Abstract
Background
Compared to distal gastrectomy (DG), pylorus-preserving gastrectomy (PPG), a peristaltic function-preserving surgery for early gastric cancer (EGC), is advantageous as it leads to a more improved nutritional status and quality of life (QOL) of patients. In recent years, total laparoscopic PPG (TLPPG), an anastomosis which is performed intracorporeally, has increasingly replaced laparoscopic-assisted PPG (LAPPG) due to its minimal invasiveness.
Aim
To evaluate the safety and feasibility of TLPPG in terms of perioperative efficacy.
Patients
Three patients underwent TLPPG in the Affiliated Hospital of Changzhi Medical College from September 2021 to March 2022.
Methods
Surgical safety analysis: Our three cases (TLPPG group) were compared to data from the CLASS-02 study, which collected data from multiple centers across China for the laparoscopic total gastrectomy (LTG group). The CLASS-02 study provides data from the most invasive type of gastric surgery, providing solid comparative data to our own.
Postoperative short-term efficacy analysis: Patient questionnaire responses provided data on postoperative nutritional and QOL status. Results from our three cases were compared to the Japanese multicenter data PGSAS-37 (PGSAS group).
Results
There were no complications or deaths occurred during or after operation in our cases. Compared to the PGSAS group, our cases scored lower for abdominal pain, dyspepsia, and weight loss.
Conclusion
Although more case information is needed, our findings demonstrate that TLPPG may be a possible and effective treatment for EGC in China, similar to that in Japan.
Key words: laparoscopic pylorus-preserving gastrectomy, post-gastrectomy syndrome, post-gastrectomy syndrome assessment scale-37, early gastric cancer
Introduction
Early gastric cancer (EGC) has been able to achieve extremely high cure rates through the use of minimally invasive (MI) and function‐preserving (FP) surgeries in east Asian countries1). Most notably, pylorus-preserving gastrectomy (PPG) aims to prevent dumping syndrome and maintains the nutritional status of patients being treated for EGC located in the middle third of the stomach1, 2). Specifically, laparoscopic assisted PPG (LAPPG) has been used in recent years to perform anastomosis intracorporeally. With current advances in technology, LAPPG has gradually been replaced by total laparoscopic PPG (TLPPG), a procedure that produces more cosmetically desirable results, less patient pain and risk of infection, and better postoperative quality of life (QOL). TLPPG has become the standard in Japan and Korea3, 4).
However, unlike Japan and Korea, China has not yet adopted TLPPG, perhaps due to the low EGC diagnosis rate and the technical complexity of the procedure. None-the-less, China still has a number of gastric cancer diagnoses every year that require optimal treatment. In 2022 alone, about 500,000 new cases presented5), of which EGC was as great as 20%6).
In order to initiate the use of TLPPG in China, it is necessary to ensure that newly learned TLPPG procedures are done properly and can yield similar results to procedures done regularly. Therefore, we performed TLPPG on three Chinese patients (TLPPG group) and quantitatively observed their postoperative conditions and QOL status to see if we could produce similar results to the CLASS-02 study7)(LTG group) in China8) the multicenter data PGSAS-37 (PGSAS group) in Japan.
Materials and methods
Patients
Three EGC patients underwent TLPPG at the Affiliated Heji Hospital of Changzhi Medical College from September 2021 to March 2022. All patients completed the PGSAS-37 questionnaire after the operation. The clinical, perioperative, pathological, and PGSAS-37 questionnaire data were retrospectively analyzed. Clinical data included time after surgery, age, gender, preoperative body mass index (BMI), pathological stage, surgical approach, extent of lymph node dissection, and combined resection. The gastric tumors were pathologically staged according to Japanese guidelines of gastric cancer treatment. The study protocol was approved by the Ethics Committee of the Affiliated Heji Hospital of Changzhi Medical College (Approval No.202005). The need for informed consent was waived in view of the retrospective and observational nature of the study.
Surgical procedure
Our TLPPG operation focused on the upper and lower incisional margins, the protection of nerves and blood vessels, and the resection and reconstruction of the stomach, which we elaborate in the following three parts:
1. At the beginning of the operation, the tumor was accurately located using laparoscopy and endoscopy. The upper and lower boundaries that were identified before operation, were marked with sutures (Figure 1).
Figure 1.
The upper and lower margins of the tumor were located by endoscopy and laparoscopy during the operation, and marked with suture.
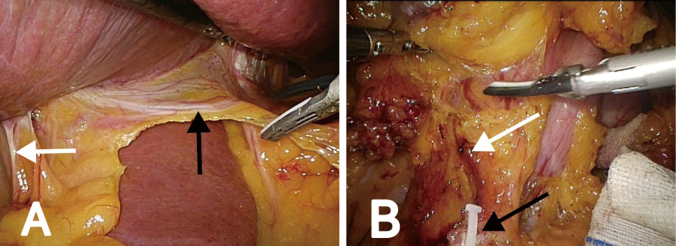
2. The hepatic branch of the vagus nerve and the pyloric branch needed to be preserved during the operation. First, the assistant held up the visceral surface of the liver, exposed the lesser omentum, and cut off the right gastric artery. The second branch of the right gastric artery was protected to preserve the blood supply to the lesser curvature of the antrum and the innervation of the pyloric branch of the vagus nerve. We cut the omentum along the right gastric vessel, hepatoduodenal ligament, and hepatic branch of the vagus nerve, and then cut off the anterior gastric branch at the distal end of the hepatic branch of the vagus nerve. In order to avoid thermal injury, the distance between the head of the ultrasonic scalpel and the vagus nerve was more than 5 mm (Figure 2A). The inferior pyloric vessels also need to be protected to preserve the blood supply to the pylorus. Along the fusion fascia, the pancreatic head, duodenal bulb, and right gastroepiploic vessels were separated and exposed layer by layer, and the No.6v and No.6a lymph nodes were cleared near the pancreas. Then, the gatrodoudenalteria was dissected by tracing the right gastroepiploic artery and the inferior pyloric artery along the GDA to identify positional relationships. Following this, the right gastroepiploic vessel was cut, and the inferior pyloric artery was retained (Figure 2B).
Figure 2.
Preservation of the vagus nerve and inferior pyloric vessels. (A) The hepatic branch of vagus nerve (black arrowhead) is located at the omentum near the liver and the pyloric branch (black arrowhead) in the middle of the hepatoduodenal ligament. (B) The severed right gastroepiploic vessels (black arrowhead) at the beginning of the vessels and the preserved inferior pyloric vessels (white arrowhead).
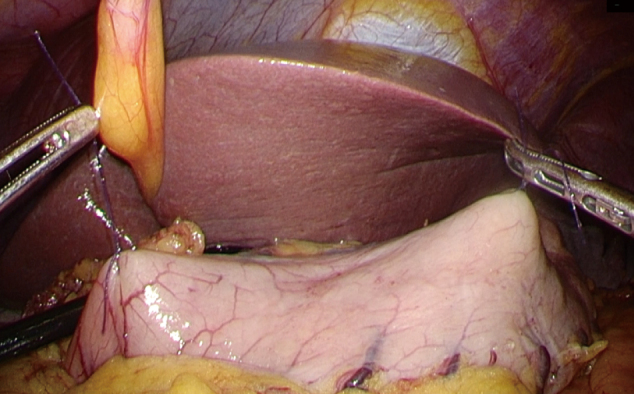
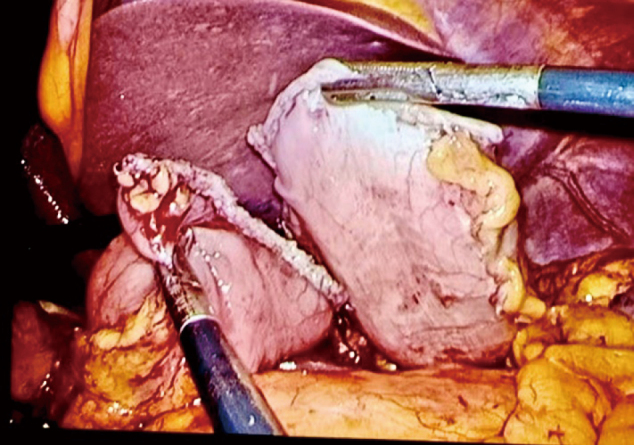
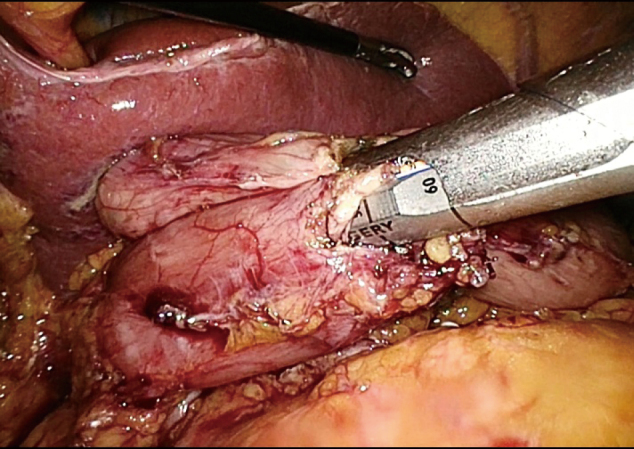
3. The middle part of the stomach was removed with two straight-line cutting closers from the upper and lower boundaries at the marked site, thus preserving the pylorus and cardia. To reduce the risk of postoperative gastric emptying disorder, a sufficient antrum length was maintained. If conditions permitted, more than 3 cm of the antrum was kept, and the specimen was transected more than 2 cm from the distal edge of the tumor. After resection, the distal and proximal ends of the stomach were obtained (Figure 3). The lateral stapler was used to anastomose the posterior wall of the proximal stomach with the anterior wall of the distal stomach (Figure 4). A 3-0 barbed thread was used to suture the common opening to achieve full-thickness suture (Figure 5).
Figure 3.
Straight-line cutting closers were used to remove the middle part of the stomach from the upper and lower edges at the marked site (A). The specimen removed through assisted small incision is shown in (B).
Figure 4.
Anastomosis was performed by an endoscopic linear stapler.
Figure 5.
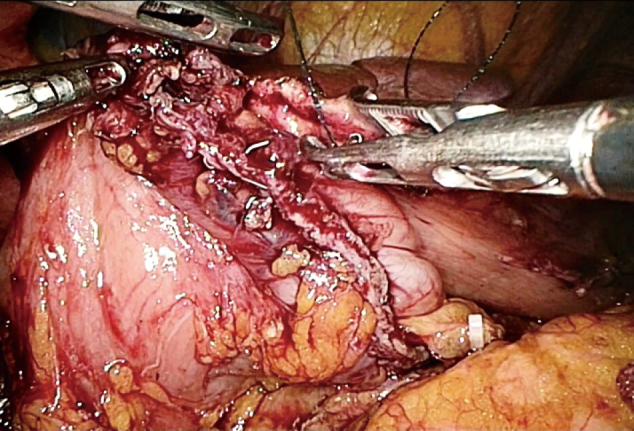
The anterior wall of the proximal and distal residual stomach was sutured and the common opening was closed.
CLASS-02 study
The Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS-02) was a prospective, multicenter, randomized clinical trial that compared the safety of laparoscopic total gastrectomy (LTG) versus open total gastrectomy (OTG) with D1+/D2 lymphadenectomy for patients with clinical stage I (T1N0M0, T1N1M0, T2N0M0) gastric cancer in the upper or middle third of the stomach. From January 2017 to September 2018, a total of 227 patients were enrolled. The primary outcome was the morbidity and mortality within 30 days following surgery. The secondary outcomes were the recovery courses and the postoperative hospital stays. We used the LTG group data of the CLASS-02 trial as the control group and compared perioperative data from the TLPPG and LTG groups.
PGS & QOL assessment
The PGSAS-379, 10) questionnaire consists of 37 questions, of which 15 are from the Gastrointestinal Symptom Assessment Scale and 22 are clinically relevant questions that have been proposed by the Japanese Postgastrectomy Syndrome Working Party (JPGSWP) (supplemental Table 1) (https://www.jsgp.jp/index.php?page=about_pgsas). These questions are summarized into nine subscales with a total of 17 primary outcomes, including assessment of esophageal reflux, abdominal pain, diet-related discomfort, dyspepsia, diarrhea, constipation, dumping syndrome, food quality, and dissatisfaction with daily life. The main findings consist of three categories: symptoms, life status, and QOL (supplemental Table 2). Higher scores for food intake per meal, daily food intake, appetite, hunger, satiety, food quality, and body weight change indicated better results, while lower scores for all other aspects indicated better results. The questionnaires were distributed by the physician at the time of the follow-up.
Supplemental Table 1.
PGSAS-37 evaluation items
| Item | Subscales | |
|---|---|---|
| Symptom | 1 Abdominal pain* | Esophageal reflux sub-scale (Items 2, 3, 5 and 16) |
| 2 Heartburn | Abdominal pain sub-scale (Items 1, 4 and 20) | |
| 3 Trans-acid | Sub-scale of diet-related irritability (Items 17-19) | |
| 4 Fasting stomachache | Sub-scale for dyspepsia (Items 6-9) | |
| 5 Nausea and vomiting | Diarrhea sub-scale (Items 11, 12 and 14) | |
| 6 Borborygmus | Constipation sub-scale (Items 10, 13 and 15) | |
| 7 Stomach distension | Dumping syndrome subscale (Items 22, 23 and 25) | |
| 8 hiccups | ||
| 9 Increased flatus | Total symptom score (over 7 subscales) | |
| 10 Constipation | ||
| 11 Diarrhea | ||
| 12 Soft stool | ||
| 13 Hard stool | ||
| 14 Urgent need to defecate | ||
| 15 Incomplete defecation | ||
| 16 Bile reflux | ||
| 17 Dysphagia | ||
| 18 Postprandial stagflation | ||
| 19 Early satiety | ||
| 20 Lower abdominal pain | ||
| 21 Number and type of early dumping syndrome | ||
| 22 Early dumping, general symptoms | ||
| 23 Early dumping, abdominal symptoms | ||
| 24 Number and type of late dumping syndrome | ||
| 25 Advanced dumping syndrome | ||
| Living status | 26 Amount of food consumed per meal | |
| 27 Daily food intake | ||
| 28 Staple food frequency | ||
| 29 Supplemental frequency | Intake quality subscale (Items 30-32) | |
| 30 Appetite | ||
| 31 Starvation | ||
| 32 Feeling of satiety | ||
| 33 The need for a meal | ||
| 34 Working capacity | ||
| QOL | 35 Symptom dissatisfaction | |
| 36 Dissatisfaction with diet | Dissatisfaction with life sub-scale (Items 35-37) | |
| 37 Dissatisfaction with one's work |
*: Abdominal pain mainly refers to stomach ache
Supplemental Table 2.
Main results in the three categories
| Category | Key findings |
|---|---|
| Symptoms | Esophageal reflux scale |
| Abdominal pain scale | |
| Sub-scale of diet-related discomfort | |
| Dyspepsia sub-scale | |
| Diarrhea subset scale | |
| Constipation subscale | |
| Dumping syndrome subscale | |
| Total symptom score | |
| Living conditions | |
| Weight | Weight change (%) |
| Diet (quantity) | Amount of food taken in per meal (%) |
| The need for extra food | |
| Diet (quality) | Intake quality sub-scale |
| Work | Service ability |
| QOL | Dissatisfaction with symptoms |
| Dissatisfaction | Dissatisfaction with one's diet |
| Dissatisfaction with one's job | |
| Dissatisfaction with daily life subscale |
The PGSAS-37 questionnaire was completed by the three TLPPG patients in our center and by 313 PPG patients from the PGSAS group. The two groups were compared in terms of PGS symptoms and QOL scores.
Results
Three cases performed successfully based on perioperative status.
We counted and described the preoperative and postoperative data of three patients, including 2 males and 1 female. The average age was 60 ± 3.6 years old, and the average BMI was 24.3 ± 1.8. The clinical stage was T1. The operation was completed under total laparoscopy, and the lymph node dissection was D1+. We followed up 3 patients after operation and filled in the PGSAS-37 questionnaire. Table 1 is the original data of 17 subscales, which provides evidence for our data comparison.
Table 1.
Original data of 17 subscales
| Case 1 | Case 2 | Case 3 | Mean | SD | ||
|---|---|---|---|---|---|---|
| Symptom | Esophageal reflux scale | 2.25 | 1.75 | 1.89 | 1.88 | 0.21 |
| Abdominal pain scale | 1.25 | 1.67 | 1.33 | 1.42 | 0.18 | |
| Diet related discomfort scale | 2.33 | 1.67 | 2.67 | 2.22 | 0.42 | |
| Dyspepsia scale | 1.67 | 1.75 | 2.00 | 1.81 | 0.14 | |
| Diarrhea scale | 2.33 | 1.00 | 2.00 | 1.78 | 0.57 | |
| Constipation scale | 1.33 | 2.33 | 2.33 | 2.00 | 0.47 | |
| Dumping syndrome scale | 2.33 | 2.00 | 1.00 | 1.78 | 0.57 | |
| Overall symptom score | 1.93 | 1.74 | 1.89 | 1.85 | 0.08 | |
| Living status | Body weight change rate (%) | -3.6% | -7.1% | -3.2% | -4.7% | 1.8% |
| Amount of food eaten at a time | 8.00 | 5.00 | 6.00 | 6.33 | 1.25 | |
| The need for extra meals | 1.00 | 2.00 | 1.00 | 1.33 | 0.47 | |
| Food intake quality scale | 3.00 | 4.67 | 4.67 | 4.11 | 0.79 | |
| service ability | 1.00 | 4.00 | 2.00 | 2.33 | 1.25 | |
| QOL | Symptom dissatisfaction | 2.00 | 1.00 | 1.00 | 1.33 | 0.47 |
| Dietary dissatisfaction | 4.00 | 1.00 | 3.00 | 2.67 | 1.25 | |
| Job dissatisfaction | 2.00 | 1.00 | 2.00 | 1.67 | 0.47 | |
| Dissatisfaction with daily life subscale | 2.67 | 1.00 | 2.00 | 1.89 | 0.68 |
The PPG group in China compared the safety of LTG and OTG for EGC using the perioperative data of 214 patients (105 cases for LTG and 109 cases for OTG). In order to demonstrate the short-term safety of TLPPG, we utilized the LTG arm of CLASS-02 trial as the control group and compared the perioperative data of the TLPPG and LTG groups. As shown in Table 2, the number of retrieved lymph nodes in the TLPPG group (17 ± 2.2) was less than that in the LTG group (35 ± 12.7). The surgery time, estimated blood loss, time to first flatus, etc. were similar between the TLPPG and LTG groups. Furthermore, no complications or deaths occurred among our patients during or after operation (Table 3). The overall complication and mortality rates were similar between the two groups. In terms of intraoperative and postoperative complications, including surgery-related and system-related complications, no difference was noted between the two groups. These results indicated that TLPPG is a safe option.
Table 2.
Surgical results & outcome
| TLPPG group (n = 3) | LTG group (n = 105) | |
|---|---|---|
| Surgical time (min) | 193 ± 12 | 230 ± 67.3 |
| Estimated blood loss (ml) | 60 ± 8 | 92 ± 109.6 |
| Time to first flatus (d) | 3.3 ± 0.5 | 3.1 ± 0.9 |
| Open conversion | 0(0%) | 2 (1.9%) |
| No. of retrieved lymph nodes | 17 ± 2.2 | 35 ± 12.7 |
| Time to ambulation (d) | 24.3 ± 1.2 | 40.6 ± 20.6 |
| Postoperative hospital stay | 10 ± 0.8 | 10.9 ± 5.1 |
Table 3.
Morbidity and mortality
| Morbidity type/mortality | TLPPG group (n = 3) | LTG group (n = 105) | ||
|---|---|---|---|---|
| No. | Rate, % (95% CI) | No. | Rate, % (95% CI) | |
| All complications | 0 | 0 | 24 | 22.9(0.15〜0.3) |
| Mortality | 0 | 0 | 1 | 1.0 (0〜2.8) |
The PGS and QOL are similar between PGSAS and TLPPG groups.
The clinicopathological features of the patients were summarized in Table 4. Because TLPPG is a less invasive procedure, the observation period after surgery was shorter than that in the PGSAS group (6.7 ± 4.1 months vs 38.4 ± 27.7 months). The preservation of the celiac branch of vagal nerve and combined organ resection in TLPPG group were less than the PGSAS group. The average age of the TLPPG group was 60.0 ± 3.6 years and that of the PGSAS group was 61.5 ± 8.7 years. There were no differences between the two groups in terms of gender, preoperative body mass index, surgical method, and lymph node dissection method.
Table 4.
Clinical and pathological characteristics results
| TLPPG group (n = 3) | PGSAS group (n = 313) | |
|---|---|---|
| Postoperative time (month) | 6.7 ± 4.1 | 38.4 ± 27.7 |
| Age | 60 ± 3.6 | 61.5 ± 8.7 |
| Gender | ||
| Male | 2 | 183 |
| Female | 1 | 126 |
| Preoperative BMI | 24.3 ± 1.8 | 22.7 ± 3.0 |
| Stages | ||
| Ⅰ | 3 | 313 |
| Ⅱ | 0 | 0 |
| Ⅲ | 0 | 0 |
| Ⅳ | 0 | 0 |
| Surgical approach | ||
| peritoneoscope | 3 | 136 |
| open abdomen | 0 | 173 |
| Degree of lymph node dissection | ||
| D1+ | 3 | 252 |
| D1 | 0 | 6 |
| D2 | 0 | 8 |
| The celiac branch reservation (absence/presence) |
||
| Absence | 1 | 213 |
| Presence | 2 | 87 |
| Combined resection (absence/presence) | ||
| absence | 1 | 12 |
| presence | 2 | 279 |
The PGSAS-37 scores for 17 symptoms were summarized in Table 5. The abdominal pain (1.42 ± 0.18 vs 1.64 ± 0.73) and dyspepsia (2.83 ± 0.47 vs 2.01 ± 0.88) scores were higher in the TLPPG group compared to the PGSAS group. Furthermore, the percentage body weight loss was lower in the TLPPG group than in the PGSAS group (4.7% vs 6.9%). There were no differences in the amount of food taken per meal, the need for additional meals, and the quality of intake between the TLPPG and PGSAS groups. Finally, the scores of QOL subscales, including dissatisfaction with symptoms, diet, work, and daily life, were also similar in both groups.
Table 5.
PGSAS-37 main symptom scores
| TLPPG group (n = 3) | PGSAS group (n = 313) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Symptom | Esophageal reflux scale | 1.88 | 0.21 | 1.70 | 0.82 |
| Abdominal pain scale | 1.42 | 0.18 | 1.64 | 0.73 | |
| Diet related discomfort scale | 2.22 | 0.42 | 2.11 | 0.87 | |
| Dyspepsia scale | 1.81 | 0.14 | 2.01 | 0.88 | |
| Diarrhea scale | 1.78 | 0.57 | 1.84 | 0.97 | |
| Constipation scale | 2.00 | 0.47 | 2.24 | 1.08 | |
| Dumping syndrome scale | 1.78 | 0.57 | 1.75 | 0.94 | |
| Overall symptom score | 2.00 | 0.14 | 1.89 | 0.67 | |
| Living status | Body weight change rate (%) | -4.7% | 1.8% | -6.9% | 7.0% |
| Amount of food eaten at a time | 6.33 | 1.25 | 7.02 | 1.87 | |
| The need for extra meals | 1.33 | 0.47 | 1.75 | 0.75 | |
| Food intake quality scale | 4.11 | 0.79 | 3.76 | 0.93 | |
| Service ability | 2.33 | 1.25 | 1.77 | 0.95 | |
| QOL | Symptom dissatisfaction | 1.33 | 0.47 | 1.80 | 0.94 |
| Dietary dissatisfaction | 2.67 | 1.25 | 2.23 | 1.11 | |
| Job dissatisfaction | 1.67 | 0.47 | 1.67 | 0.91 | |
| Dissatisfaction with daily life subscale | 1.89 | 0.68 | 1.90 | 0.83 | |
The self-nutrition indices also performed well.
We also compared the pre-operative and post-operative nutritional status of our three patients. As shown in Table 6, the BMI, albumin (ALB), and hemoglobin (HGB) level three months after operation were similar to the respective pre-operative values.
Table 6.
Self-nutrition indices results
| Preoperative | 3 months after surgery | |
|---|---|---|
| BMI | 24.3 ± 1.8 | 23.3 ± 1.1 |
| ALB(g/L) | 44.4 ± 2.8 | 45.4 ± 2.1 |
| HGB | 144.3 ± 9.0 | 142.3 ± 9.2 |
Discussion
Intracorporeal and robotic surgery for resections and anastomosis has become popular in GI surgery around the world due to recent technological advancements11). Operation with good EGC prognosis requires highly skilled MI and FP methods. However, full total operations have been increasing in Asia.
Total laparoscopic PPG (TLPPG) is one of many typical examples. This procedure prevents dumping syndrome, maintains the nutritional status, and requires a cosmetically small incision which is less painful and has a quicker recover time3). Because of these benefits, we introduced TLPPG, a technique not extensively used in China, to our clinic with the anticipation of increasing EGC diagnoses and the aim of offering better postoperative outcomes to Chinese patients with this disease.
To successfully perform the procedure and obtain ideal results, there are two major challenges that physicians must overcome. First, the operator needs to develop a scientific system for diagnosis and treatment of gastric cancer, such as preoperative positioning, intraoperative positioning, frozen section analysis of the cutting edge in the perioperative. Second, the operator needs to protect the hepatic branch and pyloric branch of the vagus nerve, as well as protect the blood vessels under the pylorus, and clean up the No.6a and No.6v lymph nodes.
Furthermore, it is worth emphasizing that: 1. A scientific diagnosis and treatment system for gastric cancer should be developed in conjunction with relevant departments, such as the Endoscopy Department, Pathology Department and other relevant disciplines. 2. The operator should be familiar with the vagus nerve and its branches. The distance between the ultrasonic scalpel head and the vagus nerve should be more than 5 mm to avoid thermal injury. 3. The operator should dissect along GDA to reveal the position relationship between the right gastroepiploic artery and the inferior pyloric artery, and then disconnect and retain these vessels.
To estimate the quality of our three TLPPG cases, we were required to measure the MI and FP. We used the CLASS-02 study (LTG) in China to estimate MI and evaluate surgical safety because LTG is the most difficult full total operation in China for EGC. There were no differences regarding blood loss, surgical time, hospital stay, and the incidence of complications compared with LTG in China. These results indicated that our initial TLPPG procedures were a safe option in our center. As for FP, we used PGSAS-37, the Japanese standard post operative score, which discusses post gastrectomy status.
Compared with other indicators, the postoperative efficacy of patients is difficult to evaluate since many symptoms cannot be quantified. In order to better quantify the postoperative state of our patients, we assessed the PGS score and QOL using the PGSAS-37 questionnaire by the Japanese national database that has been designed to evaluate functional parameters after gastrectomy. When comparing postoperative short-term efficacy, most symptom subscales and overall symptom scores were similar in both groups, with the most similar results between postoperative QOL scores. The abdominal pain, dyspepsia, and body weight loss in the PPG group yielded better results than that of the PGSAS group. Data from the PGSAS group were obtained from open and laparoscopic surgeries in multiple centers across Japan beginning in 2015.
In our center, we adopted the latest TLPPG technique, allowing us to obtain desirable patient outcomes for EGC. Abdominal pain was reduced by TLPPG since it was less invasive (Table 4), it's mainly manifested in stomach ache. The smaller incision led to less tissue damage compared to conventional laparotomy and laparoscopic-assisted surgery12), this will reduce the obstruction caused by adhesion of the stomach or intestines and reduce abdominal pains. As compared to LAPPG, the small incisions required for TLPPG remained uniform in size between patients and were independent of patient factors, leading to a potential advantage for using the technique13-15). The score of the dyspepsia subscale was also lower than that of the PGSAS group. Studies have shown that, delayed gastric emptying (DGE)16, 17) and decreased receptive relaxation function18) can cause dyspepsia. DGE is the most common and prominent complication after PPG, which may be related to factors such as blood supply to the gastric antrum19) and the retention length of gastric antrum20). According to Fukunaga19-21), preserving the infrapyloric vessel and the first branch of the right gastric vessel can greatly reduce DGE. The initial cases of PPG involved maintaining the length of the gastric antrum at 1.5 cm. With this antral length, the incidence of postoperative DGE ranged from 23% to 40%19-21). Multiple retrospective studies have shown that in order to exert the functions of the preserved gastric antrum and pylorus and to reduce DGE, the reserved length of the gastric antrum above the pyloric canal should be at least 2.5-3 cm2, 22-24). Some centers even require the preserved length of gastric antrum to be more than 4 cm25, 26).
During operation in our center, we performed nerve protection and preserved a sufficient length of gastric antrum (3-4 cm) as well as the blood vessel under the pylorus and the first branch of the right gastric vessel. Gastrointestinal contrast examination during the perioperative period and three months post-surgery showed patency, and the incidence of DGE was 0. Sufficient antrum preservation ensured a large residual gastric cavity, maximal preservation of the receptive relaxation function of the stomach, and minimal risk of dyspepsia. In addition, the rate of body weight loss was also better than the PGSAS group, primarily due to the reduction in DGE and dyspepsia, along with good nutrition absorption without obvious diet-related discomfort.
Despite observing the benefits of TLPPG in our cases, it is important to note that a weakness of this study was the extremely low number of our cases in comparison to that of previously collected data in the CLASS-02 and PGSAS-37 studies.
Conclusion
Having learned TLPPG from Professor Fukunaga, our clinic has been introduced to this innovative surgical technique and has observed its advantages. Although this method was only tested on three patients in our clinic thus far, our cases produced similar postoperative outcomes to those in Japan, suggesting that if done in larger number, more robust conclusions may be made. Never-the-less, our findings provide beginning evidence that this technique will be safe and effective for use in clinics across China.
Funding
No funding was received.
Author contributions
GW, HZP and HO designed the study; GW, HZP, MXH and WYY treated patients and collected material and clinical data from the patients; HZP, ZK and GXQ analyzed data; GW wrote the paper; All authors read and approved the final manuscript. MV and CF, native speaker, corrected English.
Conflicts of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
Not applicable.
References
- 1).Kosuga T, Tsujiura M, Nakashima S, Masuyama M, Otsuji E: Current status of function-preserving gastrectomy for gastric cancer. Ann Gastroenterol Surg, 2021; 5: 278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Oh SY, Lee HJ, Yang HK: Pylorus-Preserving Gastrectomy for Gastric Cancer. J Gastric Cancer, 2016; 16: 63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Kumagai K, Hiki N, Nunobe S, et al. : Totally laparoscopic pylorus-preserving gastrectomy for early gastric cancer in the middle stomach: technical report and surgical outcomes. Gastric Cancer, 2015; 18: 183-187. [DOI] [PubMed] [Google Scholar]
- 4).Aizawa M, Yabusaki H, Matsuki A, Bamba T, Nakagawa S: Intracorporeal hand-sewn anastomosis following pylorus-preserving gastrectomy: surgical technique and short-term surgical outcome. Langenbecks Arch Surg, 2022; 407: 1711-1720. [DOI] [PubMed] [Google Scholar]
- 5).Xia C, Dong X, Li H, et al. : Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl), 2022; 135: 584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).He J, Chen WQ, Li ZS, et al. : [China guideline for the screening, early detection and early treatment of gastric cancer (2022, Beijing)]. Zhonghua Zhong Liu Za Zhi, 2022; 44: 634-666. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 7).Liu F, Huang C, Xu Z, et al. : Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol, 2020; 6: 1590-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Yamauchi S, Orita H, Chen J, et al. : Long-term outcomes of postgastrectomy syndrome after total laparoscopic distal gastrectomy using the augmented rectangle technique. World J Gastrointest Surg, 2022; 14: 120-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Davis JL, Ripley RT: Postgastrectomy Syndromes and Nutritional Considerations Following Gastric Surgery. Surg Clin North Am, 2017; 97: 277-293. [DOI] [PubMed] [Google Scholar]
- 10).Kinami S, Takahashi M, Urushihara T, et al. : Background factors influencing postgastrectomy syndromes after various types of gastrectomy. World J Clin Cases, 2018; 6: 1111-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Lin GT, Chen JY, Chen QY, et al. : ASO Visual Abstract: Patient-Reported Outcomes of Individuals with Gastric Cancer Undergoing Totally Laparoscopic Versus Laparoscopic-Assisted Total Gastrectomy-A Real-World, Propensity Score-Matching Analysis. Ann Surg Oncol, 2023; 30: 1770-1771. [DOI] [PubMed] [Google Scholar]
- 12).Han WH, Eom BW, Yoon HM, et al. : A Comparison of Totally Laparoscopic Pylorus Preserving Gastrectomy and Laparoscopy-Assisted Pylorus Preserving Gastrectomy for Early Gastric Cancer. J Minim Invasive Surg, 2019; 22: 113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Alzahrani K, Park JH, Lee HJ, et al. : Short-term Outcomes of Pylorus-Preserving Gastrectomy for Early Gastric Cancer: Comparison Between Extracorporeal and Intracorporeal Gastrogastrostomy. J Gastric Cancer, 2022; 22: 135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Koeda K, Chiba T, Noda H, et al. : Intracorporeal reconstruction after laparoscopic pylorus-preserving gastrectomy for middle-third early gastric cancer: a hybrid technique using linear stapler and manual suturing. Langenbecks Arch Surg, 2016; 401: 397-402. [DOI] [PubMed] [Google Scholar]
- 15).Kharbutli B, Velanovich V: Gastrointestinal symptomatic outcomes of laparoscopic and open gastrectomy. World J Gastrointest Surg, 2009; 1: 56-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Yu YH, Jo Y, Jung JY, Kim BK, Seok JW: Gastric emptying in migraine: a comparison with functional dyspepsia. J Neurogastroenterol Motil, 2012; 18: 412-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Guo WJ, Yao SK, Zhang YL, et al. : Relationship between symptoms and gastric emptying of solids in functional dyspepsia. J Int Med Res, 2012; 40: 1725-1734. [DOI] [PubMed] [Google Scholar]
- 18).Hata T, Kato M, Kudo T, et al. : Comparison of gastric relaxation and sensory functions between functional dyspepsia and healthy subjects using novel drinking-ultrasonography test. Digestion, 2013; 87: 34-39. [DOI] [PubMed] [Google Scholar]
- 19).Nunobe S, Hiki N, Fukunaga T, et al. : Laparoscopy-assisted pylorus-preserving gastrectomy: preservation of vagus nerve and infrapyloric blood flow induces less stasis. World J Surg, 2007; 31: 2335-2340. [DOI] [PubMed] [Google Scholar]
- 20).Nakane Y, Michiura T, Inoue K, et al. : Length of the antral segment in pylorus-preserving gastrectomy. Br J Surg, 2002; 89: 220-224. [DOI] [PubMed] [Google Scholar]
- 21).Jiang X, Hiki N, Nunobe S, et al. : Postoperative outcomes and complications after laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Ann Surg, 2011; 253: 928-933. [DOI] [PubMed] [Google Scholar]
- 22).Kodama M, Koyama K, Chida T, Arakawa A, Tur G: Early postoperative evaluation of pylorus-preserving gastrectomy for gastric cancer. World J Surg, 1995; 19: 456-460; discussion 461. [DOI] [PubMed] [Google Scholar]
- 23).Morita S, Katai H, Saka M, et al. : Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg, 2008; 95: 1131-1135. [DOI] [PubMed] [Google Scholar]
- 24).Park DJ, Lee HJ, Jung HC, et al. : Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg, 2008; 32: 1029-1036. [DOI] [PubMed] [Google Scholar]
- 25).Imada T, Rino Y, Takahashi M, et al. : Postoperative functional evaluation of pylorus-preserving gastrectomy for early gastric cancer compared with conventional distal gastrectomy. Surgery, 1998; 123: 165-170. [PubMed] [Google Scholar]
- 26).Lee SW, Bouras G, Nomura E, et al. : Intracorporeal stapled anastomosis following laparoscopic segmental gastrectomy for gastric cancer: technical report and surgical outcomes. Surg Endosc, 2010; 24: 1774-1780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.