Abstract
Pomelo (Citrus maxima), the largest citrus fruit, provides a variety of nutrients that have several health benefits, including antioxidant and antidiabetic functions. Antioxidants help combat oxidative stress by neutralizing reactive oxygen species (ROS) and reducing cellular damage. On the other hand, antidiabetic properties involve mechanisms such as enhancing insulin secretion, improving insulin sensitivity, inhibiting carbohydrate digestion and absorption, and regulating glucose metabolism. However, there is a lack of data on the comparative analysis of the physicochemical composition, bioactive properties, and antidiabetic effects of pomelo fruits grown in Bangladesh. To address this issue, the most common and popular high-yielding five cultivars of pomelo fruits grown in Bangladesh including LOCAL, BARI-2 (BARI: Bangladesh Agricultural Research Institute, Batabi Lebu-2), BARI-3, BARI-4, and BARI-6 were evaluated concerning proximate, minerals, and physicochemical properties with their antidiabetic and antioxidant properties. Research has revealed that all pomelo varieties contained a significant amount of proximate compositions and major minerals (Ca, Mg, K, Na, and Fe). The highest juice yield (75.37 ± 0.33 %), vitamin C content (79.56 ± 2.26 mg/100 mL of fresh juice), and carotenoid content (919.33 ± 0.62 μM β-Carotene Equivalent/g DM) were found in BARI-3 pomelo fruit and adhered to the sequence (p < 0.05): BARI-3 > LOCAL > BARI-4 > BARI-6 > BARI-2; BARI-3 > LOCAL > BARI-2 > BARI-4 > BARI-6, and BARI-3 > BARI-2 > BARI-6 > LOCAL > BARI-4, respectively. The anthocyanin content and inhibitory activity of α-glucosidase were found to be at their peak in the BARI-2 pomelo variety and the values were 50.65 ± 2.27 μg cyanidin 3-glucoside equivalents/100 g DM and 85.57 ± 0.00 μM acarbose equivalents/g DM, respectively. BARI-3 pomelo variety showed highest DPPH antioxidant capacity (170.47 ± 0.01 μM Trolox equivalents/g DM), while the BARI-6 pomelo variety exhibited the highest total phenolic content (6712.30 ± 1.84 μg gallic acid equivalents/g DM), and ferric-reducing antioxidant power activity (183.16 ± 0.01 μM Fe(II) equivalents/g DM). Therefore, this study explores the nutritional value and bioactivity of five popular pomelo varieties in Bangladesh, offering valuable insights for utilizing high-value citrus resources and understanding their health-promoting functions.
Keywords: Antidiabetic, Antioxidant, Bioactive properties, Pomelo, Proximate composition, Nutraceutical potential
Highlights
-
•
Five pomelo varieties were analyzed for nutritional content and phytochemicals.
-
•
Pomelo pulp was rich in minerals and phytochemicals, making it ideal for functional food.
-
•
All pomelo varieties exhibited significant antioxidant and antidiabetic activities.
-
•
The evaluated parameters were varied with the pomelo varieties studied.
Nomenclature
- BARI
Bangladesh Agricultural Research Institute
- C3GE
cyanidin 3-glucoside Equivalents
- AE
Acarbose Equivalents
- TPC
Total Phenolic Content
- TFC
Total Flavonoid Content
- GAE
Gallic acid Equivalent
- QE
Quercetin equivalents
- TE
Trolox Equivalents
- CE
β-Carotene Equivalents
- DM
Dry matter
- FRAP
Ferric-Reducing Antioxidant Power
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- HDPE
High-density Polyethylene
1. Introduction
The remarkable surge in the field of nutrition and health has necessitated the extensive exploration of diverse plant-based food sources for potential health benefits [1]. One such fruit that has gained significant attention is the pomelo (Citrus maxima), renowned for its unique flavor, bright color, and potential health-promoting properties [2]. In recent years, scientific interest in the nutritional composition and bioactive components of the pomelo has soared, aiming to unravel its therapeutic potential and offer valuable insights for public health and food industries [3,4]. Pomelo, commonly known as the king of citrus, belongs to the Rutaceae family and is primarily cultivated in Southeast Asian countries. Bangladesh, a country blessed with a favorable climate and fertile soil, has a rich diversity of agricultural produce. Amongst the wide range of fruits cultivated, pomelo stands out for its distinctive characteristics and potential health benefits. It is locally known as “Batabi lebu/Jambura” or “Badam Jamir”. People in Bangladesh primarily consume flesh and juice of pomelo fruit as fresh, enjoying them as a delicious and nutritious snack. The fruit is often peeled and eaten raw, or its segments are added to fruit salads for enhanced flavor and texture. Its tangy-sweet taste makes it a favorite among both adults and children. With its growing popularity and consumption worldwide, researchers have directed their efforts toward unraveling the complex composition of pomelo and understanding its various bioactive components [5,6]. Unfortunately, pomelo fruits grown in Bangladesh are under-researched. Most of the literatures are restricted to India, China, Thailand, and Vietnam. However, BARI has developed some new high-yielding commercial varieties cultivated in different regions in Bangladesh that have become a preferred choice among consumers for their refreshing flavor and health-promoting properties. Therefore, it is important to explore the nutritional information of pomelo fruit grown in Bangladesh. Accordingly, mostly cultivated five pomelo fruits are considered to investigate their nutritional profile and nutraceutical potential.
The nutritional profile of fruits plays a crucial role in promoting human health and preventing various diseases like diabetes, cancer, and other cardiovascular and degenerative diseases [7,8]. Pomelo is an excellent addition to a balanced diet because it is low in calories and high in fiber food. It contains essential vitamins such as vitamin C, B-complex, and essential oils which are vital for maintaining overall health and boosting the immune system [9]. Furthermore, the mineral composition of pomelo, including potassium, calcium, and magnesium, contributes to healthy bodily functions such as nerve transmission, muscle contraction, and bone strength [10]. Apart from its nutritional composition, pomelo also possesses a remarkable array of bioactive compounds. The presence of flavonoids such as naringin, hesperidin, dihydrochalcone, and neohesperidin provides antioxidant properties, helping to avoid conditions brought on by oxidative stress [11]. These bioactive compounds have demonstrated the potential to combat inflammation, reduce the risk of cardiovascular diseases, and even exhibit anticancer properties [12]. Importantly, some of these flavonoids have also been found to have inhibitory effects on α-glucosidase, an enzyme responsible for breaking down complex carbohydrates into simpler sugars. In the context of antidiabetic effects, inhibiting α-glucosidase activity can slow down the digestion and absorption of carbohydrates, thereby reducing the postprandial rise in blood glucose levels [13]. This is particularly beneficial for individuals with diabetes or those at risk of developing diabetes, as it aids in managing blood sugar levels. Exploring the variability in the content of these compounds among distinct cultivars of pomelo will allow us to identify potential differences in their health-promoting effects.
Antioxidants play a vital role in shielding the body from oxidative damage caused by harmful free radicals [13,14]. In recent years, the importance of dietary antioxidants in promoting human health has gained significant attention. Pomelo with its rich antioxidant content has the potential to act as a natural defense against various chronic diseases [15]. Evaluating the antioxidant capacity of the five pomelo fruit varieties will provide valuable insights into their scavenging capacity of free radicals and shield against damage caused by oxidative stress. While several studies have explored the nutritional and antioxidant potential of various fruits and vegetables, limited research has been conducted on the pomelo fruit varieties found in Bangladesh. Understanding the nutraceutical potential of these varieties is crucial, as it can lead to the creation of nutritionally beneficial foods, dietary aids, and even pharmaceutical applications.
Therefore, this work aims to shed light on the nutraceutical potential of five distinct pomelo varieties in Bangladesh. By evaluating the nutritional composition and antioxidant capacity of pomelo fruit varieties, we strive to provide a comprehensive understanding of their health-promoting properties. The findings of this study may aid in the advancement of novel dietary interventions and promote the consumption of selected pomelo varieties for improved health and well-being.
2. Materials and methods
2.1. Sample collection and preparation
Five cultivars of pomelo (Citrus maxima) fruits with uniform maturity and without any defects were used in this study. The cultivars were local (LOCAL), BARI Batabi Lebu-2 (BARI-2), BARI Batabi Lebu-3 (BARI-3), BARI Batabi Lebu-4 (BARI-4), BARI Batabi Lebu-6 (BARI-6) (Fig. 1). The Local variety was procured from the Dinajpur, Bangladesh local market. Conversely, BARI-2, BARI-3, BARI-4, and BARI-6 were collected from the Bangladesh Agricultural Research Institute (BARI), Joydebpur, Gazipur, Bangladesh (24° 05' 16.3" N, 90° 91' 81.7" E). According to the local harvest schedule each year, fruits were harvested in November, after 7 months of growth (blooming). The aforementioned variety was chosen as the test sample due to its widespread availability and excellent productivity among pomelo cultivars in Bangladesh.
Fig. 1.
Selected five varieties of pomelo fruits, and separated pulp from them.
The selected pomelo fruits were washed and peeled to separate the pulp manually. The portion of each fruit pulps were blended into juice using a stainless-steel manual press squeezer to get its single-strength juice, which was further used for physicochemical analysis. For the analysis of bioactive compounds, the pomelo pulps were placed onto the freeze-dryer plate and spread evenly. The plates were then kept in the freezer for pretreatment at −60 °C for 8 h. After that, the frozen samples were immediately transferred into the freeze dryer (BK-FD 12S, BIOBASE MEIHUA TRADING Co., Ltd, Jinan, Shandong, China). The drying was performed at a pressure of less than 10 Pa for 36–40 h. After drying, the sample was crushed using a grinder apparatus (Jaipan, JFM 1300) and passed across a sieve (Mesh No 50) to get the powder sample. The obtained pomelo pulp powder (PPP) samples were then sealed in high-density polyethylene (HDPE) bags, and kept at −18 °C for analysis.
2.2. Proximate composition analysis
The proximate composition including ash, moisture, crude fiber, and fat of five different varieties of pomelo fruit pulp was determined using the AOAC technique [16]. The Lowry method [17], which is based on the biuret reaction was employed to assess the protein content of pomelo pulp powder (PPP). The total amount of carbohydrates in the PPP was determined by deducting the value of moisture, ash, fat, fiber, and protein from 100 [18], and the equation was:
| (1) |
2.3. Mineral content analysis
The traditional method was employed to ascertain the key mineral content of pomelo pulp powder (PPP), including calcium (Ca), magnesium (Mg), iron (Fe), sodium (Na), and potassium (K) [19]. The titration method was used to measure the calcium and magnesium contents while Na2-EDTA served as a complexing agent. The redox titration was used to measure the iron content in PPP. The Na and K contents were evaluated using the emission spectrophotometer (Daihan Scientific Co., Korea).
2.4. Physiochemical analysis
2.4.1. Determination of juice yield, pH, total soluble solids (TSS), titratable acidity (TA), ripening index (RI)
The pH of fruit juice was measured with a digital pH meter (Hanna, Korea), which was calibrated utilizing three different standard pH buffers 4, 7, and 9. The TSS value of pomelo juice was calculated using a digital refractometer (HI 96800, Hanna Instruments, UK) with a precision of 0.2 % ⁰Brix. The titrimetric AOAC method was used to calculate the sample's titratable acidity [20]. The ripening index (RI) of fruits was determined by using the ratio TSS and TA [21].
2.4.2. Determination of vitamin C content in pomelo
The sample's vitamin C concentration was ascertained using the approach [22]. Briefly, 10 mL of 20 % metaphosphoric acid and 4 mL of juice sample were mixed properly. After that, Whatman No. 1 filter paper was used to filter the mixture. Then, 1 mL of filtrate juice was transferred into a beaker and 10 mL of distilled water was added. Afterward, 2 mL of the resultant mixture was taken into a new beaker, shaken properly, and titrated until a pink color appeared against 2, 6-dichlorophenolindophenol dye solution. The amount of vitamin C content was determined in line with the subsequent equation (2):
| (2) |
2.5. Bioactive compounds analysis
2.5.1. Extraction of bioactive compounds
The process of extracting bioactive substances was done according to Islam et al. [23]. Briefly, 2.5 g of PPP and 50 mL of 80 % methanol was combined in a glass conical flux at 1:20 (g/mL). The extraction process was done in a shaking water bath at 100 rpm for 1 h at room temperature. Subsequently, following a 10 min centrifugation (general centrifuge MF-300, HumanLab Instrument Co., Korea) at 4000 rpm, the sample was then filtered with Whatman No. 1 paper. Finally, the extract was collected in a Falcon tube and refrigerated at 4 °C for analysis.
2.5.2. Estimation of total phenolic content (TPC)
The TPC of PPP was assessed according to the modified method published by Kabir et al. [24]. In brief, 0.5 mL of PPP extract was mixed with 0.5 mL of Folin-Ciocalteu reagent, and neutralized with 1 mL of 7.5 % sodium bicarbonate. Then, the mixture was vortexed with the addition of 8 mL of distilled water. After centrifuging for 10 min at 4000 rpm, the mixture was kept at room temperature for 35 min in the dark. Finally, the results were articulated as equal in milligrams of gallic acid per gram of dry matter (mg GAE/g DM), with absorbance measured at 750 nm using a spectrophotometer (UV-1900i, Shimadzu, Japan).
2.5.3. Estimation of total flavonoid content (TFC)
The TFC of the PPP extract was assessed employing the colorimetric approach with some adjustments as reported by Islam et al. [13]. In short, a falcon tube was used to combine 1 mL of the extracts 0.3 mL NaNO2 (5 %, w/v) solution, and 4 mL of distilled water. After 5 min of resting, 0.3 mL AlCl3 (10 %, w/v) was added and let to stand for 1 min. Finally, 2.4 mL of distilled water and 2 mL of 1 M NaOH were added and mixed well. The mixture was centrifuged for 10 min at 4000 g, and then it was left for 15 min in the absence of light. The quercetin calibration curve was used to determine the TFC (mg QE/g DM) based on the 510 nm absorbance reading (R2 = 0.993).
2.5.4. Estimation of total carotenoid content (TCC)
The amount of TCC in PPP was determined using the way explained by Hasan et al. [21]. In this assay, a conical flask was used to combine 5 g of PPP with 50 mL of n-hexane-acetone-ethanol solution (at a ratio of 50:25:25, v/v). Then the mixture was transferred in a shaking water bath and the extraction was carried out at room temperature for 10 min at 100 rpm. After that, the resultant solution was centrifuged at 6500 rpm for 5 min. The volume of collected supernatant was adjusted using the extraction solvent to 50 mL and the absorbance was measured at 450 nm. Lastly, the outcomes of total carotenoids were articulated as equal in μM β-carotene per gram of dry matter (μM β-Carotene/g DM) using β-carotene as standard.
2.5.5. Estimation of total anthocyanin content (TAC)
The quantification of TAC was performed utilizing a spectrophotometric pH differential according to the article of Hasan et al. [25]. Briefly, the extracts of 1 mL were combined with a potassium chloride buffer of 0.025 M (pH 1.0). After 15 min of incubation, the mixture's absorbance was recorded at 510 and 700 nm. Again, extracts were combined with the same ratio of sodium acetate buffer 0.4 M (pH 4.5), and incubated for 15 min before measuring the absorbance at the same wavelengths. Finally, the following equation-3 was used to estimate the amount of TAC:
| (3) |
Where A is absorbance (at pH 1.0 and 4.5), MW stands for cyanidin 3-glucoside's molecular weight (449.2 g/mol), Ɛ is cyanidin 3-glucoside's molar extinction coefficient (26,900 L mol−1 cm−1), path-length (cm) is represented by Ɩ, and 1000 is used for conversion (g to mg). The findings of TAC were represented as μg of cyanidin 3-glucoside equivalents/100 g DM (μg C3GE/100 g DM).
2.5.6. Estimation of antioxidant activity (DPPH and FRAP assay)
DPPH radical scavenging activity and Ferric-reducing antioxidant power (FRAP) assay were used to determine the antioxidant properties of the PPP. The process of Islam et al. [23] was followed to evaluate the ability of PPP to scavenge DPPH radicals. After 15 min of stirring, the absorbance of a DPPH solution in 80 % methanol was adjusted to 0.650 at 515 nm using a spectrophotometer. Then the extracted sample (50 μL) and DPPH solution (1.950) were combined, vortexed, and left at room temperature in the dark for 30 min. Afterward, the resultant mixture's absorbance was calculated using Trolox as a standard and presented as μM Trolox equivalents per gram of dry matter (μM TE/g DM).
The FRAP assay was performed by the determination process by Hasan et al. [25]. Concisely, in an acetate buffer (pH 3.6), 20 mM iron (III) chloride solution, 10 mM TPTZ solution, and 40 mM HCl were mixed at a ratio of 10:1:1 (v/v), respectively, to make the FRAP reagent. Subsequently, 25 μL of the PPP extract was diluted with the FRAP reagent (1.975 mL) and maintained 4 min of incubation time. As a final point, the mixture's absorbance was measured at 593 nm and the findings were presented as μM iron (II) sulfate equivalents per gram of dry matter (μM Fe(II)E/g DM) utilizing a standard iron (II) sulfate solution.
2.5.7. Estimation of antidiabetic activity
To determine the antidiabetic activity of PPP extract using the Islam et al. [13] procedure, the inhibitory activity of α-glucosidase was assessed. Briefly, after mixing 50 μL of PPP extract with 100 μL of 0.1 U/mL α-glucosidase solution (in 0.1 M, pH 6.9 phosphate buffer), the mixture was incubated at 25 °C for 10 min. Subsequently, the mixture was incubated for 5 min with 50 μL of a 5 mM p-Nitrophenyl-D-glucopyranoside (pNPG) solution (0.1 M, pH 6.9 phosphate buffer). Finally, at 405 nm, the absorbance was measured and the results were reported as μM Acarbose equivalents per gram of dry matter (μM AE/g DM) using Acarbose as standard.
2.6. Statistical analysis
All the analyses were performed in this study in triplicate and the results were presented as means ± standard deviation. The statistical software (SPSS, version 26.0) was used to perform one-way analysis of variances (ANOVA). The mean comparison was carried out using Duncan's multiple range test for significant effect (p ≤ 0.05).
3. Results and discussion
3.1. Proximate composition
The proximate compositions of the selected five pomelo samples are presented in Table 1. The results of pomelo fruit pulp have shown that the moisture levels did not vary significantly (p > 0.05) among all varieties. The range of moisture content was found from 90.33 ± 1.84 to 87.56 ± 1.36 %, the highest moisture value in BARI-3 and lowest in LOCAL variety, respectively. The results were consistent with Toh et al. [26], who noticed 90.46 ± 0.09 % and 90.04 ± 0.06 % moisture in white and pink pomelo pulp respectively. In another study on four different types of Thai pomelo, the moisture content (88.9–87.5 %) was similar to the values discovered in this investigation [27]. There was no significant difference (p > 0.05) in the ash content between any of the pomelo fruit varieties. The pomelo fruit var. LOCAL (0.62 ± 0.01 %) had the maximum ash content, revealing that the local variety contains a considerably higher number of minerals. Whereas, the lowest amount of ash content is found in the BARI-3 (0.32 ± 0.03 %) pomelo variety.
Table 1.
Proximate composition of five different varieties of pomelo fruit.
| Pomelo varieties | Proximate composition (%) |
|||||
|---|---|---|---|---|---|---|
| Moisture | Ash | Protein | Fat | Fiber | Carbohydrate | |
| LOCAL | 87.56 ± 1.36a | 0.62 ± 0.01a | 0.45 ± 0.001b | 0.036 ± 0.01a | 1.26 ± 0.04a | 10.07 ± 0.03a |
| BARI-2 | 88.16 ± 1.96a | 0.49 ± 0.80a | 0.46 ± 0.001a | 0.041 ± 0.03a | 1.49 ± 0.05a | 9.26 ± 0.03a |
| BARI-3 | 90.33 ± 1.84a | 0.32 ± 0.03a | 0.44 ± 0.002c | 0.034 ± 0.01a | 1.20 ± 0.12a | 7.68 ± 0.01a |
| BARI-4 | 89.54 ± 1.15a | 0.43 ± 0.13a | 0.42 ± 0.001d | 0.042 ± 0.02a | 1.34 ± 0.04a | 8.23 ± 0.02a |
| BARI-6 | 88.75 ± 1.09a | 0.54 ± 0.08a | 0.39 ± 0.002e | 0.032 ± 0.01a | 1.48 ± 0.25a | 8.81 ± 0.02a |
Each value is expressed as mean ± SD and different small letters within the same column indicate significant (p ≤ 0.05) differences among various samples.
The BARI-2 variety showed significantly (p < 0.05) the highest protein content, followed by LOCAL, BARI-3, BARI-4, and BARI-6. The fat, fiber, and carbohydrate contents of selected five pomelo samples were closely comparable and no significant (p > 0.05) difference was observed. However, the range of total fat, fiber, and carbohydrate contents was 0.042 ± 0.02 to 0.032 ± 0.01 %, 1.49 ± 0.05 to 1.20 ± 0.12 %, and 10.07 ± 0.03 to 7.68 ± 0.01 %, respectively among the varieties. The amount of fiber found in this study was higher than Kongkachuichai et al. [27], who found 0.99 to 0.73 % in five Thai pomelo varieties. The fiber in food samples not only decreased or delayed the absorption of glucose into the blood but also increased insulin sensitivity by reducing hepatic liver content [28]. Therefore, the observed variance in proximate composition could potentially be attributed to a combination of regional variation, edaphic variables, climate fluctuations, and the genetic mix of individual fruit cultivars [29].
3.2. Mineral composition
The trace elements level in fruit pulp may fluctuate depending on the mineral composition of the soil in which it was cultivated, as well as agricultural techniques like the quantity and kinds of fertilizer applied, and weather conditions [30]. The mineral compositions such as Ca, Mg, K, Na, and Fe of pomelo pulp powder (PPP) were determined and summarized in Table 2. It was observed that all varieties of pomelo fruits possessed an excessive amount of Ca content, followed by K, Fe, Na, and Mg. The Ca content in selected five pomelo varieties ranged between 349.80 ± 39.67 to 126.53 ± 12.34 mg/100 g, where the highest Ca content was found in the BARI-2 pomelo variety. These findings were higher than Ani & Abel. [31], who found 132.76 ± 2.38 mg/100 g Ca in pomelo fruit juice. Calcium, which constitutes the preponderant mineral content in fruits, potentially confers health benefits through decreasing blood pressure as described by Hasan et al. [25]. The LOCAL variety contained significantly (p < 0.05) higher amounts of Mg, K, and Na among all the selected pomelo varieties, whereas BARI-6 exhibited higher Fe content. The results of Mg, K, Na, and Fe analysis for five pomelo varieties grown in Bangladesh showed the contents ranged from 20.30 ± 0.14 to 17.21 ± 0.10 mg/100 g, 191.67 ± 11.46 to 149.83 ± 11.51 mg/100 g, 103.99 ± 0.79 to 62.40 ± 0.47 mg/100 g, and 143.57 ± 5.74 to 115.47 ± 5.66 mg/100 g, respectively. The values of Mg, K, Na, and Fe content found in this study were significantly much higher than those reported earlier in the Bangladeshi local pomelo variety [32], and Israel pomelo cultivar honey [10]. As a result, pomelo pulp has significant potential for use as a mineral-rich component in dietary formulation.
Table 2.
Mineral content of five different varieties of pomelo fruit.
| Pomelo varieties | Mineral content (mg/100 gm) |
||||
|---|---|---|---|---|---|
| Ca | Mg | K | Na | Fe | |
| LOCAL | 326.00 ± 50.94b | 20.30 ± 0.14a | 191.67 ± 11.46a | 103.99 ± 5.79a | 117.56 ± 5.36b |
| BARI-2 | 349.80 ± 39.67a | 20.25 ± 0.29a | 150.00 ± 10.25b | 83.19 ± 4.64b | 115.47 ± 5.66b |
| BARI-3 | 127.60 ± 13.34c | 18.60 ± 0.23b | 191.18 ± 9.52a | 62.40 ± 4.47c | 117.24 ± 4.64b |
| BARI-4 | 126.53 ± 12.34c | 17.21 ± 0.17c | 149.83 ± 11.51b | 83.33 ± 3.42b | 143.47 ± 5.74a |
| BARI-6 | 127.99 ± 11.17c | 17.24 ± 0.10c | 150.00 ± 8.63b | 82.53 ± 4.62b | 143.57 ± 6.46a |
Each value is expressed as mean ± SD and different small letters within the same column indicate significant (p < 0.05) differences among various samples.
3.3. Physicochemical characteristics
3.3.1. Fruit juice yield
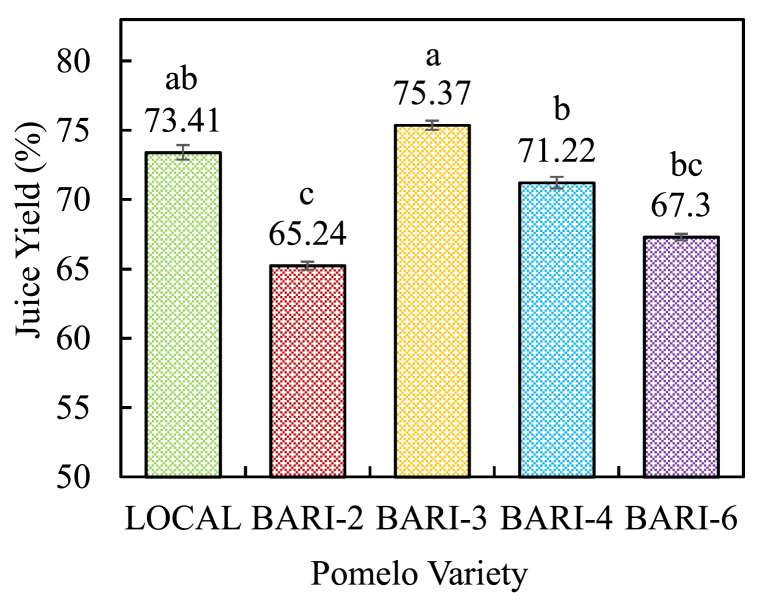
In the food sector, fruits having a high juice content may reduce the cost of juice manufacturing. Thus, the influence of particular pomelo cultivars on juice yield was investigated and the results are depicted in Fig. 2. Each variety exhibited a different juice yield percentage ranging from 75.37 ± 0.33 to 65.24 ± 0.29 %. Among the five-pomelo variety, BARI-3 had the highest yield (75.37 ± 0.33 %) followed by LOCAL (73.41 ± 0.51 %), BARI-4 (71.22 ± 0.42 %), BARI-6 (67.30 ± 0.25 %), and BARI-2 (65.24 ± 0.29 %). The results were closely similar to Chen et al. [33], who found 45.35 ± 3.11 to 73.41 ± 0.51 % juice yield in six Chinese pomelo cultivars. Another researcher earlier reported that the juice yield from pomelo fruit pulp was 71.532 ± 7.980 %, and the value agreed with the present investigation [34]. El Kantar et al. [35] reported that juice yield increased 37 % due to pulse electric field treatment and the value was 54 % before treatment and increased to 74 %. The findings in this study revealed that the pomelo variety affects the juice yield.
Fig. 2.
Juice Yield in different varieties of pomelo fruits.
3.3.2. pH, total Soluble Solids (TSS), titratable acidity (TA), ripening index (RI)
To examine the discrepancy in physicochemical composition among five specific varieties of pomelo, the findings were compiled and are displayed in Table 3. The acceptability of fruit juice was largely affected by its pH value. The pH value among selected pomelo varieties varied from 3.80 ± 0.02 to 3.61 ± 0.02. The lowest pH value was found in the BARI-3 pomelo variety and the highest value was detected in the LOCAL variety. The findings of pH values were agreed with Chen et al. [33], who noticed the pH values range from 4.75 ± 0.01 to 3.24 ± 0.00 in six cultivars of pomelo juices.
Table 3.
Physiochemical composition (pH, Total Soluble Solids (TSS), Titratable Acidity (TA), and Ripening Index (RI) of five different varieties of pomelo fruit.
| Pomelo varieties | Physiochemical composition |
||||
|---|---|---|---|---|---|
| pH | TSS (0Brix) | TA (%) | RI (TSS/TA) | Vitamin C mg/100 mL of fresh juice | |
| LOCAL | 3.80 ± 0.02a | 10.90 ± 0.10a | 0.91 ± 0.02ab | 11.98 ± 5.00a | 78.35 ± 2.27ab |
| BARI-2 | 3.76 ± 0.01a | 9.60 ± 0.10a | 0.88 ± 0.01b | 10.91 ± 10.00ab | 71.16 ± 4.50b |
| BARI-3 | 3.66 ± 0.02a | 8.30 ± 0.40ab | 0.93 ± 0.01b | 9.53 ± 40.00ab | 79.56 ± 2.26a |
| BARI-4 | 3.73 ± 0.02a | 10.50 ± 0.10a | 0.76 ± 0.01c | 13.82 ± 10.00a | 65.56 ± 2.26c |
| BARI-6 | 3.69 ± 0.01a | 8.20 ± 0.15ab | 1.10 ± 0.01a | 7.45 ± 15.00b | 63.97 ± 2.22cd |
Each value is expressed as mean ± SD and different small letters within the same column indicate significant (p < 0.05) differences among various samples.
The fruit TSS of all selected pomelo varieties ranged from 10.90 ± 0.10 to 8.20 ± 0.150Brix. Similar to the pH value, the higher TSS value was found from the LOCAL variety, whereas the lowest TSS value was presented by the BARI-6 pomelo variety. The values were within the range of Buaban et al. [36], who found 9-130Brix in Thai pomelo fruits. As stated by Shamsudin et al. [37], fruit juices containing elevated concentrations of TSS were shown to have the greatest potential for consumer acceptance; the extent to which these values varied was also influenced by the extraction process employed.
The appropriate concentration of TA may influence the sensorial attributes of juice [38], while higher TA causes sourness stimulation leading to unacceptable for consumers [39]. The obtained TA value from selected pomelo samples ranged from 1.10 ± 0.01 to 0.76 ± 0.01 %. The obtained results corroborate those that have been previously reported in Indian pomelo 1.24 to 0.2 % [40], and in Chinese pomelo 0.86 ± 0.09 to 0.54 ± 0.05 % [41]. The percentage of TA was found to be higher in pink fruit pulp including LOCAL, BARI-2, BARI-3, and BARI-6, while lower TA was found in white pulp mentioned as BARI-4 (Fig. 1). This statement is supported by Cheong et al. [42], who found higher organic acid content in pink pomelo.
The RI is a measure used to determine the degree of ripeness of fruits that indicates the maturity stage of a fruit and helps in determining the optimal time for harvesting, storage, and consumption. The ratio of TSS/TA was used to calculate the RI of selected pomelo samples and was presented in Table 3. The RI value of selected pomelo samples ranged from 13.82 ± 10.00 to 7.45 ± 15.00. The heights TSS/TA value was found from BARI-4 pomelo varieties whereas the lowest value was experienced from BARI-6 varieties, and the values were directly opposite to the TA value. The results of RI among the selected pomelo varieties showed the following trends BARI-4 > LOCAL > BARI-2 > BARI-3 > BARI-6. The findings of RI value in this study were lower than Zhang et al. [43], who found two-fold higher TSS/TA value in southern Chinese pomelo fruits cultivated under acidic soil after integrated use of lime with Mg fertilizer. The specific RI may vary depending on the type of fruit being evaluated and environmental conditions. These statements are in agreement with Buaban et al. [36], who found a lower RI value in pomelo fruit harvested in the cool period whereas a higher TSS/TA value was found in the hot period. However, in general, the ripening index takes into account various factors such as color, texture, aroma, firmness, sugar content, acidity, and starch content of the fruit.
3.3.3. Vitamin C content
Vitamin C is an essential nutrient substance present in citrus fruits, which has exhibited tremendous effects on the quality of fruits, anti-aging, and disease resistance ability [15]. The results of the vitamin C content of five different pomelo varieties were studied and presented in Table 3. The vitamin C content of pomelo fruit pulp ranged from 79.56 ± 2.26 to 63.97 ± 2.22 mg/100 mL of fresh juice, where the BARI-3 showed a significant (p < 0.05) higher vitamin C content followed by LOCAL, BARI-2, BARI-4, and BARI-6. In this study, the pomelo fruits grown in Bangladesh had a higher content of vitamin C than the pomelo cultivar from India (55.07 ± 1.41 mg/100 mL of fresh juice) [38], China (<57.8 mg/100 g of edible portion) [44], and Malaysia (<42.21 mg/100 g of fresh weight) [26]. However, a significant relationship between vitamin C content and antioxidant activity was found, which can contribute to living cells by terminating chain reactions through an antioxidative defense mechanism [25]. Therefore, the inclusion of pomelo fruit pulp in the diet could be of great relevance to human health due to its high vitamin C concentration in comparison to other vitamin C-rich fruits.
3.4. Bioactive compounds
3.4.1. Total phenolic content (TPC) and total flavonoid content (TFC)
Citrus fruits are rich in phenolics and flavonoids, which have the potential to reduce the risk of inflammation, carcinogenesis, and cardiovascular diseases reported in many epidemiological and intervention studies [33,44]. The TPC and TFC of PPP of selected varieties were exhibited in Table 4. The amount of TPC and TFC ranged from 6712.30 ± 1.84 to 5701.30 ± 1.18 μg GAE/g DM and 351.32 ± 0.02 to 288.54 ± 0.07 μg QE/g DM, respectively. The results displayed a significant difference (p < 0.05) in TPC and TFC of selected five different varieties of pomelo fruits. The TPC content among all selected pomelo varieties was found maximum in BARI-6 (6712.30 ± 1.84 μg GAE/g DM) and minimum in LOCAL (5701.30 ± 1.18 μg GAE/g DM) pomelo variety. Fruits contain phenolic compounds that are important in controlling cholesterol accumulation leading to a reduction in the risk of heart disease [31]. On the contrary, the highest TFC was detected in BARI-4 (351.32 ± 0.02 μg QE/g DM) and the lowest in BARI-3 (288.54 ± 0.07 μg QE/g DM). In this study, the TPC values were higher and TFC values were lower than Gupta et al. [3], who noticed 1680 μg QE/g of TPC and 1233.86 μg QE/g TFC in pomelo fruit pulp. The TPC and TFC contribute to the antioxidant activity of fruits as described by Islam et al. [13], and the rank of TPC and TFC in this study were as follows: BARI-6 > BARI-4 > BARI-3 > BARI-2 > LOCAL and BARI-4 > BARI-6 > LOCAL > BARI-2 > BARI-3. Phenolics and flavonoids are the most abundant group of compounds found in plant matter, which is largely affected by the growth of plants, variety, fertilizer applied, and environmental conditions. According to Chen et al. [33], it was also verified that the contents of TPC and TFC of pomelo fruits were significantly influenced by their cultivars. Therefore, the selected pomelo varieties including BARI and LOCAL can be excellent dietary sources of phenolics and flavonoids.
Table 4.
Bioactive compounds and functional properties of five different varieties of pomelo fruits.
| Pomelo varieties | Bioactive compounds |
Antioxidant function |
Antidiabetic function |
||||
|---|---|---|---|---|---|---|---|
| TPC (μg GAE/g DM) | TFC (μg QE/g DM) | Carotenoid (μM β-CE/g DM) | Anthocyanin (μg C3GE/100 g DM) | DPPH (μM TE/g DM) | FRAP (μM Fe(II)E/g DM) | α-glucosidase (μM AE/g DM) | |
| LOCAL | 5701.30 ± 1.18e | 347.29 ± 0.00c | 204.87 ± 0.02d | 15.20 ± 0.77d | 117.79 ± 0.01e | 110.97 ± 0.02e | 85.07 ± 0.00c |
| BARI-2 | 5982.37 ± 0.90d | 305.64 ± 0.06d | 731.75 ± 0.11b | 50.65 ± 2.27a | 153.95 ± 0.00c | 168.94 ± 0.01c | 85.57 ± 0.00a |
| BARI-3 | 6357.27 ± 0.55c | 288.54 ± 0.07e | 919.33 ± 0.62a | 37.52 ± 0.95b | 170.47 ± 0.01a | 173.54 ± 0.02b | 85.42 ± 0.00b |
| BARI-4 | 6540.73 ± 0.86b | 351.32 ± 0.02a | 80.64 ± 0.02e | 17.53 ± 2.91e | 134.24 ± 0.01d | 139.45 ± 0.03d | 84.48 ± 0.01d |
| BARI-6 | 6712.30 ± 1.84a | 348.24 ± 0.06b | 566.07 ± 0.06c | 28.56 ± 0.92c | 162.00 ± 0.00b | 183.16 ± 0.01a | 82.56 ± 0.02e |
Each value is expressed as mean ± SD and different small letters within the same column indicate significant (p0.05) differences among various samples.
3.4.2. Total carotenoid content (TCC), and total anthocyanin content (TAC)
A significant (p < 0.05) variation in TCC and TAC was observed in different varieties of selected pomelo fruits and the results were presented in Table 4. Depending on the selected pomelo varieties, the TCC varied from 919.33 ± 0.62 to 80.64 ± 0.02 μM β-Carotene/g DM. The highest TCC was observed in BARI-3 (919.33 ± 0.62 μM β-Carotene/g DM), while BARI-4 (80.64 ± 0.02 μM β-Carotene/g DM) was the lowest TCC. The results of TCC were comparable to the findings of Wang et al. [7], Liu et al. [45], and Zhu et al. [46], who noticed 1.54 ± 0.10 μg β-Carotene/g fresh weight, 19.66 ± 5.03 μg β-Carotene/g DM, and 1.347 ± 0.096 μg β-Carotene/g DM, respectively. The hue of the majority of most citrus fruits is mainly triggered by carotenoid accumulation and the red or pink pigment in pomelo pulp was affected by mainly lycopene [36]. In this study, LOCAL, BARI-2 and BARI-3 cultivars represented a pink pigment hue (Fig. 1). According to Jiang et al. [47], the content and composition of carotenoids in fruits largely depend on their cultivars and species. However, carotenoids and their derivatives serve as the precursors to vitamin A, which are beneficial for reproduction and sexual behavior [48], can boost the immune system [49], and reduce chronic diseases [6]. As a result, in contrast to carotenoid-rich cereals and vegetables, pomelo fruit pulp was an exceptional alternative source of natural carotenoids, according to the results of this study.
The TAC of selected pomelo varieties was detected and varied from 50.65 ± 2.27 to 17.53 ± 2.91 μg C3GE/100 g DM. The higher anthocyanin content was found according to the following order: BARI-2 > BARI-3 > BARI-6 > LOCAL > BARI-4. The lower anthocyanin content was found in BARI-4 (17.53 ± 2.91 μg C3GE/100 g DM) pomelo varieties, which may be due to the less pink color (Fig. 1) of pomelo than other varieties. These findings were in alignment with Hasan et al. [25], who observed lower anthocyanin content for less yellowish color formation in selected jujube fruits. The results of TAC in this study agreed with the studies of Koponen et al. [50], who found 2–66 mg/100 g in fruits, 1–611 mg/100 g in berries, and 3–75 mg/100 g of fresh weight in vegetables. Anthocyanins exhibit an extensive array of biological activities like other polyphenols including anti-inflammatory and antioxidant activity, which are linked to their capacity to exert neuroprotective, cardioprotective, anti-carcinogenic, and antidiabetic effects [51]. Therefore, a conclusion could be drawn that, pomelo fruit pulp rich in anthocyanin could be of great interest due to their potential usage in dietary supplements and their positive impact on human health.
3.4.3. Antioxidant activity (DPPH and FRAP assay)
In this study, two in-vitro tests such as DPPH and FRAP were used to assess the antioxidant activity of selected pomelo pulps, and the experimental results are shown in Table 4. BARI-3 variety had the highest DPPH value (170.47 ± 0.01 μM TE/g DM), followed by BARI-6, BARI-2, and BARI-4, while LOCAL exhibited the lowest DPPH value (117.79 ± 0.01 μM TE/g DM). Furthermore, BARI-6 had the highest FRAP antioxidant activity (183.16 ± 0.01 μM Fe(II)E/g DM), while the lowest FRAP antioxidant activity was showed LOCAL (110.97 ± 0.02 μM Fe(II)E/g DM) among all pomelo varieties studied. The LOCAL pomelo variety experienced the lowest antioxidant content for both the DPPH and FRAP assay. However, the rank of antioxidant activity for DPPH and FRAP among the selected pomelo variety were as follows: BARI-3 > BARI-6 > BARI-2 > BARI-4 > LOCAL and BARI-6 > BARI-3 > BARI-2 > BARI-4 > LOCAL, respectively. The results of antioxidant activity were comparable to the findings of Mäkynen et al. [4], who noticed antioxidant activity by DPPH (13.77 ± 0.66 to 0.41 ± 0.27 mg ascorbic acid equivalent/g dried extract), FRAP (616.89 ± 7.09 to 345.78 ± 2.41 μM ascorbic acid equivalent/g dried extract), and TEAC (1139.87 ± 139.86 to 356.17 ± 1.58 μM TE/g dried extract). Although the antioxidant activity of pomelo fruit was lower than other citrus fruit grown in Bangladesh such as jujube fruits and ranged from 404.13 to 358.27 μM TE/g DM and 1866.63 to 428.18 μM Fe(II)E/g DM in DPPH and FRAP assay respectively [25]. The results in this study were comparable to the findings of Deng et al. [11], who found 1590.2 to 678.9 μM TE/g fresh weight oxygen radical absorbance capacity from different varieties of pomelo and grapefruit. According to the previous study, it was specified a positive relationship between the antioxidant activity of citrus fruit with their TPC, TFC, and vitamin C content was observed [33]. Therefore, the findings in this study could be claimed that pomelo fruit pulps are a rich source of biologically active substances, which could be used as potential antioxidant medications, functional foods, and fruit juices.
3.4.4. Antidiabetic activity
The α-glucosidase is a key enzyme, which is responsible for the digestion of carbohydrates leading to the formation of glucose resulting in increasing postprandial glucose levels. Thus, inhibitors of α-glucosidase are commonly suggested by health professionals as an effective approach for controlling type-2 diabetes by diminishing glucose levels in the blood [25]. Therefore, the sourcing of natural anti-diabetic agents is gaining more attention from the researchers due to omitting the side effects of synthetic anti-diabetic drugs [13]. Accordingly, the inhibitory activity of α-glucosidase of selected five different varieties of PPP was evaluated and presented in Table 4. The α-glucosidase inhibitory activity exhibited a range of values varied from 85.57 ± 0.00 to 82.56 ± 0.02 μM AE/g DM. BARI-2 pomelo variety showed higher α-glucosidase inhibitory activity followed by BARI-3, LOCAL, BARI-4 (p < 0.05), and BARI-6 presented the lowest α-glucosidase inhibitory activity (p < 0.05). The results of α-glucosidase inhibitory activity were comparable to the findings of Deng et al. [11], who noticed IC50 values of α-glucosidase and α-amylase inhibitory activity of different pomelo and grapefruit cultivars varied from 2514 to 1053 mg of fresh fruit/mL and 1788 to 707.1 mg of fresh fruit/mL respectively. Another study reported that the percentage inhibitory activity of α-glucosidase and α-amylase in pomelo fruit juice was 72.83 to 70.68 % and 79.75 to 75.55 % respectively [52]. Phenolic and flavonoid compounds provide inhibitory activity towards the α-glucosidase enzyme as reported by Islam et al. [23], and Yin et al. [53], respectively. It was reported that a higher amount of flavonoid compounds such as neohesperidin, Naringenin, hesperidin, and naringin were contained in pomelo fruit and the values were 25.4 ± 0.12, 12.04 ± 0.12, 11.90 ± 0.21, and 9.20 ± 0.19 mg/g dried extract, respectively [9]. Earlier studies verified that the antidiabetic effect of neohesperidin on α-glucosidase and α-amylase enhanced postprandial hyperglycemic situations [52]. Therefore, the present study indicated that the α-glucosidase inhibitory activity of PPP varied with their varieties and the higher inhibitory activities make the pomelo fruit pulp a useful diabetic diet.
3.4.5. Correlation analysis
A Pearson correlation analysis was conducted to measure the relationship between different variables, where alterations in one variable are linked to changes in another, either moving in the same direction (positive correlation) or the opposite direction (negative correlation) [54]. In this study, the correlation among phenolic, flavonoid, carotenoid, anthocyanin, antioxidant and antidiabetic activity of pomelo fruits were evaluated and presented in Table 5.
Table 5.
Correlation analysis among phenolic, flavonoid, carotenoid, anthocyanin, antioxidant and antidiabetic activity of pomelo fruits.
| TPC | TFC | Carotenoid | Anthocyanin | DPPH | FRAP | α-glucosidase | |
|---|---|---|---|---|---|---|---|
| TPC | 1 | ||||||
| TFC | 0.152 | 1 | |||||
| Carotenoid | 0.092 | −0.875 | 1 | ||||
| Anthocyanin | −0.037 | −0.800 | 0.844 | 1 | |||
| DPPH | 0.561 | −0.658 | 0.874 | 0.708 | 1 | ||
| FRAP | 0.632 | −0.489 | 0.775 | 0.709 | 0.960 | 1 | |
| α-glucosidase | −0.696 | −0.625 | 0.200 | 0.330 | −0.161 | −0.328 | 1 |
The correlation analysis conducted on pomelo fruits revealed significant associations among various biochemical components and bioactivities. The TPC exhibited positive correlations with the antioxidant activity of DPPH (r = 0.561, p < 0.05) and FRAP (r = 0.632, p < 0.05) while showing negative correlations with α-glucosidase inhibitory activity (r = −0.696, p < 0.05). The TFC displayed a weak positive correlation solely with TPC (r = 0.152, p < 0.05). These findings agreed with Chen et al. [55], who observed a similar correlation among TPC, TFC, antioxidant, and antidiabetic activities. The carotenoid and anthocyanin content exhibited a positive correlation with DPPH and FRAP activity respectively. Anthocyanin content revealed moderate positive correlations with carotenoids (r = 0.844, p < 0.05), indicating a potential relationship between pigment composition and fruit hue. Further investigation is needed to confirm the extent of this correlation and its implications on pomelo fruit quality and pulp color. However, this study can partially confirm the pigment development (Fig. 1) with their presence in pomelo fruits. Antioxidant assays, DPPH and FRAP, exhibited a strong positive correlation (r = 0.960, p < 0.05). Conversely, α-glucosidase inhibitory activity demonstrated negative correlations with TPC, TFC, DPPH, and FRAP assay, whereas weak positive correlations with carotenoids and anthocyanin content. The correlation coefficients between TCC and TAC showed a weak association with both antioxidant and antidiabetic activities. These findings align with the observations made by Hasan et al. [25], who suggested that the reduced presence of anthocyanin could account for the diminished correlation with antioxidant and antidiabetic activities. Overall, these findings elucidate intricate relationships among various phytochemicals and bioactivities in pomelo fruits, shedding light on their potential health-promoting properties, particularly in managing oxidative stress and diabetes.
4. Conclusion
The purpose of this study was to investigate the nutritional differences in five varieties of pomelo fruits grown in Bangladesh. The results indicate that the proximate composition, minerals content, physicochemical properties, antioxidant, and antidiabetic activity in pomelo fruit pulp varied with their varieties. BARI-2 was an excellent source of calcium, magnesium, and anthocyanin contents whereas, the LOCAL variety represented the highest potassium and sodium contents. BARI-3 contained the highest juice percentage and was a rich source of vitamin C and carotenoids. The highest TFC, TSS, and RI were exhibited by the BARI-4 pomelo variety and the BARI-6 variety showed the highest TA content. The TPC and FRAP antioxidant activity was highest in the BARI-6 variety while BARI-2 represented the highest α-glucosidase inhibitory activity. Therefore, the findings of this study will have an impact on the manufacturing of various value-added food products such as fruit juice, juice powder, jelly, confectionery, vinegar, and food additives. To summarize, the high mineral and phytochemical content of pomelo fruit grown in Bangladesh may have a substantial potential for application as functional foods or functional additives in the food, feed, and pharmaceutical industries. Nevertheless, future research is advised to identify the exact phytochemical molecule compounds present in pomelo fruit pulp by HPLC or LC-MS method.
Data share statement
Data described in the manuscript, code book, and analytic code will be made available upon request.
CRediT authorship contribution statement
S. M Kamrul Hasan: Writing – review & editing, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization. Md. Rakibul Islam: Writing – original draft, Methodology, Investigation, Data curation. Md. Mahfuzar Rahman: Writing – original draft, Methodology, Investigation, Formal analysis. Md. Rafikul Islum: Writing – original draft, Methodology, Investigation, Formal analysis. Maisha Mahrukh Esha: Writing – original draft, Methodology, Investigation, Formal analysis.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:S M Kamrul Hasan reports financial support was provided by University of Grants Commission (UGC) of Bangladesh.
Acknowledgments
We thankfully acknowledged the University Grants Commission (UGC) of Bangladesh for supporting this work.
References
- 1.Medawar E., Huhn S., Villringer A., Veronica Witte A. The effects of plant-based diets on the body and the brain: a systematic review. Transl. Psychiatry. 2019;9(1) doi: 10.1038/s41398-019-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan T., Kong L., Zhang X., Wang Y., Zhou J., Fu Z., Pan H., She W., Yu Y. Fruit quality and volatile constituents of a new very early-ripening pummelo (Citrus maxima) cultivar ‘Liuyuezao.’. Front. Plant Sci. 2023;13(January):1–15. doi: 10.3389/fpls.2022.1089009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A.K., Dhua S., Sahu P.P., Abate G., Mishra P., Mastinu A. Variation in phytochemical, antioxidant, and volatile composition of pomelo fruit (Citrus grandis (L.) osbeck) during seasonal growth and development. Plants. 2021;10(9) doi: 10.3390/plants10091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mäkynen K., Jitsaardkul S., Tachasamran P., Sakai N., Puranachoti S., Nirojsinlapachai N., Chattapat V., Caengprasath N., Ngamukote S., Adisakwattana S. Cultivar variations in antioxidant and antihyperlipidemic properties of pomelo pulp (Citrus grandis [L.] Osbeck) in Thailand. Food Chem. 2013;139(1–4):735–743. doi: 10.1016/j.foodchem.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Al-Juhaimi F.Y., Ghafoor K., Mohamed Ahmed I.A., Özcan M.M., Uslu N., Babiker E.E. The effect of different solvent concentrations on total phenol, antioxidant activity values, and phenolic compounds of pomelo (Citrus grandis L. Osbeck) fruits. J. Food Process. Preserv. 2021;45(10) doi: 10.1111/jfpp.15840. [DOI] [Google Scholar]
- 6.Zhao Y., Yang X., Hu Y., Gu Q., Chen W., Li J., Guo X., Liu Y. Evaluation of carotenoids accumulation and biosynthesis in two genotypes of pomelo (Citrus maxima) during early fruit development. Molecules. 2021;26(16) doi: 10.3390/molecules26165054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang F., Lin J., Xu L., Peng Q., Huang H., Tong L., Lu Q., Wang C., Yang L. On higher nutritional and medical properties of a carotenoid-rich mutant pomelo (Citrus maxima (L.) Osbeck) Ind. Crop. Prod. 2019;127(July 2018):142–147. doi: 10.1016/j.indcrop.2018.10.065. [DOI] [Google Scholar]
- 8.Hasan S.K., Scampicchio M., Ferrentino G., Kongi M.O., Hansen L.D. Thermodynamics and kinetics of the Fenton reaction in foods. Thermochim. Acta. 2019;682 doi: 10.1016/j.tca.2019.178420. [DOI] [Google Scholar]
- 9.Anmol R.J., Pharm M., Marium S., Pharm M., Hiew F.T., Han W.C., Kwan L.K., Khai A., Wong Y., Khan F., Pharm M., Sarker M.R., Chan S.Y., Kifli N. Phytochemical and therapeutic potential of citrus grandis (L .) osbeck : a review. Journal of Evience-Based Integrative Medicine. 2021;26:1–20. doi: 10.1177/2515690X211043741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czech A., Zarycka E., Yanovych D., Zasadna Z., Grzegorczyk I., Kłys S. Mineral content of the pulp and peel of various citrus fruit cultivars. Biol. Trace Elem. Res. 2020;193(2):555–563. doi: 10.1007/s12011-019-01727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng M., Dong L., Jia X., Huang F., Chi J., Muhammad Z., Ma Q., Zhao D., Zhang M., Zhang R. The flavonoid profiles in the pulp of different pomelo (Citrus grandis L . Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars and their in vitro bioactivity. Food Chem. X. 2022;15(February) doi: 10.1016/j.fochx.2022.100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi L., Ma S., Ren D. vol. 16. Springer; Netherlands: 2017. (Phytochemistry and Bioactivity of Citrus Flavonoids : a Focus on Antioxidant , Anti-inflammatory , Anticancer and Cardiovascular Protection Activities. In Phytochemistry Reviews). Issue 3. [DOI] [Google Scholar]
- 13.Islam R., Kamal M., Kabir R., Hasan M., Haque A.R., Hasan S.M.K. Phenolic compounds and antioxidants activity of banana peel extracts: testing and optimization of enzyme-assisted conditions. Measurement: Food. 2023;10(March) doi: 10.1016/j.meafoo.2023.100085. [DOI] [Google Scholar]
- 14.Kamrul H.S., Schiraldi A., Cosio M.S., Scampicchio M. Food and ascorbic scavengers of hydrogen peroxide: a reaction calorimetry investigation. J. Therm. Anal. Calorim. 2016;125:729–737. doi: 10.1007/s10973-015-5170-3. [DOI] [Google Scholar]
- 15.Nie Z., Wan C., Chen C., Chen J. Comprehensive evaluation of the postharvest antioxidant capacity of majiayou pomelo harvested at different maturities based on PCA. Antioxidants. 2019;8(5) doi: 10.3390/antiox8050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AOAC Official methods of analysis of AOAC international. J. AOAC Int. 1995;78(3):585–892. [Google Scholar]
- 17.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. doi: 10.1016/s0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 18.FAO . Food and Agriculture Organization; Rome: 2004. FAO Products Year Report. 2004. [Google Scholar]
- 19.Nielsen S.S. third ed. Springer; 2017. Food Analysis Laboratory Manual.http://link.springer.com/openurl?genre=book&isbn=978-3-319-44127-6 [Google Scholar]
- 20.AOAC AOAC official method 942.15: acidity (titratable) of fruit products. Off. Methods Anal. AOAC Int. (OMA) 1996 [Google Scholar]
- 21.Hasan K., Islam R., Hasan M., Sarker S.H., Biswas M.H. Effect of alginate edible coatings enriched with black cumin extract for improving postharvest quality characteristics of guava (Psidium guajava L.) fruit. Food Bioprocess Technol. 2022;15(9):2050–2064. doi: 10.1007/s11947-022-02869-2. [DOI] [Google Scholar]
- 22.AOAC . Official Methods of Analysis. AOAC International; Washington DC: 1990. Official method 967.21. Vitamin C (ascorbic acid) in vitamin preparations and juices. [Google Scholar]
- 23.Islam M.R., Haque A.R., Kabir M.R., Hasan M.M., Khushe K.J., Hasan S.M.K. Fruit by-products: the potential natural sources of antioxidants and α-glucosidase inhibitors. J. Food Sci. Technol. 2021;58(5):1715–1726. doi: 10.1007/s13197-020-04681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabir M.R., Hasan M.M., Islam M.R., Haque A.R., Hasan S.M.K. Formulation of yogurt with banana peel extracts to enhance storability and bioactive properties. J. Food Process. Preserv. 2021;45(3):1–10. doi: 10.1111/jfpp.15191. [DOI] [Google Scholar]
- 25.Hasan S.M.K., Kabir M.R., Kabir M.R., Islam M.R., Akhter M.J., Moury J.Y. Proximate composition, minerals, phytochemicals, and functional activities of jujube fruits grown in Bangladesh. Journal of Agriculture and Food Research. 2022;8(March) doi: 10.1016/j.jafr.2022.100302. [DOI] [Google Scholar]
- 26.Toh J.J., Khoo H.E., Azrina A. Comparison of antioxidant properties of pomelo [Citrus Grandis (L) Osbeck] varieties. Int. Food Res. J. 2013;20(4):1661–1668. [Google Scholar]
- 27.Kongkachuichai R., Charoensiri R., Sungpuag P. Carotenoid, flavonoid profiles and dietary fiber contents of fruits commonly consumed in Thailand. Int. J. Food Sci. Nutr. 2010;61(5):536–548. doi: 10.3109/09637481003677308. [DOI] [PubMed] [Google Scholar]
- 28.Niero M., Bartoli G., De Colle P., Scarcella M., Zanetti M. Impact of dietary fiber on inflammation and insulin resistance in older patients: a narrative review. Nutrients. 2023;15(10) doi: 10.3390/nu15102365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain M., Maqbool M., Ishtiaq M. Nutritional evaluation of some selected fruits (pyrus Malus , Psidium Guajava , Musa Paradisiaca , citrus maxima) available in local market of Sargodha city of Pakistan. Transactions in Physical and Biochemical Sciences. 2021;1(1):47–56. [Google Scholar]
- 30.Uthman A., Garba Y. Citrus mineral nutrition and health benefits : a review. Citrus Research-Horticultural and Human. 2023;1–15 https://doi:10.5772/intechopen.107495 [Google Scholar]
- 31.Ani P.N., Abel H.C. Nutrient, phytochemical, and antinutrient composition of Citrus maxima fruit juice and peel extract. Food Sci. Nutr. 2018;6(3):653–658. doi: 10.1002/fsn3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haque M.N., Saha B.K., Karim M.R., Bhuiyan M.N.H. Evaluation of nutritional and physico-chemical properties of several selected fruits in Bangladesh. Bangladesh J. Sci. Ind. Res. 2009;44(3):353–358. doi: 10.3329/bjsir.v44i3.4410. [DOI] [Google Scholar]
- 33.Chen J., Luo W., Cheng L., Wu J., Yu Y., Li L., Xu Y. Influence of cultivar and turbidity on physicochemical properties, functional characteristics and volatile flavor substances of pomelo juices. Foods. 2023;12(5) doi: 10.3390/foods12051028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdullah N., Chin N.L. Optimising tropical fruit juice quality using thermosonication-assisted extraction via blocked face-centered composite design. Processes. 2021;9(1):1–15. doi: 10.3390/pr9010003. [DOI] [Google Scholar]
- 35.El Kantar S., Boussetta N., Lebovka N., Foucart F., Rajha H.N., Maroun R.G., Louka N., Vorobiev E. Pulsed electric field treatment of citrus fruits: improvement of juice and polyphenols extraction. Innovative Food Sci. Emerging Technol. 2018;46:153–161. doi: 10.1016/j.ifset.2017.09.024. [DOI] [Google Scholar]
- 36.Buaban P., Beckles D.M., Mongkolporn O., Luengwilai K. Lycopene accumulation in pummelo (citrus maxima [Burm.] Merr.) is influenced by growing temperature. Int. J. Fruit Sci. 2020;20(2):149–163. doi: 10.1080/15538362.2019.1605559. [DOI] [Google Scholar]
- 37.Shamsudin R., Buang S., Aziz N.A. Effect of different extraction methods on the physicochemical properties of pomelo juice. Chemical Engineering Transactions. 2015;44:265–270. doi: 10.3303/CET1544045. [DOI] [Google Scholar]
- 38.Basumatary B., Nayak P.K., Chandrasekar C.M., Nath A., Nayak M., Kesavan R.K. Impact of thermo sonication and pasteurization on the physicochemical, microbiological and anti-oxidant properties of pomelo (Citrus maxima) juice. Int. J. Fruit Sci. 2020;20(S3):S2056–S2073. doi: 10.1080/15538362.2020.1848751. [DOI] [Google Scholar]
- 39.Wei H., He C., Zhang S., Xiong H., Ni H., Li Q. Effects of four storage conditions on the sugar content, acidity, and flavor of “Guanxi” honey pomelo. J. Food Process. Preserv. 2021;45(1) doi: 10.1111/jfpp.15088. 0–2. [DOI] [Google Scholar]
- 40.Gupta A.K., Koch P., Mishra P. Optimization of debittering and deacidification parameters for Pomelo juice and assessment of juice quality. J. Food Sci. Technol. 2020;57(12):4726–4732. doi: 10.1007/s13197-020-04687-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun X., Xu S., Lu H. Non-destructive identification and estimation of granulation in honey pomelo using visible and near-infrared transmittance spectroscopy combined with machine vision technology. Appl. Sci. 2020;10(16) doi: 10.3390/APP10165399. [DOI] [Google Scholar]
- 42.Cheong M.W., Liu S.Q., Zhou W., Curran P., Yu B. Chemical composition and sensory profile of pomelo (Citrus grandis (L.) Osbeck) juice. Food Chem. 2012;135(4):2505–2513. doi: 10.1016/j.foodchem.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Zhang S., Yang W., Atif M., Ji Z., Tong L., Zhang X., Li X., Wang W., Zhang F., Wu L. Scientia Horticulturae Integrated use of lime with Mg fertilizer significantly improves the pomelo yield , quality , economic returns and soil physicochemical properties under acidic soil of southern China. Sci. Hortic. 2021;290(July) doi: 10.1016/j.scienta.2021.110502. [DOI] [Google Scholar]
- 44.Yin J., Hu X., Hou Y., Liu S., Jia S., Gan C., Ou Y., Zhang X. Comparative analysis of chemical compositions and antioxidant activities of different pomelo varieties from China. Food Chemistry Advances. 2023;2(October 2022) doi: 10.1016/j.focha.2022.100180. [DOI] [Google Scholar]
- 45.Liu C., Yan F., Gao H., He M., Wang Z., Cheng Y., Deng X., Xu J. Features of citrus terpenoid production as revealed by carotenoid, limonoid and aroma profiles of two pummelos (Citrus maxima) with different flesh color. J. Sci. Food Agric. 2015;95(1):111–119. doi: 10.1002/jsfa.6689. [DOI] [PubMed] [Google Scholar]
- 46.Zhu C., Peng C., Qiu D., Zeng J. Metabolic profiling and transcriptional analysis of carotenoid accumulation in a red-fleshed mutant of pummelo (citrus grandis) Molecules. 2022;27(14) doi: 10.3390/molecules27144595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang C.C., Zhang Y.F., Lin Y.J., Chen Y., Lu X.K. Illumina® sequencing reveals candidate genes of carotenoid metabolism in three pummelo cultivars (Citrus maxima) with different pulp color. Int. J. Mol. Sci. 2019;20(9) doi: 10.3390/ijms20092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W., Ye Q., Jin X., Han F., Huang X., Cai S., Yang L. A spontaneous bud mutant that causes lycopene and β-carotene accumulation in the juice sacs of the parental Guanxi pummelo fruits (Citrus grandis (L.) Osbeck) Sci. Hortic. 2016;198:379–384. doi: 10.1016/j.scienta.2015.09.050. [DOI] [Google Scholar]
- 49.Guo F., Yu H., Xu Q., Deng X. Transcriptomic analysis of differentially expressed genes in an orange-pericarp mutant and wild type in pummelo (Citrus grandis) BMC Plant Biol. 2015;15(1):1–12. doi: 10.1186/s12870-015-0435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koponen J.M., Happonen A.M., Mattila P.H., Törrönen A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007;55(4):1612–1619. doi: 10.1021/jf062897a. [DOI] [PubMed] [Google Scholar]
- 51.Merecz-Sadowska A., Sitarek P., Kowalczyk T., Zajdel K., Jęcek M., Nowak P., Zajdel R. Food anthocyanins: malvidin and its glycosides as promising antioxidant and anti-inflammatory agents with potential health benefits. Nutrients. 2023;15(13):3016. doi: 10.3390/nu15133016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sapkota B., Devkota H.P., Poudel P. Citrus maxima (Brum.) Merr. (Rutaceae): bioactive chemical constituents and pharmacological activities. Evid. base Compl. Alternative Med. 2022;2022 doi: 10.1155/2022/8741669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin Z., Zhang W., Feng F., Zhang Y., Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness. 2014;3(3–4):136–174. doi: 10.1016/j.fshw.2014.11.003. [DOI] [Google Scholar]
- 54.Sapkota B.K., Khadayat K., Sharma K., Raut B.K., Aryal D., Thapa B.B., Parajuli N. Phytochemical analysis and antioxidant and antidiabetic activities of extracts from Bergenia ciliata, Mimosa pudica, and Phyllanthus emblica. Advances in Pharmacological and Pharmaceutical Sciences. 2022;2022 doi: 10.1155/2022/4929824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X., He X., Sun J., Wang Z. Phytochemical composition, antioxidant activity, α-glucosidase and acetylcholinesterase inhibitory activity of quinoa extract and its fractions. Molecules. 2022;27(8):2420. doi: 10.3390/molecules27082420. [DOI] [PMC free article] [PubMed] [Google Scholar]