Abstract
Most mammalian rotaviruses contain tripeptide amino acid sequences in outer capsid proteins VP4 and VP7 which have been shown to act as ligands for integrins α2β1 and α4β1. Peptides containing these sequences and monoclonal antibodies directed to these integrins block rotavirus infection of cells. Here we report that SA11 rotavirus binding to and infection of K562 cells expressing α2β1 or α4β1 integrins via transfection is increased over virus binding to and infection of cells transfected with α3 integrin or parent cells. The increased binding and growth were specifically blocked by a monoclonal antibody to the transfected integrin subunit but not by irrelevant antibodies. In our experiments, integrin activation with phorbol ester did not affect virus binding to cells. However, phorbol ester treatment of K562 parent and transfected cells induced endogenous gene expression of α2β1 integrin, which was detectable by flow cytometry 16 h after treatment and quantitatively correlated with the increased level of SA11 virus growth observed after this time. Virus binding to K562 cells treated with phorbol ester 24 h previously and expressing α2β1 was elevated over binding to control cells and was specifically blocked by the anti-α2 monoclonal antibody AK7. Virus growth in α4-transfected K562 cells which had also been induced to express α2β1 integrin with phorbol ester occurred at a level approaching that in the permissive MA104 cell line. We therefore have demonstrated that two integrins, α2β1 and α4β1, are capable of acting as cellular receptors for SA11 rotavirus.
Rotaviruses, members of the family Reoviridae, are the major etiological agents of severe acute gastroenteritis in infants and young children worldwide and are important pathogens in most mammalian species. It is anticipated that the long-awaited introduction of the first vaccine against human rotavirus into use in North America in 1998 will lead to a reduction in the most severe human illness associated with this virus, but other vaccination and therapeutic approaches are required. Novel strategies may be devised following the identification of cellular receptors for rotavirus and from an understanding of the process of viral entry into cells, particularly into intestinal epithelial cells. These are essential steps for productive rotavirus infection and major determinants of host cell tropism.
The nonenveloped, icosahedral rotavirus particle consists of a genome of 11 segments of double-stranded RNA (48) in a triple-layered protein capsid (66). The outermost layer of each virion is composed of trimers of the 37-kDa glycoprotein VP7 and 60 spikes of the 88-kDa protein VP4, probably as dimers, which extend about 12 nm above the VP7 surface and interact extensively with VP7 throughout the outer surface (47, 65, 66).
Both VP4 and VP7 independently elicit neutralizing, protective antibodies and are virulence determinants (26, 46). VP4 is an important determinant of host cell tropism (27), receptor binding, and cell penetration (37). Proteolytic cleavage of VP4 into two subunits, VP8* (28 kDa) and VP5* (60 kDa) (16), results in increased infectivity (9, 17) and rapid internalization of virus, although it does not appear to affect virus binding to cells (28). Most animal rotaviruses, including the simian strain SA11 and the rhesus rotavirus strain RRV, are able to hemagglutinate (2) and bind to the cell surface via sialic acid (19) by using VP8* (18, 38). However, sialic acid binding does not appear to be essential for the infection of these rotaviruses, since sialic acid-independent mutants of RRV retain their infectivity (42). VP5* contains a long hydrophobic domain including a putative cell fusion region at amino acids (aa) 384 to 401 related by sequence to that of Sindbis virus (39).
VP7 appears also to play a role in rotavirus cell attachment, since it has been identified as the infected cell lysate protein which bound to MA104 cell monolayers (52), and it may interact with VP4 to influence receptor-binding specificity (6, 37, 43).
Previously, we have implicated integrins α2β1, α4β1, and αxβ2 in rotavirus cell entry (12). Integrins are αβ heterodimeric, transmembrane glycoproteins important in cell adhesion and signalling. Most human and animal rotaviruses (87%), including SA11, contain the amino acid sequence DGE at aa 308 to 310 of VP5*. The peptide DGE(A) has been reported to act as a ligand in type I collagen for the α2β1 integrin (54). Many rotaviruses, including SA11, contain the sequence LDV at aa 237 to 239 and the related sequence LDI at aa 269 to 271 in VP7. In the first connecting segment (CS1) of the independently spliced IIICS domain of fibronectin, LDV is the minimal essential sequence for a major site of adhesion of fibronectin to the α4β1 and α4β7 integrins on a range of cell types (34). In addition, at aa 253 to 255 in VP7, all mammalian rotaviruses contain the sequence GPR, which is a ligand for the αxβ2 integrin in the N-terminal domain of fibrinogen (36). In our experiments, peptides RDGEE, LDVT, and GPRP and monoclonal antibodies (MAbs) directed to α2, α4, αx, β1, and β2 integrin subunits specifically blocked rotavirus infection in an additive and dose-dependent manner (12). MAbs directed to α1, α3, α5, α6, αL, αM, and β4 did not block SA11 rotavirus infection (10, 12). However, it was not possible to determine from these studies if these integrins interact directly with rotavirus or are involved in virus attachment or in cell penetration.
In this study, we have addressed these issues for the α2β1 and α4β1 integrins by comparing the levels of binding and replication of SA11 rotavirus in cells not expressing these integrins with those in the same cells stably transfected with either of these integrins or with the α3β1 integrin. Cells transfected with the α3β1 integrin acted as a negative control, since we have shown previously that MAbs to α3 do not affect rotavirus infection of cells (10, 12). The erythroleukemic cell line K562 was chosen on the basis of its human origin, its lack of expression of α2β1, α3β1, and α4β1 integrins (23, 57), and its resistance to infection with SA11 rotavirus (S. L. Londrigan, M. J. Hewish, M. J. Thomson, G. M. Sanders, and B. S. Coulson, unpublished results). K562 cells express endogenous α5β1 (57). As a result of α2, α3, or α4 transfection, K562 cells also express integrins α2β1 (8, 22), α3β1 (22), and α4β1 (40) on their surface in a constitutively inactive form, which is unable to bind extracellular matrix proteins. However, the conditions under which functional activation of transfected α2β1 and α4β1 integrins is achieved are established for K562 cells; they include treatment with the phorbol ester phorbol 12-myristate 13-acetate (PMA) (31, 32). PMA treatment of K562 cells also induces megakaryocytic differentiation and concomitant endogenous surface expression of α2β1 integrin, which is in at least partially active form, since it is capable of mediating Mg2+-dependent adhesion to collagen (7). Thus, via integrin transfection and phorbol ester treatment, the role of α2β1 and α4β1 integrins in rotavirus cell entry is amenable to analysis with K562 cells.
MATERIALS AND METHODS
Cells.
The nonadherent human myelogenous leukemic cell line K562, on which surface expression of only one β1 integrin (α5β1) has been detected (23, 57), was grown in Dulbecco's modification of Eagle's medium (DMEM) which also contained 2 mM l-glutamine (Life Technologies, Inc., Gaithersburg, Md.), 20 mM HEPES (Boehringer, Mannheim, Germany), 26.6 μg of gentamicin (Cidomycin; Roussel UCLAF Australia) per ml, and 2 μg of Fungizone (amphotericin B) (Apothecon, Princeton, N.J.) per ml and was supplemented with 20% (vol/vol) fetal calf serum (FCS; Commonwealth Serum Laboratories, Parkville, Victoria, Australia). Cell transfectants were prepared by transfecting α2 (α2-K562 cells), α3 (α3-K562 cells), or α4 (α4-K562 cells) integrin subunit cDNA into K562 cells. After selection with 1 mg of G418 sulfate (Life Technologies, Inc.) per ml, cells stably expressing each integrin were cloned by cell sorting. The expression profile of the transfected alpha subunit in each transfectant was similar to that of the previously described K562 cells transfected with these integrin subunits (8, 15, 62). Transfectants were grown in the above-supplemented DMEM with the addition of 500 μg of G418 per ml. African green monkey kidney epithelial (MA104) cells were propagated in DMEM with l-glutamine and antibiotics as above and supplemented with 10% (vol/vol) FCS.
Virus.
Simian rotavirus SA11, serotype P5B[2], G3 (25), was originally obtained from H. Malherbe (University of Texas Health Science Center, San Antonio, Tex.). Virus was activated with 10 μg of porcine pancreatic trypsin type IX (Sigma, St. Louis, Mo.) per ml for 20 min at 37°C and propagated in MA104 cells by using DMEM containing 1 μg of trypsin per ml as described previously (53). All experiments were performed with SA11 at a multiplicity of infection of 3.
MAbs.
Mouse MAbs to human integrin subunits used in blocking experiments and flow cytometry were as follows: AK7, RMAC11, and P1E6, directed against the α chain (CD49b) of α2β1 integrin (CD49b/CD29; VLA-2) (20, 45, 61) (from Pharmingen, San Diego, Calif.; T. D'Apice, St. Vincent's Hospital, Melbourne, Australia; and Life Technologies, respectively); ASC-6, directed against the α chain (CD49c) of α3β1 integrin (56), which was donated as ascites fluid by A. Skubitz (University of Minnesota Medical School, Minneapolis, Minn.) as part of the participation of one of us (B.S.C.) in the Sixth International Workshop on Human Leucocyte Differentiation Antigens; and P4C2 and P4G9, directed against the α chain (CD49d) of α4β1 integrin (30), donated as hybridoma cell supernatant fluids (SNF) by D. Leavesley (Department of Renal Medicine, The Royal Adelaide Hospital, Adelaide, South Australia) and E. Wayner (Clinical Research Division, Fred Hutchinson Cancer Research Center, Seattle, Wash.). Control MAbs were MOPC 21 (purified; Cappel, ICN Pharmaceuticals, Aurora, Ohio), ST-3:1 (immunoglobulin G3 [IgG3]; hybridoma cell SNF directed to human rotavirus ST-3), and RV-5:2 (IgG2b; hybridoma cell SNF directed to human rotavirus RV-5). Control MAbs were matched with test MAbs for determination of isotype and protein concentration, and those directed to rotaviruses did not cross-react with SA11 by fluorescent focus reduction assay or enzyme immunoassay (13). All MAbs except P4C2, P4G9, and ST-3:1 were IgG1.
Rotavirus cell-binding assays.
K562 parent or transfected cells were washed once with DMEM by centrifugation at 450 × g for 7 min and then resuspended in ice-cold DMEM at 5 × 105 cells/ml. Confluent monolayers of MA104 cells (5 × 105) were washed twice with cold DMEM. Trypsin-activated SA11 cooled to 4°C was bound to cells on ice for 1 h, and then the cells were washed with cold DMEM. Cold DMEM containing 1 μg of porcine trypsin per ml was added to the cells, which were subjected to two rounds of freeze-thaw to release bound virus and stored at −70°C. Thawed aliquots were treated with 10 μg of porcine trypsin per ml for 20 min at 37°C, and viral titers were determined by indirect immunofluorescent staining (IIF) of MA104 cell monolayers inoculated with serial dilutions of the samples as described previously (11). Virus binding was expressed as a percentage of the titer of infectious virus bound to control cells.
For MAb blocking experiments, before virus inoculation, cells were treated for 2 h at 37°C with anti-integrin MAbs or isotype-matched control antibodies, at 10 μg/ml for purified MAbs AK7, ASC-6, and MOPC 21 and at a 1:8 or 1:16 dilution in DMEM for MAbs in hybridoma cell SNF (P4C2 and ST3:1, respectively). Antibody remained on the cells during attachment of SA11 rotavirus.
Rotavirus growth curve determinations.
MA104 and K562 parent and transfected cells were prepared as for binding assays. The cells were inoculated with trypsin-activated SA11 rotavirus and incubated at 37°C for 1 h, and the virus inoculum was replaced with an equal volume of warm DMEM containing 1 μg of trypsin per ml. Parent and transfected K562 cells were seeded in aliquots of 1 ml into 24-well plates (Nunclon Delta SI), and all cells were incubated at 37°C in a humidified incubator in 5% (vol/vol) CO2–95% (vol/vol) air. Infection was terminated by freezing at −70°C at 1, 24, 48, or 72 h postinfection (p.i.). Samples were frozen and thawed twice to release intracellular virus and then stored at −70°C. Viral titers were determined as in binding experiments and expressed as the number of fluorescing cell-forming units (FCFU) per milliliter (11). In MAb blocking experiments, cells were pretreated with MAbs as described for the virus-binding experiments. The titer of virus attributable to interaction with α2β1 or α4β1 integrin on transfected K562 cells was determined by subtracting the mean titer of virus bound to K562 cells from the mean titer of virus bound to integrin-transfected cells. The percent blocking by MAbs of the virus titer attributable to interaction with α2β1 or α4β1 integrin on transfected K562 cells was determined by expressing as a percentage the ratio of the titer of virus attributable to interaction with integrin on transfected K562 cells in the presence of anti-integrin MAb to the titer of virus attributable to interaction with integrin on transfected K562 cells in the presence of control MAb.
Treatment with phorbol ester.
Washed cells were treated with 20 nM PMA (Sigma) in DMEM for 15 min at 37°C (32). PMA was removed by washing twice with cold DMEM (for virus-binding experiments) or DMEM at room temperature (for virus growth assays and flow cytometry).
Flow cytometry.
Cell surface expression of integrins was detected by indirect immunofluorescent staining of 3 × 105 cells. Confluent MA104 cell monolayers were washed twice with phosphate-buffered saline (PBS), and cells were detached by incubation at 37°C for 10 min in PBS containing 0.75 mM EDTA. Detached MA104 cells were suspended in DMEM plus 1% (vol/vol) FCS for 30 min at 37°C to allow restitution of surface proteins. Parent and transfected K562 cells were washed twice in PBS containing 1% (vol/vol) FCS and 0.1% (wt/vol) NaN3 (PBS-FCS-Az). All cells were incubated for 45 min on ice with MAbs to integrin subunits or isotype-matched control MAbs diluted in PBS-FCS-Az to 10 μg/ml (purified MAbs) or 1:8 (hybridoma SNF), then washed as above, and reacted for 45 min on ice with fluorescein isothiocyanate-conjugated sheep anti-mouse F(ab′)2 fragments (Silenus, Melbourne, Australia) diluted 1:50 in PBS-FCS-Az. The cells were fixed with 1% (vol/vol) ultrapure formaldehyde (Polysciences, Warrington, Pa.) before being subjected to analysis of cellular fluorescence on a FACSort flow cytometer (Becton Dickinson). A positive relative linear median fluorescence intensity (RLMFI) (defined as median fluorescence intensity with anti-integrin MAb/median fluorescence intensity with control MAb) was defined as ≥1.20 (60). Monitoring of surface expression of integrin subunits α2, α3, and α4 on the K562 parent and transfected cells showed that the parent K562 cells expressed the β1 integrin subunit but not the α2, α3, or α4 subunits, consistent with their known endogenous expression of α5 (57), whereas the α2-K562 cells expressed α2β1 but not α3 or α4, the α3-K562 cells expressed α3 but not α2 or α4, and the α4-K562 cells expressed α4 but not α2 or α3. Testing at 5-month intervals during the course of the experiments showed that the levels of expression of these integrin subunits were stable.
Statistical analysis.
The unpaired, two-tailed Student t test was used to assess the statistical significance of differences in virus binding and growth. Significance was set at the 95% level. In graphs, error bars indicate the 95% confidence interval of the mean.
RESULTS
Binding of infectious SA11 rotavirus to MA104 cells, untransfected K562 cells, and K562 cells transfected with α2, α3, or α4 integrin subunits, and effect of PMA treatment.
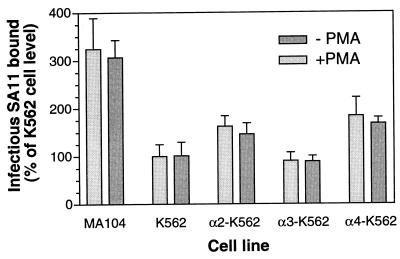
We developed an assay for binding of infectious SA11 rotavirus to K562 and MA104 cells, in which infectious virus attached to cells on ice was quantitated by IIF titer determination. Preliminary evaluation showed that an additional treatment, with 10 μg of trypsin per ml, of harvests of cells containing cell-bound virus (previously treated with trypsin to render it fully infectious) was necessary for assay reproducibility. This was presumably because cellular proteins associated with harvested virions were digested by the trypsin, allowing the virions to attach to and infect MA104 cells and be detected by IIF. As shown in Fig. 1, in each of three experiments, α2-K562 and α4-K562 cells bound significantly more virus (P ≤ 0.0007) than did parent K562 cells whereas the titer of rotavirus bound to α3-K562 cells was not significantly different from that bound to parent cells (P ≥ 0.24). The α2-K562 cells, the α4-K562 cells, and the α3-K562 cells bound 1.5- to 2.2-fold, 1.5- to 1.8-fold, and 0.8- to 1.1-fold more virus than did the parent cells, respectively. MA104 cells bound 1.2- to 3.2-fold more virus than did K562 cells in the three experiments, which was a significant difference (P < 0.0001).
FIG. 1.
Binding of infectious SA11 rotavirus to untransfected K562 cells, to K562 cells transfected with α2, α3, or α4 integrin subunits, and to MA104 cells, with and without PMA treatment of cells. Virus binding to cells was initiated 20 min after PMA treatment ended. Results shown are representative of three experiments.
Treatment of α2-K562 and α4-K562 cells with the phorbol ester PMA induces functional activation of the transfected integrin within 15 min (31, 32). As shown in Fig. 1, PMA treatment had no effect on the binding of SA11 rotavirus to these cells or to MA104, α3-K562, and K562 cells (P > 0.25).
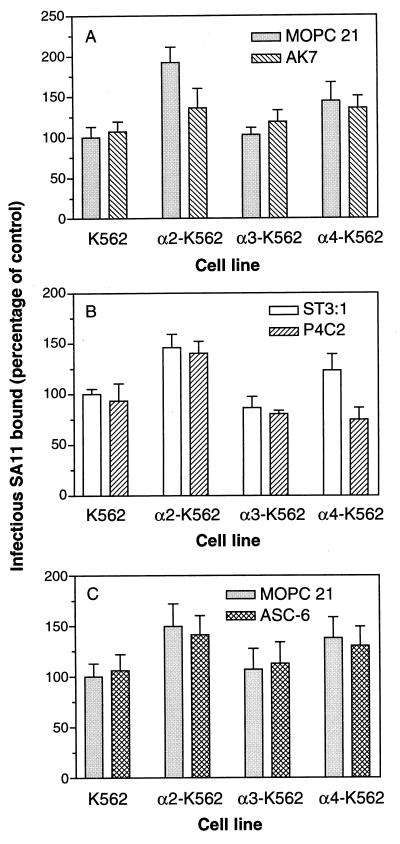
The increase in binding of SA11 rotavirus to α2- and α4-transfected K562 cells is abrogated by function-blocking MAb directed to the transfected integrin subunit.
Function-blocking MAbs to α2, α3, and α4 were tested for their ability to block SA11 binding to the K562 parent and transfected cells, in order to determine whether the increased binding to α2-K562 and α4-K562 cells over parent and α3-K562 cells was specific for the transfected integrin subunit. The anti-α2 MAb AK7, which blocks cell adhesion to collagen (20), the anti-α4 MAb P4C2, which blocks adhesion to fibronectin (30), and the anti-α3 MAb ASC-6, which blocks adhesion to laminins 1 and 5 (56), were compared with control MAbs MOPC 21 and ST-3:1 for their ability to block cellular binding of infectious SA11 rotavirus. As shown in Fig. 2A, MAb AK7 significantly blocked SA11 virus binding to α2-K562 cells, by 33 to 39% (P ≤ 0.0007), but did not significantly affect the binding to K562, α3-K562, or α4-K562 cells (0.36 ≤ P ≤ 0.78). SA11 binding to α4-K562 cells was blocked 33 to 39% by MAb P4C2 (P ≤ 0.003), but this MAb did not significantly affect binding to K562 and α2-K562 cells (0.19 ≤ P ≤ 0.45 [Fig. 2B]) and did not block virus binding to α3-K562 cells (0.19 ≤ P ≤ 0.23 [Fig. 2B]). The anti-α3 MAb ASC-6 (Fig. 2C) and control MAbs MOPC 21 and ST-3:1 did not affect the binding of SA11 to any of these cell lines.
FIG. 2.
Binding of infectious SA11 rotavirus to α2-K562 and α4-K562 cells is significantly and specifically reduced by function-blocking MAbs to the α2 and α4 integrin subunits, respectively. Cells were treated with anti-α2 MAb AK7 or control MAb MOPC 21 (A), anti-α4 MAb P4C2 or control MAb ST-3:1 (B), or anti-α3 MAb ASC-6 or control MAb MOPC 21 (C) prior to virus addition. Results shown are representative of three experiments.
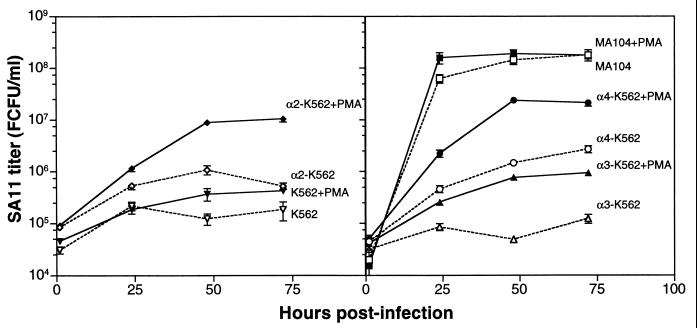
Time course of growth of SA11 rotavirus in untransfected K562 cells, in K562 cells transfected with α2, α3, or α4 integrin subunits, and in MA104 cells.
To determine whether the binding of SA11 rotavirus to α2β1 on α2-K562 cells and to α4β1 on α4-K562 cells leads to an increase in the productive infection of these cell lines, SA11 virus replication in K562 parent and transfected cells was measured at 1, 24, 48, and 72 h p.i. (Fig. 3). The level of virus replication in MA104 cells was also determined at the same time points and with the same multiplicity of infection to provide a measure of the virus yield obtained in cells permissive to SA11 rotavirus (Fig. 3). At 1 h p.i., significantly higher virus titers were associated with α4-K562 cells than with K562 and α3-K562 cells (P < 0.005). In two of three experiments, at 1 h P.I. significantly higher virus titers were associated with α2-K562 cells than with K562 and α3-K562 cells (P < 0.0011) and with K562 cells than with α3-K562 cells (P < 0.0023). By 24 h p.i., the SA11 rotavirus titers in α2-K562 and α4-K562 cells were increased over the titers in α3-K562 and K562 cells, and this difference in titer widened over time to 48 to 72 h p.i.
FIG. 3.
Replication of SA11 rotavirus in untransfected K562 cells, in K562 cells transfected with α2, α3, or α4 integrin subunits, and in MA104 cells, with and without PMA treatment of cells. Virus infection of cells was initiated 20 min after PMA treatment ended. Results shown are representative of three experiments.
At 48 h p.i., SA11 virus grew to a titer of 1.4 × 108 FCFU/ml in the susceptible MA104 cells. In contrast, the SA11 virus titer at this time in the infection-resistant K562 cells was 1.2 × 105 FCFU/ml. In α2-K562 and α4-K562 cells, SA11 grew to titers of 1.1 × 106 and 1.5 × 106 FCFU/ml, respectively, whereas α3-K562 cells produced an SA11 virus titer of 4.9 × 104 FCFU/ml. At 48 h p.i., SA11 virus grew to a significantly higher titer in α2-K562 and α4-K562 cells than in K562 and α3-K562 cells (P < 0.0001). Virus titers at this time were 1.9- to 8.9-fold and 2.5- to 12.0-fold higher in α2-K562 and α4-K562 cells, respectively, than in K562 cells, and titers in α3-K562 cells were 0.2 to 0.4 times those in K562 cells (P < 0.0001). In two of three experiments, titers of virus were significantly higher in α4-K562 cells than in α2-K562 cells (P < 0.0001).
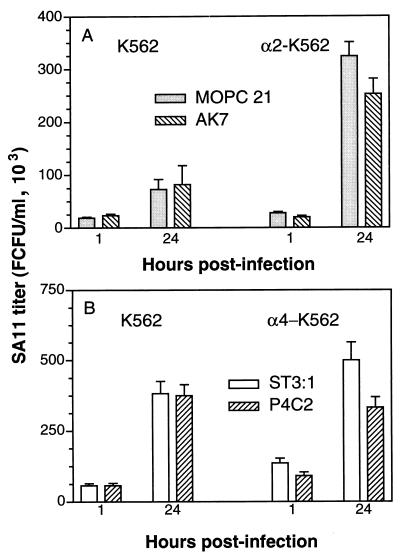
The increased SA11 virus growth in α2- and α4-transfected K562 cells is abrogated by function-blocking MAb directed to the transfected integrin subunit.
In our previous studies, treatment of MA104 cells with the anti-α2 MAb AK7 at 12 μg/ml reduced the number of MA104 cells infected with SA11 rotavirus by 60% (12). As shown in Fig. 4A, at 10 μg/ml, MAb AK7 also reduced the titer of SA11 associated with α2-K562 cells but not that of SA11 associated with K562 cells, at 1 and 24 h p.i. Infection of α3-K562 and α4-K562 cells by SA11 was not affected by MAb AK7 at 10 μg/ml (data not shown). The titer of SA11 associated with α2-K562 cells at 1 h p.i. was significantly lower in cells treated with MAb AK7 than in cells treated with irrelevant MAb MOPC 21 (P = 0.0004), whereas the virus titer in K562 cells treated with MOPC 21 was significantly higher than that in AK7-treated cells (P = 0.004). At 24 h p.i., virus titers in control and AK7-treated K562 cells were not significantly different (P = 0.49) but titers in AK7-treated α2-K562 cells were again significantly decreased with respect to those in MOPC 21-treated α2-K562 cells (P = 0.0008).
FIG. 4.
SA11 rotavirus association with α2-K562 and α4-K562 cells 1 h p.i. and replication in these cells are significantly and specifically reduced by function-blocking MAbs to the α2 and α4 integrin subunits, respectively, whereas virus association with and replication in K562 cells are not affected by these MAbs. Prior to virus infection, K562 and α2-K562 cells were treated with anti-α2 MAb AK7 or control MAb MOPC 21 (A) and K562 and α4-K562 cells were treated with anti-α4 MAb P4C2 or control MAb ST-3:1 (B). Results shown are representative of three experiments.
At 1 h p.i., the titer of SA11 attributable to association with the transfected α2 integrin after treatment with MOPC 21 MAb was 9,100 ± 4,983 (mean ± standard deviation [SD]), which was significantly greater than the titer of −3,560 ± 7,069 obtained after treatment with MAb AK7 (P = 0.001). At 24 h p.i., these titers were 250,900 ± 43,900 and 170,900 ± 56,600, respectively (P = 0.007). Overall, AK7 treatment significantly reduced the infectious virus titer attributable to the transfected α2 integrin subunit, to negligible at 1 h p.i. and by 32% at 24 h p.i.
Treatment with the anti-α4 MAb P4C2 significantly (P < 0.0001) reduced the titer of SA11 associated with α4-K562 cells, but not with K562 cells, by 33% at 1 h p.i. and by 34% at 24 h p.i. (Fig. 4B). This MAb did not block infection of α2-K562 or α3-K562 cells (data not shown). At 1 h p.i., 44% of the SA11 virus titer attributable to the α4 integrin subunit was blocked by MAb P4C2, and at 24 h p.i., all the SA11 virus replication attributable to the α4 integrin subunit was blocked by this α4-specific MAb.
In contrast to the effects of the anti-α2 and anti-α4 MAbs on SA11 growth in α2-K562 and α4-K562 cells, respectively, treatment of K562, α2-K562, α3-K562, and α4-K562 cells with the anti-α3 MAb ASC-6 did not affect the infectious yield of SA11 at 1 or 24 h p.i. (data not shown).
Effect of PMA treatment on SA11 rotavirus growth in untransfected K562 cells and in K562 cells transfected with α2, α3, or α4 integrin subunits.
PMA treatment of K562, α2-K562, α3-K562, and α4-K562 cells had no effect on cellular binding of SA11 rotavirus (see above), suggesting that PMA activation of integrins was not necessary for their recognition by SA11. However, in addition to integrin activation, PMA treatment of K562 cells is known to induce megakaryocytic differention of these cells, accompanied by endogenous gene expression of α2β1 integrin (7). As shown in Fig. 3, SA11 rotavirus grew to significantly higher titers in PMA-treated K562, α2-K562, α3-K562, and α4-K562 cells than in untreated cells (P ≤ 0.0001 at 48 h p.i.). In two experiments, virus titers were increased 3.0- to 7.2-fold, 8.2- to 14-fold, 12- to 16-fold, and 16- to 40-fold in K562, α2-K562, α3-K562, and α4-K562 cells, respectively, over those in untreated cells at 48 h after PMA treatment and virus infection. In PMA-treated α4-K562 and α2-K562 cells, virus titers reached means of 2.4 × 107 (SD = 3.0 × 106) and 9.0 × 106 (SD = 7.0 × 105) FCFU/ml, respectively, at 48 h p.i. Titers in the PMA-treated α4-K562 cells were within 1 log unit of those in PMA-treated MA104 cells (mean, 1.9 × 108 FCFU/ml; SD, 2.0 × 107 FCFU/ml). Thus, the combination of α4 expression via transfection and PMA treatment converted K562 cells from SA11 resistant to a level of permissiveness to SA11 infection approaching that of MA104 cells. PMA treatment also slightly enhanced SA11 virus infection of MA104 cells at 24 and 48 h p.i. (P ≤ 0.013), but virus titers in MA104 cells with or without PMA treatment were indistinguishable by 72 h p.i. (P = 0.91). Titers of infectious virus associated with all cell lines at 1 h p.i. were not significantly or reproducibly affected by PMA treatment, consistent with the lack of effect of PMA on virus binding to cells.
PMA treatment of untransfected K562 cells and of α2-, α3-, and α4-transfected K562 cells induces α2 integrin subunit expression.
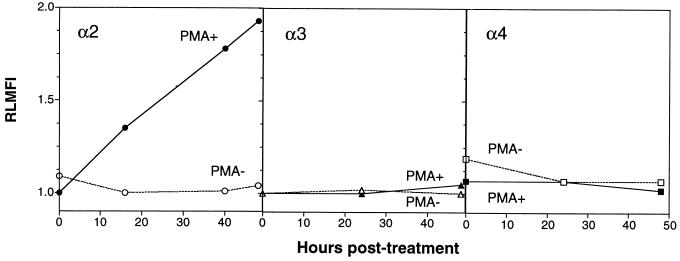
As increased SA11 growth in PMA-treated K562 cells was independent of transfection with α2, α3, or α4 integrin subunits, it appeared likely that this growth increase resulted from changes induced by PMA which were unrelated to activation of transfected integrins. We therefore examined the effect of PMA treatment on parent K562 cell surface expression of α2, α3, and α4 integrin subunits by flow cytometry over time (Fig. 5). Surface expression of integrins α3 and α4 was not detected at 0, 16, 40, or 48 h after PMA treatment. However, expression of α2 integrin was evident at 16 h after PMA treatment, and levels increased until 48 h after treatment. The kinetics of α2 integrin expression were consistent with the kinetics of SA11 virus titer increases observed up to 48 h p.i. (Fig. 3). The effect of PMA treatment on expression of the α2, α3, and α4 integrin subunits on α2-K562, α3-K562, and α4-K562 cells at 48 h after treatment was also examined and compared with the effects on the levels of integrin expression on K562 and MA104 cells (Table 1). PMA treatment of all the integrin-transfected cell lines led to surface expression of α2 but not α3 or α4 integrin subunits. The levels of α2 integrin expression induced by PMA in K562, α3-K562, and α4-K562 cells, expressed as the RLMFI, ranged from 1.78 to 2.48. In α2-K562 cells, the combination of α2 transfection and induction with PMA produced a higher α2 RLMFI of 3.19. However, the highest α2 RLMFI (4.07) was recorded in MA104 cells. The rank order in ability of SA11 to replicate in all cell lines studied which expressed α2 but not α4 was very similar to the rank order of their levels of α2 integrin expression, as follows: MA104> α2-K562 + PMA > α2-K562 − PMA > K562 + PMA > α3-K562 + PMA (Fig. 1 and 3; Table 1). Notably, PMA-treated α4-K562 cells expressed both α2 and α4, consistent with their supporting the highest titers of SA11 among the K562-derived cell lines (Fig. 3).
FIG. 5.
Time course of expression of α2, α3, and α4 integrin subunits on K562 cells, with and without treatment with PMA. Integrin expression was determined by flow cytometry with anti-integrin MAbs P1E6 (α2), ASC-6 (α3), and P4C2 (α4) and control MAbs MOPC 21 and ST-3:1.
TABLE 1.
Effect of PMA treatment of K562, α2-K562, α3-K562, α4-K562, and MA104 cells on surface expression of α2, α3, and α4 integrin subunits 48 h later
| Cell line | Cell surface expression of given integrin subunit (RLMFI)a:
|
|||||
|---|---|---|---|---|---|---|
| α2
|
α3
|
α4
|
||||
| −PMA | +PMA | −PMA | +PMA | −PMA | +PMA | |
| K562 | 1.04 | 2.19 | 1.00 | 1.05 | 1.01 | 1.03 |
| α2-K562 | 1.79 | 3.19 | 1.20 | NDb | 0.97 | ND |
| α3-K562 | 1.02 | 1.78 | 11.0 | ND | 0.89 | ND |
| α4-K562 | 1.11 | 2.48 | 1.00 | ND | 7.40 | ND |
| MA104 | 4.07 | ND | ND | ND | 1.03 | ND |
The MAbs used in flow cytometry were RMAC11 (α2), ASC-6 (α3), and P4C2 (α4). Expression of α4 on MA104 cells was determined with MAb P4G9. −PMA, no PMA treatment of cells; +PMA, cells treated with PMA. Positive RLMFI values, indicating that the integrin is expressed, are shown in boldface type. The range of the given RLMFI was a maximum of ±0.20.
ND, not done.
SA11 rotavirus shows an increase in binding to K562 cells 24 h after their treatment with PMA, which is blocked by a MAb to the α2 integrin subunit, AK7.
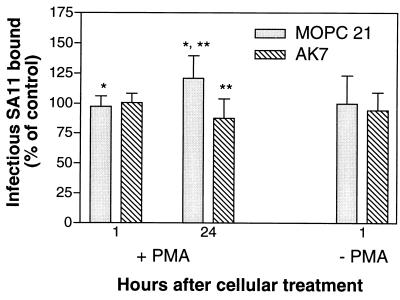
The above results suggested that the increased binding and growth of SA11 rotavirus in PMA-treated K562 cell lines was due to increased expression of the α2 integrin subunit. To test this directly, the ability of MAb AK7 to block virus bound to cells at 1 h after PMA treatment, when no α2 integrin is detectable by flow cytometry, and at 24 h after PMA treatment, when α2 integrin is detectable, was tested (Fig. 6). MAb AK7 reduced the titer of SA11 bound to PMA-treated cells 24 h after treatment by 29% (P = 0.007), to a titer indistinguishable from that in the controls, but had no effect on the bound virus titer 1 h after treatment (titer reduced by 6%; P = 0.55). In a repeat experiment, MAb AK7 reduced the titer of SA11 bound to PMA-treated cells by 33% at 24 h after treatment and by 5% at 1 h after treatment (data not shown). In the absence of PMA, MAb AK7 did not affect virus binding to cells (Fig. 6). Control MAb MOPC 21 did not affect the binding ability of virus, since the increase in virus binding to PMA-treated cells from 1 to 24 h after treatment with this MAb (1.2-fold; P = 0.017) was similar to that observed in the absence of any MAb (1.3-fold; data not shown). As expected, the PMA-treated cells at 24 h after treatment expressed α2 integrin detectable by flow cytometry with MAb AK7 (2.21 < RLMFI < 2.45) but did not express this integrin at 1 h after treatment (0.92 < RLMFI < 1.06). Mock-treated K562 cells did not express α2 integrin at 1 or 24 h after treatment (0.98 < RLMFI < 1.05).
FIG. 6.
PMA treatment of K562 cells 24 h previously produces increased SA11 rotavirus binding, which is blocked by anti-α2 integrin MAb AK7. Cells were mock treated (−PMA) or treated with PMA (+PMA) as described in Materials and Methods. Aliquots (0.5 ml) of −PMA and +PMA cells (1 × 106) were treated with 10 μg of MAb (AK7 or MOPC 21) per ml for 2 h at 37°C, cooled on ice for 20 min, and assayed for infectious SA11 rotavirus binding. Immediately after treatment, additional aliquots (0.5 ml) of −PMA and +PMA cells (5 × 105) were washed with PBS-FCS-Az, stained with MAbs MOPC 21 and AK7, and processed for flow cytometry. Remaining −PMA and +PMA cells were maintained at a density of 5 × 105 cells/ml in DMEM for 24 h, and aliquots were treated with MAbs, cooled, and assayed for infectious SA11 rotavirus binding or processed for flow cytometry, as above. ∗, P = 0.017; ∗∗, P = 0.007.
Thus, SA11 bound specifically to the PMA-induced α2 integrin on K562 cells. Expression of this integrin is the most likely basis for the increased virus growth in PMA-treated K562 cell lines.
DISCUSSION
In this study we have demonstrated that two integrins, α2β1 and α4β1, are capable of acting as cellular receptors for SA11 rotavirus. For α2β1, this was shown in two ways. First, nonpermissive cells transfected with the α2 integrin subunit supported significantly elevated levels of SA11 virus attachment and growth, which could be blocked by an anti-α2 antibody, but not by MAbs to α3 or α4 integrins or by an irrelevant MAb. Second, the same cells, when induced to express α2β1 by PMA treatment, also supported significantly elevated levels of SA11 virus attachment and growth. In K562 cells not expressing α2 integrin prior to PMA treatment, this increased virus binding could be blocked by an anti-α2 antibody but not by an irrelevant MAb. This suggests that the major effect of PMA on SA11 cell entry was via induction of α2 integrin expression. However, PMA has pleiotropic effects on K562 cells (7, 31, 32), so that other enhancing effects of PMA on SA11 cell entry, including activation of transfected integrin or induced α2, cannot be ruled out.
Overall, infectious SA11 rotavirus bound to α2β1 integrin expressed on the cell surface either by transfection or by induction of endogenous expression, and this binding led to productive infection of the cell. It is likely that α2β1 will prove to be a receptor for many rotaviruses, since the anti-α2 MAb AK7 and type I collagen block infection of MA104 cells by all rotavirus strains we have tested so far. These include SA11, RRV, and human strains RV-5, RV-4, K8, and 116E (12; M. J. Thomson and B. S. Coulson, unpublished results). From MA104 cells, SA11 rotavirus immunoprecipitates two proteins indistinguishable from two of those specifically precipitated by anti-β1 integrin MAbs, and MAb AK7 at 10 μg/ml blocked the binding of SA11 to these cells by 35% (S. L. Londrigan, P. Witterick, and B. S. Coulson, unpublished results).
A peptide (RDGEE) containing a putative α2β1 integrin ligand sequence DGE(A) (54) and a sequence related to the newly proposed α2β1 integrin ligand sequence GER (33) has been shown to block SA11 infection of MA104 cells (12). Together with our demonstration here that SA11 binds to α2β1 integrin, this suggests that the binding of SA11 to α2β1 may have similar properties to the binding of this integrin to its natural ligand, type I collagen. In support of this, anti-α2 MAbs AK7, RMAC11, Gi9, and P1E6 block the adhesion of type I collagen (which contains the sequence DGEA) to α2β1, with MAb P1E6 reducing adhesion the least (20, 29). This blocking pattern correlates with the relative abilities of these MAbs to block SA11 infection, since all were able to block infection but mAb P1E6 was the least effective (10, 12). In addition, the anti-β1 MAb 4B4, which inhibits the attachment of various cells to integrin ligands, also blocked SA11 infection of MA104 cells, whereas the anti-β1 MAb K20, which does not affect cell attachment to ligands (58), did not affect SA11 infectivity (10).
The α4β1 integrin was shown to be a SA11 receptor by using nonpermissive cells transfected with the α4 integrin subunit, which supported significantly elevated levels of SA11 virus attachment and growth over parent cells. The increases in virus binding and infectious-virus yield were blocked by an anti-α4 antibody but not by irrelevant MAbs. We have also found that RD cells express α4 integrin (Londrigan, Hewish, et al., unpublished) and that RRV infectivity in these cells is reduced by the function-blocking anti-α4 MAb P4C2 (Thomson and Coulson, unpublished).
The viral protein responsible for binding to α4β1 has not been identified. Previously, we showed that VP7 contains the α4 integrin ligand sequence LDV (12). Interestingly, VP4 of murine rotaviruses also contains the sequence LDV, at aa 538 to 540. Another α4 integrin ligand sequence, IDA, found in the C-terminal HepII domain of fibronectin (44), is present in VP4 at aa 538 to 540 in 33 (43%) of the 77 group A mammalian and avian rotavirus sequences in GenBank, including SA11, RRV, and some human strains (21). Further studies, including blocking of SA11 binding with peptides and MAbs directed to VP4 and VP7, are needed to determine the virus proteins and sequences required for rotavirus-integrin binding.
The highest level of SA11 replication was within 1 log unit of the yield in MA104 cells and occurred when both α2β1 and α4β1 integrins were being expressed in the K562 cells. This highest level was a 2.7-fold increase over the titer in cells expressing only α2β1. Thus, these integrins appear to contribute independently to rotavirus-cell interactions, but when both are present, α2β1 may be more important than α4β1. This is consistent with our previous observations that integrin ligand peptides and anti-integrin MAbs exhibited additive blocking of SA11 infectivity (12).
Although the transfected integrins α2 and α4 are expressed on K562 cells in an inactive form, PMA activation of these integrins did not affect virus-cell attachment. Thus, integrin activation by PMA was not required for SA11 rotavirus binding to α2β1 and α4β1. In addition to PMA treatment, activation in these cells is mediated by divalent cations, activating MAbs to β1 and integrin ligands (3, 32, 67). The RDGEE and LDV/IDA sequences in VP4 and VP7 may be accessible for integrin binding, allowing SA11 rotavirus to activate the integrins itself. We are further investigating the effects of integrin activation on rotavirus interactions with transfected cells.
A minority of rotaviruses, including SA11 and RRV, utilize terminal sialic acids for virus-cell binding, and the infectivity of these rotaviruses in neuraminidase-treated cells is reduced (19). However, sialic acid is not essential for the infectivity of these rotaviruses, since sialic acid-independent RRV mutants are infectious (42). GM3-related monosialogangliosides from piglet intestine bound infectious OSU porcine rotavirus but were not sufficient to confer permissivity to OSU or human rotavirus Wa infection on CHO cells (49). Interestingly, GM3 associates with α2β1 integrin to promote platelet adhesion to collagen (63). CHO cells do not express α2 or α4 integrins, and so the block in CHO cells to rotavirus replication may be due to lack of appropriate integrin expression. In K562 cells not expressing α2 or α4 integrins, very little SA11 replication occurred (Fig. 3). Infection of cells with SA11, neonatal calf diarrhea virus (NCDV), and Wa rotavirus is also blocked by Ricinus communis agglutinin I (5, 55); therefore, some rotaviruses may also recognize β-d-galactose. This lectin binds to both α2 and β1 integrin subunits (23), which are heavily N glycosylated and contain terminal sialic acid residues (24). We hypothesize that binding of rotaviruses to cells involves initial carbohydrate recognition, particularly on integrins and integrin-associated glycosphingolipids, followed by a closer protein-protein interaction of virus with integrins via integrin ligand sequences on VP4 and possibly VP7. Since VP4 is the viral spike protein and contains the sialic acid-binding domain, binding of VP4 to cellular carbohydrates and integrins might precede any VP7 binding.
Rotavirus binding to integrin could facilitate virus-cell membrane interactions, including membrane permeabilization induced by VP5 or VP7 (51), fusion (39), and endocytosis. Integrin-ligand binding and the NPXY internalization signal present in the cytoplasmic domain of β1 integrins are required for integrin assembly into focal adhesions and the induction of “outside-in” cell signalling (59). Thus, rotavirus-integrin binding may induce a signal required for virus internalization. This is the case for adenovirus endocytosis, which requires phosphoinositide-3-OH kinase activation following αv integrin binding to the virion penton base (35).
Rotavirus replication is detected primarily in the mature enterocytes of the middle and upper villus epithelium of the small intestine. The α2β1 integrin is present at sites of proliferating or differentiating epithelium, for example, on the migratory front of Caco-2 colonic adenocarcinoma cells (1). In the small bowels of children and adults, it is expressed in the crypt basal domain and at the lateral domain of crypt and lower villus enterocytes, where it mediates intercellular adhesion (4). Thus, assuming that rotavirus also uses α2β1 integrin as a receptor in vivo, access of rotavirus to this integrin might be prevented by intercellular tight junctions. Apical expression of β1 integrins in the intestinal cell line T84 is induced following breaching of the tight junctions by neutrophil transmigration (41), suggesting one mechanism by which rotavirus may gain access to α2β1 integrin. This problem of access also applies to echovirus type 1 binding to α2β1 integrin (its cellular receptor) in the intestine and to adenovirus-mediated gene delivery to the intestine via integrins (14). Once α2β1 integrin was accessed in the lower villus, rotavirus-infected cells might migrate to the middle villus region before sufficient viral antigen is synthesized to be detectable by IIF, explaining the observed virus tropism for enterocytes on the middle and upper villus.
Rotavirus usage of α4β1 integrin as a receptor on α4-transfected K562 cells suggests that rotavirus might use α4β1 and/or α4β7 integrins to interact with leukocytes, which are the only cells normally expressing α4 integrins. Intestine-homing memory T and B cells express α4β7 integrin which mediates the binding of the mucosal vascular addressin cell adhesion molecule 1 and is required for their efficient trafficking into mucosal lymphoid tissues and for their recirculation through the gastrointestinal tract and lamina propria. Rotavirus infection in children results in a specific circulating memory T-cell response that is mainly CD4+ and α4β7+ (50). In murine rotavirus infection, the memory B cells providing the secretory IgA response and protective humoral immunity also express α4β7 (64). Rotavirus binding to or infection of these α4β7+ cells could interfere with their homing and protective role and contribute to the observed short duration of protection against disease conferred by natural rotavirus infection. It is also possible that these cells facilitate rotavirus spread both within the intestine and to other organs.
Our demonstration that α2β1 and α4β1 can act as receptors for SA11 rotavirus provides a basis for many further studies directed at fully understanding the process of rotavirus-cell interactions and viral pathogenesis. This information will be crucial to our ability to produce more effective vaccines and antirotavirus agents.
ACKNOWLEDGMENTS
We are very grateful to T. D'Apice and A. Skubitz for monoclonal antibodies RMAC11 and ASC-6, respectively, and to E. Wayner and D. Leavesley for MAbs P4C2 and P4G9.
This work was supported by project grant 980635 from the National Health and Medical Research Council of Australia (M.J.H. and B.S.C.) and by National Institutes of Health grant GM47157 (Y.T.).
REFERENCES
- 1.Basson M D, Modlin I M, Madri J A. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Investig. 1992;90:15–23. doi: 10.1172/JCI115828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastardo J W, Holmes I H. Attachment of SA-11 rotavirus to erythrocyte receptors. Infect Immun. 1980;29:1134–1140. doi: 10.1128/iai.29.3.1134-1140.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazzoni G, Ma L, Blue M L, Hemler M E. Divalent cations and ligands induce conformational changes that are highly divergent among beta 1 integrins. J Biol Chem. 1998;273:6670–6678. doi: 10.1074/jbc.273.12.6670. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu J F. Integrins and human intestinal cell functions. Front Biosci. 1999;4:D310–D321. doi: 10.2741/beaulieu. [DOI] [PubMed] [Google Scholar]
- 5.Beisner B. Ph D. thesis. Parkville, Australia: The University of Melbourne; 1999. [Google Scholar]
- 6.Beisner B, Kool D, Marich A, Holmes I H. Characterisation of G serotype dependent non-antibody inhibitors of rotavirus in normal mouse serum. Arch Virol. 1998;143:1277–1294. doi: 10.1007/s007050050375. [DOI] [PubMed] [Google Scholar]
- 7.Burger S R, Zutter M M, Sturgill-Koszycki S, Santoro S A. Induced cell surface expression of functional alpha 2 beta 1 integrin during megakaryocytic differentiation of K562 leukemic cells. Exp Cell Res. 1992;202:28–35. doi: 10.1016/0014-4827(92)90400-3. [DOI] [PubMed] [Google Scholar]
- 8.Chan B M, Hemler M E. Multiple functional forms of the integrin VLA-2 can be derived from a single alpha 2 cDNA clone: interconversion of forms induced by an anti-beta 1 antibody. J Cell Biol. 1993;120:537–543. doi: 10.1083/jcb.120.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark S M, Roth J R, Clark M L, Barnett B B, Spendlove R S. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J Virol. 1981;39:816–822. doi: 10.1128/jvi.39.3.816-822.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulson B S. Effects of Workshop monoclonal antibodies on rotavirus infection of cells. In: Kishimoto T, Kikutani H, von dem Borne A E G K, Goyert S M, Mason D Y, Miyasaka M, Moretta L, Okumura K, Shaw S, Springer T A, Sugamura K, Zola H, editors. Leucocyte typing VI. New York, N.Y: Garland Publishing, Inc.; 1997. pp. 391–393. [Google Scholar]
- 11.Coulson B S, Fowler K J, Bishop R F, Cotton R G. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol. 1985;54:14–20. doi: 10.1128/jvi.54.1.14-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulson B S, Londrigan S L, Lee D J. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc Natl Acad Sci USA. 1997;94:5389–5394. doi: 10.1073/pnas.94.10.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coulson B S, Tursi J M, McAdam W J, Bishop R F. Derivation of neutralizing monoclonal antibodies to human rotaviruses and evidence that an immunodominant neutralization site is shared between serotypes 1 and 3. Virology. 1986;154:302–312. doi: 10.1016/0042-6822(86)90456-3. [DOI] [PubMed] [Google Scholar]
- 14.Croyle M A, Walter E, Janich S, Roessler B J, Amidon G L. Role of integrin expression in adenovirus-mediated gene delivery to the intestinal epithelium. Hum Gene Ther. 1998;9:561–573. doi: 10.1089/hum.1998.9.4-561. [DOI] [PubMed] [Google Scholar]
- 15.Elices M J, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler M E, Lobb R R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 16.Espejo R T, Lopez S, Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981;37:156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estes M K, Graham D Y, Mason B B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981;39:879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuentes-Panana E M, Lopez S, Gorziglia M, Arias C F. Mapping the hemagglutination domain of rotaviruses. J Virol. 1995;69:2629–2632. doi: 10.1128/jvi.69.4.2629-2632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukudome K, Yoshie O, Konno T. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology. 1989;172:196–205. doi: 10.1016/0042-6822(89)90121-9. [DOI] [PubMed] [Google Scholar]
- 20.Gamble J R, Matthias L J, Meyer G, Kaur P, Russ G, Faull R, Berndt M C, Vadas M A. Regulation of in vitro capillary tube formation by anti-integrin antibodies. J Cell Biol. 1993;121:931–943. doi: 10.1083/jcb.121.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GenBank . Rotavirus VP4 sequences. Bethesda, Md: National Center for Biotechnology Information; 1999. [Google Scholar]
- 22.Hemler M E, Elices M J, Chan B M, Zetter B, Matsuura N, Takada Y. Multiple ligand binding functions for VLA-2 (alpha 2 beta 1) and VLA-3 (alpha 3 beta 1) in the integrin family. Cell Differ Dev. 1990;32:229–238. doi: 10.1016/0922-3371(90)90035-u. [DOI] [PubMed] [Google Scholar]
- 23.Hemler M E, Huang C, Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987;262:3300–3309. [PubMed] [Google Scholar]
- 24.Hemler M E, Jacobson J G, Strominger J L. Biochemical characterization of VLA-1 and VLA-2. Cell surface heterodimers on activated T cells. J Biol Chem. 1985;260:15246–15252. [PubMed] [Google Scholar]
- 25.Hoshino Y, Jones R W, Kapikian A Z. Serotypic characterization of outer capsid spike protein VP4 of vervet monkey rotavirus SA11 strain. Arch Virol. 1998;143:1233–1244. doi: 10.1007/s007050050371. [DOI] [PubMed] [Google Scholar]
- 26.Hoshino Y, Saif L J, Kang S Y, Sereno M M, Chen W K, Kapikian A Z. Identification of group A rotavirus genes associated with virulence of a porcine rotavirus and host range restriction of a human rotavirus in the gnotobiotic piglet model. Virology. 1995;209:274–280. doi: 10.1006/viro.1995.1255. [DOI] [PubMed] [Google Scholar]
- 27.Kalica A R, Flores J, Greenberg H B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983;125:194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- 28.Kaljot K T, Shaw R D, Rubin D H, Greenberg H B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988;62:1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamata T, Puzon W, Takada Y. Identification of putative ligand binding sites within I domain of integrin alpha 2 beta 1 (VLA-2, CD49b/CD29) J Biol Chem. 1994;269:9659–9663. . (Erratum, 271:19008, 1996.) [PubMed] [Google Scholar]
- 30.Kamata T, Puzon W, Takada Y. Identification of putative ligand-binding sites of the integrin alpha 4 beta 1 (VLA-4, CD49d/CD29) Biochem J. 1995;305:945–951. doi: 10.1042/bj3050945. . (Erratum, 317:959, 1996.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassner P D, Hemler M E. Interchangeable alpha chain cytoplasmic domains play a positive role in control of cell adhesion mediated by VLA-4, a beta 1 integrin. J Exp Med. 1993;178:649–660. doi: 10.1084/jem.178.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi S, Hemler M E. Role of the alpha subunit cytoplasmic domain in regulation of adhesive activity mediated by the integrin VLA-2. J Biol Chem. 1993;268:16279–16285. [PubMed] [Google Scholar]
- 33.Knight C G, Morton L F, Onley D J, Peachey A R, Messent A J, Smethurst P A, Tuckwell D S, Farndale R W, Barnes M J. Identification in collagen type I of an integrin alpha2 beta1-binding site containing an essential GER sequence. J Biol Chem. 1998;273:33287–33294. doi: 10.1074/jbc.273.50.33287. [DOI] [PubMed] [Google Scholar]
- 34.Komoriya A, Green L J, Mervic M, Yamada S S, Yamada K M, Humphries M J. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J Biol Chem. 1991;266:15075–15079. [PubMed] [Google Scholar]
- 35.Li E, Stupack D, Klemke R, Cheresh D A, Nemerow G R. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loike J D, Sodeik B, Cao L, Leucona S, Weitz J I, Detmers P A, Wright S D, Silverstein S C. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proc Natl Acad Sci USA. 1991;88:1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludert J E, Feng N, Yu J H, Broome R L, Hoshino Y, Greenberg H B. Genetic mapping indicates that VP4 is the rotavirus cell attachment protein in vitro and in vivo. J Virol. 1996;70:487–493. doi: 10.1128/jvi.70.1.487-493.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackow E R, Barnett J W, Chan H, Greenberg H B. The rhesus rotavirus outer capsid protein VP4 functions as a hemagglutinin and is antigenically conserved when expressed by a baculovirus recombinant. J Virol. 1989;63:1661–1668. doi: 10.1128/jvi.63.4.1661-1668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackow E R, Shaw R D, Matsui S M, Vo P T, Dang M N, Greenberg H B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci USA. 1988;85:645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masumoto A, Hemler M E. Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem. 1993;268:228–234. [PubMed] [Google Scholar]
- 41.McCormick B A, Nusrat A, Parkos C A, D'Andrea L, Hofman P M, Carnes D, Liang T W, Madara J L. Unmasking of intestinal epithelial lateral membrane beta1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect Immun. 1997;65:1414–1421. doi: 10.1128/iai.65.4.1414-1421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendez E, Arias C F, Lopez S. Binding to sialic acids is not an essential step for the entry of animal rotaviruses to epithelial cells in culture. J Virol. 1993;67:5253–5259. doi: 10.1128/jvi.67.9.5253-5259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mendez E, Arias C F, Lopez S. Interactions between the two surface proteins of rotavirus may alter the receptor-binding specificity of the virus. J Virol. 1996;70:1218–1222. doi: 10.1128/jvi.70.2.1218-1222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mould A P, Humphries M J. Identification of a novel recognition sequence for the integrin alpha 4 beta 1 in the COOH-terminal heparin-binding domain of fibronectin. EMBO J. 1991;10:4089–4095. doi: 10.1002/j.1460-2075.1991.tb04985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connell P J, Faull R, Russ G R, D'Apice A J. VLA-2 is a collagen receptor on endothelial cells. Immunol Cell Biol. 1991;69:103–110. doi: 10.1038/icb.1991.16. [DOI] [PubMed] [Google Scholar]
- 46.Offit P A, Blavat G, Greenberg H B, Clark H F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986;57:46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasad B V, Wang G J, Clerx J P, Chiu W. Three-dimensional structure of rotavirus. J Mol Biol. 1988;199:269–275. doi: 10.1016/0022-2836(88)90313-0. [DOI] [PubMed] [Google Scholar]
- 48.Rodger S M, Schnagl R D, Holmes I H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975;16:1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolsma M D, Kuhlenschmidt T B, Gelberg H B, Kuhlenschmidt M S. Structure and function of a ganglioside receptor for porcine rotavirus. J Virol. 1998;72:9079–9091. doi: 10.1128/jvi.72.11.9079-9091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rott L S, Rose J R, Bass D, Williams M B, Greenberg H B, Butcher E C. Expression of mucosal homing receptor alpha4beta7 by circulating CD4+ cells with memory for intestinal rotavirus. J Clin Investig. 1997;100:1204–1208. doi: 10.1172/JCI119633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruiz M C, Abad M J, Charpilienne A, Cohen J, Michelangeli F. Cell lines susceptible to infection are permeabilized by cleaved and solubilized outer layer proteins of rotavirus. J Gen Virol. 1997;78:2883–2893. doi: 10.1099/0022-1317-78-11-2883. [DOI] [PubMed] [Google Scholar]
- 52.Sabara M, Gilchrist J E, Hudson G R, Babiuk L A. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J Virol. 1985;53:58–66. doi: 10.1128/jvi.53.1.58-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato K, Inaba Y, Shinozaki T, Fujii R, Matumoto M. Isolation of human rotavirus in cell cultures: brief report. Arch Virol. 1981;69:155–160. doi: 10.1007/BF01315159. [DOI] [PubMed] [Google Scholar]
- 54.Staatz W D, Fok K F, Zutter M M, Adams S P, Rodriguez B A, Santoro S A. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991;266:7363–7367. [PubMed] [Google Scholar]
- 55.Superti F, Donelli G. Characterization of SA-11 rotavirus receptorial structures on human colon carcinoma cell line HT-29. J Med Virol. 1995;47:421–428. doi: 10.1002/jmv.1890470421. [DOI] [PubMed] [Google Scholar]
- 56.Takada Y. CD49c Workshop Panel Report. In: Kishimoto T, Kikutani H, von dem Borne A E G K, Goyert S M, Mason D Y, Miyasaka M, Moretta L, Okumura K, Shaw S, Springer T A, Sugamura K, Zola H, editors. Leucocyte typing VI. New York, N.Y: Garland Publishing, Inc.; 1997. pp. 394–395. [Google Scholar]
- 57.Takada Y, Huang C, Hemler M E. Fibronectin receptor structures in the VLA family of heterodimers. Nature. 1987;326:607–609. doi: 10.1038/326607a0. [DOI] [PubMed] [Google Scholar]
- 58.Takada Y, Puzon W. Identification of a regulatory region of integrin beta 1 subunit using activating and inhibiting antibodies. J Biol Chem. 1993;268:17597–17601. [PubMed] [Google Scholar]
- 59.Vignoud L, Albiges-Rizo C, Frachet P, Block M R. NPXY motifs control the recruitment of the alpha5beta1 integrin in focal adhesions independently of the association of talin with the beta1 chain. J Cell Sci. 1997;110:1421–1430. doi: 10.1242/jcs.110.12.1421. [DOI] [PubMed] [Google Scholar]
- 60.Wasserman K, Subklewe M, Pothoff G, Banik N, Schell-Frederick E. Expression of surface markers on alveolar macrophages from symptomatic patients with HIV infection as detected by flow cytometry. Chest. 1994;105:1324–1334. doi: 10.1378/chest.105.5.1324. [DOI] [PubMed] [Google Scholar]
- 61.Wayner E A, Carter W G, Piotrowicz R S, Kunicki T J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988;107:1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weitzman J B, Pasqualini R, Takada Y, Hemler M E. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J Biol Chem. 1993;268:8651–8657. [PubMed] [Google Scholar]
- 63.Wen F Q, Jabbar A A, Patel D A, Kazarian T, Valentino L A. Atherosclerotic aortic gangliosides enhance integrin-mediated platelet adhesion to collagen. Arterioscler Thromb Vasc Biol. 1999;19:519–524. doi: 10.1161/01.atv.19.3.519. [DOI] [PubMed] [Google Scholar]
- 64.Williams M B, Rose J R, Rott L S, Franco M A, Greenberg H B, Butcher E C. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, alpha4beta7. J Immunol. 1998;161:4227–4235. [PubMed] [Google Scholar]
- 65.Yeager M, Berriman J A, Baker T S, Bellamy A R. Three-dimensional structure of the rotavirus haemagglutinin VP4 by cryoelectron microscopy and difference map analysis. EMBO J. 1994;13:1011–1018. doi: 10.1002/j.1460-2075.1994.tb06349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yeager M, Dryden K A, Olson N H, Greenberg H B, Baker T S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J Cell Biol. 1990;110:2133–2144. doi: 10.1083/jcb.110.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yednock T A, Cannon C, Vandevert C, Goldbach E G, Shaw G, Ellis D K, Liaw C, Fritz L C, Tanner L I. Alpha 4 beta 1 integrin-dependent cell adhesion is regulated by a low affinity receptor pool that is conformationally responsive to ligand. J Biol Chem. 1995;270:28740–28750. doi: 10.1074/jbc.270.48.28740. [DOI] [PubMed] [Google Scholar]