Abstract
Background
Drug provocation tests (DPT) are considered the gold standard procedure to ascertain the diagnosis of beta-lactam (BL) allergy. Regarding route of administration, current recommendations prioritize oral challenges, considering them safer, and reserving the intravenous route for drugs for which this is the only formulation.
Objective
To compare in terms of tolerance and safety two protocols of BL DPT, using an oral protocol (OR-DPT) and an intravenous protocol (IV-DPT).
Methods
A descriptive, retrospective study was performed, including adult patients who underwent IV-DPT or OR-DPT for suspected immediate or delayed hypersensitivity to BL antibiotics, over a period of 4 years (between January 2018 and December 2021). Demographical data, index hypersensivity reactions’ characteristics and tolerance to DPT were reviewed.
Results
A total of 1036 patients underwent DPT, mean age of 56.8 (standard deviation, SD, 17.8) years, 655 were women (63.2%). Immediate drug hypersensitivity reactions (DHR) had occurred in 564 of patients (54.4%). OR-DPT were performed in 439 (42.4%) and IV-DPT in 597 (57.6%). The frequency of reactions during DPT, regardless of the route used, was low (3.6%): only 16 (3.6%) in OR-DPT and 21 (3.5%) in IV-DPT. From IV-DPT, 16 out 21 DHR during DPT were immediate compared with 4 out of 16 in OR-DPT. Adjusted relative risk of developing a hypersensitivity reaction during IV-DPT versus OR-DPT was 1.13 (95% confidence interval (CI)0.57–2.22).
Conclusion
The results suggest that OR-DPT and IV-DPT are both safe procedures when adequately performed. However, IV-DPT protocols showed a higher rate of immediate DHR during DPT probably due to the selection of basal high-risk patients to undergo IV-DPT. In conclusion, IV-DPT may be considered as an option for challenges in drug-allergy studies, entailing a precise administration.
Keywords: Drug provocation test, Beta-lactam, Intravenous drug provocation test, Oral drug provocation test, Drug hypersensitivity reaction
Introduction
A drug provocation test (DPT) consists of the controlled administration of a suspected drug with the aim of confirming or ruling out hypersensitivity.1,2 Currently, DPT is considered the “gold standard” for the diagnosis of drug allergy.3 The European Network of Drug Allergy (ENDA) guidelines of the European Academy of Allergy and Clinical Immunology (EAACI)4,5 emphasize the importance of DPT in the establishment of a correct diagnosis in case of suspicion of beta-lactam (BL) hypersensitivity due to the risk of false positives with limited sensitivity and specificity with skin6, 7, 8 and in vitro9, 10, 11 testing. BL-allergy de-labelling is a cornerstone approach to avoid the unnecessary use of wide-spectrum antibiotics which is a major cause of antibiotic resistance.12
Protocols for DPT in BL allergy workup vary widely among studies in terms of steps, intervals between doses, increments and days of dosing as well as criteria for a positive result. In fact, a recent European survey13 showed significant heterogeneity in current practice, especially regarding DPT. Several factors have contributed to these differences: severity of the reaction, immediate or non-immediate reaction, population involved (adults or children) and the experience and resources available in clinics. Consensus is still lacking on whether DPT in BL hypersensitivity should be performed with escalating doses or a single dose or if the DPT should last one day or longer.14
According to the latest EAACI position paper,5 dosage for DPT ranges from 3 steps or fewer in mild immediate hypersensitivity reactions and non-immediate reactions,15, 16, 17, 18 or even a single dose,19 to schedules with dose escalating (4 or 5 steps) at the beginning with lower doses for high-risk patients with moderate to severe hypersensitivity reactions.20
Described intravenous DPT protocols (IV-DPT) usually follow the general published recommendations for oral DPT protocols (OR-DPT) in terms of number of steps, dosing escalating between steps and time interval between them, especially in severe hypersensitivity reactions, with variations in case of mild reactions.3,15, 16, 17, 18, 19, 20
Regarding the route of administration in DPT protocols, some authors recommend using the same route that elicited the index reaction. Moreover, in case of availability of an oral formulation of the drug, this route is sometimes recommended over the original route of administration.20,21 Taking into account these studies,5,20,21 the oral route has been the most accepted in DPTs, recommended in many guidelines.
Nevertheless, due to the risk during a DPT, the continuous intravenous administration using a high-precision pump could bring safety in terms of delivery control during such procedures, a reduction in the time required to complete DPT and could show a similar safety profile compared to the oral route. The aim of our study was to pose the possibility of using IV-DPT in selected situations, based on a retrospective comparison between patients that underwent OR-DPT and IV-DPT with different BL antibiotics.
Materials and methods
Patients
A descriptive, retrospective study was carried out, including all adult patients who underwent DPT (IV-DPT or OR-DPT) for suspected immediate or delayed hypersensitivity to BL antibiotics, over a period of 4 years (between January 2018 and December 2021) in our Allergy Section. The Hospital's Medical Research Committee approved the study.
Medical records were reviewed to determine if the index reaction was immediate (latency less than 1 h from the administration of the drug until the appearance of symptoms) or non-immediate (latency greater than 1 h) following the criteria published by the ENDA/EAACI.22,23 Immediate reactions were categorized according to severity using Brown's classification:24 Grade I or mild (symptoms limited to skin and subcutaneous tissue), Grade II or moderate (respiratory, cardiovascular, or gastrointestinal involvement), and Grade III or severe (hypoxia, hypotension or neurological involvement). Non-immediate reactions were classified according to their clinical characteristics as delayed urticaria, maculopapular eruptions, fixed drug eruptions, vasculitis, toxic epidermal necrolysis (TEN), and Stevens–Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP) or symmetrical drug-related intertriginous and flexural exanthema.25
Patients reporting symptoms not suggestive of an immune-mediated reaction (diarrhea, abdominal pain, cramps, paraesthesia, fungal mucous infection, vaginitis) were not considered for DPT and were not recorded in the present study.
In vitro testing
Specific immunoglobulin E (sIgE) against amoxicillin, penicilloyl G and penicillloyl V (ImmunoCAP ™, Thermo Fisher, Sweden) was determined when the reaction was recent (less than 6 months),26 the result was interpreted as positive if ≥ 0.35 KU/L.
Prick and intradermal testing
Skin tests (STs) were performed as recommended by the ENDA/EAACI drug allergy interest group.27 The reagents used were: the BL involved in the original reaction, benzylpenicilloyl polylysine (PPL, 5 x 10 −5M), minor determinant mixture (MDM, 2 x 10 −2M) (Diater, Madrid, Spain), penicillin G (10 000 UI/ml), amoxicillin AX, 20 mg/ml) (Glaxo Smithkline Beecham, Madrid, Spain), amoxicillin–clavulanic acid (AX-CLV, 20 mg/ml) (Glaxo Smithkline Beecham, Madrid, Spain), and cefuroxime (20 mg/ml) (Normon, Madrid, Spain). Prick test was considered positive if the wheal was ≥ 3 mm than the negative control. The intradermal test was positive if an increase of 3 mm in the diameter was observed when performing the immediate or non-immediate reading (48 h later).
Risk assessment
The determination of basal risk in patients was based on several key factors:
-
1.
Likelihood of the index reaction being immune-mediated.
-
2.
Severity and type (immediate or non-immediate) of the index reaction.
-
3.
Results of skin and/or in vitro tests.
-
4.
Presence of associated comorbidities, including severe ischemic cardiomyopathy, uncontrolled hypertension, neurologic stroke, chronic obstructive pulmonary disease, and diabetes.
-
5.
Medications taken by the patient, particularly beta-blockers or angiotensin-converting enzyme inhibitors, which could potentially lead to exposure or breakthrough reactions beyond medical control.
-
6.
The clinical judgment of the treating clinician.
These factors collectively informed the assessment of basal risk for each patient.
In our study, patients with immediate DHRs categorized as Grade I according to Brown's classification, or in cases where this classification was not applicable, those without severe comorbidities such as cardiovascular or neurological issues, with a good performance status, and a low likelihood of true allergy were classified as having a basal low risk. Furthermore, if their index reaction was not suggestive of anaphylaxis, if they experienced mild non-immediate reactions, and if both skin and in vitro tests yielded negative results, they fell into this low-risk category.
As the severity of DHRs increased, along with the presence of comorbidities, deterioration in performance status, positive results in skin or in vitro tests, and if there were reasonable doubts regarding their clinical history, the basal risk was elevated to moderate/high.
Drug provocation test
DPTs were performed with a BL, depending on sIgE and ST results.1 If the sIgE and the ST were negative with the culprit drug, the DPT was performed using the same drug to rule out allergy; on the contrary, if the previous tests were positive, the DPT was performed with an alternative BL,28 as long as allergy to the beta-lactam ring was not suspected. Beta-lactam ring sensitization was assessed according to PPL and MDM skin tests. If negative, we ruled out this possibility. In case of positivity, DPT was not performed and the patient was diagnosed as allergic to all beta-lactam antibiotics.
In recent years, several publications have demonstrated the safety of performing DPT without ST in mild non-immediate reactions,29, 30, 31, 32 therefore, in selected cases, the DPT was performed directly when the reaction was considered low risk and not suggestive of allergy. In cases in which the index reaction was a severe cutaneous adverse reaction (SCAR), the DPT was always performed with an alternative BL regardless of the result of the skin tests.33
Patients were informed on how to recognize the onset of a nonimmediate hypersensitivity reaction after the DPT and instructed to contact the department by phone or e-mail if a reaction occurred.
Route of administration
The administration of the drug could be oral or intravenous and the DPT was always performed under close medical supervision by the allergist, in the Day Care Hospital. Most DPT procedures, regardless of the route of administration (oral or intravenous), were conducted on an outpatient basis. However, for inpatients, the DPT was performed in hospitalization wards. All patients signed an informed consent prior to the study. Both protocols used for oral and IV challenges are in accordance with the recommendations of scientific societies3,19 and selected taking in to account the likelihood of immediate or non-immediate reaction and the severity as well as the other risk factors mentioned above.
The OR-DPT protocol was performed starting with the administration of 50% of the total dose of the antibiotic, the remaining 50% of the dose was administered after 30 min and the patient was discharged after 120 min if no reaction was observed. In the case of nonimmediate reactions, the treatment was continued for 5 days at home taking one single therapeutic dose daily of the suspected antibiotic (eg, amoxicillin 500 mg daily)20
Regarding IV-DPT, the drug was diluted in 100 ml of saline. The protocol consisted of the administration of the suspected or alternative drug at an initial rate of 10 ml per hour until completing 1% of the total dose, then the flow was increased to 50 ml per hour until reaching 10% of the dose and finally the rest of the medication was administered at maximum rate (according to the technical sheet of each medication), completing the procedure in approximately 40–50 min (Table 1). The intravenous route was prioritized mainly in patients considered as high risk,5 either by the original reaction characteristics (anaphylaxis, hypotension, dyspnea, etc), the ST results (positive to the BL involved) or due to personal risk of the patient (hypertension, ischemic heart disease, etc).
Table 1.
Intravenous drug provocation test protocol to beta-lactam antibiotics
| Step | Rate (mL/h) | Volume in ml (% of total dose) | Cumulative volume in ml (% of total dose) | Time (min) | Cumulative Time (min) |
|---|---|---|---|---|---|
| 1 | 10 | 1 (1%) | 1 (1%) | 6 | 6 |
| 2 | 50 | 8.3 (8.3%) | 9,3 (9.3%) | 10 | 16 |
| 3 | 200 | 90.7 (90.7%) | 100 (100%) | 27 | 43 |
Abbreviations: Example of IV-DPT for the administration of amoxicillin (1000 mg diluted in 100 ml of saline). The procedure could undergo minimal modifications according to the specifications in the technical data sheet of each drug, depending on the dilution volume, maximum administration speed, etc.
There was no previous established algorithm to choose one or other protocol. In general, low risk challenges were more likely to be oral, while higher risk cases underwent IV challenges, since an IV line was already in place as a security measure. Occasionally, the route of administration was chosen depending on the presentation available. For some drugs, the only route available was IV, for example some cephalosporins (ceftriaxone, ceftazidime, cefazolin, cefepime and cefoxitin), piperacillin-tazobactam, and monobactams.
If the original reaction was remote and highly suggestive of allergy, the study was repeated after 4 weeks with ST and DPT to rule out re-sensitization after the initial study.34 All repeated tests were performed using the same route. The data shown in this study regarding ST and DPT results correspond to the last ones that led to the final diagnosis.
Patient's monitoring
Patients were carefully monitored before the DPT, checking their general status and the stability of their chronic illness or comorbidities in order to prevent risk situations beyond medical control during DPT.
Patients’ vital signs (blood pressure, heart rate, oxygen saturation, and temperature) and pulmonary function tests (if patients had lung comorbidities) were routinely checked before starting DPT as well as before the administration of every dose, at the end of the procedure and in case of breakthrough reactions.
Treatment of breakthrough reactions
Reactions during DPT were treated according to symptoms and severity, using standard treatment with antihistamines, glucocorticoids, adrenaline, intravenous fluids, and bronchodilators if needed.35, 36, 37, 38
Interpretation of the results of the provocation
DPTs were considered positive if the index reaction was reproduced and congruent with allergy or if the patient suffered symptoms suggestive of hypersensitivity, either immediately or non-immediately (in the following hours or days).39,40 If allergy was confirmed, the BL was prohibited, and a detailed report was delivered explaining the alternative antibiotics that could be administered if required. When it was considered indicated, a DPT was performed with an alternative BL.
Statistical analysis
Descriptive results are presented as means and standard deviations for continuous variables and as frequencies and percentages for categorical variables. For comparisons, t-test was used for continuous variables and chi-square test for categorical ones. A trend test was also performed when a variable was of ordinal type.
The main comparison was made with the calculation of the relative risk of developing a hypersensitivity reaction during IV-DPT versus OR-DPT, with confidence interval (CI) at 95%. This was done in 2 ways: first, unadjusted, and then adjusting this comparison for differences in characteristics between the two groups. This adjustment, given the relatively small number of events of interest, was carried out by calculating a propensity score based on the variables age, sex, year of study, year of index reaction (before or after 2017), symptomatology of and drug related to the reaction. Inverse probability of treatment weighting (IPTW) method was then applied.
All of the analyses were performed with SAS® 9.4 (SAS Institute Inc., Cary, NC, USA). A p value < 0.05 was considered statistically significant.
The study was approved by the institutional Ethics Committee (IRB number: EOM (AG) 063/2021 (5911) with an exemption of the signed informed consent.
Results
Patients’ characteristics
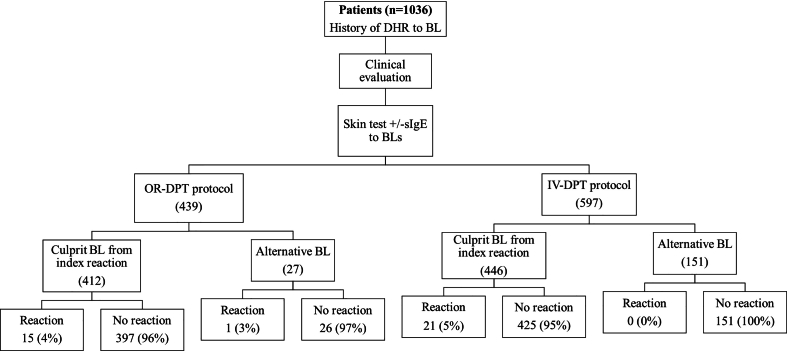
Between 2018 and 2021, 1036 patients underwent a DPT during the allergy workup to confirm or exclude hypersensitivity to BL antibiotics. The diagnostic flowchart and the distribution of DPT according to the administration route is shown in Fig. 1.
Fig. 1.
Diagnostic flowchart and distribution of DPT according to the route of administrationin the beta-lactam allergy assessment
The baseline characteristics of the patients according to the DPT protocol chosen, the index reactions and the drugs involved in them are summarized in Table 2A.
Table 2A.
Baseline characteristics of patients according to the DPT-protocol applied
| Oral n = 439 | IV n = 597 | Total DPT performed n = 1036 | p-value | |
|---|---|---|---|---|
| Sex | ||||
| Female | 293 (66.74%) | 362 (60.64%) | 655 (63.2%) | 0.044 |
| Male | 146 (33.26%) | 235 (39.36%) | 381 (36.8%) | |
| Age in years (mean, standard deviation) | 54.72 (18.36) | 58.37 (17.19) | 56.8 (17.8) | 0.001 |
| Time since index reaction (years) | ||||
| >5 | 317 (72.21%) | 381 (63.82%) | 698 (67.3%) | 0.004 |
| <5 | 122 (27.79%) | 216 (36.18%) | 338 (32.7%) | |
| Type of index reaction (% patients) | ||||
| Immediate | 164 (37.36%) | 400 (67%) | 564 (54.4%) | <0.001 |
| Nonimmediate | 198 (45.1%) | 116 (19.43%) | 314 (30.3%) | |
| Unknown | 77 (17.4%) | 81 (13.57%) | 158 (15.3%) | |
| Severity of index reaction (% patients) | ||||
| Immediate | ||||
| Mild (Brown I) | 134 (30.52%) | 230 (38.53%) | 364 (35.13%) | <0.001 |
| Moderate (Brown II) | 11 (2.51%) | 90 (15.08%) | 101 (9.74%) | |
| Severe (Brown III) | 1 (0.23%) | 58 (9.71%) | 59 (5.7%) | |
| Brown NA | 28 (6.37%) | 12 (2.01%) | 40 (3.8%) | <0.001 |
| Non-immediate | ||||
| Non-SCAR | 187 (59.93%) | 124 (20.77%) | 311 (30.0%) | <0.001 |
| SCAR | 1 (0.22%) | 0 (0%) | 1 (0.09%) | |
| Suspected BL involved in index reaction (% patients) | ||||
| Amoxicillin | 117 (26.65%) | 130 (21.78%) | 247 (23.8%) | <0.001 |
| AX-CLV | 92 (20.96%) | 142 (23.79%) | 234 (22.6%) | |
| Penicillin G | 92 (20.96%) | 121 (20.26%) | 213 (20.6%) | |
| Penicillin V | 21 (4.78%) | 27 (4.52%) | 48 (4.6%) | |
| Other BL | 15 (3.42%) | 81 (13.57%) | 96 (9.3%) | |
| Unknown | 102 (23.23%) | 96 (16.08%) | 198 (19.1%) |
Abbreviations: DHR, drug hypersensitivity reaction. DPT, drug provocation test. AX-CLV, amoxicillin-clavulanic acid. BL, beta-lactam. NA, not applicable. SCAR, severe cutaneous allergic reaction.
Of the 1036 patients included, 655 were women (63.2%) and the mean age was 56.8 (SD 17.8) years.
Of the 1036 provocations that were performed, 439 (42.4%) were OR-DPT and 597 (57.6%) IV-DPT.
The index drug hypersensitivity reaction (DHR) had occurred in the last 5 years in 32.7% of patients. 54.4% of patients were classified as having experienced an immediate DHR while in 30.3% of cases it was classified as a non-immediate reaction. In the remaining 15.3%, patients were unable to recall the time between the intake of the drug and the onset of symptoms. These 15.3% of patients were distributed in 77 OR-DPT (76 to culprit drug and 1 to alternative beta-lactam antibiotic) and 81 IV-DPT (80 to culprit drug and 1 to alternative beta-lactam antibiotic) according to the risk assessment.
The severity of the index reaction, when immediate, was severe in 5.7% (Brown's Grade III); 3.8% of patients did not fulfil criteria to be classified according to Brown's grades as they did not report systemic symptoms.
Regarding the index non-immediate reactions, almost all patients presented mild delayed symptoms, mostly as a maculopapular rash. The severe reactions recorded were consistent with Stevens-Johnson syndrome (1 case), generalized bullous fixed drug eruption (1), serum sickness like reaction/systemic vasculitis (1), and specific target organ toxicity (1). The most frequent BLs antibiotics involved in the index reaction were drugs belonging to the penicillin group: amoxicillin (23.8%), followed by amoxicillin-clavulanic acid (22.6%) and penicillin G (20.6%).
It can be noticed that in the case of moderate to severe index reactions (Brown II and III) the IV-DPT was preferred. Furthermore, there were significant differences between both groups (OR-DPT and IV-DPT) for the categories: time since index reaction, suspected BL involved in index reaction, and type of reaction.
Characteristics of patients who experienced reactions during DPT
The baseline conditions of patients who suffered reactions during DPT and the characteristics of the index DHR are summarized in Table 2B (reactions subgroup column).
Table 2B.
Comparison between baseline characteristics of patients who underwent DPT and those who presented a DHR during DPT
| Total DPT performed n = 1036 | Reactions during DPT subgroup n = 37 (3.6%) | |
|---|---|---|
| Sex | ||
| Female | 655 (63.2%) | 25 (67.6%) |
| Male | 381 (36.8%) | 12 (32.4%) |
| Age (years) | 56.8 | 50.5 |
| Time since index reaction (years) | ||
| >5 | 698 (67.3%) | 14 (37.8%) |
| <5 | 338 (32.7%) | 23 (62.2%) |
| Type of index reaction (% patients) | ||
| Immediate | 564 (54.4%) | 22 (59.4%) |
| Nonimmediate | 314 (30.3%) | 12 (32.4%) |
| Unknown | 158 (15.3%) | 3 (8.1%) |
| Severity of index reaction (% patients) | ||
| Immediate | ||
| Mild (Brown I) | 364 (35.13%) | 15 (40.5%) |
| Moderate (Brown II) | 101 (9.74%) | 4 (10.8%) |
| Severe (Brown III) | 59 (5.7%) | 2 (5.4%) |
| Brown NA | 40 (3.8%) | 1 (2.7%) |
| Non-immediate | ||
| Non-SCAR | 311 (30.0%) | 12 (32.4%) |
| SCAR | 1 (0.09%) | 0 (0%) |
| Suspected BL involved in index reaction (% patients) | ||
| Amoxicillin | 247 (23.8%) | 17 (45.9%) |
| AX-CLV | 234 (22.6%) | 11 (29.7%) |
| Penicillin G | 213 (20.6%) | 1 (2.7%) |
| Penicillin V | 48 (4.6%) | – |
| Other BL | 96 (9.3%) | 5 (13.5%) |
| Unknown | 198 (19.1%) | 3 (8.1%) |
Abbreviations: DHR, drug hypersensitivity reaction. DPT, drug provocation test. AX-CLV, amoxicillin-clavulanic acid. BL, beta-lactam. NA, not applicable. SCAR, severe cutaneous allergic reaction.
The frequency of reactions in general during DPT, regardless of the route used, was low, only 37 (3.6%) reactions were recorded after the 1036 procedures. Only 2 of them occurred after repeating the DPT in patients with remote and highly suggestive of allergy index reaction in whom re-assessment was necessary, probably due to a re-sensitization mechanism.
The mean age of patients who developed a reaction during the provocation was 50.5 (SD 16.8) years and 67.6% were women.
Recent index reactions (<5 years at the time of the study) were the most frequently related to reactions during DPT (62.2%). Patients with a history of having suffered the index reaction with amoxicillin or amoxicillin with clavulanic acid were the ones who most frequently presented adverse reactions during DPT, 45.9% and 29.7%, respectively.
The most frequent type of index reaction in this subgroup was immediate (22 cases, 59.4%). Regarding the severity of the reaction, there were 2 severe immediate reactions in the IV-DPT group (none of them lethal), requiring the use of antihistamines, systemic corticosteroids and intramuscular adrenaline; there were no severe immediate reactions in the OR-DPTs group (p = 0.307). Regarding delayed reactions, no severe reactions were observed in any of the administration routes.
Comparison between the 2 routes of administration
As shown in Fig. 1, the challenge was performed with an alternative drug 178 times (17.1%). Of those performed intravenously, 151 were with an alternative drug (25.3%) and 446 with the drug involved in the index reaction. The 21 reactions occurred with the drug of the index reaction. Regarding those carried out orally, 27 (6.1%) were with an alternative drug and 412 with the drug involved in the index reaction. Most of the reactions, 15, occurred during the provocation with the index drug, there was only 1 reaction using an alternative drug.
The reason for using an alternative drug was due to positive skin tests in 83 patients, positive sIgE in 14 patients and both in 8 patients. Almost all the DPT in these cases, where done intravenously (Table 3), due to the concern of high risk as mentioned in the risk assessment section. In the rest of the cases in which an alternative drug was used, it was due to a medical decision (need for a specific beta-lactam due to the type of infection or the results in the antibiogram or critical condition of the patient at the time of the test), or because some patients refused to receive the same drug again.
Table 3.
Results of skin test and specific Immunoglobulin E
| Positives | Median (KU/L) | Type of index reaction (immediate/non-immediate) | DPT route (intravenous/oral) | |
|---|---|---|---|---|
| sIgE Amoxicillin | 11 (1.0%) | 2.39 (0.37–11) | 11/0 | 11/0 |
| sIgE Penicilloyl G | 7 (0.67%) | 0.95 (0.37–2.34) | 6/1 | 7/0 |
| sIgE Penicillloyl V | 6 (0.57%) | 1.48 (0.50–2.42) | 5/1 | 6/0 |
| Skin test | 83 (8.0%) | 74/9 | 74/9 |
Abbreviations: sIgE Specific immunoglobulin E. DPT, drug provocation test.
Characteristics of the hypersensitivity reactions during DPT and distribution between administration routes are summarized in Table 4.
Table 4.
Characteristics of reactions during DPT according to its route of administration
| Oral n = 439 | Intravenous n = 597 | p-value | |
|---|---|---|---|
| Reaction during DPT | |||
| Yes (% patients) | 16 (3.64) | 21 (3.52) | 0.913 |
| No (% patients) | 423 (96.36) | 576 (96.48) | |
| Time since index reaction (years) | |||
| >5 | 5 (1.13) | 9 (1.5) | 0.86 |
| <5 | 10 (2.35) | 13 (2.17) | |
| Immediate hypersensitivity Reaction | |||
| Yes (% patients) | 4 (0.91) | 16 (2.68) | 0.041 |
| No (% patients) | 435 (99.09) | 581 (97.32) | |
| Severity of immediate Hypersensitivity reaction | |||
| Brown I | 4 (0.91) | 11 (1.84) | 0.333 |
| Brown II | 0 | 1 (0.17) | |
| Brown III | 0 | 2 (0.34) | |
| Non-immediate hypersensitivity Reaction | |||
| Yes (% patients) | 12 (2.73) | 5 (0.84) | 0.051 |
| No (% patients) | 427 (97.27) | 592 (99.16) | |
| Severity of non-immediate Hypersensitivity reaction | |||
| SCAR | 0 | 0 | 0.515 |
| Non-SCAR | 12 (2.73) | 5 (0.83) | |
| Culprit drug in DPT | |||
| Amoxicillin | 7 (1.59) | 10 (1.67) | |
| Amoxicillin-clavulanic acid | 8 (1.82) | 5 (0.83) | |
| Penicillin G | 0 | 1 (0.17) | |
| Piperacillin-tazobactam | 0 | 1 (0.17) | |
| Ceftriaxone | 0 | 1 (0.17) | |
| Cefazoline | 0 | 3 (0.5) | |
| Cefuroxime | 1 (0.22) | 0 |
Abbreviations: DPT, drug provocation test. SCAR, severe cutaneous allergic reaction.
A similar distribution of adverse events was observed between the 2 routes of administration: 16 reactions for the oral route (3.6%) and 21 for the intravenous route (3.5%), with no significant differences (p = 0.913).
Considering the time since the index reaction (<5 years or >5 years), we did not observe statistical differences between the route of administration (p = 0.86) but it is noticeable that the number of reactions during DPT were almost the double (OR-DPT: 5 to 10; IV-DPT 9 to13) when less than 5 years had passed since the index reaction.
Regarding the type of reactions, the immediate ones were more frequent when the DPT was performed intravenously compared to the oral route (p = 0.041): 4 out of 16 were observed in the oral route (0.91% of all OR-DPTs) and 16 out of 21 in the intravenous (2.68% of all IV-DPT). On the other hand, non-immediate reactions were more frequent when the DPT was performed orally (p = 0.051); 12 occurred after an OR-DPT and 5 after an IV-DPT. Most of the reactions were mild maculopapular eruptions, no SCAR was observed.
Whilst no statistically significant differences were found when comparing the severity of the reactions it was noticeable that there was a higher frequency of immediate reactions when the DPT was performed intravenously compared to the oral route. Cefuroxime was the only case of a BL chosen as an alternative that produced a hypersensitivity reaction during DPT. Challenges performed with cephalosporins and their results are specified in supplemental table 1. Regarding the other cases of reactions during DPT, they were produced by the same BL as in index reaction, being amoxicillin and amoxicillin with clavulanic acid the most frequent ones. When unadjusted, the relative risk of developing a hypersensitivity reaction during IV-DPT versus OR-DPT was 0.97 (95% CI 0.51–1.83). When the comparison was adjusted for the variables such as sex, year of study, time since index reaction, symptoms presented during index reaction, and culprit drug of the index reaction, the relative risk of having a hypersensitivity reaction during IV-DPT versus OR-DPT was 1.13 (95% CI 0.57–2.22).
Discussion
We present a large single centre study comparing oral (n = 439 patients) and intravenous (n = 597 patients) protocols of DPT, collecting data from 4 years (January 2018–December 2021) with an overall proportion of reactions during DPT of 3.6%.
As reported in the latest EAACI position paper,5 DPT protocols vary widely among studies and there is a lack of consensus on the most appropriate. Data regarding the use of different challenge routes is scarce. Nevertheless, the EAACI has validated 2 algorithms in an effort to standardize DPT.5,21 In these algorithms, we could find different approaches to perform DPT considering the baseline risk of the patient after performing a detailed clinical history, skin tests and in vitro tests if needed. These guidelines do not specify if the DPT should be performed through oral or intravenous route, however, it is assumed that the oral route is safer and therefore it is usually preferred. A recent guideline from the Spanish Society of Allergology and Clinical Immunology3 proposes a general protocol recommendation to perform DPT using different administration routes according to risk stratification. In both guidelines the target dose is specified as well as the dosing schedule.
It is generally accepted that to faithfully reproduce the conditions of original reactions, the same route of administration should be used for the DPT. However, the tendency is that if there is an oral formulation of the suspected BL, the DPT tends to be performed through this route but there are a lack of studies comparing oral and intravenous routes regarding safety, efficacy or time-consumption.18
In our study, we used an IV-DPT protocol based on continuous infusion divided in three steps with incremental infusion rates using a high precision pump. It was a different approach from previous studies,3,5 omitting the time of observation between increasing doses in favour of a continuous supervision of the procedure in order to identify the onset of drug reactions at low doses. It is important to note that there are some similarities between our IV-DPT protocol and those used to perform rapid drug desensitization (RDD), since in both the drug is usually administered intravenously and the infusion rate is progressively increased until the final dose is completed. This theoretical concern has been discussed previously.19 A study performed in vitro with an animal desensitization model of mast cells sensitized to house dust mites showed that the inhibition of mast cells was better with 2-fold concentration increases compared to 10-fold increases.41 Moreover, as shown in previous studies,42 in order to perform a RDD, two principles had to been followed: first, an initial starting dose around 1000–10,000th of the target dose and secondly there should be around 10–16 steps of approximately 2-fold–2.5-fold incremental doses of the drug antigen at fixed 15–30 min time intervals. We consider that using our IV-DPT protocol would not have a desensitizing effect, as the increase in infusion rate was higher than 2-fold and with time intervals between steps inferior to 15 min, not following the principles described. A long term follow up would be required to determine the possible risk of a false negative reaction during DPT when performed with the continuous IV protocol.
On the other hand, our OR-DPT protocol was divided in two steps in which the patient received 50% in each one. There are different OR-DPT regimens described in the literature, some starting with an initial dose of 1%, 5%, or 10% of the final dose,18 others 25%43 and even with a 100%19,44,45 single dose; all of them have proven to be safe when selecting patients according to risk and previous results of skin tests. We acknowledge that our two-step oral regimen, starting with a 50% dose, diverges from current practices favouring lower initial doses (10%). However, in our setting, it remains our preferred approach for low-risk patients with negative skin tests. We have found it to yield acceptable results in terms of safety and time efficiency.
OR-DPT protocols are widely used and have some advantages. Due to the ease of administration, they can be applicable in multiple settings and clinical contexts with less resources than those required to perform an IV-DPT. In addition, it is a non-invasive route of administration with the obvious advantages regarding the risk of phlebitis and infections.
In our study, IV-DPT and OR-DPT were conducted with the culprit drug from the index reaction in 74.7% (446 cases) and 94% (412 cases) respectively. While conducting DPT to alternative drugs when skin tests were positive aligns with current guidelines, this approach does not contribute significantly to enhancing our understanding of the positive predictive values of skin tests.
To address this gap in knowledge, specific studies with a prospective design are necessary. However, such studies must carefully consider ethical limitations due to the inherent risk of inducing a DHR. Balancing the need for advancing knowledge with the imperative to minimize patient risk will be crucial in designing and conducting these studies effectively.
The major result of our study is that there is no statistical difference between using OR-DPT or IV-DPT in terms of severity or frequency of reactions during DPT, even when adjusted for variables influencing the outcome.
The main advantages of using IV-DPT are related with risk management. The use of a high precision pump to perform an intravenous continuous perfusion allows us to control the exact dose of drug that the patient is receiving at any moment. In addition, it allows halting the procedure at the onset of a DHR, preventing further absorption of the drug, which cannot be interrupted in OR-DPT protocols due to the variable and continuous rate of absorption at the gastrointestinal tract. This difference is relevant especially in patients with severe original DHR which are at risk of recurrence of the reaction with low doses of drug. Also, it must be highlighted that it is less time-consuming mainly due to the suppression of observation time between steps.
In addition, IV-DPT is less time consuming than OR-DPT, which entails an improvement for the patient in terms of less work absenteeism and for the health care resources due to early discharge, thus improving the use of our Day Care Hospital. Overall, there was a low rate of DHR during DPT in this study, and more frequent when the index reaction took place in the past 5 years (62.2%), being consistent with previous published data regarding the loss of sensitivity with time.34,46 Moreover, most reactions were immediate (54%) and mild (75%).
There are several limitations in this study. First, although this study included 1036 drug provocations tests, it is a single centre study. In addition, this is a retrospective study and although both protocols were used routinely in our unit, there was a tendency to select high-risk patients to undergo IV-DPT instead of OR-DPT thus lacking randomization. Also, we are aware that some centres, due to their characteristics, may lack the required premises or equipment to perform IV-DPT.
As it can be inferred by the results, in case of moderate to severe index reactions, there was a clear disproportion in the number of patients that underwent IV-DPT protocols versus OR-DPT. Regarding the rate of DHR during DPT, some considerations must be appraised. Although the difference in the rate of DHR during DPT between both protocols did not reach statistical significance, a higher frequency occurred in the IV-DPT group that could be partially explained due to the selection bias of patients with more severe index reactions to receive the IV-DPT protocol.
Two patients developed an immediate severe DHR during the IV-DPT that required the administration of intramuscular adrenaline. In the first case, the patient suffered an anaphylactic shock with mucocutaneous involvement and hypotension during the infusion that was solved after supporting vital actions as well as treatment with adrenaline. In the second case, the patient suffered from a neoplastic disease with a bad performance status that required an allergological study. After the correct risk stratification considering the negativity of skin tests, an IV-DPT was performed. During the infusion, the patient presented isolated hypotension with diaphoresis without skin, gastrointestinal tract, and respiratory involvement and with good response to fluids and adrenaline. This patient was also classified as a severe immediate DHR during IV-DPT, despite the differential diagnosis was inconclusive, since tryptase did not raise and he recurrently developed similar reactions not related to medications.
We must stress that data regarding the comparison between both routes of administration in terms of severity of breakthrough reactions has to be taken cautiously due to unequal patient selection. In addition, the dose escalating of our OR-DPT protocol should be considered as a non-graded oral challenge. Thus, an ideal comparison design would involve using regimens which are both graded, or both not graded.
When considering the protocols chosen for OR-DPT and IV-DPT, it is evident that they deviate from the conventional approach outlined in most published guidelines,3,5 particularly in terms of initial dosage and observation intervals between steps. For OR-DPT, the decision to commence with 50% of the complete dose was based on careful patient stratification, identifying them as low-risk for DHR during DPT. Within this patient cohort, the probability of true hypersensitivity was deemed low. Therefore, rather than conducting a provocation test for diagnostic purposes, the objective was to ensure drug tolerance through the procedure.19 Conversely, IV-DPT was selected due to the capability in our setting to administer continuous infusion under direct supervision by the allergist and nursing team.
The negative result in DPT diagnoses the patients as non-allergic thus does not have much interest because any route of administration would be safe in these patients. In non-immediate reactions due to DPT there won't be differences in terms of safety and tolerability of the procedure due to the type of the DHR. In case of immediate reactions during DPT, only in 16 cases the DPT was performed with beta-lactam antibiotics that could be administered through both routes. Consequently, the low rate of DHR during DPT makes it difficult to make definitive assertions regarding the use of one protocol or another.
In conclusion, our study points out that IV-DPT seems to be safe, effective and well tolerated in a selected population of patients as OR-DPT. Considering its advantages, it could be a good choice in those patients at higher risk of having a DHR during the allergy assessment as well as in other cohorts of patients depending on the possibilities of the centre. In order to establish definitive assertions prospective randomized studies would be needed.
Abbreviations
AGEP, Acute generalized exanthematous pustulosis; AX, Amoxicillin; AX-CLV, Amoxicillin–clavulanic acid; PPL, Penicilloyl polylysine; BL, Beta-lactam; DHR, Drug hypersensitivity reaction; DPT, Drug provocation test; CI, Confidence interval; DRESS, Drug reaction with eosinophilia and systemic symptoms; ENDA, European Network of Drug Allergy; EAACI, European Academy of Allergy and Clinical Immunology; IV-DPT, Intravenous DPT protocol; MDM, Minor determinant mixture; NA, Not applicable; OR-DPT, Oral DPT protocol; RDD, Rapid drug desensitization; SCAR, Severe cutaneous adverse reaction; ST, Skin test; sIgE, Specific immunoglobulin E; SJS, Stevens-Johnson syndrome; TEN, Toxic epidermal necrolysis.
Funding
This paper has not received any funding.
Availability of data and materials
Our dataset has not been published in publicly repositories.
Statement of contribution of authors
GJMM, MGD, XVG and VC contributed to study conception, design and drafting of the manuscript and had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All the authors have participated in the clinical data collection, data analysis and interpretation and also, they have read and approved the final version of the manuscript and consented for its publication.
Ethics statement
The study was approved by the institutional Ethics Committee (IRB number: EOM (AG) 063/2021 (5911) with an exemption of the signed informed consent.
Submission declaration
The authors declare this manuscript is original, has not been published before, is not currently being considered for publication elsewhere, and has not been posted to a preprint server.
Declaration of competing interest
Gustavo-Jorge Molina-Molina MD: The author report no competing interests.
Manuel Gómez-Duque MD: The author report no competing interests.
Xavier Vidal Guitart MD PhD: The author report no competing interests.
Antònia Agustí Escasany MD PhD: The author report no competing interests.
Moisés Labrador-Horrillo MD PhD: The author report no competing interests.
Olga Luengo MD PhD: The author report no competing interests.
Anna Sala-Cunill MD PhD: The author report no competing interests.
Paula Galván Blasco MD: The author report no competing interests.
Mar Guilarte MD PhD: The author report no competing interests.
Victoria Cardona MD PhD: The author report no competing interests.
Acknowledgments
This research would not have been possible without the collaboration of our patients as well as the Pharmacology Department from Hospital Universitari Vall d’Hebron.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2024.100914.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Cephalosporin's drug provocation test (DPT).
References
- 1.Demoly P., Adkinson N.F., Brockow K., et al. International consensus on drug allergy. Allergy. 2014;69(4):420–437. doi: 10.1111/all.12350. [DOI] [PubMed] [Google Scholar]
- 2.Aberer W., Bircher A., Romano A., et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58(9):854–863. doi: 10.1034/j.1398-9995.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 3.Audicana Berasategui M.T., Ortega N., Lobera T., et al. Spanish society of allergology and clinical immunology (Seaic) vision of drug provocation tests. J Investig Allergol Clin Immunol. 2021;31(5):385–403. doi: 10.18176/jiaci.0681. [DOI] [PubMed] [Google Scholar]
- 4.Joint Task Force on Practice Parameters; American Academy of Allergy Asthma and immunology; American college of allergy, asthma and immunology; joint council of allergy, asthma and immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Romano A., Atanaskovic-Markovic M., Barbaud A., et al. Towards a more precise diagnosis of hypersensitivity to beta-lactams - an EAACI position paper. Allergy. 2020 Jun;75(6):1300–1315. doi: 10.1111/all.14122. [DOI] [PubMed] [Google Scholar]
- 6.Caubet J.C., Frossard C., Fellay B., Eigenmann P.A. Skin tests and in vitro allergy tests have a poor diagnostic value for benign skin rashes due to β-lactams in children. Pediatr Allergy Immunol. 2015;26(1):80–82. doi: 10.1111/pai.12314. [DOI] [PubMed] [Google Scholar]
- 7.Padial A., Antunez C., Blanca-Lopez N., et al. Non-immediate reactions to beta-lactams: diagnostic value of skin testing and drug provocation test. Clin Exp Allergy. 2008;38(5):822–828. doi: 10.1111/j.1365-2222.2008.02961.x. [DOI] [PubMed] [Google Scholar]
- 8.Blanca-López N., Zapatero L., Alonso E., et al. Skin testing and drug provocation in the diagnosis of nonimmediate reactions to aminopenicillins in children. Allergy. 2009;64(2):229–233. doi: 10.1111/j.1398-9995.2008.01903.x. [DOI] [PubMed] [Google Scholar]
- 9.Blanca M. Allergic reactions to penicillins. A changing world? Allergy. 1995;50(10):777–782. doi: 10.1111/j.1398-9995.1995.tb05048.x. [DOI] [PubMed] [Google Scholar]
- 10.Blanca M., Vega J.M., Garcia J., et al. Allergy to penicillin with good tolerance to other penicillins; study of the incidence in subjects allergic to beta-lactams. Clin Exp Allergy. 1990;20(5):475–481. doi: 10.1111/j.1365-2222.1990.tb03139.x. [DOI] [PubMed] [Google Scholar]
- 11.Terrados S., Blanca M., Garcia J., et al. Nonimmediate reactions to betalactams: prevalence and role of the different penicillins. Allergy. 1995;50(7):563–567. doi: 10.1111/j.1398-9995.1995.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 12.Mabilat C., Gros M.F., Van Belkum A., et al. Improving antimicrobial stewardship with penicillin allergy testing: a review of current practices and unmet needs. JAC Antimicrob Resist. 2022;4(6) doi: 10.1093/jacamr/dlac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres M.J., Celik G.E., Whitaker P., et al. A EAACI drug allergy interest group survey on how European allergy specialists deal with β-lactam allergy. Allergy. 2019;74(6):1052–1062. doi: 10.1111/all.13721. [DOI] [PubMed] [Google Scholar]
- 14.Torres M.J., Adkinson N.F., Jr., Caubet J.C., et al. Controversies in drug allergy: beta-lactam hypersensitivity testing. J Allergy Clin Immunol Pract. 2019;7(1):40–45. doi: 10.1016/j.jaip.2018.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Romano A., Gaeta F., Valluzzi R.L., et al. Diagnosing nonimmediate reactions to cephalosporins. J Allergy Clin Immunol. 2012;129(4):1166–1169. doi: 10.1016/j.jaci.2011.12.995. [DOI] [PubMed] [Google Scholar]
- 16.Picard M., Paradis L., Bégin P., Paradis J., Des Roches A. Skin testing only with penicillin G in children with a history of penicillin allergy. Ann Allergy Asthma Immunol. 2014;113(1):75–81. doi: 10.1016/j.anai.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Macy E., Ngor E.W. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258–263. doi: 10.1016/j.jaip.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Chiriac A.M., Rerkpattanapipat T., Bousquet P.J., Molinari N., Demoly P. Optimal step doses for drug provocation tests to prove beta-lactam hypersensitivity. Allergy. 2017;72(4):552–561. doi: 10.1111/all.13037. [DOI] [PubMed] [Google Scholar]
- 19.Khan D.A., Banerji A., Blumenthal K.G., et al. Drug allergy: a 2022 practice parameter update. J Allergy Clin Immunol. 2022;150(6):1333–1393. doi: 10.1016/j.jaci.2022.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Fransson S., Mosbech H., Kappel M., et al. The importance of prolonged provocation in drug allergy - results from a Danish allergy clinic. J Allergy Clin Immunol Pract. 2017;5(5):1394–1401. doi: 10.1016/j.jaip.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Messaad D., Sahla H., Benahmed S., Godard P., Bousquet J., Demoly P. Drug provocation tests in patients with a history suggesting an immediate drug hypersensitivity reaction. Ann Intern Med. 2004;140(12):1001–1006. doi: 10.7326/0003-4819-140-12-200406150-00009. [DOI] [PubMed] [Google Scholar]
- 22.Torres M.J., Blanca M., Fernandez J., et al. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy. 2003;58(10):961–972. doi: 10.1034/j.1398-9995.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 23.Romano A., Blanca M., Torres M.J., et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59(11):1153–1160. doi: 10.1111/j.1398-9995.2004.00678.x. [DOI] [PubMed] [Google Scholar]
- 24.Brown S.G.A. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371–376. doi: 10.1016/j.jaci.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Blanca M., Romano A., Torres M.J., et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009;64(2):183–193. doi: 10.1111/j.1398-9995.2008.01916.x. [DOI] [PubMed] [Google Scholar]
- 26.Sousa-Pinto B., Tarrio I., Blumenthal K.G., et al. Accuracy of penicillin allergy diagnostic tests: a systematic review and meta-analysis. J Allergy Clin Immunol. 2021;147(1):296–308. doi: 10.1016/j.jaci.2020.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brockow K., Garvey L.H., Aberer W., et al. Skin test concentrations for systemically administered drugs - an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68(6):702–712. doi: 10.1111/all.12142. [DOI] [PubMed] [Google Scholar]
- 28.Caruso C., Valluzzi R.L., Colantuono S., Gaeta F., Romano A. β-lactam allergy and cross-reactivity: a clinician's guide to selecting an alternative antibiotic. J Asthma Allergy. 2021; Jan;18(14):31–46. doi: 10.2147/JAA.S242061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Confino-Cohen R., Rosman Y., Meir-Shafrir K., et al. Oral challenge without skin testing safely excludes clinically significant delayed-onset penicillin hypersensitivity. J Allergy Clin Immunol Pract. 2017;5(3):669–675. doi: 10.1016/j.jaip.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Kuruvilla M., Thomas J. Direct oral amoxicillin challenge without antecedent penicillin skin testing in low-risk patients. Ann Allergy Asthma Immunol. 2018;121(5):627–628. doi: 10.1016/j.anai.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Tucker M.H., Lomas C.M., Ramchandar N., Waldram J.D. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of marine recruits. J Allergy Clin Immunol Pract. 2017;5(3):813–815. doi: 10.1016/j.jaip.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Garvey L.H., Savic L.C. Drug provocation testing: risk stratification is key. Curr Opin Allergy Clin Immunol. 2019;19(4):266–271. doi: 10.1097/ACI.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 33.Ardern-Jones M.R., Mockenhaupt M. Making a diagnosis in severe cutaneous drug hypersensitivity reactions. Curr Opin Allergy Clin Immunol. 2019;19(4):283–293. doi: 10.1097/ACI.0000000000000546. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg A., Confino-Cohen R. Skin testing and oral penicillin challenge in patients with a history of remote penicillin allergy. Ann Allergy Asthma Immunol. 2008;100(1):37–43. doi: 10.1016/S1081-1206(10)60402-4. [DOI] [PubMed] [Google Scholar]
- 35.Laguna J.J., Archilla J., Doña I., et al. Practical guidelines for perioperative hypersensitivity reactions. J Investig Allergol Clin Immunol. 2018;28(4):216–232. doi: 10.18176/jiaci.0236. [DOI] [PubMed] [Google Scholar]
- 36.Sheikh A., Ten Broek V., Brown S.G.A., Simons F.E.R. H1-antihistamines for the treatment of anaphylaxis: cochrane systematic review. Allergy. 2007;62(8):830–837. doi: 10.1111/j.1398-9995.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 37.Choo K.J.L., Simons E., Sheikh A. Glucocorticoids for the treatment of anaphylaxis: cochrane systematic review. Allergy. 2010;65(10):1205–1211. doi: 10.1111/j.1398-9995.2010.02424.x. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh A., Shehata Y.A., Brown S.G.A., Simons F.E.R. Adrenaline for the treatment of anaphylaxis: cochrane systematic review. Allergy. 2009;64(2):204–212. doi: 10.1111/j.1398-9995.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 39.Ibáñez M.D., Rodríguez Del Río P., Lasa E.M., et al. Prospective assessment of diagnostic tests for pediatric penicillin allergy: from clinical history to challenge tests. Ann Allergy Asthma Immunol. 2018;121(2):235–244. doi: 10.1016/j.anai.2018.05.013. e3. [DOI] [PubMed] [Google Scholar]
- 40.Hein U.R., Chantraine-Hess S., Worm M., Zuberbier T., Henz B.M., Henz B.M. Evaluation of systemic provocation tests in patients with suspected allergic and pseudoallergic drug reactions. Acta Derm Venereol. 1999;79(2):139–142. doi: 10.1080/000155599750011372. [DOI] [PubMed] [Google Scholar]
- 41.Picard M., Caiado J., Giavina-Bianchi P., Castells M. A new humanized in vitro model of IgE-mediated rapid desensitization. Clin Transl Allergy. 2014;4 [Google Scholar]
- 42.Alvarez-Cuesta E., Madrigal-Burgaleta R., Broyles A.D., et al. Standards for practical intravenous rapid drug desensitization & delabeling: a WAO committee statement. World Allergy Organ J. 2022;15(6) doi: 10.1016/j.waojou.2022.100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zambonino M.A., Corzo J.L., Muñoz C., et al. Diagnostic evaluation of hypersensitivity reactions to beta-lactam antibiotics in a large population of children. Pediatr Allergy Immunol. 2014;25(1):80–87. doi: 10.1111/pai.12155. [DOI] [PubMed] [Google Scholar]
- 44.Li J., Cvetanovski V., Fernando S. Single-step direct drug provocation testing is safe for delabelling selected non-low-risk penicillin allergy labels. Ann Allergy Asthma Immunol. 2021;127(2):232–235. doi: 10.1016/j.anai.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Iammatteo M., Blumenthal K.G., Saff R., Long A.A., Banerji A. Safety and outcomes of test doses for the evaluation of adverse drug reactions: a 5-year retrospective review. J Allergy Clin Immunol Pract. 2014 Nov-Dec;2(6):768–774. doi: 10.1016/j.jaip.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Matheu V., Perez-Rodriguez E., Sanchez-Machin I., Garcia-Robaina J.C., de La Torre Morin F. Importance of repeat testing in the diagnosis of penicillin allergy. Br J Dermatol. 2006;154(1):198. doi: 10.1111/j.1365-2133.2005.07027.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cephalosporin's drug provocation test (DPT).
Data Availability Statement
Our dataset has not been published in publicly repositories.