Abstract
In somatic cells, DNA repair is attenuated during mitosis to prevent the formation of anaphase bridges and facilitate the proper segregation of sister chromatids. Irradiation-induced γH2AX foci persist for hours in M phase somatic cells. However, we observed that anaphase bridges formed in a significant fraction of mouse zygotes irradiated during mitosis. Additionally, γH2AX signals in M phase zygotes peaked 30 min after irradiation and subsequently reduced with a half-life within 1–2 h. These results suggest that the DNA repair system may operate efficiently in M phase zygotes following irradiation, leading to the frequent formation of anaphase bridges. The absence of H2AX promoted the successful segregation of sister chromatids and enhanced the development of embryos to the blastocyst stage. The DNA repair system may be differentially regulated during the M phase of the first cell cycle to ensure the immediate elimination of damaged zygotes, thereby efficiently preventing transmission of mutations to subsequent generations.
Keywords: DNA repair, H2AX, γH2AX, M phase/mitosis, Zygotes
Among the various DNA lesions induced by genotoxic agents, double-strand breaks (DSBs) are arguably the most lethal type of DNA damage. Unrepaired or improperly processed DSBs can lead to the loss of genetic information or chromosomal translocations, resulting in the accumulation of mutations or even cell death. DSBs are primarily repaired by non-homologous end joining (NHEJ) before DNA synthesis, whereas homologous recombination (HR) becomes progressively dominant as DNA is replicated and sister chromatids become available from S phase [1]. However, during the M phase, key proteins involved in NHEJ and HR, such as ring finger protein 8 (RNF8), p53-binding protein 1 (53BP1), and X-ray repair cross-complementing protein 4 (XRCC4), are not recruited to DSBs, resulting in the suppression of NHEJ- and HR-dependent DNA repair [2,3,4,5]. Enforced recruitment of these proteins and subsequent activation of both pathways at M phase DSBs can lead to increased anaphase bridge formation and failed chromatid separation [3, 5].
DSBs activate the ataxia telangiectasia mutated (ATM) kinase and the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which phosphorylate multiple proteins involved in DNA repair, including the H2A histone family member Η2ΑΧ [6,7,8]. The resultant gamma H2AX (γH2AX) amplifies ATM signals and creates docking sites for downstream DNA repair proteins [9]. In H2AX−/− somatic cells, irradiation-induced focal formation of these downstream proteins and DNA repair are impaired [10, 11]. In interphase somatic cells, γH2AX signals intensify upon the induction of DSBs and diminish as these breaks are repaired. Accordingly, quantification of γH2AX foci or intensity is commonly used for assessing the numbers of DSBs [12, 13]. However, in M phase somatic cells, γH2AX foci form and persist in response to DNA damage induction due to the silenced NHEJ- and HR-dependent DNA repair [3].
In interphase zygotes exposed to irradiation, both ATM and DNA-PKcs are immediately activated [14] and the dynamics of γH2AX signals mirror those in irradiated interphase somatic cells (our unpublished results), indicating a functional DNA repair system. When zygotes enter M phase, DNA-PKcs becomes the primary kinase activating H2AX [14]. We observed > 90% chromatin bridge formation in 2-cell stage embryos following 10 Gy irradiation at the M phase of the first cell cycle [14]. Nevertheless, it remains unclear whether these structures originate during the M phase and how the DNA repair system is regulated during the M phase of the first cell cycle.
Materials and Methods
In vitro fertilization (IVF)
All procedures involving animals were reviewed and approved by the University of Tokyo Institutional Animal Care and Use Committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH, Bethesda, MD, USA). Spermatozoa were obtained from male ICR mice that were retired from breeding (Japan SLC, Inc., Shizuoka, Japan). The spermatozoa were preincubated in human tubal fluid (HTF) for 2 h before insemination. H2AX+/− and H2AX−/− mice were previously generated using the CRISPR/Cas9 system and confirmed by DNA sequencing, PCR, and electrophoresis (our unpublished results). H2AX+/− male and female mice were mated to produce H2AX+/+ (wild type, WT) and H2AX−/− (knock-out type, KO) female mice. H2AX-WT and H2AX-KO MII oocytes were collected from 8-week-old female littermates and superovulated, as previously described [14]. WT and maternal H2AX-deleted (hereafter designated H2AX-deleted and H2AX-KO) zygotes were obtained by IVF of WT sperm and H2AX-WT and H2AX-KO oocytes, respectively, in HTF medium. The complete absence of H2AX protein in H2AX-deleted zygotes was confirmed by immunostaining in each experiment using the mutant. Fertilized oocytes with two pronuclei, also known as zygotes, were selected and washed with potassium simplex optimized medium (KSOM) at 5 h post-insemination (HPI). Both HTF and KSOM media were covered with liquid paraffin and preincubated overnight before use in an incubator with a 5% CO2 / 95% air atmosphere at 38°C. Blastocyst formation was assessed at 96 HPI.
γ-irradiation during M phase and examination of anaphase bridges
Zygotes and 2-cell stage embryos were cultured in KSOM until 12 and 32 HPI, respectively, and then arrested at M phase in KSOM containing 0.5 μl/ml nocodazole (#140-08531, FUJIFILM Wako Pure Chemical Corp., Osaka, Japan). M phase zygotes at 15 HPI and 2-cell stage embryos at 35 HPI were then exposed to γ-rays emitted by 137Cs (Gammacell 3000 Elan; MDS Nordion, Ottawa, ON, Canada) at room temperature, with a dose rate of 6.6 Gy/min for 5 sec to reach 0.5 Gy or 1 min 31 sec to reach 10 Gy. Control (nonirradiated) embryos were subjected to the same temperature for the same time as their irradiated counterparts. One hour after irradiation, embryos were transferred to nocodazole-free KSOM to resume development for 1–1.5 h and then fixed with 3.7% paraformaldehyde (PFA) in PBS for 20 min. Fixed embryos were mounted on glass slides using VECTASHIELD mounting medium with 4′,6-diamidino-2-phenylindole (DAPI, #H-1200; Funakoshi, Tokyo, Japan).
Immunostaining
γH2AX dynamics were examined by fixing M phase zygotes at specified time points after irradiation with PBS containing 3.7% PFA and 0.2% Triton X-100. M phase WT and H2AX-deleted zygotes were arrested by nocodazole as described earlier and fixed at 15 HPI at 25°C for 20 min with PBS containing 3.7% PFA and 0.2% Triton X-100.
The fixed and permeabilized embryos were washed three times with PBS containing 0.1% bovine serum albumin (BSA) and incubated overnight at 4°C with a 1:1000 dilution in wash solution of mouse monoclonal antibody targeting H2AX phosphorylated at S139 (#05-636, clone JBW301; Sigma-Aldrich, Tokyo, Japan) or 1:500 dilution in wash solution of anti-H2AX rabbit polyclonal antibody (#20669; Abcam PLC, Cambridge, UK). Embryos were washed three times with PBS containing 0.1% BSA and incubated at 25°C for 1 h with 1:100 dilution in wash solution of either Alexa Fluor 488-labeled goat anti-mouse IgG secondary antibody (#A11001; Invitrogen, Carlsbad, CA, USA) or Alexa Fluor 647-labeled donkey anti-rabbit IgG secondary antibody (#A31573; Invitrogen). Finally, the embryos were washed and mounted on glass slides using VECTASHIELD mounting medium containing DAPI.
Image acquisition and processing
All images were captured by confocal laser scanning microscopy using a model FV3000 microscope (Olympus, Tokyo, Japan). The data were analyzed using ImageJ software (NIH, Bethesda, MD, USA).
Results
Zygotes irradiated at M phase display exceptionally high rate of formation of anaphase bridges
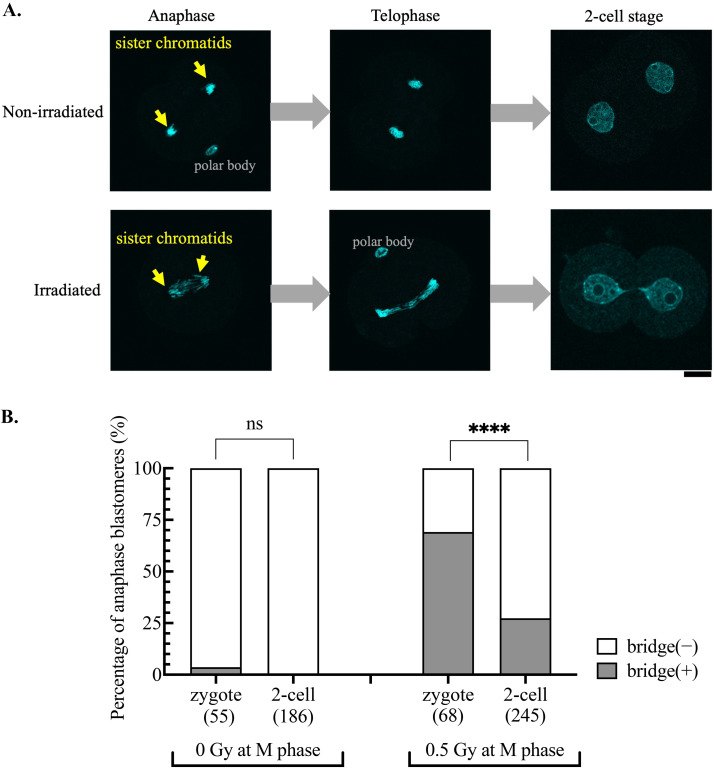
Analysis of zygotes fixed at the anaphase and telophase stages revealed that chromatid separation failure had already occurred in zygotes irradiated during M phase (Fig. 1A). Even at a low irradiation dose (0.5 Gy), anaphase bridges were observed in nearly 70% of the irradiated zygotes (Fig. 1B). This was interesting, considering that irradiation at the same dose led to the formation of anaphase bridges in < 5% of M phase somatic cells, a percentage that increased to approximately 60% only when artificially activating NHEJ- and HR-dependent DNA repair [3]. The rate of anaphase bridge formation decreased to 27% in blastomeres irradiated during the M phase of the 2-cell stage (Fig. 1B).
Fig. 1.
Exceptionally high formation rate of anaphase bridges in zygotes irradiated during mitosis. A: Representative images of nonirradiated and 10 Gy-irradiated embryos, which were fixed and stained with DAPI at anaphase and telophase of the first cell cycle and 2 h after entering the 2-cell stage. Scale bar, 20 μm. B: The percentage of anaphase cells (blastomeres) with bridges. Zygotes and 2-cell stage embryos were arrested with nocodazole and irradiated at 15 and 35 HPI, respectively. One hour after irradiation, embryos were moved to a nocodazole-free medium for further development and fixed at anaphase. The cumulative results from three independent experiments are shown. The total number of blastomeres examined for each condition is indicated in the figure. Fisher’s exact test was used for statistical analysis (ns, not significant; **** P < 0.0001).
DNA damage repair is efficient in M phase zygotes
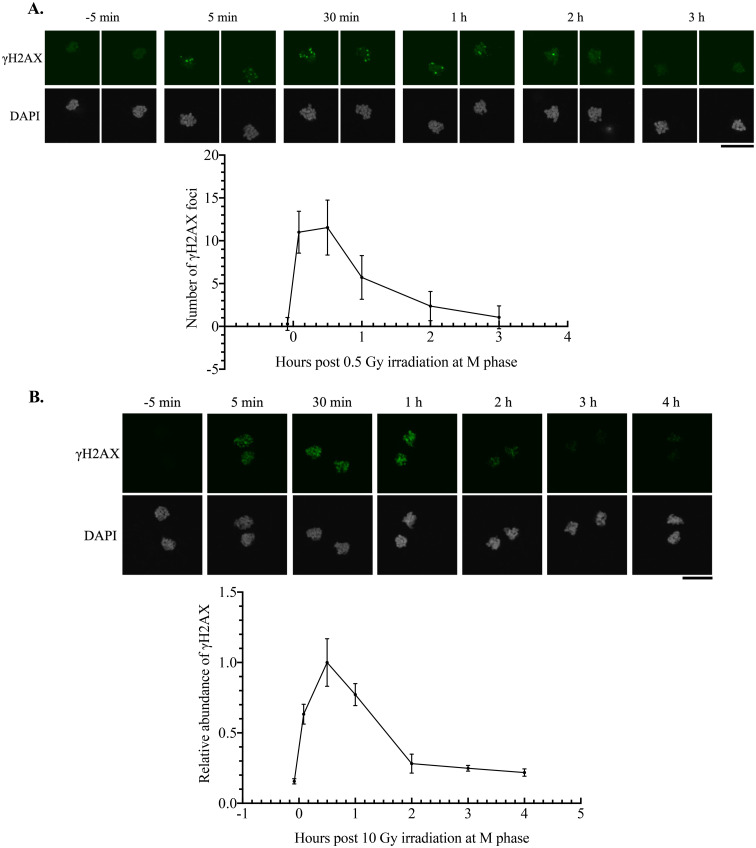
While somatic cells shut down NHEJ- and HR-dependent DNA repair as a defense against failure in sister chromatid separation, the high incidence of anaphase bridges in zygotes irradiated at M phase suggested an aberrantly active DNA repair machinery. To assess the DNA repair process, γH2AX dynamics were investigated in M phase zygotes over the timeline spanning from 5 min before irradiation to several hours post-irradiation. γH2AX signals increased immediately after irradiation with doses of 0.5 or 10 Gy. While the 0.5 Gy dose allowed the counting of foci numbers, DSBs introduced by 10 Gy were too abundant to display discrete γH2AX foci. Thus, the relative fluorescence intensity of γH2AX to DAPI was measured (Figs. 2A and B). At either irradiation dose, γH2AX signals in M phase zygotes peaked at 30 min post-irradiation, subsequently decreasing with a half-life within 1 h post-irradiation at 0.5 Gy and 2 h post-irradiation at 10 Gy (Figs. 2A and B). The γH2AX signals returned to near-background levels at 3 h post-irradiation (Figs. 2A and B).
Fig. 2.
Efficient resolution of γH2AX in irradiated M phase zygotes. A: γH2AX dynamics in zygotes irradiated with 0.5 Gy at 15 HPI (M phase). The upper panel shows representative images at each time point. Images of paternal and maternal chromosomes were taken separately to accurately count the number of γH2AX foci. Three independent experiments were conducted, and more than six embryos were examined for each condition in each experiment. B: γH2AX dynamics in zygotes irradiated with 10 Gy at 15 HPI (M phase). The upper panel shows representative images at each time point. Relative intensity of γH2AX to DAPI was measured and normalized to the average value at 30 min post-irradiation. Four independent experiments were conducted, and more than six embryos were examined for each condition in each experiment. Scale bar, 20 μm. Error bars indicate standard deviations.
Reduced formation of anaphase bridges following irradiation in the absence of H2AX
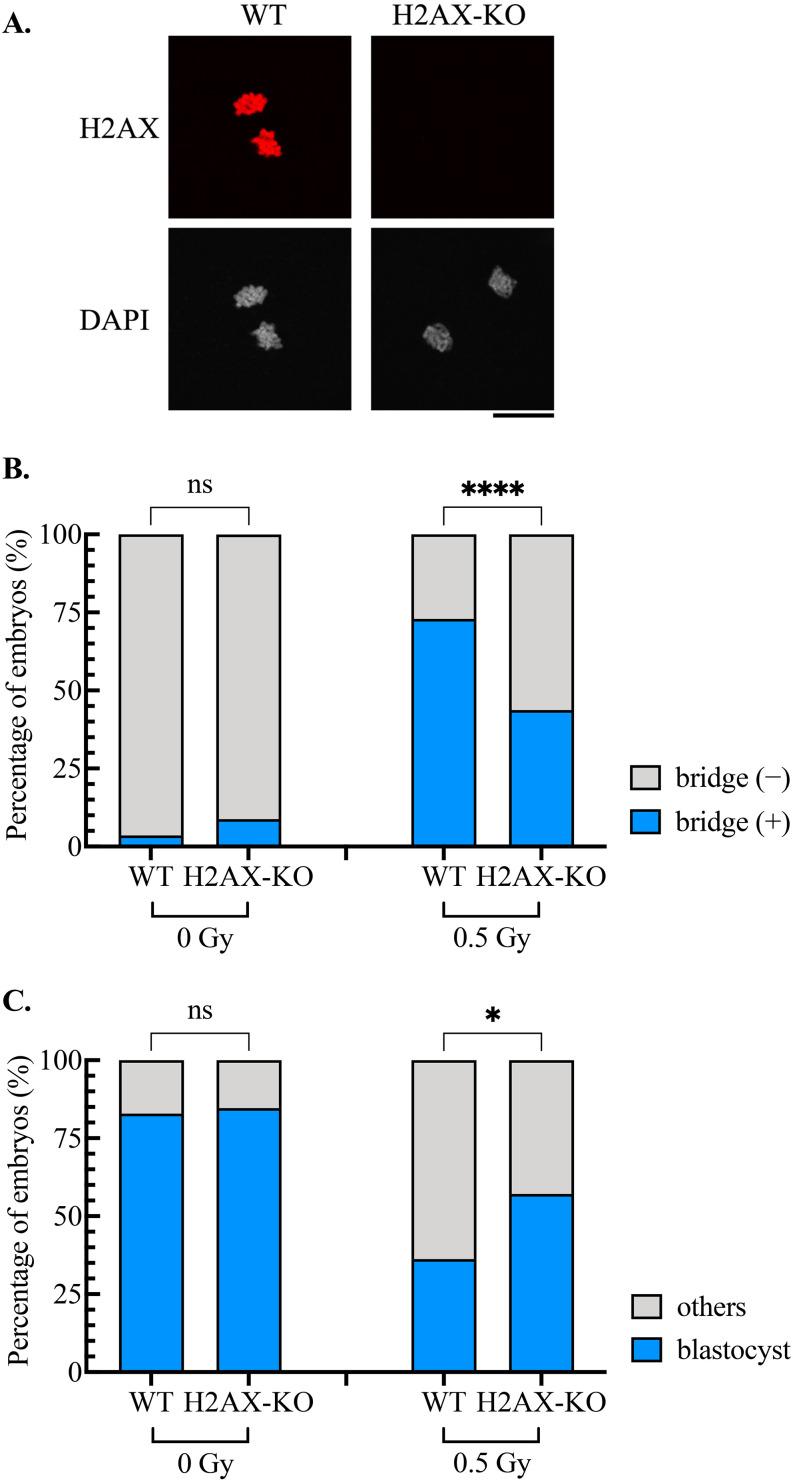
Given the high abundance of H2AX in zygotes [15] and the rapid γH2AX formation and resolution following DNA damage induction in M phase zygotes (Figs. 2A and B), we investigated the effect of the absence of H2AX on anaphase bridge formation using H2AX-deleted zygotes (Fig. 3A). Without irradiation, no significant difference in bridge formation was observed between WT and H2AX-deleted zygotes (Fig. 3B). However, upon irradiation at 0.5 Gy the number of anaphase bridges formed in H2AX-deleted zygotes was significantly lower than the number in WT zygotes (Fig. 3B). Because anaphase bridges are detrimental to cells, H2AX deletion contributed to increased blastocyst formation (Fig. 3C). These results suggest that the highly efficient DNA repair system in M phase restrains the development of damaged zygotes.
Fig. 3.
Effect of H2AX deletion on anaphase bridge formation and blastocyst development. A: Immunostaining for H2AX in wild type (WT) and H2AX-deleted (H2AX-KO) zygotes. Scale bar, 20 μm. B: Effect of H2AX deletion on anaphase bridge formation. The cumulative results from three independent experiments are shown. In total, 56 WT and 57 H2AX-KO nonirradiated zygotes, and 85 WT and 96 H2AX-KO irradiated zygotes were examined. Fisher’s exact test was used for statistical analysis (ns, not significant; **** P < 0.0001). C: Effect of H2AX deletion on blastocyst development. The cumulative results from three independent experiments are shown. In total, 70 WT and 52 H2AX-KO nonirradiated zygotes, and 58 WT and 49 H2AX-KO irradiated zygotes were examined. Fisher’s exact test was used for statistical analysis (ns, not significant; * P < 0.05).
Discussion
This study reveals an efficient functional DNA repair system in M phase zygotes. NHEJ- and HR-dependent DNA repair is suppressed in M phase somatic cells to avoid unsuccessful segregation of sister chromatids. Recently, two research teams independently reported that DNA polymerase theta (Polθ)-dependent alternative end joining (Alt-EJ) is specifically activated during mitosis for DSB repair [16, 17]. One of the teams even observed that γH2AX signals formed and then decreased post-irradiation in mitosis [17]. However, γH2AX signals were only recorded at 1 and 5 h post-irradiation in that study, and it was unclear when the decrease occurred. γH2AX signals remained higher than the background level even 5 h after irradiation [17]. The results shown in Fig. 2 suggest that DNA repair is more efficient in M phase zygotes.
A critical question is why the DNA repair system remains efficient during zygotic mitosis. Mitosis represents the most vulnerable period throughout the cell cycle, when cells are most easily killed by irradiation and other genotoxic agents [18]. Zygotes irradiated at mitosis were even more sensitive to irradiation in terms of the prevailing formation of anaphase bridges shown in this study, and the highly limited developmental potential of embryos irradiated at the first mitosis, with most losing the ability to complete the second mitosis, as we previously demonstrated [14]. Although compromising the DNA repair system via H2AX deletion may improve the survival of irradiated embryos, at least until the blastocyst stage (Fig. 3), these survivors are likely to be burdened by a high mutation load. Since all cells in an organism originate from the zygote, DNA damage in the zygote can lead to genomic instability in the organism that develops from the zygote and in subsequent generations. Thus, efficient DNA repair in M phase may act as a mechanism for screening severely damaged zygotes and avoiding the accumulation of mutations. This allows gestational carriers to discard embryos with limited developmental potential as early as possible [19].
Another important question is how efficiently DNA repair is maintained during zygotic mitosis. NHEJ-dependent DNA repair is more efficient than HR or Alt-EJ repair [20, 21]. NHEJ-dependent DNA repair may be operative in M phase zygotes. Two key factors act as switches, controlling the activation and deactivation of NHEJ.While RNF8 and 53BP1 are recruited to DSBs during interphase and facilitate NHEJ, these two proteins are phosphorylated by CDK1 during mitosis and thus excluded from DNA damaged sites [2, 3]. We examined the localization of both proteins in zygotes by immunostaining. Neither protein was detected, even in interphase zygotes (data not shown), probably because the antibodies we used were not suitable for embryos. The reported high abundance of H2AX [15] may also contribute to efficient DNA repair in zygotes, and H2AX-deleted zygotes irradiated during the M phase showed a significant decrease in the formation rate of anaphase bridges.
Cancer cells exhibit many characteristics of early embryonic cells, including continuous and rapid division. As many cancer medicines targeting mitotic cell death have been developed [22], a thorough investigation of the DNA repair system in M phase zygotes may provide valuable insights into the mechanism of action of these medicines, which is essential for improving their precision and efficacy.
Conflict of interests
The authors declare no conflicts of interest that could be perceived as prejudicing the impartiality of this research.
Acknowledgments
This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Nos. 19H05752, 21H04752, 22K15022, and 16H06276), the Japan Society for the Promotion of Science (No. 202012040), and the Hirose Foundation.
References
- 1.Rothkamm K, Krüger I, Thompson LH, Löbrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol 2003; 23: 5706–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giunta S, Belotserkovskaya R, Jackson SP. DNA damage signaling in response to double-strand breaks during mitosis. J Cell Biol 2010; 190: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orthwein A, Fradet-Turcotte A, Noordermeer SM, Canny MD, Brun CM, Strecker J, Escribano-Diaz C, Durocher D. Mitosis inhibits DNA double-strand break repair to guard against telomere fusions. Science 2014; 344: 189–193. [DOI] [PubMed] [Google Scholar]
- 4.Esashi F, Christ N, Gannon J, Liu Y, Hunt T, Jasin M, West SC. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 2005; 434: 598–604. [DOI] [PubMed] [Google Scholar]
- 5.Terasawa M, Shinohara A, Shinohara M. Canonical non-homologous end joining in mitosis induces genome instability and is suppressed by M-phase-specific phosphorylation of XRCC4. PLoS Genet 2014; 10: e1004563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007; 316: 1160–1166. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, Saha J, Beckmann PJ, Hendrickson EA, Davis AJ. DNA-PKcs promotes chromatin decondensation to facilitate initiation of the DNA damage response. Nucleic Acids Res 2019; 47: 9467–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 2001; 276: 42462–42467. [DOI] [PubMed] [Google Scholar]
- 9.Price BD, D’Andrea AD. Chromatin remodeling at DNA double-strand breaks. Cell 2013; 152: 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassing CH, Chua KF, Sekiguchi J, Suh H, Whitlow SR, Fleming JC, Monroe BC, Ciccone DN, Yan C, Vlasakova K, Livingston DM, Ferguson DO, Scully R, Alt FW. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc Natl Acad Sci USA 2002; 99: 8173–8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC, Nussenzweig A. Genomic instability in mice lacking histone H2AX. Science 2002; 296: 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sedelnikova OA, Pilch DR, Redon C, Bonner WM. Histone H2AX in DNA damage and repair. Cancer Biol Ther 2003; 2: 233–235. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell 2005; 20: 801–809. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Oda S, Suzuki MG, Mitani H, Aoki F. Cell cycle-dependent radiosensitivity in mouse zygotes. DNA Repair (Amst) 2022; 117: 103370. [DOI] [PubMed] [Google Scholar]
- 15.Nashun B, Yukawa M, Liu H, Akiyama T, Aoki F. Changes in the nuclear deposition of histone H2A variants during pre-implantation development in mice. Development 2010; 137: 3785–3794. [DOI] [PubMed] [Google Scholar]
- 16.Gelot C, Kovacs MT, Miron S, Mylne E, Haan A, Boeffard-Dosierre L, Ghouil R, Popova T, Dingli F, Loew D, Guirouilh-Barbat J, Del Nery E, Zinn-Justin S, Ceccaldi R. Polθ is phosphorylated by PLK1 to repair double-strand breaks in mitosis. Nature 2023; 621: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brambati A, Sacco O, Porcella S, Heyza J, Kareh M, Schmidt JC, Sfeir A. RHINO directs MMEJ to repair DNA breaks in mitosis. Science 2023; 381: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell 2004; 7: 637–651. [DOI] [PubMed] [Google Scholar]
- 19.Russell LB, Russell WL. An analysis of the changing radiation response of the developing mouse embryo. J Cell Physiol Suppl 1954; 43(Suppl. 1): 103–149. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Perrault AR, Takeda Y, Qin W, Wang H, Iliakis G. Biochemical evidence for Ku-independent backup pathways of NHEJ. Nucleic Acids Res 2003; 31: 5377–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scully R, Panday A, Elango R, Willis NA. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat Rev Mol Cell Biol 2019; 20: 698–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan KS, Koh CG, Li HY. Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis 2012; 3: e411. [DOI] [PMC free article] [PubMed] [Google Scholar]