEBT, or focal treatment) was 27.9% at 3 months, 39% at 6 months, and 57.6% at 1 year. There was a 50% rate of skin progression requiring additional treatment by 8.5 months. By the end of the follow-up period, 27 (24.5%) patients had died. Death was unrelated to the patient's CTCL.
Figure 1.
Cumulative incidence of progression in patients receiving various fractionation schemes of low-dose radiation.
All patients receiving TSEBT or STSEBT had low rates of physician-reported toxicities. Treatment was well tolerated. Forty-three (39.1%) patients were reported to have grade 1 toxicity, and 20 patients (18.2%) were reported to have grade 2 toxicity. Rates of grade 2 toxicity were 7 (6.4%) skin pain, 3 (2.7%) erythema, 2 (1.8%) pruritus, 3 (2.7%) lower extremity swelling, 2 (1.8%) blister formation, 7 (6.4%) alopecia, 2 (1.8%) nail ridging, and 2 (1.8%) fatigue. There were no high-grade toxicities. There were no statistically significant differences in adverse events by fractionation or total dose (P > .05).
Discussion
To our knowledge, this study adds to the literature regarding low-dose radiation total skin electron beam therapy for CTCL by evaluating the effect of dose, fractionation, and stage on time to response and time to progression. Low-dose TSEBT is routinely used to manage cutaneous t-cell lymphoma, but the optimal regimen remains to be elucidated. Over time, our clinical practice patterns have changed from 1 Gy per fraction to 1.5 Gy per fraction regimen (12 Gy in 8 fractions).3,6 Given the expected disease course of patients with CTCL, hypofractionation has been explored to provide palliation and disease control with a low toxicity profile.7 The selection of a proper dose regimen for a patient with CTCL is multifactorial. Stage and disease burden are essential clinical factors. For patients with erythroderma or very dense involve of disease, we often consider 1 Gy fractions up to 15 fractions. The first consideration for patients with limited disease burden is to use 12 Gy in 8 fractions as it is the standard.6 However, we often assess additional clinical factors such as age, performance status, and travel distance to decide final fractionation. Because of these factors and the desire to reduce the overall burden on patients, hypofractionated regimens over 3 or 4 fractions are often considered more routinely. Additionally, the COVID-19 pandemic was a driving force to consider more hypofractionated courses for this patient population.7 As we tailor our treatment to the individual needs of the patient, we have also introduced subtotal skin electron beam therapy into our practice. In our experience, we feel comfortable in offering subtotal skin electron therapy to specific patients with limited disease burden.11 Generally, patients receiving subtotal skin electron therapy receive hypofractionated regimens or 12 Gy over 8 fractions. This retrospective review compared the delivery of TSEBT among various fractionation schemes, such as 1.5 Gy fractions delivered for 8 fractions or hypofractionated regimens. The study demonstrated that the different fractionation schemes result in similar times to response and times to the subsequent treatment. However, hypofractionated regimens (>2 Gy per fraction) often lead to higher rates of CR and NCR.
Modern TSEBT delivering 30 Gy in 20 fractions over 5 weeks to the entire skin surface demonstrates overall response rates of 95% to 100%.5 Although overall response rates are high, these treatments are time-intensive and have a prolonged treatment course (5-10 weeks).5,14 Treatment with these high doses may translate into durable disease control, with times until disease progression lasting between 6 to 12 months.5,14 Multiple studies demonstrate that lower dose TSEBT (8-12 Gy) has high overall response rates (∼90%) and an acceptable side effect profile but variable times to progression or duration of clinical benefit.3,6 A 51-patient retrospective analysis from the Netherlands compared outcomes of patients receiving a conventional dose schedule of 35 Gy versus a low dose of 12 Gy, with the dose being decided upon due to overall disease burden.15 The median time to disease progression was 5.1 months for 35 Gy versus 5.3 months for 12 Gy.15 In an open clinical study, 21 patients received 10 Gy over 10 fractions (1 Gy per fraction) for various stages of mycosis fungoides/Sezary Syndrome. Ninety-five percent of patients had a complete clinical response, and the median duration of overall cutaneous response was 5.8 months.16 Compared with other skin-directed therapies recommended by the National Comprehensive Cancer Network, low-dose TSEBT therapy provides the highest efficacy and most durable response rates.17
Within the landscape of TSEBT dose/fractionation schemes, there has been a shift to shorter radiation schedules, including schedules that use modest hypofractionation (H-TSEBT). Single institution data examining H-TSEBT outcomes is notable for high overall response rates, with 31 (57.4%) courses resulting in a complete response and 23 (42.6%) resulting in a subtotal response. The median time to skin progression was 89 days.7 Another study of H-TSEBT, examining 8 Gy in 2 fractions in 6 patients, was safe, with low toxicity rates and improvement in health-related quality of life and tumor burden.18,19 We report an 89.6% overall response rate over multiple fractionation schemes delivered to 6 to 15 Gy doses. Hypofractionated doses, delivered over 2 to 4 weeks, had a median time of progression of 109 days.
The variation in times to progression or duration of clinical benefit may be attributable to several factors, including heterogeneity in clinical practice in defining or recognizing progression or clinical benefit and the unique biology underlying a patient's specific CTCL. However, despite this variation, we demonstrate that multiple low-dose TSEBT fractionation schemes are reasonable for managing CTCL.
Several weaknesses limit this retrospective study. In general, cutaneous lymphoma is a heterogeneous disease, and our study's retrospective nature does not control for variation between treatment groups. TSEBT for CTCL will be applied for patients for palliation of symptoms, such as pruritus or control of disease burden, and in combination with additional therapies such as phototherapy or other medical therapies, making interpretation of data more difficult. Timing of response to treatment is typically scheduled 6 to 8 weeks after the end of radiation. This is based on data from Hoppe et al, which demonstrated a median time to response of 7.6 weeks.3 However, follow-up may be scheduled earlier or later according to provider concerns regarding disease control, progression, toxicity, or patient preference. Follow-up could be delayed if patient traveled to receive their treatment. Because clinical response depends on the evaluation's timing, response assessment is limited in this regard.
Management of CTCL is often performed by a multidisciplinary team, including a dermatologist, hematologist/oncologist, and a radiation oncologist. When a patient has progression of disease, they may be started on new topical or systemic therapy. A limitation of this study is the lack of information regarding topical or systemic therapies in conjunction with the use of radiation therapy in management of CTCL. Therefore, the time to subsequent treatment only refers to subsequent need of radiation therapy.
Disease staging does not entirely capture the entirety of a patient's cutaneous disease burden, and measures such as the mSWAT provide a better ability to assess disease burden and response to treatment. The selection of dose regimens in this study is biased by several factors. As the proper dose regimen for CTCL remains to be elucidated, studies continue to show that low doses delivered over fewer fractions provide good palliation and disease control. Therefore, treatment decisions are affected not just by clinical factors but also by logistical ones. For instance, in patients with early-stage or limited disease burden CTCL, 12 Gy in 8 fractions or more hypofractionated regimens could be considered. When a patient has more extensive disease or erythroderma, delivering 1 Gy per fraction over 10 to 15 fractions may be more optimal to deliver the dose and keep a low toxicity profile slowly. Regardless of disease presentation, logistical factors often drive the ultimate treatment decision. For instance, a patient from out of town incurs expenses due to the cost of stay and travel. As a single institution with multiple centers seeing a high volume of patients with this rare disease entity, we are tailoring the dose regimen to meet the patient's needs.
Conclusion
Our study demonstrated a high overall response rate of TSEBT with an acceptable toxicity profile. There were no differences in response rates and median time to progression with hypofractionated regimens compared with longer fractionation schemes.
Disclosures
Aaron Mangold has received funding from Regeneron, Corbus Sun Pharma, Incyte, Pharma, Eli Lilly, Elorac, Novartis, Janssen, Soligenix, Argenx, Palvella, Abbvie, Priovant, and Merck. All other authors have no relevant conflicts of interest to disclose.
Footnotes
Sources of support: This work had no specific funding.
Research data are stored in an institutional repository and will be shared upon request.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta-Shah N, Horwitz SM, Ansell S, et al. NCCN Guidelines insights: Primary cutaneous lymphomas, version 2.2020: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2020;18:522–536. doi: 10.6004/jnccn.2020.0022. [DOI] [PubMed] [Google Scholar]
- 3.Hoppe RT, Harrison C, Tavallaee M, et al. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: Results of a pooled analysis from 3 phase II clinical trials. J Am Acad Dermatol. 2015;72:286–292. doi: 10.1016/j.jaad.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhary M, Chhabra AM, Kharod S, Marwaha G. Total skin electron beam therapy in the treatment of mycosis fungoides: A review of conventional and low-dose regimens. Clin Lymphoma Myeloma Leuk. 2016;16:662–671. doi: 10.1016/j.clml.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Morris SL, McGovern M, Bayne S, Wain M, Child F, Whittaker S. Results of a 5-week schedule of modern total skin electron beam radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:936–941. doi: 10.1016/j.ijrobp.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 6.Morris S, Scarisbrick J, Frew J, et al. The results of low-dose total skin electron beam radiation therapy (TSEB) in patients with mycosis fungoides from the UK Cutaneous Lymphoma Group. Int J Radiat Oncol Biol Phys. 2017;99:627–633. doi: 10.1016/j.ijrobp.2017.05.052. [DOI] [PubMed] [Google Scholar]
- 7.Jeans EB, Hu Y-H, Stish BJ, et al. Low-dose hypofractionated total skin electron beam therapy for adult cutaneous T-cell lymphoma. Pract Rad Oncol. 2020;10:e529–e537. doi: 10.1016/j.prro.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsayad K, Weishaupt C, Moustakis C, et al. Ultrahypofractionated low-dose total skin electron beam in advanced-stage mycosis fungoides and sézary syndrome. Int J Radiat Oncol Biol Phys. 2023;117:164–170. doi: 10.1016/j.ijrobp.2023.02.052. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe RT, Harrison C, Tavallaee M, et al. Low-dose total skin electron beam therapy as an effective modality to reduce disease burden in patients with mycosis fungoides: Results of a pooled analysis from 3 phase-II clinical trials. J Am Acad Dermatol. 2015;72:286–292. doi: 10.1016/j.jaad.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Lucido JJ, Penoncello GP, Laughlin BS, et al. Development and dosimetric characterization of a customizable shield for subtotal skin electron beam therapy. Adv Radiat Oncol. 2023;8 doi: 10.1016/j.adro.2023.101289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laughlin BS, Zaniletti I, Foster MG, et al. Tailoring Radiation Therapy for Patients With Limited Cutaneous T Cell Lymphoma: Preliminary Clinical Experience With Subtotal Skin Electron Therapy. Anticancer Res. 2024;44:1491–1497. doi: 10.21873/anticanres.16945. [DOI] [PubMed] [Google Scholar]
- 12.Team RDC . 4.0.3 ed. R Foundation for Statistical Programming; Vienna, Austria: 2010. R: A language and environment for statistical computing. [Google Scholar]
- 13.SAS Institute Inc.; Cary, NC: 2013. SAS Institute Inc. SAS 9.4. [Google Scholar]
- 14.Navi D, Riaz N, Levin YS, Sullivan NC, Kim YH, Hoppe RT. The Stanford University experience with conventional-dose, total skin electron-beam therapy in the treatment of generalized patch or plaque (T2) and tumor (T3) mycosis fungoides. Arch Dermatol. 2011;147:561–567. doi: 10.1001/archdermatol.2011.98. [DOI] [PubMed] [Google Scholar]
- 15.Smits K, Quint KD, Vermeer MH, et al. Total skin electron beam therapy for cutaneous T-cell lymphomas in the Netherlands: A retrospective analysis of treatment outcomes and selection for high or low dose schedule. Clin Transl Radiat Oncol. 2022;33:77–82. doi: 10.1016/j.ctro.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamstrup MR, Gniadecki R, Iversen L, et al. Low-dose (10-gy) total skin electron beam therapy for cutaneous T-cell lymphoma: An open clinical study and pooled data analysis. Int J Radiat Oncol Biol Phys. 2015;92:138–143. doi: 10.1016/j.ijrobp.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network. Mycosis fungoides/sezary syndrome (version 2.2022). Accessed July 16, 2023. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf
- 18.Rolf D, Elsayad K, Eich HT. Ultra-hypofractionated low-dose total skin electron beam followed by maintenance therapy: Preliminary findings from a prospective open-label study. J Am Acad Dermatol. 2021;85:1601–1603. doi: 10.1016/j.jaad.2020.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolf D, Elsayad K, Eich HT. Acute and sub-acute toxicity profile of ultra-hypofractionated low-dose total skin electron beam with two 4 Gy fractions for cutaneous T cell lymphoma. J Cancer Res Clin Oncol. 2021;147:1757–1761. doi: 10.1007/s00432-020-03449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]