Abstract
Background
Shenfu (SF) injection, a traditional Chinese medication, would improve microcirculation in cardiogenic shock and infectious shock. This study was aimed to explore the therapeutic potential of the SF injection in gut ischemia-reperfusion (I/R) injury after severe hemorrhagic shock (SHS) and resuscitation. Furthermore, we also investigated the optimal adm? inistration timing.
Methods
Twenty-four male SD rats were randomly divided into four groups: Sham group (sham, n = 6), Control group (n = 6), SF injection group (SF, n = 6), and Delayed Shenfu injection administration group (SF-delay, n = 6). In SHS and resuscitation model, rats were induced by blood draw to a mean arterial pressure (MAP) of 40 ± 5 mmHg within 1 h and then maintained for 40 min; HR, MAP ‘were recorded, microcirculation index [De Backer score, perfused small vessel density (PSVD), total vessel density (TVD), microcirculation flow index score (MFI), flow heterogeneity index (HI)] were analyzed. The blood gas index was detected, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), diamine oxidase (DAO), malondialdehyde (MDA) were measured by ELISA; ZO-1, and claudin-1 were measured by Western blotting. In addition, hematoxylin-eosin (HE) and periodic acid schiff (PAS) staining pathological sections of the intestinal mucosal tissues were also performed.
Results
SF injection increased the MAP, relieved the metabolic acidosis degree associated with the hypoperfusion, and improved the intestinal microcirculatory density and perfusion quality after I/R injury. The expression of DAO, MDA in intestinal tissue, and plasma IL-6, TNF-α significantly decreased in the SF injection group compared to the control group. The concentration of ZO-1 and claudin-1 is also higher in the SF injection group. In addition, the HE and PAS staining results also showed that SF injection could decrease mucosal damage and maintain the structure. In the SF-delay group, the degree of intestinal tissue damage was intermediate between that of the control group and SF injection group.
Conclusions
SF injection protect the intestine from I/R injury induced by SHS and resuscitation, the mechanism of which might be through improving intestinal microcirculation, reducing the excessive release of inflammatory factors and increasing intestinal mucosal permeability. Furthermore, the protection effect is more pronounced if administration during the initial resuscitation phase.
Keywords: Hemorrhagic shock, Ischemia-reperfusion injury, Resuscitation, Shenfu injection
1. Introduction
Trauma causes 5.8 million deaths worldwide each year, accounting for nearly 10 % of all deaths [1]. Shock induced by uncontrolled bleeding remains the leading cause of death among trauma patients [2]. The intestinal epithelium is susceptible to ischemia circumstances and acts as a crucial source and the target of inflammatory mediators, therefore, the gut has been proposed as the motor in critical illness [3]. The research demonstrates that in the context of hemorrhagic shock, the intestines exhibit a heightened vulnerability to ischemic harm in comparison to the heart. This highlights the importance of vigilantly assessing the microcirculatory dynamics within the intestines and similarly susceptible organs to effectively refine and improve resuscitation strategies [4]. Microcirculatory perfusion was injured earlier in the development of hemorrhagic shock, however, fluid resuscitation cannot restore microcirculation perfusion to normal level [[4], [5], [6]]. As microcirculatory perfusion is necessary for the delivery of oxygen and nutrients, the persistent impairment of which is detrimental for organ function. Hence, it is necessary to take additional treatment to restore microcirculation perfusion. The ultimate objective of resuscitation is to rectify organ malfunction and restore cell oxygen metabolism. Although microcirculation is frequently regarded as a link between systemic circulation and cells, several investigations have shown that systemic circulation resuscitation may not adequately match microcirculation improvement under microthrombi generation and vascular paralysis.
Recently, more and more attention has been paid to the protection and monitoring of microcirculation during intestinal ischemia and reperfusion [7,8]. Microcirculation is intimately related to the structure and function of the intestinal epithelium. After traumatic or hemorrhagic shock, the activated sympathetic ganglions interact with the adrenal medulla to promote intestinal pre-capillary sphincter constriction in order to maintain the perfusion of vital organs. However, this protective strategy causes a decrease in intestinal perfusion, inhibits gut peristalsis, alters mucus and tight junction expression. Additionally, a high level of reactive oxygen species creation and inflammatory factor release during reperfusion period may aggravate the intestinal mucosal injury and inhibit healing. All the above eventually damage the intestinal barrier. Furthermore, reduced secretory Immunoglobulin A disrupts the natural gut immune system, reduces gut microbial diversity [9]. The injury of intestinal mechanical and immunological barriers lead to the increase of intestinal permeability, allowing enteric flora and endotoxins translocated into the mesenteric lymph nodes and even the blood, in addition, an increase in pathogenic bacteria will also hasten this translocation [[10], [11], [12]]. Therefore, there is an urgent need to discover an effective medicine to treat intestinal ischemia-reperfusion damage, particularly microcirculation, in patients suffering from severe hemorrhagic shock.
Shenfu (SF) injection is a combination of red ginseng and aconite. It has a high concentration of active chemicals such as alkaloids, ginsenosides, and amino acids, which operate by agonizing receptors and improving cell membrane integrity [13]. As a traditional Chinese medication, SF injection has been frequently utilized in patients suffering from infectious shock and cardiogenic shock, with promising clinical efficacy [14,15]. Based on the hemorrhagic shock model, Nan Liang et al. demonstrated that SF injection may considerably enhance mean arterial blood pressure (MAP), left ventricular pressure (LVP), and the maximal rates of LVP change following resuscitation [16]. A recent animal research reveals that SF injection may protect the intestinal epithelium in hemorrhagic shock by suppressing apoptosis and oxidative stress damage [17]. However, most current studies focus merely on the impacts of hemodynamic indexes; the protective potential of SF injection in tissue microcirculation, especially in gut remains unclear. Furthermore, the process in the development of gut dysfunction may be separated between acute ischemia and reperfusion damage. But, the best moment to begin SF injection administration during the intestinal ischemia-reperfusion (I/R) injury damage process has yet to be identified. In this study, we hypothesized that SF injection could improve intestinal microcirculation perfusion, decrease the excessive release of inflammatory factors and oxidative stress, in order to protect the intestinal mucosal barrier function after intestine I/R injury induced by SHS and resuscitation.
2. Materials and methods
2.1. Shenfu injection
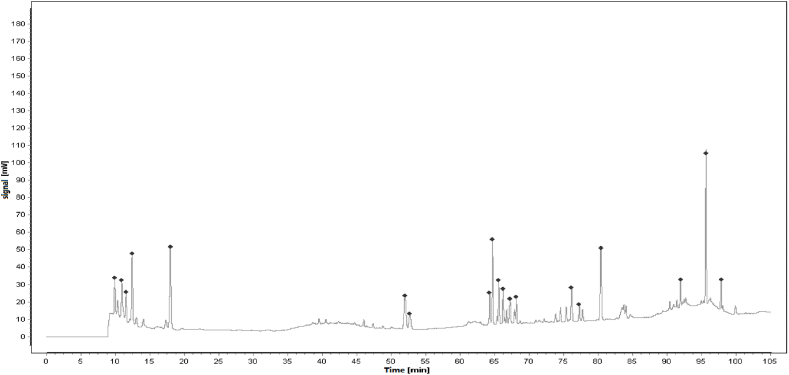
SF injection is composed of Red Ginseng (the root or rhizome of Panax ginseng C. A. Mey, 1 mg/ml) and Fuzi (the tuber of Aconitum carmichaelii Debeaux, 2 mg/ml), which were described in Table 1. The main active ingredients are ginsenosides and aconite alkaloids [18]. SF injection, which was prepared using the multi-stage countercurrent extraction and macroporous resin adsorption technology by Ya'an Sanjiu Pharmaceutical Co., Ltd. (Sichuan, China), contains ginsenoside (0.8 mg/ml) and aconitine (0.1 mg/ml). To ensure the quality of SF injection from the source, ginseng from Jingyu GAP planting base in Jilin Province and Fuzi from Jiangyou GAP planting base in Sichuan Province are used. Their quality was strictly controlled according to the standard of China Food and Drug Administration (approval No.: WS3–B-3427-98-2013) in Table 2, and was ensured by using fingerprint technology during production in Fig. 1. The workflow of SF injection preparation is presented in previous research, and 22 kinds of ginsenosides and 16 kinds of aconite alkaloids were separated as described.
Table 1.
Information of raw herbs in Shenfu injection.
| Latin scientific name | Plant parts | English name | Pinyin name | Voucher no | Voucher specimens no | Ratio |
|---|---|---|---|---|---|---|
| Panax ginseng C. A. Mey. |
Root | Red Ginseng Root | Hongshen | 13010560 | Hongshen:1305001 | 33.3 % |
| Aconitum carmichaelii Debeaux |
Tuber | Aconite Tuber | Fuzi | 13010423 | Fuzi: 1305041 | 66.6 % |
Table 2.
The quality standard of Shenfu injection (batch number: 211104AK03).
| Compound Chemical structure Content Quality control | |||
|---|---|---|---|
| Benzoylmesaconine | 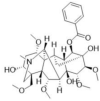 |
1.58 μg/ml | 0.50–4.50 μg/ml |
| Ginsenoside Rg1 | 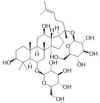 |
0.12 mg/ml | >0.04 mg/ml |
| Ginsenoside Re | 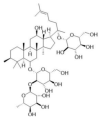 |
0.11 mg/ml | >0.02 mg/ml |
| Ginsenoside Rb1 | 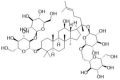 |
1.0 mg/ml | 0.6–1.8 mg/ml |
Fig. 1.
Reference Fingerprint for SF injection.
2.2. Animals preparation
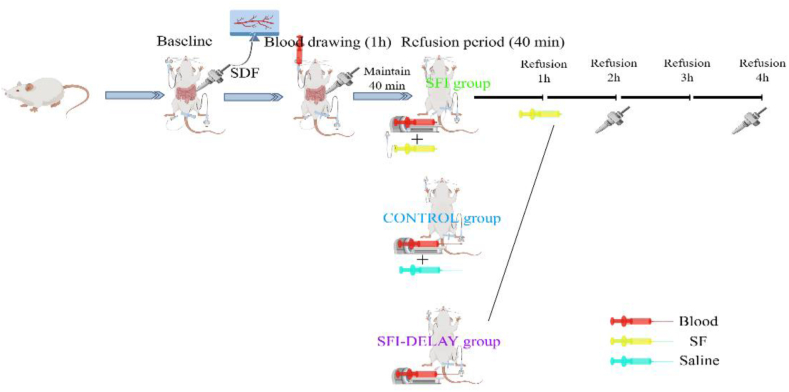
Twenty-four male Sprague-Dawley rats (4–5 months old, 400–500 g) were purchased from the Experimental Animal Center of Shandong Province (Jinan, Shandong, China) and housed in the Laboratory Animal Center of the Second Affiliated Hospital of Anhui Medical University (Hefei, China). All rats were maintained under standardized conditions with a temperature of 22 ± 1 °C, a humidity of 55 ± 5 %, a 12-h/12-h light/dark cycle, and were allowed add libitum to feed and water for 1 week before the experiment. This study was reviewed and approved by the Animal Ethics Committee of Anhui Medical University. The workflow of model establishment and drug administration is demonstrated in Fig. 2.
Fig. 2.
Flow chart of animal experiments.
2.3. Surgical preparation
Animals were fasted for 12 h prior to induction of anesthetic, but were allowed free access to water. Rats were initially anesthetized using CO2 in an induction chamber and then injected intraperitoneally with 45 mg/kg of pentobarbital. The maintenance dose was 30 mg/kg per hour throughout the procedure. After anesthesia, the rats were restrained, and the skin of the neck, lower abdomen, and left inguinal groin area was prepared and disinfected. Tracheal intubation was performed with a 14G catheter to maintain spontaneous breathing. A right internal carotid artery cannula (epidural catheter) was placed for hemorrhage control. An additional left femoral artery cannula (PE-50 catheter) and femoral vein cannula (PE-50 catheter) were used for MAP monitoring, drug administration, and fluid resuscitation, respectively. All cannulae were flushed with heparinized saline (5U/ml) to maintain patency prior to placement. After completion of the above procedures, rats were stabilized for 20 min (baseline phase). The rectal temperature was maintained at 37 ± 0.5 °C by a using the thermostatically controlled plate.
2.4. Hemorrhage and fluid resuscitation procedure
2.4.1. Hemorrhage procedure
Twenty-four rats were randomly assigned to four groups (six per group): (1) Sham group; (2) Control group: conventional volume resuscitation with an equal volume of blood loss and saline; (3) Shenfu injection group: resuscitation with an equal volume of blood loss combined with Shenfu injection (Huarun, China) immediately after shock phase; (4) Delayed Shenfu injection administration group: resuscitated with an equal volume of blood, and Shenfu injection infused at 1 h after resuscitation. The dose of Shenfu injection in this study was 10 ml/kg, which was based on the results of previous dose-response studies [19,20]. After systemic heparinization with heparinized saline (250U/ml, dose of 100U/kg), hypotension was slowly induced by blood withdrawal from the right internal carotid artery for 1 h. An initial 40 % fixed blood volume was adopted, and slight repeated blood withdrawal and infusion were performed until the MAP fell to 40 ± 5 mmHg within 1 h. Hemorrhagic shock was maintained for 40 min, followed by reperfusion. Blood was collected in heparinized syringes to prevent coagulation and stored at 4 °C.
2.4.2. Fluid resuscitation procedure
Rats in the control, SF injection, and SF-delay groups were resuscitated using autologous blood and 10 ml/kg of saline/Shenfu injection administered through a femoral vein cannula using a syringe pump within 40 min. For the rats in the SF-delay group, SF injection was administered 1 h after the completion of the initial autologous blood transfusion. All blood samples were rewarmed at room temperature for 10 min before refusion. Our goal was to achieve a MAP ≧ 80 mmHg after resuscitation; which was henceforth considered as a state of successful resuscitation. Once the target MAP was achieved, the rats were continually monitored for 4 h.
Heart rate and MAP were monitored in real-time during the experiment. In addition, a volume of 0.1 ml of blood was collected for arterial blood gas analysis at baseline, the end of the shock maintenance period, 2 and 4 h after resuscitation.
2.5. Blood and intestinal tissue extraction
Rats were euthanized by CO2 inhalation after 4 h of observation; blood was stored in sterile 1.5 ml Eppendorf tubes for 30 min at room temperature, centrifuged at 3500 rpm for 10 min at 4 °C (Eppendorf, 5702 R, Germany), and then stored at −80 °C for subsequent testing. Approximately 10 cm of intestinal segments were aseptically isolated above the ileocecal valve, taking care to avoid the microcirculation monitoring sites. The intestinal segments were then carefully rinsed with ice-cold saline to remove the luminal contents. A portion of the intestinal tissue was flash-frozen in liquid nitrogen and stored at −80 °C; another portion was fixed in 4 % formalin for 72hrs.
2.6. Microcirculatory imaging
5 cm away from the ileocecal valve was selected to observe the intestinal microcirculation using a sidestream dark-field (SDF) imaging video microscope (MicroVision Medical, 19849 D31, The Netherlands) at the baseline phase, the end of the shock maintenance period, 2 and 4 h after resuscitation. According to the roundtable consensus of the De Backer et al. [21], the following five indexes were used to separately assess intestinal microcirculatory density and perfusion quality: (1) De Backer score (density): it is calculated as the count of vessel which passed through the parcellation regions divided by the total vascular length when evenly segmented the image to 9 areas; (2) perfused small vessel density: it is calculated by measuring the density of perfused small vessels (diameter less than 20 μm) within the field of view (the total length of perfused small vessels divided by the total area of the image); (3) total vessel density: it is calculated as total vessel length divided by total area; (4) microcirculatory flow index score: scores 0–3 represent no flow, interrupted flow, vessel stasis, and normal flow; (5) flow heterogeneity index (FHI, perfusion quality): it is calculated as (MFImax-MFImin)/MFIavg. Individual videos were at least 10s long, and all microcirculatory index analyses were performed blindly using Automated Vascular Analysis software, version 5.0 (MicroVision Medical, Amsterdam, The Netherlands).
2.7. Histopathology hematoxylin and eosin (H&E) staining
After fixation for 3 days, the intestinal tissue specimens were dehydrated, hyalinized in Xylene, embedded in paraffin, sectioned (4 μm), dewaxed and hydrated sequentially, followed by H&E staining. According to the standard protocol, the tissue sections were stained with hematoxylin for 10 min and rinsed with running water for 5 min; then they were immersed in 0.7 % hydrochloric ethanol for 3 s at room temperature and washed with water until the sections turned blue (15 min), after which the nuclei were counterstained with 2 min, The sections were then immersed in 70 % alcohol, 80 % alcohol, 90 % alcohol, 95 % alcohol, anhydrous ethanol I, and anhydrous ethanol II for 30 s each, followed by xylene clear and finally neutral rubber. The degree of intestinal mucosa damage was scored according to Chiu's scoring system (grades from 0 to 8).
2.8. Periodic acid schiff (PAS) staining
Sections were prepared as described. above until staining. Tissue sections were oxidized in paraformaldehyde-lysine-periodate solutionfor 10 min at room temperature and then rinsed with PBS for 3 min. After two immersions in distilled water for 3 min each, the sections were stained with Schiff solution for 10–30mins in a light-protected environment. Then 0.5 % sodium sulfite was used for tissue differentiation. After the same rinse, counterstaining with hematoxylin was performed. Finally, the sections were immersed in 70 % alcohol, 80 % alcohol, 90 % alcohol, 95 % alcohol, anhydrous ethanol I, and anhydrous ethanol II, each for 30s each, followed by hyalinized in Xylene and sealing with neutral gum.
2.9. Enzyme-Linked immunosorbent assay (ELISA)
The level of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in serum and diamine oxidase (DAO) and malondialdehyde (MDA) in intestinal tissue were measured by ELISA. All quantitation kits were purchased from Elabscience Biotechnology CO., Ltd (Wuhan, China) (TNF-α: Cat No. E-EL-R2856c; IL-6: Cat No. E-EL-R0896c; DAO: Cat No. E-EL-R3013). Assays were performed according to the protocols provided by manufacturers. The enzyme marker detected the OD value at 450 nm wavelength, and then a standard curve was plotted. It is worth noting that the OD450 value was inversely correlated with MDA concentration in the competitive ELISA method but positively correlated with TNF-α, IL-6, and DAO in the double antibody sandwich ELISA method.
2.9.1. Western blotting
By performing the western blotting assay, the expressions of intestinal tight junction protein (ZO-1 and Claudin-1) were determined to evaluate the intestinal integrity barrier. Intestinal tissue samples were added to the RIPA lysis buffer (50μl/100 g) (Beyotime Biotechnology CO, Ltd, Shanghai, China) containing protease inhibitor PMSF (5 μl/100 g) (Beyotime Biotechnology CO, Ltd, Shanghai, China), homogenized by the ultrasonic grinder, and centrifuged at 13500 g for 10 min to obtain supernatant. Subsequently, the total protein concentrations were quantified using the BCA kit (Beyotime) according to the manufacturers' instructions.
Proteins were separated by electrophoresis on 10 % sodium dodecyl sulfate-polyacrylamide gels electrophoresis (SDS-PAGE) and then transferred to polyvinylidene difluoride (PVDF) membranes. After being washed 3 times with Tris-buffered saline-Tween (TBST) for 10 min each, the membranes were blocked with 5 % non-fat milk for 2 h and then incubated with primary antibodies at 1:1000 dilution (e.g., ZO-1 antibody [SAB5700645, Merck KGaA, Germany], Claudin-1 [SAB4503546, Merck KGaA, Germany]) overnight at 4 °C. Next, they have rewashed with TBST 3 times for 10 min and incubated with goat anti-rabbit secondary antibody (Univ CO, Ltd, Shanghai, China) at 1:1000 dilution. The target bands were observed by enhanced chemiluminescence (ELC) and detected by the Tanon-5200 Luminescence imaging system (Tanon Science, Shanghai, China). Finally, the gray values of the protein bands were analyzed by Image J software, Version1.8.0 (NIH, Bethesda, MD, USA).
2.10. Statistical analysis
Measurements and data were expressed as mean ± SD. Two-way analysis of variance was used to assess the differences among four groups, while the student's test was performed to analyze the difference in different groups. P value < 0.05 was considered with statistical significance. Statistical analysis was performed with SPSS software, Version 24 (SPSS, Chicago, IL), and GraphPad Prism software, Version 7.0 (GraphPad Software, San Diego, CA).
3. Results
3.1. Comparison of baseline
As presented in Table 3, all rats were successfully resuscitated, there were no significant differences in body weight, initial MAP, arterial blood lactate (LAC), potential of hydrogen (PH), and base excess (BE) values among four groups at baseline.
Table 3.
The baseline comparison between each group.
| Index | Sham | Control | SF | SF-delay | F | P |
|---|---|---|---|---|---|---|
| Weight (g) | 438.17 ± 24.21 | 451.33 ± 38.16 | 454.50 ± 41.38 | 436.67 ± 31.87 | 1.008 | 0.41 |
| Temperature (°C) | 36.52 ± 0.1 | 36.47 ± 0.28 | 36.40 ± 0.18 | 36.47 ± 0.18 | 0.294 | 0.78 |
| MAP (mmHg) | 131.17 ± 3.97 | 133.67 ± 12.31 | 129.33 ± 3.93 | 128 ± 9.03 | 0.547 | 0.656 |
| Lactate (mmol/L) | 0.72 ± 0.06 | 0.92 ± 0.15 | 0.89 ± 0.17 | 0.80 ± 0.19 | 2.195 | 0.12 |
| PH | 7.36 ± 0.08 | 7.34 ± 0.32 | 7.38 ± 0.32 | 7.38 ± 0.27 | 0.831 | 0.492 |
| BE (mmol/L) | −1.30 ± 2.86 | −1.45 ± 1.09 | −0.95 ± 1.01 | −0.93 ± 1.62 | 0.220 | 0.946 |
MAP: mean arterial pressure; SF: Shenfu injection.
3.2. Effect of shenfu injection in mean arterial pressure and gas analysis indexes
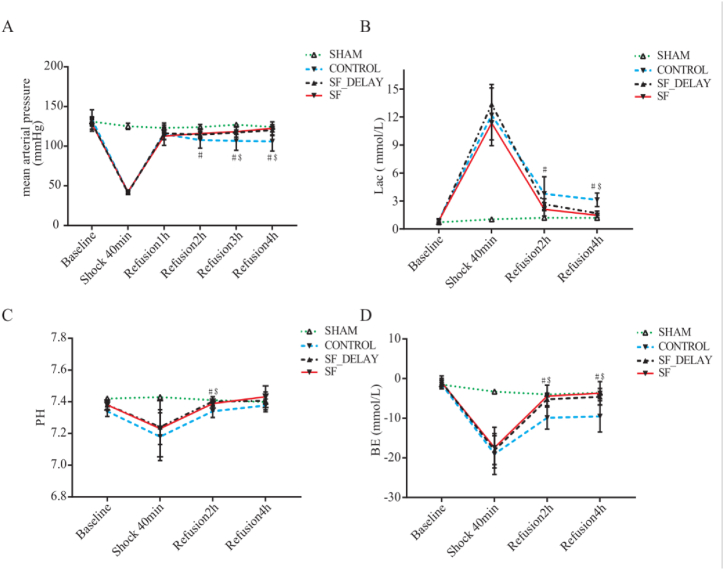
During the shock period, MAP significantly decreased to 40–45 mmHg for rats in Control group (CON), SF injection group and Delayed Shenfu injection administration group (SF-delay) groups (P < 0.001). One hour after resuscitation, there were no significant differences in MAP among those three groups; while 3 h after resuscitation, compared with CON group, MAP levels in SF injection (118.33 ± 6.77, P = 0.010) and SF-delay (117.17 ± 1.47, P = 0.019) groups began to show significant improvement. However, there were no significant differences in MAP among SF injection and SF-delay groups in all post-resuscitation periods. The change in MAP in each group of rats was presented in Table 4 and Fig. 3A.
Table 4.
The dynamic changes of arterial blood gas index and MAP at different measure time point.
| Index | Measure time point | Sham | Control | SF | SF-delay | F | Pa |
|---|---|---|---|---|---|---|---|
| Lactate (mmol/L) |
Baseline | 0.72 ± 0.06 | 0.92 ± 0.15 | 0.89 ± 0.17 | 0.80 ± 0.19 | 2.195 | 0.12 |
| Shock-40min | 1.04 ± 0.13 | 12.21 ± 3.28 | 11.35 ± 1.8 | 13.36 ± 1.75 | 45.490 | <0.001 | |
| Refusion 2 h | 1.19 ± 0.07 | 3.78 ± 1.82 | 2.1 ± 0.71 | 2.65 ± 0.59 | 6.710 | 0.03 | |
| Refusion 4 h | 1.18 ± 0.1 | 3.12 ± 0.72 | 1.46 ± 0.22 | 1.68 ± 0.22 | 28.808 | <0.001 | |
| PH | Baseline | 7.36 ± 0.08 | 7.34 ± 0.32 | 7.38 ± 0.32 | 7.38 ± 0.27 | 0.831 | 0.492 |
| Shock-40min | 7.41 ± 0.02 | 7.18 ± 0.15 | 7.24 ± 0.14 | 7.23 ± 0.11 | 4.091 | 0.020 | |
| Refusion 2 h | 7.37 ± 0.03 | 7.34 ± 0.04 | 7.39 ± 0.03 | 7.40 ± 0.03 | 4.217 | 0.018 | |
| Refusion 4 h | 7.40 ± 0.03 | 7.38 ± 0.04 | 7.43 ± 0.07 | 7.41 ± 0.06 | 1.245 | 0.320 | |
| BE (mmol/L) |
Baseline | −1.30 ± 2.86 | −1.45 ± 1.09 | −0.95 ± 1.01 | −0.93 ± 1.62 | 0.220 | 0.946 |
| Shock-40min | −3.20 ± 2.13 | −19.03 ± 5.15 | −17.42 ± 5.14 | −18.05 ± 3.62 | 9.590 | <0.001 | |
| Refusion 2 h | −3.96 ± 1.19 | −9.88 ± 2.85 | −4.42 ± 2.77 | −5.23 ± 1.58 | 7.271 | 0.001 | |
| Refusion 4 h | −3.57 ± 2.18 | −9.20 ± 4.42 | −3.68 ± 2.94 | −4.57 ± 2.09 | 3.857 | 0.014 | |
| MAP (mmHg) |
Baseline | 131.17 ± 3.97 | 133.67 ± 12.31 | 129.33 ± 3.93 | 128 ± 9.03 | 0.547 | 0.656 |
| Shock 40min | 125.17 ± 4.02 | 41.83 ± 1.72 | 42.5 ± 1.05 | 41.33 ± 2.16 | 1671.35 | <0.001 | |
| Refusion 1 h | 122.5 ± 3.62 | 115.17 ± 14.09 | 112.83 ± 4.17 | 111.17 ± 7.91 | 0.893 | 0.462 | |
| Refusion 2 h | 124.17 ± 3.43 | 107.67 ± 10.07 | 116 ± 6.29 | 114.5 ± 3.39 | 7.45 | 0.02 | |
| Refusion 3 h | 126.83 ± 1.72 | 106.83 ± 12.01 | 118.33 ± 6.77 | 117.17 ± 1.47 | 8.26 | 0.01 | |
| Refusion 4 h | 124.67 ± 0.82 | 110 ± 12.13 | 122.5 ± 8.17 | 120.33 ± 6.98 | 6.461 | 0.003 |
One-way analysis of variance results. MAP: mean arterial pressure; SF: Shenfu injection.
Fig. 3.
The dynamic changes of blood gas indexes and mean arterial pressure of each group in different periods; (A) mean arterial pressure; (B) arterial lactate concentration; (C) PH levels; (D) BE levels; # SF injection vs CON group, P < 0.05; $ SF-delay vs CON group, P < 0.05. SF is the abbreviation for Shenfu Injection.
For blood gas indexes, the values of LAC and BE were significantly increased and decreased in CON, SF injection, and SF-delay groups, respectively, when compared with sham group during the shock phase, while there were no significant differences among CON, SF injection, and SF-delay groups. Two hours after resuscitation, LAC, BE, and potential of hydrogen (PH) values of rats showed a noticeable improvement in CON, SF injection, and SF-delay groups. Regarding group comparisons, the LAC value of the SF injection group was significantly decreased compared with the CON group [2.1 ± 0.71 vs. 3.78 ± 1.82, P = 0.01]; however, no significant difference was observed between SF-delay and CON group [2.65 ± 0.59 vs. 3.78 ± 1.82, P = 0.07]. The BE and PH values of SF injection (BE: 4.42 ± 2.77 vs. −9.88 ± 2.85, P < 0.001; PH: 7.39 ± 0.03 vs. 7.34 ± 0.04, P = 0.018) and SF-delay (BE: 5.23 ± 1.58 vs. −9.88 ± 2.85, P = 0.002; PH: 7.40 ± 0.03 vs. 7.34 ± 0.04, P = 0.003) groups were all significantly elevated compared with CON groups; while no significant differences were found between SF injection and SF-delay groups (BE: 4.42 ± 2.77 vs. −5.23 ± 1.58, P = 0.531; PH: 7.39 ± 0.03 vs. 7.40 ± 0.03, P = 0.466). At 4 h after resuscitation, the BE values (SF injection vs. CON: 3.68 ± 2.94 vs. −9.20 ± 4.42, P = 0.005; SF-delay vs. CON: 4.57 ± 2.09 vs. −9.20 ± 4.42, P = 0.016) increased significantly, while the LAC (SF injection vs. CON: P < 0.001; SF-delay vs. CON: P < 0.001) values decreased significantly in SF injection and SF-delay groups when compared with those in CON group. However, no significant differences were detected in PH values in both SF injection (P = 0.071) and SF-delay (P = 0.33) groups relative to the CON group. In addition, there was also no compelling difference in the above indexes between SF injection and SF-delay groups (LAC: P = 0.331, PH: P = 0.376; BE: P = 0.622). The changes and comparisons in blood gas indexes in each group of rats are shown in Table 4 and Fig. 3B-D.
3.3. Effect of shenfu injection in intestinal microcirculation
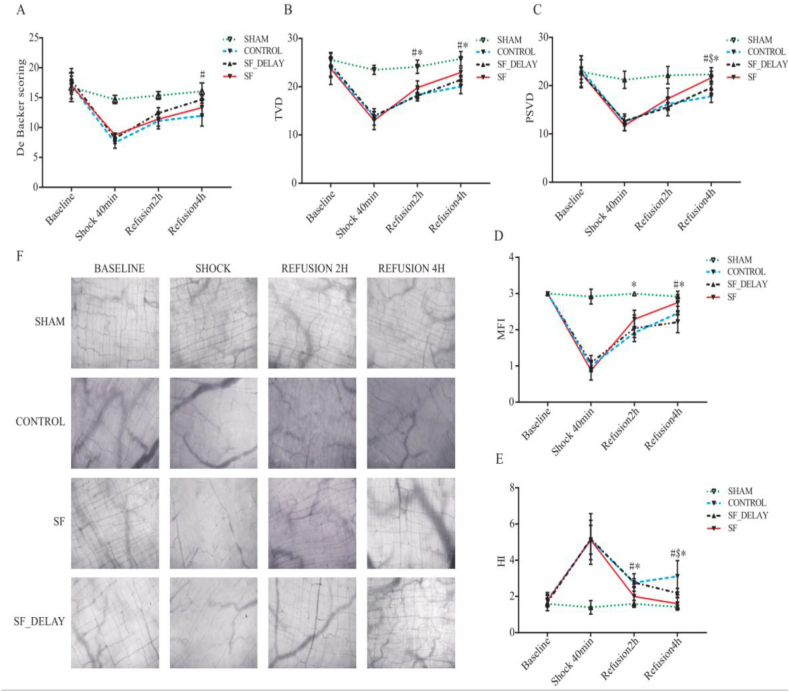
In terms of intestinal microcirculatory density, the results of AVA software found that both the De Backer score and perfused small vessel density (PSVD) and total vessel density (TVD) values presented an apparent downward trend in the shock period (Fig. 4A,C). Two hours after resuscitation, the PSVD and TVD in the SF injection group were better recovered than those in the CON and SF-delay, while a significant difference was seen only in TVD (SF injection vs. CON: 19.80 ± 1.42 vs. 18.32 ± 1.25, P = 0.047; SF injection vs. SF-delay: 19.80 ± 1.42 vs. 17.81 ± 1.28, P = 0.028). Moreover, it was worth noting that the PSVD and TVD values in the SF-delay groups were slightly lower than those in the CON group (Table 5). Four hours after resuscitation, we found that intestinal microcirculatory density in the SF injection group was significantly restored when compared with the CON group (Fig. 4F), as reflected by significantly elevated levels of De Backer score (14.70 ± 0.62 vs. 11.96 ± 1.72, P = 0.002) (Fig. 4A), PSVD (21.65 ± 1.34 vs.17.81 ± 1.27 P < 0.001) (Fig. 4C), and TVD (22.96 ± 0.91 vs.20.04 ± 1.48 P = 0.001) (Fig. 4B). Even though microcirculatory density had also improved in SF-delay, a statistically significant difference was only observed in the PSVD value (P = 0.026). Furthermore, when comparing the SF injection and SF-delay groups, there were also significant differences in the levels of PSVD (21.65 ± 1.34 vs. 19.59 ± 1.12, P = 0.012) and TVD (22.96 ± 0.91 vs. 21.43 ± 0.93, P = 0.045) (Fig. 4B,C).
Fig. 4.
The changing trend of (A) De Backer scoring, (B) TVD, (C) PSVD, (D) MFI, (E) HI in each group; (F) Status of intestinal microcirculation in different periods (20μm x 20 μm, the collection site was ileocecal valve 5 cm upward); # SF vs CON group, P < 0.05; $ SF-delay vs CON group, P < 0.05; * SF vs SF-delay group, P < 0.05. SF injection is the abbreviation for Shenfu Injection; TVD is the abbreviation for total vessel density; PSVD is the abbreviation for perfused small vessel density; MFI is the abbreviation for microcirculation flow index score; HI is the abbreviation for flow heterogeneity index.
Table 5.
The dynamic changes of each intestinal microcirculation at different measure time point.
| Index | Measure time point | Sham | Control | SF | SF-delay | F | Pa |
|---|---|---|---|---|---|---|---|
| DeBacker (1/mm) |
Baseline | 16.73 ± 2.37 | 17.10 ± 1.51 | 17.90 ± 1.97 | 17.04 ± 2.20 | 0.36 | 0.783 |
| Shock-40min | 14.69 ± 0.68 | 7.46 ± 0.92 | 8.19 ± 0.88 | 8.77 ± 0.28 | 121.715 | <0.001 | |
| Refusion 2 h | 15.38 ± 0.66 | 11.10 ± 1.33 | 12.42 ± 0.91 | 11.41 ± 1.19 | 20.53 | <0.001 | |
| Refusion 4 h | 16.06 ± 1.41 | 11.96 ± 1.72 | 14.70 ± 0.62 | 13.35 ± 1.42 | 10.097 | <0.001 | |
| TVD (mm/mm2) |
Baseline | 25.59 ± 1.37 | 24.09 ± 2.15 | 23.72 ± 3.26 | 24.51 ± 2.35 | 0.685 | 0.571 |
| Shock-40min | 23.49 ± 0.94 | 13.75 ± 1.72 | 13.00 ± 1.89 | 13.99 ± 0.82 | 73.243 | <0.001 | |
| Refusion 2 h | 24.13 ± 1.37 | 18.32 ± 1.25 | 19.80 ± 1.42 | 17.81 ± 1.28 | 31.855 | <0.001 | |
| Refusion 4 h | 25.79 ± 1.49 | 20.04 ± 1.48 | 22.96 ± 0.91 | 21.43 ± 0.93 | 24.00 | <0.001 | |
| PSVD (mm/mm2) |
Baseline | 22.88 ± 2.35 | 23.41 ± 2.81 | 22.59 ± 2.78 | 22.59 ± 2.24 | 0.151 | 0.928 |
| Shock-40min | 21.23 ± 1.78 | 12.40 ± 1.73 | 11.71 ± 1.01 | 12.69 ± 1.00 | 59.474 | <0.001 | |
| Refusion 2 h | 22.17 ± 1.81 | 16.20 ± 1.29 | 17.32 ± 2.16 | 15.57 ± 1.81 | 16.625 | 0.001 | |
| Refusion 4 h | 22.34 ± 1.40 | 17.81 ± 1.27 | 21.65 ± 1.34 | 19.59 ± 1.12 | 15.254 | <0.001 | |
| MFI | Baseline | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 | 3.00 ± 0.00 | 0.0000 | 1 |
| Shock-40min | 2.92 ± 0.14 | 1.08 ± 0.20 | 0.88 ± 0.26 | 1.00 ± 0.16 | 148.196 | <0.001 | |
| Refusion 2 h | 3.00 ± 0.00 | 2.04 ± 0.37 | 2.29 ± 0.25 | 1.92 ± 0.13 | 26.471 | <0.001 | |
| Refusion 4 h | 2.92 ± 0.14 | 2.21 ± 0.29 | 2.75 ± 0.22 | 2.47 ± 0.19 | 12.593 | <0.001 | |
| HI | Baseline | 1.59 ± 0.04 | 1.81 ± 0.40 | 1.76 ± 0.30 | 1.66 ± 0.45 | 0.524 | 0.671 |
| Shock-40min | 1.40 ± 0.37 | 5.13 ± 0.80 | 5.11 ± 1.09 | 5.17 ± 1.40 | 21.533 | <0.001 | |
| Refusion 2 h | 1.59 ± 0.09 | 2.76 ± 0.22 | 1.99 ± 0.50 | 2.77 ± 0.49 | 15.257 | <0.001 | |
| Refusion 4 h | 1.42 ± 0.10 | 3.11 ± 0.87 | 1.60 ± 0.36 | 2.18 ± 0.26 | 14.484 | <0.001 |
TVD: Total vessel density; PSVD: Perfused small vessel density; HI: Heterogeneity index; MFI: Microcirculation flow index; SF: Shenfu injection.
One-way analysis of variance results.
Furthermore, in terms of intestinal microcirculatory perfusion quality, a striking intermittent blood flow, or even the no-reflow phenomenon, was observed (Fig. 4F) during the shock period. Two hours after resuscitation, the CON and SF-delay groups experienced extensive flow stasis, whereas the SF group exhibited relatively good intestinal microcirculation flow (Fig. 4F). The analysis of AVA software demonstrated that the rats in the SF group had a higher level of MFI than the SF-delay group (2.29 ± 0.25 vs.1.92 ± 0.13 P = 0.011) and lower levels of flow heterogeneity index (HI) than CON (1.99 ± 0.50 vs.2.76 ± 0.22 P = 0.002) and SF-delay groups (P = 0.002) (1.99 ± 0.50 vs.2.77 ± 0.49 Fig. 4D,E). Four hours after resuscitation, the perfusion quality in both SF injection and SF-delay groups was significantly improved, nearly returned to baseline levels in SF injection group (Fig. 4D,E). However, flow stasis and intermittent blood flow were still presented in the CON group. In addition, the levels of MFI in SF injection group (2.75 ± 0.22 vs.2.21 ± 0.29 P < 0.001) were significantly improved than in the CON group (Fig. 4D). Moreover, the levels of HI in both the SF (1.60 ± 0.36 vs.3.11 ± 0.87, P < 0.001) and SF-delay (2.18 ± 0.26 vs.3.11 ± 0.87, P = 0.004) groups were significantly lower than those in CON group (Fig. 4E). When comparing the SF injection and SF-delay groups, there was a significant difference in the level of MFI (2.75 ± 0.22 vs.2.47 ± 0.19 P = 0.03) (Fig. 4E).
3.4. Effect of shenfu injection in histopathological changes of intestinal mucosa
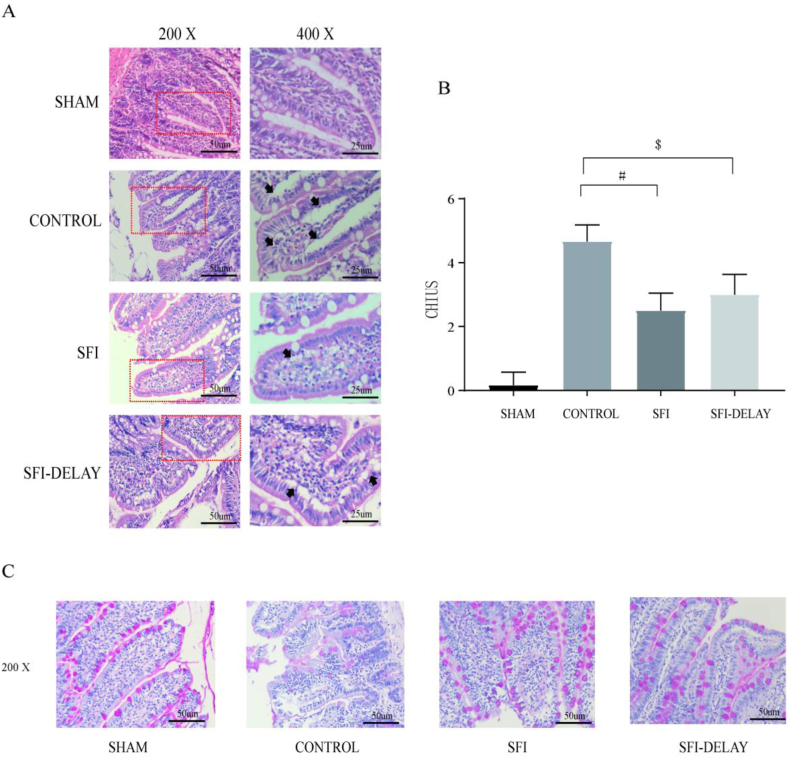
As shown in Fig. 5A, small intestinal mucosal damage was most pronounced in the CON group, as reflected by propria exfoliation, ulceration, bleeding, and the increased intestinal submucosal space. Compared with the CON group, ischemia-reperfusion injury resulted in mild intestinal mucosal damage in the SF injection and SF-delay groups. However, the increase in intestinal submucosal space and lamina propria vacuolization was more obvious in the SF-delay group under high magnification (400×) when compared with the SF injection group (Fig. 5A). Besides, Chiu's score in the SF injection group was lower than that in CON (2.5 ± 0.55 vs. 4.67 ± 0.52, P < 0.001) and SF-delay groups (3.0 ± 0.63 vs. 4.67 ± 0.52, P = 0.086) at 4 h after resuscitation; but the statistical difference existed only between the first two groups (Fig. 5B).
Fig. 5.
(A) Hematoxylin and eosin staining of intestinal mucosal in different groups; (B) the CHIUS scoring; (C) periodic acid-schiff staining of intestinal mucosal in different groups; # SF vs CON group, P < 0.05; $ SF-delay vs CON group, P < 0.05. SF injection is the abbreviation for Shenfu Injection; CHIUS is the abbreviation for intestinal mucosal injury scoring.
PAS staining is mainly used to observe the mucopolysaccharide content (purple-red granules), which reflects the number of goblet cells in the intestinal epithelium and the state of the mucus barrier. As shown in Fig. 5C, compared to the SF injection and SF-delay groups, mucin was significantly reduced in the goblet cells and showed an isolated distribution in the CON group at 4 h after resuscitation.
3.5. Effect of shenfu injection in the serum level of pro-inflammatory factors
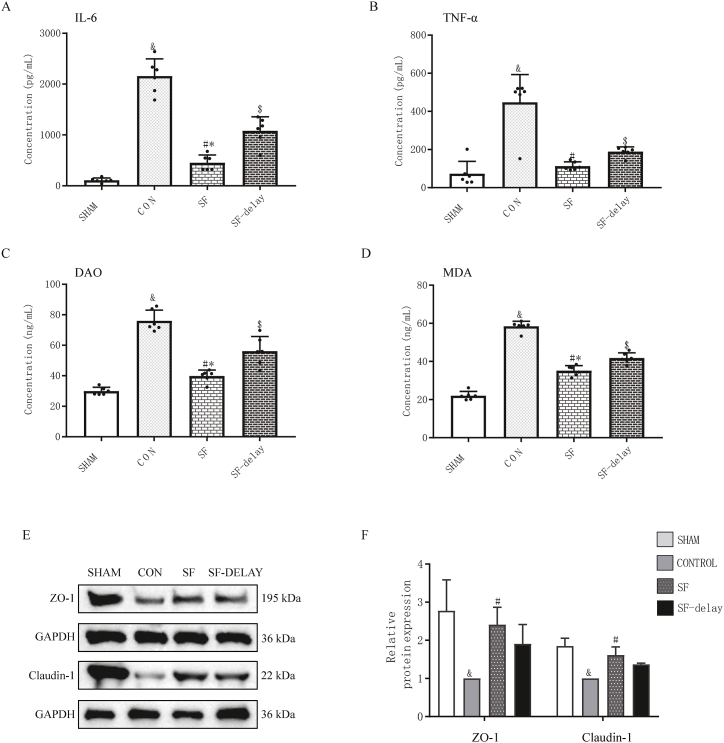
The ELISA results suggested that rats receiving SF injection presented lower levels of interleukin-6 (IL-6) (SF injection vs. CON: P < 0.001; SF-delay vs. CON: P < 0.001) (Fig. 6A) and tumor necrosis factor-α (TNF-α) (Fig. 6B) (SF injection vs. CON: P < 0.001; SF-delay vs. CON: P < 0.001) in blood compared to those who received conventional blood transfusion only; and, notably, early use of SF injection was more potent in inhibiting excessive host inflammatory response. Thus, the significant regulatory effect of SF injection on inflammatory cytokines levels in hemorrhagic shock was explicitly demonstrated.
Fig. 6.
Comparison of serum pro-inflammatory factors, DAO, MDA, ZO-1 and claudin-1 protein expressions in each group after 4 h of hemorrhagic shock resuscitation. (A) The concentration of serum IL-6; (B) the concentration of serum TNF-α; (C) the expression levels of DAO; (D) the expression levels of MDA; & indicated P < 0.05 when CON group compared with SHAM group; # indicated P < 0.05 when CON group compared with SF group; $ indicated P < 0.05 when SF-delay group compared with CON group; * indicated P < 0.05 when SF group compared to SF-delay group; (E) the expression levels of ZO-1 and Claudin-1 proteins in the intestines of each group; (F) the relative protein expression levels of ZO-1 and Claudin-1; # SF vs CON group, P < 0.05; $ SF-delay vs CON group, P < 0.05; SF vs SF-delay group, P < 0.05. DAO is the abbreviation for diamine oxidase; MDA is the abbreviation for malondialdehyde; ZO-1 is the abbreviation for Zonula Occludens Protein 1.
3.6. Effect of shenfu injection in the expression of proteins related to intestinal injury
DAO is a highly active intracellular enzyme in the upper villi of the small intestinal mucosa, closely related to the function of the intestinal chemical barrier. And malondialdehyde (MDA) is an indicator of lipid peroxidation. The expression of DAO and MDA has often been used to reflect the intestinal injury degree. The expressions of DAO and MDA in intestinal tissue was shown 4 h after resuscitation in Fig. 6C and D. Levels of DAO and MDA in other groups were higher than in the SHAM group (P < 0.001). Among all experimental groups, a substantial drop in the amount of DAO (SF injection vs. CON: P < 0.001; SF-delay vs. CON: P < 0.001) and MDA (SF injection vs. CON: P < 0.001; SF-delay vs. CON: P < 0.001) expressions were observed in SF injection and SF injection-group. Furthermore, the levels of DAO (P < 0.001) and MDA (P < 0.001) in SF injection group were also lower than those in the SF-delay group.
3.7. Effect of shenfu injection in intestinal tight junction proteins
The Western blot results demonstrated that the levels of small intestinal tight junction proteins such as Claudin-1 and ZO-1 in the CON group were markedly lower than those in the SHAM group (ZO-1, P = 0.0145; Claudin-1, P < 0.001) (Fig. 6E and F. The SF injection group expressed significantly more ZO-1 (P = 0.045) and Claudin-1(P = 0.004) than the CON group. In addition, when comparing the SF-delay group, the above protein concentrations in the SF injection group (ZO-1, P = 0.66; Claudin-1, P = 0.27) were also higher without revealing statistically significant differences (Fig. 6). The SF-delay group did not significantly differ from the CON group in ZO-1 (P = 0.23) and Claudin-1 (P = 0.06), although it had higher levels than the CON group (Fig. 6).
4. Discussion
The present study demonstrated that SF injection could protect the intestinal mucosal barrier function after intestine I/R injury induced by SHS and resuscitation through improving intestinal microcirculation perfusion, inhibiting the excessive release of inflammatory factors and oxidative stress, In addition, the administration of Shenfu injection during the resuscitation period was more effective than that administrated 1 h after the completion of blood transfusion.
The study revealed that changes occur in both macrocirculatory and microcirculatory variables within critical organs (heart) as well as non-critical organs (intestines). Comprehensive blood resuscitation significantly improved macrocirculatory metrics and augments oxygenation within the cardiac microvasculature. Nevertheless, the efficacy of restoring oxygenation in the intestinal region exhibited a lesser degree of effectiveness [4]. Therefore, it was concluded that the intestine was more vulnerable than the heart to hemorrhagic shock. Furthermore, the intestine was more severely affected by resuscitation compared to the heart. In this study, we also found that microcirculation parameters followed macrocirculatory variables in the SHS and resuscitation rat model. MAP was increased by the whole blood resuscitation and SF/saline, the gut microcirculation improved, but remained suboptimal. This all rendered the gut as “the canary of the body”. Intestinal IRI can damage both mechanical and immunological barriers, leading to increased intestinal permeability. The most serious consequence is the translocation of enteric flora and endotoxins into the mesenteric lymph nodes and bloodstream, potentially causing secondary infection.
SF injection contained a variety of alkaloids, including aconitum alkaloids and ginsenosides, which has been shown to have a bidirectional regulatory impact on vascular tone due to the exciting actions of α-and β-adrenergic receptors of various alkaloids [22]. In 2017, Qin et al. demonstrated that SF injection substantially reduced the arterial blood lactate level following cardiopulmonary resuscitation in the swine model [23]. Furthermore, several clinical investigations have shown that SF injection to improve systemic macrocirculation dysfunction in critically ill patients. Based on active standardized therapy techniques, Liu et al. administered SF injection to septic patients by continuous intravenous dripping at a dosage of 100 ml every 12 h [24]. The results of blood gas and continuous cardiac output monitoring by pulse indicator showed that 24 h after SF injection administration, blood lactate and oxygen consumption of patients were significantly reduced, and the systemic vascular resistance index and central venous oxygen saturation were significantly increased [24]. Yang et al. revealed the beneficial impact of SF injection on auricular microcirculatory blood flow and blood flow velocity as early as 2003 [25]. Following that, Jiang and Xu et al. found that SF injection would significantly improved microcirculation after cardiogenic shock and septic shock, respectively [26,27]. Li et al. from Beijing Chaoyang Hospital recently showed that SF injection may successfully increase the cerebral microvascular blood flow index of pigs after hemorrhagic shock via controlling iNOS expression in cortical tissue [6]. In the present study, we observed that intestinal microcirculation was injuried at 40 min after shock, characterized as extensive zones of tiny intestine artery stasis, stoppage, and no discernible blood flow. And the gut microcirculation parameters after volume resuscitation of HS were obviously decreased when compared with the Sham group. However, resuscitation combined application of SF injection can significantly ameliorate microcirculation disturbance after HS in SF, and SF-delay groups compared with the CON group, which revealed that SF injection exhibited outstanding effect in correcting the tissue microvascular hypoperfusion after SHS and resuscitation. In addition, we found that IL-6 and TNF-α were significantly decreased in SF and SF-delay groups when compared with the CON group in this study. IL-6 and TNF-α were considered to be substantially correlated with the degree of intestinal I/R damage and micro-vascular permeability in a large number of published investigations. Based on animal models, SF injection could lessen the excessive release of inflammatory factors during the pathophysiological process of sepsis [20,[28], [29], [30]]. And SF injection would effectively reduce serum levels of IL-6, IL-8, and TNF-α in elderly patients with severe pneumonia after 7 days of consecutive administration [31]. Furthermore, Jin et al. demonstrated that CRP, TNF-α, and IL-1 peaks in patients with acute myocardial infarction accompanied by cardiogenic shock dropped considerably following SF injection treatment (100 ml per 24 h) [32]. These findings are also consistent with the findings of our investigation. Therefore, the protective effect of SF injection on intestinal microcirculation would be related to the inhibition of pro-inflammatory molecules.
As a highly active intracellular enzyme produced by the intestinal epithelial cells, the levels of DAO activity is a valuable biomarker for estimating the severity of intestinal barrier damage, while MDA is known as the end product of lipid peroxidation, which can reflect the extent of lipid peroxidation and membrane injury. Our findings demonstrated that expressions of DAO and MDA were dramatically decreased and the levels of tight junction proteins on mucosal epithelial cells were significantly increased in SF and SF-delay groups. Therefore, we have reasons to believe SF injection can enhance intestinal barrier function through decreasing DAO and MDA expression and increasing expressions of tight junction proteins on mucosal epithelial cells following SHS and resuscitation in rats.
I/R injury induced by HS could result in endothelial barrier dysfunction, which would increase microvasculature permeability and interstitial edema [5,33]. In a study by Li et al., SF injection has shown to be efficient in increasing the levels of Na+/K + -ATPase and Ca + ATPase in intestinal epithelial cells, preventing lipid peroxidation and protecting the gut from inflammatory damage after hemorrhagic shock [34]. Another study also found that microvascular endothelial was serious injured after SHS and resuscitation, including loss of normal cell morphology, mitochondrial swelling, crista rupture, cavity change, tight junction injury and cortical interstitial edema. And SF injection could reduce the microvascular endothelium damage in the cortical tissue when compared with saline group [6]. By modulating ZO-1, Occludin, Claudin-1, and vasodilator-stimulated phosphoprotein expressions in rats, SF injection could alleviate sepsis-induced gut-barrier dysfunction and liver injury and inhibit increased levels of pro-inflammatory factors and intestinal mucous membrane permeability [28]. In our study, we found that the expressions of ZO-1, Occludin, Claudin-1, and vasodilator-stimulated phosphoprotein was decreased in rats SHS and resuscitation model. And SF injection significantly improved the expressions of ZO-1, Occludin, Claudin-1, and vasodilator-stimulated phosphoprotein. Therefore, we believed that SF injection decreased intestinal mucous membrane permeability, protected intestinal barrier dysfunction. However, Xing et al. discovered that SF injection had a dose-dependent effect on the villus and glandular structure of the ileum [35]. Only a few trials have been conducted to investigate the safety of SF injection in hemorrhagic shock-related organ failure.
The optimal strategy for SF injection in treating SHS-induced acute intestinal dysfunction remains undefined. The reasons for this uncertainty could be threefold. Firstly, the pharmacokinetic features of SF injection during the hemorrhagic shock and subsequent resuscitation process were still imperfect. Li et al. found that the ginsenoside metabolized very rapidly in rats; blood concentrations of some species would not be measurable approximately 0.33 h after dosing [36]. Furthermore, Du and colleagues employed the pharmacology effect method to determine the pharmacokinetic parameters of SF injection by constructing the acute myocardial infarction models in rats [37]. The time-stock of organisms curves with the 20 mL/kg dose showed a peak at 10 min postdose followed by a rapid decline and returned to almost 0 by 1 h after administration. The above findings suggest that SF injection is fast onset and rapid metabolism. Secondly, there was also an inconsistency between intestinal injuries during the ischemic period and the reperfusion period. The former is characterized by insufficient oxygen supply induced by a sharp decrease in tissue perfusion, while the latter is associated with a series of cascade reactions, including inflammatory responses, oxidative stress with the release of large amounts of free radicals, and calcium overload. Further studies should investigate the optimal route and the duration of administration. Thirdly, considering the prognostic uncertainty and the negative effect of excessive liquid infusion on the systemic hemodynamics, coagulation, and cardiac and endothelial function, hemorrhagic shock patients are commonly treated with damage-control resuscitation strategies, which include permissive hypotension and restrictive fluid resuscitation strategy. In practical application, the SF injection was administered to patients through IV infusion after dilution into a diluent solution such as saline or 5 % dextrose. However, Su and colleagues found that the continuous infusion for 24 h (10 ml/h) of pure SF injection could improve cardiac function in cardiogenic shock patients. Further research should be conducted to determine whether SF injection can successfully reduce the requirement for high-volume fluid resuscitation and vasoactive drugs in treating hemorrhagic shock.
5. Conclusion
In conclusion, SF injection could significantly protect the gut I/R injury after SHS and resuscitation by improving intestinal microcirculation. In addition, the effect becomes more pronounced if administration during the initial resuscitation phase.
Limitations
Despite these findings, the study also has some limitations. Firstly, as the observation period in this study is short-term, the animal model cannot fully mimic the complexity of the SHS pathophysiology. Nevertheless, published studies have demonstrated that ischemia-induced intestinal damage begins after shock onset and increases progressively to peak after 3 h. Secondly, intestinal microcirculation improvement may be partly owed to the elevated MAP, although the target arterial blood pressure and volume of fluid resuscitation are almost comparable in each test group. It is important to note that separation of micro- and macrocirculation is prevalent in SHS patients. Furthermore, the intestinal microcirculation status in the control group is not necessarily improved even with normal MAP after successful resuscitation. This means that the use of SF injection indeed affects decreasing intestinal ischemia-reperfusion injury. Thirdly, SF injection is a compound liquid of traditional Chinese herbal preparations, so it is difficult to investigate the exact molecular mechanism in organ protection for severe patients. The primary purpose of this study was to preliminarily explore the potential of SF injection in the protection of intestinal injury after SHS and optimal administration time. In the future, we will use network pharmacology approaches to validate these potential mechanisms.
Funding statement
This research was funded by the National Natural Science Foundation of China (No. 82072134), the basic and clinical cooperative research promotion plan of Anhui Medical University (No. 2019xkjT028), the National Natural Incubation Fund (No. 2020GMFY05), the Anhui Provincial Department of Education University Outstanding Top Talent Cultivation funding project (No:gxyq2020007), the Key Projects of University Excellent Young Talents Support Plan (gxyqZD2018026), the Discipline Construction Fund (No: 9101001821), Postgraduate Innovation Research and Practice Program of Anhui Medical University (YJS20230085), the Anhui University Excellent Young Talents Support Plan (No. gxyqZD2018026), and the Summit Discipline Construction Fund (No: 9101001804).
Institutional review board statement
The study protocol was approved by the Ethics Association of Anhui Medical University (Approval No.20200433).
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Tianfeng Hua: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization. Zongqing Lu: Writing – original draft, Methodology, Investigation, Data curation. Minjie Wang: Formal analysis. Yijun Zhang: Methodology. Yuqian Chu: Software. Yue Liu: Supervision. Wenyan Xiao: Methodology. Wuming Zhou: Data curation. Xuanxuan Cui: Data curation. Wei Shi: Writing – review & editing. Jin Zhang: Formal analysis. Min Yang: Visualization, Validation, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ruseckaite R., McQuilten Z.K., Oldroyd J.C., et al. Descriptive characteristics and in-hospital mortality of critically bleeding patients requiring massive transfusion: results from the Australian and New Zealand Massive Transfusion Registry. Vox Sang. 2017;112(3):240–248. doi: 10.1111/vox.12487. [DOI] [PubMed] [Google Scholar]
- 2.Naghavi M., Abajobir A.A., Abbafati C., et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadatani Y., Watanabe T., Shimada S., et al. Microbiome and intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 2018;63(1):26–32. doi: 10.3164/jcbn.17-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Iterson M., Bezemer R., Heger M., et al. Microcirculation follows macrocirculation in heart and gut in the acute phase of hemorrhagic shock and isovolemic autologous whole blood resuscitation in pigs. Transfusion. 2012;52(7):1552–1559. doi: 10.1111/j.1537-2995.2011.03471.x. [DOI] [PubMed] [Google Scholar]
- 5.Alves N.G., Trujillo A.N., Breslin J.W., et al. Sphingosine-1-Phosphate reduces hemorrhagic shock and resuscitation-induced microvascular Leakage by protecting endothelial mitochondrial integrity. Shock. 2019;52(4):423–433. doi: 10.1097/SHK.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J., Li Z., Yuan W., et al. Shenfu injection improves cerebral microcirculation and reduces brain injury in a porcine model of hemorrhagic shock. Clin. Hemorheol. Microcirc. 2021;78(2):175–185. doi: 10.3233/CH-211100. [DOI] [PubMed] [Google Scholar]
- 7.Filho I.T. Hemorrhagic shock and the microvasculature. Compr. Physiol. 2018;8(1):61–101. doi: 10.1002/cphy.c170006. [DOI] [PubMed] [Google Scholar]
- 8.Dickson K., Malitan H., Lehmann C. Imaging of the intestinal microcirculation during acute and Chronic inflammation. Biol. Bull. 2020;9(12) doi: 10.3390/biology9120418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink M.P., Delude R.L. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Crit. Care Clin. 2005;21(2):177. doi: 10.1016/j.ccc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Chen P., Billiar T. In: Gut Microbiota and Pathogenesis of Organ Injury. Chen P., editor. 2020. Gut Microbiota and multiple organ dysfunction syndrome (MODS) pp. 195–202. [DOI] [PubMed] [Google Scholar]
- 11.Zuo T., Zhang F., Lui G.C.Y., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–+. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vollmar B., Menger M.D. Intestinal ischemia/reperfusion: microcirculatory pathology and functional consequences. Langenbeck's Arch. Surg. 2011;396(1):13–29. doi: 10.1007/s00423-010-0727-x. [DOI] [PubMed] [Google Scholar]
- 13.Yanling X., Yu C., Guoliang X., et al. Research progress of shenfu injection's material foundation and its mechanism on shock. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica. 2018;20(2):294–297. [Google Scholar]
- 14.Shao F., Li H., Li D., et al. Effects of Shenfu injection on survival and neurological outcome after out -of -hospital cardiac arrest: a randomised controlled trial. Resuscitation. 2020;150:139–144. doi: 10.1016/j.resuscitation.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Huang P., Guo Y., Feng S., et al. Efficacy and safety of Shenfu injection for septic shock: a systematic review and meta-analysis of randomized controlled trials. AJEM (Am. J. Emerg. Med.) 2019;37(12):2197–2204. doi: 10.1016/j.ajem.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Liang N., Huizhi M., Yang F., et al. Effects of Shenfu injection on hemodynamics in hemorrhagic shock dogs. Chin Hosp Pharm J. 2014;34(8):620–623. [Google Scholar]
- 17.Zhang M.-q., Zhang Q., Yuan W., et al. Protective effect of shenfu injection on vascular endothelial damage in a porcine model of hemorrhagic shock. Chin. J. Integr. Med. 2022;28(9):794–801. doi: 10.1007/s11655-021-2876-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J., Song W., Xu S., et al. Shenfu injection promotes vasodilation by enhancing eNOS activity through the PI3K/akt signaling pathway in vitro. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu W., Jiang R.-l., Wang L.-c., et al. Effect of Shenfu injection on intestinal mucosal barrier in a rat model of sepsis. AJEM (Am. J. Emerg. Med.) 2015;33(9):1237–1243. doi: 10.1016/j.ajem.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 20.Liu X., Ai F., Li H., et al. Anti-inflammatory effects of shenfu injection against acute lung injury through inhibiting HMGB1-NF-κb pathway in a rat model of endotoxin shock. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/9857683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Backer D., Hollenberg S., Boerma C., et al. How to evaluate the microcirculation: report of a round table conference. Crit. Care. 2007;11(5) doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian Y., Li P., Lv B., et al. Heart function and thoracic aorta gene expression profiling studies of ginseng combined with different herbal medicines in eNOS knockout mice. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-15819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin Q., Wu C.-j., Yang J., et al. Effects of shenfu injection on cerebral metabolism in A porcine model of cardiac arrest. Chin. J. Integr. Med. 2017;23(1):33–39. doi: 10.1007/s11655-016-2616-9. [DOI] [PubMed] [Google Scholar]
- 24.Yang L., Li K., Fan K., et al. Effect of shenfu injection on hemodynamics in early stage of septic shock by PICCO monitor. J. Emerg. Tradit. Chin. Med. 2017;26(11):1993–1995. [Google Scholar]
- 25.Yang F., Zheng Y., Li D., et al. Effect of shenfu injection on microcirculation. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2003;20(1):91–100. [PubMed] [Google Scholar]
- 26.Li J., Lanbin Y., Rong Y., et al. Dose-dependent effect of shenfu injection on microcirculatory indexes of early- and mid-stage cardiogenic shock rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 2017;28(1):55–60. [Google Scholar]
- 27.Song X., Lan Y., Guo H., et al. Eeffects of shenfu injection combined with norepinephrine on sublingual microcirculation in septic shock rabbits. Chinese Journal of Microcirculation. 2020;30(1):5–10. [Google Scholar]
- 28.Liu F., Liu J., Liu Y., et al. Shen-Fu Decoction could ameliorate intestinal permeability by regulating the intestinal expression of tight junction proteins and p-VASP in septic rats. J. Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113562. [DOI] [PubMed] [Google Scholar]
- 29.Jin S., Jiang R., Lei S., et al. Shenfu injection prolongs survival and protects the intestinal mucosa in rats with sepsis by modulating immune response. Turk. J. Gastroenterol. 2019;30(4):364. doi: 10.5152/tjg.2019.18418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., Liu F., Liang T., et al. Efficacy of Shenfu decoction on sepsis in rats with condition induced by cecal ligation and puncture. J. Tradit. Chin. Med. 2020;40(4):621–628. doi: 10.19852/j.cnki.jtcm.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Lv S.J., Lai D.P., Wei X., et al. The protective effect of Shenfu injection against elderly severe pneumonia. Eur. J. Trauma Emerg. Surg. 2017;43(5):711–715. doi: 10.1007/s00068-016-0713-2. [DOI] [PubMed] [Google Scholar]
- 32.Jin Y.Y., Gao H., Zhang X.Y., et al. Shenfu Injection inhibits inflammation in patients with acute myocardial infarction complicated by cardiac shock. Chin. J. Integr. Med. 2017;23(3):170–175. doi: 10.1007/s11655-016-2749-x. [DOI] [PubMed] [Google Scholar]
- 33.Sawant D.A., Tharakan B., Hunter F.A., et al. The role of intrinsic apoptotic signaling in hemorrhagic shock-induced microvascular endothelial cell barrier dysfunction. Journal of Cardiovascular Translational Research. 2014;7(8):711–718. doi: 10.1007/s12265-014-9589-x. [DOI] [PubMed] [Google Scholar]
- 34.Liang Y., Li C., Yuan W., et al. Protective effect of Shenfu on gut epithelium in a porcine model of hemorrhagic shock. J. Invest. Med. 2021;69(7):1360–1366. doi: 10.1136/jim-2021-001939. [DOI] [PubMed] [Google Scholar]
- 35.Xing X., Jiang R., Wang L., et al. Shenfu injection alleviates intestine epithelial damage in septic rats. AJEM (Am. J. Emerg. Med.) 2015;33(11):1665–1670. doi: 10.1016/j.ajem.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Zhengguang L. Shandong University; 2014. The Therputeeutic Effects of Shenfu Injection on the Multiple Organ Dysfunction Syndrome in Elderly. [Google Scholar]
- 37.Ting D., Rongjin S., Guoliang X., et al. Study on determining pharmacokinetics parameters of Shenfu injection by pharmacology effect method. Chin. J. Clin. Pharmacol. Therapeut. 2012;17(1):69–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.