Abstract
We have investigated the initial steps in the interaction between infectious salmon anemia virus (ISAV) and cultured cells from Atlantic salmon (SHK-1 cell line). Using radioactively or fluorescently labelled viral particles we have studied the binding and fusion kinetics and the effect of pH on binding, uptake, and fusion of ISAV to SHK-1 cells and liposomes. As pH in the medium was reduced from 7.5 to 4.5, the association of virus to the cells was nearly doubled. The same effect of pH was observed when fusion between ISAV and liposomes was analyzed. In addition, the binding of ISAV to intact SHK-1 cells and to cell membrane proteins blotted onto filters was neuraminidase sensitive. However, the increased binding induced by low pH was not neuraminidase sensitive, probably reflecting activation of a fusion peptide at low pH. By using confocal fluorescence microscopy, the increased fusion of fluorescently labelled ISAV with the plasma membrane due to low pH could be demonstrated. When vacuolar pH in the cells was raised during inoculation with chloroquine or ammonium chloride, both electron and confocal microscopy showed accumulation of ISAV in endosomes and lysosomes. Production of infectious virus could be increased by lowering the extracellular pH during infection. Furthermore, chloroquine present during virus inoculation also caused a reduction in the synthesis of viral proteins in ISAV-infected cells as well as in the production of infective virus. These results indicate that ISAV binds to sialic acid residues on the cell surface and that the fusion between virus and cell membrane takes place in the acid environment of endosomes. This provides further evidence for a high degree of similarity between ISAV and influenza virus and extends the basis for the classification of this virus as a member of the Orthomyxoviridae family.
Infectious salmon anemia (ISA) is a viral disease causing severe problems in the salmon farming industry in Norway (46) and in other north Atlantic salmon farming districts (25). The disease is caused by an orthomyxo-like viral agent (10, 31) which has been isolated in a cell line (SHK-1) developed from Atlantic salmon head kidney (6). Infected fish are characterized by anemia, congestion of liver, spleen, and foregut, and hemorrhagic liver necrosis, and the mortality is usually high (8, 11). Genetic, morphological, and biochemical studies of the causative virus put this virus into the Orthomyxoviridae family (10, 19, 23, 31). These viruses are enveloped with a genome consisting of 8 single-stranded RNA segments with negative polarity. The envelope contains two major glycoproteins that mediate binding, fusion, and neuraminidase and acetylesterase activities. In addition the envelope contains an ion channel (M2) involved in viral uncoating and Golgi transport of newly synthesized glycoproteins (13).
Enveloped viruses enter their host cells by attachment to receptor molecules on the plasma membrane. These cellular receptors are major determinants for host range and tissue tropism of the virus. The virus may either enter the cytoplasm by fusion at the plasma membrane as do paramyxoviruses, retroviruses, and herpesviruses or, like influenza and Semliki Forest virus, be dependent on endocytosis and acidification for effective cellular entry (22). When these virus particles are internalized by endocytosis, the viral fusion proteins become activated by the low pH of the endosomes and fusion follows. The genome can then be released into the cytosol of the infected cell.
ISA virus (ISAV) has been shown to agglutinate erythrocytes from several fish species but not cells from mammals or birds (10). ISAV displays receptor-destroying activity on fish erythrocytes except on cells from Atlantic salmon. The viral enzyme cleaves acetylesterase substrates but not sialidase substrates. A similar enzymatic activity is associated with influenza C virus (17). Influenza C virus uses the same molecule as a hemagglutinin, esterase, and fusion factor for both sialic acid attachment and cleavage (14). In influenza C virus this enzymatic activity has also been demonstrated to be essential for infection (44). The biological significance of these findings is presently not clear.
Although ISAV has been classified as an orthomyxo-like virus, nothing is presently known about cellular receptors, mode of entry, and the molecules responsible for these processes. A preliminary report gave indications that propagation of ISAV in SHK-1 cells was inhibited by increased vacuolar pH (7). We have investigated the kinetics for binding and uptake of viral particles in the SHK-1 cell line and have studied the effects of various treatments on the infection process. Binding to cellular proteins has been studied by a virus overlay method. The effect of vacuolar pH on viral entry has been studied by confocal and electron microscopy, by in vivo labelling of viral proteins, and by measuring production of infectious particles.
MATERIALS AND METHODS
Chemicals.
Leibovitz's L-15 medium (L-15 medium), RPMI 1640 without l-methionine (d-RPMI), fetal bovine serum, HEPES, l-glutamine, and gentamicin were from Bio Whittaker, Wokingham, United Kingdom, and 2-mercaptoethanol was from Gibco BRL, Uxbridge, United Kingdom. Ammonium chloride, chloroquine (C 6628), bafilomycin A1 (B 8281), neuraminidase type V from Clostridium perfringens (N-2876), and dithiothreitol (D 9779) were from Sigma, St. Louis, Mo., and concanamycin A (27689) was from Fluka, Buchs, Switzerland. l-[35S]methionine (Redivue; AG1094; 370 MBq/ml; specific activity >37 TBq/mmol), Amplify (NAMP100), and Hyperfilm-MP (RPN1675) were purchased from Amersham International, Buckinghamshire, United Kingdom. ExelGel sodium dodecyl sulfate (SDS; 12.5%) and a LMW calibration kit were from Pharmacia Biotech, Uppsala, Sweden. Octadecyl rhodamine B chloride (R18) was obtained from Molecular Probes.
Cells and virus.
The SHK-1 cell line (6) was grown at 20°C in L-15 medium supplemented with fetal bovine serum (5%), l-glutamine (4 mM), gentamicin (50 μg/ml), and 2-mercaptoethanol (40 μM) (complete medium [CM]). ISAV (strain Glesvaer/2/90) had been passaged four to seven times in SHK-1 cells and was stored at −80°C until it was used. Generally inoculation of cells with virus was performed on cultures grown to 70 to 80% confluency. After removal of growth medium (CM), virus was added (multiplicity of infection, 0.1) in a small volume of binding medium (BM) (CM without serum) and allowed to absorb at 15°C for 4 h unless otherwise stated, followed by the addition of CM. Infection was allowed to proceed at 15°C until the cytopathic effect (CPE) was evident (approximately 7 days). The cell culture supernatant was cleared of cell debris by low-speed centrifugation at 4,000 × g for 20 min at 4°C. Virus was pelleted by centrifugation at 104,000 × g for 2 h at 4°C. The pelleted virus was finally resuspended in phosphate-buffered saline (PBS) and stored at −20°C.
Labeling of ISAV with 125I.
One Iodogen-coated bead, prewashed in 500 μl of PBS and dried, was added to a solution of 1 mCi of carrier-free Na125I diluted in 100 μl of PBS and allowed to react for 5 min. Approximately 100 μg of protein of pelleted ISAV dissolved in PBS was added to the reaction vessel. The reaction was allowed to proceed for 10 min before it was stopped by removal of the bead. A dialysis cassette was used to remove excess Na125I or unincorporated 125I from the iodinated ISAV.
Labeling of ISAV with R18.
The fluorescent probe R18 was incorporated into the viral bilayer by injecting 15 μl of a 1.4 mM R18 solution in ethanol under vigorous mixing into 1 ml of buffer (145 mM NaCl, 10 mM HEPES [pH 7.4]) containing 1 mg of pelleted ISAV protein (1). After incubation for 1 h in the dark at room temperature, unbound probe was removed by centrifuging 0.5 ml of the virus suspension through a small column (6 by 1 cm) containing Sephadex G-75. This dye shows strong self-quenching at high concentrations and has been used as a marker for virus-cell fusion. When the dye redistributes and is diluted into the cell membrane, an increase in fluorescence can be observed.
Fluorescence microscopy.
SHK-1 cells were grown to 80% confluence on 10-mm-diameter glass coverslips in 24-well tissue culture plates. Then, 5 to 10 μl of R18-labelled ISAV, diluted in 200 μl of serum-free L-15 medium (pH 4.5 or 7.4), was added to each well. The virus was allowed to absorb for 4 h at 4°C. The cells were then washed six times with PBS, before 500 μl of fresh medium (pH 4.5 or 7.4) was added. The cells were then chased for 4 h at 15°C before being washed once with PBS and fixed with 2% paraformaldehyde in PBS (pH 7.4) for 10 min. After fixation, the cells were washed three times in PBS, mounted, and examined in a Leica confocal microscope.
Preparation of liposomes.
Distearoylphosphatidylcholine (DSPC) and distearoyl-phosphatidylglycerol (sodium salt) (DSPG) were a gift from Nattermann Phospholipid (Cologne, Germany). The phospholipids (DSPC/DSPG ratio, 2:1 [wt/wt]) were dissolved in a mixture of chloroform and methanol (volume ratio, 2:1). Thin lipid films were formed after removal of the organic solvent by rotary evaporation, and these films were further dried by flushing with nitrogen gas for at least 15 min. Glass beads were added, and the films were hydrated above the phase transition temperature in a 20 mM citrate buffer, pH 4, a 20 mM citrate buffer, pH 5.6, or a 20 mM phosphate buffer, pH 7.4. After 2 h of hydration, the liposomes were sonicated for 15 min. Thereafter, the liposome suspensions were extruded above the phase transition temperature with a Lipex extruder (Lipex Biomembranes, Vancouver, Canada); passage three times through a two-stack 0.2-μm-thick polycarbonate membrane (Nucleopore; Costar, Cambridge, Mass.) was followed by 10 passages through a two-stack 0.1-μm-thick polycarbonate membrane. The mean diameter of the liposomes was measured by photon correlation spectroscopy (N4 MD; Coulter, Hialeah, Fla.), and the phospholipid concentration was determined according to the method of Rouser et al. (39).
Analysis of viral fusion.
Fusion between R18-labelled ISAV and target membranes was performed as described by Hoekstra et al. (18). The method relies on relief of fluorescence self-quenching of octadecyl rhodamine B chloride when the label is diluted into unlabelled membranes after fusion. This allows kinetic and quantitative analysis of the fusion process. The liposomes were diluted to a lipid concentration of 0.3 mM in the buffers mentioned in “Preparation of liposomes.” Ninety microliters was added to a cuvette containing 10 μl of octadecyl R18-labelled ISAV. The increase in rhodamine fluorescence was measured with a Perkin-Elmer (Buckinghamshire, United Kingdom) LS50B luminescence spectrometer (exitation, 560 nm; emission, 590 nm; slits, 5 nm) combined with the Time Drive application in a FL Winlab software package (Bodenseewerk Perkin-Elmer GmbH, Uberlingen, Germany).
Electron microscopy.
To visualize virus binding and internalization, SHK-1 cells were washed once with L-15 medium and allowed to adsorb ISAV at 0°C in L-15 medium, pH 7.4, for 4 h. The cells were then washed and fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4). They were further postfixed with 2% osmium tetroxide and 1.5% potassium ferrocyanide in 0.1 M cacodylate buffer (pH 7.2), followed by staining with 1% uranyl acetate and 1% tannic acid (all solutions in distilled water). Upon dehydration through increasing alcohol concentrations the specimens were embedded in Epon plastic resin. Ultrathin sections were cut and poststained with 0.2% lead citrate (35). Sections were examined in a JEOL 1200 electron microscope at 80 kV.
Immunolabeling with gold.
To detect fusion at the plasma membrane, SHK-1 cells were allowed to adsorb virus for 4 h at 0°C in binding medium (pH 7.4). The cells were then washed and incubated with biotinylated anti-ISAV monoclonal antibody (MAb) (9) diluted 1:200 in BM for 1 h at 0°C. After being washed, the samples were incubated with streptavidin-conjugated colloidal gold (10 nm) (diluted 1:50 in serum-free L-15) for 1 h at 0°C. The cells were washed, and the pH of the BM was reduced to 4.5 for 10 min at 15°C. The cells were fixed and prepared for electron microscopy as described above. The sections were observed in a Philips CM 100 electron microscope at 80 kV.
Binding of ISAV to SHK-1 cells.
SHK-1 cells were cultured to confluence in six-well tissue culture plates. The cell monolayers was washed once in 3 ml of BM before 1 ml of cold BM with 125I-ISAV was added to each well. The dishes were gently shaken for various times at 0°C. After incubation, free virus was removed and the cell monolayers were washed twice with 3 ml of cold BM. Cells were then lysed by adding 1 ml of lysis buffer (0.05 N NaOH in 1% SDS). The lysed cells were then counted in a scintillation counter (Packard Cobra) containing scintillation fluid. To measure the effect of pH on virus binding, binding media with variable pHs were prepared by adding appropriate amounts of 1 N NaOH or 1 N HCl to L-15 medium. The cells were incubated with radiolabelled ISAV for 3 h at 0°C in BM with pH ranging from 4.5 to 8.5. The cells were washed, lysed, and counted as described above. To measure the neuraminidase-resistant association of ISAV with SHK-1 cells after low-pH treatment, radioactive ISAV was allowed to bind to cells for 3 h at 0°C in pH 4.5 BM. The cells were washed free from unattached virus before being treated with neuraminidase (5 mg/ml) in L-15 medium and were further washed, lysed, and counted as described above.
Isolation of total membrane fraction from SHK-1 cells and tissue.
Membranes from SHK-1 cells were isolated from four 175-cm2 tissue culture flasks (600 ml) confluent with cells. The cells were washed three times with PBS before a total volume of 5 ml of homogenization buffer (0.25 M sucrose, 10 mM EDTA, 1 mM HEPES, pH 7.3) was added. The cells were then scraped off and homogenized with a 10-ml syringe attached to a 25-gauge cannula by passing the cell suspension up and down through the syringe approximately 15 times. The homogenate was centrifuged at 800 × g for 7 min to pellet nuclei and whole cells. The supernatant was centrifuged in an SW41 rotor for 1 h at 100,000 × g. The pellet was solubilized in 1 ml of nonreducing SDS sample buffer and analyzed for protein by the method of Lowry (26). Liver and kidney were isolated from Atlantic salmon and rainbow trout (Oncorhynchus mykiss). The organs were homogenized in homogenization buffer with a Dounce homogenizer. The plasma membrane was isolated from the homogenate, and the protein concentration was determined as described for SHK-1 cells.
PAGE, blotting, and virus overlay.
Membrane fractions were processed further for SDS-polyacrylamide gel electrophoresis (PAGE). Slab gels were blotted onto Immobilon filters with a Mini Trans-Blot apparatus (Bio-Rad). Approximately 50 μg of protein was loaded into each well on a 4 to 15% precast polyacrylamide gel. Protein blots were treated with block solution (PBS containing 0.1% Tween 80 and 5% bovine serum albumin) for 1 h at 4°C. The blots were washed three times for 15 min each with block solution and incubated with 125I-ISAV (106 cpm in 2 ml of PBS) at 4°C overnight. The blots were washed three times for 15 min each with block solution and air dried. Virus binding was detected by autoradiography with an Agfa RP1L film. Protein blots were treated with 2.5 U of neuraminidase from C. perfrigens/ml for 2 h at 37°C. Periodate oxidation was performed on protein blots in a 0.1 M sodium periodate solution for 2 h at room temperature. The blots were then blocked and incubated with ISAV as described above. Virus binding was detected by autoradiography. To investigate whether the binding of virus to sialic acid-rich glycoproteins occurred, 10 μg of bovine mucin was dot blotted onto Immobilon membranes and a virus overlay, as for the protein blots, was performed. The effect of neuraminidase treatment on protein-protein interactions on the filters was tested by Western blotting the filters with anti-rab5 (a small GTPase regulator of endosomal membrane traffic) antibodies (a kind gift from M. Zerial, EMBL, Heidelberg, Germany). rab5 antigens were visualized by enhanced chemiluminescence (Pierce).
Quantitation of infective virus.
SHK-1 cells grown in 25-cm2 tissue culture flasks (50 ml) were infected with ISAV and treated with inhibitors in the following way. Virus diluted with BM was added to monolayers in a volume of 1 ml, with or without inhibitors. After virus adsorption for 4 h the inoculate was removed and the cultures were washed twice with BM. CM with or without inhibitors was added, and the cultures were incubated for another 4-h period. The cells were washed twice before the addition of 5 ml of CM without inhibitors. In some experiments, the latter incubation period was omitted. The cell cultures were examined microscopically for CPE every day. Supernatants (1 ml) were removed at the given time points (see Fig. 8) and replaced with fresh medium. Cell debris was removed from the supernatants by centrifugation, and the samples were stored at −80°C until they were assayed for virus infectivity. The infectious virus titer was determined by end point dilution of virus supernatants on SHK-1 cells grown in 96-well tissue culture plates using 4 parallel wells per dilution. The cells were incubated at 15°C for 1 week, and infected cells were visualized by immufluorescence with a fluorescein isothiocyanate-conjugated anti-ISAV MAb as described by Falk et al. (10). The 50% tissue culture infective dose was estimated by the method of Karber (21).
FIG. 8.
Effect of pH perturbants on ISAV production in SHK-1 cells. (A) Chloroquine (10 or 100 μM) was present during inoculation (4 h) or after inoculation with virus as detailed in Materials and Methods. The infectious virus titer of the medium was determined at the indicated times after infection. TCID50, 50% tissue culture infective dose. (B) Comparison of the effect of chloroquine (100 μM) or bafilomycin A1 (1 μM) on ISAV production in SHK-1 cells. The inhibitors were present during (4 h) or after inoculation (4 h). The infectious titer of the medium was determined at day 2 p.i.
Protein synthesis in ISAV-infected cells.
SHK-1 cells grown to 70 to 80% confluency in 24-well tissue culture plates were infected with ISAV and treated with inhibitors essentially as described above, except that the medium volume during inoculation and treatment was always 1 ml. Three days postinfection (p.i.), the L-15 medium was removed and the cells were washed once with methionine-free medium (d-RPMI supplemented with 15 mM HEPES and 50 μg of gentamicin/ml). Protein labelling was performed by incubating the monolayers with 0.5 ml of d-RPMI (20 μCi/ml) for 24 h. After the cells were washed twice with PBS, the radiolabelled monolayers were dissolved in a small volume of sample buffer (50 mM Tris-HCl [pH 6.8], 1% SDS, 50 mM dithiothreitol, 8 mM EDTA, 0.01% bromophenol blue). After being heated for 5 min at 95°C, the samples received an extra dose of dithiothreitol to a final concentration of 50 mM, and SDS-PAGE was performed with 12.5% ExelGel SDS (Pharmacia). After fixation (40% ethanol and 10% acetic acid), fluorography was carried out in Amplify for 20 min. The gels were dried overnight at 25°C and exposed to Hyperfilm-MP at −80°C for 2 days.
Low-pH treatment during inoculation.
SHK-1 cells grown in 25-cm2 flasks (50 ml) were each inoculated with ISAV diluted in 1 ml of serum-free L-15 adjusted with 1 N HCl to pH 7.5, 7.0, 6.5, and 6.0. After incubation for 4 h at 15°C, the inoculates were removed and 5 ml of fully supplemented L15 medium, pH 7.5, was added to all flasks. Supernatants (1 ml) were removed at days 3 and 7 p.i., and the infectious virus titers were determined as described above.
Presentation of results.
Results from representative experiments are shown. Each experiment were repeated at least three times.
RESULTS
Binding of ISAV to SHK-1 cells at 0°C.
To examine the binding kinetics of ISAV with SHK-1 cells at 0°C, trace amounts of 125I-labelled ISAV were incubated with monolayers for various periods of time at 0°C and pH 7.4. As shown in Fig. 1A, the virus bound to cells in a time-dependent manner and a plateau was reached after approximately 4 h. To assess the effect of pH on virus binding, 125I-labelled virus was incubated with SHK-1 cells for 3 h at 0°C in medium adjusted to various pH values ranging from 4.5 to 8.5. We found that binding increased with decreasing pH (Fig. 1B). ISAV seemed to be tightly associated with cells at 0°C, as incubation with virus-free medium or PBS failed to elute the virus (not shown). When cells were allowed to bind virus at pH 7.4, neuraminidase treatment (5 mg of enzyme/ml for 90 min at 0°C) released up to 50% of the cell-associated virus within 90 min without affecting the integrity or viability of the cells (Fig. 1C). When cells were pretreated with neuraminidase before addition of virus, the same reduction in binding of ISAV was observed (not shown). However when virus was associated with the cells at an acid pH, it could not be released by neuraminidase treatment (Fig. 1C).
FIG. 1.
Interaction of ISAV with SHK-1 cells. (A) Kinetics of ISAV binding to SHK-1 cells at pH 7.4. The cells were incubated with trace quantities of 125I-labelled virus at 0°C. Cell-associated radioactivity was determined at the indicated times. Shown is a typical experiment (wells in triplicate). (B) Effect of pH on ISAV binding to SHK-1 cells at 0°C. The cells were incubated with 125I-labelled virus for 3 h at 0°C in L-15 medium adjusted to the indicated pH. After 3 h the cells were washed three times with binding medium and solubilized in 0.1 N NaOH–1% SDS and cell-associated virus radioactivity was determined. Data are expressed as percentages of control binding at pH 7.4 in each experiment. Values are the means of three different experiments +/− standard deviations (SD). (C) Effect of pH on neuraminidase-sensitive binding of ISAV to SHK-1 cells. Trace quantities of 125I-labelled ISAV were allowed to bind to SHK-1 cells for 3 h at 0°C and pH 4.5 or 7.4. After being washed, cells were treated with neuraminidase (5 mg/ml) in L-15 medium for 90 min at 0°C. The cells were washed twice with L-15 medium before cell-associated radioactivity was determined. Data are expressed as percentages of control. Values are the means of three different experiments +/− SD.
Binding of ISAV to membrane fractions.
We analyzed total membrane fractions from various sources for their abilities to bind 125I-labelled viral particles. SDS-PAGE and virus overlay of total membrane fractions from SHK-1 cells and from liver tissue from rainbow trout and Atlantic salmon were performed. Figure 2A shows that membrane proteins from both species and from SHK-1 cells were able to bind 125I-labelled ISAV under these conditions. When the protein blots were treated with 0.1 M periodate (oxidation of carbohydrates) or with neuraminidase, all bands disappeared (not shown). The effect of this treatment on the protein blot was checked by Western blotting the same samples with an antibody to a small, membrane-bound GTPase enriched in plasma membrane and endosomes, rab5 (32). When salmon liver membranes were blotted with this antibody before and after neuraminidase treatment, the rab5 signal at 26 kDa was found to be even stronger after treatment, demonstrating that the enzyme had no proteolytic effect on the filters (the 35-kDa band is nonspecific binding). We also tested the blotting of the peripheral membrane protein caveolin (or VIP21) with the same result (not shown).
FIG. 2.
Binding of 125I-labelled ISAV to membrane proteins. (A) Membrane fractions (50 μg of protein/lane) from SHK-1 cells (lanes 1 and 2), salmon liver (lanes 3 and 4), and rainbow trout liver (lanes 5 and 6) were separated by SDS-PAGE, electroblotted, blocked with 5% bovine serum albumin in PBS containing Tween 80 for 1 h, and incubated overnight with 125I-labelled virus. Binding was detected with autoradiographic film for 24 h. (B) Membrane fractions (50 μg/lane) from salmon liver electrophoresed and blotted with rab5 antibody before (control) or after neuraminidase treatment (5 mg/ml in L15 medium for 90 min) of the filter. (C) Mucin (100 μg), dissolved in 10 μl of SDS sample buffer was applied to a nitrocellulose filter and blocked for 1 h with 5% dry milk. The filter was then incubated with trace amounts of 125I-labelled ISAV overnight. After a wash, binding was detected by autoradiography.
In a similar fashion we investigated the binding of 125I-labelled ISAV to mucin blotted onto membranes. ISAV displayed strong binding to mucin (Fig. 2C), which was abolished upon periodate treatment (not shown).
pH-dependent fusion of ISAV with cell membranes.
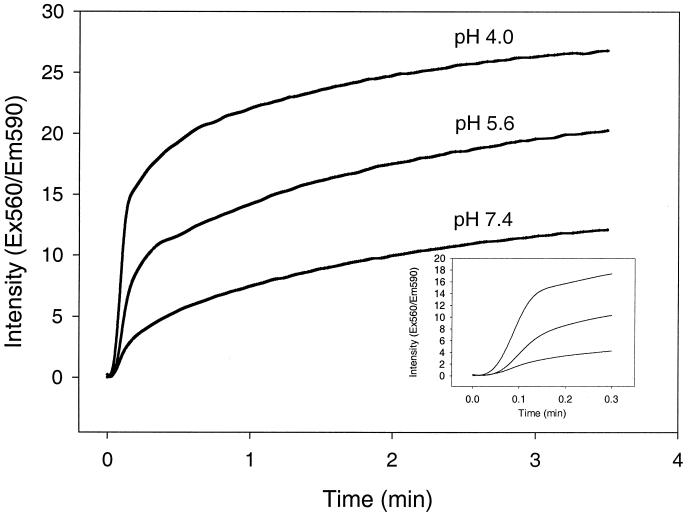
The fusogenic properties of ISAV were investigated by fluorescence microscopy with SHK-1 cells as the target membranes and quantitative spectrofluorometry with liposomes as the target membranes. R18-labeled virus was incubated with cell monolayers at a pH of either 7.4 or 4.5. Fusion was monitored as the altered distribution of R18 fluorescence in cellular membranes, as determined by confocal microscopy. Figure 3A shows confocal fluorescence micrographs of SHK-1 cells incubated with R18-labelled ISAV for 4 h before fixation. At pH 7.4, small perinuclear vesicular structures inside the cells resembling endosomes and lysosomes were labelled. When cells were incubated with virus at pH 4.5, the fluorescence showed a more uniform distribution (Fig. 3B), indicating direct fusion with the plasma membrane. When cells were allowed to internalize ISAV at pH 7.4 in the presence of the lysosomotropic drug chloroquine or ammonium chloride (Fig. 3C and D, respectively), strong vesicular staining was observed, indicating a pH-dependent accumulation of ISAV in vesicles. The fusion process was also analyzed quantitatively at various pH values by spectrofluorometry. Figure 4 shows the kinetics of fusion between R18-labelled ISAV and DSPC and DSPG (ratio, 2:1 [wt/wt]) liposomes at pH 4.0, 5.6, and 7.4. It is evident that low pH triggers a more efficient fusion process between ISAV and liposomes. When linear regression analysis was performed on the data from the initial rapid phase of the fusion process (Fig. 4, inset), it could be demonstrated that the rate of fusion increased 2.7- and 5.8-fold at pH 5.6 and 4.0, respectively, compared to the rate of fusion at pH 7.4.
FIG. 3.
Fusion of fluorescent ISAV to SHK-1 cells. SHK-1 cells were grown on glass coverslips and incubated with R18-labelled ISAV for 4 h at pH 7.5 (A) or 4.5 (B) or in the presence of 0.1 mM chloroquine (C) or 0.1 mM ammonium chloride (D). The cells were washed and fixed with 2% paraformaldehyde in PBS for 10 min and photographed with a Leica confocal fluorescence microscope.
FIG. 4.
Fusion of R18-labelled ISAV with liposomes. Liposomes were diluted to a lipid concentration of 0.3 mM in the three different buffers mentioned in “Preparation of liposomes.” The solution (90 μl) was added to a cuvette containing 10 μl (1.4 mg of viral protein/ml) of R18-labelled ISAV. The increase in rhodamine fluorescence was measured with a Perkin-Elmer LS50B luminescence spectrometer (excitation [Ex], 560 nm; emission [Em], 590 nm). Each sample was assayed three times, and the average intensity values were plotted against time.
Ultrastructural studies of ISAV entry.
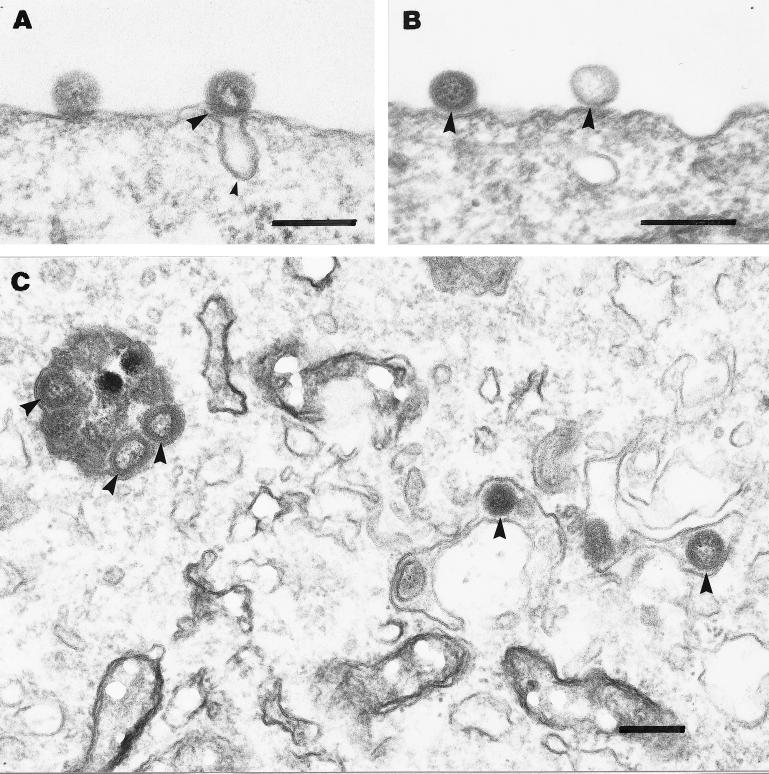
To investigate the cellular pathway of entry for ISAV, we prepared electron micrographs of cells incubated with pelleted virus for various time intervals. Figure 5A and B show the association of ISAV with the plasma membrane after 4 h of adsorption at 0°C. Virus was found to be associated either with invaginations or flat plasma membrane stretches without any visible intracellular coating. Figure 5C shows cells incubated with ISAV for 4 h at 0°C, and further chased for another 4 h at 15°C, in the presence of 0.1 mM chloroquine. Under these conditions, viral particles were found to be accumulating in endosomal vesicles. Figure 6A shows the internalization and transport to endosomes (Fig. 6B) of ISAV labelled with gold-conjugated anti-ISAV antibody. To test if ISAV is translocated from acidic organelles to the cytoplasm, we incubated SHK-1 cells with ISAV at low pH (4.5). Under these conditions, both virus-virus and virus-cell fusion could be observed (Fig. 6C). In addition, after scrutinizing a large number of cells chased for 2 h at 15°C after the initial binding at 4°C, we observed that endocytosed virus escaped from endosomal structures through fusion with the endosomal/lysosomal membrane (Fig. 7). Continuity between vesicular and viral membranes could be observed in these samples.
FIG. 5.
Electron micrographs of the early steps in ISAV infection. (A and B) Association of ISAV (big arrowheads in all panels) with the plasma membrane after 4 h of binding at 4°C. Virus was found associated with either invaginations presumably representing caveolae (small arrowhead) (A) or flat plasma membrane stretches without any visible coating (B). Further incubation of cells for 4 h with chloroquine (0.1 mM) at 15°C led to intracellular accumulation of ISAV in vesicular and tubular structures (C). Bars, 200 nm.
FIG. 6.
Effect of pH on fusion of ISAV with the plasma membrane. ISAV was bound to SHK-1 cells for 4 h at 0°C in BM (pH 7.4). The cells were then washed and incubated with biotin anti-ISAV MAb (diluted 1:200 in serum-free L-15 medium) for 1 h at 0°C. After being washed, the samples were incubated with streptavidin-conjugated colloidal gold (10 nm) (diluted 1:50 in serum-free L-15 medium) for 1 h at 0°C. The cells were then chased for 2 h before fixation. (A) Association of ISAV with an invagination of the plasma membrane of SHK-1 cells. (B) Internalized ISAV in a vesicular structure within an SHK-1 cell. (C) Samples exposed to acidic binding medium (pH 4.5) for 10 min after binding and immunolabelling. The fusion of ISAV with the plasma membrane is shown. In some cases, clear continuity between the cell and virus membrane was observed. The virus particles also seemed to aggregate and fuse with each other at the plasma membrane. Bars, 200 nm.
FIG. 7.
ISAV entry from endosomes and lysosomes. ISAV was bound to SHK-1 cells for 4 h at 0°C in BM (pH 7.4). After being washed the cells were chased for 2 h at 15°C before fixation. Shown is internalized virus fusing with the vesicular membrane. Clear continuity between the virus membrane and the vesicular membrane was observed. Bar, 200 nm.
Effect of NH4Cl, chloroquine, and bafilomycin A1 on development of CPE.
Indications of CPE (presence of vacuolated cells and some detached cells) were evident in ISAV-infected SHK-1 cells 5 to 6 days p.i. and was fully developed at day 7 (increased number of detached cells and only some cells still adherent). When cells are incubated in the presence of weak bases such as ammonium chloride and chloroquine, the pHs of intracellular organelles are elevated due to entrapment of the protonated form of the bases (the unprotonated form diffuses through the membrane). This can be used to inhibit processes in the endosomal/lysosomal system that depend on low pH (such as protein degradation and activation of fusion peptides). The presence of either NH4Cl (10 mM) or chloroquine (100 μM) during inoculation with ISAV (4 h) clearly delayed the development of CPE at least 1 to 2 days (results not shown). The antibiotic bafilomycin A1 inhibits the vacuolar ATPase and will therefore also raise the intraluminal pH of endosomes and lysosomes (5). We tested the effect of 1 μM bafilomycin A1 during inoculation and found that it had the same effect as the lysosomotrophic bases. Chloroquine and bafilomycin A seemed to have a stronger effect than NH4Cl and were therefore used in the subsequent experiments.
The presence of chloroquine and bafilomycin A1 during inoculation inhibits ISAV production.
To investigate whether the observed effects of the various inhibitors on the development of CPE in ISAV-infected cells also would result in reduced virus production, the amount of infective virus released to the medium after inoculation (4 h) of SHK-1 cells with virus in the presence or absence of inhibitors was determined. The inhibitors were also added to infected cultures for a time period of 4 h subsequent to inoculation to test the possibility that effects on virus production occurred at steps following virus entry.
Figure 8A show the time course of production of ISAV in SHK-1 cells and the effect of chloroquine (10 and 100 μM) added either together with the virus or after the inoculation period. The infectious virus titer of the medium was clearly reduced when chloroquine was present during inoculation in the highest concentration, while a negligible effect could be observed when the inhibitor was added at the end of the inoculation period. The reduction in infectious virus titer was largest at day 3 p.i. (>1.6 log10 units). The presence of 10 μM chloroquine during inoculation had no effect on virus production.
Bafilomycin A1 (1 μM) had an effect on virus production similar to that of chloroquine (100 μM), as shown in Fig. 8B. The infectious virus titer of the medium at 2 days p.i. was reduced by more than 1.2 log10 units when the inhibitors were present during inoculation.
Chloroquine inhibits synthesis of ISAV-induced proteins.
In vivo labelling of proteins of ISA virus-infected SHK-1 cells revealed the appearance of two polypeptides (approximately 70 and 25 kDa) that were not seen in noninfected cells (Fig. 9). The appearance of these polypeptides was reduced when the cells were inoculated with virus in the presence of chloroquine (100 μM), but not, or to only a minor degree, when chloroquine was added after the inoculation period. No effect of chloroquine on the protein synthesis in noninfected cells could be observed.
FIG. 9.
Effect of chloroquine on synthesis of viral polypeptides in SHK-1 cells. The polypeptides were analyzed by SDS-PAGE and autoradiography on day 4 p.i. following a 24-h incubation with [35S]methionine. Lanes 1 to 3, ISAV-infected cells; lanes 4 to 6, noninfected cells. Lanes 1 and 4, no chloroquine; lanes 2 and 5, chloroquine (100 μM) present during inoculation (4 h); lanes 3 and 6, chloroquine (100 μM) present after inoculation (4 h). Lines at the left indicate positions of molecular mass markers (70 [upper] and 25 kDa [lower]).
Low-pH treatment during inoculation enhances ISAV production.
Acidification of the medium during inoculation increased the amount of virus produced by the SHK-1 cells as shown in Table 1. At day 3 p.i., an increase in virus titer of 0.3 log10 units was observed when the inoculation pH was reduced from pH 7.5 to 7.0 or 6.5. At day 7 p.i., the highest virus titer was observed in flasks inoculated at pH 6.5, with cells showing an increase in titer of 0.7 log10 units compared to cells inoculated at pH 7.5. This difference represents a fivefold increase in virus production.
TABLE 1.
Effect of low-pH treatment during inoculation on virus productiona
| pH of inoculation medium | Virus titer (log10TCID50/ml) on day p.i.:
|
|
|---|---|---|
| 3 | 7 | |
| 7.5 | 3.6 | 5.1 |
| 7.0 | 3.9 | 5.7 |
| 6.5 | 3.9 | 5.8 |
| 6.0 | 3.2 | 5.3 |
After removal of the inoculate, fully supplemented medium of pH 7.5 was added. The infectious virus titer was measured in the supernatants at the indicated times after infection. TCID50, 50% tissue culture infective dose.
DISCUSSION
Terminal sialic acids on the cell surface, either on glycoproteins or glycolipids, have been demonstrated to be crucial for attachment to host cells for a large number of different viruses, including influenza viruses, paramyxoviruses, coronaviruses, rotaviruses, polyomaviruses, and reoviruses (24). Only for influenza viruses has this interaction been described in molecular detail, characterizing the type of sialic acids and the glycoconjugate backbone necessary for binding (28, 48, 49). Infection with ISAV causes development of disease in Atlantic salmon and replication without causing disease in other salmonid species (33, 34), but nothing is known about cellular receptors or the mode of entry for this virus. However, both genetic (23, 31) and biochemical (10) studies of this virus place it as an orthomyxo-like virus.
In this report we demonstrate neuraminidase-sensitive binding of labelled ISAV to both intact cells and membrane proteins from SHK-1 cells and liver. Liver is strongly affected during ISA and may therefore contain viral receptors (8). As has been demonstrated for influenza virus (15, 16, 27, 29, 45), prebound ISAV could be released from cells treated with neuraminidase. The percentage of bound virus released from the cells was smaller than those that have been observed for influenza virus when the same amount of enzyme was used. In the virus overlay assay, the binding of ISAV was completely abolished. This may be due to additional binding sites on the cells not displayed on the filter assay (e.g., glycolipids) with different sensitivity to neuraminidase or to a higher degree of nonspecific binding when intact cells are used. Another explanation for the reduced removal by neuraminidase may be that virus had been internalized. Cells that were fixed and observed by electron microscopy after 4 h of binding at 0°C contained internalized virus particles (not shown). In a previous study, we have demonstrated the endocytosis of fluid phase markers such as horseradish peroxidase at low temperature in SHK-1 cells (38). Agglutination assays with ISAV and erythrocytes from different species indicate that the receptor determinant is different from that used by influenza A, B, or C virus (10). A large structural diversity in sialoglycoproteins has been described previously (37), and certain types of sialoconjugates have only been found in salmonid species (20).
When influenza virus hemagglutinin is exposed to the low pH of endosomes, an apolar fusion peptide is exposed on the surface of the hemagglutinin molecule (4). This apolar peptide is responsible for intercalation into the lipid membrane to initiate fusion (41). Previous studies have also shown that a productive infection by influenza virus also can be initiated at the plasma membrane at low pH (29). When ISAV was incubated with cells at reduced pH, binding to the cells increased with reduced pH. This increase may also be due to virus aggregation (as could be observed by electron microscopy; Fig. 6) and therefore represents not only increased association with the cells. As the association at low pH also became insensitive to neuraminidase treatment, this also indicates that the binding is different from that observed at neutral pH. This was further corroborated by the experiments with R18-labelled ISAV. At low pH, lipid dye was redistributed from the virus into the plasma membrane directly upon binding at low temperature. This was not observed at physiological pH. The increased staining in cells treated with lysosomotropic drugs may be due to both impaired transport out of the endosomal compartment (2) and the increase in the endosomal volume upon alkalinization (30). The enlarged endosomal compartment would therefore contain a much larger number of fluorescent viral particles than those of control cells. This method has also been used to quantitatively analyze fusion between virus and cells at various pHs (3). In a quantitative fusion assay of R18-labelled ISAV and liposomes, it could also be demonstrated that fusion could be accelerated by lowering the pH. The initial rate of fusion increased severalfold upon lowering the pH from 7.4 to 4.0. This rate of fusion is directly comparable to what has been demonstrated for the fusion of influenza virus to liposomes (43, 47). At 37°C, this process is completed within 2 min (42).
A fourth line of evidence for a low-pH-dependent step in ISAV infection was that low pH not only increases association and fusion but also increases the production of infective virus. When cells were inoculated at pH 6.5, up to five times more virus was produced at 7 days p.i. than was produced at pH 7.5. However, if ISAV is exposed to low pH for a prolonged time (pH 4, 30 min) a loss of infectivity is observed (10). This may be due to irreversible conformational changes in the hemagglutinin molecule, as have been described for influenza virus (40), which reduce infectivity (12).
Our studies support a role for endosomes and lysosomes in the mode of entry for ISAV. First, R18-labelled ISAV clearly accumulated in perinuclear vesicles when the cells were treated with cloroquine or ammonium chloride during inoculation with virus. Furthermore, when treated cells were observed with the electron microscope, the accumulation of viral particles in endosome-like structures could be observed. Interestingly, at low pH both virus-cell fusion and virus-virus fusion could be observed in the same samples but not in untreated cells. Similar phenomena have been observed with influenza virus (29). Vacuolar pH perturbants, such as bafilomycin A1 and chloroquine, present during inoculation not only increased the number of visible viral particles in the cell but also reduced the synthesis of both viral proteins and infective particles notably. When these perturbants were applied after inoculation, they had no effect, as has been demonstrated for other orthomyxoviruses (36, 50).
Our data therefore support the following route of entry for ISAV into SHK cells: (i) binding of viral particles to neuraminidase-sensitive determinants on cell surface glycoproteins or glycolipids; (ii) internalization of ISAV and transport to endosomes and lysosomes; (iii) low-pH-dependent fusion with endosomal/lysosomal membranes. We are not proceeding with more-detailed molecular descriptions of the components involved in this pathway.
ACKNOWLEDGMENT
This study was supported by the Norwegian Research Council.
REFERENCES
- 1.Bagai S, Lamb R A. Quantitative measurement of paramyxovirus fusion: differences in requirements of glycoproteins between simian virus 5 and human parainfluenza virus 3 or Newcastle disease virus. J Virol. 1995;69:6712–6719. doi: 10.1128/jvi.69.11.6712-6719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg T, Tolleshaug H. The effects of ammonium ions and chloroquine on uptake and degradation of 125I-labeled asialo-fetuin in isolated rat hepatocytes. Biochem Pharmacol. 1980;29:917–925. doi: 10.1016/0006-2952(80)90222-1. [DOI] [PubMed] [Google Scholar]
- 3.Blumenthal R, Bali-Puri A, Walter A, Covell D, Eidelman O. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J Biol Chem. 1987;262:13614–13619. . (Erratum, 263:588, 1988.) [PubMed] [Google Scholar]
- 4.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 5.Crider B P, Xie X S, Stone D K. Bafilomycin inhibits proton flow through the H+ channel of vacuolar proton pumps. J Biol Chem. 1994;269:17379–17381. [PubMed] [Google Scholar]
- 6.Dannevig B H, Falk K, Namork E. Isolation of the causal virus of infectious salmon anaemia (ISA) in a long-term cell line from Atlantic salmon head kidney. J Gen Virol. 1995;76:1353–1359. doi: 10.1099/0022-1317-76-6-1353. [DOI] [PubMed] [Google Scholar]
- 7.Dannevig B H, Falk K, Press C M. Propagation of infectious salmon anaemia (ISA) virus in cell culture. Vet Res. 1995;26:438–442. [PubMed] [Google Scholar]
- 8.Evensen O, Thorud K E. A morphological study of the gross and light microscopic lesions of infectious anaemia in Atlantic salmon (Salmo salar) Res Vet Sci. 1991;51:215–222. doi: 10.1016/0034-5288(91)90017-i. [DOI] [PubMed] [Google Scholar]
- 9.Falk K, Namork E, Dannevig B H. Characterization and applications of a monoclonal antibody against infectious salmon anaemia virus. Dis Aquat Organisms. 1998;34:77–85. doi: 10.3354/dao034077. [DOI] [PubMed] [Google Scholar]
- 10.Falk K, Namork E, Rimstad E, Mjaaland S, Dannevig B H. Characterization of infectious salmon anemia virus, an orthomyxo-like virus isolated from Atlantic salmon (Salmo salar L.) J Virol. 1997;71:9016–9023. doi: 10.1128/jvi.71.12.9016-9023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falk K, Press C M, Landsverk T, Dannevig B H. Spleen and kidney of Atlantic salmon (Salmo salar L.) show histochemical changes early in the course of experimentally induced infectious salmon anaemia (ISA) Vet Immunol Immunopathol. 1995;49:115–126. doi: 10.1016/0165-2427(95)05427-8. [DOI] [PubMed] [Google Scholar]
- 12.Guinea R, Carrasco L. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J Virol. 1995;69:2306–2312. doi: 10.1128/jvi.69.4.2306-2312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henkel J R, Apodaca G, Altschuler Y, Hardy S, Weisz O A. Selective perturbation of apical membrane traffic by expression of influenza M2, an acid-activated ion channel, in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1998;9:2477–2490. doi: 10.1091/mbc.9.9.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrler G, Durkop I, Becht H, Klenk H D. The glycoprotein of influenza C virus is the haemagglutinin, esterase and fusion factor. J Gen Virol. 1988;69:839–846. doi: 10.1099/0022-1317-69-4-839. [DOI] [PubMed] [Google Scholar]
- 15.Herrler G, Gross H J, Milks G, Paulson J C, Klenk H D, Brossmer R. Use of a sialic acid analogue to analyze the importance of the receptor-destroying enzyme for the interaction of influenza C virus with cells. Acta Histochem Suppl. 1990;40:39–41. [PubMed] [Google Scholar]
- 16.Herrler G, Rott R, Klenk H D. Neuraminic acid is involved in the binding of influenza C virus to erythrocytes. Virology. 1985;141:144–147. doi: 10.1016/0042-6822(85)90190-4. [DOI] [PubMed] [Google Scholar]
- 17.Herrler G, Rott R, Klenk H D, Muller H P, Shukla A K, Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO J. 1985;4:1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoekstra D, de Boer T, Klappe K, Wilschut J. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984;23:5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- 19.Hovland T, Watanabe K, Endresen K. Observation of infectious salmon anemia virus in Atlantic salmon, Salmo salar L. J Fish Dis. 1994;17:291–296. [Google Scholar]
- 20.Iwasaki M, Inoue S, Troy F A. A new sialic acid analogue, 9-O-acetyl-deaminated neuraminic acid, and alpha-2,8-linked O-acetylated poly(N-glycolylneuraminyl) chains in a novel polysialoglycoprotein from salmon eggs. J Biol Chem. 1990;265:2596–602. [PubMed] [Google Scholar]
- 21.Karber G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Arch Exp Pathol Pharmacol. 1931;162:480–483. [Google Scholar]
- 22.Kielian M, Jungerwirth S. Mechanisms of enveloped virus entry into cells. Mol Biol Med. 1990;7:17–31. [PubMed] [Google Scholar]
- 23.Krossoy B, Hordvik I, Nilsen F, Nylund A, Endresen C. The putative polymerase sequence of infectious salmon anemia virus suggests a new genus within the Orthomyxoviridae. J Virol. 1999;73:2136–2142. doi: 10.1128/jvi.73.3.2136-2142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lentz T L. The recognition event between virus and host cell receptor: a target for antiviral agents. J Gen Virol. 1990;71:751–766. doi: 10.1099/0022-1317-71-4-751. [DOI] [PubMed] [Google Scholar]
- 25.Lovely J E, Dannevig B H, Falk K, Hutchin L, MacKinnon A M, Melville K J, Rimstad E, Griffiths S G. First identification of infectious salmon anaemia virus in North America with haemorrhagic kidney syndrome. Dis Aquat Organ. 1999;35:145–148. doi: 10.3354/dao035145. [DOI] [PubMed] [Google Scholar]
- 26.Lowry O, Rosenbrough N, Farr A, Randall R. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Marsh M, Helenius A, Matlin K, Simons K. Binding, endocytosis, and degradation of enveloped animal viruses. Methods Enzymol. 1983;98:260–266. doi: 10.1016/0076-6879(83)98153-3. [DOI] [PubMed] [Google Scholar]
- 28.Martin J, Wharton S A, Lin Y P, Takemoto D K, Skehel J J, Wiley D C, Steinhauer D A. Studies of the binding properties of influenza hemagglutinin receptor-site mutants. Virology. 1998;241:101–111. doi: 10.1006/viro.1997.8958. [DOI] [PubMed] [Google Scholar]
- 29.Matlin K S, Reggio H, Helenius A, Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 31.Mjaaland S, Rimstad E, Falk K, Dannevig B H. Genomic characterization of the virus causing infectious salmon anemia in Atlantic salmon (Salmo salar L.): an orthomyxo-like virus in a teleost. J Virol. 1997;71:7681–7686. doi: 10.1128/jvi.71.10.7681-7686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- 33.Nylund A, Alexandersen S, Rolland J B, Jakobsen P. Infectious salmon anemia virus (ISAV) in brown trout. J Aquat Anim Health. 1995;7:236–240. [Google Scholar]
- 34.Nylund A, Kvenseth A M, Krossoy B, Hodneland K. Replication of the infectious salmon anaemia virus (ISAV) in rainbow trout, Oncorhynchus mykiss (Walbaum) J Fish Dis. 1997;20:275–279. [Google Scholar]
- 35.Parton R G, Prydz K, Bomsel M, Simons K, Griffiths G. Meeting of the apical and basolateral endocytic pathways of the Madin-Darby canine kidney cell in late endosomes. J Cell Biol. 1989;109:3259–3272. doi: 10.1083/jcb.109.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez L, Carrasco L. Involvement of the vacuolar H(+)-ATPase in animal virus entry. J Gen Virol. 1994;75:2595–2606. doi: 10.1099/0022-1317-75-10-2595. [DOI] [PubMed] [Google Scholar]
- 37.Reuter G, Gabius H J. Sialic acids structure-analysis-metabolism-occurrence-recognition. Biol Chem Hoppe-Seyler. 1996;377:325–342. doi: 10.1515/bchm3.1996.377.6.325. [DOI] [PubMed] [Google Scholar]
- 38.Rode M, Berg T, Gjøen T. Effect of temperature on endocytosis and intracellular transport in the cell line SHK-1 derived from salmon head kidney. Comp Biochem Physiol A. 1997;117:531–537. [Google Scholar]
- 39.Rouser G, Fleischer S, Yamamoto A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5:494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 40.Skehel J J, Bayley P M, Brown E B, Martin S R, Waterfield M D, White J M, Wilson I A, Wiley D C. Changes in the conformation of influenza virus hemagglutinin at the pH optimum of virus-mediated membrane fusion. Proc Natl Acad Sci USA. 1982;79:968–972. doi: 10.1073/pnas.79.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skehel J J, Daniels R S, Hay A J, Ruigrok R, Wharton S A, Wrigley N G, Weiss W, Willey D C. Structural changes in influenza virus haemagglutinin at the pH of membrane fusion. Biochem Soc Trans. 1986;14:252–253. doi: 10.1042/bst0140252. [DOI] [PubMed] [Google Scholar]
- 42.Stegmann T, Doms R W, Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- 43.Stegmann T, Morselt H W, Scholma J, Wilschut J. Fusion of influenza virus in an intracellular acidic compartment measured by fluorescence dequenching. Biochim Biophys Acta. 1987;904:165–170. doi: 10.1016/0005-2736(87)90100-3. [DOI] [PubMed] [Google Scholar]
- 44.Strobl B, Vlasak R. The receptor-destroying enzyme of influenza C virus is required for entry into target cells. Virology. 1993;192:679–682. doi: 10.1006/viro.1993.1087. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki Y. Gangliosides as influenza virus receptors. Variation of influenza viruses and their recognition of the receptor sialo-sugar chains. Prog Lipid Res. 1994;33:429–457. doi: 10.1016/0163-7827(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 46.Thorud K, Djupvik H O. Infectious anemia in Atlantic salmon (Salmo salar L.) Bull Eur Assoc Fish Pathologists. 1988;8:109–111. [Google Scholar]
- 47.van Meer G, Davoust J, Simons K. Parameters affecting low-pH-mediated fusion of liposomes with the plasma membrane of cells infected with influenza virus. Biochemistry. 1985;24:3593–3602. doi: 10.1021/bi00335a030. [DOI] [PubMed] [Google Scholar]
- 48.von Itzstein M, Wu W Y, Kok G B, Pegg M S, Dyason J C, Jin B, Van Phan T, Smythe M L, White H F, Oliver S W, et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 49.Weis W, Brown J H, Cusack S, Paulson J C, Skehel J J, Wiley D C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988;333:426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimura A, Kuroda K, Kawasaki K, Yamashina S, Maeda T, Ohnishi S. Infectious cell entry mechanism of influenza virus. J Virol. 1982;43:284–293. doi: 10.1128/jvi.43.1.284-293.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]